Abstract
The Candida albicans ALS (agglutinin-like sequence) family encodes large cell-surface glycoproteins that function in adhesion of the fungus to host and abiotic surfaces. Monoclonal antibodies (mAbs) specific for each Als protein were developed to study Als localization on the C. albicans surface. An anti-Als4 mAb demonstrated that Als4 covers the surface of yeast cells, with a greater abundance of Als4 on cells grown at 30°C compared to 37°C. On germ tubes, Als4 is localized in a restricted area proximal to the mother yeast. Immunolabeling with several anti-Als mAbs showed overlapping localization of Als1 and Als4 on yeast cells and Als1, Als3 and Als4 on germ tubes. Overlapping localization of Als proteins was also observed on yeast and hyphae recovered from mouse models of disseminated and oral candidiasis. Differences between Als localization in vivo and in vitro suggested changes in regulation of Als production in the host compared to the culture flask. Characterization with the anti-Als mAbs reveals the simultaneous presence and differences in relative abundance of Als proteins, creating an accurate image of Als representation and localization that can be used to guide conclusions regarding individual and collective Als protein function.
Keywords: Candida albicans, ALS gene, gene family, cell surface protein, monoclonal antibody, protein localization
Introduction
C. albicans is a commensal fungus of humans. It is isolated frequently from the oral mucosa, gastrointestinal and genital tracts of normally healthy individuals. However, C. albicans can overgrow its niche and cause disease when the normal microbiota is altered, or in cases of immune compromise. The close association between C. albicans and its host is mediated in part by fungal factors, some of which are encoded by gene families (Jones et al., 2004). One example is the Als (agglutinin-like sequence) family of large cell-surface glycoproteins (reviewed by Hoyer et al., 2008). In C. albicans, there are eight different genetic loci encoding Als proteins. ALS genes have a similar organization. Each has a relatively conserved 5′ domain that encodes a peptide binding site (Salgado et al., 2011), a central domain that consists of head-to-tail copies of repeated sequence units, and a 3′ domain of relatively variable length and sequence. Mature Als proteins are glycosylated heavily and localized in the C. albicans cell wall. Als protein function is discussed most commonly in the context of adhesion to host and abiotic surfaces (reviewed in Hoyer et al., 2008).
The presence of the ALS gene family raised many possibilities regarding the functional relationship between the Als proteins. For example, it was unknown whether one or more Als proteins are found at the same time on the surface of a C. albicans cell. It was also unknown whether each Als protein is found in a similar relative abundance or if some are more plentiful than others. Finally, it was also unclear whether the Als proteins are represented evenly over the cell surface, or have a specialized localization. The answers to these questions provide clues into the individual and collective function of the Als proteins. Study of ALS gene expression patterns provided some initial insight into these relationships. Analysis of C. albicans cells from disease models and human clinical specimens showed that transcription of all ALS genes could be detected, but that genes differed with respect to maximal expression levels (reviewed in Hoyer et al., 2008). Some genes could reach high transcriptional levels while others were always relatively quiet. In cultured C. albicans cells, transcription of some ALS genes was affected by stage of culture growth and/or cellular morphology. Detecting simultaneous transcription of many ALS genes argued against the idea that a single Als protein is found on the C. albicans surface at a time.
To further explore these ideas, as well as to determine the arrangement of Als proteins on individual cells in a population, efforts shifted toward raising a monoclonal antibody (mAb) specific for each Als protein. Consistent with the strong transcriptional activity of ALS3 in cultures of C. albicans germ tubes and hyphae (Hoyer et al., 1998b; Argimon et al., 2007), anti-Als3 immunolabeling showed an intense coating of protein on the cell surface (Beucher et al., 2009; Coleman et al., 2009). This coating was also found on hyphae isolated from a murine model of disseminated candidiasis (Coleman et al., 2009). Anti-Als3 immunolabeling on yeast cells was not detectable, consistent with the low ALS3 transcriptional activity in this morphological form. In contrast, anti-Als1 immunolabeled both yeast and germ tubes/hyphae (Coleman et al., 2010). ALS1 transcription rises sharply as yeast cells from a saturated culture are placed into fresh medium, whether the cells are destined to grow as yeast or germ tubes. For yeast cultures, the inoculum cells become coated with Als1, except in the bud scar. The Als1 signal weakens on the surface of cells from subsequent generations until the protein becomes undetectable by immunolabeling. Because Als1 is stable on the yeast cell surface, the culture population is quite heterogeneous with respect to Als1 presence. On germ tubes in culture, Als1 is localized proximal to the mother yeast and persists as long hyphae form. In cells recovered from a mouse model of disseminated candidiasis, Als1 is found over a much more extensive area of the hypha surface compared to cultured cells (Coleman et al., 2010).
In order to further these studies of Als protein localization, we developed a mAb specific for Als4. Here, we describe the mAb and use it to characterize the protein’s localization on cultured C. albicans cells of various morphologies and on fungal cells recovered from animal models of candidiasis.
Materials and methods
Production of mAbs
The method for producing Als-specific mAbs was described by Coleman et al. (2009, 2010) and is summarized here for convenience. mAbs were raised against Pichia pastoris-produced, hexa-His-tagged fragments from the N-terminal domain of Als proteins (including 328 to 332 amino acids, depending on which protein; Coleman et al., 2009). Briefly, Als N-terminal fragments were secreted into the culture supernatant, cleaving the secretory signal sequence from each protein, and purified by His-Trap column chromatography according to the manufacturer’s instructions (GE Healthcare). Proteins were analyzed by mass spectrometry to verify their predicted molecular mass, and visualized by SDS-PAGE and silver staining to confirm the presence of a single protein band. Als N-terminal domain fragments were used to immunize BALB/c mice and splenic lymphocytes were used to raise monoclonal antibodies. ELISA of hybridoma culture supernatant was used for initial antibody specificity testing. Positive clones were identified by their absorbance readings above background values. Typical results showed an absorbance reading of approximately 0.3 for the reaction between the anti-Als4 supernatant and its corresponding immunogen with background values (≤0.1). Purification of the mAb from the culture supernatant and reanalysis by ELISA typically produced absorbance readings of approximately 1.5 with background of ≤ 0.1. A mAb specific for Als4 (called 4-A1) was isotyped using the Monoclonal Antibody Isotyping kit (Pierce) and was IgG1 with a kappa light chain. mAb-antigen binding kinetics were measured on a Biacore 3000 Surface Plasmon Resonance system using an indirect capture method, as described previously (Coleman et al., 2009). Surface plasmon resonance was used to measure binding kinetics between the anti-Als4 mAb and its immunogen. Replicate experiments showed a dissociation constant (Kd) of 0.8 and 0.9 nM (mean = 0.85 nM).
Candida strains
Many of the C. albicans strains used in this work were derived from SC5314 (Gillum et al., 1984)., such as CAI4 (iro1-Δura3::imm434/iro1-Δura3::imm434; Fonzi & Irwin, 1993) and CAI12, which is a CAI4 derivative (ura3/URA3; Porta et al., 1999). Strain 2034 (als4Δ/als4Δ; Zhao et al., 2005) was constructed from CAI4. Reintegration of the small ALS4 allele from strain SC5314 into 2034 generated strain 2093 (Zhao et al., 2005).
