Abstract
Understanding how colon cancer cells survive within the inflammatory milieu of a tumor, and developing approaches that increase their sensitivity to inflammatory cytokines, may ultimately lead to novel approaches for colon cancer therapy and prevention. Analysis of a number of chemopreventive and therapeutic agents reveal that HDAC inhibitors are particularly adept at sensitizing colon cancer cells TNF or TRAIL mediated apoptosis. In vivo data are consistent with an interaction between SAHA and TNF in inducing apoptosis, as AOM-induced colon tumors express elevated levels of TNF and are more sensitive to SAHA administration. Cell cycle analysis and time-lapse imaging indicated a close correspondence between SAHA-induced prophase arrest and TNF or TRAIL-induced apoptosis. Prophase arrest induced by the Aurora kinase inhibitor VX680 likewise sensitized cells to TNF and TRAIL, with siRNA analysis pointing to Aurora kinase A (and not Aurora kinase B) as being the relevant target for this sensitization. We propose that agents that promote prophase arrest may help sensitize cancer cells to TNF and other inflammatory cytokines. We also discuss how circumvention of an early mitotic checkpoint may facilitate cancer cell survival in the inflammatory micro-environment of the tumor.
Keywords: Colon cancer, TNF, TRAIL, HDAC inhibitors, Aurora kinase inhibitors
1. Introduction
Colon cancers are frequently infiltrated by immune and inflammatory cells that play a complex role in regulating lesion growth and progression. Infiltrating cells can express high levels of Cox-2 and are therefore likely to stimulate cancer cell proliferation and lesion angiogenesis [1,2]. In addition, reactive oxygen species and other genotoxic molecules generated by inflammatory cells have been proposed to establish a mutagenic environment in which cancer progression is accelerated [3–6]. Cytokine signals generated by infiltrating cells orchestrate many of these events. A number of studies have demonstrated a role for TNF in colon cancer development. Tumor formation in an inflammation-driven mouse colon cancer model is reduced in animals lacking the p55 TNF receptor (TNFR1)[7] or through the use of the TNF inhibitor, etanercept [7]. The interplay between infiltrating cells and colon cancer development appears to feature the transcription factor NF-κB as playing an important role of protecting transformed cells from apoptosis [8,9].
Although tumor infiltrating cells can promote colon tumor growth and progression, there are aspects of the immune and inflammatory response that can suppress colon cancer growth. The adaptive immune response is likely to control lesion growth, primarily through the actions of CD8 T cells. Cancers with elevated levels of CD8 positive cells tend to have a better clinical outcome [10–12], presumably through their direct cytotoxic effects on cancer or stromal cells. Infiltration of NK cells has also been associated with improved survival [13]; NK cells can induce apoptosis through the Fas pathway [14]. The anti-cancer function of the Fas pathway is supported by the finding that genetic deletion of Fas or Fas ligand enhances tumor development in the mouse ApcMin/+ model [15,16]. Although the impact of endogenous TRAIL on colon cancer progression is not clear, expression of the TRAIL death receptors on cancer cells provides a potential avenue for treatment [17–19].
The ability of tumor infiltrating immune cells to specifically target cancer cells has raised the possibility that they may serve as a conduit for cancer therapy. Efforts have been made to stimulate the activities of cells infiltrating colon cancers in patients, and these efforts have met with some success. “GOLFIG” chemo-immunotherapy, in which gemcitabine, oxalipatin, levofolinic acid and 5-fluorouracil are combined with GM-CSF has generated promising results, significantly improving patient outcome [20–22]. The actions of the DNA targeting chemotherapeutic agents are likely to work in parallel with the immune stimulant, which appears to function by neutralizing the effects of regulatory T cells in the lesions [20].
Whether cytokines generated by infiltrating immune and inflammatory cells promote or suppress lesion growth is governed by poorly understood lesion variables. Perhaps the best example of a dual-role cytokine in cancer is TNF. TNF was originally identified as the mediator of tumor necrosis in animals treated with endotoxin [23–25]. TNF was in fact envisioned as a potential therapy, but its efficacy was limited by its toxicity [26,27]. In addition, TNF can stimulate a variety of angiogenic factors, and can activate the pro-survival transcription factor NF-κB, both of which may counteract its anti-cancer actions [28,29]. TNF has also been found to promote the transformation of NIH3T3 cells in vitro [30]. As a result of these diverse effects, it is not clear whether increasing or decreasing the expression of TNF within cancer tissues would be beneficial.
One approach to developing new colon cancer therapies is to identify treatments that specifically increase the sensitivity of cancer cells to infiltrating cells. TNF and other cytokines produced within the tumor microenvironment may be particularly effective as anti-cancer agents if their effects can be tipped in favor of apoptosis. Likewise, TRAIL-based therapies may be enhanced by agents that sensitize cells to TRAIL-induced apoptosis. Recent research has shown that a wide spectrum of cancer cell types can be sensitized to TRAIL and TNF induced apoptosis by histone deacetylase (HDAC) inhibitors [31–37]. This sensitization appears to arise in part through the simultaneous activation of both the mitochondrial and receptor-mediated death pathways [33]. However, HDAC inhibitors also effect cell cycle progression and treatment of cells grown in culture causes them to arrest in early mitosis. Mitotic arrest arises through alterations in the expression of cell cycle regulatory genes and through direct effects on mitotic chromatin condensation [38,39]. In this report we assess the interplay between the cell cycle effects of the HDAC inhibitor SAHA and cancer cell sensitization to cytokine. We find that cells arrested in prophase by SAHA are acutely sensitive to TNF or TRAIL. In addition, arresting cells in prophase through Aurora kinase A inhibition likewise enhances their cytokine sensitivity. These results suggest that agents that arrest cancer cells in prophase may enhance the anti-cancer activities of infiltrating immune and inflammatory cells. We also propose that alterations in early mitotic check point proteins in colon cancer cells, such as CHFR and Aurora kinase A [40–43], may arise in part to increase the resistance of transformed cells to the elevated levels of cytokines expressed in cancer tissue.
2. Materials and Methods
2.1. Cell Culture
The HCT116 and HT-29 colon cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA). All cell lines were cultured in a humidified 37°C incubator at 5% CO2 using McCoy's 5A medium with 10% fetal bovine serum, non-essential amino acids, and antibiotic/antimycotic (Life Technologies, Carlsbad, CA). For time-lapse microscopy, cells were transferred to a 37°C incubator in McCoy's 5A medium with 25 mM HEPES (Life Technologies) at ambient CO2 24 hours prior to imaging. Drug treatments were performed approximately 24 hours after passing. VX-680 was purchased from Selleck Chemicals (Houston, TX) and SAHA from Cayman Chemical (Ann Arbor, MI). All others chemicals used for cell treatment were purchased from Sigma-Aldrich (St. Louis, MO). TNF and TRAIL were obtained from Pierce Protein Research Products (Rockford, IL).
2.2. Caspase 3 Activity
Cells were lysed by two rounds of freeze-thawing in lysis buffer containing 10 mM Tris-HCl (pH 7.5), 0.1 M NaCl, 1 mM EDTA and 0.01% TRITON X-100. Cells were then scraped into tubes and centrifuged at 10,000 × g for 10 minutes. For assays performed on 96 well plates, cells were lysed directly on the plate and centrifuged at 4,000 × g for 10 minutes. To perform the assay, 50 μl of cell lysis supernatant was mixed with 50 μl of 2× reaction mix (10 mM PIPES pH 7.4, 2 mM EDTA, 0.1% CHAPS, 10 mM DTT) containing 200 nM of the fluorogenic substrate Acetyl-Asp-Glu-Val-Asp-7-Amino-4-methylcoumarin (DEVD-AMC; Enzo Life Sciences, Farmington, NY). The fluorescence was quantified using a microplate reader (excitation/emission 360/460 nm) at the start of the reaction and after one hour. Protein concentrations were determined using the BioRad Protein Assay reagent (BioRad, Hercules, CA, USA). Caspase activity was determined by dividing the change in fluorescence after one hour by the total protein content of the reaction mixture.
2.3. Immunofluorescence microscopy
Cells were cultured in 24-well plates on glass cover slips. After treatment, cells were washed with cold phosphate buffered saline (PBS), fixed with 4% paraformaldehyde for 10 minutes at room temperature, and then permebealized with 0.5% TRITON X-100 in PBS. Cells were blocked in 5% serum (in PBS) and then incubated on a shaker for 1 hour with diluted primary antibody solutions (1:100; 5% serum) against Aurora kinase A (#610938, BD, Franklin Lakes, NJ, USA), Aurora kinase B (ab2254, Abcam, Cambridge, MA, USA), cleaved caspase 3 (#9961, Cell Signaling Technology), cleaved caspase 8 (#9496, Cell Signaling Technology, Danvers, MA, USA), or phospho-histone H3 (Ser 28)(sc-12927, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Appropriate secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA) were selected for a 45-minute incubation. Cover slip inserts were then mounted on slides for imaging.
