Abstract
OBJECTIVE
HIV-1 infected patients have an increased risk for atherothrombosis and cardiovascular disease, but the mechanism behind these risks is poorly understood. We have previously reported that expression of tissue factor (TF) on circulating monocytes is increased in persons with HIV infection and that TF expression is related to immune activation, to levels of HIV in plasma, and to indices of microbial translocation. In this study, we explore the activation state of platelets in HIV disease.
METHODS
Here, using flow cytometry-based assays, we measured platelet and platelet microparticle (PMP) activation in samples from HIV-1 infected donors and controls.
RESULTS
Platelets and PMPs from HIV-1 infected patients are activated (as reflected by expression of CD62 P-selectin) and also more frequently expressed the procoagulant tissue factor (TF) than did platelets and PMPs obtained from controls. Expression of these proteins was directly related to expression of TF on monocytes, to markers of T cell activation (CD38 and HLA-DR) and to plasma levels of soluble CD14, the coreceptor for bacterial lipopolysaccharride. Platelet and microparticle expression of TF was not related to plasma levels of HIV but expression of P-selectin was; neither TF nor P-selectin expression was related to CD4 T cell count.
CONCLUSIONS
Platelets and microparticles are activated in HIV infection and this activated phenotype may contribute to the increased risk for cardiovascular and thrombotic events in this population although a role for other confounding cardiovascular risks cannot be completely excluded.
Keywords: tissue factor, platelets, HIV-1, immune activation
Introduction
Platelets, anuclear cell fragments, participate in the localization of coagulation reactionsin the intravascular compartment. Platelet reactivity, especially within ruptured atheromatous plaques, has been implicated in the pathogenesis of atherothrombosis and cardiovascular disease 1, 2. Many agents aimed at preventing cardiovascular disease target platelet activation 2, 3. Upon activation, platelets increase expression of the adhesion molecule, P-selectin, thereby promoting adhesion to cell surfaces and clot formation 2, 3. Platelets also contain pre-mRNA for the procoagulant tissue factor. Tissue factor on platelets 4, and on other surfaces, forms a complex with factor VII to serve as a cofactor for factor VIIa to activate factor X and drive clot formation 5.
P-selectin, a glycoprotein found in the α-granules of platelets, binds to P-selectin glycoprotein ligand-1 (PSGL-1) which is expressed by the endothelium and leukocytes. This interaction is integral to the recruitment of pro-inflammatory leukocytes to sites of injury and thrombosis, such as occurs in the settings of endotoxin-induced sepsis and atherosclerosis 3, 6–9. P-selectin expressed by platelets captures microparticles, monocyte and platelet fragments, in both pathologic and non-pathologic thrombi to activate leukocytes and cause up-regulation of tissue factor expression by endothelial cells and monocytes 3, 10.
Patients with human immunodeficiency virus (HIV) infection have an increased risk of thrombotic cardiovascular disease 11, 12. The SMART study, a clinical trial of continued versus interrupted antiretroviral treatment, found that the risk of death, including deaths due to cardiovascular events, was linked to higher plasma levels of the inflammatory cytokine interleukin-6 (IL-6), C-reactive protein, and D-dimers (markers of clot formation and lysis) 13. We have earlier shown that HIV infection is associated with systemic translocation of microbial products such as bacterial lipopolysaccharide and bacterial DNAs from the intestinal lumen. These microbial products can bind to innate immune pattern recognition molecules such as Toll-like receptors (TLRs), resulting in activation of both innate 14 and adaptive immune cells 15. Plasma levels of these products are correlated with indices of immune activation 16, 17.
We have also shown that freshly isolated monocytes from HIV-infected patients more frequently express cell surface tissue factor than do monocytes from uninfected controls. Monocyte TF expression correlates with markers of immune activation, with plasma levels of HIV RNA, with plasma levels of the LPS co-receptor CD14, and with levels of D-dimers 18.
Platelets, which have an important role in arterial thrombosis, interact with leukocytes 1, 2 and express functional Toll-like receptor 4 19. We hypothesized that platelets of HIV-infected patients might also be activated as a result of sustained exposure to microbial elements in circulation. In the present report we show that platelets and microparticles in HIV infection are activated to express both P-selectin and tissue factor and that expression of these markers correlate with indices of T cell activation and microbial translocation/monocyte activation that are recognized predictors of morbidity in chronic HIV infection 20, 21.
Methods
All procedures were conducted in accordance with policies of the Case Western Reserve University/University Hospitals of Cleveland Institutional Review Board.
Peripheral blood was drawn, with consent, from HIV infected patients (n=46) and from healthy controls (n=18). All samples were conveniently collected from the populations available, in the morning, over a two month time span. Blood was collected in glass tubes for preparation of serum, and in EDTA-coated or citrate-anticoagulated plastic tubes for plasma. Anticoagulated (citrate) blood was centrifuged at 180x g for 10 minutes to separate platelet-rich plasma from cells. Peripheral blood mononuclear cells were isolated on a ficoll-hypaque cushion.
