Abstract
The orphan nuclear receptor TR3 (NR41A, Nur77) is overexpressed in most lung cancer patients and is a negative prognostic factor for patient survival. The function of TR3 was investigated in non-small cell lung cancer A549 and H460 cells, and knockdown of TR3 by RNA interference (siTR3) inhibited cancer cell growth and induced apoptosis. The prosurvival activity of TR3 was due, in part, to formation of a p300/TR3/Sp1 complex bound to GC-rich promoter regions of survivin and other Sp-regulated genes (mechanism 1). However, in p53 wild-type A549 and H460 cells, siTR3 inhibited the mTORC1 pathway and this was due to activation of p53 and induction of the p53-responsive gene sestrin 2 which subsequently activated the mTORC1 inhibitor AMPKα (mechanism 2). This demonstrates that the pro-oncogenic activity of TR3 in lung cancer cells was due to inhibition of p53 and activation of mTORC1. 1,1-Bis(3′-indolyl)-1-(p-hydroxyphenyl)methane (DIM-C-pPhOH) is a recently discovered inhibitor of TR3 which mimics the effects of siTR3. DIM-C-pPhOH inhibited growth and induced apoptosis in lung cancer cells and lung tumors in murine orthotopic and metastatic models, and this was accompanied by decreased expression of survivin and inhibition of mTORC1 signaling, demonstrating that inactivators of TR3 represent a novel class of mTORC1 inhibitors.
Keywords: TR3, Nur77, NR4A1, mTORC1, c-DIMs, lung cancer
INTRODUCTION
NR4A1 (TR3, Nur77), NR4A2 (Nurr1), and NR4A3 (Nor-1) are orphan nuclear receptors (Gronemeyer et al., 2004; McKenna et al., 2009) and were initially identified as immediate early genes induced by nerve growth factor in PC-12 cells (Milbrandt, 1988). NR4A genes are induced by multiple stressors in various tissues, and there is increasing evidence that NR4A receptors are essential for maintaining cellular homeostasis and they play a role in vascular function, metabolic pathways, inflammation, steroidgenesis and the central nervous system (reviewed in Maxwell and Muscat, 2006; Pearen and Muscat, 2010).
TR3 is highly expressed in multiple cancer cell lines and tumors, and higher levels of nuclear TR3 have been observed in colon, bladder and pancreatic tumors compared to non-tumor tissues (Maruyama et al., 1995; Ke et al., 2004; Chintharlapalli et al., 2005; Li et al., 2006; Zhang, 2007; Cho et al., 2007; Cho et al., 2010; Lee et al., 2010b). With few exceptions, knockdown of TR3 by RNA interference or a related technique in cancer cells resulted in growth inhibition, induction of apoptosis, or decreased angiogenesis indicating that TR3 is a pro-oncogenic factor (Bras et al., 2000; Kolluri et al., 2003; Ke et al., 2004; Zeng et al., 2006; Wu et al., 2010; Azoitei et al., 2010; Lee et al., 2010b). Many different classes of apoptotic agents induce apoptosis in cancer through TR3-dependent pathways (reviewed in Zhang, 2007). One of the major mechanisms associated with these effects involves nuclear export of TR3 and formation of a proapoptotic TR3-bcl-2 complex which can decrease mitochondrial membrane potential, resulting in the release of cytochrome c and activation of the extrinsic apoptosis pathway (Li et al., 2000; Lin et al., 2004; Kolluri et al., 2008). Cytosporone B (CsnB) and related analogs inhibit cancer cell and tumor growth through both nuclear export of TR3 and activation of nuclear TR3 and, unlike most other apoptosis-inducing agents, CsnB binds directly to TR3 (ligand binding domain) (Zhan et al., 2008; Liu et al., 2010).
1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes (C-DIMs) activate multiple nuclear receptors and the p-methoxyl derivative (DIM-C-pPhOCH3) activates nuclear TR3 and induces proapoptotic genes including p21, fas and TRAIL and induces apoptosis (Chintharlapalli et al., 2005; Cho et al., 2007; Lee et al., 2009; Cho et al., 2010). In contrast, the p-hydroxy C-DIM analog (DIM-C-pPhOH) deactivates nuclear TR3 and also induces apoptosis and inhibits pancreatic cancer cell and tumor growth (Lee et al., 2010b). Both TR3 knockdown (siTR3) and DIM-C-pPhOH decreased expression of survivin and induced apoptosis in pancreatic cancer cells. Constitutive expression of survivin and other specificity protein 1 (Sp1)-regulated genes (e.g. bcl-2) involved a p300/TR3/Sp1 complex bound to the proximal GC-rich region of the survivin promoter (Lee et al., 2010b), and deactivation of TR3 and downregulation of survivin was due to loss of p300 from this complex, and similar results were observed in this study in lung cancer cells (mechanism 1). In this study, another TR3-dependent pro-oncogenic pathway was identified in non-small cell lung cancer (NSCLC) A549 and H460 cells that are wild-type for p53. siTR3 and DIM-C-pPhOH inhibited mTORC1 signaling through activation of AMPKα and this was due to upstream activation of p53 and sestrin 2. Thus, endogenous TR3 maintains p53, sestrin 2 and AMPKα in a repressed state, thereby facilitating enhanced mTORC1 activity in cancer cells. These results are consistent with the pro-oncogenic function of TR3; however, this study also demonstrates the potential clinical importance of drugs such as DIM-C-pPhOH that block mTORC1 signaling through deactivation of TR3.
RESULTS
1. TR3 expression and prognostic significance in lung cancer patients
The orphan nuclear receptor TR3 is overexpressed in several different cancer cell lines and tumors and, in this study, we investigated expression of this receptor in NSCLC tumors by immunostaining and also determined the prognostic significance of TR3 expression. Figures 1A-1C show the typical Immunostaining of TR3 in normal lung tissue and adenocarcinoma (A), squamous cell carcinoma and large cell carcinoma (B). Relatively low TR3 staining was observed in normal lung tissue compared to lung tumors. Moreover, among the 59 control and 59 lung cancer patients (Table 1), many of the lung cancer patients (62.7%) exhibited high TR3 staining, whereas high TR3 staining was not observed in normal lung tissue (Fig. 1C). Lung tumor tissue samples also exhibited moderate (33.9%) and low (3.4%) staining, whereas in non-tumor tissue, only low (38.2%) and non-detectable (61.8%) TR3 staining was observed (Supplemental Table S1). A Kaplan-Meier plot of survival probability over time for 58 lung cancer patients with high TR3 expression vs. ≤ moderate TR3 shows that high expression of TR3 is associated with increased mortality for NSCLC patients (Fig. 1D). Moreover, univariate and multivariate analyses of TR3 expression vs. survival probability for patients with different stages of lung cancer invariably showed that high TR3 expression was inversely correlated with patient survival (Table 1; Supplemental Fig. S1).
