Abstract
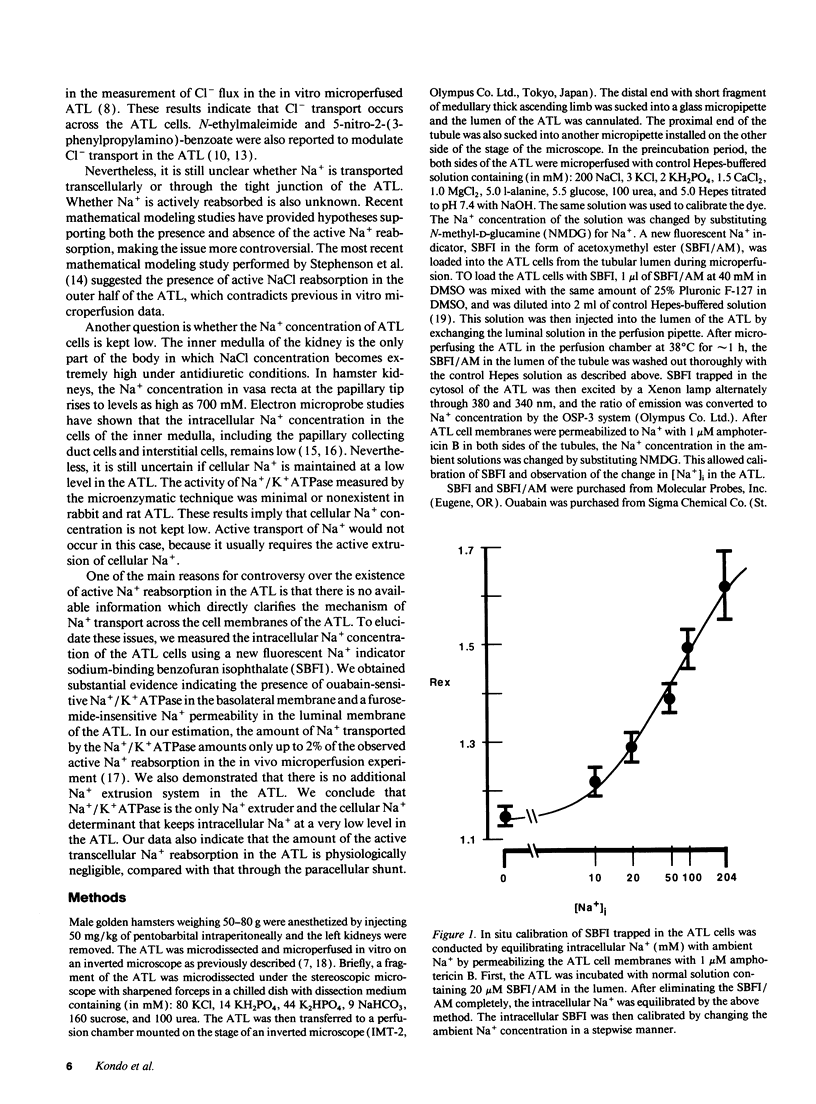
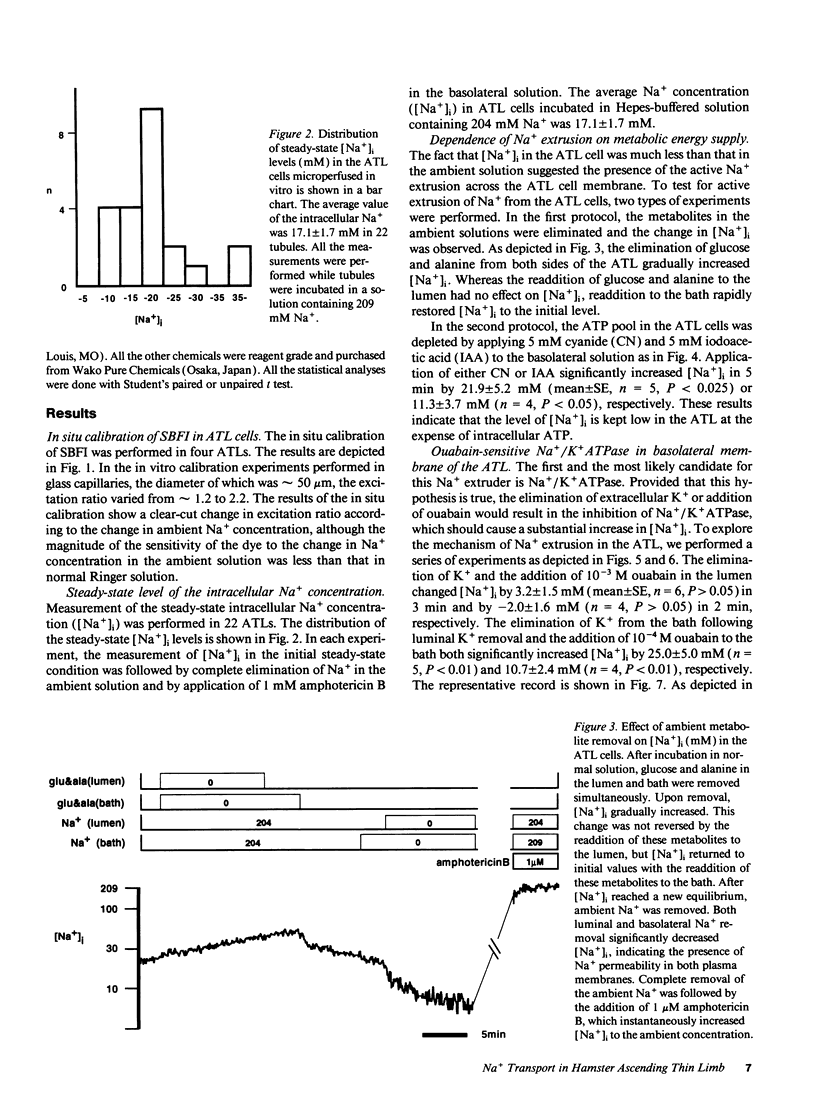
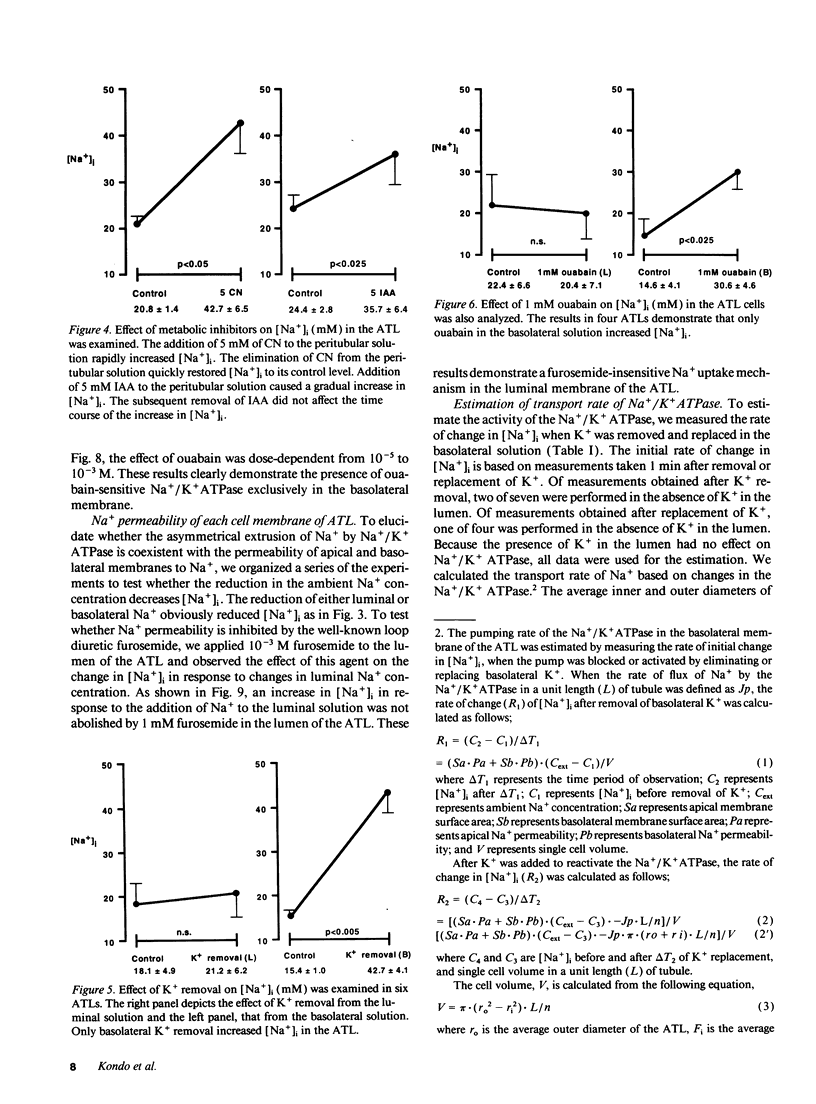
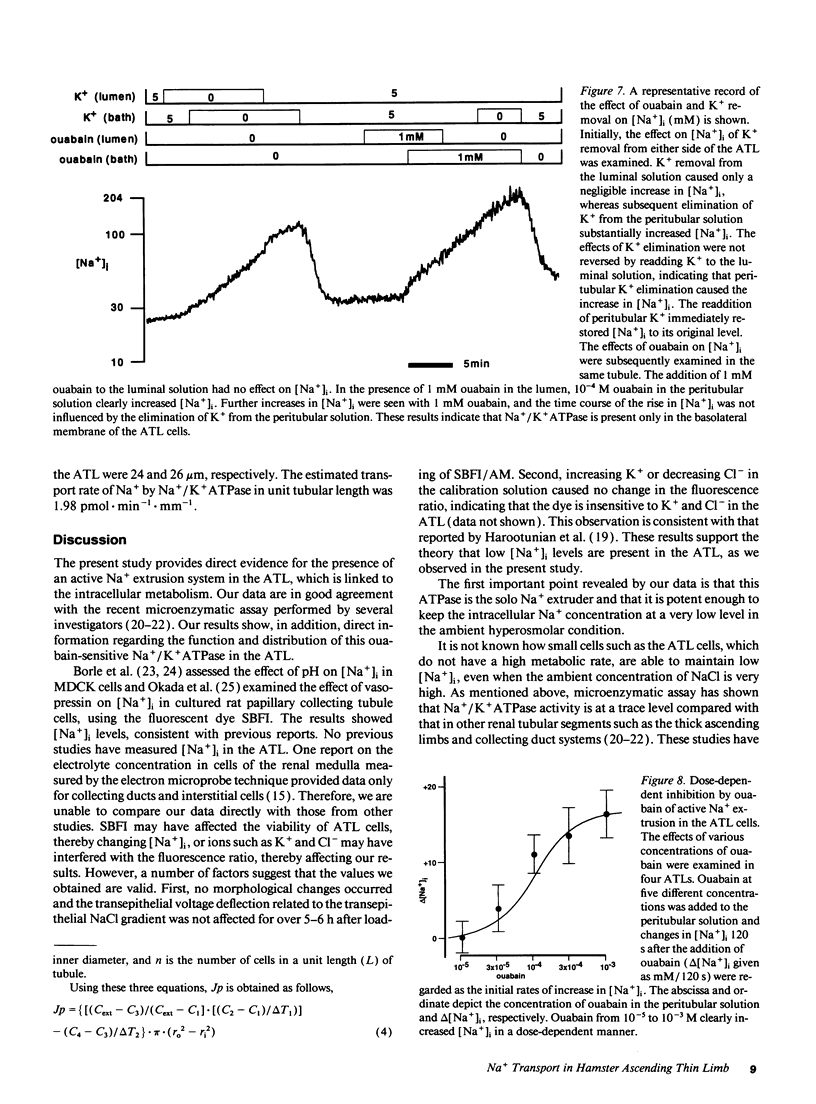
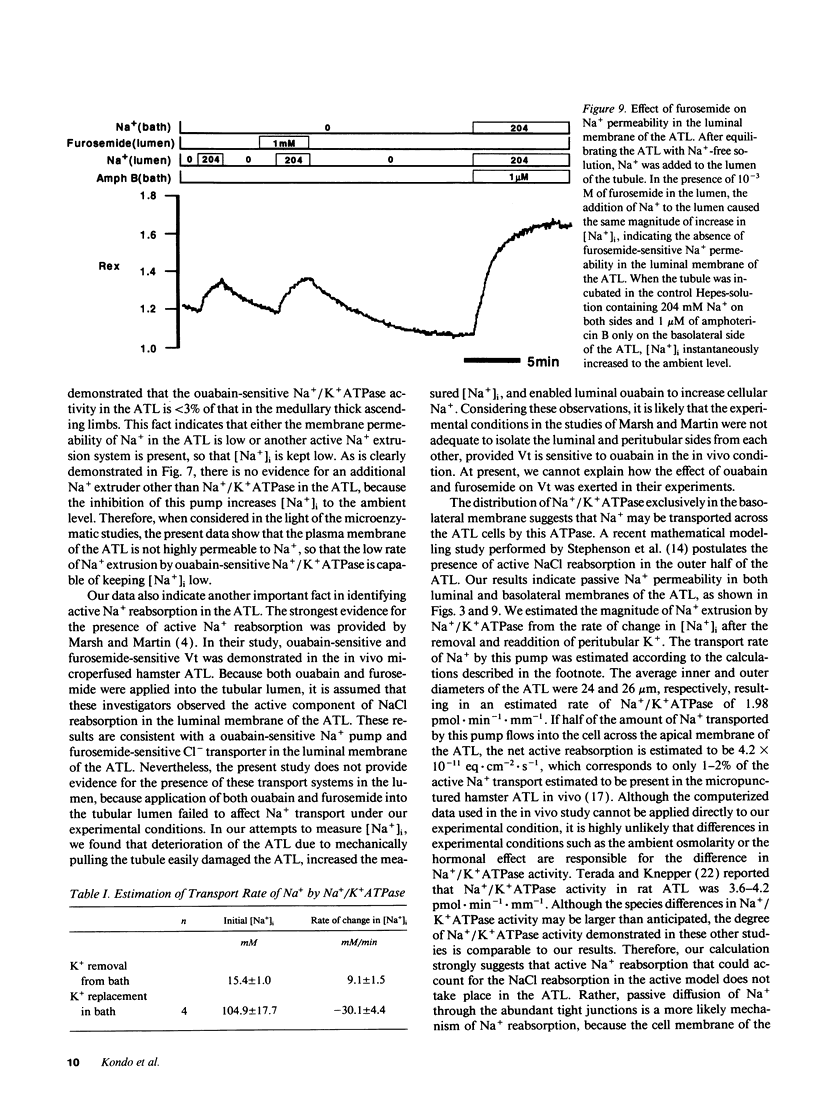
The mechanisms of Na+ transport across cell membranes were investigated in the in vitro microperfused hamster ascending thin limb (ATL) of Henle's loop using a fluorescent Na+ indicator sodium-binding benzofuran isophthalate. The intracellular Na+ concentration ([Na+]i) of the ATL cells was 17.1 +/- 1.7 mM (n = 22) when the ATL was microperfused in vitro with Hepes-buffered solution containing 204 mM Na+. Elimination of metabolites such as glucose and alanine from the basolateral solution increased [Na+]i. Applying either 5 mM cyanide or 5 mM iodoacetic acid to the bath also increased [Na+]i. The elimination of K+ and the addition of 10(-4) M ouabain in the bath increased [Na+]i by 25.0 +/- 5.0 mM (n = 5) in 3 min and by 10.7 +/- 2.4 mM (n = 4), respectively. The elimination of luminal and basolateral Na+ resulted in a decrease in [Na+]i, indicating Na+ permeability of both the luminal and basolateral cell membranes. The luminal Na+ permeability was not affected by furosemide. The presence of luminal Na+ permeability and the basolateral Na+/K+ ATPase suggests the presence of net active reabsorption of Na+, which is not a physiologically important amount, in our