Abstract
This study examined the time course of object naming in 21 individuals with primary progressive aphasia (PPA) (8 agrammatic (PPA-G); 13 logopenic (PPA-G)) and healthy age-matched speakers (n=17) using a semantic interference paradigm with related and unrelated interfering stimuli presented at stimulus onset asynchronies (SOAs) of −1000, −500, −100 and 0 ms. Results showed semantic interference (SI) (i.e. significantly slower RTs in related compared to unrelated conditions) for all groups at −500, −100 and 0 ms, indicating timely spreading activation to semantic competitors. However, both PPA groups showed a greater magnitude of SI than normal across SOAs. The PPA-L group and six PPA-G participants also evinced SI at −1000 ms, suggesting an abnormal time course of semantic interference resolution, and concomitant left hemisphere cortical atrophy in brain regions associated with semantic processing. These subtle semantic mapping impairments in non-semantic variants of PPA may contribute to the anomia of these patients.
Keywords: primary progressive aphasia, semantic interference, word interference paradigms, naming deficits in primary progressive aphasia, Free Surfer, cortical thickness
Introduction
Primary progressive aphasia (PPA) is a clinical dementia syndrome cause by neurodegenerative disease, which affects the language network and language functions while preserving attention, memory and other aspects of cognitive processing during the initial stages (Mesulam, 2003). One of the most prominent deficits seen in PPA is anomia manifested by word finding and object naming difficulty (Mesulam, 1982). The most severe anomia is seen in the semantic subtype of PPA (PPA-S), where word comprehension is also severely impaired (Adam, Patterson, Rogers, Nestor, Salmon, Acosta-Cabronero, et al., 2006; Gorno-Tempini, Dronkers, Rankin, Ogar, Phengrasamil, Rosen, et al., 2004; Mesulam, Rogalski, Wieneke, Cobia, Rademaker, Thompson, & Weintraub, 2009a; Mesulam, Wieneke, Rogalski, Cobia, Thompson, & Weintraub, 2009b; Neary, Snowden, Gustafson, Passant, Stuss, Black, et al., 1998). However, patients with agrammatic (PPA-G) and logopenic (PPA-L) variants of PPA, who have relatively spared word comprehension, also have naming impairments thought to stem from faulty word retrieval (e.g., phonological and/or articulatory deficits) rather than faulty semantic processes (Mesulam et al., 2009a, Mesulam et al., 2009b).
According to models of spoken word production, to name an object it is necessary to recognize the object, access the meaning or semantic representation of the object, selectively link the two representations, and activate the phonological form of the associated lexical item. These interrelated processes must unfold rapidly and in a manner that protects them from disruptive interference (Levelt 1992; Levelt, Roelofs, & Meyer, 1999; Rapp & Goldbrick, 2000; Roelofs 1992; also see Mesulam et al., 2009b). One method for investigating these aspects of naming is the picture-word interference paradigm, an adaptation of the Stroop task (Stroop 1935), which involves presentation of pictures to name coupled with lexical probes or interfering stimuli (IS, hereafter) presented with variable time relationships to the object. Normally, when the IS is semantically (categorically, but not associatively) related to the target picture, a semantic interference effect occurs. That is, picture naming is slowed compared to when the IS is unrelated to the target item. For example, naming a picture of a fox is slower in the presence of the IS goat compared to globe. This slowing occurs because the lexical-semantic network associated with the IS is automatically activated when it is encountered; in turn, the target picture activates its network, which includes the IS. Thus, competition occurs and the need to deactivate the IS is required (La Heif 1988; Schriefers, Meyer & Levelt, 1990; Starreveld & La Heij, 1995, 1996). Whether the source of the semantic interference effect is associated with semantic and/or lexical competition is unclear. Some suggest that both the IS and target are automatically lexicalized and, hence, available for production. The phonological form of the latter must, therefore, be inhibited in order for the target word to be produced (Bloem & La Heij; 2003; Bloem, van den Bogart, & La Heij, 2004; Finkbeiner & Caramazza, 2006; Janssen, Schrim, Mahon, & Caramazza, 2008; Mahon, Costa, Peterson, Vargas, & Caramazza, 2007). In either case, competition must be resolved before naming of the target item can be accomplished.
A parameter of importance for this task is the temporal relation between presentation of the probe and the picture to be named, that is, stimulus-onset asynchrony (SOA). At some SOAs semantically related IS hinder naming through the process of competitive interference as described above, whereas, at others the probe could conceivably facilitate naming by bringing the correct word closer to a retrieval threshold. Semantic interference effects are the most common and have been shown in healthy speakers when IS are presented at SOAs ranging from −300 ms to +100 ms (i.e., up to 300 ms prior to picture presentation or 100 ms following it) (Glaser & Düngelhoff, 1984; La Heij, Dirkx, & Kramer, 1990; Lupker, 1979; Rayner & Springer, 1986; Starreveld & LaHeij, 1995, 1996; Underwood, 1976).
Semantic interference is a normally occurring phenomenon. However, its magnitude and duration can be influenced by brain damage. In fact, the SI paradigm can be used to test the robustness of information processing routines that underlie object naming. Only a few studies have used the picture-word interference paradigm for this purpose in neurologically impaired patients with naming deficits. In a study by Hashimoto and Thompson (2010), involving 11 individuals with mild aphasia resulting from stroke, aphasic participants showed abnormally heightened semantic interference effects at SOAs of −300 and 0 ms. But at SOA = +300, semantic interference effects disappeared as in normal age-matched speakers. In another study examining semantic interference with one aphasic individual, Wilshire, Keall, Stuart, and O’Donnell (2007) also found abnormal effects, albeit patterns that differed from those seen by Hashimoto and Thompson (2010). That is, the semantic interference effect was absent at SOA = 0 ms, but trends toward semantic interference were found at SOAs of +200 and +400 ms. Interestingly, none of the patients in Hashimoto and Thompson (2010) or in Wilshire et al. (2007) showed strong evidence pointing to semantic deficits in off-line testing.
Abnormal patterns of semantic interference also were seen in a group of individuals with primary progressive aphasia (PPA) studied by Vandenberghe, Vandenbulcke, Weintraub, Johnson, Porke, Thompson, and Mesulam (2005). Like the stroke-induced aphasic speakers studied by Hashimoto and Thompson (2010) and Wilshire et al. (2007), abnormal semantic interference effects were found for the PPA speakers even though no word comprehension deficits were noted on behavioral testing. In contrast to unimpaired participants, who showed semantic facilitation (i.e., faster naming for related compared to unrelated words) at −750 ms SOA, the PPA group showed semantic interference in this condition. This pattern suggested that individuals with PPA successfully activate semantic representations in response to IS, however, deactivation is slowed compared to normal, leaving the competitor network active when the target is to be named. Alternatively, this pattern could suggest a delay in the time course of lexical activation, that is, later than normal rise time. In either case, the subsequent iterative process of linking the target object to its name was abnormally vulnerable to the interference of competing representations.
Priming studies provide some insights into how lexical-semantic activation proceeds in aphasic speakers. Individuals with Broca’s aphasia resulting from stroke, typically involving anterior brain tissue, have shown reduced levels of activation and/or slow rise time; whereas, those with Wernicke’s aphasia, associated with posterior brain lesions, show greater activation than normal as well as delayed deactivation (Blumstein, & Milberg, 2000; Janse, 2006; Prather, Zurif, Love, & Brownell, 1997; Swinney, Zurif, & Nicol, 1989; Yee, Blulmstein, & Sedivy, 2008). Both priming and word-interference paradigms operate on the premise that the functional architecture of the lexical processing system involves spreading activation across linguistic units or nodes (Dell, 1986; Masson, 1995), which results in competition among potential candidates. Hence, activation of primes in priming paradigms and that for the IS presented in word-interference paradigms involve similar processes; however, the word-interference paradigm requires an addition step – naming of a related or unrelated competitor – following this activation.
The present experiment was designed to examine the effects of semantic interference on naming in individuals with PPA-G and PPA-Land to examine patterns of cortical atrophy in participants with abnormal semantic interference effects. We enrolled PPA patients and age-matched healthy controls in a word interference paradigm with SOAs of −1000, −500, −100, and 0 ms. Based on similarities between cerebrovascular lesion sites and the location of peak atrophy involving the inferior frontal gyrus, we hypothesized that reduced or delayed activation may be seen for the PPA-G group, akin to the performance of individuals with stroke-induced Broca’s aphasia. In the case of reduced activation, semantic interference effects would be either absent or weak across all SOAs, whereas a pattern of delayed activation would result in semantic interference effects only in SOA = −500 and/or −1000 ms conditions. Conversely, we conjectured that PPA-L participants, who often show involvement of posterior brain structures (Gorno-Tempini et al., 2004; Gorno-Tempini, Brambati, Ginex, Ogar, Dronkers, Marcone et al., 2008; Mesulam et al., 2009b), may show patterns of excessive IS-induced activation, or delayed deactivation, similar to those observed in patients with stroke-induced Wernicke’s aphasia. In the former case, the magnitude of semantic interference would exceed that of normal control participants across conditions, whereas in the latter, semantic interference effects would be present in longer SOA conditions, i.e., at −1000 ms. We also entertained the possibility that both patient groups would show normal patterns of semantic interference if the source of their naming deficit stemmed strictly from post-semantic phonological and/or articulatory impairments.
Method
Participants
Participants included 21 individuals with PPA (8 PPA-G and 13 PPA-L) and 17 cognitively intact volunteers. All were recruited through the Cognitive Neurology and Alzheimer’s Disease Center (CNADC) at Northwestern University (Chicago, IL) and tested in the Aphasia and Neurolinguistics Research Laboratory at Northwestern (Evanston, IL). The three participant groups were matched for age (PPA-G: M = 62 years, sd = 6.13; PPA-L: M = 64 years, sd = 7.29; control group: M = 63 years; sd = 6.4) (χ2(2, N = 38) = .626; p = .731, Kruskal-Wallis Test) and education (PPA-G: M = 17 years, range = 14–20; PPA-L: M = 16 years, range = 12–20; controls: M = 16 years, range = 11–20) (χ2(2, N = 38) = .353; p = .838, Kruskal-Wallis Test). All were monolingual English-speaking, passed a pure-tone audiometric hearing screening, and were right handed, with the exception of two participants with PPA who were left handed (PPA-G1 and PPA-L8). All also presented a negative history of prior neurological or psychiatric deficits. Compensation for participation in the study was provided and informed consent was obtained prior to participation. The Institutional Review Board at Northwestern University approved this study.
The diagnosis of PPA was based on neurological, neuropsychological and neurolinguistic testing, showing an absence of neurological signs other than progressive language deficits. Symptom onsets ranged from 2 – 10 years prior to testing, however, the two PPA groups were matched for symptom duration (PPA-G: M = 4 years, range = 2–5; PPA-L: M = 4 years, range = 2–10) (Z = −.876, p = .381, Mann-Whitney U Test) (see Table 1).
Table 1.