Figure 1.
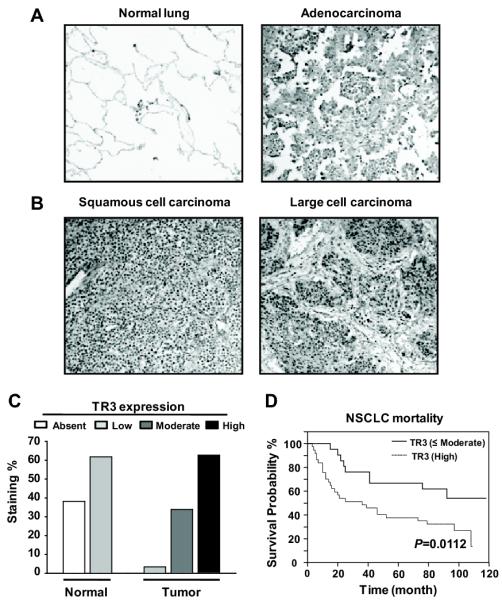
Immunohistochemical analysis of TR3 in 59 human non-small cell lung carcinomas (NSCLC) and 59 self-matching normal adjacent lung tissues. (A and B) Representative TR3 staining is shown for normal lung, adenocarcinoma, squamous cell carcinoma, and large cell carcinoma (×100). (C) Histogram of immunohistochemical score distribution obtained from the analysis showing enhanced expression of TR3 in lung tumors, but low or non-detectable TR3 staining was observed in normal lung tissues. (D) Association of TR3 overexpression with poor clinical outcome for NSCLC patients. Kaplan-Meier survival analysis in patients with NSCLC (n=58) according to TR3 expression.
Table 1.
Cox proportional-hazards regression model analysis of prognostic factors in patients with NSCLC.
| Variables | Hazards ratio (95% CI) | Comparison | P |
|---|---|---|---|
| Univariate analysis | |||
| TR3 | 2.57 (1.21-5.47) | High / ≤Moderate | 0.0151* |
| Gender | 1.42 (0.67-3.02) | Female / Male | NS |
| Age | 0.82 (0.40-1.65) | ≥65 / <65 | NS |
| pT factor | 3.24 (1.45-7.27) | T3-4 / T1-2 | 0.0045* |
| pN factor | 2.45 (1.27-4.75) | Positive / Negative | 0.0082* |
| Cancer type | 1.67 (0.73-3.81) | Non-ADC / ADC | NS |
| Cancer stage | 2.64 (1.34-5.20) | II-IV / I | 0.0052* |
| Multivariate analysis | |||
| TR3 | 2.59 (1.05-6.38) | High / ≤Moderate | 0.0397* |
| Gender | 1.78 (0.75-4.20) | Female / Male | NS |
| Age | 1.12 (0.49-2.55) | ≥65 / <65 | NS |
| pT factor | 5.96 (1.53-23.27) | T3-4 / T1-2 | 0.0106* |
| pN factor | 3.16 (0.78-12.87) | Positive / Negative | NS |
| Cancer type | 2.09 (0.13-1.75) | Non-ADC / ADC | NS |
| Cancer stage | 0.77 (0.16-3.78) | II-IV / I | NS |
Abbreviations: ADC, adenocarcinoma; non-ADC, carcinosarcoma, large cell carcinoma, and squamous cell carcinoma; CI, confidence interval; NS, no significance.
Statistically significant.
2. TR3 knockdown inhibits mTORC1 signaling
TR3 is highly expressed in pancreatic cancer cells and knockdown of the receptor by RNA interference (siTR3) decreased cell growth and induced apoptosis (Lee et al., 2010b), and siTR3 also decreased proliferation of A549 and H460 NSCLC cell lines (Fig. 1A). In pancreatic cancer cells, siTR3 also decreased survivin, bcl-2 and other growth promoting and prosurvival genes and it was shown that expression of survivin was dependent on formation of a p300/TR3/Sp1 complex at the proximal GC-rich survivin promoter, and knockdown of TR3, Sp1 or p300 by RNA interference decreased survivin and induced apoptosis (Lee et al., 2010b). Transfection of A549 or H460 cells with siTR3 also decreased expression of survivin and other Sp1-regulated genes (EGFR, bcl-2 and c-myc) as previously reported in pancreatic cancer cells, and the decreased survivin expression was not due to decreased Sp1 protein (data not shown). siTR3 decreased transactivation in H460 cells transfected with two-GC-rich survivin promoter constructs, a GAL4-Sp1 chimera, a GAL4-luc reporter plasmid, and a concensus GC-rich construct (Supplemental Figures S2A and S2B). Gel mobility shift assays using a concensus GC-rich oligonucleotides showed that Sp1 binding using nuclear extracts from H460 cells transfected with siTR3 was unchanged, whereas there was a significant decrease in p300 binding (Supplemental Fig. S2C), and this was consistent with results previously observed in pancreatic cancer cells (Lee et al., 2010b). Knockdown of Sp1 (siSp1) or p300 (sip300) decreased transactivation in cells transfected with GC-rich constructs (Supplemental Fig. S2D), decreased expression of survivin, and induced PARP cleavage in H460 cells (Supplemental Fig. S2E) and p53-null H1299 lung cancer cells (Supplemental Fig. S2F). These data support the role of p300/TR3/Sp1 complex in expression of survivin and maintenance of lung cancer survival as previously observed in pancreatic cancer cells (Lee et al., 2010b).
The induction of apoptosis in A549 and H460 cells transfected with siTR3 was significant (Fig. 2B) but appreciably lower than observed in pancreatic cancer cells (Lee et al., 2010b), and we hypothesized that TR3 may also regulate other growth promoting and antiapoptotic pathways and, based on results of preliminary screening, we focused on the mTORC1 pathway. Figure 2C illustrates that in A549 and H460 cells transfected with siTR3, there was significantly decreased phosphorylation of three key phospho-proteins regulated by mTORC1, namely phosphorylated p70S6K, pS6RP and 4E-BP1, whereas levels of the corresponding unphosphorylated proteins were unchanged. Moreover, the effects of siTR3 on the mTORC1 pathway were different from the p300/TR3/Sp1-dependent regulation of survivin and apoptosis (Lee et al., 2010b) (Supplemental Fig. S2) since knockdown of Sp1 in A549 cells did not affect expression of p70S6K, S6RP or 4E-BP1 (phosphorylated/unphosphorylated) (Fig. 2D).
Figure 2.