Flow cytometry
Platelets, from platelet-enriched citrated plasma, were identified in plasma by small size and low complexity on forward versus side scatter plots. These elements were positively identified as platelets, or platelet microparticles, by expression of the surface marker glycoprotein 1b (CD42b) using a phycoerythrin (PE) conjugated monoclonal antibody (BD Pharmingen, San Diego, CA). In selected experiments we confirmed that these labelled elements were neither monocyte derived-MPs nor monocyte PMP aggregates by the absence of CD14 expression (not shown). Surface expression of P-selectin (CD62P) and tissue factor was measured using conjugated monoclonal antibodies to CD62P (Allophycocyanin (APC)-conjugated - BD Pharmingen) and to tissue factor (fluorescein isocyanate (FITC)-conjugated (American Diagnostica, Stamford, CT).
Red blood cells within whole blood preparations were lysed by incubation in FACS Lyse buffer (BD Biosciences, San Diego, CA) for 15 minutes on ice. Monocytes were identified by light scatter characteristics and by reactivity with anti-CD14 Peridinin-chlorophyll-protein Complex (PerCP), and with anti-HLA-DR APC (BD Pharmingen). CD4+ and CD8+ T cells were identified by binding of anti-CD3 APC and anti-CD4 Pacific Blue, or anti-CD8 APC-Cy7 and levels of T cell activation were measured using anti-CD38 PE and anti-HLA-DR FITC (BD Pharmingen). Fluorochrome-conjugated monoclonal antibodies of appropriate isotype were used to establish gating. Cell preparations were stained for 30 minutes, then washed in phosphate buffered saline containing 1% fetal calf serum and 0.1% sodium azide, and resuspended in 1% paraformaldehyde. Platelet preparations were stained for 30 minutes on ice, then washed and resuspended in 1% paraformaldehyde before analysis. Cells, platelets and PMPs were acquired and analyzed using an LSR II flow cytometer (Becton-Dickinson, San Jose, CA) and FACSDiva software version 6.1.1 (BD Biosciences, San Diego, CA).
Measurement of D-dimers and Soluble CD14
Whole blood was collected into EDTA containing tubes and plasma was prepared by centrifugation for 10 minutes at 1610×g and was then frozen at −80°C until assay. Levels of D-dimers were measured using the Asserachrom D-DI immunoassay (Diagnostic Stago Asnieres, France) and soluble CD14 was measured by ELISA using the Quantikine soluble CD14 kit (R&D Systems Minneapolis, MN, USA) following the manufacturer’s instructions.
Statistical Methods
Differences between variables among patients and controls were tested with a Mann-Whitney’s U test. We tested associations between pairs of continuous variables with Spearman’s rank correlation. We processed the analyses using Graphpad Prism v. 5.02 (GraphPad Software, Inc., La Jolla, CA). All tests are two-sided, and a p-value of 0.05 was considered nominally significant.
Results
Platelets in HIV infected subjects have high surface expression of P-selectin and tissue factor
Previous studies have shown that HIV infected patients have an increased risk for thrombotic and cardiovascular events 11, 12, 22–24 and since platelets and microparticles play a major role in coagulation, we prepared these anuclear fragments from the plasma of 46 HIV infected donors and 18 healthy controls and measured surface expression of P-selectin and tissue factor by flow cytometry. All of our samples were collected from patients and controls in the University Hospital/ Case Western Reserve University population. Among the patients and controls for whom we have information, 67% of each group was male. The median ages for the patient and control populations were 42 years (range 26–65) and 33 years (range 20–61) respectively (p=0.015). Forty one percent of patients had plasma RNA levels >400copies/ml. The median plasma HIV RNA level for the entire patient population was 50 copies/ml (range = 50–590,000copies/ml) and the median CD4+ T cell count was 400 cells/ul (range = 6–1,063 cells/ul). Seventy three percent of the patients were being treated with antiretroviral therapy. A greater proportion of the patient population reported that they were current smokers (29%), compared to 17% of the controls While the HIV-infected group had a greater number of patients who were defined as obese (Body Mass Index, BMI> 30, N=7 for patients, N=0 for controls), the median BMI of each group was not statistically different (patients =25.8, controls 24.5, p=0.11). Among the HIV+ group, 6 patients reported daily treatment with aspirin, 5 patients reported using statins, 4 patients had a positive screen for Hepatitis B and 6 patients were positive for Hepatitis C. Among the healthy controls, 1 control reported taking aspirin daily, 2 were receiving statins, and none was diabetic or known to be infected with Hepatitis B or C. A greater proportion of the HIV infected population was African American (51%) compared to 6% among uninfected controls. Demographic information is summarized in Table 1.
Table 1.
