Abstract
Microglia are a heterogeneous group of monocyte-derived cells serving multiple roles within the brain, many of which are associated with immune and macrophage like properties. These cells are known to serve a critical role during brain injury and to maintain homeostasis; yet, their defined roles during development have yet to be elucidated. Microglial actions appear to influence events associated with neuronal proliferation and differentiation during development, as well as, contribute to processes associated with the removal of dying neurons or cellular debris and management of synaptic connections. These long-lived cells display changes during injury and with aging that are critical to the maintenance of the neuronal environment over the lifespan of the organism. These processes may be altered by changes in the colonization of the brain or by inflammatory events during development. This review addresses the role of microglia during brain development, both structurally and functionally, as well as the inherent vulnerability of the developing nervous system. A framework is presented considering microglia as a critical nervous system-specific cell that can influence multiple aspects of brain development (e.g., vascularization, synaptogenesis, and myelination) and have a long term impact on the functional vulnerability of the nervous system to a subsequent insult, whether environmental, physical, age-related, or disease-related.
Keywords: microglia, ontogeny, developmental neurotoxicity, brain macrophages, pruning
1. Introduction
Microglia are resident cells of the brain involved in regulatory processes critical for development, maintenance of the neural environment, injury, and repair. These cells were initially included in the early description of neuroglia provided by Virchow in the mid-nineteenth century. This was followed by the observation of non-neural cells in the brain attributed to infiltration by mesodermally-derived cells during development. In 1899, cells with rod-shaped nuclei were identified and named Staebchenzellen (Nissl, 1899). Morphologically distinct from neurons and astrocytes, these cells were classified in 1913 as a third element of the central nervous system (CNS; Cajal, 1913). A further distinction was made between microglia and oligodendrocytes by del Rio Hortega (1932). Around this time the morphological classifications of microglia fell into three types: ramified, intermediate forms, and amoeboid (del Rio Hortega, 1932; Kershman, 1939). More recent studies have used a similar classification, but these terms (i.e., quiescent/surveying, reactive/activated, and amoeboid/phagocytic) have evolved to include a functional connotation.
Microglia are bone marrow-derived cells from the monocyte-macrophage lineage (Davoust et al., 2008; Ginhoux et al., 2010; Ling et al., 1980) and serve as brain immune cells to orchestrate innate immune responses; however, they are distinct from other tissue macrophages due to their relatively quiescent phenotype and tight regulation by the CNS microenvironment. Microglia actively survey the surrounding parenchyma via dynamic movement of processes while the soma remains static. This allows each cell to sample its environment over the course of a few hours (Nimmerjahn et al., 2005). The physical extent of this surveillance depends upon the distribution of microglia, the level of ramification of individual cells, and the rate of process remodeling. This surveillance allows the cells to rapidly respond to changes in the environment, including alterations in brain activity or disruption to the brain parenchyma. Microglia often provide the first line of defense against invading microbes and, via interactions with neurons they can be the first to detect critical changes in neuronal activity and health. In the mature brain, microglia are capable not only of actively monitoring but also controlling the extracellular environment, walling off areas of the CNS from non-CNS tissue, and removing dead or damaged cells. In response to tissue damage, as can occur with trauma, stroke, or chemical exposure, microglia can be beneficial in the healing phase but may become more detrimental over the course of the injury depending upon various regulatory processes. While a number of mechanisms have been proposed microglia responsiveness, many are related to the presence of inwardly-rectifying potassium (K+) channels (Kettenmann et al., 1993), as well as receptors for adenosine triphosphate (ATP; Langosch et al, 1994; Walz et al., 1993), calcitonin gene-related peptide (Priller et al., 1995), and multiple neurotransmitter receptors (for review see Pocock and Kettenmann, 2007). In addition, microglia express pattern-recognition receptors (van Rossum and Hanisch, 2004) that can drive the rapid response to insult. The loss of ligand and receptor activation can also regulate microglia functional state. For example, healthy neurons maintain microglia in an inactive state via secreted and membrane bound signals including CD200, CX3CL1 (fractalkine), neurotransmitters and neurotrophins (Biber et al., 2007; Pocock and Kettenmann, 2007). Thus, with neuronal injury and the loss of these regulatory signals, microglia may shift out of a quiescent state.
The dynamic behavior of microglia and their specific receptor expression, reflect the potential key roles these cells play in CNS immune functioning (Perry et al., 1985; Raivich, 2005). In many models of neurodegeneration and neurotoxicity, early events of synaptic degeneration and neuronal loss are accompanied by an inflammatory response including activation of microglia, perivascular monocytes, and recruitment of leukocytes and systemic macrophages. In culture, microglia have been shown to be capable of releasing several potentially cytotoxic substances, such as reactive oxygen intermediates, nitric oxide, proteases, arachidonic acid derivatives, excitatory amino acids, and cytokines; however, they also produce various neurotrophic factors. Microglial functions are associated with a number of neurodegenerative diseases (Amor et al., 2010) and have been implicated in response to neurotoxic exposures (Harry and Kraft, 2008; Kraft and Harry, 2011); however, it remains unclear as to whether the changes in microglia are an initiating event or a reactive event.
While a substantial and often overwhelming body of literature is available on the response of microglia in the adult, there is a relative paucity of studies on the developmental features of these cells. The evidence of microglia activation in the developing brain of patients with neurodevelopmental disorders (e.g., autism) and linkage to human disease processes that have a developmental basis (schizophrenia) have raised questions as to whether developmental neuroinflammation actively contributes to the disease process. While much of the available data represents associative rather than causative factors, it raises interesting questions regarding the role of these “immune-type” cells during normal brain development and changes that may occur with developmental disorders. Within the area of developmental neurotoxicology, the potential for environmental factors or pharmacological agents to directly alter microglia function presents a new set of questions regarding the impact on brain development. It is our attempt in the following review to provide the background for features that are known about microglia during brain development, both structurally and functionally, as well as a discussion of the inherent vulnerability of the developing nervous system to inflammation/infection. While there are a number of issues related to microglia that are outside the focus of this review, we hope to set the framework for consideration of microglia as a critical nervous system-specific cell that can impact multiple aspects of brain development (e.g., vascularization, synaptogenesis, and myelination). A framework for consideration is developed, based upon the concept that changes, which occur in microglia during their maturation into nervous system resident cells, may have a long term impact on the functional vulnerability of the nervous system to a subsequent insult, whether environmental, physical, age-related, or disease-based.
2. Mononuclear phagocytes - macrophages
In 1892, Metchnikoff introduced the term “macrophage system,” using the term “macrophage” to denote cells capable of ingesting large particles and “microphages” to denote cells that took up smaller particles such as bacteria. This concept was expanded by Aschoff (1924) to include all cells that can take up vital dyes for the “reticuloendothelial system”. Based upon morphology, function, and kinetics, highly phagocytic mononuclear cells and their precursors were grouped into the mononuclear phagocyte system (van Furth et al., 1972). Mononuclear phagocytes originate from precursor cells in the bone marrow and are then transported via the peripheral blood as monocytes to eventually become tissue specific macrophages. Specifically, monocyte-macrophage development closely follows the development of hemopoiesis. In mice, cells positive for the macrophage-specific plasma membrane glycoprotein, F4/80, are present in the yolk sac between gestational days (GD) 9 and 10 (Gordon et al., 1992), in liver at GD 10–11, in spleen and around the developing neuroectoderm at GD 12, and in bone marrow at GD 16. Developing macrophages are phagocytic, proliferate profusely, and are found outside the blood vessels between mesenchymal cells. As individual organs develop, macrophages become resident cells of the connective tissue (Morris et al., 1991). Microglia were originally included in the mononuclear phagocyte system due to the fact that they are found in the central nervous system (CNS) only after the tissue has become vascularized (Andersen and Matthiessen, 1966; Hume et al., 1983).
3. Colonization of the brain by microglia
Data from the use of adult congenic bone marrow chimera (Ginhoux et al., 2010) and parabiotic mice (Ajami et al., 2007; Ginhoux et al., 2010) confirmed that postnatal microglia represent a defined population of cells that colonize the brain prior to birth and are maintained independently of blood-borne hematopoietic progenitors. The precise origin of microglia during brain development remains in debate; however, immature yolk sac macrophages have been identified as the predominant source (Ginhoux et al., 2010). The initial colonization of the brain by microglia corresponds to the vascularization of the CNS initiated from the encompassing perineural vascular plexus. It demonstrates a caudal-cephalic gradient commencing at the myelencephalon and ascending through the metencephalon, mesencephalon, diencephalon, and telecephalon (Marin-Padilla, 1985). During this time, cell-cell communication continues between microglia and vascular sprouts. This communication allows for migration of microglia towards developing vessels and stimulates angiogenic activity (Rymo et al., 2011). Microglia precursors do not appear to rely solely on the vascular system for penetration into the brain parenchyma as cells appear in regions of the developing CNS that are devoid of vascularization (Hurley and Streit, 1995). Alternative routes of entry for microglia include via the brain ventricles or across meninges (review Cuadros and Navascues, 1998).
In the human brain, microglia colonization coincides not only with vascularization but also with radial glia formation, neuronal migration, and myelination. This tightly orchestrated process displays a distinct pattern of microglia migration along radial glia, white matter tracts, and the vasculature (Rezaie and Male, 1999). As early as the mid-to-late first trimester, a limited number of amoeboid microglia are present (Andjelkovic et al., 1998; Choi, 1981; Fujimoto et al., 1989). A large accumulation of amoeboid cells with short processes in the germinal matrix is observed at 13–24 weeks of gestation (Hutchins et al. 1990). Further work demonstrated that fetal microglia located at highly vascularized sites are associated with the expression of intracellular adhesion molecule (ICAM)-2 on cerebral endothelium (Rezaie et al., 1997) and that microglia progenitors are closely associated with the parenchymal wall of penetrating radial blood vessels (Rezaie et al., 2005). They invade the parenchyma of the telencephalic wall inwards from the marginal layer and then align within the subplate region of the developing telencephalon. This colonization of the subplate coincides with the development of subplate neurons, synaptogenesis, and thalamocortical projection fibers transiently residing in the region (Kostovic and Judas, 2002).
Concurrent with the developmental features of neuronal populations, microglia begin to take on a ramified morphology (Kostovic and Judas, 2002). While the exact underlying mechanism responsible for the shift in microglia is not known, it has been speculated that the association between neuron and microglia during development may be related to the maturation of neurons and their expression of factors that serve to maintain microglia in a surveillance phenotype. Thus, one would expect that these factors would be released from mature neurons fostering differentiation of microglia from the immature amoeboid phenotype to process-bearing cells. Alternatively, or in conjunction, the decline in apoptosis of excess neurons with development removes signals from the immediate environment that otherwise would maintain microglia in an amoeboid phagocytic phenotype. With brain growth and maturation, the ability of microglia to migrate to their final destination would be bolstered by a more rounded shape with minimal processes/appendages allowing for contact related movement. In addition, with brain maturation, the shear increase in cell-cell contact requirements for microglia may foster a morphological transition of cells that would be more conducive to performing this task thus, an extension of processes. While each of these hypotheses is speculative, they are somewhat supported by what is currently known regarding microglia in the mature brain or in culture, as well as, the region specific developmental shift in microglia. As an example, up to the beginning of the third trimester, ramified microglia are observed in the cortex while amoeboid microglia predominate in the germinal matrix the ventricular zone, subventricular zone, and corpus callosum (Billiards et al., 2006; Chan et al., 2007). It is not until 35 weeks of gestation that fully developed microglia are observed (Esiri et al., 1991). With birth, and during the first few postpartum weeks, microglia disseminate throughout all parts of the brain, occupying defined spatial territories without significant overlap (Rezaie and Male, 2003) suggesting a defined area of surveillance for each cell. The number of nascent round and amoeboid microglia decreases and an increase is seen in highly ramified cells bearing long, thin, branched processes (Monier et al., 2006; Wu et al., 1994).
In mice, microglia colonization of the brain follows a similar pattern to that observed in the human. Between GD 10 and 15, when capillary sprouts perforate the CNS, focal degeneration and disintegration of subadjacent glial endfeet stimulates the penetration of endothelial cells and attracts monocytes and macrophages from the blood circulation (Marin-Padilla, 1985). Myeloid cells expressing the hematopoietic marker, cluster of differentiation (CD) 45, and the adult macrophage/microglia markers, CD11b, F4/80, and the fractalkine receptor, CX3CR1 that binds the chemokine, CX3CL1 (fractalkine or neurotactin), can be detected in the brain beginning at GD 9.5 (Ginhoux et al., 2010). F4/80 positive cells found in the vicinity of CNS capillaries are characterized by a round or irregular-shaped cell body, with or without pseudopodia (Imamoto and Leblond, 1978). Similar to the F4/80 cells in the leptomeninges, these cells often display vacuolated cytoplasm. These cells maintain a highly proliferative state throughout embryonic life (Ginhoux et al., 2010) and the expression of CD34 and CD45 (PTPRC, protein tyrosine phosphatase, receptor type, C) support the hypothesis that the cells are myeloid progenitors (Davoust et al., 2006). It is thought that many of these cells represent precursors of ramified, brain-resident microglia (Imamoto and Leblond, 1978). In rats, this influx of F4/80 positive cells occurs slightly later, at GD 15–16. By GD 18–19, ramified F4/80+ cells appear in the brain parenchyma. Similar to the mouse and human, these cells display a different morphology than those seen earlier in gestation, becoming more ramified as they fully differentiate (Kaur and Ling, 1991; Perry et al., 1985). Morphologically, they possess little cytoplasm and several long processes with multiple branches covered with very fine protrusions. Whether the increase in ramified microglia with development represents a transformation of the amoeboid cells is still in question. However, there is an inverse correlation between the population numbers of ramified and amoeboid mononuclear cells over time (Ling and Tan, 1974). Again, as mentioned earlier, this would be consistent with the hypothesis that microglia mature to a ramified phenotype with the maturation of neuronal populations. While outside of the focus of this review, this timing also coincides with the maturation of the astrocyte and oligodendrocyte populations suggesting that a natural interdependence exists between these cells during their maturation and coincident brain development.
