Abstract
Hsp90 is a chaperone protein that interacts with client proteins that are known to be in the cell cycle, signaling and chromatin-remodeling pathways. Hsp90 inhibitors act additively or synergistically with many other drugs in the treatment of both solid tumors and leukemias in murine tumor models and humans. Hsp90 inhibitors potentiate the actions of anti-cancer drugs that target Hsp90 client proteins, including trastuzumab (Herceptin™) which targets Her2/Erb2B, as Hsp90 inhibition elicits the drug effects in cancer cell lines that are otherwise resistant to the drug. A Phase II study of the Hsp90 inhibitor 17-AAG and trastuzumab showed that this combination therapy has anticancer activity in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. In this review, we discuss the results of Hsp90 inhibitors in combination with trastuzumab and other cancer drugs. We also discuss recent results from yeast focused on the genetics of drug resistance when Hsp90 is inhibited and the implications that this might have in understanding the effects of genetic variation in treating cancer in humans.
Keywords: Hsp90, cancer, drug resistance, geldanamycin
1. INTRODUCTION
Heat shock protein 90 Hsp90, a protein of molecular weight 90 KDa that is conserved from yeast to humans, is a molecular chaperone with over 200 identified client proteins. Hsp90 is an especially promising target for anti-cancer drugs as many of its client proteins are present in pathways that are often disrupted in many types of cancers [1]. A list of Hsp90 client proteins can be found at (http://www.picard.ch/downloads/Hsp90facts.pdf). Client proteins include apoptotic factors, protein kinases, transcription factors, and signaling proteins. Some client proteins, like steroid receptors [2–6], epidermal growth factor receptor (EGFR) family members [7], the MET oncogene [8, 9], Raf-1 kinases [10], AKT kinases [11], BCR-ABL fusion proteins in leukemia [8, 12–14], mutant p53 [15], cyclin dependent kinase 4 (CDK4) [16–18], hypoxia-inducible factor 1α(HIF1α), matrix metalloproteinase 2 (MMP2) [19], and chromatin-remodeling proteins such as the histone deacetylases (HDACs) [20–24] and SMYD3 [25, 26] are often mutated in cancer cells.
While Hsp90 is required in all cells, tumor cells are especially sensitive to Hsp90 inhibitors as they are “oncogene addicted” and require especially high levels of Hsp90 [27–31]. Genetically unstable cancer cells live under a multitude of stresses, including mutated and amplified signaling and client proteins, chromosome and microsatellite instability and aneuploidy, hypoxia, low pH, and low nutrient concentrations [32–36]. Cancer cells can survive and thrive in stressed microenvironments by quickly selecting for adaptive mutations and chromosomal rearrangements that increase their survival and proliferative abilities.
Unfortunately, the effectiveness of anti-cancer drugs that specifically target individual cancer promoting proteins or signaling pathways may be gradually decreased, or even totally lost, due to the genetic and epigenetic variation in cancer cells, as they become drug resistant. One strategy to address this problem is to identify targets, such as Hsp90, the proteasome [37], and the autophagosome [38–40], that affect multiple signaling pathways or the basic machinery required for cancer cells to survive under stress.
Inhibition of Hsp90 functions affects multiple oncogenic substrates simultaneously and has shown obvious anti-cancer effects in vitro and in vivo. One Hsp90 inhibitor, 17-allylamino, 17-demethoxygeldanamycin (17- AAG), a geldanamycin analog, has completed phase II clinical trials in a number of cancers [41–47] (see http://www.clinicaltrials.gov for a list of many clinical trials). Geldanamycin and its derivatives, as well as structurally different compounds like radicicol [48], are N-terminal Hsp90 inhibitors that interfere with the ATP-binding domain of Hsp90. Many C-terminal Hsp90 inhibitors are under preclinical development including several novobiocin- [49] and coumarin-based inhibitors [50].
Chemotherapy and radiation therapy [51] remain the most commonly used treatments for cancer, but new and more specific anti-cancer drugs are emerging. However, due to the rapid genetic and epigenetic changes in adaptation to stress induced by anti-cancer drugs, cancer cells are often able to become resistant to single or multiple anti-cancer agents [52–54]. The development of resistance is especially serious with chemotherapy and radiation therapy, and a critical goal of cancer therapy is to more effectively combat this resistance. Drug resistance can be induced by decreasing the uptake of water-soluble drugs, changing the activity of cytotoxic drugs by covalent modifications, by oxidation [55–58], glutathionylation [59], and glucuronidation [60], and by increasing the efflux of hydrophobic drugs [52–54].
2. HSP90 AND THE EVOLUTION OF NEW PHENOTYPES
Hsp90 aids in the folding of many signaling proteins under basal conditions, and in environmental stress, such as in cancer cells. In all eukaryotes studied, from fungi to mammals, Hsp90 and its orthologs are among the most abundant proteins comprising 1–2% of the total proteins under normal conditions [61]. Hsp90 is unique among the protein chaperones as its client proteins are primarily signaling molecules, such as nuclear-hormone receptors, tyrosine kinases, and chromatin-remodeling proteins [62–64]. It is termed a “heat shock protein”, but actually Hsp90 has high constitutive protein levels that are induced approximately 2 fold during environmental stress [65–68]. For example, yeast, which is presumably similar to human cells in this respect, has 445,000 molecules of Hsp82 (stress-induced Hsp90) per cell and upon stress this amount may be increased by two fold. This is in comparison to many kinases and transcription factors in both yeast and human cells which have fewer than 10,000 molecules per cell (http://yeastgfp.yeastgenome.org/). During f stress, Hsp90 protein levels are higher, but its chaperone activity is functionally titrated by the increase in the level of unfolded signaling proteins, co-chaperones, and post-translational modifications [69].
Hsp90 has been postulated to have a major role in facilitating the rapid evolution of new traits. In Drosophila and Arabidopsis, it is viewed as a “capacitor” for morphological evolution because reducing Hsp90 levels during early development produces a multitude of new phenotypes by unmasking hidden phenotypic variation in adults [69–72]. It has been proposed that the variation is unmasked because numerous signaling molecules that are involved in morphological development are targets of Hsp90 and, consequently, have altered activity when Hsp90 levels are reduced [71, 72]. Several generations of selection of the unmasked new phenotype enriches the polymorphisms that contribute to the phenotype by genetic rearrangement, ultimately leading to a stable phenotype even in the absence of stress [71, 72].
Our laboratory has shown in Drosophila that reduction of Hsp90 activity can epigenetically unmask new phenotypes, even in the absence of genetic variation [73]. We thus propose that epigenetic induction of new phenotypes by stress can facilitate the genetic rearrangement required to permanently stabilize the new phenotype in the selected population [74–77]. We also propose that epigenetic induction of new phenotypes by stress is mutagenic and that this can allow the stochastic induction of new mutations that can stabilize the new phenotype in the selected population [74–77]. Recently, Gangjaraju and colleagues showed that Hsp90 reduction epigenetically activates transposons in Drosophila by inactivation of the Piwi protein, an Argonaute-family protein that is involved in the microRNA pathway of RNA-directed chromatin repression [78]. In other words, Hsp90 can facilitate evolution of the organism, as well as the cancer cell, by both epigenetic and genomic mechanisms.
In 2005, Cowen and Lindquist showed that high levels of Hsp90 facilitated the evolution of drug resistance in diverse species of fungi by altering the activities of mutated drug resistance genes [70]. We also proposed that Hsp90 might have a similar effect in the development of drug resistance in cancer cells [79, 80].
3. SYNERGISTIC EFFECTS OF HSP90 INHIBITORS AND OTHER ANTI-CANCER DRUGS
Recent preclinical and clinical studies explored the effects of a combination of Hsp90 inhibitors and other anti-cancer agents in cancer therapy. Based on the different therapeutic mechanisms of conventional anti-cancer drugs, Hsp90 inhibitors exerted different effects in these combinational studies. Additive or synergistic effects were observed in most cases (Table 1).
Table 1.
Additive/Synergetic Effects of Hsp90 Inhibitors and Other Anti-cancer Drugs
| Hsp90 inhibitors |
Drugs in combination |
Interaction | Cancer cell type |
Mechanism; clinical trial (if performed) |
References |
|---|---|---|---|---|---|
| GA/17-AAG | cisplatin | synergetic | Colon (in HCT116 cell); antagonize in HT29 cell; solid tumors | schedule-dependent; p53, JNK pathway involved; phase 2 for solid tumors | [91, 92, 174] |
| radicicol | cisplatin, oxaliplatin | synergistic | Colon; Glioma | MLH1 proficient/deficient; no change in apoptosis/cell cycle | [94, 175] |
| GA | cisplatin | synergistic | pediatric solid tumor (neuroblastoma, ostersarcoma); hepatoma; | depletion of Akt, IGF1R; cell cycle arrest; | [93, 95] |
| 17-AAG | Taxol | Synergistic (schedule dependent: together) | breast cancer (mice Xenograft tumors (BT-474) and cell culture) | down-regulate Akt and Her2 | [82, 85] |
| 17-AAG | TRAIL receptor antibody (HHGS-ETR1/2) | synergistic | Hodgkin’s lymphoma, colon, prostate | down-regulate Akt, Erk, cell cycle arrest and death; suppression of NF-κB pathway (RIP and IKK degradation) | [101–104, 176] |
| 17-AAG | bortezomib (PS-341, proteasome inhibitor) | synergistic | breast (MCF-7 cell), leukemia, multiple myeloma | administration: simultaneously better than sequential addition; accumulation of aggregates | [41, 97, 98, 177] |
| 17-AAG | UCN-01 (7-hydroxystaurosporine), chk1 inhibitor | synergistic | leukemia (cell culture, U937 et al_) | interruption of RAF/MEK and Akt pathways | [117] |
| 17-AAG | imatinib mesylate/PD1 80970 | enhancement | leukemia (CML) | decrease Bcr-Abl | [114, 177–179] |
| 17-AAG | PKC412 (FLT3 tyrosine kinase inhibitor) | synergistic | AML (cell culture) | down regulate FLT3, Akt, Erk,STAT5; selective for AML with mutated FLT3 | [113] |
| 17-AAG | GTP14564 (FLT3 tyrosine kinase inhibitor against) | synergistic | leukemia (leukemias with FLT3 mutations) | reduced level of FLT3, STAT5, enhanced G0/G1 arrest and apoptosis in leukemia with FLT mutations | [180] |
| 17-AAG | LY294002 (PI3K inhibitor) | synergistic | malignant glioma | down regulate PI3K/Akt | [116, 177, 181] |
| 17-AAG | gemcitabine (only effective in S phase cells) | sensitize | ovarian tumor, myeloid leukemia cell line | 17-AAG arrests cells in G1 and G2; deplete chk1; phase 1 for solid tumors | [174, 182] |
| GA, Radicicol | topoisomerase II poison (etoposide) (VP16), | synergistic | colon (HCT 116) | DNA damage; topoisomerase II activity increase | [120, 183] |
| 17-AAG | LBH589 (HDACI) | synergistic | CML, AML | attenuate levels of mutant Bcr-Abl | [111] |
| 17-AAG | histone deacetylase inhibitors (HDACIs): suberoylanilide hydroxamic acid (SAHA) and sodium butyrate (SB) | synergistic | leukemia cell lines: human U937, human promyelocyt ic (HL-60) and lymphoblastic (Jurkat) leukemia cells | Multiple perturbations in signaling, cell cycle, and survival pathways that culminate in mitochondrial injury and apoptosis. Through ERK activation and p21CIP1, not Akt | [21, 110, 184] |
| 17-AAG | ATO (arsenic trioxide) | synergistic | leukemia | abrogate Akt activation, increased ROS generation | [118, 177] |
| 17-AAG | cytarabine | synergistic | leukemia (AML) | Chk1 depletion | [119, 185] |
| 17-AAG | doxorubicin | synergistic | breast cancer (cell culture) | not RB and schedule dependent | [85, 186] |
| 17-AAG | trastuzumab (Herceptin), first humanized antibody, target ErbB2 | enhancement at lower doses in TR sensitive cells; no enhancement in TR resistant cells (same as 17-AAG) | breast | reduce ErbB2 levels; phase 2 clinical trial for breast cancer | [42, 115, 187] |
| radicicol | Emunin, NZ28 (inhibit heat shock response) | sensitize | MM.1s myeloma | reduced Hsp70 level | [121] |
Preclinical data from different cancer cell lines and tumor xenograft models indicate that Hsp90 inhibitors show additive or synergistic effects in killing cancer cells when combined with most conventional cytotoxic agents (such as taxanes, cisplatin, gemcitabine and cytarabine), proteasome inhibitors, HDAC inhibitors, and new molecular targeting agents in schedule-and-cell-type-dependent manners (Table 1).