2.4. Time-lapse imaging
HT-29 cells stably expressing H2B-GFP were used for live cell imaging. Time-lapse videos were performed using a Personal DV microscope (Applied Precision, LLC) using a 60× oil-immersion objective. Images were taken every 8 min as z-stacks of 0.5 μm. Videos were deconvolved and quick projected using Softworks (Applied Precision, LLC).
2.5. siRNA transfection
Transfection of HT29 cells was performed as described previously [44,45] with the exception that 2.5 μl of Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used in place of Dharmafect 4 (Dharmacon Products, Lafayette, CO). Smart-pool siRNA and non-targeting control siRNA was obtained from Dharmacon for these experiments.
2.6. Flow Cytometry
Floating and adherent cells were combined and analyzed by flow cytometry. Adherent cells were harvested using a trypsin-EDTA solution, centrifuged together with the floating cells at 100 × g for 5 minutes, and resuspended in 1 mL of cold PBS. Cells were then fixed by adding 3 mL of cold 100% ethanol while gently mixing and stored at 4°C for 2 hr. Cells were then washed in PBS with 5 mM EDTA, resuspended in PBS and separated into two tubes, with one used as an unstained control. Cells were stained with 30 ug/ml propidium iodide (Sigma-Aldrich) and 0.3 mg/ml RNase A in a PBS solution for one hour in the dark and filtered prior to analysis on a FACSCalibur instrument (BD Biosciences, San Jose) using CellQuest software (BD Biosciences) for cell cycle analysis.
2.7. Treatment of tumor-bearing mice
A/J Mice, purchased from Jackson Laboratory (Bar Harbor, ME), were housed in a ventilated, temperature controlled room with a 12-h light: 12-h dark cycle. Mice were allowed free access to laboratory rodent chow (Laboratory Rodent Diet 5001; PMI Nutrition International, Richmond IN) and water. At 6 weeks of age, mice were injected i.p. with 10mg/kg azoxymethane (AOM) weekly for five weeks [46]. Twenty-four weeks after the final dose, animals were provided SAHA in the drinking water at 0.5 mg/ml for 48 hours (approximately 100 mg/kg/day at 4 ml per day). Colons were then obtained from euthanized animals, with exophytic tumors clipped from the normal adjacent tissue for separate analysis. Extracts were prepared from normal and tumor tissue, and analyzed for RNA expression and caspase 3 activity using previously described methodologies [47]. Briefly, cytosolic extracts were employed for caspase activity determination (using 50 μl in the assay described above). For histone acetylation analysis, the nuclear fraction was extracted with 1% SDS and sonicated prior to immunoblot analysis. RNA was prepared by grinding normal tissue and isolated tumors in TRIzol reagent (Invitrogen, Grand Island, NY). Reverse transcription was performed using the ABI High-Capacity cDNA reverse transcription kit (Applied Biosystems Inc., Foster City, CA) following the manufacturer's protocol. Real-time quantitative PCR was performed using an Applied Biosystems 7500 Fast Real-Time PCR system and software (Carlsbad, CA).
2.8. Statistics
Data are shown as means ± SEM (n ≥ 3). The effects of multiple treatments were analyzed with repeated measures ANOVA and a Tukey's post hoc test. Comparison between two treatments was performed using a Student's t test. A Fisher's exact test was employed to determine the significance of the association between apoptosis and the cell cycle phase. Probability values < 0.05 were considered significantly different. Significant changes of note are indicated in the figures by asterisks.
3. Results
3.1. HDAC inhibitors sensitize colon cancer cells to cytokine treatment
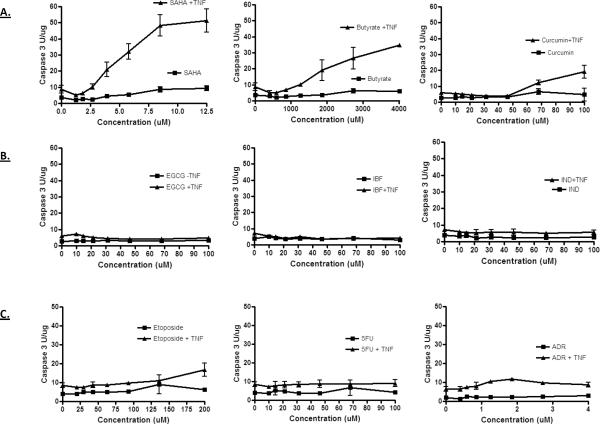
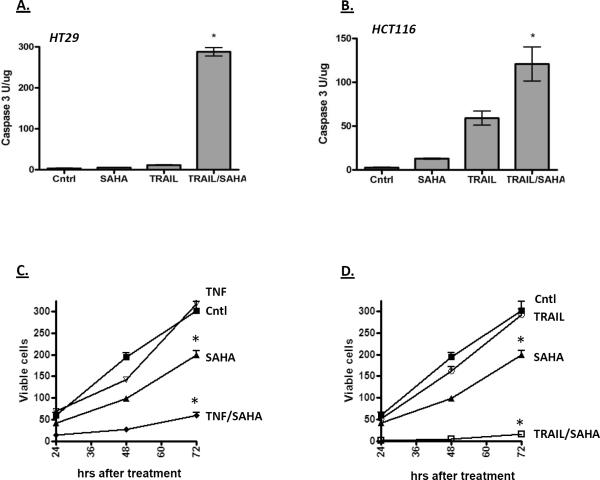
HDAC inhibitors including SAHA and butyrate have been shown to sensitize colon cancer cells to cytokines [31–37]. To determine whether this is a general activity of anticancer agents, the HT29 colon cancer cell line was treated with a number of different chemotherapeutic (etoposide, 5-fluorouracil and Adriamycin) and chemopreventive (curcumin, epigallocatechin gallate, ibuprofen and indomethacin) agents for 18 hours in the presence or absence of TNF, and then tested for apoptosis using a fluorgenic caspase 3 assay. As shown in Figure 1, the HDAC inhibitors increased caspase activity robustly when combined with TNF. Curcumin had a similar effect above 50 μM, whereas the other chemopreventive and chemotherapeutic agents tested did not. Although many of the agents tested here can induce growth arrest and apoptosis at later time points, these data indicate that HDAC inhibitors are particularly adept at acutely sensitizing the cells to TNF. SAHA was also found to sensitize HT29 and HCT116 colon cancer cells to TRAIL induced apoptosis (Figure 2A and 2B) and reduced the number of viable cells in the culture (Figure 2C and 2D). Finally, the growth rate of the surviving cells was significantly lower following treatment of TNF or TRAIL with SAHA, suggesting that the combination treatment has a sustained affect on the ability of the cancer cells to proliferate (Figure 2C and 2D).
Figure 1.
TNF sensitizes colon cancer cells to HDAC inhibitors. The HT-29 colon cancer cells line was treated with increasing concentration of the indicated agents for 18 hours in the presence or absence of TNF (100 ng/ml): SAHA, butyrate, curcumin, epigallocatechin gallate (EGCG), ibuprofen (IBF), indomethacin (IND), etoposide, 5-fluorouracil (5FU) and Adriamycin (Adr). Extracts were then prepared from cells and tested for caspase activity using the DEVD-AMC fluorgenic substrate.
Figure 2.
SAHA enhances caspase activation by TRAIL in HT-29 (A) and HCT116 cells (B). Cells were incubated with SAHA (10 μM) and TRAIL (100 ng/ml) for 18 hours as indicated, and then assayed for caspase activity as described in Figure 1. The increase in caspase activity in the combination treatment was significantly higher than the individual treatments (ANOVA; p < 0.01). C and D) Combining SAHA with TNF (C) or TRAIL (D) reduces viable cell number and slows proliferation after drug removal. HT-29 cells were treated with TNF, TRAIL and SAHA as indicated for 18 hours, after which the medium was replaced with normal growth medium. Viable cells were counted 24, 48 and 72 hours after the switch to normal growth medium. Combining SAHA with TNF or TRAIL resulted in a significant decrease in cell number and reduced the rate of cell growth after return to normal growth medium (ANOVA, p < 0.01).