Microglial colonization of brain regions during development has often been considered as a response of the cells towards programmed neuronal death or axonal degeneration (Perry et al., 1985; Rakic and Zecevic, 2000). It has been proposed that programmed cell death stimulates F4/80 cells to migrate into the CNS (Gordon et al., 1992; Ling and Wong, 1993; Perry et al., 1985; for review Mallat et al., 2005; Schlegelmilch et al., 2011). In support of this role, transitory phagocytes have been demonstrated to remove naturally occurring dead neurons in the cerebral cortex of the adult rat (Ferrer et al., 1990). Similarly, these cells, which display an amoeboid morphology and phenotype, contribute to the highly regulated process of developmental neuronal death that occurs around time of birth (Wakselman et al., 2008). It is estimated that only 30% of microglial progenitors persist and differentiate into ramified microglia in rodents (Wang et al., 2002). Microglia gradually accumulate, dramatically increasing in number between postnatal days (PND) 5–15. By PND 15, the entire parenchyma is filled with ramified microglia occupying a defined territory (Lawson et al., 1990; Perry et al., 1985) (Fig. 1). Work by Schmid et al. (2009) suggested that the CNS environment serves as a major contributor to the differentiation of these cells to a neural specific phenotype, thus separating them from mononuclear phagocytes. Once committed to the CNS, microglia continue to maintain some features common to their blood-borne monocyte cells of origin, such as phagocytosis and the expression of Fc (fragment, crystallizable region) receptors and complement receptor 3 (CR3).
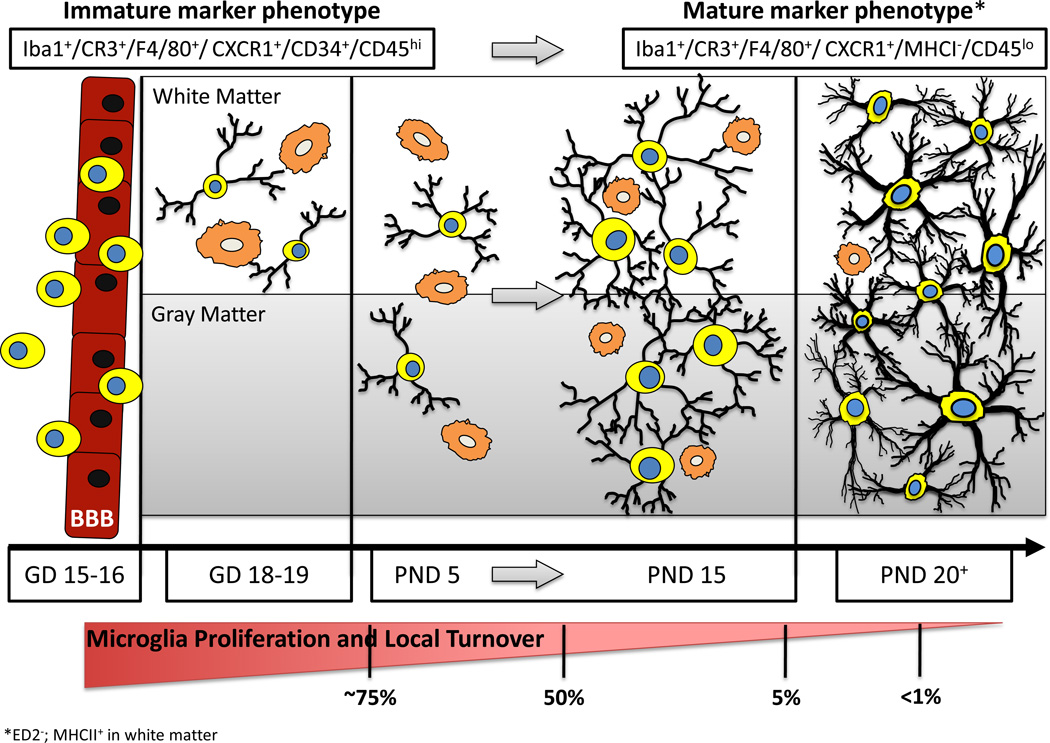
Figure 1.
Developmental Stages Leading to Establishment of a Mature Population of Brain Microglia. Prior to late gestation (GD15-16), bone marrow derived mononuclear cells cross the blood-brain barrier (BBB) and take up residence in the brain parenchyma. These cells typically possess little cytoplasm and have small or nonexistent appendages (indicated as process-free yellow cells with blue nuclei). At early stages of colonization (GD18–19), these cells are identifiable in white matter regions (or along vascular/ ventricular margins) possessing either an amoeboid/ phagocytic morphology (orange cells) or long, highly-branched processes. During early postnatal stages (~PND5), these highly proliferative mononuclear cells are observed in both white matter and gray matter regions of the brain with either morphological phenotype. During a critical period of postnatal microglia development (PND5– PND15), the number of microglia increases dramatically. During this time, an increased ratio of ramified versus amoeboid microglia becomes apparent, with the cells having noticeably more complex process arbors and cytoplasmic material. The microglia present towards the latter part of this maturation window (~PND15) are well distributed throughout the brain, facilitating surveillance of the majority of the parenchyma; they exhibit a significantly reduced proliferative capacity; they begin to exhibit more heterogeneity (both within and across brain regions) in terms of their morphology, orientation, and process field organization; and they begin to express a somewhat modified constellation of cell surface markers. These maturation features continue such that by PND20 the adult population of microglia is fairly well established. In the absence of stimulation, these cells are highly ramified with complex process arbors encompassing the entire brain parenchyma, and they possess little to no evidence of proliferation or turnover from the systemic population. * These marker lists are not comprehensive (e.g., mature microglia express ED2− and white matter microglia are MHCII+).
4. Proliferation and Stability of the Microglia Population
Peripheral mononuclear cells maintain a high level of proliferation in the adult animal. For monocytes-macrophages within the leptomeninges, 60–70% of the cells are replaced in chimeric mice by donor bone marrow cells within a 2-month period. Twenty percent of the proliferative population was observed in the choroid plexus and 20–40% in the perivascular area (Sedgwick et al., 1991). Coincident with a tremendous increase in the number of microglia present in the parenchyma, radiolabeled tracer studies support earlier anatomical work demonstrating that microglia develop primarily after birth and mainly between PND 5 to 20 in mice (Perry et al., 1985; Lawson et al., 1990). Injections of 14[C]TdR between PND 10 and 14 resulted in the labeling of approximately 50% of microglia when assessed at PND 45. This significantly decreased to 5% with labeling at PND 15–19 and sparse labeling, if any, with labeling ≥ PND 20 (Korr, 1980). Similar observations were reported using 3[H]TdR (Imamoto and Leblond, 1978). These data, with regards to the low proliferative level of adult microglia, were confirmed with a 30 day infusion of 3[H]TdR in adult rats (McCarthy and Leblond, 1988) and estimated to be 0.24% following 48 hr of labeling with methyl-1’,2’ 3[H]TdR (Lawson et al., 1992). Upon initial examination, 5–10% of the donor cells were seen in the Virchow-Robin space situated along capillaries or along small vessels between the basement membrane of the endothelial cells and the astroglial foot processes contacting the glia limitans (Graeber et al., 1989; Lasserman et al., 1991). While these were initially thought to be microglia, upon further scrutiny, they were identified as lymphocytes (De Groot et al., 1992; Lassman et al., 1991; Sedgwick et al., 1991). Chimeric mice examined 1 year past marrow transfer still exhibited only scattered and rare (<1%) ramified, microglia-like cells expressing the donor MHC class I haplotype (Sedgwick et al., 1991). Thus, parenchymal microglia constitute a heterogeneous cell population which are long-living cells with an extremely low turnover rate.
The slow turn-over rate for mature microglia raises an issue related to changes that may occur in this critical neural cell population. While this has not been a primary issue of investigation there is limited data suggesting that microglia maintain a history of previous events. Thus, if this history alters the appropriate functioning of microglia then the effects could be long lasting. Additionally, a simple change in the number of microglia colonizing the brain during development, either too many or too few, could have a significant impact on not only the establishment of the nervous system network but also on critical cell specific processes later in life.
5. Brain localization of mature microglia and peripheral macrophage-like cells
Mature microglia constitute 15–20% of the total cell population of the brain (Carson et al., 2006). In the normal adult rodent brain, “resting” microglia are ramified (highly branched) with a small amount of perinuclear cytoplasm and a small, dense and heterochromatic nucleus. In addition, the processes are continuously active and constantly interact with the surrounding microenvironment (Nimmerjahn et al., 2005). These cells are located outside of the vascular basement membrane; however, the cytoplasmic processes can be found intermingled with astrocytic foot processes in the glia limitans (Lassmann et al., 1991). The presence of microglia in the vicinity of blood vessels has lead to the use of the term “juxtavascular microglia”, which is a description of parenchymal microglia which happen to be located near a cerebral blood vessel.
Microglia are present in all major regions of the mature brain; however, a clear specificity is observed across regions. This specificity involves morphological differences between white matter and the parenchyma and between regions of grey matter. Microglia within the myelin white matter tracts demonstrate a unique morphology characterized by cells that align their cytoplasmic extensions in parallel with, or at right angles to, nerve processes. In the grey matter, cells display a more diffuse ramified shape, showing processes extending in multiple directions. Even within the different grey matter regions, microglia can display distinct morphologies (Fig. 2). In addition, different regions display variation in microglia density and distribution. These observations have been reported for the human (Mittelbronn et al., 2001) and the mouse (Lawson et al., 1990). In mice, a higher relative number of microglia is observed in the dentate gyrus of the hippocampus, the substantia nigra, and portions of the basal ganglia, with the highest number of microglia present in the olfactory telencephalon (Lawson et al., 1990). Microglia show a slightly different phenotype in brain regions lacking a blood-brain barrier (BBB), including the circumventricular organs (Perry and Gordon, 1987) and Kolmer cells of the choroid plexus suggesting that serum factors influence microglia morphology.
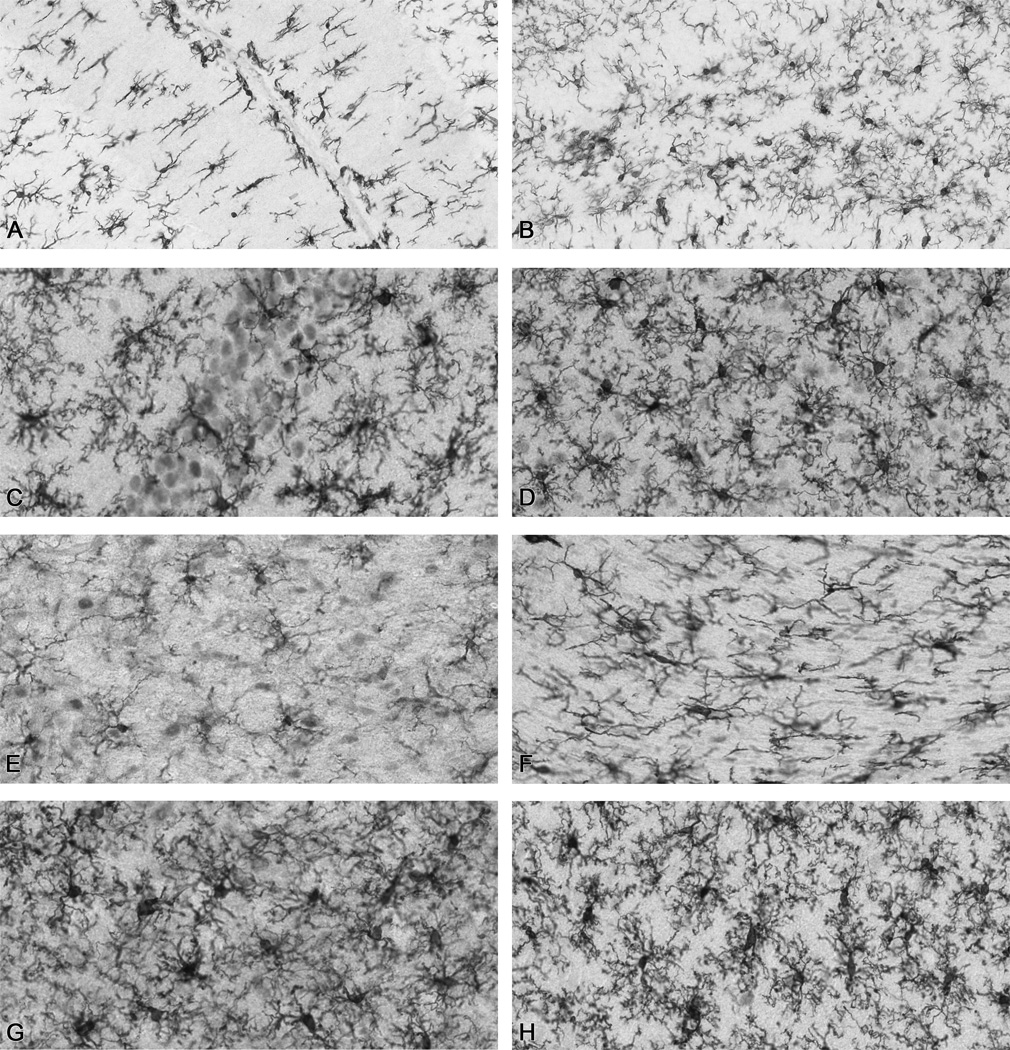
Figure 2.
Representative Iba-1+ microglia in various regions of the rat brain. (A) cerebellum (B) dentate gyrus (C) hippocampal CA1 (D) substantia nigra (E) hypothalamus (F) corpus callosum (G) parietal cortex (H) enthorinal cortex. Images from 40µm sections were scanned at 20× magnification using an Aperio Scanscope T2 Scanner (Aperio Technologies, Inc., Vista, CA) and viewed using Aperio Imagescope v. 6.25.0.1117).
Regional differences do not appear to be limited to morphology. For example, very few parenchymal, ramified microglia-like cells are found to express the donor MHC class I haplotype (Matsumoto and Fujiwara, 1987): rather, positive cells are localized preferentially in the white matter (Mattiace et al., 1990; Streit et al., 1989). With further investigation into the heterogeneity of microglia one would assume that a significant number of factors, both cell membrane and secreted, will be found to be differentially-expressed across the various subpopulations.