Summary of PPA participant demographic data.
| Participant | Age | Gender | Education | Handedness | Symptom Duration (years) |
|---|---|---|---|---|---|
| PPA-G 1 | 60 | M | 15 | L | 4.5 |
| PPA-G 2 | 62 | M | 20 | R | 5 |
| PPA-G 3 | 59 | M | 12 | R | 3.1 |
| PPA-G 4 | 52 | F | 18 | R | 1.5 |
| PPA-G 5 | 61 | F | 18 | R | 5 |
| PPA-G 6 | 72 | M | 20 | R | 5 |
| PPA-G 7 | 62 | F | 16 | R | 3 |
| PPA-G 8 | 69 | F | 14 | R | 3.5 |
| PPA-L 1 | 69 | M | 15 | R | 2.5 |
| PPA-L 2 | 66 | M | 19 | R | 9.5 |
| PPA-L 3 | 63 | M | 18 | R | 2.5 |
| PPA-L 4 | 64 | F | 16 | R | 2.8 |
| PPA-L 5 | 59 | M | 14 | R | 7 |
| PPA-L 6 | 58 | M | 16 | R | 2 |
| PPA-L 7 | 65 | F | 13 | R | 5 |
| PPA-L 8 | 66 | M | 12 | L | 2.5 |
| PPA-L 9 | 75 | F | 16 | R | 2.5 |
| PPA-L 10 | 60 | M | 18 | R | 2 |
| PPA-L 11 | 48 | M | 16 | R | 6 |
| PPA-L 12 | 62 | M | 20 | R | 3 |
| PPA-L 13 | 76 | F | 16 | R | 2 |
| Mean (SD) | |||||
| PPA-G | 62 (6) | 17 (3) | 3.8 (1.3) | ||
| PPA-L | 64 (7) | 16 (2) | 3.8 (2.4) | ||
| Control | 63 (6) | 16 (3) | N/A | ||
Note: A = Agrammatic; L = Logopenic
To rule out memory, attention, and executive function deficits the Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975), Wechsler Memory Scales-III (Faces I and II) (Wechsler, 1997), Facial Recognition Test (Benton, Hamsher, Varney, & Spreen, 1998), Trail Making Test (Partington & Leiter, 1949), and a 10-item version of the Visual Verbal Test (Feldman & Drasgow, 1959; also see Wickland, Johnson, & Weintraub, 2004) were administered (see Table 2 for participant scores). Both PPA groups generally performed within normal limits on these measures, with no significant differences found between them and the control groups based on the Kruskal-Wallis Test, with the exception of the MMSE (χ2(2, N = 38) = 18.160; p = .05). On this test, both PPA groups performed more poorly than the controls, likely due to the patients’ compromised language ability (Golper, Rau, Erskine, Langhans, & Houlihan, 1987; Osher, Wicklund, Rademaker, Johnson, & Weintraub, 2008). A motor speech examination also was administered; only four participants (PPA-G6, PPA-G8; PPA-L7, PPA-L11) presented with mild impairments, which did not affect speech intelligibility or ability to perform the experimental task.
Table 2.
Summary of neuropsychological test scores for agrammatic (PPA-G 1 – PPAG 8) and logopenic (PPA-L 1 – PPA-L 13) participants across measures.
| MMSE | WMS-III | Visual Perception Facial |
Processing Speed |
Visual Verbal Shift |
Visual Verbal Sort |
||
|---|---|---|---|---|---|---|---|
| Participant | (30) | Immediate (48) |
Delayed (48) |
Recognition (54) |
Trail Test A |
(10) | (20) |
| PPA-G 1 | 28 | N/A | N/A | 48 | 41 | 10 | 20 |
| PPA-G 2 | 28 | 39 | 39 | 48 | 44 | 10 | 20 |
| PPA-G 3 | 20 | 28 | 31 | 51 | 108 | 8 | 18 |
| PPA-G 4 | 30 | 37 | 39 | 45 | 25 | 8 | 18 |
| PPA-G 5 | 30 | 31 | 38 | 42 | 64 | 6 | 16 |
| PPA-G 6 | 28 | 35 | 41 | 52 | 34 | 10 | 20 |
| PPA-G 7 | 26 | 34 | 36 | 53 | 28 | 6 | 16 |
| PPA-G 8 | 28 | 41 | 36 | 52 | 55 | 7 | 17 |
| PPA-L 1 | 30 | N/A | N/A | 45 | 37 | 9 | 18 |
| PPA-L 2 | 24 | N/A | N/A | N/A | 48 | N/A | N/A |
| PPA-L 3 | 27 | N/A | N/A | 48 | 29 | 8 | 18 |
| PPA-L 4 | 26 | N/A | N/A | 46 | 28 | 8 | 18 |
| PPA-L 5 | 24 | 31 | 48 | 39 | 87 | 8 | 18 |
| PPA-L 6 | 23 | 42 | 44 | 44 | 49 | 9 | 19 |
| PPA-L 7 | 19 | 26 | 29 | 41 | N/A | N/A | N/A |
| PPA-L 8 | 29 | 38 | 39 | 47 | 36 | 9 | 19 |
| PPA-L 9 | 28 | 30 | 37 | 49 | 17 | N/A | N/A |
| PPA-L 10 | 28 | 37 | 40 | 50 | 35 | 9 | 19 |
| PPA-L 11 | 29 | 39 | 39 | 50 | 33 | 7 | 17 |
| PPA-L 12 | 29 | 38 | 37 | 48 | 31 | 10 | 20 |
| PPA-L 13 | 24 | 33 | 37 | 47 | 25 | 10 | 20 |
| Mean (SD) | |||||||
| PPA-G | 27.3 (3.2) | 35.0 (4.5) | 37.1 (3.2) | 48.9 (3.9) | 49.9 (26.9) | 8.4 (1.7) | 18.4 (1.7) |
| PPA-L | 26.2 (3.2) | 34.9 (5.2) | 38.9 (5.2) | 46.2 (3.4) | 37.9 (17.8) | 8.7 (0.9) | 18.6 (1.0) |
| Control | 29.7* (0.6) | 37.25 (3.8) | 39.1 (3.0) | 46.9 (3.9) | 30.4 (8.9) | 9.0 (0.8) | 18.9 (1.0) |
To detail the language impairment of the PPA participants, a battery of neurolinguistic tests was administered. See Tables 3 and 4 for a summary of results. Aphasia Quotients (AQs) derived from administration of the Western Aphasia Battery (WAB, Kertesz, 2006) ranged from 73.9 to 85 (M = 79.5) for the PPA-G participants, which was significantly lower than those for the PPA-L participants, ranging from 78.6 to 97.1 (M = 90.2) (Z = −3.259, p< .01, Mann-Whitney U Test). WAB fluency scores also differed between groups: fluency scores for the PPA-G group ranged from 4 to 6 (M = 5.1)) and for the PPA-L group from 5 to 10 (M = 8.2) (Z = −3.232, p< .01, Mann-Whitney U Test).
Table 3.
Summary of language test scores for agrammatic (PPA-G 1 – PPAG 8) and logopenic (PPA-L 1 – PPA-L 13) participants.
| Western Aphasia Battery | BNT | NNB | PPVT | PPT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant | AQ (100) |
F (10) |
Comp (10) |
Rep (10) |
Nam (10) |
(%) | Noun (%) |
N:V | (%) | Words (%) |
Pictures (%) |
| PPA-G 1 | 80.4 | 6 | 9.6 | 7.2 | 8.4 | 98.3 | 100.0 | 1.1 | 97.2 | N/A | 100.0 |
| PPA-G 2 | 82.3 | 4 | 9.1 | 8 | 10 | 98.3 | 93.3 | 1.5 | 100.0 | 100.0 | 100.0 |
| PPA-G 3 | 79.9 | 4 | 8.85 | 7.8 | 9.3 | 81.7 | 95.0 | 1.1 | 94.4 | 90.4 | 94.2 |
| PPA-G 4 | 78.8 | 5 | 8.5 | 7.6 | 9.3 | 76.7 | 93.3 | 1.4 | 97.2 | 98.1 | 98.1 |
| PPA-G 5 | 75.3 | 4 | 8.25 | 8.8 | 8.6 | 88.3 | 98.3 | 1.0 | 100.0 | 88.5 | 96.2 |
| PPA-G 6 | 80.6 | 6 | 9 | 5.9 | 9.4 | 73.3 | 85.0 | 1.5 | 88.9 | 98.1 | 96.2 |
| PPA-G 7 | 73.9 | 6 | 7.85 | 5 | 9.1 | 45.0 | 86.0 | 1.2 | 94.4 | 94.2 | 94.2 |
| PPA-G 8 | 85 | 6 | 10 | 8.6 | 8.9 | 93.3 | 100.0 | 1.1 | 88.9 | 88.5 | 94.2 |
| PPA-L 1 | 92 | 9 | 9.2 | 9 | 9.8 | 98.3 | 96.7 | 0.9 | 97.2 | 98.1 | 98.1 |
| PPA-L 2 | 85.6 | 8 | 10 | 6.6 | 9.2 | 93.3 | 90.0 | 1.0 | NA | NA | 100.0 |
| PPA-L 3 | 97.1 | 10 | 9.85 | 8.9 | 9.5 | 96.7 | 100.0 | 1.0 | 100.0 | 100.0 | 96.2 |
| PPA-L 4 | 93 | 9 | 9.2 | 9 | 9.3 | 90.0 | 83.3 | 1.1 | N/A | N/A | 96.2 |
| PPA-L 5 | 90.5 | 9 | 9.85 | 7.4 | 9 | 86.7 | 96.7 | 1.0 | 97.2 | 98.1 | 100.0 |
| PPA-L 6 | 86.9 | 6 | 9.45 | 9 | 10 | 90.0 | 95.0 | 1.1 | 97.2 | 100.0 | 100.0 |
| PPA-L 7 | 78.6 | 6 | 7.4 | 8.8 | 8.1 | 83.3 | 83.3 | 1.2 | 83.3 | 94.2 | 78.8 |
| PPA-L 8 | 90.4 | 9 | 9.8 | 9 | 7.4 | 35.0 | 73.3 | 0.9 | 97.2 | 92.3 | 92.3 |
| PPA-L 9 | 97.2 | 10 | 10 | 9 | 9.4 | 88.3 | 98.3 | 1.1 | 97.2 | 94.2 | 94.2 |
| PPA-L 10 | 93.2 | 9 | 9.7 | 8.9 | 9 | 98.3 | 100.0 | 1.1 | 100.0 | 96.2 | 96.2 |
| PPA-L 11 | 83.2 | 5 | 9.8 | 9 | 8.8 | 98.3 | 91.7 | 1.1 | 100.0 | 96.2 | 96.2 |
| PPA-L 12 | 95.8 | 9 | 9.8 | 10 | 9.1 | 40.0 | 86.7 | 0.9 | 88.9 | 98.1 | 98.1 |
| PPA-L 13 | 88.8 | 8 | 9.6 | 7.2 | 9.6 | 83.3 | 98.3 | 1.0 | 97.2 | 100.0 | 98.1 |
| Mean (SD) | |||||||||||
| PPA-G | 79.5** (3.6) | 5.1*** (1.0) | 8.9* (0.7) | 7.4* (1.3) | 9.1 (0.5) | 81.9 (17.6) | 93.9 (5.8) | 1.2* (0.2) | 95.1 (4.4) | 94.0 (4.9) | 96.6 (2.5) |
| PPA-L | 90.2 (5.5) | 8.2 (1.6) | 9.5 (0.7) | 8.6 (0.9) | 9.1 (0.7) | 83.2 (21.0) | 91.8 (8.1) | 1.0 (0.1) | 96.0 (5.2) | 97.0 (2.6) | 95.7 (5.6) |
| Control | 99.8 (0.6) | 10.0 (0.0) | 10.0 (0.1) | 10.0 (0.1) | 9.6 (1.6) | 98.2 (2.2) | 99.5 (1.1) | 1.0 (0.0) | 99.0 (1.7) | 98.9 (1.8) | 98.1 (1.4) |
Note: AQ = Aphasia Quotient; F = Fluency; Comp = Auditory Comprehension; Rep = Repetition; Nam = Naming; BNT = Boston Naming Test; NNB = Northwestern Naming Battery; Noun = noun naming subtest; N:V = Noun:Verb ratio; PPVT = Peabody Picture Vocabulary Test; PPT = Pyramids and Palm Tree Test.
p< .05,
p< .01,
p< .001 (reflect significant differences between PPA-G and PPA-L groups).