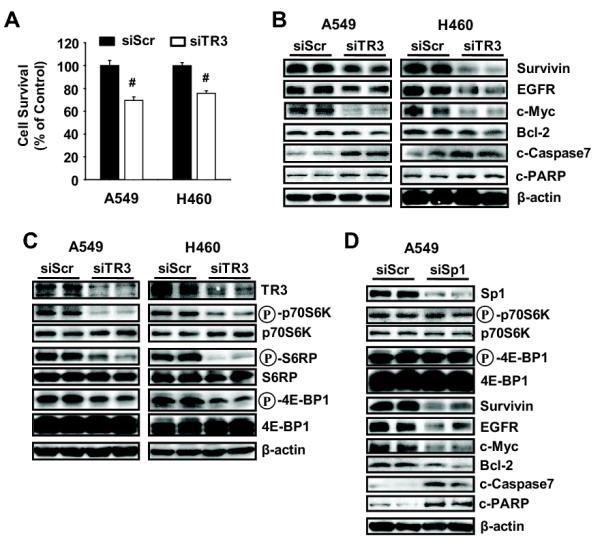
Knockdown of TR3 inhibits cell growth and induces apoptosis in NSCLC cells. (A) Cell survival. After transfection with either siScr or siTR3 for 4 days, the number of cells in each well was counted. The experiment was repeated three times with four replicates each and the data are presented as means with SD. #P<0.001 vs siScr. (B-D) Cells were transfected with an indicated siRNA for 72 hours, and whole cell lysates were analyzed by Western blot analysis. β-Actin was used as a loading control and the experiment was repeated three times with similar results. TR3 knockdown (C) varied between 60-80% in this study and similar results were observed in subsequent experiments using siTR3.
3. TR3 inhibits p53 → sestrin (induction) → AMPKα (activation) by inactivating 53
Phosphorylated AKT and AMPKα are potential upstream activators and deactivators, respectively, of mTORC1 and transfection of siTR3 into A549 and A460 cells did not affect expression of phospho-AKT, whereas there was an increase in phospho-AMPKα in both cell lines (Fig. 3A). Moreover, in A549 cells transfected with siTR3, siTR3 increased phosphorylation of AMPKα and decreased phosphorylation of 4E-BP1 and p70S6K , whereas knockdown of AMPKα alone had minimal effects on phospho-p70S6K or 4E-BP1 (Figures 2C and 3A). However, siAMPKα inhibited the effects of siTR3 on phosphorylation of 70S6K and 4E-BP1 confirming that inactivation of TR3 resulted in activation of AMPKα and inhibition of the mTORC1 pathway.
Figure 3.
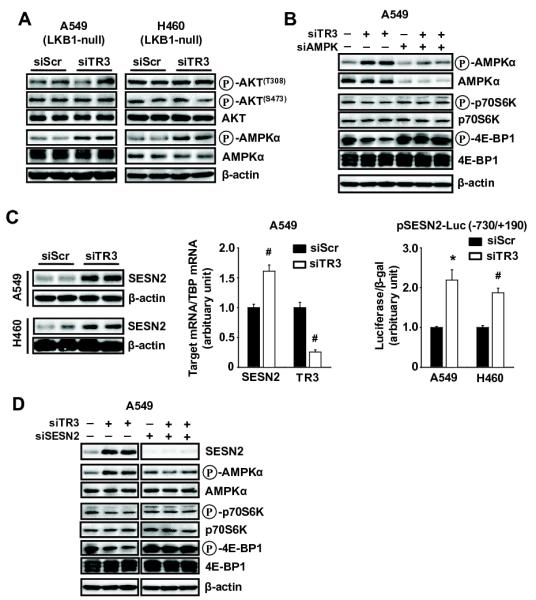
Knockdown of TR3 inhibits mTORC1 signaling through sestrin2-dependent but LKB1-independent activation of AMPKα in NSCLC cells. (A, B, and D) Cells were transfected with indicated siRNAs for 72 hr, and whole cell lysates were analyzed by Western blot analysis. (C) Cells were transfected with either siScr or siTR3 for 72 hr (left panel) or for 60 hr (middle panel), and sestrin2 protein and mRNA levels were determined by western blot analysis and real-time PCR, respectively, as described in the Materials and Methods. β-Actin was used as a loading control and TBP was used as an internal control. Sestrin2 and TR3 mRNA levels are presented as means with SD of 3 experiments. #P<0.001 vs siScr. (C, right panel) Cells were cotransfected with each siRNA and pSESN2-Luc (−730/+190), and luciferase activity (relative to β-galactosidase activity) was determined. The corresponding empty vector was used as a control, and the results are presented as means with SD of 3 experiments. *P<0.005 and #P<0.001 vs siScr.
Both A549 andH460 cells are LKB1 null which cannot be an upstream regulator of mTORC1; therefore, the effects of siTR3 on expression of sestrin 2, another potential upstream regulator of AMPKα were examined (Budanov and Karin, 2008). Transfection of A549 and H460 cells with siTR3 resulted in increased levels of sestrin protein, mRNA and luciferase activity in cells transfected with pSESN2-luc, a construct containing the −730 to +190 region from the sestrin 2 promoter (Fig. 3C). The role of sestrin 2 in mediating TR3-dependent activation of AMPKα and inhibition of mTORC1 was determined by RNA interference in A549 cells transfected with siTR3 and siSENS2 alone or in combination (Fig. 3D). The effects of siTR3 alone were consistent with activation and inactivation of AMPKα and mTORC1 signaling, respectively. Sestrin 2 knockdown alone had minimal effects, whereas in cells cotransfected with siTR3 plus siSENS2, the loss of sestrin 2 expression abrogated the effects of siTR3 on AMPKα-independent inhibition of the mTORC1 pathway. In addition, we also showed that overexpression of TR3 in H460 cells decreased SESN2 and phosphorylation of AMPKα and activated mTORC1 signaling (Supplemental Fig. S3A); however, the effects of TR3 overexpression were not observed in cells cotransfected with siSESN2 (Supplemental Fig. S3B).