6. Microglia phenotypes
During development, in the normal brain, and in the brain following injury, microglia display varied morphologies. The morphological differences in microglia are suggested to represent different functional states; thus, efforts have been made to classify the various morphological phenotypes. Much of our available data on morphological differences is derived from studies on the mature brain and changes that can occur with injury. Functional changes of microglia are often accompanied by a morphological transformation leading from cells with thin, ramified processes to cells with larger somata and shorter, coarser cytoplasmic processes. This can eventually progress to amoeboid cells with morphology similar to macrophages. The concept of a stereotypic and linearly graded phenotypic reaction of microglia in transition between a process-bearing, or ramified, appearance and a rounded, or amoeboid morphology has been challenged (Hanisch and Kettenmann, 2007; Ransohoff and Perry, 2009). Rather, ramified cells are not required to follow a set progression and can immediately differentiate into amoeboid cells or can return to a ramified morphology without assuming intermediate morphological phenotypes.
In vivo, the transformation of resident microglia into those with a phagocytic phenotype is strictly regulated and occurs in response to stimuli such as cell death or accumulated debris, excess aberrant protein, or the presence of viral or bacterial pathogens. Alternatively, a differential microglia response can be induced by a variety of input signals and may not necessarily result in a shift to an amoeboid phenotype. It is now becoming evident that in the developing brain, many of the standards for microglia morphology/activation may require readdressing. Given the predominantly amoeboid morphological phenotype of microglia in the fetal brain and their ability to secrete cytokines, including interleukin 1 (IL-1) and tumor necrosis factor alpha (TNFα)(Giulian et al., 1988; Munoz-Fernandez and Fresno, 1998), it was initially thought that immature microglia existed in an activated state and therefore were unable to mount a full host-response to injury. However, studies have demonstrated both a pro-inflammatory cytokine response and phagocytic activity following injury in the perinatal brain (Hagan et al., 1996; Silverstein et al., 1997). In the case of direct injury, the microglia demonstrate similar morphologies to those seen in the adult. When more subtle insults are examined there is also a similar pattern. In the case of stress, both prenatal (Gomez-Gonzalez and Escobar, 2010) and adult (Sugama et al., 2011), microglia shift to a bushy/ramified morphology suggesting a similar functional role under stressful conditions regardless of age. In the adult rodent, ischemia can induce microglia to display either a more ramified and bushy appearance or an amoeboid morphology depending on the level of damage and distance from the infarct site(s). In the immature rodent, ischemia-induced changes in capillary flow or, presumably, altered CNS vascularization can retain the microglia in an amoeboid phenotype for longer and delay the normal ramification process (Masuda et al., 2011). It is likely that the nature of the injury drives the prominent morphology of the microglia. In the case of the adult, this consists of a transient shift to the amoeboid phenotype, while in the young, the cells retain this rounded morphology for prolonged periods of time.
In order to try to understand similarities, differences, and the impact of any specific type of microglia response, efforts are needed to fully characterize the anatomical and functional phenotype of responding microglia in each specific situation. This is where the use of the generic term “microglia activation” has served to hinder further understanding of these diverse and dynamic nervous system specific cells. More recently, there has been a renewed effort to identify the morphological characteristics and proliferative component of microglia responses. While claims are often made with regards to an increase in the number of microglia, such conclusions are somewhat difficult to support given the difficulty to definitively determine that the apparent increase in number is not simply reflective of an increase in the ability to immunologically detect the cells. One framework for examining the morphological change in microglia assumes that the transition between a process-bearing, or ramified, appearance and a rounded, or amoeboid, appearance occurs along a dynamic continuum with multiple, functionally-distinct, intermediate stages. The morphological differences in microglia are suggested to represent different functional states; thus, efforts have been made to classify the various morphological phenotypes. One approach has been to utilize the Sholl method of analysis that has been very successful in evaluating neuronal arborization (Sholl, 1953). While this approach would be useful to identify ramified from amoeboid cell morphology, it is often found to be limited in distinguishing between the various ramified morphologies that microglia can assume. As an alternative approach, morphometric procedures have been developed that involve calculation of a form factor (FF) or an index of ramification (IR) to assess the shape of immuno-labeled microglia (Wilms et al., 1997 and Heppner et al., 1998, respectively). Wilms et al. (1997) used a ratio of the cellular area to perimeter to estimate process complexity: FF = [(4Π)(cell area)] / (cell perimeter)2; while, Heppner et al. (1998) drew a polygon inscribed by the outermost cellular process endings (convex area) in relation to the cell area: IR = (cell area) / (convex area). For both of these measures, values closer to one indicate more amoeboid morphology with a value of “1” describing a perfectly circular cell. These parameters have been used to estimate the morphology of microglia in various culture systems, including isolated microglia cultures (Heppner et al., 1998), microglia co-cultured with astrocytes or other cells (Wilms et al., 1997), and slice cultures (Kraft et al., 2011). This type of quantification has been used in vitro to support the notion that morphological “activation” of these cells does not always correlate with changes in immunological and physiological properties (e.g., MHCII expression) and that these properties are independently modified by the stimulating agent (Beyer et al., 2000). This type of quantification has also been used to assess the degree of microglia “reaction” following transient global ischemia in vivo (Soltys et al., 2005). Using 2–4 planes of focus of microglia to capture the full process arborization, the authors applied a principal component analysis to score nine parameters of microglia morphology, including FF, a “ramification factor” based on the ratio of terminal process segments to primary processes, and a measurement titled “solidity” similar to IR described above. The FF and solidity (IR) were reliable for discriminating between four microglia morphologies (i.e., ramified, hypertrophic, bushy, or amoeboid). Overall this general approach provides a quantitative assessment of morphological differences in microglia and can be used to establish a morphology scoring system (Fig. 3). While this approach has not been applied to the developing brain, it offers a method to assess changes that occur with time and one that has been successfully used to characterize microglia responses in vitro (Kraft et al., 2011) or to assign a ramification score in vivo (Funk et al., 2011).
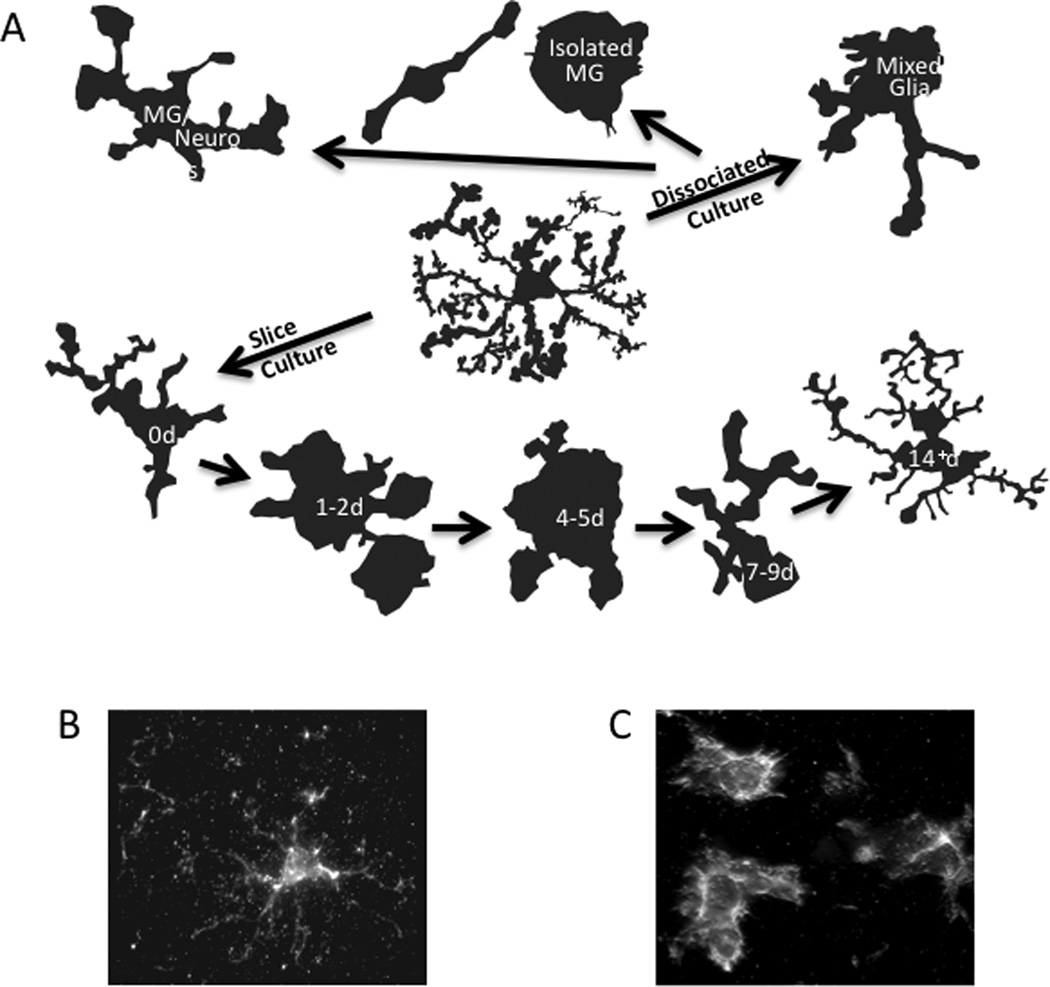
Figure 3.
Morphologies of microglia in (A) primary and slice cultures and (B) in vivo normal and (C) in vivo activated representing cells can be rated based upon the field of process arborization versus cell body to determine various stages/types of microglia.
Brain macrophages exist in various states of activation within an injured tissue and retain the capability to shift their functional phenotype within specific stages of the inflammatory response (Stout et al., 2005). Whether this is represented in the cell morphology is not yet known. In efforts to characterize functional changes and thus activation states, data from peripheral macrophages have been recently adapted and proposed to describe the various stages that brain macrophages undergo during neurodegeneration (Colton and Wilcock, 2010). They include the classical pro-inflammatory phenotype (e.g., TNFα, IL-6, IL-12, IL-1β and nitric oxide synthase (NOS)2) to alternatively-activated (e.g., IL-4, transforming growth factor beta (TGFβ)), anti-inflammatory (e.g.,TGFβ, IL-10), and tissue repair and reconstruction (eg. Arginase 1 (AG1), mannose receptor (MRC), and Chitinase-3-like-1 (Ym1 in rodents)), phenotypes. Molecular profiles of microglia during stages of injury or disease are just now being generated, with only a limited amount of publications. However, it is likely that this approach could be applied to the developing brain to generate region specific molecular profiles representative of the functional phases of microglia and to determine if microglia phenotypes are similar between the young and the mature brain.
7. Resident microglia versus blood-borne macrophages
7.1. Sources of brain macrophages
The brain has two sources of phagocytic cells, or brain macrophages, namely, the resident microglia and blood-derived monocytes entering the brain upon vascular injury. Once colonization of the brain is complete and peripheral monocytes are hindered from crossing the blood-brain-barrier, those remaining in the brain assume characteristics unique to the CNS. However, mature microglia continue to share phenotypic characteristics and lineage-related properties with bone marrow-derived monocytes and macrophages. They also share innate immunological functions with other mononuclear phagocytes, such as monocytes, macrophages, and dendritic cells, and they express MHC antigens, T- and B- lymphocyte markers, and other immune cell antigens (McGeer and McGeer, 1995; Williams et al., 1994). Microglia can serve as antigen-presenting cells (APCs), although far less effectively than cells in peripheral organs (Carson et al., 1998).
At the interface between the CNS parenchyma and the blood another macrophage resides. "Perivascular microglia" or "perivascular macrophages" are terms routinely used to denote mononuclear cells between the first layer of the basal membrane and the glia limitans (i.e., limited to the perivascular space). A subset of these cells is in intimate association with the vascular element, is a component of the neurovascular unit, and fit the morphological definition of “pericytes”. Unlike microglia, these elongated cells are not ramified and, rather than residing in the parenchyma, they are components of the vascular wall and can be specifically labeled in the rat with an ED2 antibody (Graeber and Streit, 1990). Pericytes serve to regulate capillary blood flow, clearance and phagocytosis of debris, and form a mode of communication between the cell membrane and paracrine signaling. They have a critical influence on the blood-brain-barrier with the integration of endothelial and astrocyte functions at the neurovascular unit (Armulik et al., 2010; Winkler et al., 2011). In contrast to juxtavascular microglia, perivascular microglia exhibit a rapid turnover rate from the systemic population. Accordingly, as these structural cells cannot be removed via vascular perfusion, they can present a confounding variable in the interpretation of such changes in the absence of cellular localization.
Under normal conditions, following various pharmacological intervention/stimulants, or in the early stages of neurological disorders or neurodegenerative diseases, the job of “brain macrophage” falls to the resident microglia. However, with any compromise of the blood-brain barrier, this task is accomplished by both the resident microglia and infiltrating blood-borne monocytes. With the entry of blood-derived monocytes, the brain not only has multiple sources of phagocytic cells, or brain macrophages, but also gains a strong presence of antigen presenting cells (APCs) (Hickey and Kimura, 1988; Carson et al., 1998). When peripheral monocytes enter the brain upon injury they assume the morphology and protein expression of microglia, thus, hindering the ability to discriminate between the two cell types of brain macrophages (Flugel et al., 2001). In addition, the infiltration of peripheral monocytes, localized damage to the vasculature would allow access of activated pericytes. With a physical injury such as trauma or ischemia, or during autoimmune events, the barrier is disrupted and blood-borne immune cells gain access to the brain parenchyma, although this entry into the brain is limited and delayed as compared to peripheral tissues (Andersson et al., 1992). As with an autoimmune disease, this entry can occur regardless of the presence or absence of tissue damage if the blood-brain barrier is compromised (Matsumoto and Fujiwara, 1987). Alternatively, it has been suggested that infiltrating monocytes may differentiate to the microglial morphology and remain in the brain tissue for an extended period of time. In this state they can persist until destroyed by senescence or prompted to move back into the circulation. In addition to the secondary contribution of cells from the circulating blood, components of plasma such as fibrinogen can, in and of themselves, initiate a response of microglia (Adams et al., 2007; Ransohoff and Perry, 2009; Ryu et al., 2009). A large proportion of the evidence supporting a neurotoxic function of microglia is derived from animal models that allow entry of blood-borne monocytes (Kokovay and Cunningham 2005; Liberatore et al., 1999; McCoy et al., 2006; Rodriquez et al., 2007; Wu et al., 2003; Zhang et al., 2005). Thus, a distinction of central versus peripheral source of brain phagocytic cells becomes critical in understanding any biological outcome.