estimation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck F., Dörge A., Rick R., Thurau K. Intra- and extracellular element concentrations of rat renal papilla in antidiuresis. Kidney Int. 1984 Feb;25(2):397–403. doi: 10.1038/ki.1984.30. [DOI] [PubMed] [Google Scholar]
- Beck F., Dörge A., Rick R., Thurau K. Osmoregulation of renal papillary cells. Pflugers Arch. 1985;405 (Suppl 1):S28–S32. doi: 10.1007/BF00581776. [DOI] [PubMed] [Google Scholar]
- Borle A. B., Bender C. Effects of pH on Ca2+i, Na+i, and pHi of MDCK cells: Na(+)-Ca2+ and Na(+)-H+ antiporter interactions. Am J Physiol. 1991 Sep;261(3 Pt 1):C482–C489. doi: 10.1152/ajpcell.1991.261.3.C482. [DOI] [PubMed] [Google Scholar]
- Borle A. B., Borle C. J., Dobransky P., Gorecka-Tisera A. M., Bender C., Swain K. Effects of low extracellular Ca2+ on cytosolic free Ca2+, Na+, and pH of MDCK cells. Am J Physiol. 1990 Jul;259(1 Pt 1):C19–C25. doi: 10.1152/ajpcell.1990.259.1.C19. [DOI] [PubMed] [Google Scholar]
- Garg L. C., Knepper M. A., Burg M. B. Mineralocorticoid effects on Na-K-ATPase in individual nephron segments. Am J Physiol. 1981 Jun;240(6):F536–F544. doi: 10.1152/ajprenal.1981.240.6.F536. [DOI] [PubMed] [Google Scholar]
- Harootunian A. T., Kao J. P., Eckert B. K., Tsien R. Y. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J Biol Chem. 1989 Nov 15;264(32):19458–19467. [PubMed] [Google Scholar]