Demographic information of the study cohort
| Gender | Age (Median yrs) |
Tobacco use |
Antiretroviral usage |
Ethnicity (# individuals, self reported information) |
BMI (median) |
|
|---|---|---|---|---|---|---|
| Controls | 7 females 11 males |
33 range [20–61] |
Non-smokers 15 Smokers 3 |
Not applicable | 1- African American 12- Caucasian 2 -Hispanic/Latin 2-Asian 1 –African |
24.5 range [16.7– 28.3] |
| Patients | 14 females 28 males |
42 range [26–65] |
Non-smokers 33 Smokers 13 |
55 on HAART | 19 -African American 18- Caucasian |
25.8 range [18.9– 42.1] |
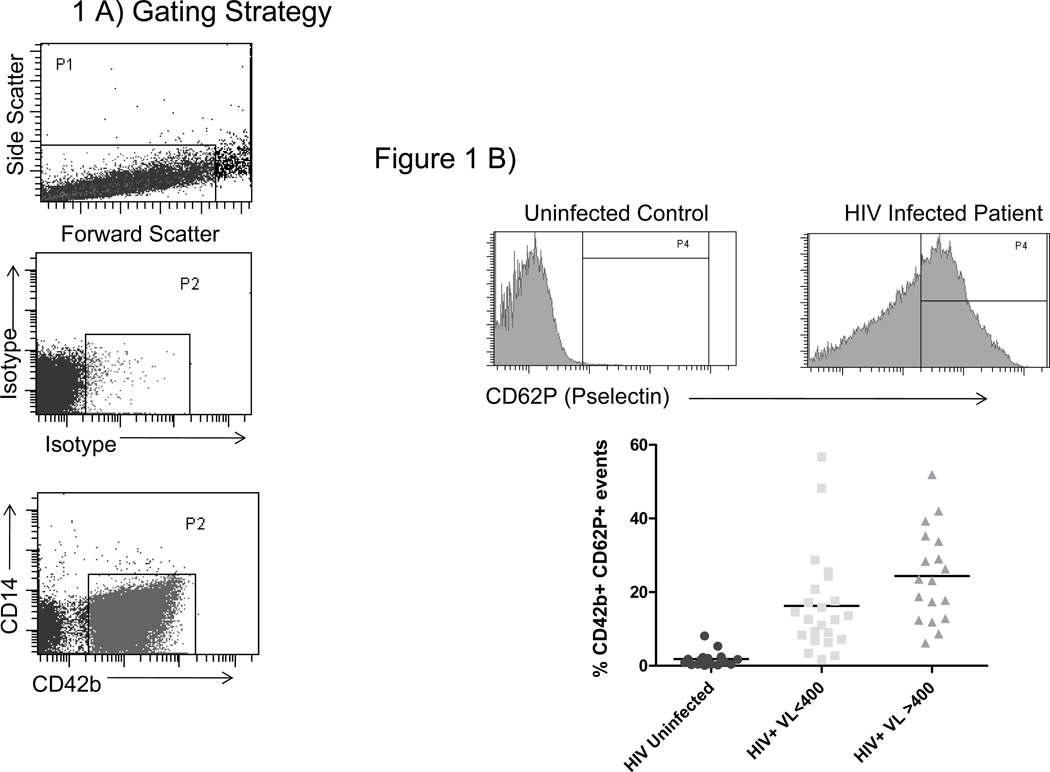
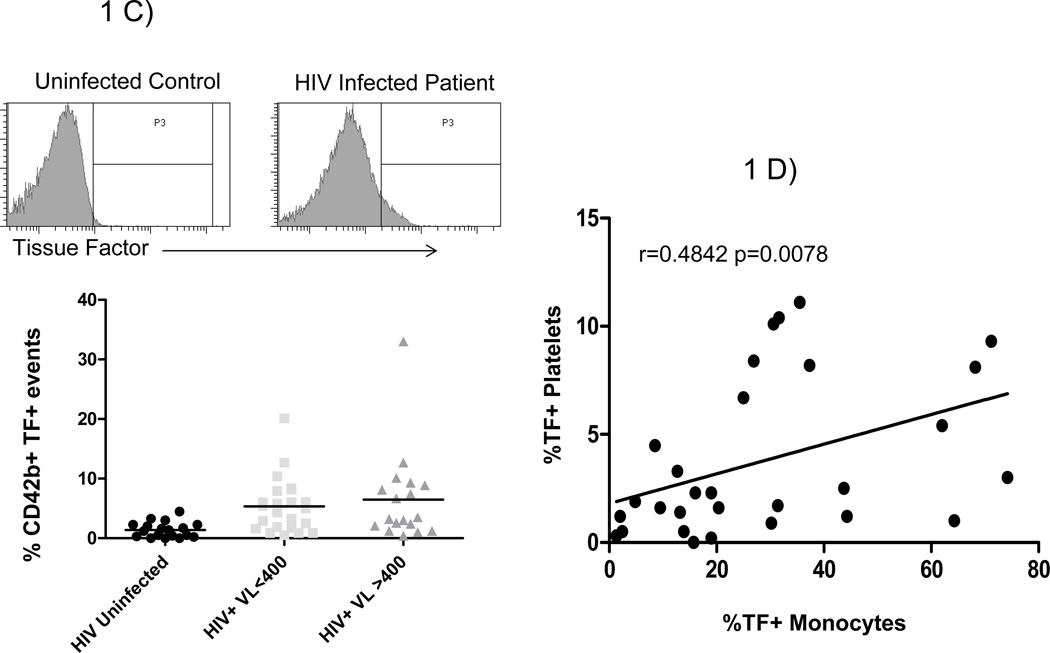
Platelets and microparticles were identified by low forward and side scatter characteristics, and by expression of CD42b (Figure 1A). Among healthy controls, these elements rarely expressed P-selectin (median 1.1% positive) or tissue factor (median 1.2% positive). The proportions of platelets and microparticles expressing P-selectin or tissue factor (17.3% and 4.7% respectively) in HIV-infected patient samples were 10 significantly greater (p <0.001 for both) than among controls. Platelets and microparticles obtained from patients with detectable viremia (n =18) more frequently had detectible surface P-selectin than did platelets and PMPs obtained from patients with virologic control (HIV RNA<400 copies/ml) (n = 23, 23.3 versus 12.6%, p =0.022, Figure 1B). The proportions of TF+ platelets and PMPs were similar among patients with uncontrolled viremia and patients with virologic suppression (3.4% vs 5.0%, respectively, p=0.7, Figure 1C). The proportions of TF+ and CD62P+ platelets and microparticles were directly related when samples from all donors were analyzed (r=0.53 p<0.001, data not shown).
