Abstract
Objective
To examine the predictive ability of stage of hypoxic-ischemic encephalopathy (HIE) for death or moderate/severe disability at 18 months among neonates undergoing hypothermia.
Study design
Stage of encephalopathy was evaluated at <6 hr of age, during study intervention and at discharge among 204 participants in the NICHD Neonatal Research Network Trial of whole body hypothermia for HIE. HIE was examined as a predictor of outcome by regression models.
Results
Moderate and severe HIE occurred at <6 hrs of age among 68% and 32% of 101 hypothermia group infants and 60% and 40% of 103 control group infants, respectively. At 24 and 48 hrs of study intervention, infants in the hypothermia group had less severe HIE than infants in the control group. Persistence of severe HIE at 72 hrs increased the risk of death or disability after controlling for treatment group. The discharge exam improved the predictive value of stage of HIE at < 6hrs for death/disability.
Conclusions
On serial neurological examinations, improvement in stage of HIE was associated with cooling. Persistence of severe HIE at 72 hours and an abnormal neurological exam at discharge was associated with a greater risk of death or disability.
Keywords: Neurological examinations, neonates, clinical biomarker, death, disability
Clinical encephalopathy has been examined over the past three decades as an early predictor of neurological and developmental outcome among term infants with intrapartum distress or hypoxia-ischemia. The Sarnat evaluation of staging of encephalopathy by clinical examination correlates well with subsequent neurodevelopmental impairment in infancy and childhood 1–4. Other scoring systems adapted from the Sarnat evaluation are also used to predict outcome 5–6.
Hypothermia to a target temperature of 33–34°C for 72 hours has currently been shown to reduce death or disability at 18 months or increase the rate of survivors without disabilities 7–11. These trials have all included moderate/severe stage of encephalopathy 1 as eligibility criteria at study random assignment at < 6 hours of age with three trials having the additional criteria for abnormal amplitude integrated electroencephalography 7, 9, 11. The major trials have established a relationship between the stage of hypoxic-ischemic encephalopathy (HIE) at < 6 hours of life and early childhood outcome. Relatively less is known about the relationship between stage of encephalopathy at other times in the neonatal period and later outcome. In the Cool Cap trial, infants with persistent moderate encephalopathy on day four had a more favorable prognosis after selective head as cooling compared with standard care 12. The National Institute of Child Health and Human Development (NICHD) Neonatal Research Network (NRN) randomized controlled trial (RCT) data of serial neurological examinations provided us an opportunity to examine the evolution of encephalopathy as a clinical biomarker and its relationship with outcome. Neurological examinations over the first few days may serve as a marker of brain injury after hypoxia-ischemia as well as response to treatment. We hypothesized that the evolution of encephalopathy over the first three days may be a better predictor of death or disability than the stage of HIE at < 6 hours of age and that failure to improve the stage of encephalopathy over the 72 hr intervention would be associated with a higher frequency of death or disability compared with those without persistent encephalopathy, and finally that the neurologic status at discharge would be a better predictor of outcome than the stage of HIE at < 6 hours of age.
METHODS
This is a secondary analysis of the NICHD NRN RCT of whole body hypothermia for term infants with HIE. As part of the eligibility criteria, a modified Sarnat neurological examination was performed at ≤ 6 hours of age. Additional examinations were performed at 24, 48 and 72 hours during the study intervention period and at discharge by a certified physician examiner. The certification process was as follows: each NRN site principal investigator was considered the gold standard examiner after orientation and review of the examination with the study subcommittee. Additional physician examiners reviewed the definitions of the components of the examination from the study manual of procedures and then performed neurological examinations on 3 term infants, including two infants with abnormal findings, independent of the site PI and within one hour of the examination performed by the PI. A hard copy of the examinations was sent to the study Data Coordinating Center at RTI International, Research Triangle Park, North Carolina. The Study lead investigator (SS) compared the examinations of the physician examiners with the site PI and a physician was certified if all 3 examinations achieved concordance with that of the site PI regarding stage of HIE.