Overexpression of ALS4 was accomplished using plasmid 1105 (Green et al., 2005), which is a modified version of CIp10 (Murad et al., 2000). Plasmid 1105 encodes the C. albicans TPI1 promoter and terminator sequences, separated by a polylinker that includes the restriction sites (5′ – 3′) XhoI-SmaI-NotI-BglII. The XhoI-BglII sites allow cloning for overexpression of any full-length ALS gene due to lack of these restriction sites in any of the ALS gene coding regions. The smaller allele of ALS4 from strain SC5314 was amplified by PCR using primers ALS4Xho (5′ CCC CTC GAG ATG CTT TTA CAA TTT TTG TTG CTA AGC 3′) and ALS2/4Bgl (5′ CCC AGA TCT TCA CTA AAA GAA TAT AGA AAT AGC AGT AAG TAA CAC ATA C 3′) and Pfu polymerase according to manufacturer’s instructions. The XhoI-BglII-digested fragment was ligated into XhoI-BglII-cut plasmid 1105. The PTPI1-ALS4 overexpression construct was linearized with StuI and transformed into C. albicans strain CAI4. Southern blotting demonstrated that the construct integrated into the RP10 locus of CAI4.
C. albicans strains of diverse origin and clade assignment (detailed in Coleman et al., 2009) were also used. These included strains WO-1, GC15, GC23, 1–28, 2309, SqF087, CrA038 and OKP77. A set of diverse Candida species was assembled including Candida dubliniensis strains CD36 (from Derek Sullivan, Trinity College, Dublin, Ireland), CM1 and 16F (from Richard Barton, University of Leeds, UK), as well as isolates purchased from the American Type Culture Collection (Candida glabrata ATCC 2001, Candida krusei ATCC 14243, Candida parapsilosis ATCC 22109, Candida lusitaniae ATCC 42720, Candida tropicalis ATCC 201380 and Candida guilliermondii ATCC 6260).
C. albicans culture conditions
Routine culture used YPD liquid or YPD agar plates (per liter: 10 g yeast extract, 20 g peptone, 20 g glucose, with 20 g Bacto agar added for plates). Isolates are stored at −80°C and streaked to YPD agar for use. Plates are incubated at 37°C for 24 h and stored for no more than one week at 4°C. A single colony was added to 20 ml YPD liquid for each starter culture. Starter cultures were incubated for 16 h and 200 rpm shaking. Experiments that required 37°C used cells from a 37°C starter culture; for 30°C experiments, a 30°C starter culture was used. Starter culture cells were washed in Dulbecco’s PBS without calcium or magnesium (DPBS), counted and inoculated at 1 × 106 cells ml−1 into the appropriate growth medium for the experiment. For germ tube growth, cells were inoculated into prewarmed (37°C) germ tube-inducing growth medium at a density of 5 × 106 cells ml−1. Media included RPMI 1640 without L-glutamine, YPD with 10% fetal bovine serum, DPBS with 10% fetal bovine serum, and the medium described by Lee et al. (1975). Additional cell culture details accompany individual experiments in the Results as appropriate (see below).
Covalent and immunolabeling of the C. albicans cell surface
Anti-Als immunolabeling methods have been described previously (Coleman et al., 2009, 2010). For routine immunolabeling, C. albicans cells were fixed in DPBS containing 3% paraformaldehyde. Fixed cells were washed in DPBS and non-specific binding blocked with normal goat serum. Cells were incubated with anti-Als mAb and then with a secondary FITC-conjugated antibody. Fluorescence was detected by microscopy, using one of two different microscope systems. The first was an Olympus BX50 FluoView confocal microscope using Melles Griot argon (488 nm) and krypton (568 nm) lasers. The second was a Zeiss Axiovert 200M microscope equipped with a mercury X-Cite illuminator, an ApoTome Structured Illumination Optical Sectioning system with a VH ApoTome grid under a weak ApoTome filter setting, standard excitation and emission filters for FITC and rhodamine, a Plan-Apochromat x63/1.40 numerical aperture oil objective lens, and an AxioCam Mrc 5 color camera. Methods for electron microscopy detection of immunogold labeling were described previously (Coleman et al., 2009).
Identification of the inoculum cells in a culture was accomplished by covalent labeling of the C. albicans cell surface with Alexa 594 carboxylic acid, succinimidyl ester (Molecular Probes A-20004) as described previously (Coleman et al., 2010). Alexa dyes were also used for direct labeling of mAbs for multi-color imaging of Als proteins on the C. albicans cell surface. Anti-Als1 and anti-Als3 mAbs were labeled with Alexa 633 (Molecular Probes A20170) and Alexa 594 (Molecular Probes A10239), respectively, according to manufacturer’s instructions. C. albicans germ tubes were grown at 37°C for 1 h in RPMI 1640 medium, then fixed in DPBS with 3% paraformaldehyde. Germ tubes were washed in DPBS, then labeled with anti-Als4, followed by a FITC-conjugated secondary antibody as described above. Following washing, the germ tubes were incubated with 0.018 mg ml−1 Alexa 633-conjugated anti-Als1 and Alexa 594-conjugated anti-Als3 in DPBS for 1 h at 4°C. Germ tubes were washed three times in DPBS, and suspended in 50 μl DPBS. Ten μl of the cell suspension was mixed with ProLong Gold antifade reagent (Molecular Probes P36930) in a 1:1 ratio, placed on a glass slide, coverslipped and allowed to set for 24 h. Slides were examined using the Zeiss Axiovert 200M microscope (described above).
Murine models of candidiasis
All animal experiments were conducted under the approval of the Institutional Animal Care and Use Committee of the University of Illinois. The mouse model of disseminated candidiasis involved inoculation of 5 × 105 C. albicans cells into the lateral tail vein of a 7-week-old BALB/cByJ mouse (Jackson Laboratories). Mice were euthanized 28 h post-inoculation and C. albicans analyzed in homogenized kidney tissue.
Background information for the mouse model of oral candidiasis was taken from the Candida literature (Kamai et al., 2001; de Repentigny et al., 2002; Clancy et al., 2009; Conti et al., 2009) and assembled into the method presented here. Six-week-old male C57BL/6J mice (approximately 23 g; Jackson Laboratories) were immunosuppressed with 225 mg kg−1 cortisone acetate (Sigma) in saline with 0.1% Tween 80 (Sigma) administered on days −1, 1 and 3 relative to the day of inoculation with C. albicans. On day −1, mice were anesthetized with isoflurane administered in an induction chamber and a sterile swab used to sample the oral cavity. Plating of the swab on Sabouraud dextrose agar (per liter: 20 g glucose, 10 g Bacto peptone, 17 g Bacto agar, pH 6.9) with 20 μg ml−1 chloramphenicol (SDA + Cml) was used to ensure that the mouse was free of C. albicans prior to the start of the experiment. A calcium alginate urethral swab soaked in a suspension of C. albicans yeasts (1 × 108 cells ml−1) was used to inoculate the oral cavity. Mice were anesthetized with an intraperitoneal injection of 200 mg kg−1 ketamine, placed on a heating pad, and the C. albicans-soaked swab placed sublingually for 60 min. Mice were housed separately and provided food and water ad libitum. Infection proceeded for 24 h or for 5 days. At each time point, mice were sedated with isofluorane and euthanized with an intraperitoneal injection of Fatal Plus (Vortech).