Table 4.
Summary of additional language test scores.
| NAVS | NAT | Narrative Measures | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Participant | SCT-C | SCT-NC | SPPT-C | SPPT-NC | C | NC | WPM | MLU | Prop. Gram. Sentences |
| PPA-G 1 | 100.0 | 86.7 | 100.0 | 93.3 | N/A | N/A | 63.1 | 6.5 | 46.7 |
| PPA-G 2 | 66.7 | 60.0 | 66.7 | 6.7 | 80.0 | 33.3 | 25.2 | 4.0 | 10.0 |
| PPA-G 3 | 93.3 | 66.7 | N/A | N/A | N/A | N/A | 55.4 | 5.0 | 60.0 |
| PPA-G 4 | 60.0 | 66.7 | 80.0 | 26.7 | 100.0 | 33.3 | 110.3 | 7.6 | 57.5 |
| PPA-G 5 | 73.3 | 73.3 | 100.0 | 13.3 | 100.0 | 33.3 | 36.0 | 5.4 | 58.3 |
| PPA-G 6 | 86.7 | 53.3 | 80.0 | 20.0 | 100.0 | 33.3 | 77.1 | 11.3 | 21.8 |
| PPA-G 7 | 93.3 | 86.7 | N/A | N/A | 33.3 | 0.0 | 47.6 | 7.1 | 10.7 |
| PPA-G 8 | 100.0 | 100.0 | 100.0 | 86.7 | 66.7 | 60.0 | 40.0 | 6.8 | 62.5 |
| PPA-L 1 | 93.3 | 86.7 | 100.0 | 100.0 | 86.7 | 66.7 | 104.5 | 9.5 | 92.0 |
| PPA-L 2 | 100.0 | 100.0 | 100.0 | 80.0 | N/A | N/A | 100.8 | 7.9 | 72.0 |
| PPA-L 3 | 100.0 | 100.0 | 100.0 | 100.0 | N/A | N/A | 141.1 | 7.9 | 88.2 |
| PPA-L 4 | 100.0 | 93.3 | 100.0 | 93.3 | N/A | N/A | 98.1 | 7.8 | 94.7 |
| PPA-L 5 | 100.0 | 80.0 | 66.7 | 53.3 | 100.0 | 53.3 | 86.0 | 6.3 | 85.7 |
| PPA-L 6 | 100.0 | 73.3 | 100.0 | 86.7 | 100.0 | 73.3 | 118.7 | 10.5 | 78.1 |
| PPA-L 7 | 86.7 | 66.7 | 86.7 | 100.0 | 86.7 | 46.7 | 19.1 | 5.4 | 84.6 |
| PPA-L 8 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 83.0 | 7.6 | 54.6 |
| PPA-L 9 | 100.0 | 100.0 | 100.0 | 86.7 | 100.0 | 93.3 | 157.7 | 13.9 | 70.4 |
| PPA-L 10 | 80.0 | 60.0 | 86.7 | 66.7 | 86.7 | 80.0 | 58.9 | 8.6 | 88.9 |
| PPA-L 11 | 100.0 | 100.0 | 100.0 | 80.0 | 100.0 | 100.0 | 64.3 | 9.2 | 57.1 |
| PPA-L 12 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 105.8 | 10.1 | 92.3 |
| PPA-L 13 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 93.3 | 94.1 | 10.7 | 81.3 |
| Mean (SD) | |||||||||
| PPA-G | 84.2* (15.5) | 74.2* (15.7) | 87.8 (14.2) | 41.1* (38.5) | 80.0 (26.7) | 32.2* (19.1) | 56.8* (27.0) | 6.7* (2.2) | 40.9** (22.9) |
| PPA-L | 96.9 (6.4) | 89.2 (14.5) | 95.4 (10.0) | 88.2 (14.9) | 96.0 (6.4) | 80.7 (20.0) | 94.8 (35.5) | 89.2 (14.5) | 80.0 (13.1) |
| Control | 100.0 (0.0) | 100.0 (0.0) | 100.0 (0.0) | 100.0 (0.0) | 100.0 (0.0) | 100.0 (0.0) | 131.8 (19.6) | 11.1 (2.1) | 93.8 (43.7) |
Note: NAVS = Northwestern Assessment of Verbs and Sentences; SCT-C = Sentence Comprehension Test, Canonical sentences; SCT-NC = Sentence Comprehension Test, Noncanonical sentences; SPPT-C = Sentence Production Priming Test, Canonical sentences; SPPT-NC = Sentence Production Priming Test, Noncanonical sentences; NAT = Northwestern Anagram Test; C = Canonical sentences; NC = Noncanonical sentences; WPM = Words per Minute; MLU = Mean Length of Utterance.
p< .05,
p< .01 (reflect significant differences between PPA-G and PPA-L groups).
Confrontation naming was evaluated using the Boston Naming Test (BNT, Kaplan, Goodglass, & Weintraub, 2001) and the Northwestern Naming Battery, a test of category-specific naming and word processing (NNB, Thompson & Weintraub, experimental version). On both tests, all participants showed naming difficulty. Scores ranged from 45% to 98.3% (M = 81.9%) for the PPA-G group and from 35 to 98.3% (M = 83.2%) for the PPA-L group on the BNT (Z = −.511, p = .609, Mann-Whitney U Test) and from 85 to 100% (M = 93.9%) for the PPA-G group and 73.3 to 100% (M= 91.8%) for the PPA-L group on the noun naming subtest of the NNB (Z = −.474, p = .636, Mann-Whitney U Test). Noun to verb ratios also were calculated based on a subset of items from the NNB noun and verb naming subtests, with results showing significantly greater ratios for the PPA-G compared to the PPA-L participants, indicating greater difficulty naming verbs compared to nouns.
Both PPA groups showed relatively preserved single word comprehension tested using a 36-item subset of the Peabody Picture Vocabulary Test (PPVT, Dunn & Dunn, 2007) (i.e., moderately difficult items, #157–192). Scores ranged from 89.9 to 100% (M = 95.1%) for the PPA-G group and from 83.3 to 100% (M = 96%) for the PPA-L group (Z = −.695, p = .487, Mann-Whitney U Test). To evaluate semantic knowledge the Pyramids and Palm Trees Test (PPT; Howard & Patterson, 1992) was administered, (both word and picture versions). On the word version, scores ranged from 88.5 to 100% correct (M = 94%) and 92.3 to 100% correct (M = 97%) for the PPA-G and PPA-L groups, respectively (Z = −1.292, p = .196, Mann-Whitney U Test); scores on the picture version ranged from 94.2 to 100% correct (M = 96.6%) and from 78.8 to 100% correct (M = 95.7) for the two groups, respectively (Z = −.223, p = .824, Mann-Whitney U Test). Performance on the picture version was within normal limits for both patient groups (χ2(2, N = 38) = .828; p = .661, Kruskal-Wallis Test), however, the PPA-G group performed significantly more poorly than the control group on the word version (Z = −2.380, p< .05, Mann-Whitney U Test).
Sentence comprehension and production were tested using the Northwestern Assessment of Verbs and Sentences (NAVS, Thompson, experimental version). The Sentence Comprehension Test (SCT) and the Sentence Production Priming Test (SPPT) of the NAVS examined comprehension and production, respectively, of both canonical and noncanonical sentence structures. Both production and comprehension of noncanonical sentences were more difficult for the PPA-G participants (SCT: M = 74.2%; SPPT: M = 41.1%) compared to PPA-L group (SCT: M = 89.2%; SPPT: M = 88.2%) (SCT: Z = −2.055, p< .05; SPPT: Z = −2.456, p< .05, Mann-Whitney U Test). The Northwestern Anagram Test (NAT) (Thompson, Mesulam & Weintraub, 2009, http://www.soc.northwestern.edu/NorthwesternAnagramTest/; also see Weintraub, Mesulam, Wieneke, Rademaker, Rogalski, & Thompson, 2009) also was administered to evaluate sentence production. Like the NAVS SPPT, the NAT examines production of both canonical and noncanonical sentences; however, rather than spoken responses, participants are required to create sentences that match pictures by ordering randomly presented word cards. Results showed patterns similar to those derived from the SPPT; the PPA-G, compared to the PPA-L, group performed significantly worse on noncanonical sentences (M = 32.2% vs. 81.7%) (Z = −3.071, p< .01, Mann-Whitney U Test), but there was no significant group difference in performance on canonical sentences (Z = −1.314, p = .189, Mann-Whitney U Test).
Finally, aspects of spontaneous speech were evaluated by collecting and analyzing narrative language samples derived by asking participants to tell the story of Cinderella from a wordless picture book (after Thompson, Ballard, Tait, Weintraub, & Mesulam, 1997; Thompson, Shapiro, Tait, Jacobs, Schneider, & Ballard, 1995). The narrative data showed that, although the PPA-G and PPA-L groups produced fewer words per minute than controls participants, words per minute was lower in the PPA-G group (M = 56.8 vs. 94.8) (Z = −2.317, p< .05, Mann-Whitney U Test), who also produced fewer grammatically correct sentences (M = 40.9% vs. 80%) (Z = −3.187, p< .01, Mann-Whitney U Test).