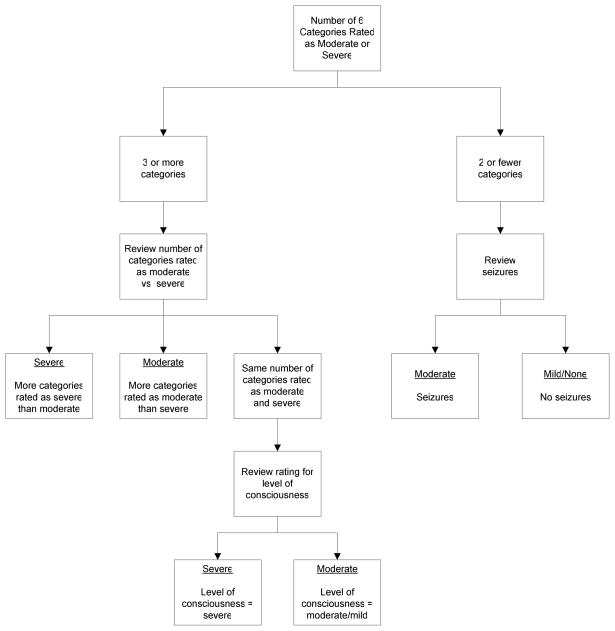
Whole body hypothermia was performed for 72 hours at 33.5°C as described previously 8. For study eligibility, encephalopathy was defined as the presence of moderate or severe encephalopathy in at least 3 of the following 6 categories: 1) level of consciousness: moderate (lethargy), severe (stupor or coma), 2) spontaneous activity: moderate (decreased), severe (no activity), 3) posture: moderate (distal flexion, complete extension), severe (decerebrate), 4) tone: moderate (hypotonia), severe (flaccid), 5) primitive reflexes, suck: moderate (weak), severe (absent) or Moro: moderate (incomplete), severe (absent) and 6) autonomic nervous system, pupils: moderate (constricted), severe (deviated, dilated or non-reaction to light) or heart rate: moderate (bradycardia), severe (variable) or respiration: moderate (periodic breathing), severe (apnea) (Figure 1; available at www.jpeds.com). Additional neurological findings (presence of clinical seizures, increased tone, sustained clonus, fisted hand, abnormal movements and absence of gag reflex) were noted during the evaluations at 24, 48 and 72 hours ( ± 12 hour window). The discharge evaluation (performed within 3 days of discharge) was expanded to include the presence of an asymmetric tonic neck reflex and need for gavage or gastrostomy tube feeds. The presence of any of these findings was coded as abnormal.
Figure I. Classification of Infants According to HIE Grade.
Note: The six categories are level of consciousness, spontaneous activity, posture, tone, primitive reflexes, and autonomic system.
All surviving infants were evaluated at 18–22 months of age by certified examiners trained to reliability and unaware of treatment assignment. Severe disability was defined as any one of the following: Bayley II Mental Developmental Index (MDI) < 70, Gross Motor Function Classification System (GMFCS) level 3–5, hearing impairment requiring hearing aids, or blindness. Moderate disability was defined as MDI 70–84 and one or more of the following: GMFCS level 2, hearing impairment with no amplification, or a persistent seizure disorder.
This study was performed in the 16 centers of the NICHD NRN. The institutional review board of each participating center approved the main trial protocol, and written informed consent was obtained from the parents of eligible infants prior to random assignment of study intervention.
Logistic and ordered logistic regression models were used for bivariate comparisons of HIE stage and length of time in HIE stage by treatment group (hypothermia vs. control). In addition, a series of logistic regression models were conducted using SAS PROC GLIMMIX to examine the primary outcome, death or disability, by HIE stage, or length of time in HIE stage, after controlling for treatment group and HIE stage at random assignment. Goodness-of-fit between models was compared by the area-under-the-curve c statistic. Clinical center was included as a random effect to account for clustering of infants within center. Any comparisons regarding stage of HIE at the different time points were focused on the modified Sarnat evaluation. The exposure of infants to medications that may influence the neurological examination (anticonvulsants, analgesics/sedatives and neuromuscular blocking agents) was noted at the different time points and compared between the 2 groups. Medication use was not dictated by the study protocol but was per usual care at the clinical centers. Serum concentrations of medications were not obtained or documented as part of the study protocol.
RESULTS
Of the 208 infants who participated in the RCT, 3 infants (control group) were lost to follow-up. One infant (hypothermia group) qualified for the study with clinical seizures but was missing a neurological exam at randomization that would have assigned the infant to either moderate or severe stage of encephalopathy. Therefore, this analysis included a total of 101 infants in the hypothermia group and 103 infants in the control group. The number of infants who died or had missing examinations included the following: hypothermia group at 24 hours: 3 died, 3 missing exams; at 48 hours: 8 died, 4 missing exams; at 72 hours: 13 died, 3 missing exams and at discharge: 19 died and 5 missing examinations. In the control group, at 24 hours: 3 died, 3 missing exams; at 48 hours 8 died, 5 missing exams; at 72 hours, 10 died, 4 missing exams and at discharge: 28 died and 9 missing examinations. Among study infants, 64 of 174 infants had their 72 hour exam performed after the study intervention ended. Of these 64 infants, 27 were in the hypothermia group and the neurologic examination was performed within 4.3 ± 3.6 hours of initiation of rewarming, and in the control group, 37 infants had their examination performed 5.4 ± 6.6 hours after the 72 hour study intervention period
Exposure to medications prior to study baseline, at baseline and at 24, 48 and 72 hours of study intervention included: anticonvulsants, range 44 to 58% of the infants, analgesics/sedatives from 16 to 36% and neuromuscular blocking agents from 4 to 10% of infants. The frequency of exposure to each of these categories of medications, at each of these time points was comparable between the hypothermia and control group of infants (data not shown).