Combination therapies of Hsp90 inhibitors and other drugs are now in phase II clinical trials. A recently completed phase II study of 17-AAG, an Hsp90 inhibitor, and trastuzumab, showed that this combination therapy has significant anticancer activity in patients with HER2-positive metastatic breast cancer progressing on trastuzumab [42]. In this study, 31 breast cancer patients progressing on trastuzumab were enrolled with a median age of 53 years and a minimum Karnofsky performance status (KPS) of 90% [42]. The KPS attempts to quantify cancer patients' general well-being and activities of daily life and is used in oncological randomized controlled trials as a measure of quality of life. The KPS runs from 0% (dead) to 100% (healthy with no problems).
The exciting results with 17-AAG and trastuzumab in treating trastuzumab-resistant breast cancer, combined with the other Hsp90 combination preclinical trials in rodents, suggests that many more clinical trials will be attempted in the near future.
3.1. Taxanes
Paclitaxel (Taxol®) is a mitotic inhibitor used in cancer chemotherapy. It stabilizes microtubules, thereby causing mitotic arrest and apoptosis [81]. Taxol is one of the two clinically available taxanes and is used in against a broad range of cancers. Hsp90 inhibitors, such as 17-AAG and geldanamycin (GA), sensitize lung and breast cancer cells to paclitaxel induced cytotoxicity both in vitro and in vivo [82–86]. Low doses of 17-AAG enhance paclitaxel cytotoxicity by drastic reduction of paclitaxel 50% inhibitory concentration (IC50) values and significantly increase induction of apoptosis.
The synergistic effects of 17-AAG and other drugs are dependent on the cell type [82, 84, 85]. In cells expressing retinoblastoma (RB), or high level of ErbB2 or Akt, that are clients of Hsp90, concurrent exposure to17-AAG and paclitaxel is required for the synergistic activity of the two drugs. Exposure of these cells to 17-AAG causes a G1 growth arrest [82, 85, 87], whereas paclitaxel arrests the cells in mitosis. Thus, in future development of combinational treatment strategy, the administration schedule should be considered if cell cycle dependent changes are involved in modulating the activity of the drug.
3.2. Cisplatin
The compound cis-PtCl2(NH3)2 (cisplatin), also known as Peyrone's salt [88], is used to treat several types of cancers, including sarcomas, carcinomas, lymphomas, and germ cell tumors. Cisplatin crosslinks DNA and consequently trigger apoptosis [89, 90]. It has been widely used alone or in combined regimes with other anti-cancer drugs for the therapy of a variety of tumors and often shows synergistic anti-cancer effects in different cancer types [91–95]. Of the 17-AAG and cisplatin combinations, synergistic anti-cancer activities were observed in several colon cancer cell lines [91, 92], pediatric solid tumor cells cultures (neuroblastoma and osteosarcoma) [95], and hepatoma cell cultures and xenograft models [93].
Radicicol, another widely-used Hsp90 inhibitor, also sensitizes colon cancer cells to cisplatin via the interaction of Hsp90 with MLH1, a protein crucial for DNA mismatch repair [94]. It has been proposed that synergistic interactions depend on the effect exerted by 17-AAG on cisplatin-induced signaling through the JNK stress-induced and the p53 DNA-damage-induced pathways [91, 92]. Cisplatin and Hsp90 inhibitors like 17-AAG, might be important in inducing cytoprotective effects, thereby lowering the toxicity of chemotherapeutic agents such as gemcitabine [96].
3.3. Proteasome Inhibitors
Bortezomib (PS-341; Velcade™) is the first proteasome inhibitor approved for the treatment of relapsed multiple myeloma (MM) and mantle cell lymphoma (MCL). In MM, complete responses have been obtained in patients with otherwise rapidly advancing disease [41, 97, 98]. The attributing mechanisms include increased protein misfolding, coupled to impaired protein clearance by suppression of the chymotryptic activity of the 20S proteasome. The marked anti-cancer activity of a combination of Hsp90-and-proteasome inhibitors might arise from their complementing abilities to simultaneously trigger intracellular accumulation of unfolded proteins and preventing their cellular protection functions [41]. More importantly, combined Hsp90-and-proteosome-inhibitors treatment overcomes the drug resistance of primary MM cells which are resistant to cytotoxic chemotherapy and bortezomib [41].
3.4. Death Receptor Ligands: Tumor Necrosis Factor (TNF) and Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL)
TRAIL binds to the death receptors DR4 (TRAIL-RI) and DR5 (TRAIL-RII) and induces caspase-8-dependent apoptosis. It also binds the receptors DcR1, a decoy receptor and DcR2, which contains a truncated death domain and activates NFκB. The apoptosis-inducing “death receptor” ligands, TRAIL and TNF, are promising candidates for cancer treatment but display variable cytotoxicity and drug resistance in different cell lines [99]. Combination of 17-AAG with “death receptor” targeting agents can synergistically increase their anti-tumor activities and abolishes the drug resistance of TRAIL/TNF in Glioma [100]. In TRAIL/TNF-resistant cancer cell lines, such as prostate LNCaP cells, and colon HT29 and RKO cells, pre- or co-exposure to17-AAG with TRAIL/TNF induced high levels of apoptosis. This was also observed with TNF-resistant lung H23 and H460 cells [46–48]. In all instances, synergistic induction of apoptosis by pre- or co-exposure to17-AAG with TRAIL/TNF was induced primarily through down regulation of NFκB or Akt cell survival pathways [101–103]. Synergistic effects between 17-AAG and anti-TRAIL monoclonal antibodies have also been observed [104].
3.5. Histone Deacetylase Inhibitors
HDAC) inhibitors, or more accurately “protein deacetylase inhibitors” because they often target proteins other than histones, are a group of compounds that inhibit the deacetylation of many proteins, including histones and Hsp90 [105, 106]. HDAC inhibitors can induce apoptosis in cancer cell lines and some HDAC inhibitors are under clinical evaluation [107–109]. Co-administration of 17-AAG with HDAC inhibitors, like sodium butyrate (SB), suberoylanilide hydroxamic acid (SAHA), or LBH589, can synergistically induces apoptosis in leukemia cells [110, 111]. Moreover, a combination treatment of 17-AAG and LBH589 is effective in imatinib mesylate (IM)-resistant primary chromic myeloid leukemia blast crisis (CML-BC) and acute myeloid leukemia (AML) cells [111]. The detailed mechanisms of these synergistic effects are unclear, but they likely involve perturbations of survival pathways and cell cycle progression. HDAC inhibitors also leads to Hsp90 hyper-acetylation that inhibits its ATP-binding and chaperoning activities [105].
3.6. Protein Kinase Inhibitors
Several protein kinase inhibitors (PKIs) act synergistically with Hsp90 inhibitors in killing tumor cells. Leukemic cells with FLT3 tyrosine kinase gain-of-function mutations are synergistically and selectively sensitive to 17-AAG and FLT3 tyrosine kinase inhibitors, midostaurin (PKC412) and GTP14564 [112, 113]. Imatinib, a BCR-ABL tyrosine kinase inhibitor, also shows synergistic effects with 17-AAG in imatinib-resistant CML cells over-expressing BCR-ABL and P-glycoprotein [114]. 17-AAG combined with trastuzumab, the humanized antibody against receptor tyrosine kinase ErbB2, inhibits proliferation of trastuzumab-resistant breast tumor cell line JIMT-1 [115].
The molecular mechanisms of these synergistic effects are the pronounced reduction in protein level and activity of these kinases, which are all Hsp90 “client” proteins. Additional synergistic interactions occur when 17-AAG is combined with Chk1 inhibitor UCN-01 or PI3K inhibitor LY294002, and interference with the Akt survival pathway and cell cycle progression are thought to contribute to the phenomenon [116, 117].
3.7. Other Drugs and treatments
Hsp90 inhibitors also synergistically act with many other anti-cancer drugs, including doxorubincin, topoisomerase II inhibitors, cytarabine, arsenic trioxide and compounds that inhibit the induction of heat shock proteins, via different mechanisms [86, 118–121]. Of the other anti-cancer treatments, such as ionizing irradiation, adding Hsp90 inhibitors also enhances the cancer-killing effects synergistically [122–124]. Generally, treatment with 17-AAG provides a means of reversing the drug or radiation resistance in cancer cells.
4. NATURAL VARIATION EFFECTS HSP90-DEPENDENT DRUG RESISTANCE AND SENSITIVITY
Hsp90 … provide[s] at least two routes to the rapid evolution of new traits: (i) Acting as a potentiator, Hsp90’s folding reservoir allows individual genetic variation to immediately create new phenotypes; when the reservoir is compromised, the traits previously created by the potentiated variants disappear. (ii) Acting as a capacitor, Hsp90’s excess chaperone activity buffers the effects of other variants, storing them in a phenotypically silent form; when the Hsp90 reservoir is compromised, the effects of these variants are released, allowing them to create new traits. Jarosz and Lindquist (2010) [69]
In 1958, Schabel suggested that model organisms such as yeast and bacteria can be used to understand drug resistance in cancer [125]. For the past two decades, the Lindquist laboratory [69], the Picard laboratory [126–128], and other laboratories (e.g., [65, 129, 130]), based on Schabel’s advice have used the yeast Saccharomyces cerevisiae to understand how Hsp90 affects resistance or sensitivity. In a previous review [80], we discussed how the Lindquist laboratory’s Hsp90-based drug-resistance studies might apply to drug resistance in cancer.