p53 activates sestrin 2 (Budanov and Karin, 2008), and TR3 directly interacts with p53 and inhibits the transcriptional activity by blocking p53 acetylation (Budanov and Karin, 2008). Functional interactions between TR3 and p53 were examined in A549 cells (p53 wild-type) transfected with siTR3 and sip53 alone or in combination (Fig. 4A). Transfection with siTR3 induced sestrin 2 and only slightly increased p53 protein levels and affected phosphorylation of AMPKα, p70S6K and S6RP as indicated in Figures 2C, 3B and 3D. sip53 alone decreased p70S6K and S6RP phosphorylation and, in combination with siTR3, sip53 decreased the effects of siTR3 on sestrin 2 protein expression and phosphorylation of AMPKα, 70S6K and S6RP. In contrast, transfection of siTR3 into p53-null H1299 lung cancer cells did not affect expression of sestrin 2 or the phospho-proteins associated with AMPKα-dependent inhibition of mTORC1 (Fig. 4B), whereas p53-independent inactivation of the TR3/p300/Sp1 complex by siTR3 resulted in downregulation of survivin and induction of apoptosis (Supplemental Fig. S4A). However, we observed that overexpression of p53 in H1299 cells cotransfected with siTR3 increased SESN2/phospho-AMPKα and inhibited mTORC1 signaling, whereas this was not observed in cells cotransfected with siTR3 overexpressing mutant p53 (Supplemental Fig. S4B). Inactivation of TR3 by RNA interference also significantly induced luciferase activity in cells transfected with a p53-responsive construct (Fig. 4C), and in A549 cells transfected with siTR3, results of a ChIP assay showed enhanced recruitment of p53 to the p53 response element in the sestrin 2 promoter (Fig. 4D). Supplemental Figure S5A confirms that both p53 and TR3 are coimmunoprecipitated in A549 and H460 cells as previously reported (Zhao et al., 2006). We also demonstrate that TR3 overexpression (a) decreased luciferase activity in A549 and H460 cells transfected with the p53-luc construct, (b) decreased p53 binding to the SESN2 promoter in a ChIP assay in H460 cells, and (c) decreased luciferase activity in H460 cells transfected with the SESN2-luc construct (Supplemental Figs. S5B, S5C and S5D). Thus, TR3 is not only required for basal expression of survivin and survival genes via a p300/TR3/Sp1 complex but also activates the mTORC1 pathway through inactivation of p53 (Fig. 4E), suggesting that agents or drugs that inactivate or block TR3 signaling will be highly effective as inhibitors of cancer cell and tumor growth and survival.
Figure 4.
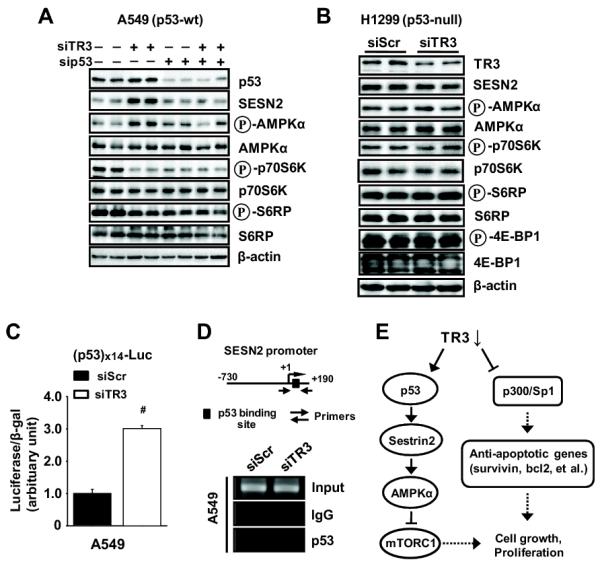
Knockdown of TR3 regulates sestrin2-AMPKα-mTORC1 through induction of p53 transcriptional activity. (A and B) Cells were transfected with the indicated siRNAs for 72 hr, and whole cell lysates were analyzed by western blot analysis. β-Actin was used as a loading control and the experiment was repeated three times with similar results. (C) Cells were cotransfected with each siRNA and p53x14-Luc, and luciferase activity (relative to β-galactosidase activity) was determined. The corresponding empty vector was used as a control, and the results are presented as means with SD of 3 experiments. #P<0.001 vs siScr. (D) ChIP assay. Cells were transfected with either siScr or siTR3 for 60 hr, and binding of p53 with the sestrin2 promoter region containing p53 binding site as indicated was determined as described in the Materials and Methods. The experiment was repeated three times with similar results. (E) Schematic diagram summarizing knockdown of TR3 inhibits cell growth through dual-targeting of mTORC1 signaling and Sp1 in human lung cancer cells expressing wild-type p53.
4. DIM-C-pPhOH inactivates TR3 and TR3-dependent responses
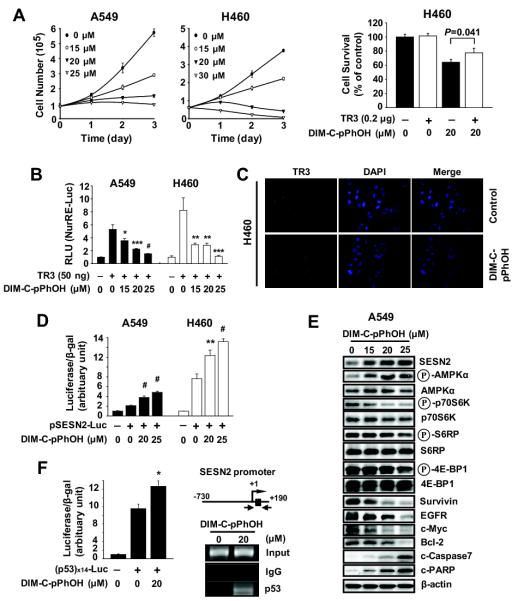
DIM-C-pPhOH inactivates TR3 in pancreatic cancer cells and the effects of this compound and TR3 knockdown (siTR3) were comparable (Lee et al., 2010b). Results in Figure 5A show that DIM-C-pPhOH inhibited A549 and H460 cell proliferation, and overexpression of TR3 partially reversed the growth inhibitory effects of DIM-C-pPhOH. DIM-C-pPhOH also decreased transactivation in A549 and H460 cells transfected with NuRE-luc and TR3 (Fig. 5B) and immunostaining demonstrated that DIM-C-pPhOH did not affect TR3 expression or induce nuclear translocation (Fig. 5C). Moreover, western blot analysis of lysates from cells treated with DMSO or 20 μM DIM-C-pPhOH showed that TR3, p300 and Sp1 expression were observed only in the nucleus, and levels were similar in both treatment groups (data not shown). We also observed similar effects of DIM-C-pPhOH and siTR3 on disruption of the TR3/p300/Sp1-mediated expression of survivin and survival in lung cancer cells. DIM-C-pPhOH decreased transactivation in cells transfected with Gal4-Sp1/Gal4-luc or GC-rich constructs (GC3-TATA and GC3-TK-luc) (Supplemental Fig. S6A). DIM-C-pPhOH also decreased survivin mRNA levels and luciferase activity in cells transfected with survivin constructs (Supplemental Fig. S6B). In a gel shift assay, DIM-C-pPhOH decreased p300 but not Sp1 binding to a GC-rich probe (Supplemental Fig. S6C), and the effects of DIM-C-pPhOH on inhibition of cell growth, decreased survivin expression, and induction of apoptosis were also observed in p53-null H1299 cells (Supplemental Fig. S6D). Thus, like siTR3, DIM-C-pPhOH inactivates the TR3/p300/Sp1 survival complex (Fig. 4E). The complementarity between siTR3 and DIM-C-pPhOH was also observed for the activation of sestrin 2 and AMPKα and the subsequent inhibition of the mTORC1 pathway. DIM-C-pPhOH induced luciferase activity in A549 and H460 cells transfected with pSENS2-luc (Fig. 5D) and induced sestrin 2 and phospho-AMPKα and decreased phosphorylated p70S6K, S6RP and 4E-BP1 expression in A549 cells (Fig. 5E). DIM-C-pPhOH also induced luciferase activity in cells transfected with p53-luc constructs and, in a ChIP assay, DIM-C-pPhOH enhanced recruitment of p53 to the p53 response element region of the sestrin 2 promoter (Fig. 5F) as observed for siTR3 (Fig. 4D). Results in Supplemental Figures S7A and S7B also show in H460 cells transfected with siTR3 that DIM-C-pPhOH in combination with siTR3 enhances inhibition of growth and mTORC1 signaling. Thus, both siTR3 and DIM-C-pPhOH decrease or deactivate TR3, respectively, to inhibit growth and survival of lung cancer cells by inhibition of the mTORC1 pathway via p53/sestrin-dependent activation of AMPKα.
