Abstract
Avian influenza viruses are a source of genetic material that can be transmitted to humans through direct introduction or reassortment. Although there is a wealth of information concerning global monitoring for antiviral resistance among human viruses of the N1 and N2 neuraminidase (NA) subtypes, information concerning avian viruses of these and other NA subtypes is limited. We undertook a surveillance study to investigate the antiviral susceptibility of avian influenza N6 NA viruses, the predominant subtype among wild waterfowl. We evaluated 73 viruses from North American ducks and shorebirds for susceptibility to the NA inhibitor oseltamivir in a fluorescence-based NA enzyme inhibition assay. Most (90%) had mean IC50 values ranging from <0.01 to 5.0 nM; 10% were from 5.1 to 50.0 nM; and none were >50.0 nM. Susceptibility to oseltamivir remained stable among all isolates collected over approximately three decades (P≤0.74). Two isolates with I222V NA substitution had moderately reduced susceptibility to oseltamivir in vitro (IC50, 30.0 and 40.0 nM). One field sample was a mixed population containing an avian paramyxovirus (APMV) and H4N6 influenza virus, as revealed by electron microscopy and hemagglutination inhibition assays with a panel of anti-APMV antisera. This highlights the importance of awareness and careful examination of non-influenza pathogens in field samples from avian sources. This study showed that oseltamivir-resistant N6 NA avian influenza viruses are rare, and must be tested both phenotypically and genotypically to confirm resistance.
Keywords: avian N6 subtype, natural reservoir, neuraminidase inhibitors, resistance, oseltamivir
1. Introduction
Influenza A viruses are segmented negative-sense RNA viruses that have established waterfowl as their reservoir host (Webster et al. 1992). These viruses are divided into 16 hemagglutinin (HA) and 9 neuraminidase (NA) subtypes (Fouchier et al. 2005). The NAs are each further divided into two groups based on their genetic relatedness: group-1 NAs (N1, N4, N5, and N8) and group-2 NAs (N2, N3, N6, N7, and N9) (Russell et al. 2006). Surveillance of influenza in North America (Krauss et al. 2004) and in Europe (Fouchier et al. 2005; Munster et al. 2005) has established the immense diversity of influenza A virus subtypes that are found among wild aquatic birds and has revealed the importance of mallard ducks, gulls, and shorebirds in the perpetuation of all known subtypes of influenza A viruses. These studies have been of great importance by revealing the role of genetic material from avian viruses in influenza virus evolution and the emergence of pandemics in humans, lower animals, and domestic poultry. Genes arising from the avian pool can reassort and cross over to other species (Castrucci et al. 1993). Evidence of this occurred with the human pandemic 2009 H1N1 virus, in which the NA gene was an avian-like NA of Eurasian swine genetic lineage (Cohen, 2009; Garten et al. 2009). Most influenza subtypes are found in the mallard duck (Anas platyrhynchos), which has been implicated as a major host and source of influenza A viruses (Kim et al. 2010).
Monitoring the susceptibility of human influenza viruses to available anti-influenza drugs (M2 blockers amantadine and rimantadine and NA inhibitors [NAIs] oseltamivir and zanamivir) is a part of surveillance studies. Very little has been done concerning monitoring the antiviral susceptibility of influenza viruses isolated from their natural reservoirs, although these viruses can reassort and potentially enter the human population. It is the continuous evolution of influenza viruses in the natural aquatic reservoir that results in the random acquisition of NA mutations that can affect NAI susceptibility or even potentially lead to drug resistance. In addition, the genetic stability of functional sites in the NA gene can differ in different NA subtypes (Russell et al. 2006). Currently, continued monitoring of antiviral susceptibility among influenza viruses isolated from waterfowl should be considered an important part of surveillance.
NAIs have been designed based on the N9 NA structure (Varghese and Colman, 1991; von Itzstein et al. 1993). Recent reports suggest that group-1 NAs contain an extra cavity, named the 150 cavity, in the catalytic site and have an open conformation (Li et al. 2010; Russell et al. 2006). Group-2 NAs (such as N6 NA) lack this 150 cavity and have a closed conformation (Li et al 2010; Russell et al. 2006). Research suggests that these NA structural differences affect oseltamivir binding (Collins et al. 2009). NAIs have been tested and shown to be active against all 9 NA subtypes in vitro; however, only a limited number of strains were tested in each NA subtype from avian origin (Govorkova et al. 2001; Gubareva et al. 1995) until recently in our studies (Stoner et al. 2010). In that study we evaluated the susceptibilities of N1 NAs from 123 influenza viruses obtained from wild waterfowl and domestic swine to NAIs and sequenced the NAs of the identified outliers. Currently, there is a lack of information concerning the NAI susceptibility and confirmation of molecular markers of resistance in viruses of NA subtypes other than those detected in humans (e.g., viruses of N1 and N2 NA subtypes).
The prevalence of different HA and NA subtypes of influenza A viruses varies among aquatic birds, and one of the more prevalent subtypes among influenza viruses isolated from wild ducks is the H4N6 subtype (21.9%), followed by the H1N4 (18.8%) and H10N7 (16.7%) subtypes during 2001 to 2006 in North America (Krauss et al. 2007). Here, we studied avian influenza viruses of the N6 NA subtype isolated from 1976 to 2010 from North American ducks and shorebirds for their susceptibility to oseltamivir in fluorescence-based NA inhibition assay. In addition, we provide cautionary insight regarding mixed field virus samples when using traditional influenza screening of isolates obtained from wild aquatic birds and testing NAI susceptibility.
2. Materials and methods
2.1. NAIs
Oseltamivir carboxylate (oseltamivir; [3R,4R,5S]-4-acetamido-5-amino-3-[1-ethylpropoxy]-1-cyclohexane-1 carboxylic acid) was provided by Hoffmann-La Roche (Basel, Switzerland). Zanamivir (4-guanidino-Neu5Ac2en) was provided by the GlaxoSmithKline (Research Triangle Park, NC). Peramivir ([1S,2S,3R,4R,1’S]-3-[1′-acetylamino-2′-ethyl]butyl-4-[(aminoimino)-methyl]amino-2-hydroxycyclopentane-1-carboxylic acid) was provided by BioCryst Pharmaceuticals (Birmingham, AL). The compounds were dissolved in distilled water, and aliquots of stock were stored at −20°C until used.