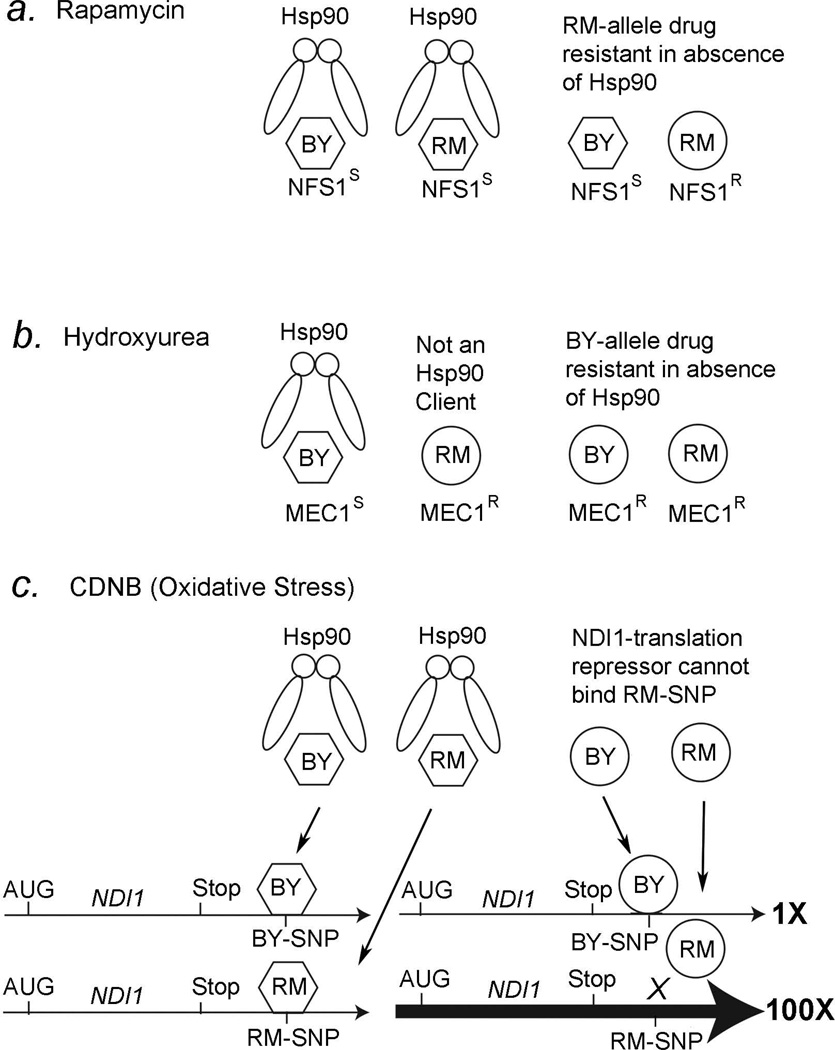
Natural variation Saccharomyces cerevisiae can affect the growth rate of the yeast cells [69]. Jarosz and Lindquist have reported that Hsp90 can act either as a “potentiator” or a “capacitor” for drug resistance and considered how this might affect the rapid evolution of new traits in general. Using recombinant inbred lines of bakers’ yeast (BY4716) and red wine yeast (RM11-1a) in the presence of anti-fungals, osmotic stressors, and other small molecules, they compared the growth rates in the presence or the absence of Hsp90 [69]. Hsp90 was inhibited by the Hsp90 inhibitors radicicol and geldanamycin [69]. Mechanistic models for the Hsp90-mediated potentiation or capacitation that may explain three of the findings described by Jarosz and Lindquist, rapamycin, hydroxyurea, and 1-chloro-2,4-nitrobenzine (CDNB), are shown in Figure 1.
Figure 1. Hsp90 and drug resistance in yeast.
A, RM11-1a yeast are resistant to rapamycin in the absence of Hsp90. We propose that both BY4716 and RM11-1a Nfs1 proteins are clients for Hsp90 which helps it maintain a rapamycin sensitive phenotype (hexagon), but Nfs1 protein forms a rapamycin-resistant structure (circle) in the absence of Hsp90.
B, BY4716 yeast are sensitive to hydroxyurea in the presence of Hsp90. We propose that the BY4716 Mec1 protein is a client for Hsp90 and it forms a structure that confers hydroxyurea sensitivity (hexagon), but the RM11-1a Mec1 protein is not a client for Hsp90 and forms a structure that confers resistance to hydroxyurea (circle). In the absence of Hsp90, we propose that the BY4716 Mec1 protein folds into a structure that confers resistance to hydroxyurea (circle).
C, RM11-1a yeast are resistant to oxidative stress (CDNB) in the absence of Hsp90. The BY4716 and RM11-1a NDI1 genes have a SNP in the 3’UTR that affects binding to a hypothetical 3’UTR binding protein in the absence of Hsp90 (circle), but not in the presence of Hsp90 (hexagon). See text for more details. (see [69]).
The immunosuppressant rapamycin can prolong the life of mice [131–133] and Drosophila [134] and is also useful for treating breast and skin cancers [135–138]. Jarosz and Lindquist found that BY4716 and RM11-1a yeast, and all recombinant inbred lines made from these two strains, have identical growth rates in the presence of Hsp90, but RM11-1a yeast have a ~3-fold increase in growth rate in the absence of Hsp90 compared with BY4716 [69]. The recombinant inbred lines made from BY4716 and RM11-1a indicates that the NFS1 gene must have the RM11-1a genotype to confer rapamycin resistance (Fig. 1a). Nfs1 protein is a cysteine desulfurase that acts as a sulfur donor in tRNA thiolation [139], and yeast mutations in this same pathway confer rapamycin resistance [140].
Jarosz and Lindquist [69] have proposed that the Nfs1 protein is a client for Hsp90 and that Hsp90 folds the Nfs1 into a form that makes both RM11-1a and BY4716 yeast sensitive to rapamycin (Fig. 1a, left). However, in the absence of Hsp90, Nfs1 with the RM11-1a genotype folds into a new conformation that is now resistant to rapamycin, but the BY4716 genotype protein remains in the rapamycin-sensitive conformation (Fig. 1a, right). In other words, Hsp90 functions as a capacitor for the rapamycin resistant phenotype in the RM11-1a strain but not the BY4716 strain. In the absence of Hsp90, such as during stress, the previously hidden phenotype of rapamycin resistance is revealed by the new of the Nfs1-resistant (NFS1R) conformation in the RM11-1a strain (Fig.1a).
Hydroxyurea is used to treat a variety of cancers, from leukemia to breast cancer [141–144]. It is also used in combination with other drugs to treat head and neck cancer [145]. One mechanism of action is thought to be through the inhibition of deoxyribonucleotide synthesis [146, 147]. Jarosz and Lindquist found that RM11-1a yeast are more resistant to hydroxyurea than BY4716 yeast in the presence of Hsp90, but that both BY4716 and RM11-1a yeast are resistant to hydroxyurea in the absence of Hsp90 [69]. Analyses of the RM11-1a and BY4716 recombinant inbred lines indicate that the MEC1 gene from BY4716 confers the sensitivity to hydroxyurea (Fig. 1b, left). Mec1 is a component of several checkpoint and DNA repair pathways in yeast [148–151], and therefore likely repairs the DNA damage induced by hydroxyurea.
Jarosz and Lindquist [69] further propose that Hsp90 functions as a capacitor in BY4716 yeast to make the Mec1 protein sensitive to hydroxyurea. However, according to their model, Hsp90 is not a chaperone for the Mec1 protein from RM11-1a yeast, but is a chaperone for Mec1 protein in BY4716 yeast (Fig. 1b). In the absence of Hsp90, such as in stressful environments, the Mec1 protein in BY4716 yeast folds into a different conformation that is now more resistant to hydroxyurea (Fig. 1b, right). Since the Mec1 protein in RM11-1a yeast is not a client for Hsp90, according to their model, it confers resistance to hydroxyurea regardless of whether Hsp90 is present or not (Fig. 1b, right). This result is important because it suggests that what might be a client protein for Hsp90 in one genetic background might not be a client in another genetic background. If this is true in humans, which is likely, this would suggest a possible reason why Hsp90 inhibitors are more effective in some cancer patients than others when used in combination with other drugs (Fig. 1b).
CDNB, a.k.a., DNCB (2,4-dinitro-1-chlorobenzine), is a redox cycling quinone that produces superoxide anions in its free radical state [152]. Paper were published in the 1970s and 1980s [153–157] that attempted to correlate skin-hypersensitivity caused by CDNB administration with cancer prognosis, with the concept of cancer being an autoimmune disease. We could not find any citations after 1987 in this regard. When exposed to CDNB, RM11-1a yeast show a remarkable 1500-fold increase in growth rate as compared to BY4716 yeast in the absence of Hsp90, and a 1500-fold increase in growth rate compared with both RM11-1a and BY4716 yeast in the presence of Hsp90 [69]. This example is illustrative for two reasons, the first being the causative genetic polymorphism maps to the 3’ untranslated region of the NDI1 gene (Fig. 1c, bottom left). The Ndi1 protein encodes an NADH-quinone (Q) oxidoreductase that protects against oxidative stress [158–160]. CDNB produces oxidative stress both by directly producing free radicals, when in its free radical form, and by titrating GSH levels [161–169]. Interestingly, overexpression of Ndi1 increases lifespan in Drosophila [170], which is consistent with the free-radical theory of aging [171]. The second reason is that it suggests that Hsp90 functions to regulate NDI1 expression in an indirect rather than a direct manner.
How might Hsp90 affect expression of NDI1 in RM11-1a yeast but not BY4716 yeast? We propose that Hsp90 is a chaperone for a hypothetical 3’UTR binding protein that binds to the NDI1 3’UTR when it has either the RM11-1a or the BY4716 genotype (Fig. 1c, left). In the absence of Hsp90, according to our model, the 3’UTR binding protein folds into a different conformation (a circle) that no longer binds to the NDI1 3’UTR with the RM11-1a genotype, but it can still bind to the NDI1 3’UTR with the BY4716 genotype (Fig. 1c, right). We propose that the 3’UTR binding protein is a translational repressor that also decreases the NDI1 mRNA levels when it is bound. Therefore, the NDI1 gene has much higher expression in RM11-1a yeast compared with BY4716 yeast (Fig. 1c, right, thick arrow). This model would explain why CDNB resistance maps to the 3’UTR of the NDI1 gene and not the hypothetical 3’UTR binding protein.
A fascinating finding of Jarosz and Lindquist is that the clustering of the genotype and the phenotype in 11 different yeast strains is improved in the absence of Hsp90 [69]. Genetic clustering was done by comparing the whole genome sequences of the 11 yeast strains. In the presence of Hsp90, there was no significant clustering of the phenotypes for resistance to 100 different growth conditions, including alternative carbon sources, oxidative stressors, antifungal drugs, small molecule drugs, and DNA damaging agents. However, in the absence of Hsp90, the phenotypes cluster as well as the genotypes. They conclude, “It is difficult to imagine how environmental stress in general, and Hsp90 in particular, could have such as strong impact on genotype-phenotype correlations unless it acted through the evolutionary history of these strains to influence the retention of a broad swath of genetic variation” [69]. In other words, this is the best evidence to suggest that Hsp90 plays a critical role as a capacitor for phenotypic variation, such as in drug resistance in yeast, and probably also drug resistance in cancer. We predict that cancer cell phenotypes, such as growth rates in drug containing media, will cluster with the genotypes better when Hsp90 is inhibited. Understanding this relationship will be needed for facilitating personalized medicine approaches to treating cancer in humans with Hsp90 inhibitors used in combination with other drugs.
5. SUMMARY AND FUTURE STUDIES
Hsp90 has a unique role in evolution by maintaining the activity of mutant proteins and serving as a capacitor to buffer phenotypic variation [69, 71, 73, 172, 173]. The role of Hsp90 in evolution of drug resistance requires study in greater detail. This review collates numerous studies that show that Hsp90 often acts synergistically with other anti-cancer drugs.