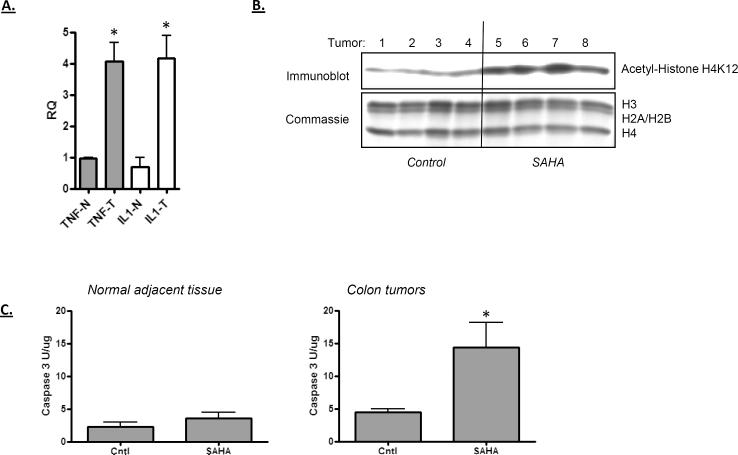
An experiment was run in the mouse AOM colon cancer model to determine whether a similar proapoptotic interaction between SAHA and cytokines may occur in vivo. As shown in Figure 3A, AOM-induced colon tumors express elevated level of cytokine, with significantly increased TNF and IL-1β expression in the tumors relative to adjacent normal tissue. Treatment of mice with SAHA (for 48 hours) increased the level of histone acetylation in the tumors (Figure 3B). The level of caspase activity within the tumors was likewise increased by the SAHA treatment, whereas no significant change in the adjacent normal tissue was observed. Although the sensitivity of the tumors in this model may arise from a number of variables, these data are consistent with the interplay between cytokine and SAHA in promoting apoptosis in vivo.
Figure 3.
A) AOM-induced mouse colon tumors express elevated levels of inflammatory cytokines TNF and IL-1. RNA was extracted from tumors (T) or adjacent normal tissue (N) and quantified for mRNA expression. Actin was used as a control RNA. Tumors showed an increased expression of TNF and IL-1 (Student t test; p < 0.01, n = 5). B) Treatment with SAHA increases histone acetylation in colon tumors. Histone proteins were isolated from tumors of four control animals (tumors 1–4) or four SAHA-treated animals (tumors 5–8). Histone acetylation was then analyzed by immunoblotting using an antibody specific for acetylated-histone H4. Gel loading was normalized by Coomassie staining of the histone proteins. C) Treatment of mice with SAHA increases caspase activation in colon tumors. Protein extracts prepared from normal tissue or colon tumors (as indicated) were assayed for caspase activity as described in Figure 1. SAHA increased caspase activity significantly in tumor tissue only (Student's t test; p < 0.01).
3.2. Mitotic effects of HDAC inhibitors and cytokine sensitivity
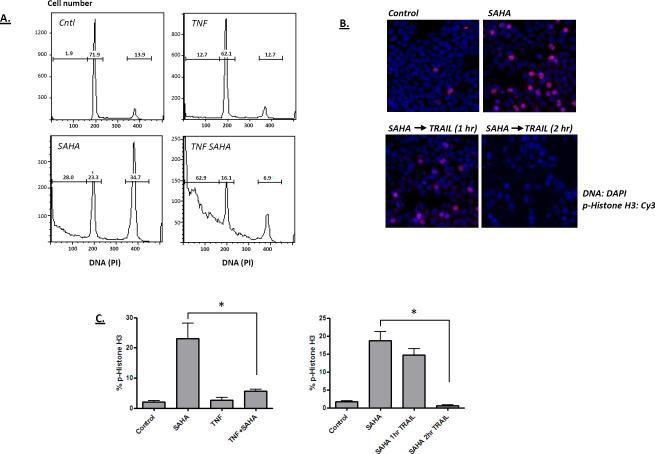
The mechanism by which HDAC inhibitors sensitize colon cancer cells to cytokine-induced apoptosis may include a range of effects, including altered expression of anti-apoptosis proteins such as cFlip and the inhibition of NF-κB. HDAC inhibitors are also known to interfere with mitosis by activating the expression of cell cycle inhibitors and by interfering with sister chromatid adhesion [48]. To assess the contribution of this mitotic effect on colon cancer cell sensitivity to cytokine, the influence of SAHA and TNF on the cell cycle distribution of HT29 cells was determined (Figure 4A). SAHA was found to increase the percentage of cells in the culture in G2/M phase, whereas TNF alone had little effect on the cell cycle distribution. When TNF and SAHA were combined, the number of sub-diploid cells was increased, accompanied with a large reduction in the number of G2/M phase cells. To more specifically determine the sensitivity of mitotic cells to cytokine treatment, cells were stained for the mitotic marker, phospho-histone H3 serine 28. Figure 4B shows that cells treated with SAHA (18 hours) show an increase in the number of cells in mitosis, which rapidly disappear from the culture following treatment with TRAIL (Figures 4B and 4C). A similar effect was observed following TNF treatment of HT29 cells arrested with SAHA (Figure 4C). The loss of mitotic cells from the culture may be a result of their rapid apoptosis.
Figure 4.
A) Cell cycle effects of TNF and SAHA. HT-29 cells were treated with TNF (100 ng/ml) or SAHA (10 μM) as indicated (for 18 hours), and then assayed for cell cycle distribution by propidium iodine labeling and flow cytometric analysis. SAHA treatment alone generated an increase in G2/M phase cells, with the number of sub-diploid cells greatest in the TNF/SAHA combination treatment. B) Influence of SAHA and TNF on cells in mitosis. HT-29 cells were treated with SAHA for 18 hours, as indicated. TRAIL was then introduced into the culture for 1 or 2 hours. Cells were fixed and analyzed for the expression of the mitosis marker: histone H3 phosphorylated at serine 28. Phospho-histone H3 staining is red and DNA/DAPI is shown in blue. C) TNF and TRAIL reduce the number of M phase cells accumulated after SAHA treatment. The left panel shows the percent of phospho-histone H3 expressing cells when TNF is included with SAHA. The right panel shows the results of Figure 4B quantified. The reduction in SAHA-arrested cells by TNF or TRAIL treatment was significant (ANOVA; p < 0.01).
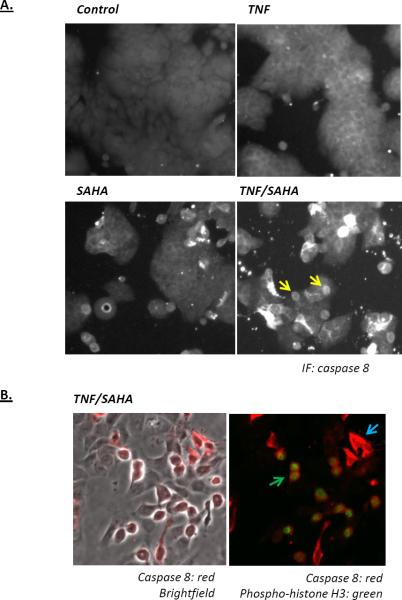
To examine the interaction between mitosis and apoptosis in more detail, HT29 cells were treated with SAHA in the absence or presence of TNF, and then analyzed for caspase 8 activation. As show in Figure 5A, active caspase 8 staining increased following treatment with TNF or SAHA, but was highest when both TNF and SAHA were present. Inspection of the cells treated with both SAHA and TNF showed that rounded cells expressed higher levels of caspase 8 (see arrows in Figure 5A). Since cells arrested in mitosis become round, cells were co-stained for active caspase 8 and phospho-histone H3 (Figure 5B). The results of this staining show that all of the mitotic cells expressed active caspase 8 (Figure 5B, green arrow). Some non-mitotic cells also activated caspase 8, but this occurred only in a subpopulation of the non-mitotic cells (Figure 5B, blue arrow).
Figure 5.
Immunofluorescence analysis of caspase 8 activation in HT-29 cells treated with SAHA and TNF. A) HT-29 cells were treated as indicated for 18 hours. Cells were then fixed and stained for active caspase 8. The arrows in the TNF/SAHA panel indicate rounded, potentially mitotic cells that stain positively for active caspase 8. B) Dual immunofluorescent staining for active caspase 8 and the mitosis marker phospho-histone H3 (Ser28). The left shows active caspase 8 staining (red) on a brightfield image and the left panel is the same image with active caspase 8 (red) and phosphohistone H3 in green. All cells showing phospho-histone H3 staining co-stained for active caspase 8; one such cell indicated by the green arrow. A sub-population of non-mitotic cells also stained for active caspase 8, with one example indicated by the blue arrow.