2.2. Viruses and cells
The 73 avian influenza viruses of the N6 NA subtype were obtained from the St. Jude Children’s Research Hospital influenza repository and the University of Georgia (Table 1). All isolates were from Canada and the United States. Viruses were isolated from 1976 to 2010 from apparently healthy wild waterfowl and were isolated from fecal samples or from oropharyngeal or cloacal swabs obtained from Anseriformes (Ducks), Anas platyrhynchos (Mallard), Anas acuta (Pintail), Anas dícors (Blue-wing Teal), Aythya valisineria (Canvasback), and Bucephala albeola (Bufflehead), and from Charadriiformes (shorebirds), Arenaria intepres (Ruddy Turnstone) and Larus argentatus (Herring Gull). The HA subtypes of isolates used in this study were H2, H3, H4, H6, and H13. Virus stocks were cultivated from the original archived stocks for each virus by passaging once in 10-day-old embryonated chicken eggs. These virus stocks were frozen at −80°C until used.
TABLE 1.
Susceptibility of avian influenza A viruses of the N6 NA subtype to the NAI oseltamivir.
| N6 NA influenza viruses | Subtype | Year of isolation | Origin | Mean IC50 ± SD (nM) b |
|---|---|---|---|---|
| Isolated from ducks a | ||||
| A/Canvasback Duck/Alberta/102/1976 | H3N6 | 1976 | Canada | 0.35±0.08 |
| A/Mallard Duck/Alberta/100/1976 | H3N6 | 1976 | Canada | 0.04±0.09 |
| A/Mallard Duck/Alberta/22/1976 | H3N6 | 1976 | Canada | 0.06±0.12 |
| A/Mallard Duck/Alberta/44/1976 | H3N6 | 1976 | Canada | 1.26±0.83 |
| A/Mallard Duck/Alberta/97/1976 | H3N6 | 1976 | Canada | <0.01c |
| A/Mallard Duck/Alberta/20/1976 | H4N6 | 1976 | Canada | 0.11±0.08 |
| A/Mallard Duck/Alberta/37/1976 | H4N6 | 1976 | Canada | 0.11±0.22 |
| A/Pintail Duck/ Alberta/1/1976 | H4N6 | 1976 | Canada | 0.05±0.13 |
| A/Pintail Duck/Alberta/53/1976 | H4N6 | 1976 | Canada | 7.4±0.42 |
| A/Pintail Duck/Alberta/211/1979 | H4N6 | 1979 | Canada | 0.09±0.22 |
| A/Pintail Duck/Alberta/358/1979 | H3N6 | 1979 | Canada | <0.01 |
| A/Mallard Duck/Minnesota/1041/1980 | H6N6 | 1980 | USA | 1.28±0.05 |
| A/Mallard Duck/Alberta/145/1981 | H4N6 | 1981 | Canada | 0.68±0.71 |
| A/Mallard Duck/Alberta/635/1985 | H6N6 | 1985 | Canada | 0.2±0.28 |
| A/Blue-wing Teal Duck/Alberta/888/1985 | H4N6 | 1985 | Canada | 0.7±0.1 |
| A/Mallard Duck/Alberta/697/1985 | H4N6 | 1985 | Canada | 5.33±0.99 |
| A/Pintail Duck/Alberta/623/1985 | H4N6 | 1985 | Canada | 0.05±0.07 |
| A/Mallard Duck/Alberta/17/1989 | H4N6 | 1989 | Canada | 0.28±0.04 |
| A/Mallard Duck/Alberta/131/1991 | H2N6 | 1991 | Canada | 0.13±0.01 |
| A/Mallard Duck/Alberta/132/1991 | H2N6 | 1991 | Canada | <0.01 |
| A/Mallard Duck/Alberta/135/1991 | H2N6 | 1991 | Canada | 0.25±0 |
| A/Mallard Duck/Alberta/25/1992 | H3N6 | 1992 | Canada | 0.14±0.01 |
| A/Mallard Duck/Alberta/35/1992 | H3N6 | 1992 | Canada | 5.32±0.2 |
| A/Mallard Duck/Alberta/183/1992 | H4N6 | 1992 | Canada | 0.12±0.01 |
| A/Mallard Duck/Alberta/119/2000 | H4N6 | 2000 | Canada | 0.06±0.08 |
| A/Mallard Duck/Alberta/35/2001 | H4N6 | 2001 | Canada | 1.28±0.08 |
| A/Pintail Duck/Alberta/269/2001 | H4N6 | 2001 | Canada | 4.35±0.21 |
| A/Mallard Duck/Alberta/76/2002 | H3N6 | 2002 | Canada | 3.90±0.16 |
| A/Pintail Duck/Alberta/166/2003 | H3N6 | 2003 | Canada | 7.52±0.37 |
| A/Mallard Duck/Alberta/237/2003 | H4N6 | 2003 | Canada | 0.10±0.13 |
| A/Mallard/Alberta/33/2004 | H4N6 | 2004 | Canada | 0.35±0.08 |
| A/Mallard Duck/Alberta/201/2005 | H4N6 | 2005 | Canada | 0.41±0.12 |
| A/Mallard Duck/Alberta/205/2005 | H4N6 | 2005 | Canada | 0.27±0.15 |
| A/Pintail Duck/Alberta/66/2005 d | H4N6 | 2005 | Canada | 2.10±0.01 |
| A/Blue-wing Teal Duck/Alberta/376/2007 | H3N6 | 2007 | Canada | 0.04±0.09 |
| A/Mallard Duck/Minnesota/Sg-000185/2007 | H3N6 | 2007 | USA | 1.18±0.01 |
| A/Bufflehead Duck/Alberta/418/2007 | H4N6 | 2007 | Canada | 0.47±0.08 |
| A/Northern Pintail Duck/Alberta/335/2007 | H4N6 | 2007 | Canada | 2.37±0.1 |
| A/Blue-wing Teal Duck/Minnesota/Sg-00899/2008 | H4N6 | 2008 | USA | 0.13±0.02 |
| A/Mallard Duck/Alberta/121/2008 | H4N6 | 2008 | Canada | 0.03±0.07 |
| A/Mallard Duck/Alberta/572/2008 | H4N6 | 2008 | Canada | 0.11±0 |
| A/Mallard Duck/Minnesota/Sg-00569/2008 | H4N6 | 2008 | USA | 0.12±0.01 |
| A/Mallard Duck/Minnesota/Sg-00971/2008 | H4N6 | 2008 | USA | 0.42±0.04 |
| A/Mallard Duck/Minnesota/Sg-01051/2008 | H4N6 | 2008 | USA | 0.12±0.01 |
| A/Northern Pintail Duck/Minnesota/Sg-00898/2008 | H4N6 | 2008 | USA | 0.15±0.01 |
| A/Mallard Duck/Minnesota/AI10-1708/2010 | H13N6 | 2010 | USA | <0.01 |
| Overall duck isolates | 1.07±1.93 | |||
|
| ||||
| Isolated from shorebirds/gulls a | ||||
| A/Gull/Maryland/684/1977 | H3N6 | 1977 | USA | <0.01 |
| A/Gull/MA/18/1980 | H13N6 | 1980 | USA | <0.01 |
| A/Gull/MA/50/1980 | H13N6 | 1980 | USA | 0.28±0.05 |