Stimuli
Stimuli included forty black and white line drawings of either living (n = 20) or non-living items (n = 20). The living items were selected from the categories of birds/mammals or fruits/vegetables (10 each) and the nonliving items were either tools or clothing (10 each). An additional 10 filler items (also black and white line drawings), selected from a variety of other categories such as food items (e.g., pizza) and physical phenomenon (e.g., volcano) also were included for a total of 50 items. For each item, IS consisting of written words (all nouns) were selected, four of which were semantically related to the target (or filler) item and four that were not. For each of the four SOAs, one semantically related and one semantically unrelated IS was randomly selected and paired with each stimulus picture, for a total of 160 related target pairs, 160 unrelated target pairs, and 80 filler pairs (40 related and 40 unrelated). For example, for the target item penguin, robin, goose, walrus, and seal were selected as semantically related IS and handle, weather, comic, and receipt were selected as unrelated IS. Ten healthy volunteers (age 25 – 46) rated all word pairs for semantic, categorical rather than associative, relatedness (1 = not related; 7 = highly related). Only semantically related pairs with a mean ranking of 5.5 and above and unrelated pairs with a ranking of 2.5 or lower were used in the experiment. None of the IS overlapped phonologically with the target items. All items (both targets and IS) ranged from 1 – 3 syllables in length and were checked for frequency using the CELEX and MRC databases. Target (but not filler1) items were matched for frequency by category (i.e., living and non-living things) (target picture: t(38) = .899, p =.375) and with IS across categories; (t(318) = .062, p = .950). However, overall, the mean frequency of the semantically related IS (M = 0.6285) was significantly lower than that of unrelated IS (M = 1.1311) across SOAs. See Appendix A for a complete list of simuli.
Appendix A.
Target and corresponding interfering stimuli (IS), with mean frequencies and standard deviations (SDs)
| Target-Living | Related IS | Unrelated IS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SOA −1000 | SOA −500 | SOA −100 | SOA 0 | SOA −1000 | SOA −500 | SOA −100 | SOA 0 | ||
| 1 | apple | kiwi | melon | mango | cherry | Battle | echo | jargon | parcel |
| 2 | bear | elk | goat | moose | Ape | Stamp | lawn | clock | gust |
| 3 | butterfly | centipede | locust | termite | mosquito | Museum | computer | recipe | invention |
| 4 | carrot | spinach | lettuce | parsley | cauliflower | Sulfur | dinner | goggles | title |
| 5 | corn | barley | pea | squash | wheat | Poem | ring | wax | guard |
| 6 | cow | llama | yak | mule | Ram | Drain | rug | tent | spear |
| 7 | dog | mink | weasel | hare | coyote | Grease | cave | pond | sphere |
| 8 | eagle | turkey | condor | swan | quail | Villain | label | disco | token |
| 9 | garlic | parsnip | lentil | dill | pepper | Partner | desert | terror | locker |
| 10 | giraffe | cheetah | gazelle | buffalo | antelope | Novel | mantle | cabin | lotion |
| 11 | monkey | kangaroo | gorilla | panda | Sloth | Halo | rifle | leather | thunder |
| 12 | olive | plum | pickle | turnip | Bean | Easel | target | finish | coment |
| 13 | onion | basil | ginger | cabbage | pepper | Gutter | fiddle | cartoon | record |
| 14 | owl | stork | hawk | duck | wren | Gum | moon | pad | pass |
| 15 | pear | yam | guava | beet | Lime | Loft | jar | wire | nest |
| 16 | penguin | robin | goose | walrus | Seal | Handle | weather | comic | receipt |
| 17 | pig | boar | mole | fox | Hog | Tape | yacht | text | key |
| 18 | squirrel | weasel | gecko | hamster | gopher | Symbol | beauty | trophy | textile |
| 19 | whale | trout | otter | squid | dolphin | Lodge | globe | skate | sweat |
| 20 | worm | snake | eel | mite | cricket | Net | gem | coal | pole |
| Frequency M (SD) | 1.11 (0.40) | 0.47 (0.48) | 0.6 (0.4) | 0.54 (0.42) | 0.74 (0.51) | 1.13 (0.44) | 1.24 (0.48) | 1.13 (0.43) | 1.17 (0.45) |
| Target-Nonliving | |||||||||
| 1 | blender | mixer | chopper | beater | juicer | Journey | sheriff | diaper | acorn |
| 2 | broom | rake | shovel | plough | vaccuum | Knoll | swell | neck | slang |
| 3 | dresser | trunk | cabinet | hamper | closet | Flower | nomad | shadow | bunny |
| 4 | fork | cleaver | cutlery | dagger | skewer | Thief | knee | toast | root |
| 5 | glove | jacket | vest | parka | hood | Swamp | loot | lard | mud |
| 6 | knife | sword | bayonet | lancet | scalpel | Swarm | log | sky | soup |
| 7 | pliers | tongs | sander | mallet | funnel | Cripple | recess | tennis | hygiene |
| 8 | saw | chisel | axe | hatchet | File | Ham | nod | jog | clam |
| 9 | scissors | marker | razor | eraser | sharpener | Pardon | habit | juror | theater |
| 10 | screw | nut | bolt | pin | Tack | Tag | grudge | thumb | nerve |
| 11 | shirt | jersey | fleece | mitten | Hat | Gulf | lock | host | pain |
| 12 | shoe | sneaker | heel | nylons | slipper | Glue | bay | oat | cake |
| 13 | shorts | jeans | boxers | trousers | slacks | Fang | coin | rice | bug |
| 14 | skirt | dress | kilt | sarong | Toga | Frost | bull | yeast | grave |
| 15 | spoon | cup | pot | spatula | baster | Park | junk | fist | guest |
| 16 | stapler | clip | binder | folder | Latch | Neighbor | kitten | hostage | nanny |
| 17 | stove | griddle | dryer | furnace | heater | Guide | nose | frog | dad |
| 18 | stroller | crib | wagon | carriage | buggy | Kidney | jungle | moustache | cafe |
| 19 | taxi | jeep | carriage | train | caravan | Pilot | suspect | statue | jockey |
| 20 | truck | bus | sedan | wagon | shuttle | Smile | notch | slum | wrist |
| Frequency M (SD) | 0.95 (0.65) | 0.64 (0.62) | 0.57 (0.59) | 0.62 (0.49) | 0.85 (0.66) | 1.01 (0.51) | 1.12 (0.51) | 1.17 (0.55) | 1.08 (0.55) |
| Filler | |||||||||
| 1 | bread | cereal | barley | oat | Rice | Ink | lung | mom | hut |
| 2 | cheese | yogurt | cream | butter | Milk | Vat | jam | pearl | foam |
| 3 | cookie | pie | pudding | candy | Cake | Menu | physics | fairy | ivy |
| 4 | guitar | saxophone | flute | bell | clarinet | Maple | pavement | nectar | tyrant |
| 5 | house | barn | shed | mall | garage | Waste | mask | tray | zoo |
| 6 | letter | postman | mailbox | postcard | package | Snore | boss | cart | youth |
| 7 | pizza | spaghetti | burrito | lasagna | Taco | Meadow | virus | nostril | whisker |
| 8 | ring | anklet | necklace | gold | bracelet | Mat | cone | law | wave |
| 9 | volcano | mountain | plateau | cliff | bluff | Offender | pioneer | lavender | ritual |
| 10 | wreath | corsage | stick | fabric | bouquet | Jump | claw | breath | gun |
| Frequency M (SD) | 1.33 (0.83) | 0.68 (0.69) | 0.85 (0.52) | 1.08 (0.57) | 1.02 (0.61) | 0.92 (0.45) | 1.16 (0.22) | 1.13 (0.62) | 1.21 (0.60) |
The 400 stimulus pairs were pseudo randomly divided into 10 sets of 40 items each. Care was taken to ensure that each target item did not occur more than once per set. In addition, items from the same SOA condition were separated by at least three trials on each set. The picture and word stimulus pairs were entered into Super lab (version 4.0; Cedrus, Phoenix, AZ) in three versions, which varied the inter stimulus interval (ISI): 3500 ms, 5000 ms or 7000 ms ISI, to accommodate general naming delays evinced by some PPA participants. For all healthy participants the 3500 ms version was used; the version used for the PPA participants depended on naming latency observed during administration of the BNT: eight participants (five PPA-G and 3 PPA-L) were tested with the 5000 ms version and 4 PPA-L participants were tested with the 7000 ms version; for all others the 3500 ms version was used.
Procedures
Participants were tested on a Macintosh (20 inch, iMac 5, 1) HD Version 10.4.10. They were seated at a distance of approximately 2 feet from the computer screen on which the IS and target pictures were presented. Each trial began with presentation of a cross fixation (500 ms.), followed by an IS and target picture in the four SOA conditions (see Figure 1). Participants were asked to name each picture as quickly as possible and ignore the IS to the extent possible. The acoustic waveform of each response produced was recorded through a computer microphone using Praat 5.0 software (Boersma & Weenink 2010). Prior to beginning the experiment, participants were provided with pre-training to familiarize them with all picture stimuli (both target and filler items) and to ascertain their ability to name them. We also tested all written IS to determine reading ability. Each picture was presented for naming with no response contingent feedback provided. Items that could not be named correctly within a 5 second response time were presented a second time. All participants included in the study were able to name at least 80% of the pictures on either the first or second trial. The written IS were then presented one at a time for participants to read aloud; all showed good reading ability with performance ranging from 92% to 100%. Erred items were presented a second time, with feedback provided. Finally, practice trials were presented, which required participants to name target pictures overlaid with written words. These trials used stimuli similar, but not identical, to the experimental items.
Figure 1.
Cartoon of stimulus trials. On each trial a 500 ms. cross fixation preceded presentation of an interfering stimulus (IS) and target picture to be named in each SOAs condition.
Data Analysis
Naming responses that matched the target picture were considered correct. This included responses phonologically related to the target (e.g., pinkquin for penguin) as well as false starts and self-corrections, occurring within the allotted response time. All other responses were considered incorrect. Correct responses then were analyzed for reaction time (RT), measured from picture onset (marked with an inaudible beep) to production of the first phoneme of the target word marked in the acoustic waveform. Thirty percent of the data were rescored for both accuracy and RT by an independent coder for scoring reliability purposes; overall point-to-point agreement between the primary and secondary coders was 97%. Any disagreements were resolved by consensus. Mean accuracy and RTs derived from each SOA condition were computed for related and unrelated trials for each participant group.
Cortical Thickness Measurement: Data Acquisition and Analysis
Magnetic resonance (MR) imaging, performed at the Northwestern University Department of Radiology Center for Advanced MRI (CAMRI), was undertaken for both the PPA and control participants. T1-weighted images, using 3D MP-RAGE sequences (TR = 2300 ms, TE = 2.86 ms, flip angle = 9°, FoV = 256 mm) recording 160 slices at a thickness of 1.0 mm were acquired on a 3T Siemens TRIO system using a 12-channel birdcage head coil. MR images were processed using the image analysis suite Free Surfer (version 4.5.0), which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). Cortical thickness estimates were calculated by measuring the distance between representations of the white-gray and pial-CSF boundaries across each point of the cortical surface (see Fischl, Sereno, Tootell, & Dale, 1999, for details). Statistical surface maps then were generated using a general linear model (GLM) that displayed differences in cortical thickness between PPA and control groups for each vertex along surface representations.