Figure 1. Platelets/microparticles from HIV infected patients have an activated phenotype.
A) Platelets/microparticles are defined by their low complexity and small size in plasma and by expression of CD42b. Representative histograms and summary data among platelets/microparticles from healthy controls (N=18), patients with controlled viremia (HIV RNA<400 copies/mL, N=23), and uncontrolled viremia (HIV RNA>400 copies/mL, N=18) for surface expression of B) CD62P and C) tissue factor. The proportions of platelet/PMP that expressed CD62P in each of our patient groups were significantly different from the proportion in controls (p<0.001 for both). Likewise, the proportions of TF+ platelets/PMPs in each of our patient groups were significantly different from the proportion of TF+ platelets/PMPs in controls (p>0.001 for both) D) The proportions of TF+ platelets/microparticles and TF+ monocytes are directly related (r=0.4842 p=0.0078).
We previously described an increase in surface expression of TF on monocytes from HIV infected patients 18. In this study, the proportions of monocytes and platelets/microparticles expressing tissue factor, from all donors, are directly related (r=0.4842, p=0.0078, Figure 1D), suggesting that the drivers of platelet/microparticle and monocyte expression of tissue factor may be related.
Platelet/microparticle activation is differentially related to plasma levels of the LPS coreceptor CD14 and to plasma HIV levels, but not to CD4 T cell counts
To obtain preliminary insight into the determinants of platelet activation in HIV-1 infection, we explored potential clinical and laboratory correlates of platelet activation. Unlike monocyte TF expression, there was no relationship between platelet/PMP expression of TF and the magnitude of plasma viremia or CD4+ T cell count (r=0.1066 p=0.51 and r= −0.12, p=0.46 respectively, not shown). There was a correlation between the proportion of P-selectin positive platelets/PMPs and levels of plasma viremia (r=0.45, p=0.003) but, there was no relationship between P-selectin expression and CD4+ T cell counts(r=−0.20, p=0.21, not shown). Thus, HIV replication or its consequences may drive platelet expression of P-selectin, but not of tissue factor. We speculate that monocytes may recognize and be activated by HIV-1 through surface receptors used for viral entry or by innate immune receptors expressed by monocytes that are likely absent on platelets and PMPs.
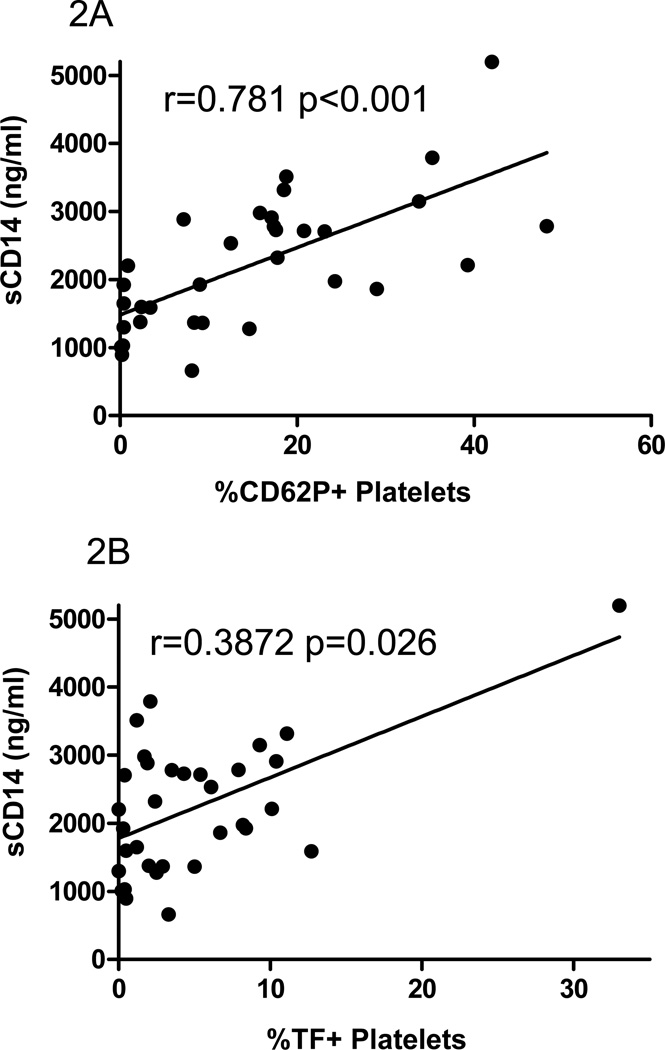
We have previously reported that the increased plasma levels of the LPS co-receptor (sCD14) in HIV infected patients correlated with the proportion of monocytes expressing TF 18. In the present study, we find higher plasma levels of sCD14 in HIV infection than in healthy controls (2603ng/ml versus 1364ng/ml, p=0.004). In all donors, plasma levels of sCD14 were directly related to platelet/microparticle expression of P-selectin (r=0.71, p<0.001, Figure 2A) and tissue factor (R=0.39, p=0.026, Figure 2B). The relationship between platelet/microparticle expression of P-selectin and sCD14 remained significant in the HIV infected patients population (r=0.456, p=0.029, not shown).