Phase II clinical trials of 17-AAG and trastuzumab have shown very promising results [42]. Since Hsp90 has over 200 client proteins, many of which are targeting in treating cancer, it is likely that 17-AAG will be used in combination with many other drugs in future human clinical studies. The classical mechanism for Hsp90 inhibitor function is that the inhibitor causes the degradation of its client proteins. For example, many HER2 positive breast cancers have an over expression of HER2 by gene amplification. In the absence of Hsp90, HER2 cannot be folded properly and is subject to ubiquitin-mediated proteolysis. If there is less HER2, than drugs that target HER2, such as the breast cancer drug trastuzumab is much more effective.
However, the classical mechanism for studying the synergistic action of Hsp90 inhibitors does not take into account the natural genetic variation in the human population. Studies of drug resistance in yeast, which are summarized here, have shown that several anti-cancer drugs are made either more or less potent when in combination with Hsp90 inhibitors, depending on the genetic variation in the yeast. Future studies in humans will need to be done to understand how genetic variation affects drug resistance, and will indicate which drugs will be most effective when used in combination with Hsp90 inhibitors.
ACKNOWLEDGEMENT
Research in our lab was supported by NIH grants R01ES012933 to D.M.R. and DK071073 to X.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Echeverria PC, Forafonov F, Pandey DP, Muhlebach G, Picard D. Detection of changes in gene regulatory patterns, elicited by perturbations of the Hsp90 molecular chaperone complex, by visualizing multiple experiments with an animation. BioData Min. 2011;4:15. doi: 10.1186/1756-0381-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Augello MA, Hickey TE, Knudsen KE. FOXA1: master of steroid receptor function in cancer. The EMBO journal. 2011 doi: 10.1038/emboj.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wayne N, Mishra P, Bolon DN. Hsp90 and client protein maturation. Methods in Molecular Biology. 2011;787:33–44. doi: 10.1007/978-1-61779-295-3_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Memarzadeh S, Cai H, Janzen DM, Xin L, Lukacs R, Riedinger M, et al. Role of autonomous androgen receptor signaling in prostate cancer initiation is dichotomous and depends on the oncogenic signal. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7962–7967. doi: 10.1073/pnas.1105243108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imamura T. Epigenetic setting for long-term expression of estrogen receptor alpha and androgen receptor in cells. Horm Behav. 2011;59:345–352. doi: 10.1016/j.yhbeh.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Biggar PH, Liangos O, Fey H, Brandenburg VM, Ketteler M. Vitamin D, chronic kidney disease and survival: a pluripotent hormone or just another bone drug? Pediatr Nephrol. 2011;26:7–18. doi: 10.1007/s00467-010-1526-x. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, Choi KJ, Seo WD, Jang SY, Kim M, Lee BW, et al. Enhancement of radiation sensitivity in lung cancer cells by celastrol is mediated by inhibition of Hsp90. International Journal of Molecular Medicine. 2011;27:441–446. doi: 10.3892/ijmm.2011.601. [DOI] [PubMed] [Google Scholar]
- 8.Walsh N, Larkin A, Swan N, Conlon K, Dowling P, McDermott R, et al. RNAi knockdown of Hop (Hsp70/Hsp90 organising protein) decreases invasion via MMP-2 down regulation. Cancer Letters. 2011;306:180–189. doi: 10.1016/j.canlet.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Ou WB, Hubert C, Corson JM, Bueno R, Flynn DL, Sugarbaker DJ, et al. Targeted inhibition of multiple receptor tyrosine kinases in mesothelioma. Neoplasia. 2011;13:12–22. doi: 10.1593/neo.101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cercek A, Saltz L. BEYOND KRAS: Other markers and potential treatment strategies for KRAS mutant and wild-type patients. Curr Treat Options Oncol. 2011;12:126–135. doi: 10.1007/s11864-011-0147-3. [DOI] [PubMed] [Google Scholar]
- 11.Bai L, Xu S, Chen W, Li Z, Wang X, Tang H, et al. Blocking NF-kappaB and Akt by Hsp90 inhibition sensitizes Smac mimetic compound 3-induced extrinsic apoptosis pathway and results in synergistic cancer cell death. Apoptosis. 2011;16:45–54. doi: 10.1007/s10495-010-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khong T, Spencer A. Targeting heat shock protein 90 induces apoptosis and inhibits critical survival and proliferation pathways in multiple myeloma. Molecular Cancer Therapeutics. 2011 doi: 10.1158/1535-7163.MCT-11-0174. [DOI] [PubMed] [Google Scholar]
- 13.Beck R, Dejeans N, Glorieux C, Pedrosa RC, Vasquez D, Valderrama JA, et al. Molecular chaperone Hsp90 as a target for oxidant-based anticancer therapies. Current Medicinal Chemistry. 2011;18:2816–2825. doi: 10.2174/092986711796011256. [DOI] [PubMed] [Google Scholar]
- 14.Beck R, Pedrosa RC, Dejeans N, Glorieux C, Leveque P, Gallez B, et al. Ascorbate/menadione-induced oxidative stress kills cancer cells that express normal or mutated forms of the oncogenic protein Bcr-Abl. An in vitro and in vivo mechanistic study. Investigational New Drugs. 2011;29:891–900. doi: 10.1007/s10637-010-9441-3. [DOI] [PubMed] [Google Scholar]
- 15.Hagn F, Lagleder S, Retzlaff M, Rohrberg J, Demmer O, Richter K, et al. Structural analysis of the interaction between Hsp90 and the tumor suppressor protein p53. Nature Structural & Molecular Biology. 2011 doi: 10.1038/nsmb.2114. [DOI] [PubMed] [Google Scholar]
- 16.Mehta PP, Whalen P, Baxi SM, Kung PP, Yamazaki S, Yin MJ. Effective Targeting of Triple- Negative Breast Cancer Cells by PF-4942847, a Novel Oral Inhibitor of Hsp 90. Clin Cancer Res. 2011;17:5432–5442. doi: 10.1158/1078-0432.CCR-11-0592. [DOI] [PubMed] [Google Scholar]
- 17.Breinig M, Mayer P, Harjung A, Goeppert B, Malz M, Penzel R, et al. Heat shock protein 90-sheltered overexpression of insulin-like growth factor 1 receptor contributes to malignancy of thymic epithelial tumors. Clin Cancer Res. 2011;17:2237–2249. doi: 10.1158/1078-0432.CCR-10-1689. [DOI] [PubMed] [Google Scholar]
- 18.Pacey S, Wilson RH, Walton M, Eatock MM, Hardcastle A, Zetterlund A, et al. A phase I study of the heat shock protein 90 inhibitor alvespimycin (17-DMAG) given intravenously to patients with advanced solid tumors. Clin Cancer Res. 2011;17:1561–1570. doi: 10.1158/1078-0432.CCR-10-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stellas D, El Hamidieh A, Patsavoudi E. Monoclonal antibody 4C5 prevents activation of MMP2 and MMP9 by disrupting their interaction with extracellular HSP90 and inhibits formation of metastatic breast cancer cell deposits. BMC Cell Biol. 2010;11:51. doi: 10.1186/1471-2121-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li D, Marchenko ND, Moll UM. SAHA shows preferential cytotoxicity in mutant p53 cancer cells by destabilizing mutant p53 through inhibition of the HDAC6-Hsp90 chaperone axis. Cell death and differentiation. 2011 doi: 10.1038/cdd.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu W, Wang J, Jin J, Qian W, Qian J, Cheng Y, et al. Heat shock protein 90 inhibition results in altered downstream signaling of mutant KIT and exerts synergistic effects on Kasumi-1 cells when combining with histone deacetylase inhibitor. Leukemia Res. 2011;35:1212–1218. doi: 10.1016/j.leukres.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Wang G, Ye Y, Yang X, Liao H, Zhao C, Liang S. Expression-based in silico screening of candidate therapeutic compounds for lung adenocarcinoma. PLoS ONE. 2011;6:e14573. doi: 10.1371/journal.pone.0014573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng Q, Chen X, Sun L, Zhao C, Sui G, Cai L. Carbamazepine promotes Her-2 protein degradation in breast cancer cells by modulating HDAC6 activity and acetylation of Hsp90. Molecular and cellular biochemistry. 2011;348:165–171. doi: 10.1007/s11010-010-0651-y. [DOI] [PubMed] [Google Scholar]
- 24.Aldana-Masangkay GI, Sakamoto KM. The role of HDAC6 in cancer. J Biomed Biotechnol. 2011;2011:875824. doi: 10.1155/2011/875824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo XG, Zou JN, Wang SZ, Zhang TC, Xi T. Novobiocin decreases SMYD3 expression and inhibits the migration of MDA-MB-231 human breast cancer cells. IUBMB Life. 2010;62:194–199. doi: 10.1002/iub.288. [DOI] [PubMed] [Google Scholar]
- 26.Ruden DM, Xiao L, Garfinkel MD, Lu X. Hsp90 and environmental impacts on epigenetic states: a model for the trans-generational effects of diethylstilbesterol (DES) on uterine development and cancer. Hum Mol Genet. 2005;14:R147–R155. doi: 10.1093/hmg/ddi103. [DOI] [PubMed] [Google Scholar]
- 27.Zuber J, Rappaport AR, Luo W, Wang E, Chen C, Vaseva AV, et al. An integrated approach to dissecting oncogene addiction implicates a Myb-coordinated self-renewal program as essential for leukemia maintenance. Genes & Development. 2011;25:1628–1640. doi: 10.1101/gad.17269211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan W, Zhang W, Jiang T. Oncogene addiction in gliomas: implications for molecular targeted therapy. J Exp Clin Cancer Res. 2011;30:58. doi: 10.1186/1756-9966-30-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folkman J, Ryeom S. Is oncogene addiction angiogenesis-dependent? Cold Spring Harbor Symposia on Quantitative Biology. 2005;70:389–397. doi: 10.1101/sqb.2005.70.042. [DOI] [PubMed] [Google Scholar]
- 30.Jonkers J, Berns A. Oncogene addiction: sometimes a temporary slavery. Cancer Cell. 2004;6:535–538. doi: 10.1016/j.ccr.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Workman P. Cancer genome targets: RAF-ing up tumor cells to overcome oncogene addiction. Expert Rev Anticancer Ther. 2002;2:611–614. doi: 10.1586/14737140.2.6.611. [DOI] [PubMed] [Google Scholar]
- 32.Tan SS, Ahmad I, Bennett HL, Singh L, Nixon C, Seywright M, et al. GRP78 up-regulation is associated with androgen receptor status, Hsp70-Hsp90 client proteins and castrate-resistant prostate cancer. The Journal of pathology. 2010 doi: 10.1002/path.2795. [DOI] [PubMed] [Google Scholar]