Results
Naming accuracy
Table 5 provides the mean percentage correct naming for the control, PPA-G, and PPA-L groups by condition. Overall, groups showed good ability to name. The mean of semantically related and unrelated trials was 97.6% and 97.9% correct, respectively, for the controls; 94.4% and 95.5% correct, respectively for the PPA-G group; and 89.5% and 91% correct, respectively, for the PPA-L group. A 3 (group) × 4 (SOA) × 2 (relatedness) ANOVA indicated a significant main effect of group (F (2, 35) = 6.694, p < .01), but not SOA (F (3, 105) = .299, p = .826). Pairwise comparisons for individual groups revealed that the PPA-L group performed statistically more poorly than the control group (p < .01), but not the PPA-G group (p = .27). A main effect of relatedness approached significance (F (1, 35) = 3.838, p = .058), with semantically related trials being more difficult, that is less accurate, than unrelated trials. There were no significant interaction effects.
Table 5.
Mean percentage correct naming (and standard deviation) for control, agrammatic PPA (PPA-G), and logopenic PPA (PPA-L) groups at each SOA.
| Stimulus Onset Asynchrony | |||||
|---|---|---|---|---|---|
| −1000 ms | −500 ms | −100 ms | 0 ms | ||
| Group | Total Accuracy | ||||
| Control | |||||
| Related | 97.4 (3.8) | 98.0 (2.6) | 97.7 (2.7) | 97.3 (2.6) | 97.6 (2.4) |
| Unrelated | 97.7 (3.2) | 98.1 (2.6) | 98.2 (2.8) | 97.7 (3.2) | 97.9 (2.6) |
| PPA-G | |||||
| Related | 92.8 (5.6) | 95.0 (4.6) | 94.1 (8.1) | 95.6 (3.7) | 94.4 (4.8) |
| Unrelated | 95.3 (4.1) | 95.9 (5.2) | 96.3 (2.7) | 94.7 (3.6) | 95.5 (2.9) |
| PPA-L | |||||
| Related | 90.8 (10.0) | 89.0 (11.6) | 88.7 (10.3) | 89.5 (10.3) | 89.5 (10.3) |
| Unrelated | 92.0 (7.0) | 90.6 (8.4) | 92.0 (7.0) | 89.5 (9.1) | 91.0 (7.4) |
Reaction time analyses
Only correct responses were analyzed for RT, which required removal of 2.2% (118/5440), 4.84% (124/2560), and 9.43% (377/40002) of the data from the control, PPA-G, and PPA-L groups, respectively. Furthermore, outliers (RTs greater than two standard deviations above the group mean) were replaced with the group mean. Less than 2% of the data for the patient groups and none for the controls were outliers.
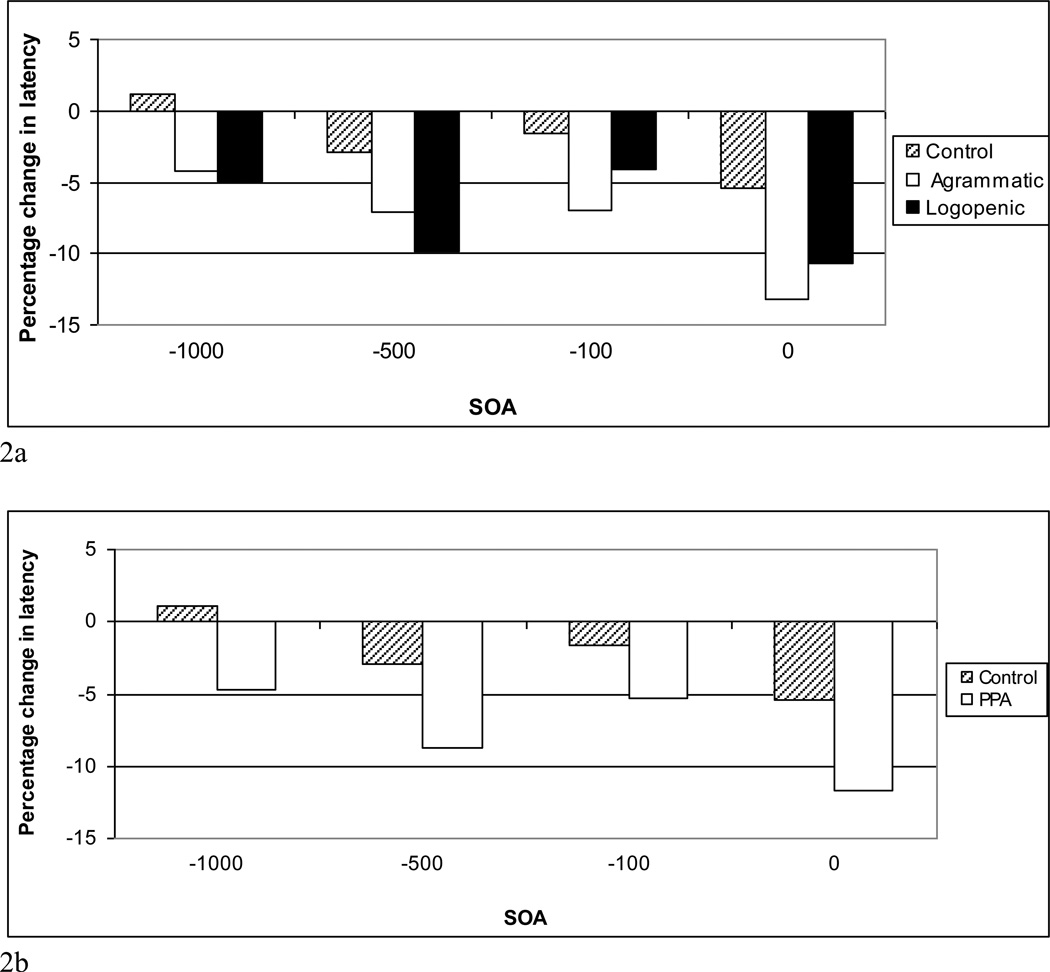
Table 6 provides the mean RTs for related and unrelated trials at each SOA for each participant group. Because we were unable to completely control for frequency of occurrence of the related and unrelated IS, i.e., the semantically related IS were significantly lower in frequency than the unrelated stimuli, we first examined the relation between IS word frequency and RT using the Pearson test (2-tailed), with results showing no significant correlation between them for either the control or PPA groups (controls: r=.064, p=.418; PPA: r=.107, p=.180). Analysis of homogeneity of variance of the RT data also showed no significant differences (p> .05). The RT data were, therefore, first analyzed using a 3 (group) × 4 (SOA) × 2 (Relatedness) × 2 (living versus non-living things) repeated measures ANOVA, which revealed main effects of group (F (2, 35) = 33.611, p < .000), SOA (F (3, 105) = 79.035, p < .000), relatedness (F (1, 35) = 41.796, p < .000), and living versus non-living things (F(1, 35) = 17.410, p<.000). Follow-up pairwise comparisons indicated that naming RTs were significantly longer for both PPA groups compared to the control group (p’s< .001) and RTs for living things were significantly longer than for non-living things for the control participants (p<.001), but not for either PPA group. In addition, there were interactions between relatedness and group (F (2, 35) = 6.163, p < .01) and between SOA and relatedness (F (3, 105) =14.981, p < .001). In order to determine how relatedness affected groups and SOAs, a 3 (group) × 4 (SOA) ANOVA was conducted with differences between related and unrelated naming RTs as the dependent variable. Results showed a main effect of SOA (F (3, 105) = 14.981, p < .001), with SI effects in the SOA = 0 ms condition being greater than those seen at the all other SOAs (p’s < .01). In addition, there was a main effect of group (F (2, 35) = 6.163, p < .01), with both PPA-G and PPA-L groups demonstrating significantly greater naming RTs for related compared to unrelated trials, that is, overall greater SI effects, than the normal controls (p’s < .01). Figure 2 displays the percentage of RT latency differences between groups, calculated using the following formula: [(unrelated-related)/unrelated) × 100]3, showing that at all SOAs the magnitude of SI effects was greater for both PPA groups compared to the healthy control participants.
Table 6.
Mean reaction time for semantically related and unrelated trials for control, agrammatic PPA (PPA-G) and logopenic PPA (PPA-L) groups at each SOA.
| Stimulus Onset Asynchrony | ||||||||
|---|---|---|---|---|---|---|---|---|
| −1000 ms | −500 ms | −100 ms | 0 ms | |||||
| RT | SD | RT | SD | RT | SD | RT | SD | |
| Control | ||||||||
| Related | 967.9 | 90.2 | 1024.1** | 77.7 | 1072.7** | 118.6 | 1183.4** | 106.1 |
| Unrelated | 978.9 | 98.8 | 995.2 | 76.3 | 1055.3 | 121.7 | 1123.1 | 137.4 |
| Difference | −11.0 | 28.9 | 17.4 | 60.3 | ||||
| PPA-G | ||||||||
| Related | 1368.3 | 199.9 | 1404.5* | 207.9 | 1488.1* | 215.5 | 1628.1** | 181.0 |
| Unrelated | 1312.4 | 199.3 | 1310.8 | 151.9 | 1390.3 | 122.0 | 1437.8 | 186.1 |
| Difference | 55.9 | 93.7 | 97.8 | 190.3 | ||||
| PPA-L | ||||||||
| Related | 1298.8** | 173.7 | 1364.2*** | 144.3 | 1416.8* | 172.9 | 1562.2*** | 184.4 |
| Unrelated | 1237.9 | 150.2 | 1242.0 | 102.1 | 1360.5 | 149.7 | 1410.7 | 143.7 |
| Difference | 60.9 | 122.2 | 56.3 | 151.5 | ||||
Note: Mean reaction times (RT) in milliseconds (ms), and standard deviation (SD) by stimulus onset asynchrony (SOA).
p< .05,
p< .01,
p< .001.
Figure 2.
(a) Magnitude of semantic interference effect (RT latency differences between related and unrelated trials) at each SOA for control, agrammatic (PPA-G), and logopenic (PPA-L) participant groups; (b) Magnitude of semantic interference effect for control and PPA groups (PPA-G and PPA-L) combined.
In order to determine whether or not the overall magnitude of SI was related to performance on neuropsychological or language tests administered prior to the study, we undertook correlational analysis, using Pearson tests. Results showed no significant correlations between the magnitude of SI (calculated by computing the mean magnitude of SI across all SOAs for each participant) and performance on any of these tests. These data are presented in Table 7.
Table 7.