Figure 2. The proportions of platelets/microparticles expressing P-selectin or tissue factor are related to plasma levels of sCD14.
Plasma samples were thawed in batch and levels of sCD14 were measured. In samples from all donors A) the proportion of P-selectin positive platelets/microparticles is directly related to plasma levels of sCD14, r=0.781 p<0.001; and B) the proportion of tissue factor positive platelets/microparticles is directly related to plasma levels of sCD14, r=0.3872 p=0.026.
Plasma levels of D-dimers, markers of coagulation and fibrinolysis, were predictive of all-cause mortality in the SMART study 13, 16. Since we had earlier linked monocyte TF expression to D-dimer levels 18, we asked if this marker also was related to platelet/PMP activation. While plasma levels of D-dimers were increased in patients compared to levels in controls (436 FEU versus 256 FEU, respectively, p=0.029) there were only modest correlations between D-dimer levels and the proportion of platelets/microparticles expressing P-selectin (r=.40, p=0.041) or tissue factor (r=0.30, p=0.115) in samples from all donors.
Platelet/microparticle activation is directly related to CD8+ T cell activation
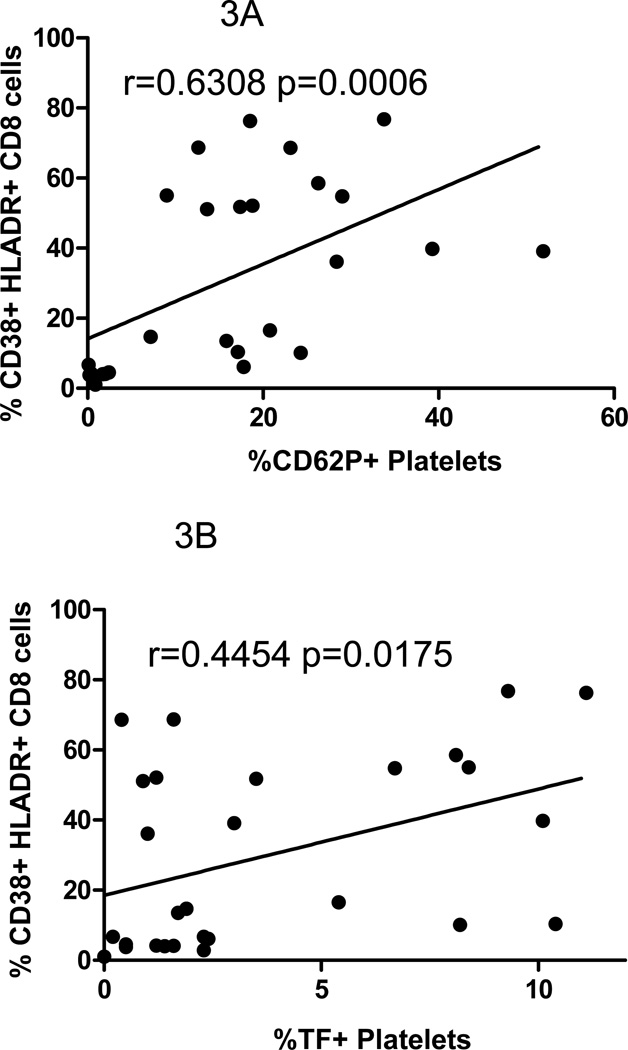
T cell activation indices have been linked to disease course in HIV infection 25, 26. We next asked if T cell and platelet/PMP activation could also be related. The expression of CD38 and HLA-DR on CD8+ T cells was strongly correlated with platelet/microparticle surface expression of both P-selectin (r=0.63, p=0.0006) and tissue factor (r=0.45, p=0.0175) (Figure 3A and B) in all donors, and the relationship between CD38 and HLA-DR on CD8+ T cells and the proportion of platelets/PMPs that express TF remained when analyzing the patient population alone (r=0.530, p=0.02).
Figure 3. The proportions of platelets/microparticles expressing P-selectin or tissue factor are related to markers of T cell activation.
CD8+ T lymphocytes were identified by size, complexity, and by expression of CD3 and CD8, and markers of activation (CD38 and HLA-DR) were measured on phenotypically gated cells. In samples from all donors A) the proportion of P-selectin positive platelets/microparticles is directly related to CD8+ T cell activation, r=0.6308 p=0.0006; and B) the proportion of TF positive platelets/microparticles is directly related to CD8+ T cell activation, r=0.4454 p=0.0175.
Discussion
Both in the pre-HAART and in the post HAART eras, HIV infected patients have experienced an increased risk of thrombosis and cardiovascular disease, 12, 27, 28 yet, the determinants of these risks are incompletely understood. In a recent study where continuous antiretroviral therapy was compared to intermittent therapy, all cause mortality, as well as morbidity due to cardiovascular disease, were linked to increased levels of D-dimers, interleukin-6, and C-reactive protein 13, implicating both inflammation and coagulation as potential mediators of these risks.