- 33.Beck R, Verrax J, Gonze T, Zappone M, Pedrosa RC, Taper H, et al. Hsp90 cleavage by an oxidative stress leads to its client proteins degradation and cancer cell death. Biochemical Pharmacology. 2009;77:375–383. doi: 10.1016/j.bcp.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Kim HL, Cassone M, Otvos L, Jr., Vogiatzi P. HIF-1alpha and STAT3 client proteins interacting with the cancer chaperone Hsp90: therapeutic considerations. Cancer Biol Ther. 2008;7:10–14. doi: 10.4161/cbt.7.1.5458. [DOI] [PubMed] [Google Scholar]
- 35.Shames DS, Minna JD. IP6K2 is a client for HSP90 and a target for cancer therapeutics development. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1389–1390. doi: 10.1073/pnas.0711993105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krukenberg KA, Southworth DR, Street TO, Agard DA. pH-dependent conformational changes in bacterial Hsp90 reveal a Grp94-like conformation at pH 6 that is highly active in suppression of citrate synthase aggregation. Journal of Molecular Biology. 2009;390:278–291. doi: 10.1016/j.jmb.2009.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crawford LJ, Walker B, Irvine AE. Proteasome inhibitors in cancer therapy. J Cell Commun Signal. 2011;5:101–110. doi: 10.1007/s12079-011-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh P, Godbole M, Rao G, Annarao S, Mitra K, Roy R, et al. Inhibition of autophagy stimulate molecular iodine-induced apoptosis in hormone independent breast tumors. Biochemical and biophysical research communications. 2011 doi: 10.1016/j.bbrc.2011.10.054. [DOI] [PubMed] [Google Scholar]
- 39.Cheong JH, Park ES, Liang J, Dennison JB, Tsavachidou D, Nguyen-Charles C, et al. Dual inhibition of Tumor Energy Pathway by 2-deoxy glucose and metformin Is Effective Against a Broad Spectrum of Preclinical Cancer Models. Molecular Cancer Therapeutics. 2011 doi: 10.1158/1535-7163.MCT-11-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao F, Wang G, Wei W, Tu Y, Tong H, Sun S. An autophagy inhibitor enhances the inhibition of cell proliferation. Mol Med Report. 2012;5:84–88. doi: 10.3892/mmr.2011.590. [DOI] [PubMed] [Google Scholar]
- 41.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Kung AL, Davies FE, et al. Antimyeloma activity of heat shock protein-90 inhibition. Blood. 2006;107:1092–1100. doi: 10.1182/blood-2005-03-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Modi S, Stopeck A, Linden H, Solit D, Chandarlapaty S, Rosen N, et al. HSP90 inhibition is effective in breast cancer: a phase II trial of tanespimycin (17-AAG) plus trastuzumab in patients with HER2-positive metastatic breast cancer progressing on trastuzumab. Clin Cancer Res. 2011;17:5132–5139. doi: 10.1158/1078-0432.CCR-11-0072. [DOI] [PubMed] [Google Scholar]
- 43.Pacey S, Gore M, Chao D, Banerji U, Larkin J, Sarker S, et al. A Phase II trial of 17-allylamino, 17-demethoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma. Investigational New Drugs. 2010 doi: 10.1007/s10637-010-9493-4. [DOI] [PubMed] [Google Scholar]
- 44.Senju M, Sueoka N, Sato A, Iwanaga K, Sakao Y, Tomimitsu S, et al. Hsp90 inhibitors cause G2/M arrest associated with the reduction of Cdc25C and Cdc2 in lung cancer cell lines. Journal of cancer research and clinical oncology. 2006;132:150–158. doi: 10.1007/s00432-005-0047-7. [DOI] [PubMed] [Google Scholar]
- 45.Schwock J, Pham NA, Cao MP, Hedley DW. Efficacy of Hsp90 inhibition for induction of apoptosis and inhibition of growth in cervical carcinoma cells in vitro and in vivo. Cancer chemotherapy and pharmacology. 2008;61:669–681. doi: 10.1007/s00280-007-0522-8. [DOI] [PubMed] [Google Scholar]
- 46.Williams CR, Tabios R, Linehan WM, Neckers L. Intratumor injection of the Hsp90 inhibitor 17AAG decreases tumor growth and induces apoptosis in a prostate cancer xenograft model. The Journal of urology. 2007;178:1528–1532. doi: 10.1016/j.juro.2007.05.120. [DOI] [PubMed] [Google Scholar]
- 47.Solit DB, Osman I, Polsky D, Panageas KS, Daud A, Goydos JS, et al. Phase II trial of 17-allylamino- 17-demethoxygeldanamycin in patients with metastatic melanoma. Clin Cancer Res. 2008;14:8302–8307. doi: 10.1158/1078-0432.CCR-08-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Day JE, Sharp SY, Rowlands MG, Aherne W, Lewis W, Roe SM, et al. Inhibition of Hsp90 with resorcylic acid macrolactones: synthesis and binding studies. Chemistry. 2010;16:10366–10372. doi: 10.1002/chem.201001119. [DOI] [PubMed] [Google Scholar]
- 49.Donnelly A, Blagg BS. Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide-binding pocket. Current Medicinal Chemistry. 2008;15:2702–2717. doi: 10.2174/092986708786242895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sgobba M, Forestiero R, Degliesposti G, Rastelli G. Exploring the binding site of C-terminal hsp90 inhibitors. J Chem Inf Model. 2010;50:1522–1528. doi: 10.1021/ci1001857. [DOI] [PubMed] [Google Scholar]
- 51.Provencio M, Sanchez A, Garrido P, Valcarcel F. New molecular targeted therapies integrated with radiation therapy in lung cancer. Clin Lung Cancer. 2010;11:91–97. doi: 10.3816/CLC.2010.n.012. [DOI] [PubMed] [Google Scholar]
- 52.Lassi K, Dawson NA. Drug development for metastatic castration-resistant prostate cancer: current status and future perspectives. Future oncology. 2011;7:551–558. doi: 10.2217/fon.11.14. [DOI] [PubMed] [Google Scholar]
- 53.Merkel TJ, DeSimone JM. Dodging drug-resistant cancer with diamonds. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002137. 73ps8. [DOI] [PubMed] [Google Scholar]
- 54.Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol. 2011;8:12–23. doi: 10.1038/nrclinonc.2010.136. [DOI] [PubMed] [Google Scholar]
- 55.Zaugg K, Yao Y, Reilly PT, Kannan K, Kiarash R, Mason J, et al. Carnitine palmitoyltransferase 1C promotes cell survival and tumor growth under conditions of metabolic stress. Genes & Development. 2011;25:1041–1051. doi: 10.1101/gad.1987211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawai M, Furuta Y, Yahara K, Tsuru T, Oshima K, Handa N, et al. Evolution in an oncogenic bacterial species with extreme genome plasticity: Helicobacter pylori East Asian genomes. BMC Microbiol. 2011;11:104. doi: 10.1186/1471-2180-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith DG, Magwere T, Burchill SA. Oxidative stress and therapeutic opportunities: focus on the Ewing's sarcoma family of tumors. Expert Rev Anticancer Ther. 2011;11:229–249. doi: 10.1586/era.10.224. [DOI] [PubMed] [Google Scholar]
- 58.Chen CH, Chang YJ, Ku MS, Chung KT, Yang JT. Enhancement of temozolomide-induced apoptosis by valproic acid in human glioma cell lines through redox regulation. J Mol Med (Berl) 2011;89:303–315. doi: 10.1007/s00109-010-0707-1. [DOI] [PubMed] [Google Scholar]
- 59.De Luca A, Moroni N, Serafino A, Primavera A, Pastore A, Pedersen JZ, et al. Treatment of doxorubicin resistant MCF7/Dx cells with nitric oxide causes histone glutathionylation and reversal of drug resistance. The Biochemical journal. 2011 doi: 10.1042/BJ20111333. [DOI] [PubMed] [Google Scholar]
- 60.de Almagro MC, Selga E, Thibaut R, Porte C, Noe V, Ciudad CJ. UDP-glucuronosyltransferase 1A6 overexpression in breast cancer cells resistant to methotrexate. Biochemical Pharmacology. 2011;81:60–70. doi: 10.1016/j.bcp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 61.Prodromou C. The 'active life' of Hsp90 complexes. Biochimica et Biophysica Acta. 2011 doi: 10.1016/j.bbamcr.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H, Burrows F. Targeting multiple signal transduction pathways through inhibition of Hsp90. J Mol Med. 2004;82:488–499. doi: 10.1007/s00109-004-0549-9. [DOI] [PubMed] [Google Scholar]
- 63.Shipp C, Watson K, Jones GL. Associations of HSP90 client proteins in human breast cancer. Anticancer Research. 2011;31:2095–2101. [PubMed] [Google Scholar]
- 64.Tan SS, Ahmad I, Bennett HL, Singh L, Nixon C, Seywright M, et al. GRP78 up-regulation is associated with androgen receptor status, Hsp70-Hsp90 client proteins and castrate-resistant prostate cancer. The Journal of pathology. 2011;223:81–87. doi: 10.1002/path.2795. [DOI] [PubMed] [Google Scholar]
- 65.Truman AW, Millson SH, Nuttall JM, Mollapour M, Prodromou C, Piper PW. In the yeast heat shock response, Hsf1-directed induction of Hsp90 facilitates the activation of the Slt2 (Mpk1) mitogen-activated protein kinase required for cell integrity. Eukaryotic Cell. 2007;6:744–752. doi: 10.1128/EC.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Becker B, Multhoff G, Farkas B, Wild PJ, Landthaler M, Stolz W, et al. Induction of Hsp90 protein expression in malignant melanomas and melanoma metastases. Exp Dermatol. 2004;13:27–32. doi: 10.1111/j.0906-6705.2004.00114.x. [DOI] [PubMed] [Google Scholar]
- 67.Galea-Lauri J, Richardson AJ, Latchman DS, Katz DR. Increased heat shock protein 90 (hsp90) expression leads to increased apoptosis in the monoblastoid cell line U937 following induction with TNF-alpha and cycloheximide: a possible role in immunopathology. J Immunol. 1996;157:4109–4118. [PubMed] [Google Scholar]
- 68.Kawagoe J, Abe K, Aoki M, Kogure K. Induction of HSP90 alpha heat shock mRNA after transient global ischemia in gerbil hippocampus. Brain Research. 1993;621:121–125. doi: 10.1016/0006-8993(93)90306-8. [DOI] [PubMed] [Google Scholar]
- 69.Jarosz DF, Lindquist S. Hsp90 and environmental stress transform the adaptive value of natural genetic variation. Science. 2010;330:1820–1824. doi: 10.1126/science.1195487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science. 2005;309:2185–2189. doi: 10.1126/science.1118370. [DOI] [PubMed] [Google Scholar]
- 71.Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–224. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- 72.Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- 73.Sollars V, Lu X, Xiao L, Wang X, Garfinkel MD, Ruden DM. Evidence for an epigenetic mechanism by which Hsp90 acts as a capacitor for morphological evolution. Nat Genet. 2003;33:70–74. doi: 10.1038/ng1067. [DOI] [PubMed] [Google Scholar]