| A/Gull/Minnesota/ 945/1980 | H13N6 | 1980 | USA | 0.83±0.04 |
| A/Herring Gull/Delaware/660/1988 | H13N6 | 1988 | USA | 30.00±0.01 |
| A/Herring Gull/Delaware/665/1988 | H4N6 | 1988 | USA | 40.00±0.01 |
| A/Herring Gull/New Jersey/136/1990 | H3N6 | 1990 | USA | 1.28±0.27 |
| A/Herring Gull/New Jersey/144/1990 | H3N6 | 1990 | USA | 0.16±0.04 |
| A/Gull/New Jersey/34/1992 | H13N6 | 1992 | USA | 0.14±0.05 |
| A/Gull/New Jersey/48/1992 | H13N6 | 1993 | USA | 0.22±0.11 |
| A/Ruddy Turnstone/New Jersey/AI01-1407/2001 | H13N6 | 2001 | USA | <0.01 |
| A/Shorebird/Delaware/231/2001 | H13N6 | 2001 | USA | <0.01 |
| A/Ruddy Turnstone/New Jersey/1321394/2005 | H3N6 | 2005 | USA | 0.12±0 |
| A/Shorebird/Delaware/518/2005 | H3N6 | 2005 | USA | 0.30±0.39 |
| A/Ruddy Turnstone/New Jersey/1321394/2005 | H3N6 | 2005 | USA | 1.48±0.01 |
| A/Ruddy Turnstone/New Jersey/Sg-00502/2008 | H4N6 | 2008 | USA | 6.70±0.01 |
| A/Shorebird/Delaware/606/2008 | H4N6 | 2008 | USA | 0.23±0.27 |
| A/Shorebird/Delaware/309/2008 | H4N6 | 2008 | USA | 0.13±0.01 |
| A/Ruddy Turnstone/New Jersey/Sg-00551/2008 | H6N6 | 2008 | USA | 0.1±0.1 |
| A/Ring-billed Gull/Minnesota/AI0-1784/2010 | H13N6 | 2010 | USA | <0.01 |
| A/Ring-billed Gull/Minnesota/AI0-1791/2010 | H13N6 | 2010 | USA | 1.13±0.13 |
| A/Ring-billed Gull/Minnesota/AI0-1820/2010 | H13N6 | 2010 | USA | 1.29±0.08 |
| A/Ring-billed Gull/Minnesota/AI0-1829/2010 | H13N6 | 2010 | USA | 0.49±0.34 |
| A/Ring-billed Gull/Minnesota/AI10-1725/2010 | H13N6 | 2010 | USA | 0.86±0.71 |
| A/Ring-billed Gull/Minnesota/AI10-1738/2010 | H13N6 | 2010 | USA | 1.01±0.96 |
| A/Ring-billed Gull/Minnesota/AI10-1778/2010 | H13N6 | 2010 | USA | 0.19±0.01 |
| A/Ring-billed Gull/Minnesota/AI10-1765/2010 | H13N6 | 2010 | USA | 0.06±0.08 |
| Overall shorebird/gull isolates | 3.22±10.30 | |||
Avian influenza viruses were all from apparently healthy wild birds and were isolated from fecal samples or from tracheal/oropharyngeal or cloacal swabs.
NA inhibition assay used MUNANA as substrate at a final concentration of 100 μM. Values are the mean of two or three independent determinations.
Values from 0.005 to 0.01 were included in calculating the IC50 values.
A/Pintail Duck /Alberta/66/2005 (H4N6) influenza virus that was sensitive to oseltamivir was counted in the total number of pintail isolates after it was purified out.
2.3. NA activity and NA inhibition assay
NA activity of the avian viruses was measured in a fluorescence-based assay using the fluorogenic substrate 2’-(4-methylumbelliferyl)-α-D-N-acetylneuraminic acid (MUNANA) (Sigma-Aldrich, St Louis, MO) (Potier et al., 1979). Fluorometric determinations were quantified with a Synergy 2 multi-mode microplate reader (BioTek Instruments, Winooski, VT) based on the release of the fluorescent product 4-methyl-umbelliferone using excitation and emission wavelengths of 360 and 460 nm, respectively. Viruses were standardized to equivalent NA enzyme activity in the linear range of the curve and incubated with an NAI at concentrations of 0.00005 to 5 μM. The concentration of NAI that reduced NA activity by 50% relative to a control mixture with no NAI (IC50) was determined by plotting the percent inhibition of NA activity as a function of the compound concentrations calculated using GraphPad Prism 4 software (GraphPad Software, La Jolla, CA). IC50 values were reported as the means of 2-3 independent determinations.
2.4. Sequencing
The RNeasy kit (Qiagen, Chatsworth, CA) was used to extract viral RNA. Universal primers to the N6 NA and M2 genes of influenza virus (Hoffmann et al. 2001) and specific RT-nested primers (FIP1, FOP2 and FOP1, FOP2) to Newcastle disease virus (NDV) (Kho et al. 2000) were used for amplification. A one-step reverse transcription PCR kit was used to generate cDNA, and PCR products were extracted and purified from a 1% agarose gel (QIAquick gel extraction and One-Step RT-PCR kits; Qiagen Inc., Valencia, CA). Sanger sequencing reactions were performed by the Hartwell Center for Bioinformatics and Biotechnology at St. Jude Children’s Research Hospital.
2.5. Hemagglutination inhibition assay
Eleven chicken post-infectious sera to reference avian paramyxoviruses (APMV) and a positive reference control antigen (B-propiolactone inactivated P/Mallard Duck/Alberta/331/88) were used. APMV sera were treated with receptor-destroying enzyme (Accurate Chemical and Scientific Corp., Westbury, NY), heat-inactivated at 56°C for 30 min, and tested by hemagglutination inhibition assay with 0.5% chicken erythrocytes according to standard procedures (Palmer et al. 1975).
2.6. Electron microscopy
Egg-grown mixed field samples of virus were prepared by fixation with 1.25% glutaraldehyde-PBS prior to negative staining with 2% phosphotungstic acid and imaged under transmission electron microscopy.