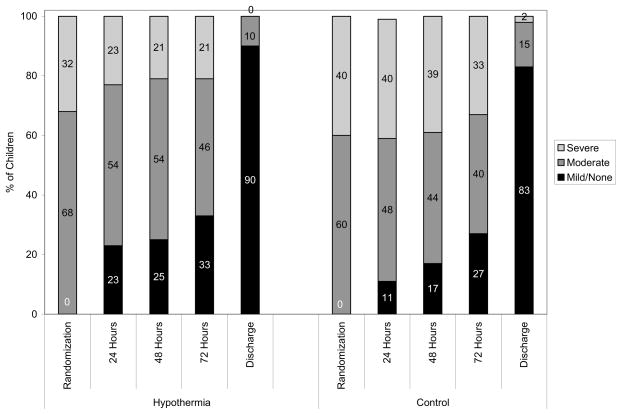
The stage of encephalopathy among infants who survived to the time points noted is shown in Figure 2. Although there were no significant differences in the stage of HIE among the two treatment groups at < 6 hours of age [OR (95% CI) = 0.70 (0.39, 1.25)], P = 0.23, those in the hypothermia group had less severe encephalopathy at 24 hours [OR (95% CI) = 0.44 (0.25, 0.77)], P = .004 and at 48 hours [(OR (95% CI) = 0.49 (0.28–0.87)], P = .01. The groups were comparable in stage of encephalopathy at 72 hours [(OR (95% CI) = 0.65 (0.38–1.14], P = 0.13 or at discharge [(OR (95% CI) = 0.58 (0.22–1.54)], P = 0.27.
Figure II.
The stage of encephalopathy at <6 hours, 24, 48, 72 and discharge
Improvement in stage of encephalopathy (from severe to moderate or moderate to mild/none) was achieved earlier among infants in the hypothermia group than those in the control group (P = .04). Among the infants in the hypothermia group, 34 (40%) experienced improvement in their stage of HIE within the first 24 hours of treatment, 14 (16%) between 48–72 hours, and 38 (44%) at ≥ 72 hours. Among the infants in the control group, 21 (24%) experienced improvement in 24 hours, 18 (20%) between 48–72 hours, and 50 (56%) at ≥ 72 hours. The groups did not differ significantly with respect to time before classification as none/mild HIE (P = .07). A deterioration (change in stage of encephalopathy from moderate to severe) occurred among 19 infants (8 hypothermia and 11 control) in the first 24 hours, 6 (2 hypothermia and 4 control) within 48 hours and 3 hypothermia infants within 72 hours. Ten infants (7 hypothermia and 3 control) initially improved in stage of HIE to mild/none and then deteriorated to moderate encephalopathy within 72 hours.
The hypothermia group had significantly lower odds of the primary outcome of death or disability at 18 months (Table I). Based on the bivariate analyses (unadjusted), infants with more severe HIE at each time point had increased odds of death/disability. Similarly, increased risk of death/disability was associated with more than 72 hours (vs. 24 hours) before improvement in the initial stage of HIE or before classification as none/mild HIE. At discharge, other than sustained clonus, the presence of any of the following was associated with significantly increased odds of death/disability: hypertonia, fisted hand, abnormal movements, absent gag reflex, or asymmetric tonic neck reflex.
Table I.