- 74.Ruden DM. Identification of Schizosaccharomyces pombe transcription factor PGA4, which binds cooperatively to Saccharomyces cerevisiae GAL4-binding sites. Mol Cell Biol. 1990;10:1432–1438. doi: 10.1128/mcb.10.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ruden DM, Garfinkel MD, Sollars VE, Lu X. Waddington's widget: Hsp90 and the inheritance of acquired characters. Semin Cell Dev Biol. 2003;14:301–310. doi: 10.1016/j.semcdb.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 76.Ruden DM, Garfinkel MD, Xiao L, Lu X. Epigenetic Regulation of Trinucleotide Repeat Expansions and Contractions and the "Biased Embryos" Hypothesis for Rapid Morphological Evolution. Curr Genomics. 2005;6:145–155. [Google Scholar]
- 77.Ruden DM, Jamison DC, Zeeberg BR, Garfinkel MD, Weinstein JN, Rasouli P, et al. The EDGE hypothesis: Epigenetically directed genetic errors in repeat-containing proteins (RCPs) involved in evolution, neuroendocrine signaling, and cancer. Frontiers in neuroendocrinology. 2008;29:428–444. doi: 10.1016/j.yfrne.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gangaraju VK, Yin H, Weiner MM, Wang J, Huang XA, Lin H. Drosophila Piwi Functions in Hsp90-Mediated Suppression of Phenotypic Variation. Nat Genet. 2010 doi: 10.1038/ng.743. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xiao L, Lu X, Ruden DM. Effectiveness of hsp90 inhibitors as anti-cancer drugs. Mini Rev Med Chem. 2006;6:1137–1143. doi: 10.2174/138955706778560166. [DOI] [PubMed] [Google Scholar]
- 80.Xiao L, Rasouli P, Ruden DM. Possible effects of early treatments of hsp90 inhibitors on preventing the evolution of drug resistance to other anti-cancer drugs. Curr Med Chem. 2007;14:223–232. doi: 10.2174/092986707779313372. [DOI] [PubMed] [Google Scholar]
- 81.Andre N, Meille C. Taxanes in paediatric oncology: and now? . Cancer Treat Rev. 2006;32:65–73. doi: 10.1016/j.ctrv.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 82.Solit DB, Basso AD, Olshen AB, Scher HI, Rosen N. Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer Res. 2003;63:2139–2144. [PubMed] [Google Scholar]
- 83.Nguyen DM, Lorang D, Chen GA, Stewart JHt, Tabibi E, Schrump DS. Enhancement of paclitaxel-mediated cytotoxicity in lung cancer cells by 17-allylamino geldanamycin: in vitro and in vivo analysis. Ann Thorac Surg. 2001;72:371–378. doi: 10.1016/s0003-4975(01)02787-4. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 84.Nguyen DM, Chen A, Mixon A, Schrump DS. Sequence-dependent enhancement of paclitaxel toxicity in non-small cell lung cancer by 17-allylamino 17-demethoxygeldanamycin. J Thorac Cardiovasc Surg. 1999;118:908–915. doi: 10.1016/s0022-5223(99)70061-9. [DOI] [PubMed] [Google Scholar]
- 85.Munster PN, Basso A, Solit D, Norton L, Rosen N. Modulation of Hsp90 function by ansamycins sensitizes breast cancer cells to chemotherapy-induced apoptosis in an RB- and schedule-dependent manner. See: E. A. Sausville, Combining cytotoxics and 17-allylamino, 17-demethoxygeldanamycin: sequence and tumor biology matters, Clin. Cancer Res., 7: 2155–2158, 2001. Clin Cancer Res. 2001;7:2155–2136. [PubMed] [Google Scholar]
- 86.Blagosklonny MV, Fojo T, Bhalla KN, Kim JS, Trepel JB, Figg WD, et al. The Hsp90 inhibitor geldanamycin selectively sensitizes Bcr-Abl-expressing leukemia cells to cytotoxic chemotherapy. Leukemia. 2001;15:1537–1543. doi: 10.1038/sj.leu.2402257. [DOI] [PubMed] [Google Scholar]
- 87.Srethapakdi M, Liu F, Tavorath R, Rosen N. Inhibition of Hsp90 function by ansamycins causes retinoblastoma gene product-dependent G1 arrest. Cancer Res. 2000;60:3940–3946. [PubMed] [Google Scholar]
- 88.Peyrone M. Ueber die Einwirkung des Ammoniaks auf Platinchlorür. Justus Liebigs Ann Chem. 1844;51:1–29. [Google Scholar]
- 89.Sorenson CM, Eastman A. Mechanism of cis-diamminedichloroplatinum(II)-induced cytotoxicity: role of G2 arrest and DNA double-strand breaks. Cancer Res. 1988;48:4484–4488. [PubMed] [Google Scholar]
- 90.Frankenberg-Schwager M, Kirchermeier D, Greif G, Baer K, Becker M, Frankenberg D. Cisplatin-mediated DNA double-strand breaks in replicating but not in quiescent cells of the yeast Saccharomyces cerevisiae. Toxicology. 2005;212:175–184. doi: 10.1016/j.tox.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 91.Vasilevskaya IA, Rakitina TV, O'Dwyer PJ. Quantitative effects on c-Jun N-terminal protein kinase signaling determine synergistic interaction of cisplatin and 17-allylamino-17-demethoxygeldanamycin in colon cancer cell lines. Mol Pharmacol. 2004;65:235–243. doi: 10.1124/mol.65.1.235. [DOI] [PubMed] [Google Scholar]
- 92.Vasilevskaya IA, Rakitina TV, O'Dwyer PJ. Geldanamycin and its 17-allylamino-17-demethoxy analogue antagonize the action of Cisplatin in human colon adenocarcinoma cells: differential caspase activation as a basis for interaction. Cancer Res. 2003;63:3241–3246. [PubMed] [Google Scholar]
- 93.Liao ZY, Zhang SH, Zhen YS. Synergistic effects of geldanamycin and antitumor drugs. Yao Xue Xue Bao. 2001;36:569–575. [PubMed] [Google Scholar]
- 94.Fedier A, Stuedli A, Fink D. Presence of MLH1 protein aggravates the potential of the HSP90 inhibitor radicicol to sensitize tumor cells to cisplatin. Int J Oncol. 2005;27:1697–1705. [PubMed] [Google Scholar]
- 95.Bagatell R, Beliakoff J, David CL, Marron MT, Whitesell L. Hsp90 inhibitors deplete key anti-apoptotic proteins in pediatric solid tumor cells and demonstrate synergistic anticancer activity with cisplatin. Int J Cancer. 2005;113:179–188. doi: 10.1002/ijc.20611. [DOI] [PubMed] [Google Scholar]
- 96.Sano M. Radicicol and geldanamycin prevent neurotoxic effects of anti-cancer drugs on cultured embryonic sensory neurons. Neuropharmacology. 2001;40:947–953. doi: 10.1016/s0028-3908(01)00018-1. [DOI] [PubMed] [Google Scholar]
- 97.Mitsiades CS, Mitsiades N, Richardson PG, Treon SP, Anderson KC. Novel biologically based therapies for Waldenstrom's macroglobulinemia. Semin Oncol. 2003;30:309–312. doi: 10.1053/sonc.2003.50065. [DOI] [PubMed] [Google Scholar]
- 98.Mimnaugh EG, Xu W, Vos M, Yuan X, Isaacs JS, Bisht KS, et al. Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol Cancer Ther. 2004;3:551–566. [PubMed] [Google Scholar]
- 99.Wajant H, Gerspach J, Pfizenmaier K. Tumor therapeutics by design: targeting and activation of death receptors. Cytokine Growth Factor Rev. 2005;16:55–76. doi: 10.1016/j.cytogfr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 100.Siegelin MD, Habel A, Gaiser T. 17-AAG sensitized malignant glioma cells to death-receptor mediated apoptosis. Neurobiology of disease. 2009;33:243–249. doi: 10.1016/j.nbd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 101.Wang X, Ju W, Renouard J, Aden J, Belinsky SA, Lin Y. 17-allylamino-17-demethoxygeldanamycin synergistically potentiates tumor necrosis factor-induced lung cancer cell death by blocking the nuclear factor-kappaB pathway. Cancer Res. 2006;66:1089–1095. doi: 10.1158/0008-5472.CAN-05-2698. [DOI] [PubMed] [Google Scholar]
- 102.Vasilevskaya IA, O'Dwyer PJ. 17-Allylamino-17-demethoxygeldanamycin overcomes TRAIL resistance in colon cancer cell lines. Biochem Pharmacol. 2005;70:580–589. doi: 10.1016/j.bcp.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 103.Ma Y, Lakshmikanthan V, Lewis RW, Kumar MV. Sensitization of TRAIL-resistant cells by inhibition of heat shock protein 90 with low-dose geldanamycin. Mol Cancer Ther. 2006;5:170–178. doi: 10.1158/1535-7163.MCT-05-0129. [DOI] [PubMed] [Google Scholar]
- 104.Georgakis GV, Li Y, Rassidakis GZ, Martinez-Valdez H, Medeiros LJ, Younes A. Inhibition of heat shock protein 90 function by 17-allylamino-17-demethoxy-geldanamycin in Hodgkin's lymphoma cells down-regulates Akt kinase, dephosphorylates extracellular signal-regulated kinase, and induces cell cycle arrest and cell death. Clin Cancer Res. 2006;12:584–590. doi: 10.1158/1078-0432.CCR-05-1194. [DOI] [PubMed] [Google Scholar]
- 105.Yu X, Guo ZS, Marcu MG, Neckers L, Nguyen DM, Chen GA, et al. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst. 2002;94:504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- 106.Chen L, Meng S, Wang H, Bali P, Bai W, Li B, et al. Chemical ablation of androgen receptor in prostate cancer cells by the histone deacetylase inhibitor LAQ824. Mol Cancer Ther. 2005;4:1311–1319. doi: 10.1158/1535-7163.MCT-04-0287. [DOI] [PubMed] [Google Scholar]
- 107.Boyle GM, Martyn AC, Parsons PG. Histone deacetylase inhibitors and malignant melanoma. Pigment Cell Res. 2005;18:160–166. doi: 10.1111/j.1600-0749.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 108.Gore SD, Carducci MA. Modifying histones to tame cancer: clinical development of sodium phenylbutyrate and other histone deacetylase inhibitors. Expert Opin Investig Drugs. 2000;9:2923–2934. doi: 10.1517/13543784.9.12.2923. [DOI] [PubMed] [Google Scholar]
- 109.Vrana JA, Decker RH, Johnson CR, Wang Z, Jarvis WD, Richon VM, et al. Induction of apoptosis in U937 human leukemia cells by suberoylanilide hydroxamic acid (SAHA) proceeds through pathways that are regulated by Bcl-2/Bcl-XL, c-Jun, and p21CIP1, but independent of p53. Oncogene. 1999;18:7016–7025. doi: 10.1038/sj.onc.1203176. [DOI] [PubMed] [Google Scholar]
- 110.Rahmani M, Yu C, Dai Y, Reese E, Ahmed W, Dent P, et al. Coadministration of the heat shock protein 90 antagonist 17-allylamino- 17-demethoxygeldanamycin with suberoylanilide hydroxamic acid or sodium butyrate synergistically induces apoptosis in human leukemia cells. Cancer Res. 2003;63:8420–8427. [PubMed] [Google Scholar]
- 111.George P, Bali P, Annavarapu S, Scuto A, Fiskus W, Guo F, et al. Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3. Blood. 2005;105:1768–1776. doi: 10.1182/blood-2004-09-3413. [DOI] [PubMed] [Google Scholar]