In plasma samples from persons with chronic HIV infection, we and others have found increased levels of microbial products; evidence of microbial translocation from the damaged gut 16, 17, 29. These levels were linked both to indices of immune activation and also to T cell homeostasis as reflected by a strong relationship to the magnitude of CD4+ T cell restoration after application of combination antiretroviral therapies 16, 17. Moreover, we have demonstrated that a broad variety of microbial TLR ligands can induce an activation phenotype on both CD4+ and CD8+ T cells in vitro that is similar to the activation phenotype seen in vivo in chronic HIV infection 15. More recently, we have shown that selected TLR ligands can induce tissue factor expression on blood monocytes and that monocytes from HIV infected donors commonly express high levels of cell surface TF 18. Soluble TF levels were also increased in HIV infection and levels of monocyte TF were related to plasma levels of the LPS coreceptor sCD14 and to plasma levels of D-dimers, potentially linking this procoagulant to microbial translocation/monocyte activation and to ongoing clot formation and fibrinolysis. Recently, Hashimoto et al have also found that in vitro exposure to LPS could induce platelets to express P-selectin 30. Thus, it is reasonable to propose that monocyte and platelet activation and increased procoagulant expression in chronic HIV infection may be linked at least in part to in vivo exposure to microbial products.
We identified platelets/microparticles by their size and light scatter characteristics and confirmed that these small anuclear fragments are of platelet origin by the concordant expression of CD42b, a glycoprotein that is uniquely expressed by platelets 31. The absence of CD14 expression assured that these elements were not microparticles derived from macrophages/monocytes, nor had they bound their membrane fragments. Based on our gating strategy, we could not discriminate between platelets and PMPs. These subcellular platelet fragments are mainly produced by activated platelets and express the same cell surface antigens as do activated platelets, including CD42b, tissue factor, and P-selectin 32–34. While we cannot distinguish activated platelets from platelet derived microparticles, these two populations are functionally and phenotypically nearly identical and the main finding of this study is that the proportion of small, CD42b+ anuclear cell fragments that express TF or P-selectin are increased in the circulation of HIV-1 infected patients, compared to these proportions in the circulation of healthy uninfected controls. High levels of P-selectin have been previously reported in plasma 35 and on the surfaces of platelets in HIV infected patients 36, in consumptive coagulopathies, 37 and in settings of direct endothelial injury and platelet activation 38. Unlike the finding reported by Holme et al 36, we did not see a relationship between platelet/microparticle expression of P-selectin and CD4+ T cell counts among our HIV infected donors 36.
Platelet/microparticle expression of TF was not directly related to clinical indices of HIV disease (plasma viremia and CD4+ T cell count), but both TF and P-selectin expression on platelets/microparticles were related to T cell activation. This work suggests that the drivers of chronic immune activation and inflammation that are independent of the magnitude of viral replication may be important determinants of morbidity in both treated and untreated HIV infection.
While our findingsrelate the chronic immune activation of HIV-1 infection to platelet activation and coagulation in HIV disease, the results of this cross sectional study may be limited by the small sample size and by significant differences in our patient and control populations. Patients tended to be older (mean age 42 years) than our controls (mean age 33 years, p=0.015) but, age has not been associated with differences in P-selectin expression in other studies 39–42 nor did we show a significant relationship between age and platelet expression of Pselectin (r=0.159, p=0.334) or TF (r=0.035, p=0.832) in our patient population. Although there were proportionally more smokers among patients (29%) than among controls (17%), in our patient population, smokers did not have higher platelet tissue factor expression (mean= 5.7 %TF+) or platelet activation (16.29% CD62P+) than did non-smokers (5.5 %TF+, 20.77%CD62P+, p=0.39 and p=0.34, respectively), suggesting smoking does not influence the increased levels of platelet activation in these patients. While we also had more obese patients among the HIV infected population (N=7) than among the controls (N=0), expression of CD62P or TF on platelets from obese patients and non-obese patients within the HIV+ population was not significantly different (p= 0.43 and p=0.42, respectively). While the proportion of African Americans was much greater in our patient population (51%) than in controls (6%), we did not see a significant difference in markers of platelet activation between African Americans and Caucasians within the HIV-infected population. The proportions of CD62P+ or TF+ platelets within the African American population were 19.4+/−3% and 6.15+/−2%, and among the Caucasian population, the proportions of CD62P+ and TF+ platelets were 18.7+/−3% and 5.1+/−3%, (p=0.75 and p=0.87, respectively.) These results suggest that race/ethnicity was not a major determinant of the increased platelet activation seen here in persons with HIV infection. Several of these indices (smoking, BMI, ethnicity) contribute to an increased risk of cardiovascular disease and platelet activation. Due to the small overall sample size in this study and the differences between our HIV+ and HIV− subjects as noted above, we cannot entirely rule out some contribution of these confounders on the differences in platelet activation reported herein. Future studies, where a larger and or more comparable groups of participants are examined, will be needed to confirm our findings that platelets and microparticles are activated in HIV infection. Conceivably this activation contributes to the apparent increased cardiovascular risk experienced by persons with HIV infection that is linked both to HIV-1 replication and to other indices of immune activation.