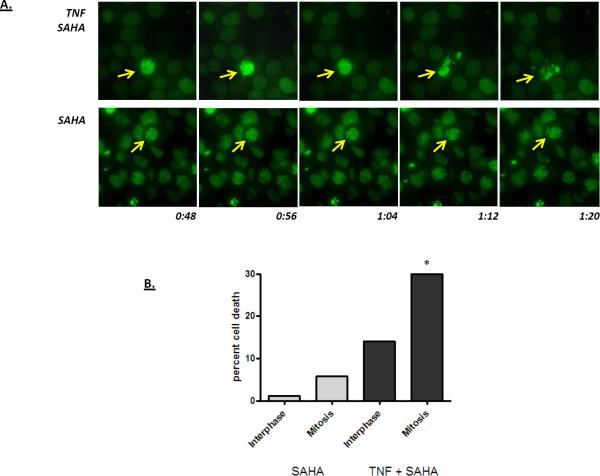
To further assess the relationship between mitotic arrest and apoptosis, HT29 cells expressing a GFP-tagged histone H2B were treated with SAHA overnight to accumulate cells in mitosis, and then treated with TNF. Time-lapse imaging was then performed. As shown in Figure 6, cells arrested in mitotic prophase were observed in the cultures treated with SAHA overnight. If the cultures not treated with TNF, these mitotic cells were stable for the duration of the experiment (Figure 6). However, cultures treated with TNF displayed an increased rate of apoptosis. Although increased apoptosis was observed in both the interphase and the arrested cells, the rate of apoptosis was significantly higher for the population of cells arrested in early mitosis (Figure 6).
Figure 6.
Time-lapse imaging of HT-29 cells expressing a histone H2B-GFP fusion protein. Cells were grown on glass cover-slips and then treated overnight with SAHA (10 μM). Cells were either left untreated (SAHA, lower panel) or were treated with TNF (TNF SAHA, upper panel), and then prepared for time-lapse imaging. A series of representative images are shown: stable chromatin condensation was typically observed in cells treated with SAHA alone whereas inclusion of TNF into the culture medium increased the rate of apoptosis of mitotic cells. The arrows in the images track an individual cell. The times at the bottom of the image indicate the length of time following TNF treatment. B) Quantification of time lapse videos showing the percent of interphase and mitotic cells undergoing apoptosis in cultures treated with SAHA and SAHA plus TNF. Mitotic cells undergo apoptosis more readily than interphase cells (p > 0.02; Fisher's exact test). Less than one percent of control cells undergo apoptosis in this assay (not shown).
3.3. Aurora kinase inhibition and cytokine sensitivity
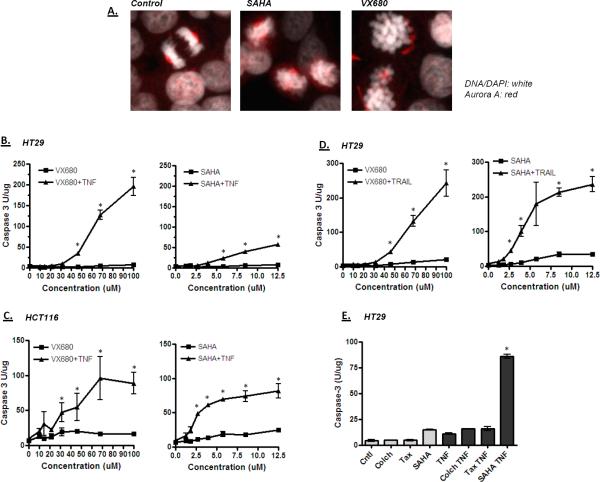
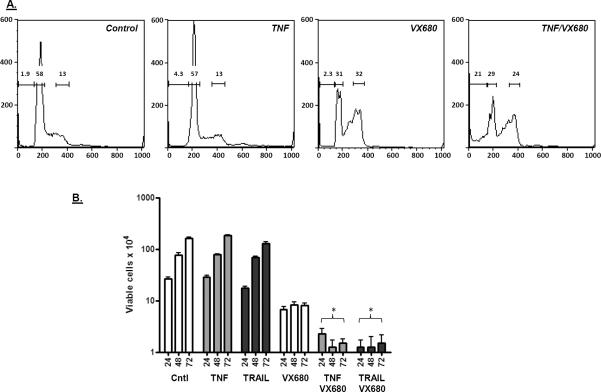
Since cells arrested in prophase by SAHA were found to be acutely sensitive to TNF and TRAIL, we determined how other mitotic blockers affected cytokine sensitivity. We first tested the Aurora kinase inhibitor VX680. As shown in Figure 7A, treatment of HT29 cells with SAHA or VX680 resulted in the accumulation of cells with condensed mitotic chromosomes, reduced centrosomal clustering of Aurora kinase A and no signs of chromosome congression on the metaphase plate. Like SAHA, VX680 was also able to sensitize colon cancer cells to cytokine (Figures 7B–D); VX680 sensitized both HT29 and HCT116 colon cancer cells to TNF or TRAIL, as determined by caspase 3 activation. This activity is not general to all mitotic inhibitors; taxol and colchicine, which arrest cells later at metaphase, did not sensitize HT29 cells to TNF (Figure 7E). To confirm the growth-inhibitory actions of VX680 in the presence of TNF or TRAIL, cells were analyzed for DNA content by flow cytometry. As shown in Figure 8A, VX680 treatment on its own induced an accumulation of cells in G2/M, and inclusion of TNF with VX680 increased the percentage of subdiploid cells over 5-fold. Finally, the number of viable cells in the culture was dramatically reduced by the TNF/VX680 and TRAIL/VX680 combinations (Figure 8B). Growth inhibition by the combination treatment persisted up to 72 hours after removal of the treatment, indicating that the growth inhibitory effect is irreversible.
Figure 7.
Effect of the Aurora kinase inhibitor VX680 on colon cancer cells. A) HT-29 cells were treated with VX680 or SAHA for 18 hours, stained for Aurora kinase A and imaged by confocal microscopy. Mitotic cells in control cultures were most frequently found at metaphase or later, with well organized Aurora kinase A staining (red) observed at the mitotic poles (left panel). SAHA and VX680 treatments generated cells with condensed chromosomes, but with poorly organized staining for Aurora kinase A. These data show similar mitotic blocks achieved by SAHA and VX680. B–D) Caspase activation shows that VX680 effectively sensitizes colon cancer cells to TNF or TRAIL. Cells were treated for 18 hours with VX680 or SAHA in the presence or absence of TNF or TRAIL as indicated, and then processed for caspase activity as described in Figure 1. B) Comparison of VX680 and SAHA for sensitizing HT-29 cells to TNF. C) Comparison of VX680 and SAHA for sensitizing HCT116 cells to TNF. D) Comparison of VX680 and SAHA for sensitizing HT-29 cells to TRAIL. E) Not all mitotic inhibitors can sensitize cells to TNF. HT-29 cells were treated with colchicine (Colch), taxol (Tax) or SAHA in the presence and absence of TNF, and the assayed for caspase activation. Only SAHA sensitized the cells to TNF. Significant differences in this figure are indicated by an asterisk (ANOVA; p < 0.01).
Figure 8.
Cell cycle effects of VX680 and TNF. A) HT-29 cells were treated with TNF (100 ng/ml) or VX680 (60 μM) as indicated (for 18 hours), and then assayed for cell cycle distribution by propidium iodine labeling and flow cytometric analysis. VX680 treatment alone increased cells in G2/M phase, with the number of sub-diploid cells greatest in the TNF/VX680 combination treatment. Gates show the percentage of subdiploid, diploid and tetraploid cells. B) Effect of VX680, TNF and TRAIL on viable cell number and cell proliferation after drug removal. HT-29 cells were treated with TNF, TRAIL or VX680 as indicated for 18 hours, after which the medium was replaced with normal growth medium. Viable cells were then counted 24, 48 and 72 hours after the switch to growth medium. Combining SAHA with TNF or TRAIL generated resulted in a significant decrease in viable cell number and reduced the rate of cell growth after return to normal growth medium (ANOVA; p < 0.001).