Death/Disability by stage of HIE and Time in HIE stage
| Variable | N | Death/Disability
|
|||
|---|---|---|---|---|---|
| N (%) | Unadjusted OR | 95% CI | P | ||
| Treatment Group | |||||
| Hypothermia | 101 | 45 (45) | 0.49 | 0.28–0.86 | 0.012 |
| Control | 103 | 64 (62) | |||
| Stage of HIE at Each Time Point | |||||
| < 6 hours of age | |||||
| Severe | 73 | 58 (79) | 6.07 | 3.11–11.82 | < 0.001 |
| Moderate | 131 | 51 (39) | REF | ||
| 24 hours | |||||
| Severe | 61 | 54 (89) | 55.93 | 15.11–207.02 | < 0.001 |
| Moderate | 98 | 41 (42) | 5.22 | 1.70–15.98 | 0.004 |
| Mild/None | 33 | 4 (12) | REF | ||
| 48 hours | |||||
| Severe | 54 | 48 (89) | 66.00 | 17.27–252.19 | < 0.001 |
| Moderate | 88 | 33 (38) | 4.95 | 1.61–15.23 | 0.005 |
| Mild/None | 37 | 4 (11) | REF | ||
| 72 hours | |||||
| Severe | 47 | 41 (87) | 81.99 | 21.64–310.65 | < 0.001 |
| Moderate | 75 | 34 (45) | 9.95 | 3.26–30.40 | < 0.001 |
| Mild/None | 52 | 4 (8) | REF | ||
| Discharge | |||||
| Severe/Moderate | 19 | 16 (84) | 11.62 | 3.20–42.23 | < 0.001 |
| Mild/None | 124 | 39 (31) | REF | ||
| Time before improvement in Stage of HIE | |||||
| Time before classification as none/mild HIE | |||||
| More than 72 hours | 112 | 73 (65) | 13.57 | 4.45–41.40 | < 0.001 |
| 48–72 hours | 29 | 2 (7) | 0.54 | 0.09–3.17 | 0.493 |
| 24 hours | 33 | 4 (12) | REF | ||
| Time before any improvement in HIE stage | |||||
| More than 72 hours | 88 | 59 (67) | 4.96 | 2.38–10.31 | < 0.001 |
| 48–72 hours | 32 | 5 (16) | 0.45 | 0.15–1.38 | 0.163 |
| 24 hours | 55 | 16 (29) | REF | ||
| Discharge Findings | |||||
| Gavage/Gastrostomy Tube Feedings | |||||
| Yes | 32 | 27 (84) | 16.97 | 5.97–48.24 | < 0.001 |
| No | 116 | 28 (24) | REF | ||
| Six Additional Findings | |||||
| Hypertonia | |||||
| Yes | 35 | 24 (69) | 5.87 | 2.56–13.48 | <0.001 |
| No | 107 | 29 (27) | REF | ||
| Clonus | |||||
| Yes | 12 | 6 (50) | 1.76 | 0.54–5.78 | 0.351 |
| No | 127 | 46 (36) | REF | ||
| Fisted hand | |||||
| Yes | 18 | 13 (72) | 5.61 | 1.87–16.87 | 0.002 |
| No | 120 | 38 (32) | REF | ||
| Abnormal movements | |||||
| Yes | 14 | 13 (93) | 28.67 | 3.62–226.90 | 0.002 |
| No | 125 | 39 (31) | REF | ||
| Gag reflex absent | |||||
| Yes | 33 | 25 (76) | 9.26 | 3.74–22.95 | < 0.001 |
| No | 107 | 27 (25) | REF | ||
| Asymmetric tonic neck reflex | |||||
| Yes | 15 | 9 (60) | 3.12 | 1.04–9.37 | 0.043 |
| No | 120 | 39 (33) | REF | ||
Note: REF=reference category; Moderate and severe HIE stage at discharge are combined because only one child had severe HIE at discharge.
The stage of HIE at < 6 hours of age was a significant predictor of death/disability, after controlling for treatment group (Table II, Model 1). However, among survivors to 72 hours, after adding stage of HIE at 72 hours, the stage of HIE at < 6 hours of age was no longer significant (Table II, Models 2 vs. 3). The addition of stage of HIE at 72 hours significantly improved the model fit as measured by the area under the ROC curve (AUC) beyond that of stage of HIE at < 6 hours of age and treatment group (0.87 vs. 0.75, P < .001).
Table II.