The proposed linkage between platelet activation and microbial translocation is also limited by the fact that we did not measure LPS directly. While sCD14 levels have been correlated directly with plasma levels of LPS in some studies16, 43 other studies were unable to find such a relationship21, 44. Activation of monocytes by microbial products, such as LPS, or inflammatory cytokines, such as IL-6 44, can induce shedding of CD14. Thus, increased levels of plasma sCD14 may also reflect monocyte activation through a variety of stimuli. We have previously demonstrated a relationship between monocyte expression of TF and sCD14 18 and in this current work, we report a relationship between platelet expression of TF and Pselectin to plasma levels of sCD14, suggesting that there may be a common “driver,” such as microbial translocation or inflammatory cytokines, that may be helping to drive monocyte and platelet activation in HIV disease.
In summary, we show here that platelets/microparticles of HIV infected persons are activated in vivo to express P selectin and tissue factor, and that expression of these markers of adhesion and coagulation are related to soluble levels of the LPS receptor CD14. The proportions of TF+ monocytes and TF+ platelets/microparticles appear to be directly related, and our data suggest that they may be driven, at least in part, by sustained exposure to microbial elements or represent another consequence of monocyte activation. Both platelet/microparticle and monocyte TF expression were strongly related to levels of the LPS coreceptor sCD14 while monocyte TF, 18 but not platelet/microparticle TF, was strongly correlated to plasma levels of HIV. We have recently found that monocytes can be directly activated by HIV in vitro to express tissue factor (unpublished); this is not likely the case for platelets. Additional study is needed to clarify the mechanisms of TF upregulation and platelet activation in HIV infection and to ascertain the relationship among these indices and in vivo thrombosis and cardiovascular morbidity. We suspect that the recognized increases in cardiovascular morbidities that are complicating the course of HIV infection may be at least in part, a result of the sustained exposure of these patients to the proinflammatory environment that persists even among HIV infected persons in whom viremia is well controlled by antiretroviral therapies 17.
Acknowledgements
E.M. M.K. and N.F. performed experiments. R.A. provided patient samples. B.R. provided statistical method support. All authors contributed to the analysis of data and writing of the paper. This work was funded by grants from the National Institutes of Health AI -07164, AI-67039, AI-68636, the Fasenmyer fund and the Center for AIDS Research at Case Western Reserve University AI 36219.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This data has been presented, in part, at the Conference on Retroviruses and Opportunistic Infections, February 2009, Montreal, Canada
The authors have no competing financial interests.
References
- 1.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008 Aug 28;359(9):938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 2.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007 Dec 13;357(24):2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 3.Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov. Feb;9(2):154–169. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 4.Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007 Jun 15;109(12):5242–5250. doi: 10.1182/blood-2006-06-030619. [DOI] [PubMed] [Google Scholar]
- 5.Jude B, Zawadzki C, Susen S, Corseaux D. Relevance of tissue factor in cardiovascular disease. Arch Mal Coeur Vaiss. 2005 Jun;98(6):667–671. [PubMed] [Google Scholar]
- 6.Klintman D, Li X, Thorlacius H. Important role of P-selectin for leukocyte recruitment, hepatocellular injury, and apoptosis in endotoxemic mice. Clin Diagn Lab Immunol. 2004 Jan;11(1):56–62. doi: 10.1128/CDLI.11.1.56-62.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangell P, Mihaescu A, Wang Y, Schramm R, Jeppsson B, Thorlacius H. Critical role of P-selectin-dependent leukocyte recruitment in endotoxin-induced intestinal barrier dysfunction in mice. Inflamm Res. 2007 May;56(5):189–194. doi: 10.1007/s00011-007-6163-x. [DOI] [PubMed] [Google Scholar]
- 8.Palabrica T, Lobb R, Furie BC, et al. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992 Oct 29;359(6398):848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 9.Norman KE, Katopodis AG, Thoma G, et al. P-selectin glycoprotein ligand-1 supports rolling on E- and P-selectin in vivo. Blood. 2000 Nov 15;96(10):3585–3591. [PubMed] [Google Scholar]
- 10.Maly M, Vojacek J, Hrabos V, Kvasnicka J, Salaj P, Durdil V. Tissue factor, tissue factor pathway inhibitor and cytoadhesive molecules in patients with an acute coronary syndrome. Physiol Res. 2003;52(6):719–728. [PubMed] [Google Scholar]