2.7. Statistical analysis
Differences in mean IC50 values were detected using the non-parametric Kruskal-Wallis rank sum test. This test is distribution-free and minimizes the influence of extreme values in a statistical analysis. Box-and-whisker plots were used to identify any extreme values and to graphically depict groups of influenza viruses of the N6 NA subtype isolated from different years. The mean IC50 value is indicated on the y-axis instead of log10IC50 values. Log10IC50 values were not used in the box-and-whisker plots because higher or extreme values of (20.0-40.0 nM) would be obscured, and none of the values reached as high as 100.0 nM. The box is bounded by the 25th and 75th percentiles. The line in the middle of the box is the median. The interquartile range (IQR), calculated as the difference between the 75th and 25th percentile, was used to determine the length of the whiskers. The whiskers are either the minimum (or maximum) value of the data point or 1.5 times the IQR below (or above) the 25th (or 75th) percentile, whichever is smaller.
3. Results
3.1. Oseltamivir susceptibility among N6 NA subtype
We examined antiviral susceptibility among 73 influenza viruses of the N6 NA subtype isolated from the two main groups of migratory waterfowl (ducks and gulls/shorebirds) in the phenotypic NA enzyme inhibition assay (Table 1). Influenza viruses were isolated from North America from different avian species: 31 strains from Mallard Duck, 10 strains from Pintail Duck, 3 strains from Blue-winged Teal, 1 strain from Bufflehead Duck, and 1 strain from Canvasback Duck, 9 strains from shorebirds, and 18 strains from gulls. Currently there are no generally accepted criteria for the definition of antiviral resistance to NAIs based on phenotypic assays; therefore, we used baseline mean IC50 values determined for oseltamivir among avian influenza viruses of the N1 NA subtype: high susceptibility (mean IC50<5.0 nM), moderate susceptibility (mean IC50 = 5.1-50.0 nM), and reduced susceptibility (mean IC50> 50.0 nM) (Stoner et al. 2010). Most of the viruses tested (90%, N=66) had mean IC50 values that were <5.0 nM; 10% (N=6) had mean IC50 values = 5.1-50.0 nM; and none were >50.0 nM (Table 1). The mean IC50 among avian viruses of the N6 NA subtype ranged from <0.01 to 7.5 nM for duck isolates and from <0.01 to 1.5 nM for gull/shorebird viruses. Comparison of the oseltamivir susceptibility of viruses isolated from ducks and shorebirds revealed no significant difference (P<0.69) in mean IC50 values (1.07 nM for ducks and 3.22 nM for gulls/shorebirds) (Fig. 1A-C). However, we identified two gull isolates (A/Herring Gull/Delaware/660/1988 [H13N6] and A/Herring Gull/Delaware/665/1988 [H4N6]) that had mean IC50 values of 30.0 nM and 40.0 nM, respectively. These two isolates possessed 10- to 13-fold greater IC50 values than the other gull/shorebird isolates tested and thus exhibited moderately reduced susceptibility to oseltamivir in vitro (Fig. 2B). These isolates were highly susceptible to zanamivir, with mean IC50 values of 1.7 nM for A/Herring Gull/Delaware/660/1988 (H13N6) and 1.3 nM for A/Herring Gull/Delaware/665/1988 (H4N6) viruses.
FIG.1.
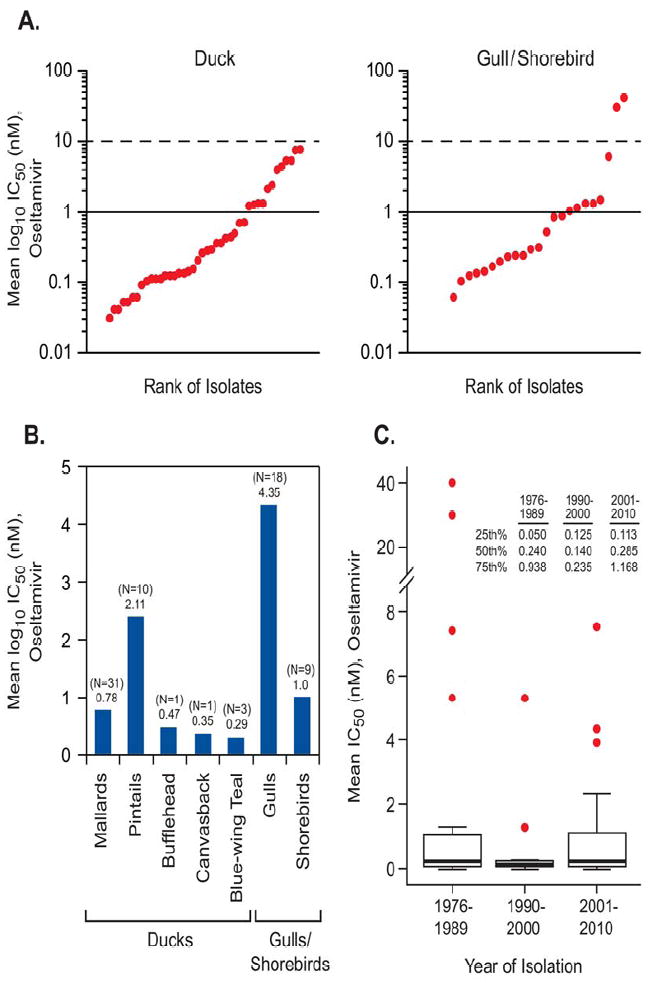
Plots showing the IC50 (nM) ranges of oseltamivir for avian influenza viruses of the N6 subtype isolated from various species of aquatic birds and tested by NAI assay. In panel A isolates are ranked in order by the IC50. Forty-six duck and 27 gull and shorebird isolates were analyzed by the Kruskal-Wallis rank sum test. Panel B shows the number of isolates collected and tested for each group (N = number of isolates) and analyzed by their mean IC50 value (below the N number of isolates). The Kruskal-Wallis test comparing the two groups (ducks and gulls/shorebirds) showed their mean IC50 values (ducks: 1.07 nM; gulls/shorebirds: 3.22 nM) were not significantly different (P ≤ 0.69). Panel C shows quantile box plots illustrating the mean IC50 values for oseltamivir for all avian viruses isolated in different years. The range of isolation years is shown on the X axis. Viruses collected were from various wild birds from the United States and Canada. Results within the graph were analyzed by the Kruskal-Wallis test. No statistical difference was observed among viruses isolated in different years (P<0.74).
FIG. 2.