Figure 5.
DIM-C-pPhOH, the TR3 deactivator, inhibits mTORC1 signaling through activation of the p53/sestrin2/AMPKα axis in NSCLC cells. (A, left panel) Cells were treated with either DMSO or DIM-C-pPhOH for 3 days, and the number of cells in each well was counted on days 1, 2, and 3. (A, right panel) Cells were transfected with either Flag-empty or Flag-TR3 for 24 hr, and treated with DIM-C-pPhOH (20 μM) for another 24 hr. The number of cells in each well was counted and the data are presented as means with SD of 3 experiments. (B) Cells were cotransfected with NurRE-Luc (0.1 μg) and 50 ng of Flag-TR3 for 5 hr, and treated with DIM-C-pPhOH for 18 hr. Luciferase activity (relative to β-galactosidase activity) was determined, and the corresponding empty vector was used as a control. *P<0.05, **P<0.01, ***P<0.005, and #P<0.001 vs DMSO + TR3. (C) Subcellular localization of TR3. Cells were treated with either DMSO or 20 μM of DIM-C-pPhOH for 12 hr, and endogenous TR3 was stained and visualized as described in the Materials and Methods. (D and F, left panel) Cells were transfected with pSESN2-Luc (−730/+190) or p53x14-Luc for 5 hr, and treated with DIM-C-pPhOH for another 18 hr. *P<0.05, **P<0.005 and #P<0.001 vs DMSO. (E) Cells were treated with DIM-C-pPhOH for 24 hr, and whole cell lysates were analyzed by western blot analysis. (F, right panel) Cells were treated with DIM-C-pPhOH for 12 hr, and the ChIP assay was performed.
5. DIM-C-pPhOH inhibits lung tumor growth
The effects of DIM-C-pPhOH on the growth of orthotopic lung tumors derived from A549 cells was carried out to complement the in vitro studies with siTR3 and DIM-C-pPhOH in lung cancer cells (Figs. 2-5). DIM-C-pPhOH (30 mg/kg/d) decreased lung tumor weights and volumes and this was accompanied by increased apoptosis (TUNEL staining) in the tumors from animals treated with DIM-C-pPhOH compared to tumors from the control (corn oil) mice (Fig. 6A and Supplemental Table S3). Treatment with DIM-C-pPhOH also decreased survivin and increased cleavage of caspases 3 and 7 and PARP (Fig. 6B) which is associated with inactivation of the p300/TR3/Sp1 complex (Figs. 2, S2 and S3). DIM-C-pPhOH also inhibited mTORC1 signaling through activation of sestrin 2 and AMPKα and this was accompanied by decreased phosphorylation of 4E-BP1 and p7056K (Fig. 6C). The effects of DIM-C-pPhOH (30 mg/kg/d) were also investigated in a metastatic mouse model for lung cancer where cells were introduced by tail vein injection (Figs. 6C and 6D). In this study, DIM-C-pPhOH also decreased tumor weights and volumes and tumor burden (Fig. 6D and Supplemental Table S4). These data clearly demonstrate that in vivo deactivation of TR3 by DIM-C-pPhOH results in tumor growth inhibition by inhibiting at least two TR3-mediated pro-oncogenic pathways (Fig. 4E).
Figure 6.
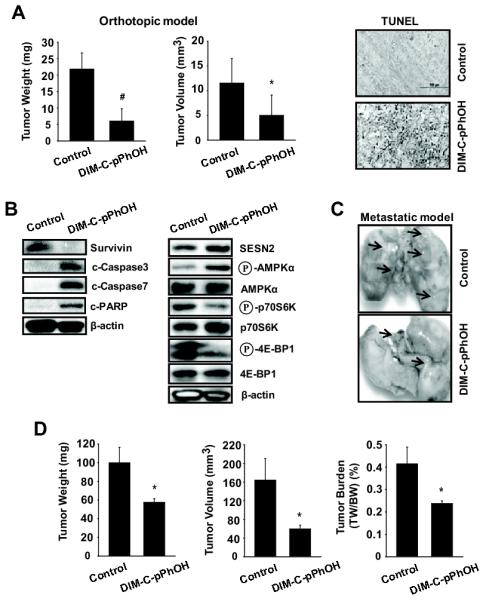
DIM-C-pPhOH inhibits tumor growth and lung metastasis in vivo. (A and B) The orthotopic mouse model of lung cancer. A549 cells were orthotopically implanted into athymic nude mice, and each mouse was dosed 3 times a week by oral gavage with either corn oil (control) or DIM-C-pPhOH (30 mg/kg/day) for 4 weeks starting 7 days after implantation. Median tumor weights and volumes (A, left panel) were calculated as described in the Materials and Methods. The data are presented as means with SD (n=10 per group). *P<0.05 and #P<0.001 vs control group. (A, right panel) TUNEL staining. Tumor sections were stained using the DeadEnd colorimetric kit as described in Materials and Methods. The apoptotic tumor cells are stained. Images were collected at high (×100) magnification. (B) Protein expression in tumor lysates. Tumor lysates from tumor samples were further analyzed by western blot analysis, and β-actin was used as a loading control. (C and D) The metastatic mouse model of lung cancer. A549 (2 × 106) cells were inoculated into athymic nude mice via the tail vein for 4 weeks before treatment, and each mouse was dosed 3 times a week by oral gavage with either corn oil (control) or DIM-C-pPhOH (30 mg/kg/day) for 4 weeks. (C) Lung micrographs show development of multiple tumor foci (arrows). (D) Metastatic tumor weight, volume, and burden were calculated.