7.2. Identification of microglia from macrophages
Compared to resident microglia, cells of the peripheral immune system provide an enriched source of cytokine and inflammatory factors, and thus, exhibit exaggerated activation phenotypes as compared to those of microglia. When during life this distinction become prominent is not known but one would expect that the CNS environment would drive the distinction of resident microglia early within the colonization period. Two seminal studies demonstrated that the resident microglia of the CNS are not identical to macrophages that acutely infiltrate the CNS (Hickey and Kimura, 1988; Sedgwick et al., 1991).
Turn-over rate is one basis for evaluation of the cells. As compared to the infrequent replacement of resident microglia, a few-week turnover rate is observed for perivascular microglia, pericytes, and menigneal or choroid plexus macrophages (Hickey and Kimura, 1988). A second characteristic, and one that can be experimentally used to determine cell populations, is the fact that, while both peripheral macrophages and resident microglia express the leukocyte common antigen, CD45, much lower levels are observed in the resident microglia (Carson et al., 1998; Sedgwick et al. 1991). CD45, a protein tyrosine phosphatase expressed by all nucleated cells of hemopoietic lineage, is an inhibitory receptor for CD22, a molecule expressed by B cells and neurons that can restrict LPS-induced TNFα production by microglia (Mott et al., 2004). Development of a flow cytometry method to discriminate the two cell populations led to the observation that resident microglia display a CD11b+/CD45low phenotype with undetectable levels of CD14 (Becher and Antel, 1996; Peterson et al., 1995). This was in contrast to the CD11b+/CD45high profile of peripheral mononuclear cells. Further flow cytometric characterization found that freshly-isolated human microglia displayed a profile of CD11b+/CD45low/CD4−/CD11chigh/MHC class II+/CD26−/CD14− (Dick et al., 1997).
Further distinction between the two cell types is based upon anatomical differences of cultured cells (Giulian et al., 1995). These authors reported that the presence of fetal bovine serum or the extracellular matrix protein, laminin, decreased the number of process-bearing microglia in culture. This same exposure had no effect upon cultured peripheral mononuclear phagocytes. Consistent with the early description of microglia as having “wavy processes beset with spines” (Rio-Hortega, 1932), the microglia were distinguishable by their surface coverage of spines, with <3% showing the ruffled membrane morphology present on most peritoneal macrophages. In addition, contact between astrocytes and microglia promoted and increased microglia ramification while the presence of astrocytes did not alter mononuclear phagocytes (Giulian et al., 1995).
Probably the most definitive method to identify if the microglia response is due to infiltrating monocytes is through the use of congenic bone marrow chimera (Ginhoux et al., 2010) or parabiotic mice (Ajami et al., 2007; Ginhoux et al., 2010). To generate bone marrow chimera mice, young adult animals are whole body irradiated with the head shielded and the bone marrow reconstituted with a tagged cell population obtained from a donor. This then allows for the anatomical evaluation of “tagged” cells within the brain parenchyma. The parabiotic procedure eliminates the confounder of irradiation but, as with the chimera mice, this procedure is also not conducive for examining the developing brain.
8. Microglia functions potentially impacting brain development
It has been speculated that microglia perform specialized functions critical to the development of the brain. While a number of these may be unique to development, a greater number are assumed based upon data from the adult brain or from cells in culture. For example, microglia appear to influence events associated with neuronal proliferation and differentiation during development. Neurogenesis of embryonic cortical cells is enhanced in culture by microglia (Aarum et al., 2003). The exogenous addition of microglia or conditioned medium from microglia cultures promotes the differentiation of basal forebrain progenitors into cholinergic neurons (Jonakait et al., 1996, 2000). Similarly, Antony et al. (2011) suggested that microglia are an important component of embryonic neural precursor differentiation in the cortex. In the absence of microglia, proliferation of cortical precursors and astrogenesis is reduced, while an increase in microglia enhances the differentiation of cortical precursors into astrocytes. Additionally, the release of soluble factors by microglia can serve to direct neural progenitor cell differentiation toward a neuronal phenotype, possibly via the expression of the innate pattern recognition receptors, toll-like-receptors (TLRs; Rolls et al., 2007). Like the mature microglia, fetal microglia are known to secrete cytokines including IL-1 and TNFα (Giulian et al., 1988; Munoz-Fernandez and Fresno, 1998). However, during development, cytokine patterning occurs in a region specific manner in the brain and influences normal cell maturation (Brenneman et al., 1992). A role for microglia in development is not limited to cell proliferation and survival (e.g., microglia have been reported to modulate axon pathfinding, possibly via modification of the extracellular matrix by thrombospondin; Chamak et al., 1994).
As an example of the critical nature of microglia during development, in drosophila, CNS morphogenesis requires a hemocyte-derived macrophage population that clears cellular debris associated with normal programmed cell death (Sears et al., 2003). In addition to a clearance response, microglia have been shown to promote apoptosis of embryonic cerebellar Purkinje cells (Marin-Teva et al., 2004) and early postnatal hippocampal neurons (Wakselman et al., 2008). While clearance of excess cellular debris and aberrant proteins from programmed cell death is critical, the distribution of microglia in the developing mammalian brain suggests a more heterogeneous role for microglia (Rezaie and Male, 2003; Rezaie et al., 2005). As one example, microglia are a primary source of free radicals in the brain and while they can be damaging, endogenously produced free radicals are also an essential component of development and brain homeostatic processes. During development, in addition to phagocytosis of apoptotic neurons, microglia-mediated respiratory burst may help to regulate the numbers of neurons integrated, in a nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent manner. Microglia can serve in a neuroprotective role with the production of multiple regulatory factors including neurotrophins such as neuronal growth factor (NGF), brain derived neurotrophic factor (BDNF), neurotrophin-3 (NT3), glial derived neurotrophic factor (GDNF) and cytokines with neurotrophic activity (reviewed in Garden and Moller, 2006). In addition, macrophage colony stimulating factor acts as a neurotrophic factor supporting neuronal survival and neurite outgrowth indirectly through microglia (Michaelson et al., 1996).
8.1. Microglia and synapse remodeling
An additional function of microglia includes a significant contribution to synaptic stripping or remodeling events (Stevens, 2007; Trapp, 2007; Wake, 2009), although the extent to which the action of these cells is directly causative is debated (Perry and O’Connor, 2010). However, the data suggest that microglia play a significant role in monitoring synaptic function and that they contribute to synapse maturation or elimination. Process-bearing “activated” or “reactive” microglia have been reported in the thalamus, cerebellum, olfactory bulb, and hippocampus during postnatal synaptic remodeling (Perry et al., 1985; Dalmau et al., 1998; Fiske and Brunjes, 2000). Work by Tremblay et al. (2010) demonstrated that sensory input from 8–10 days postnatal alters microglial contact with dendritic spines and thus, alters the subsequent developmental loss of these synapses.
In a more recent study, the co-localization of neuronal postsynaptic protein within microglia cytoplasm suggested that microglia maintain the ability to engulf and remove dendritic spines during development (Paolicelli et al., 2011). This specific phagocytosis may occur via signaling of the neuronal chemokine, fractalkine (CX3CL1), to its receptor on microglia (CX3CR1) (Hughes et al., 2002; Nishiyori et al., 1998), as microglia number was reduced and synaptic pruning delayed in the developing brain of mice deficient for CX3CR1 (Paolicelli et al, 2011). In these mice, the CX3CR1-dependent deficiency in synaptic pruning led to a transient excess of dendritic spines and immature synapses. Functionally, this manifested as increased seizure susceptibility and a reduction in synapse efficiency as measured by hippocampal long-term potentiation (LTP). The classical complement cascade has also been implicated in synapse elimination by microglia (Stevens et al., 2007), as they express CR3, can stimulate phagocytosis, and are spatially associated with complement factor, C1q, at synapses (Gasque et al., 2002). Complement component C3 expression and localization to the synapse is correlated with the peak of synaptic remodeling in the dorsal lateral geniculate nucleus (Stevens et al., 2007). This has led to the speculation that C3 serves to tag the extranumerary synaptic inputs for CR3-mediated phagocytosis by microglia (Schafer and Stevens, 2010). In mice deficient for C1q, the deficit in removal of excess excitatory synapses during development led to the acquisition of epileptiform activity later in life (Chu et al., 2010). It is likely that similar processes occur with remodeling of synaptic circuits, as microglia–synapse interactions at dendritic spines has been shown to occur in an experience-dependent manner (Wake et al., 2009; Tremblay et al., 2010). Work from Zhong et al. (2010) reported that both LTP and long-term depression (LTD) are influenced by microglia in a TNFα–dependent manner. Exogenous TNFα modulates synaptic strength in cultured hippocampal neurons by promoting surface expression of AMPA-type glutamate receptors (Beattie et al., 2002), which involves the upregulation of β3 integrin expression (Cingolani et al., 2008). Microglia-neuron signaling occurs in both directions with neuronal activity directly activating microglia (Hung et al., 2010). As an example, prolonged inhibition of spontaneous neuronal activity in hippocampal cells stimulates TNFα release, while activity-regulated signals decrease TNFα release (Stellwagen and Malenka, 2006). It was proposed that this represents synaptic scaling, where the strength of synapses is modulated in response to changes in network activity. This suggests that microglia can detect the functional state of neurons and initiate an appropriate response targeted to the specific neuronal activation pattern. Thus, one can propose that alterations in microglia functioning during synapse formation and maturation of the brain can have significant long-term effects on the final established neural circuitry.
8.2. Microglia and myelination
Microglia are observed within the myelin tracts during early development. Both inflammation and changes in white matter microglia have been associated with deficits in oligodendroglia progenitor cells or myelination as occurs with periventicular leukomalacia (reviewed by Deng et al., 2008; Harry et al., 2006) or in receptor-dependent events. As one example of the latter, TREM2 is an orphan receptor expressed in a subset of microglia. This receptor is implicated in limiting the pro-inflammatory phenotype of macrophages and promoting the expression of molecules associated with presenting antigen to T-lymphocytes. A loss of function mutation in TREM2 underlies altered myelination and early onset dementia in Nasu-Hakola disease (Klunemann et al., 2005). The transmembrane adaptor molecule DAP12 mediates TREM2-triggered intracellular signaling, which signals via immunoreceptor tyrosine-based activation motifs (ITAM) (Lanier and Bakker 2000; Prada et al., 2006). Work by Thrash et al. (2009) demonstrated that, in PND 1 mice, TREM2+/DAP12+ microglia are found adjacent to oligodendrocytes prior to the initiation of myelination. Given that TREM2 functions to inhibit microglial and macrophage cytokine expression and promotes microglial phagocytosis of cellular debris (Takahashi et al., 2007), it is possible that the close apposition of TREM2+/DAP12+ microglia to oligodendrocytes prior to myelination represents a regulatory cell-cell interaction required for normal brain development.
9. Examples of microglia as a target for developmental disruption
The overall body of data is now leading to an appreciation of these cells as a critical component in the formation of neural circuitry and as a potential target for developmental disruption. The contributory role of microglia to the developing vascular and axonal networks has been recently reviewed (Pont-Lezica et al., 2011) and includes a discussion of results from Paolicelli et al. (2011) showing that microglial CX3CR1 is required for spine elimination during a specific developmental period in the juvenile hippocampus. Similarly, microglial expression of CSF-1R, the receptor for IL-34, in the postnatal mouse occurs at times that coincide with peak expansion of microglial cell numbers. The expression of this receptor is essential for appropriate brain development, most notably in the cortex and olfactory bulb (Erblich et al., 2011). The phagocytic action of microglia on amyloid-β (Aβ) fibrils exhibits age dependence in that brain microglia cultured from PND 0 brain, but not from PND 180 brain, phagocytose Aβ in vitro (Floden and Combs, 2011). This phagocytic action appears to involve IL-34 interactions with microglia (Mizuno et al., 2011). Depletion of microglia in the transcriptional factor, PU.1 did not affect NPC survival or neurogenesis in vitro, but did result in inhibited precursor cell proliferation and astrogenesis (Antony et al., 2011). Overall, the data suggests that microglial actions may be most critical during postnatal brain maturation rather than during embryonic stages of development.
The contributions of microglia during development can be impacted by changes in experience during the postnatal period. Using a model of early life stress, Chocyk et al. (2011) demonstrated that maternal separation resulted in an increase in active caspase 9+ microglia within the substantia nigra and the ventral tegmental area of juvenile male rats, potentially resulting in a decrease in microglia in these regions in the adult. Peak expression of the lipopolysaccharide binding protein (LBP) occurs at 2–3 weeks postnatally in close proximity to both the post-synapse and microglial processes. Wei et al. (2011) reported that early life stress induces a loss of LBP and results in an increased anxiety level and impaired memory in the adult. However, prenatal stress exposure leads to slightly increased anxiety-like behavior in the adult (Kohman et al., 2010) with no deficits in learning and memory performance observed with or without LPS challenge. Early handling of male rat pups daily between PND 2 and PND 10 produced a 3-fold increase in mRNA levels for the anti-inflammatory cytokine, IL-10, within the nucleus accumbens (Schwarz et al., 2011). These authors concluded that neonatal handling can shift glia into a predominantly anti-inflammatory state and that this will serve to block morphine-induced glial activation. Further work showed a 5-fold decrease in relative methylation of the IL-10 gene in microglia of handled rats suggesting an early-life epigenetic programming of IL-10 expression that would manifest upon challenge in the adult (Schwarz et al., 2011). Prior to our current knowledge of epigenetic programming, this feature would have been considered as a long-term preconditioning.