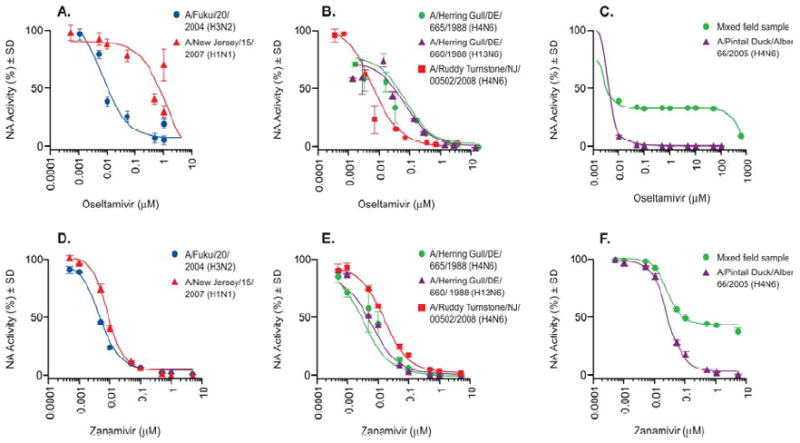
Oseltamivir susceptibility of reference influenza viruses and N6 NA isolates with the I222V NA mutation. Panels A and D show reference human influenza viruses that are known to be sensitive (
 ) or resistant (
) or resistant (
 ) to oseltamivir. Reference panel control virus A/Fukui/20/2004 (H3N2) was susceptible to both oseltamivir and zanamivir, and A/New Jersey/15/2007 (H1N1) was oseltamivir-resistant but zanamivir-sensitive. Panels B and E show A/Herring Gull/Delaware/665/1988 (H4N6) (
) to oseltamivir. Reference panel control virus A/Fukui/20/2004 (H3N2) was susceptible to both oseltamivir and zanamivir, and A/New Jersey/15/2007 (H1N1) was oseltamivir-resistant but zanamivir-sensitive. Panels B and E show A/Herring Gull/Delaware/665/1988 (H4N6) (
 ) and A/Herring Gull/Delaware/660/1988 (H13N6) (
) and A/Herring Gull/Delaware/660/1988 (H13N6) (
 ) which are both moderately sensitive to oseltamivir. Panel E shows A/Herring Gull/ Delaware/665/1988 (H4N6) (
) which are both moderately sensitive to oseltamivir. Panel E shows A/Herring Gull/ Delaware/665/1988 (H4N6) (
 ) and A/Herring Gull/Delaware/660/1988 (H13N6) (
) and A/Herring Gull/Delaware/660/1988 (H13N6) (
 ), which both were highly susceptible to zanamivir. A control wild type virus, A/Ruddy Turnstone/ New Jersey /00502/2008 (H4N6) (
), which both were highly susceptible to zanamivir. A control wild type virus, A/Ruddy Turnstone/ New Jersey /00502/2008 (H4N6) (
 ) was run as a control (no I222V NA mutation) and is shown in Panels B and E. Panels C and F show the susceptibility of mixed field samples to NAIs oseltamivir and zanamivir. Panels C and F show the mixed field sample (
) was run as a control (no I222V NA mutation) and is shown in Panels B and E. Panels C and F show the susceptibility of mixed field samples to NAIs oseltamivir and zanamivir. Panels C and F show the mixed field sample (
 ), which was oseltamivir- and zanamivir-resistant, and A/Pintail Duck/Alberta/66/2005 (H4N6) isolate (
), which was oseltamivir- and zanamivir-resistant, and A/Pintail Duck/Alberta/66/2005 (H4N6) isolate (
 ), which was oseltamivir- and zanamivir-sensitive.
), which was oseltamivir- and zanamivir-sensitive.
3. 2. Analysis of oseltamivir susceptibility over time and HA subtype
Of particular interest was whether the data over time would reveal a significant increase in the number of isolates with a mean IC50 value outside the interquartile range or, alternatively, whether a significant increase in the mean IC50 over time would be evident. Viruses originating in North America were analyzed by year of isolation to determine whether NAI susceptibility had varied over the 34 years between 1976 and 2010 (Fig. 1C). Statistical analysis revealed that the medians for each of the decades of isolation were as follows: 1976 to 1989 = 0.24, 1990-2000 = 0.14, and 2001-2010 = 0.29. Compared with the other isolates, IC50 values of 30.0 and 40.0 nM (A/Herring Gull/ Delaware Delaware/660/1988 [H13N6] and A/Herring Gull/Delaware/665/1988 [H4N6]) are much higher and are real differences and not outliers. We accounted for extreme values by using the nonparametric Kruskal-Wallis test. Thus, nearly all avian isolates remained highly susceptible to oseltamivir over time with no significant difference in IC50 values between the isolation years among any of the aquatic birds. The differences between years of isolation did not show evidence of an upward linear trend but remained stable from year to year (P<0.74). In this study, we wanted to address the question of whether different combinations of HA subtype would reveal a trend of HA subtype correlated to the IC50 value of oseltamivir among the avian isolates. All 16 HA subtypes have been isolated from wild aquatic birds (Krauss et al. 2004; Munster et al. 2006), and their functions are related to the HA/NA balance (Kaverin et al. 1998; Wagner et al. 2002).
3.3. Sequence analysis
Currently, the NAI resistance–associated molecular markers for influenza viruses of the N6 NA subtype are unknown and have not been confirmed by phenotypic NA enzyme inhibition assay. We analyzed 256 sequences of the N6 NA subtype from the NCBI database and from the St. Jude Children’s Research Hospital database and screened for the presence of mutations encoding amino acid substitutions at key residues shown to confer resistance to NAIs in the N1 and N2 NA subtypes. We found that all NA catalytic and framework residues were conserved among influenza viruses isolated from North America, with the exception of the two gull isolates (A/Herring Gull/ Delaware/660/1988 [H13N6] and A/Herring Gull/Delaware/665/1988 [H4N6]). These two isolates possess the I222V NA mutation (I222V, N2 numbering; and I223V, N1 numbering). The I222V NA mutation has been shown to confer reduced susceptibility of human influenza viruses of the N1 and N2 NA subtypes to oseltamivir in phenotypic assays (Baz et al. 2006; CDC, 2009; Pizzorno et al. 2011).