DISCUSSION
TR3 and other NR4A receptors are involved in multiple pathways involved in cellular homeostasis and are particularly important in response to different forms of stress (Maxwell and Muscat, 2006; Pearen and Muscat, 2010). There is also increasing evidence that TR3 is overexpressed in multiple tumor types and exhibits pro-oncogenic activities (Maruyama et al., 1995; Bras et al., 2000; Kolluri et al., 2003; Ke et al., 2004; Chintharlapalli et al., 2005; Li et al., 2006; Zeng et al., 2006; Zhang, 2007; Cho et al., 2007; Cho et al., 2010; Wu et al., 2010; Azoitei et al., 2010; Lee et al., 2010b) and results summarized in Figure 1 and Supplemental Figure 1 suggest that overexpression of TR3 is a negative prognostic factor for lung cancer patient survival. Thus, drugs that target TR3 may be important for treating cancer and the effective use of TR3 agonists or antagonists will be dependent on understanding the role of these receptors in critical disease-specific pathways.
Among the earliest studies on the role of TR3 in cancer was the observation that various apoptosis-inducing agents induced nuclear export of TR3 and this resulted in formation of a proapoptotic mitochondrial TR3-bcl-2 complex (Li et al., 2000; Kolluri et al., 2003; Lin et al., 2004; Zhang, 2007; Kolluri et al., 2008; Wu et al., 2010). This pathway is being extensively exploited for drug development. The importance of TR3 nuclear export has been confirmed in recent studies in liver cancer which show that endogenous suppression of TR3 nuclear export by the chromodomain helicase/adenosine triphosphatase DNA binding protein 1-like oncogene is an important determinant for liver cancer (Chen et al., 2009; Chen et al., 2010). A recent study showed that TR3 knockdown or treatment with DIM-C-pPhOH inhibited growth and induced apoptosis in pancreatic cancer cells and downregulated expression of survivin (Lee et al., 2010b). Mechanistic studies showed that endogenous expression of survivin was dependent on formation of p300/TR3/Sp1 complex bound to the proximal GC-rich region of the survivin gene promoter and inactivation of this complex by siTR3 or DIM-C-pPhOH resulted in loss of p300 and decreased survivin expression. Similar results were observed in p53-positive A549 and H460 and p53-negative H1299 lung cancer cells where both siTR3 and DIM-C-pPhOH inhibited cell growth, induced apoptosis, and decreased expression of survivin and other Sp1-regulated genes (Figs. 2B, 2D, 5C, S2 and S6). DIM-C-pPhOH was also highly effective as an inhibitor of lung tumor growth in orthotopic and metastatic in vivo models (Fig. 6). Thus, identification of a novel endogenous p300/TR3/Sp1-dependent prosurvival pathway in pancreatic (Lee et al., 2010b) and lung cancer cells is consistent with the anticancer activities observed for DIM-C-pPhOH as a TR3 deactivator.
TR3 binds and inactivates p53 (Zhao et al., 2006), and the role of this interaction in A549 and H460 cells that express wild-type p53 was investigated. Results of preliminary pathway screening studies with siTR3 identified the mTORC1 signaling pathway as another TR3-regulated circuit in p53 wild-type lung cancer cells, and siTR3 decreased phosphorylation of the diagnostic mTORC1 downstream targets, namely p70S6K, S6RP and 4E-BP1 (Fig. 2C). mTORC1 has become an increasingly important target for anticancer drugs such as rapamycin because of its central role in regulating multiple downstream pro-oncogenic factors (Bjornsti and Houghton, 2004; Hay and Sonenberg, 2004; Shaw and Cantley, 2006; Guertin and Sabatini, 2007; Yang and Guan, 2007). The phosphorylated forms of 4E-BP1 and p70S6K are key downstream targets of mTORC1 that regulate initiation and translation of multiple genes such as cyclin D1 and cyclin A that are critical for normal cell growth and in a cancer context, many of the the 4E-BP1- and p70S6K-regulated genes are pro-oncogenic (Hay and Sonenberg, 2004; She et al., 2010). Dephosphorylation of 4E-BP1 and p70S6K in cells transfected with siTR3 is consistent with a role for TR3 in regulating mTORC1 in lung cancer cells, and our results for DIM-C-pPhOH (Fig. 5), a TR3 deactivator, demonstrate that this compound represents a novel inhibitor of mTORC1 that acts by deactivation of TR3.
mTORC1 can be inhibited by LKB1 or by inactivation of PI3K; however, both A549 and H460 cells are LKB1-negative, and siTR3 did not affect phosphorylation of AKT (Fig. 3A). In the lung cancer cell lines, siTR3 activated AMPKα and inhibited mTORC1 through induction of sestrin 2 and these responses were blocked in cells cotransfected with siAMPKα (Fig. 3B), thus demonstrating a connection between TR3 and AMPKα-dependent inhibition of mTORC1. In contrast, siTR3 did not affect AMPKα phosphorylation in p53-null H1299 lung cancer cells (Fig. 4B); however, overexpression of p53 in H1299 cells cotransfected with siTR3 inhibited mTORC1 signaling (Fig. S4B). These results indicate that the effects of siTR3 and DIM-C-pPhOH on activation of AMPKα and inhibition of mTORC1 are p53-dependent.
TR3 binds to and inactivates p53 (Zhao et al., 2006); however, loss of TR3 (by siTR3) or inactivation of TR3 in lung cancer cells treated with DIM-C-pPhOH resulted in increased p53-dependent transactivation (Figs. 4A, 4C and 5F) and this was not due to increased acetylation (data not shown). Loss of or inactivation of TR3 was accompanied by activation of the p53-responsive gene sestrin 2, and recruitment of p53 to the p53-responsive region of the sestrin 2 promoter (Figs. 3-5). A recent report showed that regulation of mTORC1 signaling by p53 is through p53-dependent activation of sestrins which, in turn, activate AMPKα (Budanov and Karin, 2008; Lee et al., 2010a). Results of studies in lung cancer cells transfected with siTR3 alone or siAMPKα, siSESN2 and sip53 alone or in combination with siTR3 (Figs. 3B, 3D and 4A) demonstrate that TR3 regulates the mTORC1 pathway and this is due to direct interactions of TR3 with p53 (Fig. S5A) which inactivate the tumor suppressor gene (Zhao et al., 2006). Moreover, overexpression of TR3 blocks interaction of p53 with the sestrin 2 promoter, inhibits activation of constructs containing p53 and sestrin 2 promoter inserts (Supplemental Figs. S5B and S5D), and activates mTORC1 signaling (Supplemental S3A). Thus, inactivation of p53 by TR3 blocks the activation of sestrin 2 and AMPKα, resulting in activation of mTORC1 (Fig. 4E) and this defines another pro-oncogenic function of TR3 in p53 wild-type cancer cells.