Translation of the changes in microglia in the adult brain to the developing brain may not be easy. In the adult, the normal response examined is a shift in morphology and an elevation in pro-inflammatory cytokines. In the immature brain, the microglia either continue to display an amoeboid phenotype and maintain high levels of cyotkine production, the roles are different than what occurs with adult injury. In addition, as in the adult brain, determining whether any observed microglia change represents a direct effect upon microglia as an underlying mechanism or rather if the cells are simply responding to their environment. One interesting study examined the morphological differences in microglia as a function of thyroid hormone status. Alterations in thyroid hormone (TH) signaling during development are associated with detrimental effects on the brain, including changes in synapse formation and myelination. Lima et al (2001) reported that isolated rat microglia express TH receptors (TRα1 and TRβ1), and that changes in thyroid status, either a deficit or over expression, significantly alter the maturation of microglia (Flavia et al., 2001). Within the first 1–2 weeks of postnatal life, hypothyroid animals showed a deficit in the density of microglia and a delay in process extension. In contrast, hyperthyroidism accelerated the extension of microglial processes and increased the density of cortical microglial cell bodies. How this altered timing for the shift of microglia from the fetal amoeboid morphology to process bearing affects microglia function and/ or their ability to respond remains unknown. What this data does demonstrate is that subtle changes in the neural environment, in the absence of a cell death response, can shift the morphology of developing microglia.
Exposures to xenobiotics during development can directly target microglia. As one example, early work in this area by Harry and co-workers demonstrated that a direct exposure to the known neurotoxicant, trimethyltin (TMT), could activate microglia in culture to produce TNFα and shift the phagocytic capability of the cells, but in the absence of an induction of iNOS (Maier et al. 1997). While a number of compounds have been shown to stimulate microglia in culture, including mercury, the studies with TMT reveal a direct translation from in vitro to in vivo models. The stimulatory effects of TMT, as indicated by morphology and the elevation of pro-inflammatory cytokines were observed in vivo when the adolescent mouse was exposed to TMT (Bruccoleri et al., 1998; McPherson et al., 2011). Of interest was the observation that both in vivo and in vitro models showed a similar temporal progression of responses and that they occurred in the presence and absence of dying neurons, respectively.
Acute alcohol exposure in adolescent rats results in morphological changes and proliferation of hippocampal microglia that persist for at least 4 weeks post-exposure. This occurred without accompanying alterations in expression of ED-1, MHC-II, or TNFα (McClain et al., 2011). It was suggested that this response represents a "priming" of microglia, as MHC-II and TNFα levels are increased when these exposures are repeated using an intermittent paradigm (Ward et al., 2009; Alfonso-Loeches et al., 2010). Early postnatal ethanol exposure (PND 3–5) results in depleted microglial cell numbers and associated loss of Purkinje cells in the cerebellum that may be inhibited by PPAR-γ activation (Kane et al., 2011). In adolescent rats, ethanol exposure, in combination with 3,4-methyleneddioxymethamphatamine (MDMA), resulted in an accumulation of CD11b+ microglia at bordering zones of the hippocampal subgranular zone (SGZ) and concomitant decreases in adult neurogenesis and memory function (Hernandez-Rabaza et al., 2010). Sensitivity of microglia in the developing brain has also been noted following exposure to manganese (Mn). Studies by Moreno et al. (2009; 2011) demonstrated that juvenile exposures (PND 20–34) resulted in exaggerated Iba1+ morphological changes and microglial NOS2 expression, altered dopamine content, and associated evidence of nitrosative stress in the basal ganglia, as compared to adult exposures (weeks 12–20). Interestingly, mice pre-exposed as juveniles that also received adult Mn did not exhibit morphological microglial activation and had elevated striatal nitrotyrosine adducts, in contrast to those without pre-exposure (Moreno et al., 2009). This finding reiterates the potential for microglial priming during development as a modifying factor for the subsequent response of these cells to insult. The late postnatal period also appears to represent a sensitive window for modification of microglial function following toluene exposure, as analyses at PND 21 following exposures from either GD 14–18, PND 2–6, or PND 8–12 reveal enhanced Iba1 content and pro-inflammatory cytokine signaling (e.g., NF-κB, TLR4, TNFα) in the PND 8–12 group (Tin-Tin Win-Shwe et al., 2011). Similarly, changes in microglia following chemical-induced excitotoxicity at PND 7 was examined by Drouin-Oullet et al. (2011) and appeared to involve transient increases in microglial activation markers (Iba1, PK11195, and ED-1) and the cytokine, IL-1β. The long-term consequences were examined at PND 56, which revealed amphetamine-induced hyperactivity as well as deficits in spatial learning and social interactions, in parallel with elevated microglial mGluR5 expression. Although all of these changes were inhibited by minocycline, the potential contribution of infiltrating cells following the intra-hippocampal injections or direct effects of minocycline on the injured neurons were not evaluated. Overall, the existing data suggests a critical regulatory role for microglia in brain development that is much expanded from initial considerations of microglia in the context of their standard, immune-mediated responses.
10. Is the vulnerability of the developing nervous system to infection/inflammation related to microglia?
Maternal immune activation has been considered a risk factor for long-term alterations in the neurobehavior of the offspring. Epidemiological evidence shows an association between maternal infection and neurodevelopmental abnormalities, as well as increased risk for developing cerebral palsy, schizophrenia, and autism (Ellman and Susser, 2009). As an example, intrauterine inflammation during pregnancy is associated with an increased risk of preterm delivery and altered neurological functioning (Saliba and Henrot, 2001). The link between maternal infection and adverse effects in the offspring is thought to be related to an induction of a fetal inflammatory response involving increased production of inflammatory mediators including IL-1, IL-6, IL-8, and TNFα in chorionic membranes, amniotic fluid, and fetal blood. The current literature, with regards to fetal brain levels of pro-inflammatory cytokines, suggests that the maternal immune response elevates the availability of cytokines to the fetus (Goepfert et al., 2004). In animal models of either maternal infection or activation of an immune response in the absence of infection, cytokine levels are altered in the fetal brain (Boksa, 2010; Cai et al., 2003; Meyer et al., 2009; Pang et al., 2006). In these prenatal models, the elevated brain cytokine levels in the offspring may result from a direct effect; however, it must also been considered that they may indirectly result from the maternal immune response, including not only pro-inflammatory cytokines such as TNFα, IL-1, and IL-6, but also peptides such as corticotropin releasing hormone, adrenocorticotropin hormone, monoamines, glucocorticoids, free radicals, and opioid peptides (Weigent and Blalock, 1997).
Early-life infection has been associated with altered stress reactivity, disease susceptibility, and vulnerability to neurological disorders (Brown and Patterson, 2011). Initial work examining the role of inflammation in determining adverse effects on the developing nervous system was based upon the associations observed between intrauterine inflammation, periventicular leukomalacia of the developing brain, and cerebral palsy (Huleihel et al., 2004 for review). Using an LPS model for intrauterine inflammation in the rat, namely an intracervical injection of 0.1 mg/kg LPS on GD15, Poggi et al., (2005) demonstrated an earlier proficiency in forelimb placement and surface righting tests in LPS-exposed offspring, with no differences observed in locomotor activity, rotorod, or anxiety when tested as young adults. These results are in sharp contrast to reports in the literature of a broad spectrum of neurobehavioral effects on the offspring following relatively severe systemic maternal infection. In these models, deficits in paired-pulse and latent inhibition, open field exploration, and social interactions occurred in the absence of hippocampal neuronal death (Smith et al., 2007). With exposure paradigms that resulted in a loss of hippocampal neurons, neurobehavioral alterations extended to include deficits in Morris water maze performance (Samuelsson et al., 2006). Further work demonstrated altered non-spatial hippocampal processing with gestational inflammation (Ito et al., 2010). Although these studies suggest that the developing brain is vulnerable to inflammation, the broad spectrum of neurobehavioral alterations reported may also represent high-dose, toxic effects of maternal infection or associated systemic effects. Work focused on the response of the early postnatal brain to inflammation has also demonstrated persistent modifications to nervous system function. As an example, an intracerebral injection of LPS on PND5 produced neuropathology, delayed reflex responses, hyperactivity in open-field and plus-maze tests, and impaired passive avoidance performance in pre-weaning animals (Pang et al., 2006).
11. Priming, preconditioning, or early life reprogramming of microglia
There is a significant amount of evidence regarding what is often termed “priming” and “preconditioning” events that serve to either exacerbate or provide neuroprotection from a secondary insult, respectively. In these states, the constitutive level of proinflammatory mediators would not be altered; however, upon subsequent challenge, an exaggerated response would be induced. The phenomena of priming represents a phenotypic shift of the cells toward a more sensitized state. Thus, primed microglia will respond to a secondary “triggering” stimulus more rapidly and to a greater degree than would be expected if non-primed. It has been considered that microglia in the aging brain exist in a primed state based upon the shift in morphology to fragmented processes and a elevation in basal levels of pro-inflammatory cytokines (Streit). Exactly how long this primed state will last has not been determined; however, data from microglia suggest that it can extend over an expanded period of time. Preconditioning can also represent changes that would occur not only over the short term but may be long lasting. Again, preconditioned microglia would be phenotypically shifted in a manner such that they could more readily respond to an injury or insult. However, conceptually it is normally considered as a enhanced response to promote recovery and foster repair. While mechanisms of precondition in the brain are not well understood one aspect proposed is that the prevention of inflammation serves as an underlying mechanism in the adult brain. In this regard, while the normal characteristics of preconditioning in which a below threshold event confers protection or tolerance to a subsequent above threshold insult usually last for only a few days. The additional recruitment of a reprogramming event likely accounts for the long term changes in the response threshold of the system observed by Schwarz et al. (2011). Much of our understanding of preconditioning is derived from cardiac ischemia (Das and Das, 2008) however, one could assume that there are likely correlates. What we know from these data is that preconditioning adaptation occurs in a biphasic pattern with a 2–3 hr early period and a 24–96 hr late period. It is thought that the early period depends on adenosine, opioids, and prostaglandins released with the acute insult. These molecules then serve to activate G-protein-coupled receptor, K(ATP) channel, protein kinase C, tyrosine kinase, and members of the MAP kinase family as well as generate oxygen-free radicals. The late period is less potent and requires newly synthesized proteins such as iNOS, COX-2, manganese superoxide dismutase and heat shock proteins. Stimulation of each of these molecules has been demonstrated in acute neuroinflammatory conditions in the brain and thus, are likely to contribute to establishing preconditioning. One could speculate that the rapid dynamics occurring with brain development and the normal requirements for up-regulation of a number of these signaling factors may impart a different view with regards to “preconditioning” or “priming” of microglia. In any case, mechanisms that would have a substantial impact on the microglia establishing its normal signaling pattern, threshold for activation, or down-regulatory mechanisms would likely result in a shift in the response capability of these cells in the adult brain. Additionally, changes that occur in the establishment of the microglia cell population in the brain may change the healthy lifespan of the cells i.e., shift the temporal and spatial pattern of microglia senescence. Alternatively, either process could reflect changes in developmental programming.
In both the adult and developing animal, low-dose LPS exposure can provide preconditioning that manifests as a reduction in neuroinflammation and induced neuroprotection. Lin et al (2009) demonstrated that a low dose of LPS (0.05 mg/kg) given 24 hours prior to a hypoxic/ischemic insult in rat pups significantly reduced microglia and macrophage activation, TNFα expression, and reactive oxygen species production. These animals also showed less brain damage and learning and memory deficits. Galic et al. (2008) examined age related vulnerabilities to LPS in rats to determine critical age periods. Postnatal injection of LPS did not induce permanent changes in microglia or hippocampal levels of IL-1β or TNFα; however, when LPS was given during the critical postnatal periods, PND 7 and 14, an increased sensitivity to drug-induced seizures was observed in 8-week-old rats. This was accompanied by elevated cytokine release and enhanced neuronal degeneration within the hippocampus after limbic seizures. This persistent increase in seizure susceptibility occurred only with LPS injection at postnatal day 7 or 14 and not with injections during the first day of life or at PND 20. Similar long-lasting effects were observed for pentylenetetrazol-induced seizures when PND 11 or 16 rat pups were subjected to LPS and hyperthermic seizures (Auvin et al., 2009). These results again highlight this early postnatal period as a "critical window" of development vulnerable to long-lasting modification of microglia function by specific stimuli. Work by Bilbo and co-workers demonstrated LPS-induced deficits in fear conditioning and a water maze task following infection of PND4 rats with Escherichia coli. In the young adult, an injection of LPS induced an exaggerated IL-1β response and memory deficits in rats neonatally exposed to infection (Bilbo et al., 2005). Consistent with the earlier work by Galic et al., (2008), an age dependency for vulnerability was detected with E. coli.-induced infection at PND 30 not showing an increased sensitivity to LPS in later life (Bilbo et al., 2006).
One hypothesis for developmental sensitivity is the heterogeneous roles for inflammatory factors and pro-inflammatory cytokines during development, including their timing-, region- and situation-specific neurotrophic properties. Many of the pro-inflammatory cytokines are lower at birth with a subsequent rapid elevation occurring during the first few weeks of life. In an examination of the developing mouse cortex between PND 5 and 11, mRNA levels for TNFα, IL-1β, and TNFp75 receptor remained relatively constant while a significant increase in mRNA levels of CR3, macrophage-1 antigen (MAC-1), IL-1α, IL-1 receptor 1 (IL-1)R1, TNFp55 receptor (TNFp55R), IL-6, and gp130 occurred (Fig. 2). This data suggests that an upregulation of interleukins and cytokine receptors may contribute to enhanced cytokine signaling during normal cortical development.
The critical role of microglia in response to infection, either early in development (e.g., at PND 0; Hornig et al., 1999) or specifically at late postnatal stages (e.g., at PND 14, but not before PND 1 or after PND 20; Galic et al., 2008), has been reviewed by Bilbo and Schwarz (2009), who indicate that early changes in the neuroinflammatory profile of microglia, such as exaggerated production of IL-1β, is linked to adult susceptibility to depression, seizures, and other neurobehavioral consequences. One hypothesis put forward using a model reliant on postnatal exposure to LPS suggests that these types of exposure may "reprogram" neuroimmune responses such that adult stress results in hyperactivation of the hypothalamic pituitary adrenal (HPA) axis (Mouihate et al., 2010) and corticosterone changes (Bilbo and Schwarz, 2009).