- 112.Yao Q, Nishiuchi R, Li Q, Kumar AR, Hudson WA, Kersey JH. FLT3 expressing leukemias are selectively sensitive to inhibitors of the molecular chaperone heat shock protein 90 through destabilization of signal transduction-associated kinases. Clin Cancer Res. 2003;9:4483–4493. [PubMed] [Google Scholar]
- 113.George P, Bali P, Cohen P, Tao J, Guo F, Sigua C, et al. Cotreatment with 17-allylamino-demethoxygeldanamycin and FLT-3 kinase inhibitor PKC412 is highly effective against human acute myelogenous leukemia cells with mutant FLT-3. Cancer Res. 2004;64:3645–3652. doi: 10.1158/0008-5472.CAN-04-0006. [DOI] [PubMed] [Google Scholar]
- 114.Radujkovic A, Schad M, Topaly J, Veldwijk MR, Laufs S, Schultheis BS, et al. Synergistic activity of imatinib and 17-AAG in imatinib-resistant CML cells overexpressing BCR-ABL--Inhibition of P-glycoprotein function by 17-AAG. Leukemia. 2005;19:1198–1206. doi: 10.1038/sj.leu.2403764. [DOI] [PubMed] [Google Scholar]
- 115.Zsebik B, Citri A, Isola J, Yarden Y, Szollosi J, Vereb G. Hsp90 inhibitor 17-AAG reduces ErbB2 levels and inhibits proliferation of the trastuzumab resistant breast tumor cell line JIMT-1. Immunol Lett. 2006;104:146–155. doi: 10.1016/j.imlet.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 116.Premkumar DR, Arnold B, Jane EP, Pollack IF. Synergistic interaction between 17-AAG and phosphatidylinositol 3-kinase inhibition in human malignant glioma cells. Mol Carcinog. 2006;45:47–59. doi: 10.1002/mc.20152. [DOI] [PubMed] [Google Scholar]
- 117.Jia W, Yu C, Rahmani M, Krystal G, Sausville EA, Dent P, et al. Synergistic antileukemic interactions between 17-AAG and UCN-01 involve interruption of RAF/MEK- and AKT-related pathways. Blood. 2003;102:1824–1832. doi: 10.1182/blood-2002-12-3785. [DOI] [PubMed] [Google Scholar]
- 118.Pelicano H, Carew JS, McQueen TJ, Andreeff M, Plunkett W, Keating MJ, et al. Targeting Hsp90 by 17-AAG in leukemia cells: mechanisms for synergistic and antagonistic drug combinations with arsenic trioxide and Ara-C. Leukemia. 2006;20:610–619. doi: 10.1038/sj.leu.2404140. [DOI] [PubMed] [Google Scholar]
- 119.Mesa RA, Loegering D, Powell HL, Flatten K, Arlander SJ, Dai NT, et al. Heat shock protein 90 inhibition sensitizes acute myelogenous leukemia cells to cytarabine. Blood. 2005;106:318–327. doi: 10.1182/blood-2004-09-3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barker CR, McNamara AV, Rackstraw SA, Nelson DE, White MR, Watson AJ, et al. Inhibition of Hsp90 acts synergistically with topoisomerase II poisons to increase the apoptotic killing of cells due to an increase in topoisomerase II mediated DNA damage. Nucleic Acids Res. 2006;34:1148–1157. doi: 10.1093/nar/gkj516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zaarur N, Gabai VL, Porco JA, Jr., Calderwood S, Sherman MY. Targeting heat shock response to sensitize cancer cells to proteasome and Hsp90 inhibitors. Cancer Res. 2006;66:1783–1791. doi: 10.1158/0008-5472.CAN-05-3692. [DOI] [PubMed] [Google Scholar]
- 122.Harashima K, Akimoto T, Nonaka T, Tsuzuki K, Mitsuhashi N, Nakano T. Heat shock protein 90 (Hsp90) chaperone complex inhibitor, radicicol, potentiated radiation-induced cell killing in a hormone-sensitive prostate cancer cell line through degradation of the androgen receptor. Int J Radiat Biol. 2005;81:63–76. doi: 10.1080/09553000400029460. [DOI] [PubMed] [Google Scholar]
- 123.Enmon R, Yang WH, Ballangrud AM, Solit DB, Heller G, Rosen N, et al. Combination treatment with 17-N-allylamino-17-demethoxy geldanamycin and acute irradiation produces supra-additive growth suppression in human prostate carcinoma spheroids. Cancer Res. 2003;63:8393–8399. [PubMed] [Google Scholar]
- 124.Bisht KS, Bradbury CM, Mattson D, Kaushal A, Sowers A, Markovina S, et al. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Res. 2003;63:8984–8995. [PubMed] [Google Scholar]
- 125.Schabel FM., Jr. Utility of drug-resistant organisms in cancer chemotherapy studies. Annals of the New York Academy of Sciences. 1958;76:442–453. doi: 10.1111/j.1749-6632.1958.tb54863.x. discussion 53–6. [DOI] [PubMed] [Google Scholar]
- 126.Wider D, Peli-Gulli MP, Briand PA, Tatu U, Picard D. The complementation of yeast with human or Plasmodium falciparum Hsp90 confers differential inhibitor sensitivities. Molecular and biochemical parasitology. 2009;164:147–152. doi: 10.1016/j.molbiopara.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 127.Abbas-Terki T, Donze O, Briand PA, Picard D. Hsp104 interacts with Hsp90 cochaperones in respiring yeast. Molecular and cellular biology. 2001;21:7569–7575. doi: 10.1128/MCB.21.22.7569-7575.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Louvion JF, Abbas-Terki T, Picard D. Hsp90 is required for pheromone signaling in yeast. Molecular biology of the cell. 1998;9:3071–3083. doi: 10.1091/mbc.9.11.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rodriguez-Caban J, Gonzalez-Velazquez W, Perez-Sanchez L, Gonzalez-Mendez R, Valle NR. Calcium/calmodulin kinase1 and its relation to thermotolerance and HSP90 in Sporothrix schenckii: an RNAi and yeast two-hybrid study. BMC Microbiol. 2011;11:162. doi: 10.1186/1471-2180-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000532. e1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, et al. Rapamycin extends maximal lifespan in cancer-prone mice. The American journal of pathology. 2010;176:2092–2097. doi: 10.2353/ajpath.2010.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li S. The possible cellular mechanism for extending lifespan of mice with rapamycin. Biol Proced Online. 2009;11:1–2. doi: 10.1007/s12575-009-9015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harrison B, Tran TT, Taylor D, Lee SD, Min KJ. Effect of rapamycin on lifespan in Drosophila. Geriatr Gerontol Int. 2010;10:110–112. doi: 10.1111/j.1447-0594.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 135.Athar M, Kopelovich L. Rapamycin and mTORC1 inhibition in the mouse: skin cancer prevention. Cancer Prev Res (Phila) 2011;4:957–961. doi: 10.1158/1940-6207.CAPR-11-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gruppuso PA, Boylan JM, Sanders JA. The physiology and pathophysiology of rapamycin resistance: implications for cancer. Cell Cycle. 2011;10:1050–1058. doi: 10.4161/cc.10.7.15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Macaskill EJ, Bartlett JM, Sabine VS, Faratian D, Renshaw L, White S, et al. The mammalian target of rapamycin inhibitor everolimus (RAD001) in early breast cancer: results of a preoperative study. Breast cancer research and treatment. 2011;128:725–734. doi: 10.1007/s10549-010-0967-z. [DOI] [PubMed] [Google Scholar]
- 138.Wong SW, Tiong KH, Kong WY, Yue YC, Chua CH, Lim JY, et al. Rapamycin synergizes cisplatin sensitivity in basal-like breast cancer cells through up-regulation of p73. Breast cancer research and treatment. 2011;128:301–313. doi: 10.1007/s10549-010-1055-0. [DOI] [PubMed] [Google Scholar]
- 139.Leidel S, Pedrioli PG, Bucher T, Brost R, Costanzo M, Schmidt A, et al. Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature. 2009;458:228–232. doi: 10.1038/nature07643. [DOI] [PubMed] [Google Scholar]
- 140.Ramanathan A, Schreiber SL. Multilevel regulation of growth rate in yeast revealed using systems biology. J Biol. 2007;6:3. doi: 10.1186/jbiol56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cole DR, Beckloff GL, Rousselot LM. Clinical Results with Hydroxyurea in Cancer Chemotherapy; Preliminary Report. New York state journal of medicine. 1965;65:2132–2136. [PubMed] [Google Scholar]
- 142.Fishbein WN, Carbone PP, Freireich EJ, Misra D, Frei E., 3rd Clinical Trials of Hydroxyurea in Patients with Cancer and Leukemia. Clin Pharmacol Ther. 1964;5:574–580. doi: 10.1002/cpt196455574. [DOI] [PubMed] [Google Scholar]
- 143.Sears ME. Phase Ii Studies of Hydroxyurea (Nsc-32065) in Adults: Cancer of the Breast. Cancer Chemother Rep. 1964;40:43. [PubMed] [Google Scholar]
- 144.Origenes ML, Jr., Beatty EC, Jr., Brubaker C, Hammond D, Hartmann JR, Shore N, et al. Trial of Hydroxyurea (Nsc-32065) in Cancer in Children. Cancer Chemother Rep. 1964;37:41–46. [PubMed] [Google Scholar]
- 145.Kao J, Genden EM, Gupta V, Policarpio EL, Burri RJ, Rivera M, et al. Phase 2 trial of concurrent 5-fluorouracil, hydroxyurea, cetuximab, and hyperfractionated intensity-modulated radiation therapy for locally advanced head and neck cancer. Cancer. 2011;117:318–326. doi: 10.1002/cncr.25374. [DOI] [PubMed] [Google Scholar]
- 146.Hoglund L, Pontis E, Reichard P. Deoxyribonucleotide metabolism in hydroxyurea-resistant V79 hamster cells. European journal of biochemistry / FEBS. 1991;196:239–245. doi: 10.1111/j.1432-1033.1991.tb15810.x. [DOI] [PubMed] [Google Scholar]
- 147.Young CW, Schochetman G, Karnofsky DA. Hydroxyurea-induced inhibition of deoxyribonucleotide synthesis: studies in intact cells. Cancer Research. 1967;27:526–534. [PubMed] [Google Scholar]
- 148.Kozhina TN, Kozhin SA, Korolev VG. [Gene RAD31 is identical to gene MEC1 of yeast Saccharomyces cerevisiae] Genetika. 2011;47:610–614. [PubMed] [Google Scholar]
- 149.Donnianni RA, Ferrari M, Lazzaro F, Clerici M, Tamilselvan Nachimuthu B, Plevani P, et al. Elevated levels of the polo kinase Cdc5 override the Mec1/ATR checkpoint in budding yeast by acting at different steps of the signaling pathway. PLoS genetics. 2010;6 doi: 10.1371/journal.pgen.1000763. e1000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Carballo JA, Cha RS. Meiotic roles of Mec1, a budding yeast homolog of mammalian ATR/ATM. Chromosome research : an international journal on the molecular, supramolecular and evolutionary aspects of chromosome biology. 2007;15:539–550. doi: 10.1007/s10577-007-1145-y. [DOI] [PubMed] [Google Scholar]
- 151.Morrow DM, Tagle DA, Shiloh Y, Collins FS, Hieter P. TEL1, an S. cerevisiae homolog of the human gene mutated in ataxia telangiectasia, is functionally related to the yeast checkpoint gene MEC1. Cell. 1995;82:831–840. doi: 10.1016/0092-8674(95)90480-8. [DOI] [PubMed] [Google Scholar]