Correlations between (a) the overall magnitude of semantic interference, and (b) semantic interference at the SOA = −1000 ms and performance on pre-test neuropsychological and language measures.
| Magnitude of SI | SI at −1000 ms | ||||
|---|---|---|---|---|---|
| r | P | r | P | ||
| Neuropsychological | |||||
| MMSE | −.037 | .874 | .080 | .730 | |
| WMS-III | |||||
| Measures | Immediate | −.174 | .519 | −.035 | .897 |
| WMS-III Delayed | −.193 | .474 | .175 | .516 | |
| Facial Recognition | .095 | .690 | −.042 | .861 | |
| Trail Test A | −.052 | .828 | −.099 | .679 | |
| Visual Verbal | |||||
| Shifts | −.161 | .523 | .226 | .367 | |
| Visual Verbal | |||||
| Points | −.183 | .468 | .214 | .394 | |
| Language | WAB-AQ | .009 | .969 | .104 | .652 |
| Measures | WAB-F | .177 | .443 | .046 | .844 |
| WAB-AC | −.108 | .643 | .000 | .999 | |
| WAB-R | .027 | .907 | −.090 | .697 | |
| WAB-N | −.247 | .281 | .163 | .479 | |
| BNT | −.193 | .401 | −.055 | .814 | |
| NNB-Noun | .085 | .715 | −.286 | .208 | |
| NNB-N:V | −.372 | .096 | .371 | .098 | |
| PPVT | −.191 | .434 | −.007 | .977 | |
| PPT-Word | −.252 | .312 | .373 | .127 | |
| PPT-Picture | −.206 | .370 | .030 | .898 | |
Note: Pearson correlation (2-tailed)
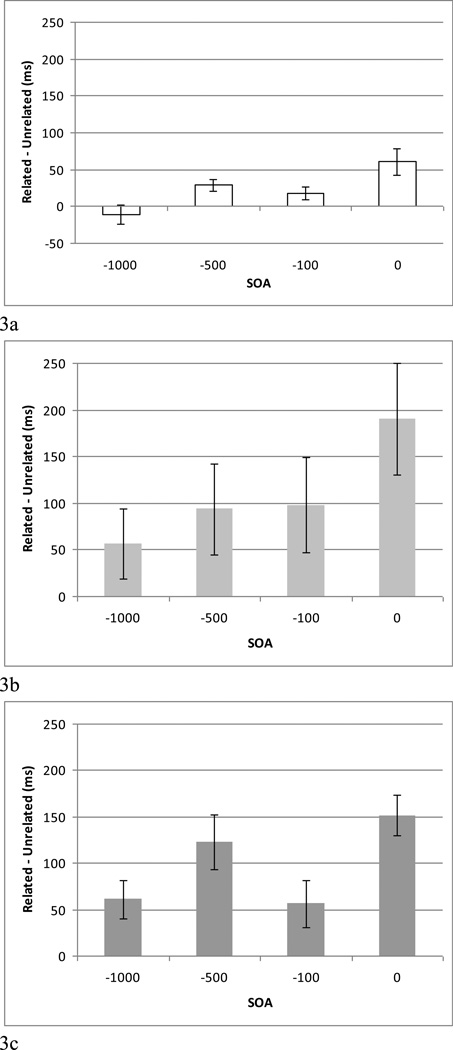
Additional analyses were undertaken to examine SI effects within each participant group using a 4 (SOA) × 2 (Relatedness) ANOVA. For the control group, significant main effects of SOA (F (3, 48) = 44.480, p < .001) and Relatedness (F (1, 16) = 8.986, p < .01) and an interaction between SOA and Relatedness (F (3, 48) = 7.418, p < .001) were found. Post-hoc analyses using a paired t-test (one-tail) revealed SI effects, that is, significantly slower naming RTs for semantically related compared to unrelated trials, at SOAs of 0 (p < .01), −100 (p < .01), and −500 ms (p < .01), but not at SOA −1000 ms. Inspection of the individual data derived from the SOA = −1000 ms condition showed that none of the control participants demonstrated slower mean RTs in the related compared to the unrelated condition, with 10 (of 17) showing the opposite pattern: faster RTs in the semantically related compared to the unrelated condition, which was significant for three participants (p< .01). No significant RT differences between related and unrelated conditions were found for the remaining control participants (p> .05). For the PPA-G group, there also were main effects of SOA (F (3, 21) = 10.400, p < .001) and Relatedness (F (1, 7) = 5.678, p < .05) as well as an interaction between SOA and Relatedness (F (3, 21) = 6.510, p < .01). Post-hoc analyses indicated significant SI at SOAs of 0 (p < .01), −100 (p < .05) and −500 ms (p < .05) but not at SOA −1000 ms (p = .089). Notably, however, six of the eight PPA-G participants (all but PPA-G1 and PPA-G5) showed semantic interference effects at −1000 ms (i.e., greater mean RTs in the related compared to the unrelated condition, ranging from 11.78 ms (PPA-G3; RT related = 1340.96 ms; unrelated = 1329.18 ms) to 282.08 ms (PPA-G2; RT related = 1553.59 ms; unrelated = 1271.51 ms) (PPA-G group mean RT difference = 55.94 ms). These RT differences were significant for all participants (p< .05), with the exception of PPA-G3, which approached significance (p = .0654).
For the PPA-L group, main effects of SOA (F (3, 36) = 39.628, p < .001) and Relatedness (F (1, 12) = 49.613, p < .001) and a significant interaction between SOA and Relatedness (F (3, 36) = 3.997, p < .05) also were found, with post-hoc analyses indicting SI effects for the PPA-L group at all SOAs: SOA 0 (p < .001), −100 (p < .05), −500 (p < .001), and −1000 ms (p < .01). Importantly, a SI effect at −1000 ms was found for 10 of the 13 PPA-L participants (all except for PPA-L2, PPA-L6, and PPA-L10). That is, mean RTs for related trials were longer than for unrelated trials for these participants, ranging from 8.01 ms (PPA-L3; related = 1084.52 ms; unrelated = 1076.51 ms) to 144.69 ms (PPA-L4; related = 1177.28 ms; unrelated = 1032.59 ms) (PPA-L group mean RT difference = 60.9 ms). For all participants RTs for related trials were significantly slower than unrelated trials (p< .05). SI affects at each SOA (calculated by subtracting RTs for unrelated trials from related trials) for participant group are shown in Figure 3.
Figure 3.
Semantic interference effects (mean RT for related-unrelated trials) for the (a) control group, (b) agrammatic (PPA-G) group, and (c) logopenic (PPA-L) group.
Once again, we examined for correlations between SI and the PPA participants’ performance (both PPA-G and PPA-L) on our pre-test neuropsychological and language measures. Results revealed no significant correlations between these variables (see Table 7). We also queried whether symptom duration or age were correlated with SI effects at SOA = −1000 ms and found no significant correlations for either (symptom duration: r=.173, p=.453; age: r=.051, p=.828). In addition, in order to determine that the abnormal SI effects seen at −1000 ms were not driven by disease severity we compared WAB AQ scores for those who did (n=16) and did not (n=5) show this effect. Results indicated no significant differences between groups (M = 85.13 and 86.72 for participants with normal and abnormal semantic interference, respectively; p = .840 (Mann-Whitney U Test)).
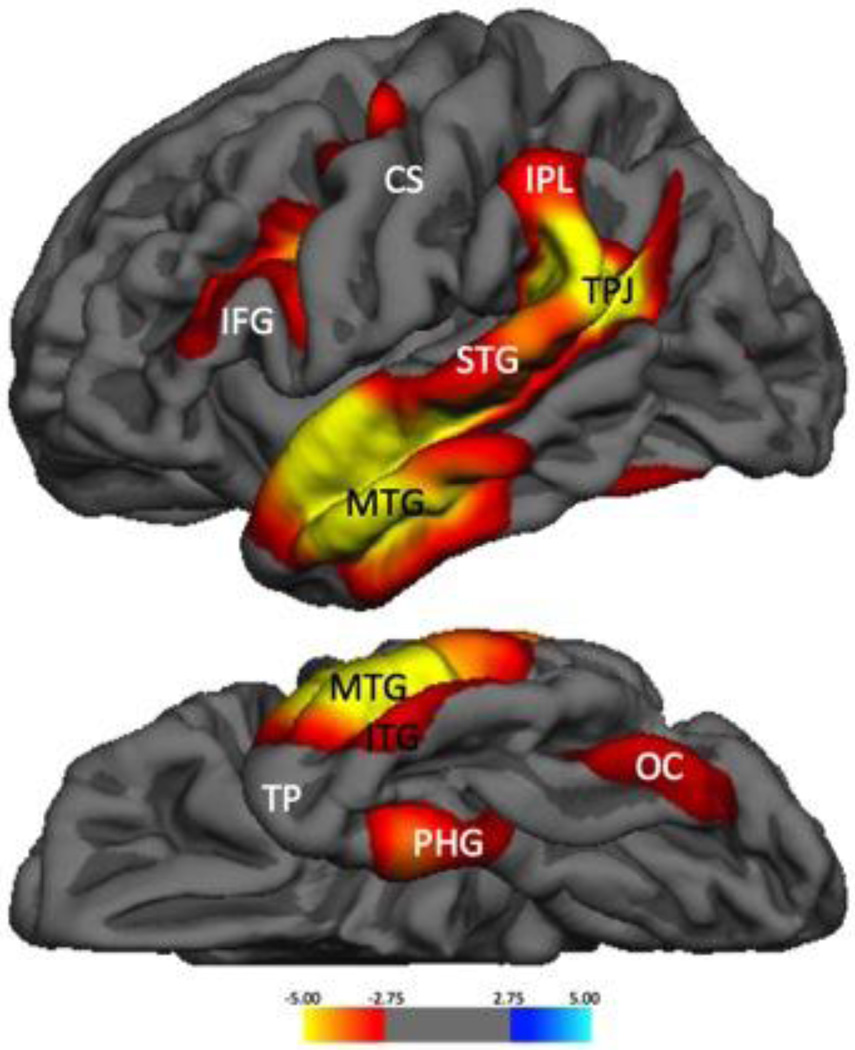
Cortical Thinning
Because participants from both the PPA-G and PPA-L groups evinced abnormal SI effects at −1000 ms, we examined atrophy patterns for patients who showed abnormal effects (n=13; five PPA-G and eight PPA-L participants4). Two participants were left-handed (PPA-G1, PPA-L8) and therefore were not included in this analysis and an MR scan was not obtained for two of the participants with abnormal effects (PPA-G4, PPA-L5). Notably, because of the low number of PPA participants with no semantic interference at −1000 ms and the fact that MR scans were obtained for only three of them (one PPA-G and two PPA-L participants5) we did not perform cortical thickness analysis for this small group.Figure 4 shows the regional distribution of cortical thinning (atrophy) for the participants showing abnormal SI compared to controls (FDR, p = 0.01). Results showed asymmetric cortical thinning, with atrophy constrained to the left hemisphere, most severely in the perisylvian region, with extensive atrophy in the lateral temporal cortex. Notably, significant cortical thinning also was found in the left inferior frontal gyrus, parahippocampal gyrus and in the posterior fusiform gyrus.
Figure 4.