- Hogg R. J., Kokko J. P. Comparison between the electrical potential profile and the chloride gradients in the thin limbs of Henle's loop in rats. Kidney Int. 1978 Nov;14(5):428–436. doi: 10.1038/ki.1978.147. [DOI] [PubMed] [Google Scholar]
- Imai M., Kokko J. P. Mechanism of sodium and chloride transport in the thin ascending limb of Henle. J Clin Invest. 1976 Nov;58(5):1054–1060. doi: 10.1172/JCI108556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M., Kokko J. P. Sodium chloride, urea, and water transport in the thin ascending limb of Henle. Generation of osmotic gradients by passive diffusion of solutes. J Clin Invest. 1974 Feb;53(2):393–402. doi: 10.1172/JCI107572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M., Kondo Y., Koseki C., Yoshitomi K. Dual effect of N-ethylmaleimide on Cl- transport across the thin ascending limb of Henle's loop. Pflugers Arch. 1988 May;411(5):520–528. doi: 10.1007/BF00582373. [DOI] [PubMed] [Google Scholar]
- Isozaki T., Yoshitomi K., Imai M. Effects of Cl- transport inhibitors on Cl- permeability across hamster ascending thin limb. Am J Physiol. 1989 Jul;257(1 Pt 2):F92–F98. doi: 10.1152/ajprenal.1989.257.1.F92. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Doucet A., Morel F. Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol. 1979 Aug;237(2):F114–F120. doi: 10.1152/ajprenal.1979.237.2.F114. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Frömter E. Axial heterogeneity of sodium-bicarbonate cotransport in proximal straight tubule of rabbit kidney. Pflugers Arch. 1987 Nov;410(4-5):481–486. doi: 10.1007/BF00586529. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Imai M. Effect of glutaraldehyde on renal tubular function. II. Selective inhibition of Cl- transport in the hamster thin ascending limb of Henle's loop. Pflugers Arch. 1987 May;408(5):484–490. doi: 10.1007/BF00585073. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Yoshitomi K., Imai M. Effect of Ca2+ on Cl- transport in thin ascending limb of Henle's loop. Am J Physiol. 1988 Feb;254(2 Pt 2):F232–F239. doi: 10.1152/ajprenal.1988.254.2.F232. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Yoshitomi K., Imai M. Effect of pH on Cl- transport in TAL of Henle's loop. Am J Physiol. 1987 Dec;253(6 Pt 2):F1216–F1222. doi: 10.1152/ajprenal.1987.253.6.F1216. [DOI] [PubMed] [Google Scholar]
- Kondo Y., Yoshitomi K., Imai M. Effects of anion transport inhibitors and ion substitution on Cl- transport in TAL of Henle's loop. Am J Physiol. 1987 Dec;253(6 Pt 2):F1206–F1215. doi: 10.1152/ajprenal.1987.253.6.F1206. [DOI] [PubMed] [Google Scholar]
- Koyama S., Yoshitomi K., Imai M. Effect of protamine on ion conductance of ascending thin limb of Henle's loop from hamsters. Am J Physiol. 