- 152.Lee J, Kannagi M, Ferrante RJ, Kowall NW, Ryu H. Activation of Ets-2 by oxidative stress induces Bcl-xL expression and accounts for glial survival in amyotrophic lateral sclerosis. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2009;23:1739–1749. doi: 10.1096/fj.08-121046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Katada H, Nishikawa K, Yoneda T, Kitamura H, Nakajima A, Mikami R. [Role of immune skin reactions in progressive lung cancer during the administration of OK-432--relation to reactions to DNCB, PPD and Su-PS] Gan To Kagaku Ryoho. 1987;14:61–70. [PubMed] [Google Scholar]
- 154.Munzarova M, Kovarik J, Hlavkova J, Popelinsky L, Lauerova L. DNCB and PPD skin tests and prognosis in 152 patients with breast cancer. A prospective 2-year follow-up. Neoplasma. 1985;32:45–50. [PubMed] [Google Scholar]
- 155.Munzarova M, Kovarik J, Ninger E, Zemanova D, Lauerova L, Kolcova V, et al. DNCB and PPD skin testing in breast cancer. Neoplasma. 1983;30:385–389. [PubMed] [Google Scholar]
- 156.Cunningham TJ, Daut D, Wolfgang PE, Mellyn M, Maciolek S, Sponzo RW, et al. A correlation of DNCB-induced delayed cutaneous hypersensitivity reactions and the course of disease in patients with recurrent breast cancer. Cancer. 1976;37:1696–1700. doi: 10.1002/1097-0142(197604)37:4<1696::aid-cncr2820370413>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 157.Sadoff L, Glovsky M, Alenty A, Catalona WJ, Taylor PT, Chretien PB. DNCB test in cancer patients. The New England journal of medicine. 1972;287:47–48. doi: 10.1056/NEJM197207062870117. [DOI] [PubMed] [Google Scholar]
- 158.Yamashita T, Nakamaru-Ogiso E, Miyoshi H, Matsuno-Yagi A, Yagi T. Roles of bound quinone in the single subunit NADH-quinone oxidoreductase (Ndi1) from Saccharomyces cerevisiae. The Journal of biological chemistry. 2007;282:6012–6020. doi: 10.1074/jbc.M610646200. [DOI] [PubMed] [Google Scholar]
- 159.Seo BB, Wang J, Flotte TR, Yagi T, Matsuno-Yagi A. Use of the NADH-quinone oxidoreductase (NDI1) gene of Saccharomyces cerevisiae as a possible cure for complex I defects in human cells. The Journal of biological chemistry. 2000;275:37774–37778. doi: 10.1074/jbc.M007033200. [DOI] [PubMed] [Google Scholar]
- 160.Seo BB, Matsuno-Yagi A, Yagi T. Modulation of oxidative phosphorylation of human kidney 293 cells by transfection with the internal rotenone-insensitive NADH-quinone oxidoreductase (NDI1) gene of Saccharomyces cerevisiae. Biochimica et Biophysica Acta. 1999;1412:56–65. doi: 10.1016/s0005-2728(99)00051-1. [DOI] [PubMed] [Google Scholar]
- 161.Lee R, Britz-McKibbin P. Differential rates of glutathione oxidation for assessment of cellular redox status and antioxidant capacity by capillary electrophoresis-mass spectrometry: an elusive biomarker of oxidative stress. Analytical Chemistry. 2009;81:7047–7056. doi: 10.1021/ac901174g. [DOI] [PubMed] [Google Scholar]
- 162.Xu S, He Y, Vokurkova M, Touyz RM. Endothelial cells negatively modulate reactive oxygen species generation in vascular smooth muscle cells: role of thioredoxin. Hypertension. 2009;54:427–433. doi: 10.1161/HYPERTENSIONAHA.109.133983. [DOI] [PubMed] [Google Scholar]
- 163.Raftos JE, Dwarte TM, Luty A, Willcock CJ. Direct measurement of the rate of glutathione synthesis in 1-chloro-2,4-dinitrobenzene treated human erythrocytes. Redox Rep. 2006;11:9–14. doi: 10.1179/135100006X100986. [DOI] [PubMed] [Google Scholar]
- 164.Tanaka T, Nakamura H, Yodoi J, Bloom ET. Redox regulation of the signaling pathways leading to eNOS phosphorylation. Free Radical Biology & Medicine. 2005;38:1231–1242. doi: 10.1016/j.freeradbiomed.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 165.Fujise H, Higa K, Kanemaru T, Fukuda M, Adragna NC, Lauf PK. GSH depletion, K-Cl cotransport, and regulatory volume decrease in high-K/high-GSH dog red blood cells. American journal of physiology Cell physiology. 2001;281:C2003–C2009. doi: 10.1152/ajpcell.2001.281.6.C2003. [DOI] [PubMed] [Google Scholar]
- 166.Elliott SJ, Doan TN, Henschke PN. Reductant substrate for glutathione peroxidase modulates oxidant inhibition of Ca2+ signaling in endothelial cells. The American journal of physiology. 1995;268:H278–H287. doi: 10.1152/ajpheart.1995.268.1.H278. [DOI] [PubMed] [Google Scholar]
- 167.Den Boer PJ, van Loon AA, Mackenbach P, van der Schans GP, Grootegoed JA. Effect of glutathione depletion on the cytotoxicity of xenobiotics and induction of single-strand DNA breaks by ionizing radiation in isolated hamster round spermatids. J Reprod Fertil. 1990;88:259–269. doi: 10.1530/jrf.0.0880259. [DOI] [PubMed] [Google Scholar]
- 168.Mkoji GM, Smith JM, Prichard RK. Glutathione redox state, lipid peroxide levels, and activities of glutathione enzymes in oltipraz-treated adult Schistosoma mansoni. Biochemical Pharmacology. 1989;38:4307–4313. doi: 10.1016/0006-2952(89)90530-3. [DOI] [PubMed] [Google Scholar]
- 169.Harlan JM, Levine JD, Callahan KS, Schwartz BR, Harker LA. Glutathione redox cycle protects cultured endothelial cells against lysis by extracellularly generated hydrogen peroxide. The Journal of clinical investigation. 1984;73:706–713. doi: 10.1172/JCI111263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sanz A, Soikkeli M, Portero-Otin M, Wilson A, Kemppainen E, McIlroy G, et al. Expression of the yeast NADH dehydrogenase Ndi1 in Drosophila confers increased lifespan independently of dietary restriction. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9105–9110. doi: 10.1073/pnas.0911539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hekimi S, Lapointe J, Wen Y. Taking a "good" look at free radicals in the aging process. Trends in Cell Biology. 2011 doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rutherford SL, Carpenter AT. The effect of sequence homozygosity on the frequency of X-chromosomal exchange in Drosophila melanogaster females. Genetics. 1988;120:725–732. doi: 10.1093/genetics/120.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rutherford SL, Henikoff S. Quantitative epigenetics. Nat Genet. 2003;33:6–8. doi: 10.1038/ng0103-6. [DOI] [PubMed] [Google Scholar]
- 174.Hubbard J, Erlichman C, Toft DO, Qin R, Stensgard BA, Felten S, et al. Phase I study of 17-allylamino-17 demethoxygeldanamycin, gemcitabine and/or cisplatin in patients with refractory solid tumors. Investigational New Drugs. 2011;29:473–480. doi: 10.1007/s10637-009-9381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ohba S, Hirose Y, Yoshida K, Yazaki T, Kawase T. Inhibition of 90-kD heat shock protein potentiates the cytotoxicity of chemotherapeutic agents in human glioma cells. J Neurosurg. 2010;112:33–42. doi: 10.3171/2009.3.JNS081146. [DOI] [PubMed] [Google Scholar]
- 176.Oikonomou E, Koc M, Sourkova V, Andera L, Pintzas A. Selective BRAFV600E inhibitor PLX4720, requires TRAIL assistance to overcome oncogenic PIK3CA resistance. PLoS ONE. 2011;6:e21632. doi: 10.1371/journal.pone.0021632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Takahashi S. Combination therapy with arsenic trioxide for hematological malignancies. Anticancer Agents Med Chem. 2010;10:504–510. doi: 10.2174/1871520611009060504. [DOI] [PubMed] [Google Scholar]
- 178.Nimmanapalli R, Fuino L, Bali P, Gasparetto M, Glozak M, Tao J, et al. Histone deacetylase inhibitor LAQ824 both lowers expression and promotes proteasomal degradation of Bcr-Abl and induces apoptosis of imatinib mesylate-sensitive or -refractory chronic myelogenous leukemia-blast crisis cells. Cancer Res. 2003;63:5126–5135. [PubMed] [Google Scholar]
- 179.Gilbert JA, Adhikari LJ, Lloyd RV, Rubin J, Haluska P, Carboni JM, et al. Molecular markers for novel therapies in neuroendocrine (carcinoid) tumors. Endocr Relat Cancer. 2010;17:623–636. doi: 10.1677/ERC-09-0318. [DOI] [PubMed] [Google Scholar]
- 180.Yao Q, Nishiuchi R, Kitamura T, Kersey JH. Human leukemias with mutated FLT3 kinase are synergistically sensitive to FLT3 and Hsp90 inhibitors: the key role of the STAT5 signal transduction pathway. Leukemia. 2005;19:1605–1612. doi: 10.1038/sj.leu.2403881. [DOI] [PubMed] [Google Scholar]
- 181.Reka AK, Kuick R, Kurapati H, Standiford TJ, Omenn GS, Keshamouni VG. Identifying inhibitors of epithelial-mesenchymal transition by connectivity map-based systems approach. J Thorac Oncol. 2011;6:1784–1792. doi: 10.1097/JTO.0b013e31822adfb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Arlander SJ, Eapen AK, Vroman BT, McDonald RJ, Toft DO, Karnitz LM. Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. J Biol Chem. 2003;278:52572–52577. doi: 10.1074/jbc.M309054200. [DOI] [PubMed] [Google Scholar]
- 183.Yao Q, Weigel B, Kersey J. Synergism between etoposide and 17-AAG in leukemia cells: critical roles for Hsp90, FLT3, topoisomerase II, Chk1, and Rad51. Clin Cancer Res. 2007;13:1591–1600. doi: 10.1158/1078-0432.CCR-06-1750. [DOI] [PubMed] [Google Scholar]
- 184.Nguyen A, Su L, Campbell B, Poulin NM, Nielsen TO. Synergism of heat shock protein 90 and histone deacetylase inhibitors in synovial sarcoma. Sarcoma. 2009;2009 doi: 10.1155/2009/794901. 794901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Al Shaer L, Walsby E, Gilkes A, Tonks A, Walsh V, Mills K, et al. Heat shock protein 90 inhibition is cytotoxic to primary AML cells expressing mutant FLT3 and results in altered downstream signalling. Brit J Haematol. 2008;141:483–493. doi: 10.1111/j.1365-2141.2008.07053.x. [DOI] [PubMed] [Google Scholar]
- 186.Huang HC, Yang Y, Nanda A, Koria P, Rege K. Synergistic administration of photothermal therapy and chemotherapy to cancer cells using polypeptide-based degradable plasmonic matrices. Nanomedicine. 2011;6:459–473. doi: 10.2217/nnm.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Widakowich C, Dinh P, de Azambuja E, Awada A, Piccart-Gebhart M. HER-2 positive breast cancer: what else beyond trastuzumab-based therapy? Anticancer Agents Med Chem. 2008;8:488–496. doi: 10.2174/187152008784533062. [DOI] [PubMed] [Google Scholar]