Since endogenous TR3 activates mTORC1 through inhibition of p53/sestrin 2-dependent activation of AMPKα, an mTORC1 inhibitor, this suggests that drugs such as DIM-C-pPhOH that inactivate TR3 and thereby enhance p53-dependent inhibition of mTORC1 in vitro (Fig. 5E) and in vivo (Fig. 6B) will be highly effective anticancer agents. Thus, identification of the role of TR3 as a prognostic factor (Fig. 1) and as an important regulator of mTORC1 signaling and survival pathways in lung cancer (Fig. 4E) suggests that subsets of lung cancer patients that overexpress TR3 and are wild-type for p53 would benefit from clinical treatment with TR3 inactivators such as DIM-C-pPhOH alone or in combination therapy. Drugs such as DIM-C-pPhOH that inactivate TR3 represent a new class of mTORC1 inhibitors, and our ongoing studies are focused on developing other novel potent inhibitors of this orphan receptor and its downstream pro-oncogenic pathways.
MATERIALS AND METHODS
Immunohistochemical analysis
The tissue microarray slides containing 59 cases of human NSCLC tissues (IMH-305) and 59 cases of self-matching normal adjacent lung tissues (IMH-340) were obtained from Imgenex (San Diego, CA). Immunohistochemical staining for TR3 was performed on paraffin-embedded specimens by using standard avidin-biotin complex (ABC) method described previously (Lee et al., 2010b). Immunostaining intensity was scored as absent, low, moderate, or high by three independent investigators without prior knowledge of the clinical follow-up data. There was no specific staining when secondary antibody was used alone as a negative control.
Cell lines and plasmids
A549, H460, and H1299 human NSCLC cell lines were obtained from the American Type Culture Collection (Manassas, VA). Cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS), and 10 ml/L 100x antibiotic antimycotic solution (Sigma-Aldrich). Cells were maintained at 37°C in the presence of 5% CO2. All the plasmids used in this study except the sestrin2 promoter (−730/+190) reporter construct (pSESN2-Luc) and p53X14-luciferase reporter construct (p53X14-Luc) pCMV-p53 wild-type and pCMV-p53-mt135 mutant (p53-mt135) were previously described (Lee et al., 2010b). The pSESN2-Luc and p53X14-Luc were purchased from SwitchGear Genomics, Inc. and Stratagene, respectively, and the wild-type and mutant p53 expression plasmids were purchased from Clontech (Mountain View, CA).
Transfection of siRNA oligonucleotides
Cells (1.5 × 105 cells/well) were plated in 6-well plates in DMEM media supplemented with 10% FBS. After 16 hr, the cells were transfected with 200 nM of each siRNA duplex for 6 hr using LipofectAMINE 2000 reagent (Invitrogen) following the manufacturer’s protocol. After transfection, cells were collected for cell proliferation assay, western blot analysis, and quantitative real-time PCR assay. The sequences of siRNA oligonucleotides used were as follows: TR3-1 5′-CAG UCC AGC CAU GCU CCU dTdT-3′; TR3-2 5′-CGC UC AUG CCA GCA UUA U-3′; TR3-3 5′-GGC UUG AGC UGC AGA AUG A-3′; Sp1 5′-AUC ACU CCA UGG AUG AAA UGA dTdT-3′; and p300 5′-AAC CCC UCC UCU UCA GCA CCA dTdT-3′. The siRNA for AMPKα (sc-45312), sestrin2 (sc-106544), and p53 (sc-29435) were purchased from Santa Cruz (Santa Cruz, CA). As a negative control, a nonspecific scrambled small inhibitory RNA (siScr) oligonucleotide was used (Qiagen).
Subcellular localization assay
Cells were seeded onto coverslips in 12-well plates, then treated with either the vehicle (DMSO) or the compound. After 12 hr, cells were fixed in 1% formalin in PBS (pH 7.4) after washing with PBS and permeabilized by immersing the cells in 0.2% Triton X-100 solution in PBS for 10 min. Cells were then incubated with anti-TR3 rabbit IgG, followed by antirabbit IgG conjugated with FITC (Santa Cruz). For nuclear counterstaining, cells were mounted in mounting medium including DAPI (Vector Lab., CA). Fluorescent images were collected and analyzed using a Zeiss Axioplan2 fluorescence microscope (Carl Zeiss, Jena, Germany).
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed using the ChiP-IT Express Magnetic Chromatin Immunoprecipitation kit (Active Motif) according to the manufacturer’s protocol. After treatment with the compound or siRNAs, cells were fixed with 1% formaldehyde for 10 min, and the cross-linking reaction was stopped by addition of 0.125 M glycine. After washing twice with phosphate-buffered saline, cells were scraped and pelleted. Collected cells were then hypotonically lysed, and nuclei were collected. Nuclei were then sonicated to desired chromatin length (~500 bp). The chromatin was immunoprecipitated with each target antibody and protein G magnetic beads at 4°C overnight with gentle agitation. The beads were collected and washed, and the chromatin-protein complex was eluted. Cross-linking was then reversed and purified DNA was subjected to PCR amplification. The sestrin2 primers which contains a p53 binding site were 5′-CAG ACC TCT GAT TGG CTG GAC CG-3′ (sense), and 5′-CAG GGG TTT TCA CGG CCT CGG AA-3′ (antisense) and encompass a region containing a p53 binding site. PCR products were analyzed by electrophoresis on a 2% agarose gel in the presence of ethidium bromide.
In Vivo Experiments
A549 human NSCLC cells were used in both the orthotopic and metastatic models and a detailed description of the procedures is provided in the Supplemental Materials and Methods section.
Statistical analysis
The significance of correlations was determined using the χ2 test and Cox proportional hazard regression model. To evaluate the degree of association between variables, hazard ratio, odds ratio, and corresponding 95% confidence intervals (CI) were analyzed. Association of TR3 expression with clinical outcome for NSCLC patients was examined using Kaplan-Meier survival analysis, and P<0.05 was considered significant. Statistical significance of differences in protein levels, luciferase activity, and cell and tumor growth between groups were analyzed using either Student’s t-test or analysis of variance (ANOVA) with Scheffe’s test. The results are expressed as means with SD for three experiments for each group unless otherwise indicated, and a P<0.05 was considered statistically significant. All statistical tests were two-sided.