The mouse head was sagittally sectioned from ventral to dorsal along the midline splitting the mandibular symphysis and mandibular and maxillary incisors. The right side of the head was fixed in 10% neutral buffered formalin (pH 7.2) for histopathology analysis. The oral mucosa was dissected from the left side of the head to evaluate cfu g−1 of tissue. The left half of the tongue was also included in this sample. To isolate the oral mucosa, a feline periosteal elevator was used to free the palatal mucosa and gingival from the underlying bone. The pharyngeal, buccal, and labial mucosa were harvested using a combination of blunt and sharp dissection with a feline periosteal elevator and tenotomy scissors. The tissue sample was homogenized in 0.5 ml sterile saline and a portion plated onto SDA + Cml. Plates were incubated for 24 h and colonies counted manually. The remainder of the homogenate was passed over a 100 um nylon cell strainer (BD Falcon 352360) placed on the top of a 50 ml conical tube. DPBS (50 ml) was passed through the strainer to wash free C. albicans cells and smaller tissue debris into the conical tube. The tube was centrifuged to pellet the C. albicans cells. The pellet was washed twice with sterile DPBS and then fixed in 3% paraformaldehyde. After 10 min, the suspension was washed twice with DPBS and stored at 4°C until immunolabeled.
Results and Discussion
Anti-Als4 immunolabeling of C. albicans budding yeasts
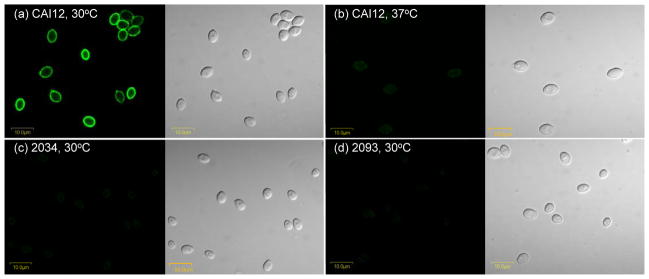
Anti-Als4 specificity was initially demonstrated using ELISA and surface plasmon resonance assays (see above). The mAb selected from these processes was called anti-Als4 4-A1. Its specificity was demonstrated further in the context of analyzing the localization of Als4 on the surface of C. albicans yeast cells (Fig. 1). Growth conditions for the yeasts were selected based on previous transcriptional data that showed growth-stage dependence of ALS4 expression in yeast cultures with the strongest expression observed in cells from saturated cultures (Hoyer et al., 1998a; Zhao et al., 2005). Yeast cells placed into fresh growth medium initially have abundant ALS4 mRNA. By mid-log phase of a growth curve, ALS4 mRNA is not detectable by Northern blot. ALS4 transcription increases in cultures as they reach saturation. Previous studies also showed a temperature-dependence for ALS4 expression with transcripts present at nearly 1000-fold higher copy number at 30°C compared to 37°C (Zhao et al., 2005). Consistent with these predictions from transcriptional data, immunolabeling of CAI12 cells from a 16 h culture showed a strong surface-localized signal for 30°C-grown cells and a barely detectable signal for cells grown at 37°C (Fig. 1a and b). Surface labeling that was observed was attributed to antigens in the outer flocculant layer of the yeast cell surface, as demonstrated by electron microscopy (Fig. 2). Immunolabeling of cells from strain 2034 (als4Δ/als4Δ) was not detectable (Fig. 1c). Reintegration of a wild-type ALS4 allele in strain 2093 did not restore cell-surface immunolabeling (Fig. 1d). This result was unexpected, given the previous phenotypic characterization of strain 2093 (Zhao et al., 2005). Deletion of ALS4 results in a strain that has reduced adhesion to monolayers of vascular endothelial cells; the reduction in adhesion just crosses the threshold of statistical significance. Reintegration of a wild-type copy of ALS4 restores the wild-type phenotype (Zhao et al., 2005). Real-time PCR measurements of transcript levels showed that strain 2093 has a fold change of 0.24 ± 0.14 compared to the CAI12 control, consistent with replacement of only one copy of the gene in the mutant isolate (Zhao et al., 2005). The ALS4 allele reintegrated to create strain 2093 is the shorter of the two ALS4 alleles from C. albicans strain SC5314. In SC5314, ALS4 alleles differ by the number of copies of a 108-bp tandemly repeated sequence in the central domain of the coding region. In SC5314, one ALS4 allele has 36 copies of the repeated sequence while the other allele only has 18 copies (Braun et al., 2005). It is possible that the shorter allele makes a protein for which the N-terminal domain is not sufficiently surface-exposed to be recognized by the anti-Als4 mAb.
Fig. 1.
Immunolabeling of C. albicans yeasts with anti-Als4 MAb. Yeasts of C. albicans control strain CAI12 were grown in YPD for 16 h at either 30°C (a) or 37°C (b). C. albicans strains 2034 (als4Δ/als4Δ; c) and 2093 (als4Δ/als4Δ::ALS4; d) were grown at 30°C for 16 h. All cells were immunolabeled with anti-Als4 and a FITC-conjugated secondary antibody. Panels with a dark background have laser (488 nm) illumination; other panels are illuminated with white light. Als4 was detected on the surface of CAI12 cells grown at 30°C and just barely detected on yeasts grown at 37°C. Als4 was not detected on the surface of the deletion mutant strain 2034 (c) nor on the surface of the ALS4 reintegrant strain 2093 (d). Cells were imaged using an Olympus BX50 FluoView microscope.
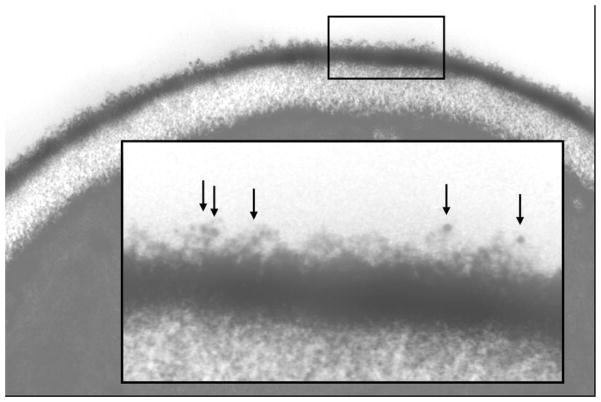
Fig. 2.