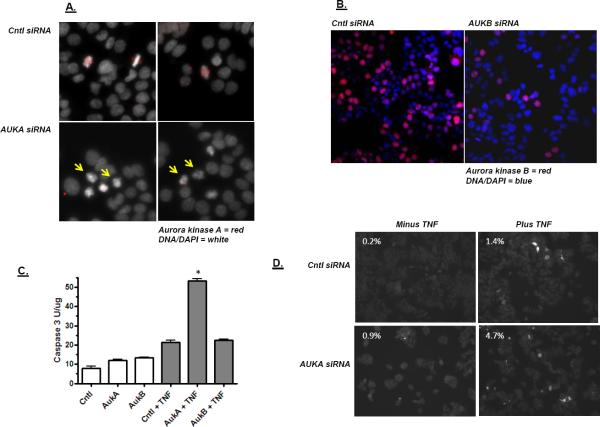
Aurora kinases A and B are structurally-related kinases that play distinct roles in mitosis, but both can be inhibited by VX680 [49]. To determine the contribution of these kinases individually to TNF-induced apoptosis, an RNAi approach was taken. Aurora kinase A is normally localized near centrosomes where it mediates mitotic spindle formation [50–52]. Knockdown of Aurora kinase A with siRNA lead to a reduction Aurora kinase A localization at the centrosome and increased the number of cells with condensed chromosomes blocked in early mitotsis (Figure 9A). Aurora B binds to chromosomes where it facilitates chromatin condensation for mitosis [53,54]. As shown in Figure 9B, Aurora kinase B siRNA generates a partial knockdown, but cells do not condense their chromosomes. Cells with Aurora kinase A or B knockdown were then tested for their sensitivity to TNF. As shown in Figure 9C, Aurora kinase A knockdown increased the sensitivity of the cells to TNF whereas Aurora kinase B knockdown did not. In addition, cell staining showed that Aurora kinase A knockdown cells treated with TNF activated caspase 3 more frequently (Figure 9D). This finding suggests that the relevant kinase target for cytokine sensitization is Aurora kinase A. This sensitization may stem from the fact that Aurora kinase A inhibition blocks cells at a relatively early stage of mitosis.
Figure 9.
Effect of Aurora kinase A and B knockdown on TNF sensitivity. A) Images of cells 48 hours following the transfection with control, non-targeting siRNA (Cntl siRNA) or Aurora kinase A siRNA (AUKA siRNA). Two independent panels are shown for each transfection reaction. Aurora kinase A appears as an organized foci in mitotic cells of the control cultures. Mitotic cells with condensed chromatin but no strong Aurora kinase A foci appear following Aurora kinase A siRNA transfection. B) Images of cells 48 hours following the transfection with control, non-targeting siRNA (Cntl siRNA) or Aurora kinase B siRNA (AUKB siRNA). Aurora kinase B knockdown is estimated at 80% by cell count. C) Effect of Aurora kinase A and B knockdown on caspase activation by TNF. 48 hours after siRNA transfection, cells were treated with TNF for four hours. Extracts were then prepared and tested for caspase activity as described in Figure 1. Aurora kinase A siRNA increased TNF activation of caspase significantly (ANOVA; p > 0.01). D) Cells were processed as described in 9C for Aurora kinase A knockdown, but were analyzed for active caspase 3 staining. The percentage of cells staining positively for active caspase 3 is indicated in each panel.
4. Discussion
Immune and inflammatory cells are frequently found infiltrating colon cancers and earlier colonic lesions and their presence is likely to play a complex role in regulating tumor growth and progression. On the one hand, inflammatory mediators can promote cancer progression through the generation of growth-stimulating factors and DNA-reactive metabolites [6,55–58]. However, cytotoxic T cells and death-inducing cytokines generated by infiltrating cells have the ability to suppress lesion growth [10,59,60]. Cytokines appear to be important for this anticancer effect since anti-tumor T cell immunity can be inhibited when TNF is absent (even though viral immunity remains intact) [61]. Consistent with the potential anti-cancer activity of immune and inflammatory cells, evidence has been obtained that stimulating these cells can be effective component of colon cancer treatment. A recently developed colon cancer treatment protocol that combines granulocyte macrophage colony stimulating factor and IL-2 with standard chemotherapeutic agents fluorouracil and oxaliplatin (referred to as GOLFIG) has been found to significantly increase patient survival [20,22]. Identifying agents that specifically promote cancer cell killing by inflammatory cytokines could help target cell killing to neoplastic lesions, and may be particularly useful in colon cancer treatment protocols that include immune and inflammatory cell stimulation. Here we show that HDAC and Aurora kinase inhibitors are well suited for sensitizing cells to TNF and TRAIL. The HDAC inhibitor SAHA was also found to target cell killing to tumor tissue in the mouse AOM model, consistent with its interaction with TNF over-expressed in these lesions. In addition to potential cancer treatment applications, agents that promote apoptosis of cancer cells in the presence of cytokines could be beneficial for cancer prevention, particularly in cases where colon cancer development is associated with a strong and chronic inflammatory component. Thus, HDAC and Aurora kinase A inhibitors (in some form) may ultimately be beneficial for reducing colon cancer development in patients with inflammatory bowel disease.
The ability of HDAC inhibitors to sensitize cancer cells to cytokine treatments has been proposed to occur through a variety of different mechanisms, including increased death receptor expression, anti-apoptotic gene expression and NF-κB activation [31,32,34,36,37,62,63]. It is difficult to say at this point whether there is a common mechanism underlying all of the reported changes. However, one consequence of HDAC inhibition that has not been previously examined for its impact on cytokine sensitization is mitotic arrest. HDAC inhibitors can induce cell cycle arrest at mitosis, a response that likely stems from the activation of Cdk inhibitory proteins such as p21WAF1 [64,65]. In addition, HDACs are required for properly condensing mitotic chromosomes and associate directly with components of the mitotic machinery where they may participate directly in spindle assembly and chromosome segregation [48,66–69]. Our studies show that mitotic arrest, and specifically arrest at prophase, constitutes the primary pathway to apoptosis in colon cancer cells treated with SAHA and TNF or TRAIL. This finding is significant since it suggests that agents that target prophase may be generally effective for sensitizing cells to cytokine-induced apoptosis. Consistent with this possibility we found that induction of prophase arrest through VX680 or Aurora kinase A siRNA knockdown likewise sensitizes colon cancer cells to cytokine induced apoptosis. Given the range of anti-mitotic agents available, it is possible that one will have the cellular and pharmacological properties well suited for colon cancer treatment and/or chemopreventive applications. With regard to the chemopreventive applications, it should be noted that the aminosalicylate mesalazine has been reported to inhibit progression through mitosis (although S phase effects have also been reported)[70–72]. Mesalazine has also been reported to reduce the risk of colon cancer ulcerative colitis patients [73–75], and although the details of this chemopreventive activity is not fully understood, this finding generally supports the potential value of mitotic-targeting agents for the prevention of inflammation-associated cancer.
Although it is not clear how arrest in early mitosis sensitizes cancer cells to death ligand, there are numerous reports of apoptotic proteins being involved in mitosis and vice versa. One potentially relevant finding is that the expression of caspase 3 mRNA peaks approximately one hour before the mitotic cyclin, cyclin B [76]. The increase in mRNA expression correlates with an increase in caspase activity. Interestingly, caspase 3 appears to be involved in regulating the mitotic spindle checkpoint such that its inhibition results in a premature breach of this checkpoint [76]. Arresting cells at an early stage of mitosis pharmacologically may therefore prolong this endogenous capsase 3 activation pathway in a manner that complements receptor-mediated apoptosis signaling. The potential interplay between mitosis and apoptosis is also supported by the finding that numerous mitotic proteins are caspase targets. For instance, CENP-C and INCENP are caspase targets and cleavage of these proteins results in the mislocalization of Aurora B kinase and a disruption of the chromosomal passenger complex [77]. It is possible that disruption of the passenger complex during early mitosis amplifies the apoptotic signal activated by death receptor activation. Additional analyses will however be required to determine how mitotic events sensitize cells to death ligands, and whether more specific mitotic manipulations might be available to specifically target cancer cells.
The primary intention of our studies is to develop treatment approaches that selectively target cancer cell apoptosis by complementing the activity of death ligands expressed at elevated levels in cancer tissue. The ability of SAHA to induce apoptosis selectively in mouse colon tumors is consistent with this effect. However, given the critical role of apoptosis in inflammation, the interaction between TNF and SAHA may also influence the course of an inflammatory response. SAHA and other HDAC inhibitors have been reported to possess promising anti-inflammatory activities [78]. For instance, SAHA has been reported to suppresses colonic inflammation in the mouse DSS model [79,80]. Whether the TNF sensitizing activity of SAHA plays a role in its anti-inflammatory actions is unclear, but enhancing apoptosis of damaged cells and/or infiltrating inflammatory cells could plausibly constitute part of this effect (along with other previously noted effects)[81,82]. Although TNF is involved in mounting an inflammatory response, evidence has been obtained that both TNF and TRAIL help resolve the inflammatory response by promoting apoptosis of neutrophils, lymphocytes and other infiltrating cells [83–85]. Although the extent to which long-term SAHA treatment will alter the inflammatory signaling within a colon tumor is unknown, it is possible that resolution will ultimately result in a smaller, less aggressive lesion.