3.4. Susceptibility of mixed field virus sample to NAIs
In the course of examining the susceptibility of wild bird isolates to NAIs, one sample was surprisingly resistant to oseltamivir, as well as to zanamivir and peramivir, with mean IC50 of ≥500.0 μM (Fig. 2C, F). The pattern of the IC50 curve that was observed with this field sample was much different than that of known oseltamivir-resistant A/New Jersey/15/2007 (H1N1) human influenza virus (Fig. 2A, D). Additionally, both A/Fukui/20/2004 (H3N2) and A/New Jersey/15/2007 (H1N1) viruses yielded sigmoid-shaped inhibition curves and were susceptible to zanamivir, while the mixed field sample appeared to be unaffected and again exhibited an unusual inhibition curve. Electron microscopic examination revealed two populations of virions in this virus field sample. One population contained classic morphologies of influenza virus, which is “kidney bean,” and the other contained a “herring bone” coiled genome that is characteristic of APMV (S1). We did not detect NDV using sequence analysis and specific RT-nested primers (FIP1, FOP2 and FOP1, FOP2) to NDV. Seven of 11 APMV strain-specific antisera were positive in HI assays with titers of 640–1280 in comparison with a positive reference antigen used (P/Mallard Duck/Alberta/331/1988), whose HI titer was 1280. Due to cross-reactivity between different serotypes of APMV, we were unable to determine to which group of APMV the virus belonged. We conducted two passages on limiting dilutions in the presence of APMV antisera followed by plaque purification in MDCK cells and isolated the influenza A/Pintail Duck/Alberta/66/2005 (H4N6) virus that was susceptible to oseltamivir, zanamivir, and peramivir. Thus, we identified a mixed virus population containing proportions of susceptible H4N6 influenza virus and APMV.
4. Discussion
Ongoing surveillance of known and novel mutations involved in NAI susceptibility of human influenza viruses is an informative aspect of risk assessment for a pandemic. Little is known about avian influenza viruses whose genetic material is the primary reservoir of novel influenza viruses. It is possible that influenza viruses harbored by aquatic birds can develop resistance to NAIs by acquisition of naturally occurring mutations during virus evolution, allowing them to infect humans and result in the emergence of a pandemic strain. The overall results of our study showed that avian influenza viruses of the N6 NA subtype are highly susceptible to the NAI oseltamivir and that natural resistance is rare among this subtype, although we did detect two viruses in both ducks and shorebirds that had mean IC50 values (>10.0 nM, 3%) that were higher than all other isolates tested. This suggests that if larger and diverse sample populations of isolates were collected from more birds and tested, then a wider distribution of NAI susceptibility and resistance could be detected. We found that shorebirds/ gulls had the highest mean IC50 values among the waterfowl tested and oseltamivir susceptibility did not change over time (P≤0.74).
Previous studies have shown that NA-dependent resistance varies with NA subtype and that mutations in NA may produce different resistant phenotypes (Gubareva et al. 2001; 2004). Different drug-resistant NA mutant viruses have arisen after oseltamivir treatment of humans infected with viruses containing either N1 or N2 NA subtypes (Aoki et al. 2007; Baz et al. 2006). Oseltamivir-resistant N6 NA viruses have not been identified to date, and there are no functionally defined genetic markers for oseltamivir resistance among influenza viruses of the N6 NA subtype. Our sequence analysis verified an I222V NA residue change in two virus isolates (A/Herring Gull/Delaware/660/1988 and A/Herring Gull/Delaware/665/1988). Phenotypic analysis showed that these two viruses had a ~6-fold higher mean IC50 value than overall N6 NA viruses and thus can be classified as moderately susceptible to oseltamivir based on cutoff values (5.1-50 nM) previously described (Stoner et al. 2010). Interestingly, substitutions of I to R, K or V, at the 222 NA residue of human H1N1 2009 viruses confer moderately reduced susceptibilities to both zanamivir and oseltamivir but, when combined with the H275Y NA substitution, cause even higher levels of resistance to oseltamivir and peramivir than the H275Y substitution alone (Deyde et al. 2010; Hurt et al. 2009; Pizzorno et al. 2011). The genotypic assay was applied for the detection of previously characterized resistance-conferring mutations in avian influenza viruses (Orozovic et al. 2011). Among 5490 avian NA sequences from the NCBI database and from a European bird observatory, 6 of 55 influenza viruses analyzed of the N6 NA subtype (10.91%) had the NA catalytic residue change R152K, and 2 viruses (4%) had the I222V NA framework mutation (Orozovic et al. 2011). The researchers based their conclusions on the potential role of NA mutations in the resistance phenotype only on genetic analysis but did not confirm this resistance by NA enzyme inhibition assay. This demonstrates that evaluation of NAI susceptibility must be based on both phenotypic and genotypic assays.
Oseltamivir phosphate is a prodrug that is extensively metabolized in the human liver to oseltamivir carboxylate the active form of the drug. The active form is excreted and cannot be removed from waste water by sewage treatment (Fick et al. 2007). Therefore, oseltamivir carboxylate can enter the local aquatic environment in areas where oseltamivir is used for therapeutic use, such as Japan, where it is frequently used to treat seasonal influenza. There is some concern that wild birds harboring influenza A viruses can come into contact with waterways and sewage containing water enriched with anti-influenza drugs, where drug selection can occur and create resistant viruses (Fick et al. 2007; Ghosh et al. 2010a; Ghosh et al. 2010b; Sacca’ et al. 2009; Singer et al. 2007; Söderström et al. 2009). However, to date, there has been no change in the NAI susceptibility of either N1 (Stoner et al., 2010) or N6 in influenza viruses from wild birds after the introduction of the NAIs, at least in the United States.
Many pathogens, such as bacteria, viruses, and mycoplasma, possess NA activity and can be isolated from wild birds. In testing the antiviral susceptibility of avian field samples, it is important to be aware that non-influenza pathogens can be mistakenly identified as NAI-resistant influenza viruses. The R292K NA mutation in H3N2 influenza viruses is the only NA mutation that has been shown to cause cross-resistance to oseltamivir, zanamivir, and peramivir (Aoki et al. 2007). Therefore, if the mean IC50 values for an influenza virus are exceptionally high for all three NAIs, a detailed characterization must be done to determine whether it is in fact an influenza virus. Our finding highlights a trap for the unwary when screening NAI susceptibility among viruses isolated from wild aquatic birds. What appears to be a resistant influenza virus may in fact be a mixed virus population containing a new circulating APMV strain that traditional primers will not detect. Depending on the APMV strain in a given sample, this can be a problem, because traditional influenza screening by PCR, serotyping with available antisera, or other methods such as a rapid chromatographic immunoassay for influenza viral antigen (BD, Sparks, MD) may not detect the unsuspected presence of APMV or other non-influenza pathogens (Miller et al. 2010; Wang et al. 1999; Warke et al. 2008). Field samples that display these features can be tested for other non-influenza pathogens, imaged by electron microscopy or molecular identification techniques.