Electron micrograph of a C. albicans yeast cell. Yeast cells of strain CAI12 were grown for 16 h at 30°C in YPD medium and immunolabeled with anti-Als4 MAb and a gold-conjugated secondary antibody, prior to fixation, embedding and sectioning. Gold particles are indicated by arrows shown in the higher-magnification inset. This methodology detects Als4 that was localized in the outermost flocculant layer of the C. albicans cell surface.
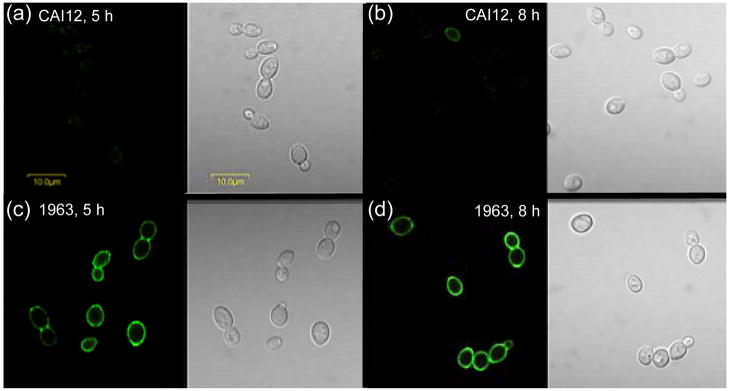
To explore whether surface-exposed Als4 can be produced by the shorter ALS4 allele, an ALS4 overexpression strain was constructed. This strain, called 1963, expresses the shorter ALS4 allele from strain SC5314 constitutively under control of the highly active promoter from the gene encoding the glycolytic enzyme triose phosphate isomerase (TPI1). Strain 1963 has the PTPI1-ALS4 construct integrated at the RP10 locus, and also two intact wild-type ALS4 alleles. In order to distinguish between Als4 produced from the wild-type alleles and Als4 produced from transcripts originating from the PTPI1-ALS4 construct, yeast cells were grown over a time course. Time points studied were those where most wild-type C. albicans cells do not have anti-Als4 surface labeling. Immunolabeling analyses showed that most cells of strain CAI12 were Als4-negative while cells from the PTPI1-ALS4 strain maintained a constant strong surface signal over the course of the experiment (Fig. 3). These results suggested that the short ALS4 allele from strain SC5134 produced protein that was visible on the C. albicans cell surface and suggested that the level of protein produced by strain 2093 was below the limit of detection for this immunolabeling assay.
Fig. 3.
Anti-Als4 immunolabeling of yeast forms over the course of culture growth to determine if Als4 produced from the strain SC5314 ALS4 smaller allele can be detected on the C. albicans cell surface. C. albicans strains CAI12 (control; a and b) and 1963 (ALS4 overexpression; c and d) were grown in YPD at 30°C. At various time points, aliquots of culture were removed, fixed with paraformaldehyde and immunolabeled with anti-Als4. For CAI12, Als4 presence on the cell surface varies with stage of growth. Many inoculum cells were Als4-positive (see Fig. 1a), but became more rare in the culture as cell divisions produce Als4-negative yeasts. At 5 h post-inoculation (a), Als4-positive CAI12 cells became more difficult to detect. At 8 h post-inoculation, ALS4 expression began to increase in strain CAI12, producing Als4-positive daughter cells (b). In contrast, strain 1963 that produced Als4 constitutively under the control of the TPI1 promoter, showed a consistently strong presence on the surface of the yeast cells at 5 h (c) and at 8 h (d). Because the native ALS4 alleles in strain CAI12 were not transcribed actively at these time points, surface Als4 in strain 1963 was derived primarily from the PTPI1-ALS4 construct and supported the conclusion that Als4 produced from the smaller ALS4 allele was detectable on the C. albicans cell surface. Cells were imaged using an Olympus BX50 FluoView microscope.
Recent work used an anti-Als5 mAb to address the issue of immunodetection limits of C. albicans cell-surface proteins (Zhao et al., 2011). Although ALS5 transcription is measured readily using real-time RT-PCR techniques, maximal transcription levels observed in wild-type cells are well below those for ALS1, ALS2, ALS3 and ALS4 (reviewed in Hoyer et al., 2008). An anti-Als5 mAb can detect Als5 on the surface of C. albicans cells overexpressing the gene, however, immunolabeling did not show a signal for wild-type cells (Zhao et al., 2011). Real-time RT-PCR was used to quantify ALS5 transcript levels and Western blotting of cell wall extracts used to demonstrate the presence of the protein. Immunolabeling signal amplification was unable to increase the signal to a visible level, helping to define a detection limit for the immunolabeling assay.
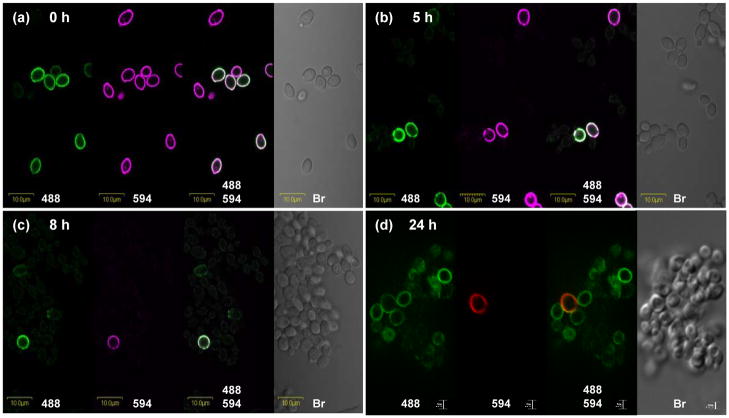
The stability of Als4 on the yeast surface was assessed by labeling the surface of inoculum yeast cells with Alexa 594 and following the cells in culture over time. This technique was introduced for the study of anti-Als1 (Coleman et al., 2010). Alexa 594 was covalently attached to the surface of yeast cells that had been grown in YPD at 30°C for 16 h; labeled cells were released into fresh YPD medium. Fig. 4a shows consistent Alexa 594 labeling of all yeast, despite the presence of Als4-negative cells among the inoculum yeast. Analysis of immunolabeled cells at 1 h post-inoculation showed buds on which Als4 presence was very weak or below the detection limit for the immunolabeling assay (data not shown). As the culture matured to 5 h (Fig. 4b) and 8 h (Fig. 4c), some Alexa 594-positive yeast maintained their Als4-positive signal, suggesting relative stability of Als4 on the C. albicans cell surface. The presence of increasing numbers of Alexa 594-negative/Als4-negative cells in the culture paralleled previously documented decreasing ALS4 transcript abundance as culture growth progressed (Hoyer et al., 1998a). At 8 h, the increasing presence of Alexa 594-negative/Als4-positive cells reflected the increasing ALS4 transcriptional activity that occurs as culture growth approaches stationary phase (Hoyer et al., 1998a). Although rare, inoculum cells were still detectable at 24 h of culture growth, although it was unclear whether the Als4 present on these cells remained from the initial inoculum time, or reflected new Als4 synthesis as the culture moved toward stationary phase (Fig. 4d).
Fig. 4.