While limited, the available data suggests that events occurring during development, especially postnatal development, have the potential to cause long term alterations in the phenotype of microglia and that this can be done in a region specific manner. Additional questions remains as to whether these “altered” microglia serve to change the normal environment and thus what subtle impact this may have on neighboring neurons and astrocytes. There is no data available to determine the impact of microglia activation during development on later life events such as the shift to a primed state for microglia in the aged brain. However, given their influence on a diverse array of processes and cell types in the formation of the brain, as well as their role in the mature brain, the fact that their phenotype can be rapidly and significantly altered raises important issues for the consideration of early life events and adult disorders or disease.
12. Potential for early life effects on microglia cell aging and senescence
The impact of early life infection has been expanded beyond childhood diseases to encompass vulnerability to diseases associated with aging, such as Parkinson’s disease (PD) and Alzheimer’s disease (AD). Given that neuroinflammation is a component of each of these diseases, one might speculate that changes occurring to developing microglia can have a long lasting effect on the ability of these cells to manifest appropriate responses in later life. Age-related changes in cytokine production and dysmorphic microglia morphology (Frank et al., 2006; Perry et al., 1993; Sheffield and Berman, 1998; Streit et al., 2004) have been proposed to underlie the microglia contribution to neurodegenerative diseases. Thus, any event that would result in a modification of the normal aging of microglia and their functional capability would be expected to have long-lasting effects upon the brain.
When one examines unique features of aging microglia, a pattern of morphological and functional change emerges. It has been proposed that microglia assume a more reactive/activated phenotype as a function of aging and show an increased expression of MHC II antigens (Finch et al., 2002). Upon further examination, microglia in the aged brain displayed morphological changes in cytoplasmic structure reflective of dystrophy and senescence, and microglia loss with aging has been demonstrated (Streit et al., 2008). While the cells appear to display a more prominent morphological phenotype with aging, the evidence of fragmentation and dystrophy suggests an overall decrease in the ability of the brain to mount a normal host response to injury and alterations to microglial neuroprotective properties such as pruning and trophic factor secretion would be expected. Microglia exhibit telomere shortening and decreased telomerase activity with aging, lending support for the hypothesized occurrence of microglial senescence in normal and pathological aging (Flanary and Streit, 2004). It has been proposed that β-amyloid promotes microglial deterioration and accelerates senescence (Streit et al., 2004), and published work suggests that microglia from AD brains have shorter telomeres, as compared to control brains (Flanary et al. 2007). If microglia are functionally impaired by senescence, they would likely demonstrate decreased production of neurotrophic factors, impaired phagocytosis, and impaired protein clearance. Cumulatively, this may result in increased neurotoxicity (Streit et al., 2008; Richartz et al., 2005). Recent work by Baker et al. (2011), demonstrated that lifetime removal of p16Ink4a-positive senescent cells in the adipose tissue, skeletal muscle, and eye resulted in a delayed onset of age-related pathologies (Baker et al., 2011). Furthermore, they demonstrated that late-life clearance of senescent cells attenuated the progression of already established age-related disorders. While this work was not conducted in the nervous system, one could envision that the inability for any organ system to clear senescent or dysfunctional cells would result in an adverse and potentially “disease-related” environment.
It has been further proposed that microglia change as a function of aging and that such change may reflect a diminished capability for the cells to perform their normal function. It has been hypothesized that changes in aging microglia drive pathogenic progression of diseases or injury through a diminution of neuroprotective functions, increase in neurotoxicity, and dysregulation of responses to signals and perturbations (Flanary et al., 2007; Streit et al., 2008; Luo et al., 2010). Microglial changes with aging have been proposed to occur in response to repeated or continual, low level stressors encountered throughout an organism’s lifetime; alternatively, re-patterning of the microglial response phenotype during development may underlie these changes. Recent work examined the age-related changes in microglia in the retina and their response to injury (Damani et al., 2011). Surveillance microglia in the aged retina showed smaller and less branched processes and slower process motility. In addition, the dynamic microglia response to injury was altered such that, in the aged retina, microglia demonstrated a slower acute response to injury as compared to microglia in the younger retina. Of additional interest was the observation that these injury responses were maintained for longer in the aged retina, suggesting a deficit in the down-regulatory signaling for resolution of the injury response. These are compelling observations in that they are somewhat similar to those reported for rats exposed to E. coli on PND 4 (Bilbo et al., 2005; 2010; Bland et al., 2010). The constitutive adult microglia morphology in these developmentally exposed rats was characterized by larger cell volumes and short, thick processes (Bland et al., 2010). This occurred in the absence of changes in total cell number. While there were no deficits in memory function in the young adult, deficits occurred upon a secondary challenge with LPS (Bilbo et al., 2005) or with aging to 16 months (Bilbo et al., 2010). Thus, it is possible that the stimulation of microglia (in this case, by E. coli) during an early stage of development critically alters the microglia cell in such a way that it maintains a shift in response capability into adulthood, which may lead to accelerated aging of the cell. If this occurs, a deficit in the normal neuroprotective functions of the microglia may be compromised and thus, represent an increased vulnerability of the brain to subsequent injury/insult.
13. Conclusions
Modifications to resident immune cells of the brain can manifest as alterations in the intrinsic function of the cells or in interactions with ongoing developmental processes. The nature of these alterations can be dependent upon the developmental age of the brain and the ongoing developmental processes occurring at the time of insult. The currently available data strongly suggests that microglia can be altered during early life, including a potential susceptible period during early postnatal development. Given the ever-increasing roles and contributions of microglia to regulating brain development, neuronal activity, and network connectivity, the impact of an altered microglia population can be substantial and diverse. In addition, microglia are long-lived in the brain, having a very slow turnover rate. Therefore, any shift that may occur in the microglia population during early life can have a significant long-term impact on their ability to clear the brain of aberrant proteins, senescent cells and debris, as well as coordinate an appropriate response to a physical injury, chemical exposure, or disease process. These alterations can occur not only as a change in the number of microglia in the brain or in specific brain regions, but also as a change in the basal activation state such as would occur under a “primed” or “preconditioned” state. Alternatively, this can be viewed as a modification to their functional capacity or injury reaction due to an early cellular reprogramming event. These long overlooked neural-specific cells are now in a position to be regarded with greater appreciation of their role in the development and maintenance of an optimally functioning nervous system.
Acknowledgements
This research was supported by the Division, National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services #1Z01ES101623 and ES021164, and the U.S. Environmental Protection Agency, Office of Research and Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The views expressed in this article are those of the authors and they do not necessarily represent the views or policies of the National Toxicology Program or the U.S. EPA.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- Aarum J, Sandberg K, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci USA. 2003;100:15983–15989. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, et al. The fibrin-derived γ377–395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med. 2007;204:571–582. doi: 10.1084/jem.20061931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetziaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci. 2010;30:8285–8295. doi: 10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen H, Matthiessen ME. The histiocyte in human foetal tissues: its morphology, cytochemistry, origin, function and fate. Z. Zellforsch. 1966;72:193–212. doi: 10.1007/BF00334275. [DOI] [PubMed] [Google Scholar]
- Andersson PB, Perry VH, Gordon S. Intracebral injection of proinflammatory cytokines or leucocyte chemotaxins induces minimal myelomonocytic cell recruitment to the parenchyma of the CNS. J Exper Med. 1992;176:255–259. doi: 10.1084/jem.176.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjelkovic AV, Nikolic B, Pachter JS, Zecevic N. Macrophages/microglial cells in human central nervous system during development: an immunohistochemical study. Brain Res. 1998;814:13–25. doi: 10.1016/s0006-8993(98)00830-0. [DOI] [PubMed] [Google Scholar]
- Antony JM, Paquin A, Nutt SL, Kaplan DR, Miller F. Endogenous microglia regulate development of embryonic cortical precursor cells. J Neurosci Res. 2011;89:286–298. doi: 10.1002/jnr.22533. [DOI] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Aschoff L. Das reliculo-endotheliale System. Ergeb Inn Med Kinderheilkd. 1924;26:1–118. [Google Scholar]
- Auvin S, Porta N, Nehlig A, Lecointe C, Vallee L, Bordet R. Inflammation in rat pups subjected to short hyperthermic seiuzures enhances brain long-term excitability. Epilepsy Res. 2009;86:124–130. doi: 10.1016/j.eplepsyres.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur nK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 479 doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, et al. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Becher B, Antel JP. Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia. 1996;18:1–10. doi: 10.1002/(SICI)1098-1136(199609)18:1<1::AID-GLIA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Beyer M, Gimsa U, Eyupoglu IY, Hailer NP, Nitsch R. Phagocytosis of neuronal or glial debris by microglial cells: upregulation of MHC class II expression and multinuclear giant cell formation in vitro. Glia. 2000;31:262–266. doi: 10.1002/1098-1136(200009)31:3<262::aid-glia70>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Bilbo SD. Early-life infection is a vulnerability factor for aging related glial alterations and cognitive decline. Neurobiol Learn Mem. 2010;94:57–64. doi: 10.1016/j.nlm.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Biedenkapp JC, Der-Avakian A, Watkins LR, Rudy JW, Maier SF. Neonatal infection-induced memory impairment after lipopolysaccharide in adulthood is prevented via caspase-1 inhibition. J Neurosci. 2005;25:8000–8009. doi: 10.1523/JNEUROSCI.1748-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Rudy JW, Watkins LR, Maier SF. A behavioural characterization of neonatal infection-facilitated memory impairment in adult rats. Behav Brain Res. 2006;169:39–47. doi: 10.1016/j.bbr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Billiards SS, Haynes RL, Folkerth RD, Trachtenberg FL, Liu LG, Volpe JJ, et al. Development of microglia in the cerebral white matter of the human fetus and infant. J Comp Neurol. 2006;497:199–208. doi: 10.1002/cne.20991. [DOI] [PubMed] [Google Scholar]
- Bland ST, Beckley JT, Young S, Tsang V, Watkins LR, Maier SF, et al. Enduring consequences of early-life infection on glial and neural cell genesis within cognitive regions of the brain. Brain Behav Immun. 2010;22:451–455. doi: 10.1016/j.bbi.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Schultzberg M, Bartfai T, Gozes I. Cytokine regulation of neuronal survival. J Neurochem. 1992;58:454–460. doi: 10.1111/j.1471-4159.1992.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Brown AS, Patterson PH. Maternal infection and schizophrenia: implications for prevention. Schizophr Bull. 2011;37:284–290. doi: 10.1093/schbul/sbq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruccoleri A, Brown HW, Harry GJ. Cellular localization and temporal elevation of TNF, IL-1, and TGFβ1 mRNA in hippocampal injury response induced by trimethyltin. J Neurochem. 1998;71:1577–1587. doi: 10.1046/j.1471-4159.1998.71041577.x. [DOI] [PubMed] [Google Scholar]
- Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975:37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- Cajal RY. Contribucion al conocimiento de la meuroglia del cerebro humano. Trab Lab Invest Biol. 1913;11:254. [Google Scholar]
- Carson MJ, Reilly CR, Sutcliff JG, Lo D. Mature microglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Chamak B, Morandi V, Mallat M. Brain macrophages stimulate neurite growth and regeneration by secreting thrombospondin. J Neurosci Res. 1994;38:221–233. doi: 10.1002/jnr.490380213. [DOI] [PubMed] [Google Scholar]
- Chan WY, Kohsaka S, Razaie P. The origin and cell lineage of microglia – New concepts. Brain Res Rev, 2007;53:344–354. doi: 10.1016/j.brainresrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Chocyk A, Dudys D, Przyborowska A, Majcher I, Mackowiak M, Wedzony K. Maternal separation affects the number, proliferation and apoptosis of glia cells in the substantia nigra and ventral tegmental area of juvenile rats. Neurosci. 2011;173:1–18. doi: 10.1016/j.neuroscience.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Choi BH. Haematogenous cells in the central nervous system of developing human embryos and fetuses. J Comp Neurol. 1981;196:683–694. doi: 10.1002/cne.901960412. [DOI] [PubMed] [Google Scholar]
- Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci U S A. 2010;107:7975–7980. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Thalhammer A, Yu LM, Catalano M, Ramos T, Colicos MA, Goda Y. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 2008;58:749–762. doi: 10.1016/j.neuron.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM. Assessing activation states in microglia. CNS & Neurological Disorders Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Cuadros MA, Navascues J. The origin and differentiation of microglial cells during development. Progress in Neurobiology. 1998;56:173–189. doi: 10.1016/s0301-0082(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Dalmau I, Finsen B, Zimmer J, Gonzalez B, Castellano B. Development of microglia in the postnatal rat hippocampus. Hippocampus. 1998;8:458–474. doi: 10.1002/(SICI)1098-1063(1998)8:5<458::AID-HIPO6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10:263–276. doi: 10.1111/j.1474-9726.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Das DK. Molecular mechanisms of preconditioning. IUBMB Life. 2008;60:199–203. doi: 10.1002/iub.31. [DOI] [PubMed] [Google Scholar]