1991 Oct;261(4 Pt 2):F593–F599. doi: 10.1152/ajprenal.1991.261.4.F593. [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Azen S. P. Mechanism of NaCl reabsorption by hamster thin ascending limbs of Henle's loop. Am J Physiol. 1975 Jan;228(1):71–79. doi: 10.1152/ajplegacy.1975.228.1.71. [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Martin C. M. Origin of electrical PD's in hamster thin ascending limbs of Henle's loop. Am J Physiol. 1977 Apr;232(4):F348–F357. doi: 10.1152/ajprenal.1977.232.4.F348. [DOI] [PubMed] [Google Scholar]
- Okada K., Ishikawa S., Saito T. Effect of vasopressin on Na+ kinetics in cultured rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1990 Nov 30;173(1):224–230. doi: 10.1016/s0006-291x(05)81045-9. [DOI] [PubMed] [Google Scholar]
- Stephenson J. L., Zhang Y., Tewarson R. Electrolyte, urea, and water transport in a two-nephron central core model of the renal medulla. Am J Physiol. 1989 Sep;257(3 Pt 2):F399–F413. doi: 10.1152/ajprenal.1989.257.3.F399. [DOI] [PubMed] [Google Scholar]
- Terada Y., Knepper M. A. Na+-K+-ATPase activities in renal tubule segments of rat inner medulla. Am J Physiol. 1989 Feb;256(2 Pt 2):F218–F223. doi: 10.1152/ajprenal.1989.256.2.F218. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Kondo Y., Imai M. Evidence for conductive Cl- pathways across the cell membranes of the thin ascending limb of Henle's loop. J Clin Invest. 1988 Sep;82(3):866–871. doi: 10.1172/JCI113691. [DOI] [PMC free article] [PubMed] [Google Scholar]