Since cancer tissue frequently maintains high levels of cytokine production, cancer cells may evolve mechanisms that prevent prophase arrest from occurring. Interestingly, there does appear to be such a mechanism in place. The checkpoint with FHA and RING finger (CHFR) protein can detect abnormalities in prophase and return cells to late interphase (also known as antephase)[86,87]. The mechanism by which CHFR controls this checkpoint is complex, but appears to involve its E3 ubiquitin ligase activity and the promotion of PLK1 and Aurora kinase A degradation [88,89]. Colon cancer cells frequently express reduced levels of CHFR due to promoter methylation silencing, which in turn increases Aurora kinase A expression [88,90]. We found that Aurora kinase A knockdown can increase cell sensitivity to TNF, indicating that reduced CHFR/increased in Aurora kinase A expression can provide some protection from inflammatory cytokines. The silencing of CHFR has been proposed to primarily play a role in promoting chromosomal instability (CIN) in colon cancer. Although CHFR may indeed serve this function in some colon cancers, it is interesting to note that CHFR silencing is found more frequently in colon cancers with microsatellite instability (MIN) than those with CIN. This suggests that CHFR silencing may provide an advantage to colon cancer cells independent of its effects on promoting CIN. One possibility is that this silencing minimizes the prophase arrest and cytokine-induced cell death in MIN cancers. MIN cancers are characterized by a more intense infiltration of immune and inflammatory cells, so CHFR silencing may provide protection from these cells [10,91]. Additional work will be needed to determine which the types of colon cancers and colon cancer cells might most effectively be treated with prophase disrupting agents.
Acknowledgements
This work was supported by NIH grant R21CA125592 to CG. We also thank Carol Norris at the University of Connecticut Flow Cytometry & Confocal Microscopy Facility for her help in confocal imaging and flow cytometry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bamba H, Ota S, Kato A, Adachi A, Itoyama S, Matsuzaki F. High expression of cyclooxygenase-2 in macrophages of human colonic adenoma. Int J Cancer. 1999;83:470–5. doi: 10.1002/(sici)1097-0215(19991112)83:4<470::aid-ijc6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–16. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 3.Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365–72. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- 4.D'Inca R, Cardin R, Benazzato L, Angriman I, Martines D, Sturniolo GC. Oxidative DNA damage in the mucosa of ulcerative colitis increases with disease duration and dysplasia. Inflamm Bowel Dis. 2004;10:23–7. doi: 10.1097/00054725-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–62. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 6.Tardieu D, Jaeg JP, Deloly A, Corpet DE, Cadet J, Petit CR. The COX-2 inhibitor nimesulide suppresses superoxide and 8-hydroxy-deoxyguanosine formation, and stimulates apoptosis in mucosa during early colonic inflammation in rats. Carcinogenesis. 2000;21:973–6. doi: 10.1093/carcin/21.5.973. [DOI] [PubMed] [Google Scholar]
- 7.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest. 2008;118:560–70. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 9.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Prall F, Duhrkop T, Weirich V, Ostwald C, Lenz P, Nizze H, et al. Prognostic role of CD8+ tumor-infiltrating lymphocytes in stage III colorectal cancer with and without microsatellite instability. Hum Pathol. 2004;35:808–16. doi: 10.1016/j.humpath.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Funada Y, Noguchi T, Kikuchi R, Takeno S, Uchida Y, Gabbert HE. Prognostic significance of CD8+ T cell and macrophage peritumoral infiltration in colorectal cancer. Oncol Rep. 2003;10:309–13. [PubMed] [Google Scholar]
- 12.Holcombe RF, Jacobson J, Dakhil SR, Stewart RM, Betzing KS, Kannan K, et al. Association of immune parameters with clinical outcome in stage III colon cancer: results of Southwest Oncology Group Protocol 9009. Cancer Immunol Immunother. 1999;48:533–9. doi: 10.1007/s002620050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–8. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Montel AH, Bochan MR, Hobbs JA, Lynch DH, Brahmi Z. Fas involvement in cytotoxicity mediated by human NK cells. Cell Immunol. 1995;166:236–46. doi: 10.1006/cimm.1995.9974. [DOI] [PubMed] [Google Scholar]
- 15.Guillen-Ahlers H, Suckow MA, Castellino FJ, Ploplis VA. Fas/CD95 deficiency in ApcMin/+ mice increases intestinal tumor burden. PLoS One. 2010;5:e9070. doi: 10.1371/journal.pone.0009070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fingleton B, Carter KJ, Matrisian LM. Loss of functional Fas ligand enhances intestinal tumorigenesis in the Min mouse model. Cancer Res. 2007;67:4800–6. doi: 10.1158/0008-5472.CAN-06-4473. [DOI] [PubMed] [Google Scholar]
- 17.Strater J, Hinz U, Walczak H, Mechtersheimer G, Koretz K, Herfarth C, et al. Expression of TRAIL and TRAIL receptors in colon carcinoma: TRAIL-R1 is an independent prognostic parameter. Clin Cancer Res. 2002;8:3734–40. [PubMed] [Google Scholar]
- 18.van Geelen CM, Westra JL, de Vries EG, Boersma-van Ek W, Zwart N, Hollema H, et al. Prognostic significance of tumor necrosis factor-related apoptosis-inducing ligand and its receptors in adjuvantly treated stage III colon cancer patients. J Clin Oncol. 2006;24:4998–5004. doi: 10.1200/JCO.2006.06.8809. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Ren X, Alt E, Bai X, Huang S, Xu Z, et al. Chemoprevention of colorectal cancer by targeting APC-deficient cells for apoptosis. Nature. 2010;464:1058–61. doi: 10.1038/nature08871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, et al. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother. 2010;33:435–41. doi: 10.1097/CJI.0b013e3181d32f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correale P, Tagliaferri P, Fioravanti A, Del Vecchio MT, Remondo C, Montagnani F, et al. Immunity feedback and clinical outcome in colon cancer patients undergoing chemoimmunotherapy with gemcitabine + FOLFOX followed by subcutaneous granulocyte macrophage colony-stimulating factor and aldesleukin (GOLFIG-1 Trial) Clin Cancer Res. 2008;14:4192–9. doi: 10.1158/1078-0432.CCR-07-5278. [DOI] [PubMed] [Google Scholar]
- 22.Correale P, Fioravanti A, Bertoldi I, Montagnani F, Miracco C, Francini G. Occurrence of autoimmunity in a long-term survivor with metastatic colon carcinoma treated with a new chemo-immunotherapy regimen. J Chemother. 2008;20:278–81. doi: 10.1179/joc.2008.20.2.278. [DOI] [PubMed] [Google Scholar]
- 23.Oettgen HF, Carswell EA, Kassel RL, Fiore N, Williamson B, Hoffmann MK, et al. Endotoxin-induced tumor necrosis factor. Recent Results Cancer Res. 1980;75:207–12. doi: 10.1007/978-3-642-81491-4_32. [DOI] [PubMed] [Google Scholar]
- 24.Green S, Dobrjansky A, Carswell EA, Kassel RL, Old LJ, Fiore N, et al. Partial purification of a serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1976;73:381–5. doi: 10.1073/pnas.73.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–70. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selby P, Hobbs S, Viner C, Jackson E, Jones A, Newell D, et al. Tumour necrosis factor in man: clinical and biological observations. Br J Cancer. 1987;56:803–8. doi: 10.1038/bjc.1987.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Creagan ET, Kovach JS, Moertel CG, Frytak S, Kvols LK. A phase I clinical trial of recombinant human tumor necrosis factor. Cancer. 1988;62:2467–71. doi: 10.1002/1097-0142(19881215)62:12<2467::aid-cncr2820621202>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Guadagni F, Ferroni P, Palmirotta R, Portarena I, Formica V, Roselli M. Review. TNF/VEGF cross-talk in chronic inflammation-related cancer initiation and progression: an early target in anticancer therapeutic strategy. In Vivo. 2007;21:147–61. [PubMed] [Google Scholar]