Distribution of cortical thinning of the lateral (top) and ventral (bottom) left hemisphere pial surface for PPA individuals showing abnormal semantic interference effects in the SOA −1000 ms. condition. Yellow indicates regions of greatest thinning – compared to a normal cohort. Significance is displayed as a log(10) p-value. IFG: inferior frontal gyrus, CS: central sulcus; IPL: inferior parietal lobule; TPJ: temporoparietal junction; STG: superior temporal gyrus, MTG: middle temporal gyrus, ITG: inferior temporal gyrus; TP: temporal pole; PHG: parahippocampal gyrus; OC: occipital cortex (posterior fusiform gyrus).
Discussion
In this experiment we examined the effects of semantic interference on object naming by presenting semantically related and unrelated interfering stimuli or word probes at SOAs of −1000, −500, −100, and 0 ms. For the control group, statistically significant semantic interference effects were found at SOAs of 0, −100, and −500 ms, but not at −1000 ms. Previous studies also have found such effects in normal speakers at SOAs up to −300 ms (Glaser & Düngelhoff, 1984; Hashimoto & Thompson, 2010; Starreveld & LaHeij, 1996). However, studies have reported no semantic interference at earlier SOAs (see, for example, Glaser and Düngelhoff (1984) who found no semantic interference at SOA = −400 ms). We suggest that the semantic interference effect found in the present study at SOA = −500 ms likely relates to the age of our control participants (mean age = 63 years; SD = 6 years) compared to those in most studies of semantic interference effects, which have included younger participants (age range approximately 18 to 30 years). Indeed, Taylor and Burke (2002) found greater SI effects for older participants (age 62–85), similar in age to our control cohort, compared to younger speakers (age 18–29 years). Semantic priming studies also show greater priming effects for older compared to younger speakers, likely due to their extensively developed lexical-semantic networks (Laver & Burke, 1993).
Another possibility is that we found semantic interference at −500 ms for the healthy control participants because of frequency differences between our related and unrelated interfering stimuli. That is, the semantically related probes were significantly lower in frequency than our unrelated pairs, which may have inflated semantic interference effects. Indeed, Miozzo and Caramazza (2003) found longer RT latencies associated with lower distractor word frequency in word-interference paradigms. However, they found no interaction between frequency and semantic relatedness. Importantly, we also found no correlation between the frequency of our interfering stimuli and reaction time for either the control or PPA participants.
In keeping with previous studies we did not find interference effects in the SOA −1000 ms condition for the control participants. In fact, in this condition, naming was faster when distractors were semantically related (967.9 ms) compared to when they were unrelated (978.9 ms), albeit not significantly so for the group. Vandenberghe et al. (2005) and La Heij et al. (1990) found similar semantic facilitation effects in neurologically intact participants at SOAs of −750 and −800 ms, respectively. These data, considered collectively, suggest that activations triggered by a semantically related interfering stimulus normally cease to exert a competitive influence on object naming within approximately 750 seconds of presentation, at which time the residual activation trace appears to facilitate naming.
Turning to the results from our PPA participants, both PPA groups evinced significant semantic interference effects at −500, −100, and 0 ms, consistent with control participants, indicating that exposure to a semantically related interfering stimulus induced spreading activation to related items that competitively interfered with subsequent naming of an object in that category. However, both PPA groups showed heightened activation, that is, a larger magnitude of semantic interference compared to age-matched controls at all SOAs. These greater semantic interference effects are similar to those found by Hashimoto and Thompson (2010). In addition, abnormal semantic interference in the −1000 ms SOA condition was noted for both logopenic and agrammatic PPA participants. For the logopenic group, the speed of object naming was slowed by a mean of 60.9 ms by a semantically related (compared to unrelated) word presented 1000 ms prior to object presentation. Interestingly, most of the agrammatic participants (six of eight) also showed this pattern, although the group effect was not significant (p = .089), with a mean RT difference between the two trial types of 55.9 ms. This finding supports those reported by Vandenberghe et al. (2005) who found semantic interference effects for PPA patients at −750 ms.
These abnormal semantic interference effects suggest excessive activation of semantic networks in aphasic individuals compared to normal speakers as well as greater vulnerability to distractor interference than normal. Interestingly, vulnerability to distractor interference has been shown to be particularly pronounced in individuals with the semantic variant of PPA, where a blurring of semantic distinctions among members of semantic categories becomes a major factor contributing to anomia, leading to characteristic semantic par aphasias and coordinate errors in word-object matching tasks (Mesulam et al., 2009a). Although the performance of individuals with the semantic variant of PPA was not examined in the present study, because their characteristically poor naming ability precluded participation, there is considerable overlap of symptoms among PPA variants. Therefore, it is possible that the abnormal semantic interference effects found in both agrammatic and logopenic participants stem from semantic mapping deficits characterized by difficulty in rapidly dissociating the interfering stimulus from the target when the two are semantically related and hence more likely to be blurred or confused. As discussed in the introduction, we point out once again that the source of such semantic mapping deficits may derive from impairments at the semantic or lexical, phonological level of word processing (or both). On Levelt’s model of lexicalization (e.g., Levelt 1992; Levelt et al., 1999), for example, when an interfering stimulus is encountered, spreading activation to semantically related items occurs automatically and, in turn, lexical detail associated with both the IS and its semantic network are accessed. Hence, inhibition of semantic competitors is required at the semantic as well as the phonological level. Where in this path processing goes awry in individuals with PPA is, however, unclear.
Interestingly, the naming impaired participants in the present study performed well on tests of single word comprehension and semantic knowledge, as did the PPA individuals studied by Vandenberghe et al (2005), on the face of it suggesting that that the source of these individuals’ naming deficits related more to word-retrieval difficulty, rather than to semantic deficits. The abnormal semantic interference effects found here, however, suggest otherwise – that naming deficits in PPA derive at least in part from impairments associated with subtle semantic processing deficits, which may not be detectable using off-line testing methods. In fact, in the present study, we found no significant correlations between any of our pre-test language measures and either the overall magnitude of semantic interference across SOAs or abnormal semantic interference at −1000 ms. Other work in our laboratories also has shown subtle abnormalities of semantic processing in PPA subtypes (e.g., PPA-G and PPA-L) that are not associated with overt abnormalities of word comprehension or object identification (Rogalski, Rademaker, Mesulam, & Weintraub, 2008).
Notably, the abnormal semantic interference effects found in the present study could not be attributed to general cognitive deficits, such as memory or executive function, in that pre-testing of these functions showed that the PPA participants performed well across all measures. In addition, no significant correlations were found between performance on any of the neuropsychological tests administered and the magnitude of semantic interference across SOAs or abnormal semantic interference at −1000 ms. Notably, we also found no significant correlations between chronological age or PPA symptom duration and abnormal semantic interference. These findings indicate that the semantic mapping deficits found in our PPA participants cannot be explained by any general cognitive compromise or decline.
The patterns of cortical atrophy found for the PPA-G and PPA-L participants who showed abnormal semantic interference effects at −1000 ms also are in line with the semantic processing deficits noted. This group of individuals showed extensive atrophy in the left lateral temporal cortex, including the superior, middle, and inferior temporal gyri as well as the temproparietal junction. Notably, previous studies with non-semantic variants of PPA also have found atrophy in these regions. For example, Wilson, Henry, Besbris, Ogar, Dronker, Jarrold et al. (2010) and Gunawardena, Ash, McMillan, Avants, Gee, & Grossman (2010) reported temporal atrophy in their cohort of logopenic and agrammatic PPA participants, respectively. In the present study we also found atrophy in the parahippocampal gyrus on the ventral surface of the brain as well as in the ventral temporal occipital region (i.e., posterior fusiform gyrus) and in portions of the inferior frontal gyrus, also in the left hemisphere. Each of these regions has been implicated in semantic processing and could provide substrates for the SI effects we observed. In addition to research associating the lateral temporal cortex with semantic processing deficits (Gorno-Tempini et al., 2004; Hodges, Patterson, Oxbury, & Funnell, 1992; Mesulam, et al., 2009a; Mesulam et al., 2009b; Mummery, Patterson, Wise, Vandenberghe, Price & Hodges, 1999; Rosen, Kramer, Gorno-Tempini, Schuff, Weiner, & Miller, 2002), several studies indicate that the ventral occipitotemporal region, including the fusiform gyrus, is involved in processing of visual word forms (Devlin, Jamison, Gonnerman, & Matthews, 2006; Hillis, Newhart, Heidler, Barker, Herskovits, & Degaonkar, 2005; Kronbichler, Bergmann, Hutzler, Staffen, Mair, Ladurner et al., 2007; Simmons, Koutstaal, Prince, Wagner, & Schachter, 2003) as well as visual objects (Haxby, Gobbini, Furey, Ishai, Schouten, & Pietrini, 2001). Price and Devlin (2011) suggest that this region is engaged in both bottom-up and top-down processing, integrating visuospatial features abstracted from sensory input with higher-level associations, including meaning representations. The IFG also is engaged for semantic processing, with numbers of neuroimaging studies using a variety of tasks, including making semantic decisions about words and generating words based on semantic relationships, reporting significant activation in this region (Costafreda, Fu, Lee, Everitt, Brammer & David, 2006; Demb, Desmond, Wagner, Vaidya, Glover, & Gabrieli, 1995; Fiez, 1997; Gabrieli, Desmond, Demb, Wagner, Stone, Vaidya et al., 1996; Kapur, Craik, Tulving, Wilson, Houle, & Brown, 1994; Klein, Milner, Zatorre, Meyer, & Evans, 1995; Peterson, Fox, Posner, Mintun, & Raichle, 1988; Poldrack, Wagner, Prull, Desmond, Glover, & Gabrieli, 1999; Wagner, Paré-Blagoev, Clark, & Poldrack, 1998). Thompson-Schill, D’Esposito, Aguirre, and Farah (1977) also found greater IFG activation associated with the selection demands of neuroimaging tasks, suggesting a role of this region is selection of competing alternatives from semantic memory. In addition, the left IFG has been implicated in response inhibition (Swick, Ashley, & Turken, 2006). These latter roles fit well with the present findings in that the abnormal SI effects may result at least in part from an inability to select from (and deactivate or inhibit) activated semantically interfering related items.
Regardless of the precise role of these regions in semantic processes associated with either normal or disordered naming, the present findings indicate that agrammatic and logopenic PPA participants show similar automatic lexical-semantic processing routines. Hence our prediction that their performances would mirror those of stroke-induced anterior and posterior aphasias was not completely supported. Priming studies show that Broca’s aphasia, which in clinico anatomical terminology is associated with a prototypical anterior aphasia with necrosed tissue in the inferior frontal region, leads to an under activation of lexical competitors and/or delayed activation; whereas, Wernicke’s aphasia, the prototypical posterior aphasia, with posterior perisylvian brain damage, leads to over activation as well as delayed deactivation of lexical competitors (Blumstein, & Milberg, 2000; Janse, 2006; Prather et al., 1997; Swinney, et al., 1989; Yee et al., 2008)6. However, neither the agrammatic or logopenic PPA individuals in the present study showed evidence of under activation. Rather both groups showed spreading activation to semantically related items, with semantic interference effects noted at all SOAs, with the exception of −1000 ms for some participants. They also did not show performance patterns suggestive of delayed activation of semantic networks in that both PPA groups showed semantic interference effects in the SOA = 0 ms and −100 ms conditions. However, the magnitude of semantic interference was abnormal for both PPA groups across all SOAs, an effect associated with over activation of semantic competitors, expected for patients with posterior but not anterior lesions. In addition, both groups showed evidence of delayed deactivation, as seen in Wernicke’s aphasia, in that semantic interference effects persisted to the SOA = −1000 ms condition, indicating a failure to completely inhibit the lexical-semantic network associated with interfering stimuli in a timely manner, leaving them active during attempts to name target pictures.