Photomicrographs of Alexa 594-labeled and anti-Als4-labeled C. albicans yeast cells to assess the persistence of Als4 on the cell surface over the course of culture growth. Cells of strain CAI12 were grown in YPD medium at 30°C for 16 h and then covalently labeled with Alexa 594 (0 h; a). These cells were inoculated into fresh YPD medium and followed over time as they grew at 30°C and 200 rpm shaking. Cells were assessed by photomicroscopy with illumination at 488 nm (to detect the FITC-labeled secondary antibody that indicates Als4 presence), at 594 nm (to detect Alexa 594 that marks cells used in the culture inoculum) and white light (Br; bright field microscopy to visualize all cells present). Time points were taken at 5 h (b), 8 h (c) and 24 h (d). Autofluorescence of yeast cells results in faint outlines of cells at 488 nm (green); bleeding of the FITC signal into the 594 channel results in faint purple signals on cells at 594 nm. Signals from 594 nm are red in (d). Cells in panels a to c were imaged using an Olympus BX50 FluoView microscope. Cells in panel d were imaged using the Zeiss Axiovert 200M microscopy system (see Materials and methods).
Like Als4, the presence of Als1 on the surface of yeast cells in a C. albicans culture varies over the course of culture growth (Coleman et al., 2010). ALS1 transcription increases sharply when yeast cells from a saturated culture are placed into fresh growth medium, and decreases steadily over the course of culture growth. As such, inoculum cells are coated heavily with Als1, and give rise to daughters that have a decreased Als1 abundance on the cell surface. As culture growth progresses, the surface of cells from subsequent generations falls below the detection limit for labeling with anti-Als1. ALS4 transcriptional pattern and protein presence suggest that ALS4 transcription initiates as yeast cells enter mid-log phase and increases as the culture reaches saturation (Hoyer et al., 1998a). This transcriptional pattern results in differential representation of Als4 on the yeast cell surface. Most cells from a saturated culture are Als4-positive. Placing these into fresh medium results in a sharp decrease in ALS4 transcription, and as the ALS4 transcript is degraded or diluted over subsequent cell divisions, the cell-surface presence of Als4 drops below the detection limit for the immunolabeling assay (Fig. 4). The result is a heterogeneous representation of Als proteins on the surface of cultured cells, with some cells in the population exhibiting a heavy coating of more than one Als protein (see below).
Anti-Als4 immunolabeling of germ tubes
Anti-Als4 was also used to detect Als4 on C. albicans germ tubes (Fig. 5). Als4 was visible as early as 20 min following inoculation of yeasts into germ tube induction medium (Fig. 5a). On longer germ tubes, Als4 was proximal to the mother yeast, in a restricted distribution (Fig. 5b). The Als4 signal persisted in this location over time as the culture matured into hyphae (data not shown). The growth temperature of the inoculum culture dictated whether mother yeast cells were labeled with anti-Als4. Growth of the inoculum cells at 37°C resulted in mother yeasts without detectable anti-Als4 signal. However, growth of the inoculum cells at 30°C gave mother yeasts with a strong Als4 presence (Fig. 5c). Growth of germ tubes in other germ tube induction media (YPD + 10% fetal bovine serum, DPBS + 10% fetal bovine serum, Lee medium) gave similar results (data not shown).
Fig. 5.
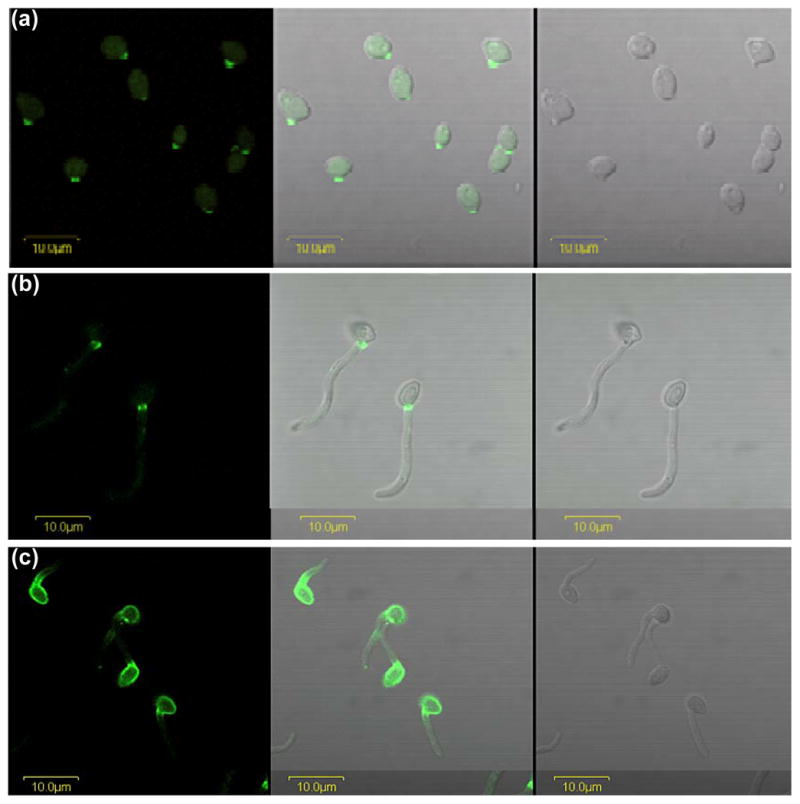
Anti-Als4 immunolabeling of germ tubes. C. albicans CAI12 was grown in YPD medium for 16 h at 37°C, washed in DPBS, counted and transferred into pre-warmed RPMI 1640 medium at a cell density of 5 × 106 cells ml−1. Cells were removed from the culture at 20 min (a) and 90 min (b), fixed in paraformaldehyde and immunolabeled with anti-Als4 and a FITC-conjugated secondary antibody. Cell-surface Als4 was visible as early as 20 min following inoculation of the RPMI 1640 culture. The restricted distribution of Als4 on germ tubes was visible at the later time point. Images were illuminated with laser only (488 nm; left panel), white light (right panel) or both (center panel). (c) Yeast cells were grown in YPD medium for 16 h at 30°C and transferred to RPMI 1640 medium at 37°C as described above. After 60 min, cells were fixed in paraformaldehyde and immunolabeled with anti-Als4. The mother yeast cells grown at 30°C had a strong Als4 presence on the surface that persisted as germ tube formation progressed. Localization of Als4 in a narrow band proximal to the mother yeast was similar to results for the 37°C-grown mother yeast. All cells were imaged using an Olympus BX50 FluoView microscope.
Anti-Als4 immunolabeling of diverse Candida isolates
Coleman et al. (2009) described a set of diverse C. albicans isolates that were also listed in Materials and methods. The isolates were selected to represent various genetic clades and were collected from different body sites in healthy or diseased humans or animals. Yeasts were grown in YPD at 30°C until culture saturation (≥ 16 h) and germ tubes were grown in RPMI 1640 for 1 h. Each of the diverse isolates had the same immunolabeling pattern as described for CAI12 (data not shown).