All other Materials and Methods are described in the Supplemental Materials and Methods.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health (R01-CA124998) and the Texas A&M AgriLife.
REFERENCES
- Azoitei N, Pusapati GV, Kleger A, Moller P, Kufer R, Genze F, et al. Protein kinase D2 is a crucial regulator of tumour cell-endothelial cell communication in gastrointestinal tumours. Gut. 2010;59:1316–1330. doi: 10.1136/gut.2009.206813. [DOI] [PubMed] [Google Scholar]
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat. Rev. Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- Bras A, Albar JP, Leonardo E, de Buitrago GG, Martinez A. Ceramide-induced cell death is independent of the Fas/Fas ligand pathway and is prevented by Nur77 overexpression in A20 B cells. Cell Death. Differ. 2000;7:262–271. doi: 10.1038/sj.cdd.4400653. [DOI] [PubMed] [Google Scholar]
- Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTORC1 signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Hu L, Chan TH, Tsao GS, Xie D, Huo KK, et al. Chromodomain helicase/adenosine triphosphatase DNA binding protein 1-like (CHD1l) gene suppresses the nucleus-to-mitochondria translocation of nur77 to sustain hepatocellular carcinoma cell survival. Hepatology. 2009;50:122–129. doi: 10.1002/hep.22933. [DOI] [PubMed] [Google Scholar]
- Chen L, Yuan YF, Li Y, Chan TH, Zheng BJ, Huang J, et al. Clinical significance of CHD1L in hepatocellular carcinoma and therapeutic potentials of virus-mediated CHD1L depletion. Gut. 2010 doi: 10.1136/gut.2010.224071. in press. [DOI] [PubMed] [Google Scholar]
- Chintharlapalli S, Burghardt R, Papineni S, Ramaiah S, Yoon K, Safe S. Activation of Nur77 by selected 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J. Biol. Chem. 2005;280:24903–24914. doi: 10.1074/jbc.M500107200. [DOI] [PubMed] [Google Scholar]
- Cho SD, Lee SO, Chintharlapalli S, Abdelrahim M, Khan S, Yoon K, et al. Activation of nerve growth factor-induced Bα by methylene-substituted diindolylmethanes in bladder cancer cells induces apoptosis and inhibits tumor growth. Mol. Pharmacol. 2010;77:396–404. doi: 10.1124/mol.109.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SD, Yoon K, Chintharlapalli S, Abdelrahim M, Pei P, Hamilton S, et al. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and independent pathways. Cancer Res. 2007;67:674–683. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov. 2004;3:950–964. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Sabatini DM. Defining the role of mTORC1 in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTORC1. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Ke N, Claassen G, Yu DH, Albers A, Fan W, Tan P, et al. Nuclear hormone receptor NR4A2 is involved in cell transformation and apoptosis. Cancer Res. 2004;64:8208–8212. doi: 10.1158/0008-5472.CAN-04-2134. [DOI] [PubMed] [Google Scholar]
- Kolluri SK, Bruey-Sedano N, Cao X, Lin B, Lin F, Han YH, et al. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Mol. Cell Biol. 2003;23:8651–8667. doi: 10.1128/MCB.23.23.8651-8667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri SK, Zhu X, Zhou X, Lin B, Chen Y, Sun K, et al. A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell. 2008;14:285–298. doi: 10.1016/j.ccr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2010a;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SO, Abdelrahim M, Yoon K, Chintharlapalli S, Papineni S, Kim K, et al. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res. 2010b;70:6824–6836. doi: 10.1158/0008-5472.CAN-10-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SO, Chintharlapalli S, Liu S, Papineni S, Cho SD, Yoon K, et al. p21 Expression is induced by activation of nuclear nerve growth factor-induced Bα (NGFI-Bα, Nur77) in pancreatic cancer cells. Mol. Cancer Res. 2009;7:1169–1178. doi: 10.1158/1541-7786.MCR-08-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- Li QX, Ke N, Sundaram R, Wong-Staal F. NR4A1, 2, 3--an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis. Histol. Histopathol. 2006;21:533–540. doi: 10.14670/HH-21.533. [DOI] [PubMed] [Google Scholar]
- Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Zeng HN, Zhang LR, Zhan YY, Chen Y, Wang Y, et al. A unique pharmacophore for activation of the nuclear orphan receptor Nur77 in vivo and in vitro. Cancer Res. 2010;70:3628–3637. doi: 10.1158/0008-5472.CAN-09-3160. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Tsukada T, Bandoh S, Sasaki K, Ohkura N, Yamaguchi K. Expression of NOR-1 and its closely related members of the steroid/thyroid hormone receptor superfamily in human neuroblastoma cell lines. Cancer Lett. 1995;96:117–122. doi: 10.1016/0304-3835(95)03921-i. [DOI] [PubMed] [Google Scholar]
- Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl. Recept. Signal. 2006;4:e002. doi: 10.1621/nrs.04002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NJ, Cooney AJ, DeMayo FJ, Downes M, Glass CK, Lanz RB, et al. Minireview: Evolution of NURSA, the Nuclear Receptor Signaling Atlas. Mol. Endocrinol. 2009;23:740–746. doi: 10.1210/me.2009-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron. 1988;1:183–188. doi: 10.1016/0896-6273(88)90138-9. [DOI] [PubMed] [Google Scholar]
- Pearen MA, Muscat GE. Minireview: Nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol. Endocrinol. 2010;24:1891–1903. doi: 10.1210/me.2010-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTORC1 signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18:39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Lin Y, Li W, Sun Z, Gao W, Zhang H, et al. Regulation of Nur77 expression by {beta}-catenin and its mitogenic effect in colon cancer cells. FASEB J. 2010 doi: 10.1096/fj.10-166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Guan KL. Expanding mTORC1 signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, Van HM, et al. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J. Exp. Med. 2006;203:719–729. doi: 10.1084/jem.20051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Du X, Chen H, Liu J, Zhao B, Huang D, et al. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat. Chem. Biol. 2008;4:548–556. doi: 10.1038/nchembio.106. [DOI] [PubMed] [Google Scholar]
- Zhang XK. Targeting Nur77 translocation. Expert. Opin. Ther. Targets. 2007;11:69–79. doi: 10.1517/14728222.11.1.69. [DOI] [PubMed] [Google Scholar]
- Zhao BX, Chen HZ, Lei NZ, Li GD, Zhao WX, Zhan YY, et al. p53 mediates the negative regulation of MDM2 by orphan receptor TR3. EMBO J. 2006;25:5703–5715. doi: 10.1038/sj.emboj.7601435. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.