Logistic Regression Models of Death/Disability by Stage of HIE at < 6 hours of age, 72 Hours, and Discharge
| Variable | Adjusted OR | 95% CI | P |
|---|---|---|---|
| All children (N=204) | |||
| Model 1: Stage of HIE at < 6 hours of age | |||
| Treatment Group | |||
| Hypothermia | 0.51 | 0.28–0.94 | 0.031 |
| Control | REF | ||
| HIE stage at < 6 hours of age | |||
| Severe | 6.08 | 3.07–12.06 | < 0.001 |
| Moderate | REF | ||
|
| |||
| Children assessed at 72 hours (N=174) | |||
| Model 2: Stage of HIE at < 6 hours of age | |||
| Treatment Group | |||
| Hypothermia | 0.41 | 0.21–0.80 | 0.009 |
| Control | REF | ||
| HIE stage at < 6 hours of age | |||
| Severe | 5.54 | 2.66–11.53 | < 0.001 |
| Moderate | REF | ||
| Model 3: HIE stage at < 6 hours of age and 72 Hours | |||
| Treatment Group | |||
| Hypothermia | 0.38 | 0.17–0.83 | 0.016 |
| Control | REF | ||
| HIE stage at < 6 hours of age | |||
| Severe | 2.25 | 0.94–5.37 | 0.068 |
| Moderate | REF | ||
| HIE stage at 72 Hours | |||
| Severe | 59.95 | 14.61–245.96 | < 0.001 |
| Moderate | 9.15 | 2.85–29.39 | < 0.001 |
| Mild/None | REF | ||
|
| |||
| Children assessed at discharge (N=143) | |||
| Model 4: Stage of HIE at < 6 hours of age | |||
| Treatment Group | |||
| Hypothermia | 0.53 | 0.26–1.08 | 0.082 |
| Control | REF | ||
| HIE stage at < 6 hours of age | |||
| Severe | 3.01 | 1.35–6.72 | 0.007 |
| Moderate | REF | ||
| Model 5: Stage of HIE at < 6 hours of age and Discharge | |||
| Treatment Group | |||
| Hypothermia | 0.57 | 0.27–1.20 | 0.139 |
| Control | REF | ||
| HIE stage at < 6 hours of age | |||
| Severe | 2.66 | 1.14–6.22 | 0.025 |
| Moderate | REF | ||
| HIE stage at Discharge | |||
| Severe/Moderate | 9.85 | 2.61–37.16 | < 0.001 |
| Mild/None | REF | ||
| Model 6: HIE stage at < 6 hours of age and Discharge Findings | |||
| Treatment Group | |||
| Hypothermia | 0.58 | 0.25–1.37 | 0.212 |
| Control | REF | ||
| HIE stage at < 6 hours of age | |||
| Severe | 1.97 | 0.75–5.20 | 0.168 |
| Moderate | REF | ||
| HIE stage/Findings at Discharge | |||
| Severe/Moderate | 8.47 | 1.76–40.88 | 0.008 |
| Mild/None + Additional Findings | 2.69 | 1.09–6.67 | 0.033 |
| Mild/None + No Additional Findings | REF | ||
| Gavage/Gastrostomy Tube Feeding at Discharge | 8.55 | 2.73–26.82 | < 0.001 |
Note: REF=reference category; Models also include research center as a random effect. Areas under ROC curve (AUC) by model: 1 (AUC=0.75), 2 (AUC=0.75), 3 (AUC=0.87), 4 (AUC=.68), 5 (AUC=0.73), and 6 (AUC=0.80).
Among survivors to discharge, the stage of HIE at < 6 hours of age and at discharge were significantly associated with death/disability while controlling for treatment in Model 5. Adding stage of HIE at discharge did not significantly improve the explanatory power of the model beyond that found with HIE stage at < 6 hours of age and treatment group (Table II, Models 4 vs. 5; AUC 0.73 vs.0.68, P = .07). However, including the additional neurological findings at discharge in Model 4 did significantly improve AUC over the base model (Table II, Models 6 vs. 4; AUC 0.80 vs. 0.68, P = .003). Those with mild/no HIE grade at discharge, but who had any of the additional findings, had significantly greater odds of death/disability than those with mild/no HIE who did not have the additional findings. Those with moderate/severe HIE at discharge had 8 times the odds of death/disability as those with mild/no HIE and no additional findings. The need for gastrostomy/gavage tube feeding at discharge was also associated with increased risk for death/disability.
Time before improvement in stage of HIE and time before classification as mild/no HIE were both significant predictors of death/disability (Table III) and both models (Model 1 and 2) had significantly higher AUC (0.84 and 0.86) than the model with treatment group and HIE grade at < 6 hours of age, (AUC 0.75, P < .001). Infants who did not improve the stage of HIE within the study intervention (72 hours) had significantly higher odds of death/disability than those who improved within the first 24 hours (P < .001).
Table III.
Logistic Regression Models of Death/Disability by Time before Improvement in HIE stage
| Variable | Adjusted OR | 95% CI | P |
|---|---|---|---|
| Model 1: Time Before Classification as Mild/None (N=174) | |||
| Treatment Group | |||
| Hypothermia | 0.38 | 0.18–0.81 | 0.013 |
| Control | REF | ||
| HIE stage at < 6 hours of age | |||
| Severe | 3.44 | 1.52–7.77 | 0.003 |
| Moderate | REF | ||
| Time before initial classification as none/mild HIE | |||
| More than 72 hours | 9.65 | 2.96–31.50 | < 0.001 |
| 48–72 hours | 0.43 | 0.07–2.75 | 0.372 |
| 24 hours | REF | ||
| Model 2: Time Before Any Improvement in HIE stage (N=175) | |||
| Treatment Group | |||
| Hypothermia | 0.43 | 0.20–0.95 | 0.036 |
| Control | REF | ||
| HIE stage at < 6 hours of age | |||
| Severe | 20.27 | 6.15–66.86 | < 0.001 |
| Moderate | REF | ||
| Time before any improvement in HIE stage | |||
| More than 72 hours | 18.61 | 5.37–64.49 | < 0.001 |
| 48–72 hours | 0.74 | 0.17–3.21 | 0.681 |
| 24 hours | REF | ||
Note: REF=reference category; Models also include research center as a random effect. Areas under ROC curve (AUC): model 1 (AUC=0.84) and model 2 (AUC=0.87).