- 29.Yan L, Anderson GM, DeWitte M, Nakada MT. Therapeutic potential of cytokine and chemokine antagonists in cancer therapy. Eur J Cancer. 2006;42:793–802. doi: 10.1016/j.ejca.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Komori A, Yatsunami J, Suganuma M, Okabe S, Abe S, Sakai A, et al. Tumor necrosis factor acts as a tumor promoter in BALB/3T3 cell transformation. Cancer Res. 1993;53:1982–5. [PubMed] [Google Scholar]
- 31.Butler LM, Liapis V, Bouralexis S, Welldon K, Hay S, Thai le M, et al. The histone deacetylase inhibitor, suberoylanilide hydroxamic acid, overcomes resistance of human breast cancer cells to Apo2L/TRAIL. Int J Cancer. 2006;119:944–54. doi: 10.1002/ijc.21939. [DOI] [PubMed] [Google Scholar]
- 32.Lakshmikanthan V, Kaddour-Djebbar I, Lewis RW, and Kumar MV. SAHA-sensitized prostate cancer cells to TNFalpha-related apoptosis-inducing ligand (TRAIL): mechanisms leading to synergistic apoptosis. Int J Cancer. 2006;119:221–8. doi: 10.1002/ijc.21824. [DOI] [PubMed] [Google Scholar]
- 33.Shankar S, Singh TR, Fandy TE, Luetrakul T, Ross DD, Srivastava RK. Interactive effects of histone deacetylase inhibitors and TRAIL on apoptosis in human leukemia cells: involvement of both death receptor and mitochondrial pathways. Int J Mol Med. 2005;16:1125–38. [PubMed] [Google Scholar]
- 34.Fandy TE, Shankar S, Ross DD, Sausville E, Srivastava RK. Interactive effects of HDAC inhibitors and TRAIL on apoptosis are associated with changes in mitochondrial functions and expressions of cell cycle regulatory genes in multiple myeloma. Neoplasia. 2005;7:646–57. doi: 10.1593/neo.04655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Facchetti F, Previdi S, Ballarini M, Minucci S, Perego P, La Porta CA. Modulation of pro- and anti-apoptotic factors in human melanoma cells exposed to histone deacetylase inhibitors. Apoptosis. 2004;9:573–82. doi: 10.1023/B:APPT.0000038036.31271.50. [DOI] [PubMed] [Google Scholar]
- 36.Nakata S, Yoshida T, Horinaka M, Shiraishi T, Wakada M, Sakai T. Histone deacetylase inhibitors upregulate death receptor 5/TRAIL-R2 and sensitize apoptosis induced by TRAIL/APO2-L in human malignant tumor cells. Oncogene. 2004;23:6261–71. doi: 10.1038/sj.onc.1207830. [DOI] [PubMed] [Google Scholar]
- 37.Giardina C, Boulares H, Inan MS. NSAIDs and butyrate sensitize a human colorectal cancer cell line to TNF-alpha and Fas ligation: the role of reactive oxygen species. Biochim Biophys Acta. 1999;1448:425–38. doi: 10.1016/s0167-4889(98)00156-6. [DOI] [PubMed] [Google Scholar]
- 38.Noh EJ, Lee JS. Functional interplay between modulation of histone deacetylase activity and its regulatory role in G2-M transition. Biochem Biophys Res Commun. 2003;310:267–73. doi: 10.1016/j.bbrc.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 39.Noh EJ, Lim DS, Jeong G, Lee JS. An HDAC inhibitor, trichostatin A, induces a delay at G2/M transition, slippage of spindle checkpoint, and cell death in a transcription-dependent manner. Biochem Biophys Res Commun. 2009;378:326–31. doi: 10.1016/j.bbrc.2008.11.057. [DOI] [PubMed] [Google Scholar]
- 40.Brandes JC, van Engeland M, Wouters KA, Weijenberg MP, Herman JG. CHFR promoter hypermethylation in colon cancer correlates with the microsatellite instability phenotype. Carcinogenesis. 2005;26:1152–6. doi: 10.1093/carcin/bgi058. [DOI] [PubMed] [Google Scholar]
- 41.Corn PG, Summers MK, Fogt F, Virmani AK, Gazdar AF, Halazonetis TD, et al. Frequent hypermethylation of the 5' CpG island of the mitotic stress checkpoint gene Chfr in colorectal and non-small cell lung cancer. Carcinogenesis. 2003;24:47–51. doi: 10.1093/carcin/24.1.47. [DOI] [PubMed] [Google Scholar]
- 42.Lam AK, Ong K, Ho YH. Aurora kinase expression in colorectal adenocarcinoma: correlations with clinicopathological features, p16 expression, and telomerase activity. Hum Pathol. 2008;39:599–604. doi: 10.1016/j.humpath.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998;17:3052–65. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Godman CA, Joshi R, Tierney BR, Greenspan E, Rasmussen TP, Wang HW, et al. HDAC3 impacts multiple oncogenic pathways in colon cancer cells with effects on Wnt and vitamin D signaling. Cancer Biol Ther. 2008;7:1570–80. doi: 10.4161/cbt.7.10.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spurling CC, Godman CA, Noonan EJ, Rasmussen TP, Rosenberg DW, Giardina C. HDAC3 overexpression and colon cancer cell proliferation and differentiation. Mol Carcinog. 2008;47:137–47. doi: 10.1002/mc.20373. [DOI] [PubMed] [Google Scholar]
- 46.Papanikolaou A, Wang QS, Delker DA, Rosenberg DW. Azoxymethane-induced colon tumors and aberrant crypt foci in mice of different genetic susceptibility. Cancer Lett. 1998;130:29–34. doi: 10.1016/s0304-3835(98)00101-3. [DOI] [PubMed] [Google Scholar]
- 47.Rigatti MJ, Verma R, Belinsky GS, Rosenberg DW, Giardina C. Pharmacological inhibition of Mdm2 triggers growth arrest and promotes DNA breakage in mouse colon tumors and human colon cancer cells. Mol Carcinog. 2011 doi: 10.1002/mc.20795. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eot-Houllier G, Fulcrand G, Watanabe Y, Magnaghi-Jaulin L, Jaulin C. Histone deacetylase 3 is required for centromeric H3K4 deacetylation and sister chromatid cohesion. Genes Dev. 2008;22:2639–44. doi: 10.1101/gad.484108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, et al. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262–7. doi: 10.1038/nm1003. [DOI] [PubMed] [Google Scholar]
- 50.Gopalan G, Chan CS, Donovan PJ. A novel mammalian, mitotic spindle-associated kinase is related to yeast and fly chromosome segregation regulators. J Cell Biol. 1997;138:643–56. doi: 10.1083/jcb.138.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kimura M, Kotani S, Hattori T, Sumi N, Yoshioka T, Todokoro K, et al. Cell cycle-dependent expression and spindle pole localization of a novel human protein kinase, Aik, related to Aurora of Drosophila and yeast Ipl1. J Biol Chem. 1997;272:13766–71. doi: 10.1074/jbc.272.21.13766. [DOI] [PubMed] [Google Scholar]
- 52.Glover DM, Leibowitz MH, McLean DA, Parry H. Mutations in aurora prevent centrosome separation leading to the formation of monopolar spindles. Cell. 1995;81:95–105. doi: 10.1016/0092-8674(95)90374-7. [DOI] [PubMed] [Google Scholar]
- 53.Giet R, Glover DM. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J Cell Biol. 2001;152:669–82. doi: 10.1083/jcb.152.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams RR, Carmena M, Earnshaw WC. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 2001;11:49–54. doi: 10.1016/s0962-8924(00)01880-8. [DOI] [PubMed] [Google Scholar]
- 55.Tardieu D, Jaeg JP, Cadet J, Embvani E, Corpet DE, Petit C. Dextran sulfate enhances the level of an oxidative DNA damage biomarker, 8-oxo-7,8-dihydro-2'-deoxyguanosine, in rat colonic mucosa. Cancer Lett. 1998;134:1–5. doi: 10.1016/s0304-3835(98)00228-6. [DOI] [PubMed] [Google Scholar]
- 56.Williams CS, Mann M, DuBois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–16. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 57.Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, Oser SM, et al. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–41. [PubMed] [Google Scholar]
- 58.Sinicrope FA. Targeting cyclooxygenase-2 for prevention and therapy of colorectal cancer. Mol Carcinog. 2006;45:447–54. doi: 10.1002/mc.20232. [DOI] [PubMed] [Google Scholar]