Anti-Als4 immunolabeling of isolates of various Candida species was also tested. Coleman et al. (2009) described the isolate collection and the information is summarized in Materials and methods. Three strains of C. dubliniensis, and one isolate each of C. glabrata, C. krusei, C. parapsilosis, C. lusitaniae, C. tropicalis and C. guilliermondii were tested. All Candida species were grown for 16 h in YPD at 30°C. Cells from this culture were also transferred to RPMI for 1 h at 37°C. Many of these Candida species do not form germ tubes or hyphae, so the morphologies observed for C. albicans cells grown under these standard conditions were not necessarily present in this work. C. tropicalis grown for 1 h in RPMI produced a positive signal with anti-Als4 suggesting that the epitope recognized by the mAb is present on this strain (Fig. 6). The anti-Als4 signal was also present on this strain for cells grown for 16 h at 30°C in YPD (data not shown).
Fig. 6.
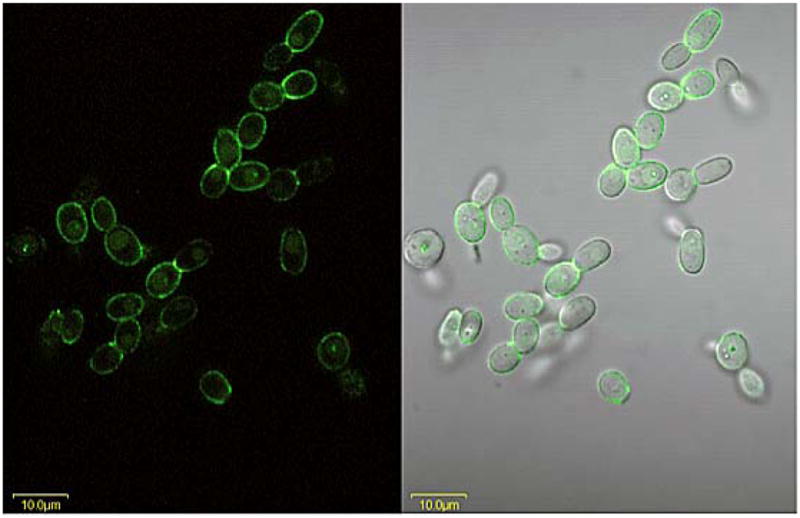
Anti-Als4 immunolabeling of C. tropicalis grown for 1 h in RPMI medium. C. tropicalis ATCC 201380 was grown for 16 h in YPD medium at 37°C and 200 rpm shaking. Cells were washed in DPBS, counted and inoculated into RPMI 1640 medium at a density of 5 × 106 cells ml−1. After 1 h, cells were collected and fixed in paraformaldehyde. Fixed cells were immunolabeled with anti-Als4 and a FITC-conjugated secondary antibody. Cells were visualized with laser light (488 nm; left panel) or laser and white light (right panel). The Als4-positive signal indicated that the anti-Als4 MAb epitope was present on C. tropicalis. C. tropicalis cells grown for 16 h in YPD were also Als4-positive (data not shown). Cells were imaged using an Olympus FluoView microscope.
Overlapping localization of Als proteins on the C. albicans surface
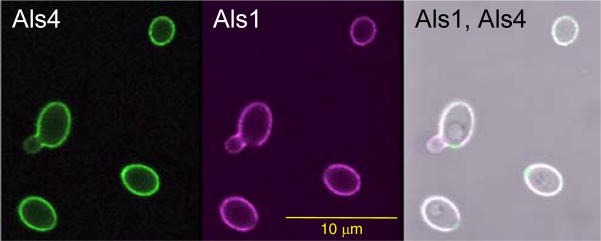
Previous work with anti-Als3 (Coleman et al., 2009) and anti-Als1 (Coleman et al., 2010) demonstrated similar localizations for these proteins and Als4 on yeast and/or germ tubes. To visualize the overlapping localization of Als proteins, yeast cells were grown at 30°C for 16 h and then placed into fresh growth medium for 1 h. Paraformaldehyde-fixed cells were immunolabeled simultaneously with Alexa 488-labeled anti-Als4 and Alexa 593-labeled anti-Als1 (Fig. 7). At this time point, ALS1 transcription has increased sharply following transfer of stationary phase cells into fresh growth medium (Coleman et al., 2010) while ALS4 transcription sharply decreases (Hoyer et al., 1998a). The net effect is yeast cells with a solid coating of both Als4 and Als1 on the surface. Lack of anti-Als1 labeling on the bud scar was noted in previous work (Coleman et al., 2010).
Fig. 7.
Overlapping localization of Als4 and Als1 on C. albicans yeast cells. CAI12 cells were grown for 16 h at 30°C, washed, counted and transferred to fresh YPD medium for 1 h at 30°C. Following fixation in paraformaldehyde, cells were immunolabeled with Alexa 488-labeled anti-Als4 and Alexa 594-labeled anti-Als1. Cells were imaged using an Olympus FluoView microscope. Als4 was visible on the surface of the yeast, with only a weak signal on the emerging bud. Als1 coated the surface of the yeast, with the exception of the bud scar. The weaker Als1 signal on the emerging bud matches previous observations for anti-Als1 immunolabeling of yeast (Coleman et al., 2010).
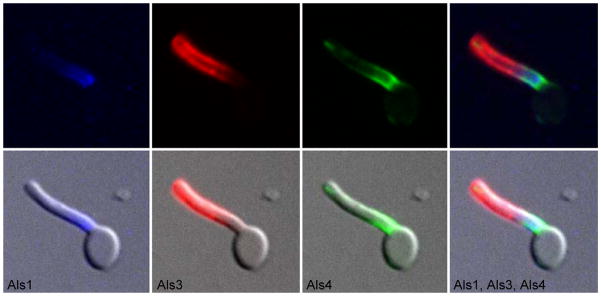
A similar multiple anti-Als mAb labeling was conducted on germ tubes. Mother yeast were grown for 16 h in YPD at 37°C and released into pre-warmed RPMI 1640 for 1 h. Paraformaldehyde-fixed germ tubes were immunolabeled with anti-Als4 and a FITC-conjugated secondary antibody, then with Alexa 633-labeled anti-Als1 and Alexa 593-labeled anti-Als3. Overlapping areas of Als protein localization were evident in the three-color micrograph (Fig. 8). Overlapping localization and stability of Als proteins argues against the contention that the ALS gene family exists for the purpose of generating antigenic variation that is a mechanism of immune evasion for the fungus. To pursue these ideas beyond the culture flask, C. albicans cells recovered from animal disease models were studied.
Fig. 8.