DISCUSSION
We have noted that infants who received whole body hypothermia for neonatal HIE had a higher likelihood of improving their stage of encephalopathy within 24 hours than infants in the control group. Persistence of the severe stage of HIE (compared with none/mild HIE) throughout the 72 hour study intervention period was associated with a greater chance of death or disability at 18 months of age. At the time of discharge, the presence of hypertonia, fisted hand, abnormal movements, absent gag reflex, an asymmetric tonic neck reflex or need for gavage tube or gastrostomy feeds increased the risk for death or disability. In a secondary analysis of the Cool Cap Study, Gunn et al have noted that milder stage of encephalopathy at random assignment of cooling, greater improvement in encephalopathy to day 4 and the intervention of cooling were associated with favorable outcome at 18 months of age 12.
The strength of this study is the information obtained from detailed neurological examinations by physician examiners certified after rigorous training both during and after whole body hypothermia. A low rate of missing data and loss to follow-up is another strength of the study. A neurological examination performed at multiple time points can be used as a clinical biomarker for prognostication. Using data from this study it is possible to counsel parents regarding the possible risk of death or disability based on the stage of HIE during and at the end of the period of whole body hypothermia. The presence of continued severe encephalopathy at the end of 72 hours of cooling may reflect a more severe injury or ongoing injury distant from the time of the hypoxic-ischemic insult, as injury continues for days or weeks 13, 14. The information obtained from this study evaluating stage of HIE at the end of 72 hours of cooling raises the question whether cooling to 72 hours at 33.5°C is adequate or whether either the duration or depth of cooling should be increased 15. This question is being addressed in an ongoing trial.
The limitations of this study are that the study did not distinguish between mild encephalopathy and no encephalopathy.
The neurological examination at < 6 hours of age has been shown to be a clinical biomarker of patient selection for neuroprotection. The results of our findings and those of the Cool Cap Study demonstrate that the neurological examination at the end of hypothermia therapy continues to be a good predictor of outcome. The effects of cooling on clinical examinations and on the amplitude integrated EEG recovery16 are highly consistent. The prevalence of abnormalities on the discharge examination is low; however, we have noted that when present, they are helpful in predicting death or disability. On the other hand, if abnormalities at discharge are not noted, some risk of disability cannot be ruled out.
There is increasing interest in identification of early biomarkers as a surrogate of childhood outcome following neonatal HIE 1–6, 17–20 It is not known whether an early/short term clinical biomarker such as early neurological examination can be used in lieu of long term outcome. Biomarkers have limitations, the home environment influences outcome following hospital discharge and the neonatal brain has the ability to recover from injury. Our research indicates that neurological findings during and following hypothermia treatment are valuable for informing such predictions.
Acknowledgments
Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (list of grants available at www.jpeds.com [Appendix 2]).
Abbreviations
- NICHD
National Institute of Child Health and Human Development
- NRN
Neonatal Research Network
- HIE
Hypoxic-ischemic encephalopathy
Appendix 1
Members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network include:
The Hypothermia Study Group
Case Western Reserve University Rainbow Children’s Hospital Principal Investigator: Avroy A. Fanaroff, MD; Co-PI: Michele C. Walsh, MD; Study Coordinator: Nancy Newman, BA; RN; Follow Up Principal Investigator: DeeAnne Wilson-Costello, MD; Follow Up Coordinator: Bonnie Siner, RN. Brown University Women & Infant’s Hospital Principal Investigator: William Oh, MD; Study Coordinator: Angelita Hensman, BSN, RNC; Follow Up Principal Investigator: Betty Vohr, MD; Follow Up Coordinator: Lucy Noel, RN. Duke University Principal Investigator: C. Michael Cotten, MD; Study Coordinator: Kathy Auten, BS; Follow Up Principal Investigator: Ricki Goldstein, MD; Follow Up Coordinator: Melody Lohmeyer, RN. Emory University Grady Memorial Hospital and Crawford Long Hospital Principal Investigator: Barbara J. Stoll, MD; Co-PI: Lucky Jain, MD; Study Coordinator: Ellen Hale, RN, BS. Indiana University Riley Hospital for Children and Methodist Hospital Principal Investigator: James A. Lemons, MD; Study Coordinators: Diana Dawn Appel, RN BSN, Lucy Miller, RN, BSN; Follow Up Principal Investigator: Anna Dusick, MD; Follow Up Coordinator: Leslie Richard, RN. Stanford University Principal Investigator: David K. Stevenson, MD; Co-PI: Krisa VanMeurs, MD; Study Coordinator: M. Bethany Ball, BS, CCRC; Follow Up Principal Investigator: Susan R. Hintz, MD. University of Alabama at Birmingham University Hospital-UAB Principal Investigator: Waldemar A. Carlo, MD; Study Coordinator: Monica Collins, RN, BSN, Shirley Cosby, RN, BSN; Follow Up Principal Investigator: Myriam Peralta-Carcelen, MD; Follow Up Coordinator: Vivien Phillips, RN, BSN. University of Cincinnati The University Hospital, Cincinnati Children’s Hospital Medical Center; Principal Investigator: Edward F. Donovan, MD; Study Coordinators: Cathy Grisby, BSN, Barb Alexander, RN, Jody Shively, RN, Holly Mincey, RN; Follow Up Principal Investigator: Jean Steichen, MD; Follow Up Coordinator: Teresa Gratton, PA. University of California-San Diego UCSD Medical Center and Sharp Mary Birch Hospital for Women Principal Investigators: Neil N. Finer, MD; Co-PI: David Kaegi, MD; Study Coordinators: Chris Henderson, CRTT, Wade Rich, RRT-NPS, Kathy Arnell, RN; Follow Up Principal Investigator: Yvonne E. Vaucher, MD, MPH; Follow Up Coordinator: Martha Fuller, RN, MSN. University of Miami Principal Investigator: Shahnaz Duara, MD; Study Coordinator: Ruth Everett, BSN; Follow Up Principal Investigator: Charles R. Bauer, MD. University of Rochester Golisano Children’s Hospital at Strong Principal Investigator: Ronnie Guillet, MD; PhD; Study Coordinator: Linda Reubens, RN; Follow Up Principal Investigator: Gary Myers, MD; Follow Up Coordinator: Diane Hust, RN. The University of Texas Southwestern Medical Center at Dallas: Parkland Hospital Principal Investigator: Abbot R. Laptook, MD; Study Coordinators: Susie Madison, RN, Gay Hensley, RN, Nancy Miller, RN; Follow Up Principal Investigator: Roy Heyne, MD, Sue Broyles, MD; Follow Up Coordinator: Jackie Hickman, RN. University of Texas Health Science Center at Houston Medical School: Children’s Memorial Hermann Hospital and Lyndon Baines Johnson General Hospital/Harris County Hospital District Principal Investigator: Jon E. Tyson, MD, MPH; Study Coordinator: Georgia McDavid, RN, Esther G. Akpa, RN, BSN, Claudia Y. Franco, RN, BNS, MSN, NNP, Patty A. Cluff, RN, Anna E. Lis, RN, BSN; Follow-Up Principal Investigators: Brenda H. Morris, MD, Pamela J. Bradt, MD, MPH. Wayne State University Hutzel Women’s Hospital & Children’s Hospital of Michigan Principal Investigator: Seetha Shankaran, MD; Study Coordinators: Rebecca Bara, RN, BSN, Geraldine Muran, RN, BSN; Follow Up Principal Investigator: Yvette Johnson, MD; Follow Up Coordinator: Debbie Kennedy, RN. Yale University New Haven Children’s Hospital Principal Investigator: Richard A. Ehrenkranz, M.D. Study Coordinator: Patricia Gettner, RN; Follow Up Coordinator: Elaine Romano, RN.
NICHD Neonatal Research Steering Committee
Brown University William Oh, MD; Case Western University Avroy A. Fanaroff, MD; Duke University Ronald N. Goldberg, MD; Emory University Barbara J. Stoll, MD; Indiana University James A. Lemons, MD; Stanford University David K. Stevenson, M.D.; University of Alabama at Birmingham Waldemar A. Carlo, MD; University of Cincinnati Edward F. Donovan, MD; University of California-San Diego Neil N. Finer, MD; University of Miami Shahnaz Duara, MD; University of Rochester Dale L. Phelps, MD; University of Texas – Dallas Abbot R. Laptook, MD; University of Texas – Houston Jon E. Tyson, MD, MPH; Wake Forest University T. Michael O’Shea, MD, MPH; Wayne State University Seetha Shankaran, MD; Yale University Richard A. Ehrenkranz, MD, Chair, Alan Jobe, University of Cincinnati
Data Coordinating Center: RTI International
Principal Investigator: W. Kenneth Poole, PhD; Coordinators: Betty Hastings and Carolyn M. Petrie, MS
National Institute of Child Health and Human Development
Program Scientist: Rosemary D. Higgins, MD, Linda L. Wright, MD; Coordinator: Elizabeth McClure, MEd
Data Safety and Monitoring Committee
Children’s National Medical Center Gordon Avery, MD; Columbia University Mary D’Alton, MD; RTI International W. Kenneth Poole, PhD (ex officio); University of Virginia John C. Fletcher, Ph.D. (deceased); University of Washington Christine A. Gleason, MD; University of Pittsburgh Carol Redmond, Ph.D.