- Davoust N, Vuaillat C, Androdias G, Nataf S. From bone marrow to microglia: barriers and avenues. Trends Immunol. 2008;29:227–234. doi: 10.1016/j.it.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Davoust N, Vuaillat C, Cavillon G, Domenget C, Hatterer E, Bernard A, et al. Bone marrow CD34+/B220+ progenitors target the inflamed brain and display in vitro differentiation potential toward microglia. The FASEB Journal. 2006;20:2081–2092. doi: 10.1096/fj.05-5593com. [DOI] [PubMed] [Google Scholar]
- del Rio Hortega P. Microglia. In: Penfield W, editor. Cytology and cellular pathology of the nervous system. New York: Hoeber; 1932. pp. 481–584. [Google Scholar]
- Deng W, Pleasure J, Pleasure D. Progress in periventricular leukomalacia. Arch Neurol. 2008;65:1291–1295. doi: 10.1001/archneur.65.10.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Dick AD, Pell M, Brew BJ, Foulcher E, Sedgwick JD. Direct ex vivo flow cytometric analysis of human microglial cell CD14 expression: examination of central nervous system biopsy specimens from HIV-seropositive patients with other neurological diseases. AIDS. 1997;11:1699–1708. doi: 10.1097/00002030-199714000-00006. [DOI] [PubMed] [Google Scholar]
- Drouin-Ouellet J, Brownell AL, Saint-Pierre M, Fasano C, Emond V, Trudeau LE, et al. Neuroinflammation is associated with changes in glial mGluR5 expression and the development of neonatal excitotoxic lesions. Glia. 2011;59:188–199. doi: 10.1002/glia.21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman LM, Susser ES. The promise of epidemiologic studies: neuroimmune mechanisms in the etiologies of brain disorders. Neuron. 2009;64:25–27. doi: 10.1016/j.neuron.2009.09.024. [DOI] [PubMed] [Google Scholar]
- Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS ONE. 2011;6:e26317. doi: 10.1371/journal.pone.0026317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri MM, Al Izzi MS, Reading MC. Macrophages, microglia cells, and HLA-DR antigens in fetal and infant brain. J Clin Pathol. 1991;44:102–106. doi: 10.1136/jcp.44.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Bennet E, Soriano ET, del Rio T, Fonseca M. Naturally occurring cell death in the cerebral cortex of the rat and removal of dead cells by transitory phagocytes. Neuroscience. 1990;39:451–458. doi: 10.1016/0306-4522(90)90281-8. [DOI] [PubMed] [Google Scholar]
- Finch CE, Morgan TE, Rozovsky I, et al. Microglia and aging in the brain. In: Streit WJ, editor. Microglia in the regenerating and degenerating CNS. New York: Spriner Verlag; 2002. pp. 275–305. [Google Scholar]
- Fiske BK, Brunjes PC. Microglial activation in the developing rat olfactory bulb. Neuroscience. 2000;96:807–815. doi: 10.1016/s0306-4522(99)00601-6. [DOI] [PubMed] [Google Scholar]
- Flanary BE, Streit WJ. Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia. 2004;45:75–88. doi: 10.1002/glia.10301. [DOI] [PubMed] [Google Scholar]
- Flanary BE, Sammons NW, Nguyen C, Walker D, Streit WJ. Evidence that aging and amyloid promote microglial cell senescence. Rejuv Res. 2007;10:61–74. doi: 10.1089/rej.2006.9096. [DOI] [PubMed] [Google Scholar]
- Flavia R, Lima S, Gervais A, Colin C, Izembart M, Neto VM, et al. Regulation of Microglial Development: A Novel Role for Thyroid Hormone. J Neurosci. 2001;21:2028–2038. doi: 10.1523/JNEUROSCI.21-06-02028.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floden AM, Combs CK. Microglia demonstrate age-dependent interaction with amyloid-β fibrils. J Alzheimers Dis. 2011;25(2):279. doi: 10.3233/JAD-2011-101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flugel A, Bradl M, Kreutzberg GW, Graeber MB. Transformation of donor-derived bone marrow precursors into host microglia during autoimmune CNS inflammation and during the retrograde response to axotomy. J Neurosci Res. 2001;66:74–82. doi: 10.1002/jnr.1198. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Fujimoto E, Miki A, Mizoguti H. Histochemical study of the differentiation of microglial cells in the developing human cerebral hemispheres. J Anat. 1989;166:253–264. [PMC free article] [PubMed] [Google Scholar]
- Galic MA, Riazi K, Helda JG, Mouihate A, Fournier NM, Spencer SJ, et al. Postnatal inflammation increases seizure susceptibility in adult rats. J Neurosci. 2008;28:6904–6913. doi: 10.1523/JNEUROSCI.1901-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GC, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Gasque P, Neal JW, Singhrao SK, McGreal EP, Dean YD, Van BJ, et al. Roles of the complement system in human neurode- generative disorders: pro-inflammatory and tissue remodeling activities. Mol Neurobiol. 2002;25:1–17. doi: 10.1385/mn:25:1:001. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Young DG, Woodward J, Brown DC, Lachman LB. Interleukin-1 is an astroglial growth factor in the developing brain. J Neurosci. 1988;8:709–714. doi: 10.1523/JNEUROSCI.08-02-00709.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Li J, Bartel S, Broker J, Li X, Kirkpatrick JB. Cell surface morphology identifies microglia as a distinct class of mononuclear phagocyte. J Neurosci. 1995;15:7712–7726. doi: 10.1523/JNEUROSCI.15-11-07712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goepfert AR, Andrews WW, Carlo W, Ramsey PS, Cliver SP, Goldenberg RL, Hauth JC. Umbilical cord plasma interleukin-6 concentrations in preterm infants and risk of neonatal morbidity. Am J Obstet Gynecol. 2004;191:1375–1381. doi: 10.1016/j.ajog.2004.06.086. [DOI] [PubMed] [Google Scholar]
- Gomez-Gonzalez B, Escobar A. Prenatal stress alters microglial development and distribution in postnatal rat brain. Acta Neuropathol. 2010;119:303–315. doi: 10.1007/s00401-009-0590-4. [DOI] [PubMed] [Google Scholar]
- Gordon S, Lawson L, Rabinowitz S, Crocker PR, Morris L, Perry VH. Antigen markers of macrophage differentiation in murine tissues. Curr Topic Microbi Immunol. 1992;18:2–37. doi: 10.1007/978-3-642-77377-8_1. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Perivascular microglia defined. Trends Neurosci. 1990;13:366. doi: 10.1016/0166-2236(90)90020-B. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan P, Poole S, Bristow AF, Tilders F, Silverstein FS. Intracerebral NMDA injection stimulates production of interleukin-1 beta in perinatal rat brain. J Neurochem. 1996;67:2215–2218. doi: 10.1046/j.1471-4159.1996.67052215.x. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;11:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Harry GJ, Kraft AD. Neuroinflammation and microglia: considerations and approaches for neurotixicity assessment. Expert Opin Drug Metab Toxicol. 2008;4:1266–1277. doi: 10.1517/17425255.4.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harry GJ, Lawler C, Brunssen SH. Maternal infection and white matter toxicity. Neurotoxicology. 2006;27:658–670. doi: 10.1016/j.neuro.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Rabaza V, Navarro-Mora G, Velazquez-Sanchez C, Ferragud A, Marin MP, Garcia-Verdugo JM, et al. Neurotoxicity and persistent cognitive deficits induced by combined MDMA and alcohol exposure in adolescent rats. Addict Biol. 2010;15:413–423. doi: 10.1111/j.1369-1600.2010.00259.x. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hornig M, Weissenböck H, Horscroft N, Lipkin WI. An infection-based model of neurodevelopmental damage. Proc Natl Acad Sci USA. 1999;96:12102–12107. doi: 10.1073/pnas.96.21.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huleihel M, Golan H, Hallak M. Intrauterine infection/inflammation during pregnancy and offspring brain damages: possible mechanisms involved. Reprod Biol Endocrinol. 2004;2:17. doi: 10.1186/1477-7827-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PM, Botham MS, Frentzel S, Mir A, Perry VH. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia. 2002;37:314–327. [PubMed] [Google Scholar]
- Hume DA, Perry VH, Gordon S. Immunohistochemical localization of a macrophage-specific antigen in developing mouse retina: phagocytosis of dying neurons and differentiation of microglial cells to form a regular array in the plexiform layers. J Cell Biol. 1983;97:253–257. doi: 10.1083/jcb.97.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung J, Chansard M, Ousman SS, Nguyen MD, Colicos MA. Activation of microglia by neuronal activity: results from a new in vitro paradigm based on neuronal-silicon interfacing technology. Brain Behav Immun. 2010;24:31–40. doi: 10.1016/j.bbi.2009.06.150. [DOI] [PubMed] [Google Scholar]
- Hurley SD, Streit WJ. Griffonia simplicifolia II lectin labels a population of radial glial cells in the embryonic rat brain. Dev Neurosci. 1995;17:324–334. doi: 10.1159/000111302. [DOI] [PubMed] [Google Scholar]
- Hutchins KD, Dickson DW, Rashbaum WK, Lyman WD. Localization of morphologically distinct microglial populations in the developing human fetal brain: implications for ontogeny. Dev Brain Res. 1990;55:95–102. doi: 10.1016/0165-3806(90)90109-c. [DOI] [PubMed] [Google Scholar]
- Imamoto K, Leblond CP. Radioautographic investigation of gliogenesis in the corpus callosum of young rats. II. Origin of microglial cells. J Comp Neurol. 1978;180:139–164. doi: 10.1002/cne.901800109. [DOI] [PubMed] [Google Scholar]
- Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus. Brain Behav Immun. 2010;24:930–941. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonakait GM, Luskin MB, Wei R, Tian XF, Ni L. Conditioned medium from activated microglia promotes cholinergic differentiation in the basal forebrain in vitro. Dev Biol. 1996;177:85–95. doi: 10.1006/dbio.1996.0147. [DOI] [PubMed] [Google Scholar]
- Jonakait GM, Wen Y, Wan Y, Ni L. Macrophage cell-conditioned medium promotes cholinergic differentiation of undifferentiated progenitors and synergizes with nerve growth factor action in the developing basal forebrain. Exp Neurol. 2000;161:285–296. doi: 10.1006/exnr.1999.7255. [DOI] [PubMed] [Google Scholar]
- Kane CJ, Phelan KD, Han L, Smith RR, Xie J, Douglas JC, Drew PD. Protection of neurons and microglia against ethanol in a mouse model of fetal alcohol spectrum disorders by peroxisomes proliferator-activated receptor-γ agonists. Brain Behav Immun. 2011;25:S137–S145. doi: 10.1016/j.bbi.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C, Ling EA. A study of the transformation of amoeboid microglial cells into microglia labelled with the isolectin Griffonea simplicifolia in postnatal rats. Acta Anat. 1991;142:118–125. doi: 10.1159/000147175. [DOI] [PubMed] [Google Scholar]
- Kershman J. Genesis of microglia in the human brain. Arch Neurol Psych. 1939;41:24–50. [Google Scholar]
- Kettenmann H, Banati R, Walz W. Electrophysiological behavior of microglia. Glia. 1993;7:93–101. doi: 10.1002/glia.440070115. [DOI] [PubMed] [Google Scholar]
- Klunemann HH, Ridha BH, Magy L, Wherrett JR, Hemelsoet DM, Keen RW, et al. The genetic causes of basal ganglia calcification, dementia, and bone cysts: DAP12 and TREM2. Neurology. 2005;64:1502–1507. doi: 10.1212/01.WNL.0000160304.00003.CA. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Tarr AJ, Day CE, McLinden KA, Boehm GW. Influence of prenatal stress on behavioral, endocrine, and cytokine responses to adulthood bacterial endotoxin exposure. doi: 10.1016/j.bbr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Kraft AD, Harry GJ. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int J Environ Public Health. 2011;8:2980–3011. doi: 10.3390/ijerph8072980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AD, Kaltenbach LS, Lo DC, Harry GJ. Activated microglia proliferate at neurites of mutant huntingtin-expressing neurons. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.02.015. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostović I, Judas M. Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec. 2002;267:1–6. doi: 10.1002/ar.10069. [DOI] [PubMed] [Google Scholar]
- Kokovay E, Cunningham LA. Bone marrow-derived microglia contribute to the neuroinflammatory response and express iNOS in the MPTP mouse model of Parkinson’s disease. Neurobiol Dis. 2005;19:471–478. doi: 10.1016/j.nbd.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Langosch JM, Gebicke-Haerter PJ, Norenberg W, Illes P. Characterization and transduction mechanisms of purinoceptors in activated rat mciroglia. Brit J Pharmacol. 1994;113:4403–4411. doi: 10.1111/j.1476-5381.1994.tb16169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier LL, Bakker AB. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol Today. 2000;21:611–614. doi: 10.1016/s0167-5699(00)01745-x. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Zimprich F, Vass K, Hickey WF. Microglial cells are a component of the perivascular glia limitans. J Neurosci Res. 1991;28:236–243. doi: 10.1002/jnr.490280211. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neurosci. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neurosci. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Lima FR, Gervais A, Colin C, Izembart M, Neto VM, Mallat M. Regulation of microglial development: a novel role for thyroid hormone. J Neurosci. 2001;21:2028–2038. doi: 10.1523/JNEUROSCI.21-06-02028.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Huang CC, Chang KF. Lipoplysaccharide preconditioning reduces neuroinflammation against hypoxic ischemia and provides long-term outcome of neuroprotection in the neonatal rat. Pediatr Res. 2009;66:254–259. doi: 10.1203/PDR.0b013e3181b0d336. [DOI] [PubMed] [Google Scholar]
- Ling EA, Tan CK. Amoeboid microglial cells in the corpus callosum of neonatal rats. Arch Histol Jpn. 1974;36:265–280. doi: 10.1679/aohc1950.36.265. [DOI] [PubMed] [Google Scholar]
- Ling EA, Wong WC. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- Ling EA, Penney D, Leblond DP. Use of carbon labeling to demonstrate the role of blood monocytes as precursors of the “amoeboid cells” present in the corpus callosum of postnatal rats. J Comp Neurol. 1980;193:631–657. doi: 10.1002/cne.901930304. [DOI] [PubMed] [Google Scholar]
- Luo XG, Ding JZ, Chen SD. Microglia in the aging brain: relevance to neurodegeneration. Mol Neurodegener. 2010;5:12. doi: 10.1186/1750-1326-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol. 2005;15:101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Maier WE, Bartenbach MJ, Brown HA, Tilson HA, Harry GJ. Induction of tumor necrosis factor alpha in cultured glial cells by trimethyltin. Neurochem Int’l. 1997;30:385–392. doi: 10.1016/s0197-0186(96)00073-3. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Embryonic vascularization of the mammalian cerebral cortex: Golgi and electron microscopic studies. J Comp Neurol. 1985;241:237–249. doi: 10.1002/cne.902410210. [DOI] [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Masuda T, Croom D, Hida H, Kirov SA. Capillary blood flow around microglial somata determines dynamics of microglial processes in ischemic conditions. Glia. 2011;59:1744–1753. doi: 10.1002/glia.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Fujiwara M. Absence of donor-type major histocompatibility complex class I antigen bearing microglia in the rat central nervous system of radiation bone marrow chimeras. J Neuroimmunol. 1987;17:71–82. doi: 10.1016/0165-5728(87)90032-4. [DOI] [PubMed] [Google Scholar]