C. albicans germ tube labeled with anti-Als1, anti-Als3 and anti-Als4 MAbs to show overlapping localization of the three proteins. C. albicans germ tubes of strain CAI12 were grown in RPMI 1640 for 1 h. Cells were fixed in paraformaldehyde and immunolabeled with anti-Als4 and a FITC-conjugated secondary antibody, Alexa 633-labeled anti-Als1, and Alexa 593-labeled anti-Als3 as indicated under each image above. Cells were imaged using the Zeiss Axiovert 200M microscopy system (see Materials and methods). All images were illuminated with a mercury lamp. Standard filter sets for Cy5 (Alexa 633-Anti-Als1), rhodamine (Alexa 594-anti-Als3) and FITC (anti-Als4-FITC secondary antibody) were used. Upper images show individual or merged fluorescent signals while lower images include both fluorescent and bright field signals. The merged images showed that Als1, Als3 and Als4 were present simultaneously on the germ tube and had overlapping localizations.
Localization of Als proteins on C. albicans isolated from animal models of candidiasis
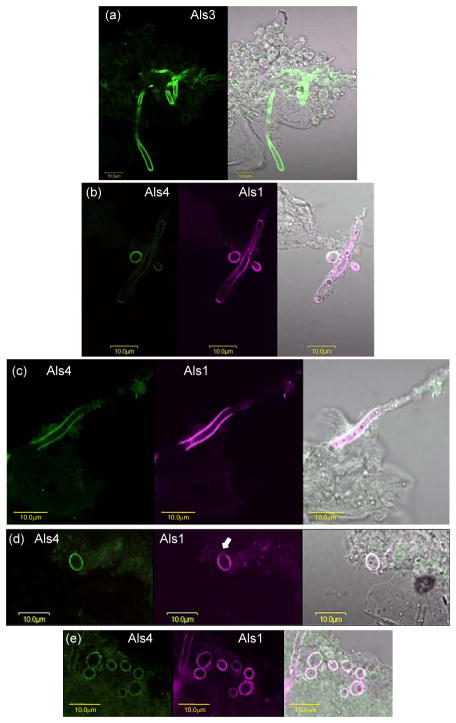
Two animal models of candidiasis were used to analyze localization of Als proteins on C. albicans cells grown in vivo. Previous work using a murine model of disseminated candidiasis showed differing localization of Als1 on hyphae, compared to results from in vitro-grown cells (Coleman et al., 2010). Mice were inoculated intravenously with C. albicans yeast cells and the infection allowed to proceed for 28 h. Mice were euthanized and the kidneys removed and homogenized. Anti-Als4 immunolabeling of the supernatant from the homogenate showed rare hyphae that were Als4-positive (Fig. 9). When detectable, these cells showed Als4 over a larger surface of the hypha than observed on cells grown in culture (Fig. 5). Because it was difficult to locate yeast cells in the kidney homogenate, Als4 localization on yeast recovered from the animal model remained unknown.
Fig. 9.
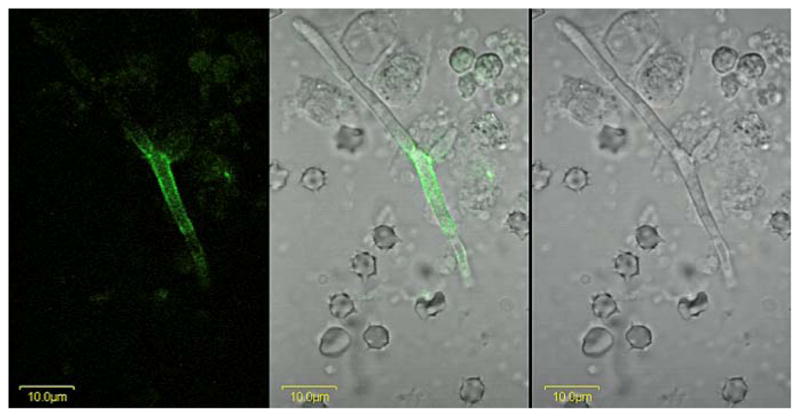
A C. albicans hypha from murine kidney tissue immunolabeled with anti-Als4. A BALB/cByJ mouse was inoculated via the lateral tail vein with 5 × 105 cells of C. albicans strain CAI12. At 28 h post-inoculation, the kidneys were removed, minced with a razor blade, and homogenized. A portion of the supernatant was treated with anti-Als4 and a FITC-conjugated secondary antibody. Cells were imaged using an Olympus BX50 FluoView microscope. Images above are illuminated with laser only (488 nm; left panel), white light (right panel) or both (center panel). Als4-positive cells were difficult to find, suggesting that they were rare in this model. Repetition of the experiment with other mice yielded the same result.
C. albicans cells were also recovered from a murine model of oral candidiasis (Fig. 10). In this model, both yeasts and hyphae are visible. Since C. albicans cells from an oral model had yet to be analyzed with anti-Als mAbs, mAbs against Als1, Als3 and Als4 were used. Fungal cells were collected from mice that were infected for 5 d. The mean cfu g−1 in the oral tissue of the five mice in this group was 9.3 × 105. As expected, anti-Als3 labeled the entire surface of hyphae from the murine model, and did not label yeast cells (Fig. 10a). Similar to the model of disseminated candidiasis, anti-Als4-positive hyphae from the oral model were rare (Fig. 10b). Anti-Als1 labeled long stretches of the hypha surface; Als1-positive hyphae were common in the oral model (Fig. 10b and c). In some instances, hyphae were positive for both Als1 and Als4 (Fig. 10c). Als4-positive and Als1-positive yeast were also easy to find. In many instances, Als1 and Als4 covered the surface of the same yeast cell (Fig. 10d and e), although cells with either Als1 or Als4 alone were detected. In some instances, anti-Als1 labeled the yeast cell surface, with the exception of the bud scar, although far more cells appeared to have the entire surface covered with Als1 (Fig. 10d and e).
Fig. 10.
C. albicans hyphae and yeast recovered from a murine model of oral candidiasis and immunolabeled with anti-Als antibodies. Immunosuppressed mice were inoculated orally with C. albicans CAI12 (see Materials and methods). After 5 days of infection, mice were euthanized and the tongue and oral mucosa homogenized. C. albicans cells were collected using a cell strainer, washed, fixed in paraformaldehyde, and immunolabeled with anti-Als mAbs. Cells were imaged using an Olympus BX50 FluoView microscope. (a). Anti-Als3 labeled hyphae, but not yeast cells. The left panel was illuminated with laser (488 nm) and the right with laser and white light. (b and c). Anti-Als1 (center panels) labeled both hyphae and yeast. Als4-positive hyphae (left panels) were more difficult to find compared to anti-Als1-labeled hyphae. In contrast to the restricted localizations of Als4 and Als1 on hyphae from culture, the proteins were detected over greater lengths of the hyphae recovered from the mouse model. (d and e). Anti-Als4 (left) and anti-Als1 (second from left) immunolabeled yeast cells. In many instances, anti-Als4 and anti-Als1 labeled the entire surface of the same yeast cell. Previous in vitro analyses demonstrated that anti-Als1 labels the yeast cell surface, with the exception of the bud scar (Coleman et al., 2010). In vivo, cells with a lack of labeling at one pole were detected (see arrow in d) although far more cells appeared to have an entire surface covered with anti-Als1 (e).