- 11.d'Arminio A, Sabin CA, Phillips AN, et al. Cardio- and cerebrovascular events in HIV-infected persons. Aids. 2004 Sep 3;18(13):1811–1817. doi: 10.1097/00002030-200409030-00010. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. Aids. 2008 Aug 20;22(13):1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008 Oct 21;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. May;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 15.Funderburg N, Luciano AA, Jiang W, Rodriguez B, Sieg SF, Lederman MM. Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS One. 2008;3(4):e1915. doi: 10.1371/journal.pone.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006 Dec;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 17.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009 Apr 15;199(8):1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Funderburg NT, Mayne E, Sieg SF, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. Jan 14;115(2):161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andonegui G, Kerfoot SM, McNagny K, Ebbert KV, Patel KD, Kubes P. Platelets express functional Toll-like receptor-4. Blood. 2005 Oct 1;106(7):2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 20.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999 Apr;179(4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 21.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. Mar 15;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangili A, Gerrior J, Tang AM, et al. Risk of cardiovascular disease in a cohort of HIV-infected adults: a study using carotid intima-media thickness and coronary artery calcium score. Clin Infect Dis. 2006 Dec 1;43(11):1482–1489. doi: 10.1086/509575. [DOI] [PubMed] [Google Scholar]
- 23.Mooser V. Atherosclerosis and HIV in the highly active antiretroviral therapy era: towards an epidemic of cardiovascular disease? Aids. 2003 Apr;17 Suppl 1:S65–S69. doi: 10.1097/00002030-200304001-00009. [DOI] [PubMed] [Google Scholar]
- 24.Periard D, Cavassini M, Taffe P, et al. High prevalence of peripheral arterial disease in HIV-infected persons. Clin Infect Dis. 2008 Mar 1;46(5):761–767. doi: 10.1086/527564. [DOI] [PubMed] [Google Scholar]
- 25.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004 Aug 15;104(4):942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997 Oct 1;16(2):83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 27.Majluf-Cruz A, Silva-Estrada M, Sanchez-Barboza R, et al. Venous thrombosis among patients with AIDS. Clin Appl Thromb Hemost. 2004 Jan;10(1):19–25. doi: 10.1177/107602960401000104. [DOI] [PubMed] [Google Scholar]
- 28.Crum-Cianflone NF, Weekes J, Bavaro M. Thromboses among HIV-Infected Patients during the Highly Active Antiretroviral Therapy Era. AIDS Patient Care STDS. 2008 Sep 10; doi: 10.1089/apc.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marchetti G, Bellistri GM, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. Aids. 2008 Oct 1;22(15):2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto K, Jayachandran M, Owen WG, Miller VM. Aggregation and Microparticle Production Through Toll-like Receptor 4 Activation in Platelets From Recently Menopausal Women. J Cardiovasc Pharmacol. 2009 Jun 12; doi: 10.1097/FJC.0b013e3181ab373d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wickenhauser C, Schmitz B, Baldus SE, et al. Selectins (CD62L, CD62P) and megakaryocytic glycoproteins (CD41a, CD42b) mediate megakaryocytefibroblast interactions in human bone marrow. Leuk Res. 2000 Dec;24(12):1013–1021. doi: 10.1016/s0145-2126(00)00063-1. [DOI] [PubMed] [Google Scholar]
- 32.Biro E, Sturk-Maquelin KN, Vogel GM, et al. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003 Dec;1(12):2561–2568. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 33.Joop K, Berckmans RJ, Nieuwland R, et al. Microparticles from patients with multiple organ dysfunction syndrome and sepsis support coagulation through multiple mechanisms. Thromb Haemost. 2001 May;85(5):810–820. [PubMed] [Google Scholar]
- 34.Nieuwland R, Berckmans RJ, McGregor S, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000 Feb 1;95(3):930–935. [PubMed] [Google Scholar]
- 35.Calza L, Pocaterra D, Pavoni M, et al. Plasma levels of VCAM-1, ICAM-1, ESelectin, and P-Selectin in 99 HIV-positive patients versus 51 HIV-negative healthy controls. J Acquir Immune Defic Syndr. 2009 Apr 1;50(4):430–432. doi: 10.1097/QAI.0b013e31819a292c. [DOI] [PubMed] [Google Scholar]
- 36.Holme PA, Muller F, Solum NO, Brosstad F, Froland SS, Aukrust P. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. Faseb J. 1998 Jan;12(1):79–89. doi: 10.1096/fasebj.12.1.79. [DOI] [PubMed] [Google Scholar]
- 37.Chong BH, Murray B, Berndt MC, Dunlop LC, Brighton T, Chesterman CN. Plasma P-selectin is increased in thrombotic consumptive platelet disorders. Blood. 1994 Mar 15;83(6):1535–1541. [PubMed] [Google Scholar]
- 38.Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost. 2005 Aug;3(8):1590–1596. doi: 10.1111/j.1538-7836.2005.01373.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen D, Giannopoulos K, Shiels PG, et al. Inhibition of intravascular thrombosis in murine endotoxemia by targeted expression of hirudin and tissue factor pathway inhibitor analogs to activated endothelium. Blood. 2004 Sep 1;104(5):1344–1349. doi: 10.1182/blood-2003-12-4365. [DOI] [PubMed] [Google Scholar]
- 40.Choudhury A, Chung I, Blann AD, Lip GY. Platelet surface CD62P and CD63, mean platelet volume, and soluble/platelet P-selectin as indexes of platelet function in atrial fibrillation: a comparison of "healthy control subjects" and "disease control subjects" in sinus rhythm. J Am Coll Cardiol. 2007 May 15;49(19):1957–1964. doi: 10.1016/j.jacc.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 41.Choudhury A, Chung I, Panja N, Patel J, Lip GY. Soluble CD40 ligand, platelet surface CD40 ligand, and total platelet CD40 ligand in atrial fibrillation: relationship to soluble P-selectin, stroke risk factors, and risk factor intervention. Chest. 2008 Sep;134(3):574–581. doi: 10.1378/chest.07-2745. [DOI] [PubMed] [Google Scholar]
- 42.Wang HB, Wang JT, Zhang L, et al. P-selectin primes leukocyte integrin activation during inflammation. Nat Immunol. 2007 Aug;8(8):882–892. doi: 10.1038/ni1491. [DOI] [PubMed] [Google Scholar]
- 43.Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11(4):225–229. doi: 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- 44.Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C. CD14 is an acute-phase protein. J Immunol. 2004 Apr 1;172(7):4470–4479. doi: 10.4049/jimmunol.172.7.4470. [DOI] [PubMed] [Google Scholar]