From our study, we conclude that avian influenza viruses carrying molecular markers for resistance are more likely to arise from natural fluctuations in point mutations or reassortment events, but these are rare. It is unknown what advantage avian influenza viruses with these mutations have in nature. The fact that they appear infrequently suggests that resistance is a rare event and thus is not maintained in influenza viruses isolated from their natural aquatic reservoir. Potential causes of these mutations include natural differences in conformation between group-1 and group-2 NA structures, phylogenetic stability, and environmental exposure to oseltamivir inducing a drug selection pressure. Our study did confirm that identification of molecular markers for resistance in the NA genes of influenza viruses isolated from wild aquatic birds is not sufficient for the evaluation of NAI susceptibility among these viruses, such as the N6 NA subtype. All genetic markers for resistance need to be carefully validated with the phenotypic functional assays before being used to categorize viruses. Moreover, in view of the interest in avian viruses as progenitors of new human pandemics, the information is of essential value to the influenza community; further sampling and monitoring of drug-resistant viruses among the avian species is required.
Supplementary Material
First report antiviral susceptibility of avian influenza viruses of N6 NA subtype.
Isolates with molecular marker for oseltamivir resistance moderately susceptible.
Avian influenza viruses of N6 NA subtype highly susceptible to the NAI oseltamivir.
Natural resistance is rare among the N6 subtype.
Acknowledgments
This study was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, contract number HSN266200700005C, and by the American Lebanese Syrian Associated Charities. We thank John Franks for technical assistance, Marie Gramer for helpful advice, David Galloway for scientific editing, Betsy Williford for graphic assistance, Cell and Tissue Imaging Shared Resource, and James Knowles for administrative assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoki FY, Boivin G, Roberts NA. Influenza virus susceptibility and resistance to oseltamivir. Antivir Ther. 2007;12:603–616. [PubMed] [Google Scholar]
- Baz M, Abed Y, McDonald J, Boivin G. Characterization of multidrug- resistant influenza A/H3N2 viruses shed during 1 year by an immunocompromised child. Clin Infect Dis. 2006;43(12):1555–1561. doi: 10.1086/508777. [DOI] [PubMed] [Google Scholar]
- Castrucci MR, Donatelli I, Sidoli L, Barigazzi G, Kawaoka Y, Webster RG. Genetic reassortment between avian and human influenza A viruses in Italian pigs. Virology. 1993;193(1):503–506. doi: 10.1006/viro.1993.1155. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis-North Carolina, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:969–972. [PubMed] [Google Scholar]
- Cohen J. Swine Flu Outbreak New Details on Virus’s Promiscuous Past. Science. 2009;324(5931):1127. doi: 10.1126/science.324_1127. [DOI] [PubMed] [Google Scholar]
- Collins PJ, Haire LF, Lin YP, Liu J, Russell RJ, Walker PA, Martin SR, Daniels RS, Gregory V, Skehel JJ, Gamblin SJ, Hay AJ. Structural basis for oseltamivir resistance of influenza viruses. Vaccine. 2009;27:6317–6323. doi: 10.1016/j.vaccine.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Deyde VM, Sheu TG, Trujillo AA, Okomo-Adhiambo M, Garten R, Klimov AI, Gubareva LV. Detection of molecular markers of drug resistance in 2009 pandemic influenza A (H1N1) viruses by pyrosequencing. Antimicrob Agents Chemother. 2010;54(3):1102–1110. doi: 10.1128/AAC.01417-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick J, Lindberg RH, Tysklind M, Haemig PD, Waldenström J, Wallensten A, Olsen B. Antiviral oseltamivir is not removed or degraded in normal sewage water treatment: implications for development of resistance by influenza A virus. PLoS One. 2007;2(10):e986. doi: 10.1371/journal.pone.0000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwann GF, Olsen B, Osterhaus AD. Characterization of a novel influenza hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005;79(5):2814–2822. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E Skepner, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RAM, Pappas C, Alpuche-Aranda CM, López-Gatell H, Olivera H, López I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. Antigenic and genetic characteristics of swine-origin 2009 A (H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh GC, Nakada N, Yamashita N, Tanaka H. Occurrence and fate of oseltamivir carboxylate (Tamiflu) and amantadine in sewage treatment plants. Chemosphere. 2010a;81(1):13–17. doi: 10.1016/j.chemosphere.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Ghosh GC, Nakada N, Yamashita N, Tanaka H. Oseltamivir carboxylate, the active metabolite of oseltamivir phosphate (Tamiflu), detected in sewage discharge and river water in Japan. Environ Health Perspect. 2010b;118(1):103–107. doi: 10.1289/ehp.0900930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorkova EA, Leneva IA, Goloubeva OG, Bush K, Webster RG. Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses. Antimicrob Agents Chemother. 2001;45:2723–2732. doi: 10.1128/AAC.45.10.2723-2732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubareva LV, Penn CR, Webster RG. Inhibition of replication avian influenza viruses by the neuraminidase inhibitor 4-guanidino-2,4-dideoxy-2,3-dehydro-N-acetylneuraminic acid. Virology. 1995;212(2):323–330. doi: 10.1006/viro.1995.1489. [DOI] [PubMed] [Google Scholar]
- Gubareva LV, Webster RG, Hayden FG. Activities of zanamivir, oseltamivir, and RWJ-270201 against clinical isolates of influenza virus and neuraminidase inhibitor-resistant variants. Antimicrob Agents Chemother. 2001;45:3403–3408. doi: 10.1128/AAC.45.12.3403-3408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubareva LV. Molecular mechanisms of influenza virus resistance to neuraminidase inhibitors. Virus Res. 2004;103:199–203. doi: 10.1016/j.virusres.2004.02.034. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol. 2001;146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- Hurt AC, Holien JK, Barr IG. In vitro generation of neuraminidase inhibitor resistance in A(H5N1) influenza viruses. Antimicrob Agents Chemother. 2009;53:4433–4440. doi: 10.1128/AAC.00334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverin NV, Gambaryan AS, Bovin NV, Rudneva IA, Shilov AA, Khodova OM, Varich NL, Sinitsin BV, Makarova NV, Kropotkina EA. Postreassortment changes in influenza A virus hemagglutinin restoring HA-NA functional match. Virology. 1998;244:315–321. doi: 10.1006/viro.1998.9119. [DOI] [PubMed] [Google Scholar]