These results demonstrated the overlapping localization of Als protein in vivo, as well as the different immunolabeling patterns for C. albicans grown in culture and cells isolated following growth in vivo. Much previous work has focused on differences in gene expression between cells grown in vivo and those derived from a culture flask (reviewed in Wilson et al., 2009); the concept of niche-specific C. albicans gene expression is recognized widely (reviewed in Kumamoto, 2008). The anti-Als mAbs provide a new approach to studying these concepts, by identifying the end-product of gene expression differences in a specific host context. The anti-Als mAbs are powerful tools to address these experimental questions regarding C. albicans virulence and commensalism and the limit of relevant information that can be derived from cells in a culture flask. Most applicable to the current discussion, however, is the demonstration with these mAbs that Als proteins share overlapping localizations both in vitro and in vivo, providing a more complete image of the cell-surface architecture of C. albicans from the viewpoint of the Als family. This image of Als protein localization and presence can be used to guide conclusions regarding individual and collective Als protein function.
Acknowledgments
We thank Liping Wang and Rachel Breitenfeld of the University of Illinois Immunological Resource Center for producing the anti-Als mAbs. Lou Ann Miller of the Center for Microscopic Imaging also contributed to this work. This research was funded by grant R01 DE14158 from the National Institute of Dental and Craniofacial Research, National Institutes of Health. The investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR16515-01 from the National Center for Research Resources, National Institutes of Health.
References
- Argimon S, Wishart JA, Leng R, et al. Developmental regulation of an adhesin gene during cellular morphogenesis in the fungal pathogen Candida albicans. Eukaryot Cell. 2007;6:682–692. doi: 10.1128/EC.00340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beucher B, Marot-Leblond A, Billaud-Nail S, Oh S-H, Hoyer LL, Robert R. Recognition of Candida albicans Als3 by the germ tube-specific monoclonal antibody 3D9.3. FEMS Immunol Med Microbiol. 2009;55:314–323. doi: 10.1111/j.1574-695X.2008.00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun BR, van Het Hoog M, d’Enfert C, et al. A human-curated annotation of the Candida albicans genome. PLoS Genet. 2005;1:36–57. doi: 10.1371/journal.pgen.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy CJ, Cheng S, Nguyen MH. Animal models of candidiasis. Methods Mol Biol. 2009;499:65–76. doi: 10.1007/978-1-60327-151-6_8. [DOI] [PubMed] [Google Scholar]
- Coleman DA, Oh S-H, Zhao X, Hoyer LL. Heterogeneous distribution of Candida albicans cell-surface antigens demonstrated with an Als1-specific monoclonal antibody. Microbiology. 2010;156:3645–3659. doi: 10.1099/mic.0.043851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman DA, Oh S-H, Zhao X, Zhao H, Hutchins J, Vernachio JH, Patti JM, Hoyer LL. Monoclonal antibodies specific for Candida albicans Als3 that immunolabel cells in vitro and in vivo and block adhesion to host surfaces. J Microbiol Meth. 2009;78:71–78. doi: 10.1016/j.mimet.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Shen F, Nayyar N, et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Repentigny L, Aumont F, Ripeau JS, Fiorillo M, Kay DG, Hanna Z, Jolicoeur P. Mucosal candidiasis in transgenic mice expressing human immunodeficiency virus type 1. J Infect Dis. 2002;185:1103–1114. doi: 10.1086/340036. [DOI] [PubMed] [Google Scholar]
- Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans genes for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Green CB, Zhao X, Hoyer LL. Use of green fluorescent protein and reverse transcription-PCR to monitor Candida albicans agglutinin-like sequence gene expression in a murine model of disseminated candidiasis. Infect Immun. 2005a;73:1852–1855. doi: 10.1128/IAI.73.3.1852-1855.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer LL, Payne TL, Hecht JE. Identification of Candida albicans ALS2 and ALS4 and localization of Als proteins to the fungal cell surface. J Bacteriol. 1998a;180:5334–5343. doi: 10.1128/jb.180.20.5334-5343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer LL, Payne TL, Bell M, Myers AM, Scherer S. Candida albicans ALS3 and insights into the nature of the ALS gene family. Curr Genet. 1998b;33:451–459. doi: 10.1007/s002940050359. [DOI] [PubMed] [Google Scholar]
- Hoyer LL. The ALS gene family of Candida albicans. Trends Microbiol. 2001;9:176–180. doi: 10.1016/s0966-842x(01)01984-9. [DOI] [PubMed] [Google Scholar]
- Hoyer LL, Green CB, Oh S-H, Zhao X. Discovering the secrets of the Candida albicans agglutinin-like sequence (ALS) gene family—a sticky pursuit. Med Mycol. 2008;46:1–15. doi: 10.1080/13693780701435317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T, Federspiel NA, Chibana H, et al. The diploid genome sequence of Candida albicans. Proc Natl Acad Sci USA. 2004;101:7329–7334. doi: 10.1073/pnas.0401648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamai Y, Kubota M, Kamai Y, Hosokawa T, Fukuoka T, Filler SG. New model of oropharyngeal candidiasis in mice. Antimicrob Agents Chemother. 2001;45:3195–3197. doi: 10.1128/AAC.45.11.3195-3197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. Niche-specific gene expression during C. albicans infection. Curr Opin Microbiol. 2008;11:325–330. doi: 10.1016/j.mib.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KL, Buckley HR, Campbell CC. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia. 1975;13:148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Murad AM, Lee PR, Broadbent ID, Barelle CJ, Brown AJ. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast. 2000;16:325–327. doi: 10.1002/1097-0061(20000315)16:4<325::AID-YEA538>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Porta A, Ramon AM, Fonzi WA. PRR1, a homolog of Aspergillus nidulans palF, controls pH-dependent gene expression and filamentation in Candida albicans. J Bacteriol. 1999;181:7516–7523. doi: 10.1128/jb.181.24.7516-7523.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado PS, Yan R, Taylor JD, Burchell L, Jones R, Hoyer LL, Matthews SJ, Simpson PJ, Cota E. Structural basis for the broad specificity to host-cell ligands by the pathogenic fungus Candida albicans. Proc Natl Acad Sci USA. 2011;108:15775–15779. doi: 10.1073/pnas.1103496108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D, Thewes S, Zakikhany K, et al. Identifying infection-associated genes of Candida albicans in the postgenomic era. FEMS Yeast Res. 2009;9:688–700. doi: 10.1111/j.1567-1364.2009.00524.x. [DOI] [PubMed] [Google Scholar]
- Zhao X, Oh S-H, Yeater KM, Hoyer LL. Analysis of the Candida albicans Als2p and Als4p adhesins suggests the potential for compensatory function within the Als family. Microbiology. 2005;151:1619–1630. doi: 10.1099/mic.0.27763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Oh S-H, Coleman DA, Hoyer LL. ALS51, a newly discovered gene in the Candida albicans ALS family, created by intergenic recombination: analysis of the gene and protein, and implications for evolution of microbial gene families. FEMS Immunol Med Microbiol. 2011;61:245–257. doi: 10.1111/j.1574-695X.2010.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]