- 59.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol. 2010;222:350–66. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deschoolmeester V, Baay M, Van Marck E, Weyler J, Vermeulen P, Lardon F, et al. Tumor infiltrating lymphocytes: an intriguing player in the survival of colorectal cancer patients. BMC Immunol. 2010;11:19. doi: 10.1186/1471-2172-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Calzascia T, Pellegrini M, Hall H, Sabbagh L, Ono N, Elford AR, et al. TNF-alpha is critical for antitumor but not antiviral T cell immunity in mice. J Clin Invest. 2007;117:3833–45. doi: 10.1172/JCI32567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shankar S, Davis R, Singh KP, Kurzrock R, Ross DD, Srivastava RK. Suberoylanilide hydroxamic acid (Zolinza/vorinostat) sensitizes TRAIL-resistant breast cancer cells orthotopically implanted in BALB/c nude mice. Mol Cancer Ther. 2009;8:1596–605. doi: 10.1158/1535-7163.MCT-08-1004. [DOI] [PubMed] [Google Scholar]
- 63.Schmudde M, Braun A, Pende D, Sonnemann J, Klier U, Beck JF, et al. Histone deacetylase inhibitors sensitize tumour cells for cytotoxic effects of natural killer cells. Cancer Lett. 2008;272:110–21. doi: 10.1016/j.canlet.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 64.Stevens FE, Beamish H, Warrener R, Gabrielli B. Histone deacetylase inhibitors induce mitotic slippage. Oncogene. 2008;27:1345–54. doi: 10.1038/sj.onc.1210779. [DOI] [PubMed] [Google Scholar]
- 65.Alao JP, Olesch J, Sunnerhagen P. Inhibition of type I histone deacetylase increases resistance of checkpoint-deficient cells to genotoxic agents through mitotic delay. Mol Cancer Ther. 2009;8:2606–15. doi: 10.1158/1535-7163.MCT-09-0218. [DOI] [PubMed] [Google Scholar]
- 66.Sakai H, Urano T, Ookata K, Kim MH, Hirai Y, Saito M, et al. MBD3 and HDAC1, two components of the NuRD complex, are localized at Aurora-A-positive centrosomes in M phase. J Biol Chem. 2002;277:48714–23. doi: 10.1074/jbc.M208461200. [DOI] [PubMed] [Google Scholar]
- 67.Warrener R, Chia K, Warren WD, Brooks K, Gabrielli B. Inhibition of histone deacetylase 3 produces mitotic defects independent of alterations in histone H3 lysine 9 acetylation and methylation. Mol Pharmacol. 2010;78:384–93. doi: 10.1124/mol.109.062976. [DOI] [PubMed] [Google Scholar]
- 68.Ishii S, Kurasawa Y, Wong J, Yu-Lee LY. Histone deacetylase 3 localizes to the mitotic spindle and is required for kinetochore-microtubule attachment. Proc Natl Acad Sci U S A. 2008;105:4179–84. doi: 10.1073/pnas.0710140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Y, Kao GD, Garcia BA, Shabanowitz J, Hunt DF, Qin J, et al. A novel histone deacetylase pathway regulates mitosis by modulating Aurora B kinase activity. Genes Dev. 2006;20:2566–79. doi: 10.1101/gad.1455006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koelink PJ, Mieremet-Ooms MA, Corver WE, Wolanin K, Hommes DW, Lamers CB, et al. 5-aminosalicylic acid interferes in the cell cycle of colorectal cancer cells and induces cell death modes. Inflamm Bowel Dis. 2010;16:379–89. doi: 10.1002/ibd.21086. [DOI] [PubMed] [Google Scholar]
- 71.Luciani MG, Campregher C, Fortune JM, Kunkel TA, Gasche C. 5-ASA affects cell cycle progression in colorectal cells by reversibly activating a replication checkpoint. Gastroenterology. 2007;132:221–35. doi: 10.1053/j.gastro.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reinacher-Schick A, Schoeneck A, Graeven U, Schwarte-Waldhoff I, Schmiegel W. Mesalazine causes a mitotic arrest and induces caspase-dependent apoptosis in colon carcinoma cells. Carcinogenesis. 2003;24:443–51. doi: 10.1093/carcin/24.3.443. [DOI] [PubMed] [Google Scholar]
- 73.Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000;14:145–53. doi: 10.1046/j.1365-2036.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 74.Rubin DT, LoSavio A, Yadron N, Huo D, Hanauer SB. Aminosalicylate therapy in the prevention of dysplasia and colorectal cancer in ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4:1346–50. doi: 10.1016/j.cgh.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 75.van Staa TP, Card T, Logan RF, Leufkens HG. 5-Aminosalicylate use and colorectal cancer risk in inflammatory bowel disease: a large epidemiological study. Gut. 2005;54:1573–8. doi: 10.1136/gut.2005.070896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hsu SL, Yu CT, Yin SC, Tang MJ, Tien AC, Wu YM, et al. Caspase 3, periodically expressed and activated at G2/M transition, is required for nocodazole-induced mitotic checkpoint. Apoptosis. 2006;11:765–71. doi: 10.1007/s10495-006-5880-x. [DOI] [PubMed] [Google Scholar]
- 77.Faragher AJ, Sun XM, Butterworth M, Harper N, Mulheran M, Ruchaud S, et al. Death receptor-induced apoptosis reveals a novel interplay between the chromosomal passenger complex and CENP-C during interphase. Mol Biol Cell. 2007;18:1337–47. doi: 10.1091/mbc.E06-05-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Glauben R, Siegmund B. Inhibition of histone deacetylases in inflammatory bowel diseases. Mol Med. 2011;17:426–33. doi: 10.2119/molmed.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glauben R, Batra A, Stroh T, Erben U, Fedke I, Lehr HA, et al. Histone deacetylases: novel targets for prevention of colitis-associated cancer in mice. Gut. 2008;57:613–22. doi: 10.1136/gut.2007.134650. [DOI] [PubMed] [Google Scholar]
- 80.Glauben R, Batra A, Fedke I, Zeitz M, Lehr HA, Leoni F, et al. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol. 2006;176:5015–22. doi: 10.4049/jimmunol.176.8.5015. [DOI] [PubMed] [Google Scholar]
- 81.Tong X, Yin L, Giardina C. Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition. Biochem Biophys Res Commun. 2004;317:463–71. doi: 10.1016/j.bbrc.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 82.Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118:724–34. doi: 10.1016/s0016-5085(00)70142-9. [DOI] [PubMed] [Google Scholar]
- 83.McGrath EE, Marriott HM, Lawrie A, Francis SE, Sabroe I, Renshaw SA, et al. TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J Leukoc Biol. 2011 doi: 10.1189/jlb.0211062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collison A, Foster PS, Mattes J. Emerging role of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) as a key regulator of inflammatory responses. Clin Exp Pharmacol Physiol. 2009;36:1049–53. doi: 10.1111/j.1440-1681.2009.05258.x. [DOI] [PubMed] [Google Scholar]
- 85.Watson RW, Redmond HP, Wang JH, Bouchier-Hayes D. Bacterial ingestion, tumor necrosis factor-alpha, and heat induce programmed cell death in activated neutrophils. Shock. 1996;5:47–51. doi: 10.1097/00024382-199601000-00010. [DOI] [PubMed] [Google Scholar]
- 86.Chin CF, Yeong FM. Safeguarding entry into mitosis: the antephase checkpoint. Mol Cell Biol. 2009;30:22–32. doi: 10.1128/MCB.00687-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Matsusaka T, Pines J. Chfr acts with the p38 stress kinases to block entry to mitosis in mammalian cells. J Cell Biol. 2004;166:507–16. doi: 10.1083/jcb.200401139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukuda T, Kondo Y, Nakagama H. The anti-proliferative effects of the CHFR depend on the forkhead associated domain, but not E3 ligase activity mediated by ring finger domain. PLoS One. 2008;3:e1776. doi: 10.1371/journal.pone.0001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yu X, Minter-Dykhouse K, Malureanu L, Zhao WM, Zhang D, Merkle CJ, et al. Chfr is required for tumor suppression and Aurora A regulation. Nat Genet. 2005;37:401–6. doi: 10.1038/ng1538. [DOI] [PubMed] [Google Scholar]
- 90.Fu Z, Regan K, Zhang L, Muders MH, Thibodeau SN, French A, et al. Deficiencies in Chfr and Mlh1 synergistically enhance tumor susceptibility in mice. J Clin Invest. 2009;119:2714–24. doi: 10.1172/JCI37405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guidoboni M, Gafa R, Viel A, Doglioni C, Russo A, Santini A, et al. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am J Pathol. 2001;159:297–304. doi: 10.1016/S0002-9440(10)61695-1. [DOI] [PMC free article] [PubMed] [Google Scholar]