- Kho CL, Mohd-Azmi ML, Arshad SS, Yusoff K. Performance of an RT-nested PCR ELISA for detection of Newcastle disease virus. J Virol Methods. 2000;86(1):71–83. doi: 10.1016/s0166-0934(99)00185-8. [DOI] [PubMed] [Google Scholar]
- Kim JK, Negovetich NJ, Forrest HL, Webster RG. Ducks: the “Trojan horses” of H5N1 influenza. Influenza Other Respi Viruses. 2010;3(4):121–128. doi: 10.1111/j.1750-2659.2009.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Walker D, Pryor SP, Niles L, Chenhong L, Hinshaw VS, Webster RG. Influenza A viruses of wild migrating aquatic birds in North America. Vector Borne Zoonotic Dis. 2004;4(3):177–189. doi: 10.1089/vbz.2004.4.177. [DOI] [PubMed] [Google Scholar]
- Krauss S, Obert CA, Franks J, Walker D, Jones K, Seiler P, Niles L, Pryor SP, Obenauer JC, Naeve CW, Widjaja L, Webby RJ, Webster RG. Influenza in migratory birds and evidence of limited intercontinental virus exchange. PLoS Pathog. 2007;3(11):e167. doi: 10.1371/journal.ppat.0030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Qi J, Zhang W, Vavricka CJ, Shi Y, Wei J, Feng E, Shen J, Chen J, Liu D, He J, Yan J, Liu H, Jiang H, Teng M, Li X, Gao GF. The 2009 pandemic H1N1 neuraminidase N1 lacks the 150-cavity in its active site. Nat Struct Mol Biol. 2010;17(10):1266–1268. doi: 10.1038/nsmb.1909. [DOI] [PubMed] [Google Scholar]
- Miller PJ, Afonso CL, Spackman E, Scott MA, Pedersen JC, Senne DA, Brown JD, Fuller CM, Uhart MM, Karesh WB, Brown IH, Alexander DJ, Swayne DE. Evidence for a new avian paramyxovirus serotype 10 detected in rockhopper penguins from the Falkland Islands. J Virol. 2010;84(21):11496–11504. doi: 10.1128/JVI.00822-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Wallensten A, Baas C, Rimmelzwaan GF, Schutten M, Olsen B, Osterhaus AD, Fouchier RA. Mallards and highly pathogenic avian influenza ancestral viruses, northern Europe. Emerg Infect Dis. 2005;11(10):1545–1551. doi: 10.3201/eid1110.050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Veen J, Olsen B, Vogel R, Osterhaus AD, Fouchier RA. Towards improved influenza A surveillance in migrating birds. Vaccine. 2006;24(44-46):6729–6733. doi: 10.1016/j.vaccine.2006.05.060. [DOI] [PubMed] [Google Scholar]
- Orozovic G, Orozovic K, Lennerstrand J, Olsen B. Detection of resistance mutations to antivirals oseltamivir and zanamivir in avian influenza A viruses isolated from wild birds. PLoS One. 2011;6(1):e16028. doi: 10.1371/journal.pone.0016028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DF, Coleman MT, Dowdle WR, Schild GC. Immunology Series No 6 Procedural Guide. U.S. Dept. of Health, Education and Welfare; 1975. Advanced laboratory techniques for influenza diagnosis; p. 32. [Google Scholar]
- Pizzorno A, Bouhy X, Abed Y, Boivin G. Generation and characterization of recombinant pandemic influenza A (H1N1) viruses resistant to neuraminidase inhibitors. J Infect Dis. 2011;203(1):25–31. doi: 10.1093/infdis/jiq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier M, Mameli L, Bélisle M, Dallaire L, Melançon SB. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal Biochem. 1979;94(2):287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- Russell RJ, Haire LF, Stevens DJ, Collins PJ, Lin YP, Blackburn GM, Hay AJ, Gamblin SJ, Skehel JJ. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature. 2006;443(7107):45–49. doi: 10.1038/nature05114. [DOI] [PubMed] [Google Scholar]
- Saccà ML, Accinelli C, Fick J, Lindberg R, Olsen B. Environmental fate of the antiviral drug Tamiflu in two aquatic ecosystems. Chemosphere. 2009;75(1):28–33. doi: 10.1016/j.chemosphere.2008.11.060. [DOI] [PubMed] [Google Scholar]
- Singer AC, Nunn MA, Gould EA, Johnson AC. Potential risks associated with the proposed widespread use of Tamiflu. Environ Health Perspect. 2007;115(1):102–106. doi: 10.1289/ehp.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderström H, Järhult JD, Olsen B, Lindberg RH, Tanaka H, Fick J. Detection of the antiviral drug oseltamivir in aquatic environments. PLoS One. 2009;4(6):e6064. doi: 10.1371/journal.pone.0006064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner TD, Krauss S, DuBois RM, Negovetich NJ, Stallknecth DE, Senne DA, Gramer MR, Swafford S, DeLiberto T, Gorvokova EA, Webster RG. Antiviral susceptibility of avian and swine influenza virus of the N1 neuraminidase subtype. J Virol. 2010;84(19):9800–9809. doi: 10.1128/JVI.00296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese JN, Colman PM. Three-dimensional structure of the neuraminidase of influenza virus A/Tokyo/3/67 at 2•2 Å resolution. J Mol Biol. 1991;221(2):473–486. doi: 10.1016/0022-2836(91)80068-6. [DOI] [PubMed] [Google Scholar]
- von Itzstein M, Wu YW, Kok GB, Pegg MS, Dyason JC, Jin B, Van Phan T, Smythe ML, White HF, Oliver SW, Colman PM, Varghese JN, Ryan DM, Woods JM, Bethell RC, Hotham VJ, Cameron JM, Penn CR. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363(6428):418–423. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- Wagner R, Matrosovich M, Klenk HD. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol. 2002;12(3):159–166. doi: 10.1002/rmv.352. [DOI] [PubMed] [Google Scholar]
- Wang C, Miguel B, Austin FW, Keirs RW. Comparison of the immunofluorescent assay and reverse transcription-polymerase chain reaction to detect and type infectious bronchitis virus. Avian Dis. 1999;43:590–596. [PubMed] [Google Scholar]
- Warke A, Appleby L, Mundt E. Prevalence of antibodies to different avian paramyxoviruses in commercial poultry in the United States. Avian Dis. 2008;52(4):694–697. doi: 10.1637/8390-070308-RESNOTE.1. [DOI] [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses (review) Microbiol Rev. 1992;56(1):152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