- Mattiace LA, Davies P, Dickson DW. Detection of HLA-DR on microglia in the human brain is a function of both clinical and technical factors. Am J Pathol. 1990;136:1101–1114. [PMC free article] [PubMed] [Google Scholar]
- McCarthy GF, Leblond CP. Radioautographic evidence for slow astrocyte turnover and modest oligodendrocyte production in the corpus callosum of adult mice infused with 3H-thymidine. J Comp Neurol. 1988;271:589–603. doi: 10.1002/cne.902710409. [DOI] [PubMed] [Google Scholar]
- McClain JA, Morris SA, Deeny MA, Marshall SA, Hayes DM, Kiser ZM, Nixon K. Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain Behav Immun. 2011;25:S120–S128. doi: 10.1016/j.bbi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of parkinson’s disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21:195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- McPherson CA, Kraft AD, Harry GJ. Injury-induced neurogenesis: consideration of resident microglia as supportive of neural progenitor cells. Neurotox Res. 2011b;19:341–352. doi: 10.1007/s12640-010-9199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metchnikoff E. Lecons sur la pathologie comparee de l’inflammation. Paris: Masson; 1892. [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009;33:1061–1079. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Michaelson MD, Bieri PL, Mehler MF, Xu H, Arezzo JC, Pollard JW. CSF-1 deficiency in mice results in abnormal brain development. Development. 1996;122:2661–2672. doi: 10.1242/dev.122.9.2661. [DOI] [PubMed] [Google Scholar]
- Mittelbronn M, Dietz K, Schluesener JH, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001;101:249–255. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Doi Y, Zizoguchi H, Jin S, Noda M, Sonobe Y, et al. Interleukin-34 selectively enhances the neuroprotective effects of microglia to attenuate oligomeric amyloid-β neurotoxicity. Amer J Pathol. 2011;179:2016–2027. doi: 10.1016/j.ajpath.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouihate A, Galic MA, Ellis SL, Spencer SJ, Tsutsui S, Pittman QJ. Early life activation of toll-like receptor 4 reprograms neural anti-inflammatory pathways. J Neurosci. 2010;30:7975–7983. doi: 10.1523/JNEUROSCI.6078-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier A, Evrard P, Gressens P, Verney C. Distribution and differentiation of microglia in the human encephalon during the first two trimesters of gestation. J Comp Neurol. 2006;499:565–582. doi: 10.1002/cne.21123. [DOI] [PubMed] [Google Scholar]
- Moreno JA, Streifel KM, Sullivan KA, Legare ME, Tjalkens RB. Developmental exposure to manganese increases adult susceptibility to inflammatory activation of glia and neuronal protein nitration. Toxicol Sci. 2009;112:405–415. doi: 10.1093/toxsci/kfp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Streifel KM, Sullivan KA, Hanneman WH, Tjalkens RB. Manganese-induced NF-kB activation and nitrosative stress is decreased by estrogen in juvenile mice. Toxicol Sci. 2011;122:121–133. doi: 10.1093/toxsci/kfr091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris L, Graham CF, Gordon S. Macrophages in haemopoietic and other tissues of the developing mouse detected by the monoclonal antibody F4/80. Development. 1991;112:517–526. doi: 10.1242/dev.112.2.517. [DOI] [PubMed] [Google Scholar]
- Mott RT, Ait-Ghezala G, Town T, Mori T, Vendrame M, Zeng J, et al. Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia. 2004;46:369–379. doi: 10.1002/glia.20009. [DOI] [PubMed] [Google Scholar]
- Munoz-Fernandez MA, Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Prog Neurobiol. 1998;56:307–340. doi: 10.1016/s0301-0082(98)00045-8. [DOI] [PubMed] [Google Scholar]
- Mouihate A, Galic MA, Ellis SL, Spencer SJ, Tsutsui S, Pittman QJ. Early life activation of toll-like receptor 4 reprograms neural anti-inflammatory pathways. J Neurosci. 2010;30:7975–7983. doi: 10.1523/JNEUROSCI.6078-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, et al. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429:167–172. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- Nissl F. Ueber einige Beziehungen zwishcen Nerven zellerkrankungen und glioesen Erscheinungen bei verschiedenen Psychosen. Arch Psychiat. 1899;32:1–21. [Google Scholar]
- Pang Y, Fan L-W, Zheng B, Cai Z, Rhodes PG. Role of interleukin-6 in lipopolysaccharide-induced brain injury and behavioral dysfunction in neonatal rats. Neurosci. 2006;141:745–755. doi: 10.1016/j.neuroscience.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Perry VH, Gordon S. Modulation of CD4 antigen on macrophages and microglia in rat brain. J Exp Med. 1987;166:1138–1143. doi: 10.1084/jem.166.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, O'Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro. 2010;2 doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Hume DA, Gordon S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience. 1985;15:313–326. doi: 10.1016/0306-4522(85)90215-5. [DOI] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Sheng WS, Anderson WR, Ulevitch RJ, et al. CD14 receptor-mediated uptake of monopsonised mycobacterium tuberculosis by human microglia. Infect Immun. 1995;63:1598–1602. doi: 10.1128/iai.63.4.1598-1602.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock JM, Kettenmann H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007;30:527–535. doi: 10.1016/j.tins.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Poggi SH, Park J, Toso L, Abebe D, Roberson R, Woodard JE, et al. No phenotype associated with established lipopolysaccharide model for cerebral palsy. J Obstet Gynecol. 2001;192:727–733. doi: 10.1016/j.ajog.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Pont-Lezica L, Bechade C, Belarif-Cantaut Y, Pascual O, Bessis A. Physiological roles of microglia during development. J Neurochem. 2011;119:901–908. doi: 10.1111/j.1471-4159.2011.07504.x. [DOI] [PubMed] [Google Scholar]
- Prada I, Ongania GN, Buonsanti C, Panina-Bordignon P, Meldolesi J. Triggering receptor expressed in myeloid cells 2 (TREM2) trafficking in microglial cells: continuous shuttling to and from the plasma membrane regulated by cell stimulation. Neuroscience. 2006;140:1139–1148. doi: 10.1016/j.neuroscience.2006.03.058. [DOI] [PubMed] [Google Scholar]
- Priller J, Haas CA, Reddington M, Kreutzberg GW. Calcitonin gene-related peptide and ATP induce immediate early gene expression in cultured rat microglia cells. Glia. 1995;15:447–457. doi: 10.1002/glia.440150408. [DOI] [PubMed] [Google Scholar]
- Rakic S, Zecevic N. Programmed cell death in the developing human telencephalon. Eur J Neurosci. 2000;12:2721–2734. doi: 10.1046/j.1460-9568.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Raivich G. Like cops on the beat, the active role of resting microglia. Trends Neurosci. 2005;28:571–573. doi: 10.1016/j.tins.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Male D. Colonisation of the developing human brain and spinal cord by microglia: a review. Microsc Res Tech. 1999;45:359–382. doi: 10.1002/(SICI)1097-0029(19990615)45:6<359::AID-JEMT4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Male D. Microglia in fetal and adult human brain can be distinguished from other mononuclear phagocytes through their lack of CD163 expression. Neuroembryology. 2003;2:130–133. [Google Scholar]
- Rezaie P, Cairns NJ, Male DK. Expression of adhesion molecules on human fetal cerebral vessels: relationship to microglial colonization during development. Brain Res Dev Brain Res. 1997;104:175–189. doi: 10.1016/s0165-3806(97)00153-3. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Dean A, Male D, Ulfig N. Microglia in the cerebral wall of the human telencephalon at second trimester. Cerebral Cortex. 2005;15:938–949. doi: 10.1093/cercor/bhh194. [DOI] [PubMed] [Google Scholar]
- Richartz E, Stransky E, Batra A, Simon P, Lewczuk P, Buchkremer G, et al. Decline of immune responsiveness: a pathogenetic factor in Alzheimer's disease? J Psychiatr Res. 2005;39:535–543. doi: 10.1016/j.jpsychires.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Rodriquez M, Alvarez-Erviti L, Blesa FJ, Rodriquez-Oroz MC, Arina A, Melero I, et al. Bone-marrow-derived cell differentiation into microglia: a study in a progressive mouse model of Parkinson’s disease. Neurobiol Dis. 2007;28:316–325. doi: 10.1016/j.nbd.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9:1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- Rymo SF, Gerhardt H, Wolfhagen Sand F, Lang R, Uv A, Betsholtz C. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS ONE. 2011;6:e15846. doi: 10.1371/journal.pone.0015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JK, Davalos D, Akassoglou K. Fibrinogen signal transduction in the nervous system. J Thromb Haemost. 2009;7:S151–S154. doi: 10.1111/j.1538-7836.2009.03438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba E, Henrot A. Inflammatory mediators and neonatal brain damage. Biol Neonate. 2001;79:224–227. doi: 10.1159/000047096. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1345–R1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Stevens B. Synapse elimination during development and disease: immune molecules take centre stage. Biochem Soc Trans. 2010;38:476–481. doi: 10.1042/BST0380476. [DOI] [PubMed] [Google Scholar]
- Schlegelmilch T, Henke K, Peri F. Microglia in the developing brain: from immunity to behavior. Curr Opin Neurobiol. 2011;21:5–10. doi: 10.1016/j.conb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Schmid CD, Melchior B, Masek K, Puntambekar SS, Danielson PE, Lo DD. Differential gene expression in LPS/IFNgamma activated microglia and macrophages: in vitro versus in vivo. J Neurochem. 2009;109:117–125. doi: 10.1111/j.1471-4159.2009.05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Hutchinson MR, Bilbo SD. Early-life experience decreases drug-induced reinstatement ofmorphing CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. J Neurosci. 2011;31:17835–17847. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears HC, Kennedy CJ, Garrity PA. Macrophage-mediated corpse engulfment is required for normal Drosophila CNS morphogenesis. Development. 2003;130:3557–3565. doi: 10.1242/dev.00586. [DOI] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19:47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Silverstein FS, Barks JDE, Hagan P, Liu X-H, Ivacko J, Szaflarski J. Cytokines and perinatal brain injury. Neurochem Inter. 1997;30:375–383. doi: 10.1016/s0197-0186(96)00072-1. [DOI] [PubMed] [Google Scholar]
- Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Soltys Z, Orzylowska-Sliwinska O, Zaremba M, Orlowski D, Piechota M, Fiedorowicz A, et al. Quantitative morphological study of microglial cells in the ischemic rat brain using principal component analysis. J Neurosci Methods. 2005;146:50–60. doi: 10.1016/j.jneumeth.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Miller KR, Lopes KO, Njie E. Microglial degeneration in the aging brain – bad news for neurons? Front Biosci. 2008;13:3423–3438. doi: 10.2741/2937. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kunhs AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Graeber MB, Kreutzberg GW. Expression of Ia antigens on perivascular and microglial cells after sublethal and lethal neuronal injury. Exp Neurol. 1989;105:115–126. doi: 10.1016/0014-4886(89)90111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Fujita M, Kitani H, Hashimoto M. Cold stress induced morphological microglial activation and increased IL-1b expression in astroglial cells in rat brain. J Neuroimmunol. 2011;233:29–36. doi: 10.1016/j.jneuroim.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4 doi: 10.1371/journal.pmed.0040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrash JC, Torbett BE, Carson MJ. Developmental regulation of TRAM2 and DAP12 expression in the mouse CNS: implications for Nasu-hakola disease. Neurochem Res. 2008;34:38–45. doi: 10.1007/s11064-008-9657-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tin-Tin Win-Shwe, Kunugita N, Yoshida Y, Nakajima D, Tsukahara S, Fujimaki H. Differential mRNA expression of neuroimmune markers in the hippocampus of infant mice following toluene exposure during brain developmental period. J Appl Toxicol. 2011 doi: 10.1002/jat.1643. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Wujek JR, Criste GA, Jalabi W, Yin X, Kidd GJ, Stohlman S, Ransohoff R. Evidence for synaptic stripping by cortical microglia. Glia. 2007;55:360–368. doi: 10.1002/glia.20462. [DOI] [PubMed] [Google Scholar]
- Tremblay MÈ, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Furth R, Cohn ZA, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull WHO. 1972;46:845–852. [PMC free article] [PubMed] [Google Scholar]
- Van Rossum D, Hanisch UK. Microglia. Metab Brain Dis. 2004;19:393–411. doi: 10.1023/b:mebr.0000043984.73063.d8. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W, Ilschner S, Ohlemeyer C, Banati R, Kettenmann H. Extracellular ATP activates a cation conductance and a K+ conductance in cultured microglial cells from mouse brain. J Neurosci. 1993;13:4403–4411. doi: 10.1523/JNEUROSCI.13-10-04403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Wu CH, Shieh JH, Wen CY. Microglial distribution and apoptosis in fetal rat brain. Dev Brain Res. 2002;139:337–342. doi: 10.1016/s0165-3806(02)00584-9. [DOI] [PubMed] [Google Scholar]
- Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, de Witte P, et al. Neuroinflammation induced in the hippocampus of ‘binge drinking’ rats may be mediated by elevated extracellular glutamate content. J Neurochem. 2009;111:1119–1128. doi: 10.1111/j.1471-4159.2009.06389.x. [DOI] [PubMed] [Google Scholar]
- Wei L, Simen A, Mane S, Kaffman A. Early life stress inhibits expression of a novel innate immune pathway in the developing hippocampus. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.239. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigent DA, Blalock JE. Production of peptide hormones and neurotransmitters by the immune system. Chem Immunol. 1997;69:1–30. doi: 10.1159/000058652. [DOI] [PubMed] [Google Scholar]
- Williams K, Ulvestad E, Antel J. Immune regulatory and effector propereties of human adult microglia studies in vitro and in situ. Adv Neuroimmunol. 1994;4:273–281. doi: 10.1016/s0960-5428(06)80267-6. [DOI] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Ziokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Wen CY, Shieh JY, Ling EA. Down-regulation of membrane glycoprotein in amoeboid microglia transforming into ramified microglia in postnatal rat brain. J Neurocytol. 1994;23:258–269. doi: 10.1007/BF01275530. [DOI] [PubMed] [Google Scholar]
- Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci USA. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]