Footnotes
The authors declare no conflicts of interest.
no reprints available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. Arch Neurol. 1976;33:696–75. doi: 10.1001/archneur.1976.00500100030012. [DOI] [PubMed] [Google Scholar]
- 2.Badawi N, Felix JF, Kurubczuk JJ, Dixon G, Watson L, Keogh JM, et al. Cerebral palsy following term newborn encephalopathy: a population-based study. Dev Med Child Neurol. 2005;47:293–98. doi: 10.1017/s0012162205000575. [DOI] [PubMed] [Google Scholar]
- 3.Ambalavanan N, Carlo WA, Shankaran S, Bann CM, Emrich SL, Higgins RD, et al. Predicting outcomes of neonates diagnosed with hypoxemic-ischemic encephalopathy. Pediatrics. 2006;118:2084–93. doi: 10.1542/peds.2006-1591. [DOI] [PubMed] [Google Scholar]
- 4.Robertson CM. Long-term follow-up of term infants with perinatal asphyxia. In: Stevenson DK, Benitz WE, Sunshine P, editors. Fetal and Neonatal Brain Injury. Cambridge University Press; 2003. pp. 829–58. [Google Scholar]
- 5.Thompson CM, Puterman AS, Linley LL, Hann FM, van der Elst CW, Molteno CD, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86:757–61. doi: 10.1111/j.1651-2227.1997.tb08581.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller SP, Latal B, Clark H, Barnwell A, Glidden D, Barkovich AJ, et al. Clinical signs predict 30-month neurodevelopmental outcome after neonatal encephalopathy. Am J Obstet Gynecol. 2004;190:93–9. doi: 10.1016/s0002-9378(03)00908-6. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Wyatt J, Azzopardi DV, Ballard R, Edwards, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: Multicenter Randomised Trial. Lancet. 2005;365:663–70. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 8.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 9.Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–58. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 10.Zhou WH, Cheng GQ, Shao XM, Liu XZ, Shan RB, Zhuang DY, et al. Selective head cooling with mild systemic hypothermia after neonatal hypoxic-ischemic encephalopathy: a multicenter randomized controlled trial in China. J Pediatr. 2010;157:367–72. doi: 10.1016/j.jpeds.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Simbruner G, Mittal RA, Rohlmann F, Muche R. neo. nEURO.network Trial Participants. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO. network RCT. Pediatrics. 2010;126:e771–8. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 12.Gunn AJ, Wyatt JS, Whitelaw A, Barks J, Azzopardi D, Ballard R, et al. Therapeutic hypothermia changes the prognostic value of clinical evaluation of neonatal encephalopathy. J Pediatr. 2008;152:55–8. doi: 10.1016/j.jpeds.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Robertson NJ, Cowan FM, Cox IJ, Edwards AD. Brain alkaline intracellular pH after neonatal encephalopathy. Ann Neurol. 2002;52:732–42. doi: 10.1002/ana.10365. [DOI] [PubMed] [Google Scholar]
- 14.Northington FJ, Ferriero DM, Graham EM, Traystman RJ, Martin LJ. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol Dis. 2001;8:207–19. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- 15.Shankaran S. Optimizing Cooling Strategies at < 6 Hours of age for neonatal hypoxic-ischemic encephalopathy (HIE) http://www.clinicaltrials.gov.NCT01192776.
- 16.Thoresen M, Hellstrom-Westas, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. 2010;126:e131–9. doi: 10.1542/peds.2009-2938. [DOI] [PubMed] [Google Scholar]
- 17.Nelson KB, Ellenberg JH. Antecedents of cerebral palsy. 1. Univariate analysis of risks. Am J Dis Child. 1985;139:1031–8. doi: 10.1001/archpedi.1985.02140120077032. [DOI] [PubMed] [Google Scholar]
- 18.Nelson KB, Ellenberg JH. Antecedents of cerebral palsy. II. Multivariate analysis of risk. N Engl J Med. 1986;315:81–6. doi: 10.1056/NEJM198607103150202. [DOI] [PubMed] [Google Scholar]
- 19.Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179:507–13. doi: 10.1016/s0002-9378(98)70387-4. [DOI] [PubMed] [Google Scholar]
- 20.Bennet L, Booth L, Gunn AJ. Potential biomarkers for hypoxic-ischemic encephalopathy. Semin Fetal Neonatal Med. 2010;15:253–60. doi: 10.1016/j.siny.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]