The lack of clear-cut distinctions in lexical-semantic processing routines for the two PPA groups may reflect the nature of the clinical pathology associated with PPA compared to vascular aphasia. In aphasias caused by cerebrovascular lesions, portions of the brain are completely and permanently destroyed. In contrast, neurodegenerative disease that causes PPA leads to a gradual and partial loss of neurons at sites of atrophy. Hence, the remaining neurons, even at sites of maximal atrophy, continue to participate in relevant language tasks (Sonty, Mesulam, Weintraub, Johnson, Parrish, & Gitelman, 2007). The deficits in these patients, therefore, may reflect contributions from the residual functioning of atrophied tissue, resulting in blurred distinctions between PPA subtypes at least in early stages of language decline.
Further research using, for example, an eye-tracking paradigm similar to that used by Yee et al. (2008), may help to clarify the nature of lexical-semantic processing deficits in PPA and perhaps elucidate differences between PPA subtypes. The time course of activation and resolution of semantic competitors can be tracked by monitoring participant’s eye movements, elucidating either over or under activation of semantic competitors as well as any delays in activation or abnormal resolution of semantic competition. A failure to resolve lexical competition would show a pattern of timely, but abnormally long-lasting, fixations on competitor items, whereas, a deficient activation pattern would show delayed fixations on competitor items. Regardless of which is correct, however, the abnormal semantic interference effects found for patients with PPA (both agrammatic and logopenic variants) suggest that naming difficulty in these patients does not result only from phonological encoding problems. Rather, faulty semantic processing is implicated as well.
Conclusion
Both agrammatic and logopenic PPA and healthy age-match participants show slowed object naming speeds when exposed to semantically related interfering stimuli 500 or 100 m sec prior to or simultaneously with presentation of an object to be named. However, this semantic interference effect was greater in magnitude and also lasted longer, detectable even in the −1000 ms SOA condition. These results show that individuals with non-semantic forms of PPA are able to activate appropriate semantic fields in response to experimental lexical probes but that they are excessively vulnerable to interference by semantic competitors during the subsequent iterative process that links an object to its name. Even PPA patients with intact word comprehension may thus have a perturbation of semantic processing that compromises naming ability.
A semantic interference paradigm examined the time course of naming in PPA.
Semantic interference at SOA = 0, −100, and −500 ms was seen in PPAs and controls.
Only PPA participants evinced abnormal SI at −1000 ms.
A greater magnitude of SI was found for PPAs compared to controls across all SOAs.
Atrophy in left perisylvian cortex was found for PPAs with abnormal SI effects.
Acknowledgments
This research was supported by the NIH grants: RO1DC01948 (C.K. Thompson), R01DC008551 (M. Mesulam), and AG13854 (Alzheimer’s Disease Core Center), Northwestern University. The authors wish to thank Dr. Darin Cobia for his assistance with data analysis. We also acknowledge the Center for Advanced MRI (CAMRI) at Northwestern University for their scanning support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Filler items were higher in frequency than the experimental targets, however, all items (both experimental and filler) were matched for number of syllables and picturability.
For one logopenic subjects, one script (160 responses) could not be analyzed due to computer error.
After Wilshire et al. (2007).
Participants PPA-G2, 3, 6, 7, and 8; PPA-L1, 3, 4, 7, 9, 11, 12, and 13.
Participants PPA-G5, PPA-L 6, and PPA-L10.
Studies showing differential priming effects for individuals with stroke-induced Broca’s and Wernicke’s aphasia provide only gross descriptions of patient lesions, therefore, they only estimate the neural correlates of these lexical-semantic processes.
References
- Benton A, Sivan A, Hamsher KS, Varney N, Spreen O. Contributions to neuropsychological assessment: A clinical manual. 2nd edition. New York: Oxford University Press; 1995. [Google Scholar]
- Bloem I, La Heij W. Semantic facilitation and semantic interference in word translation: Implications for models of lexical access in language production. Journal of Memory and language. 2003;48(3):468–488. [Google Scholar]
- Bloem I, van den Boogaard S, La Heij W. Semantic facilitation and semantic interference in language production: Further evidence for the conceptual selection model of lexical access. Journal of Memory and Language. 2004;51(2):307–323. [Google Scholar]
- Blumstein SE, Milberg WP. Language deficits in Broca’s and Wernicke’s aphasia: A singular impairment. In: Grodzinsky Y, Shapiro L, Swinney D, editors. Langauge and the brain: Representation and processing. New York: Academic Press; 2000. [Google Scholar]
- Boersma P, Weenink D. Pratt: Version 5.0. Amsterdam: Institute of Phonetic Sciences at the University of Amsterdam; 2010. [Google Scholar]
- Costafreda SG, Fu CHY, Lee L, Everitt B, Brammer MJ, David AS. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: Role of the left inferior frontal gyrus. Human Brain Mapping. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damian MF, Martin RC. Semantic and phonological codes interact in single word production. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25(2):345–361. doi: 10.1037//0278-7393.25.2.345. [DOI] [PubMed] [Google Scholar]
- Dell GS. A spreading activation theory or retrieval in sentence production. Psychological Review. 1986;93(3):283–321. [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JDE. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. Journal of Neuroscience. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Gonnerman LM, Matthews PM. The role of posterior fusiform in reading. Journal of Cognitive Neuroscience. 2006;18:911–922. doi: 10.1162/jocn.2006.18.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L, Dunn D. Peabody picture vocabulary test (4th edition) Minneapolis: NCS Pearson, Inc; 2007. [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Human Brain Mapping. 1997;5:79–83. [PubMed] [Google Scholar]
- Feldman MJ, Drasgow J. The Visual-Verbal Test. Los Angeles: Western Psychological Services; 1959. [Google Scholar]
- Finkbeiner M, Caramazza A. Now you see it, now you don’t: On turning semantic interference into facilitation in a Stroop-like task. Cortex. 2006;42(6):790–796. doi: 10.1016/s0010-9452(08)70419-2. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution inter subject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Desmond JE, Demb JB, Wagner AD, Stone MV, Vaidya CJ, Glover GH. Functional magnetic resonance imaging of semantic memory processes in the frontal lobes. Psychological Science. 1996;7:278–283. [Google Scholar]
- Glaser WR, Düngelhoff FJ. The time course of picture–word interference. Journal of Experimental Psychology: Human Perception and Performance. 1984;10(5):640–654. doi: 10.1037//0096-1523.10.5.640. [DOI] [PubMed] [Google Scholar]
- Golper LC, Rau MT, Erskine B, Langhans JJ, Houlihan J. Aphasic patients’ performance on a mental status examination. Clinical Aphasiology. 1987;17:124–135. [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Annals of Neurology. 2004;55(3):335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Perani D, Garibotto V, Cappa SF, Miller BL. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71(16):1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena D, Ash S, McMillan C, Avants B, Gee J, Grossman M. Why are patients with progressive non fluent aphasia non fluent? Neurology. 2010;75:588–594. doi: 10.1212/WNL.0b013e3181ed9c7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Thompson CK. The use of the picture-word interference paradigm to examine naming abilities in aphasic individuals. Aphasiology. 2010;24(5):580–611. doi: 10.1080/02687030902777567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker P, Herskovits E, Degaonkar M. The roles of the “visual word form area” in reading. Neuroimage. 2005;24:548–559. doi: 10.1016/j.neuroimage.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic Dementia: progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115(6):1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. The Pyramids and Palm Trees Test: A test of semantic access from words and pictures. Bury St. Edmunds, UK: Thames Valley Company; 1992. [Google Scholar]
- Janse E. Lexical competition effects in aphasia: Deactivation of lexical candidates in spoken word processing. Brain and Language. 2006;97(1):1–11. doi: 10.1016/j.bandl.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Janssen N, Schrim W, Mahon BZ, Caramazza A. Semantic interference in a delayed naming task: Evidence for the response exclusion hypothesis. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2008;34(1):249–256. doi: 10.1037/0278-7393.34.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test (2nd edition) Philadelphia: Lippincott, Williams, & Wilkins; 2001. [Google Scholar]
- Kapur S, Craik FIM, Tulving E, Wilson A, Houle S, Brown GM. Proceedings of the National Academy of Science, USA. 1994;91:2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A. The Western Aphasia Battery-Revised (WAB-R) Austin, Texas: Pro-Ed; 2006. [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Meyer E, Evans AC. The neural substrates underlying word generation: a bilingual functional imaging study. Proceedings of the National Academy of Science, USA. 1995;92:2899–2903. doi: 10.1073/pnas.92.7.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronbichler M, Bergmann J, Hutzler F, Staffen W, Mair A, Ladurner G, Wimmer H. Taxi vs. Taksi: On orthographic word recognition in the left ventral occipitotemporal cortex. Journal of Cognitive Neuroscience. 2007;19(10):1584–1594. doi: 10.1162/jocn.2007.19.10.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Heij W. Components of Stroop-like interference in picture naming. Memory & Cognition. 1988;16(5):400–410. doi: 10.3758/bf03214220. [DOI] [PubMed] [Google Scholar]
- La Heij W, Dirkx J, Kramer P. Categorical interference and associative priming in picture naming. British Journal of Psychology. 1990;81:511–525. [Google Scholar]
- Laver GD, Burke DM. Why do semantic priming effects increase in old age? A meta-analysis. Psychology and Aging. 1993;8(1):34–43. doi: 10.1037//0882-7974.8.1.34. [DOI] [PubMed] [Google Scholar]
- Levelt WJM. Accessing words in speech production: Stages, processes, and representations. Cognition. 1992;42(1–3):1–22. doi: 10.1016/0010-0277(92)90038-j. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, Meyer AS. A theory of lexical access in speech production. The Behavioral & Brain Sciences. 1999;22(1):1–75. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- Lupker SJ. The semantic nature of response competition in the picture–word interference task. Memory & Cognition. 1979;7(6):485–495. [Google Scholar]
- Lupker SJ. The role of phonetic and orthographic similarity in picture–word interference. Canadian Journal of Psychology. 1982;36(3):349–367. doi: 10.1037/h0080652. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Costa A, Peterson R, Vargas KA, Caramazza A. Lexical selection is not by competition: A reinterpretation of semantic interference and facilitation effects in the picture–word interference paradigm. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2007;33(3):503–535. doi: 10.1037/0278-7393.33.3.503. [DOI] [PubMed] [Google Scholar]
- Masson MEJ. A distributed-memory model of semantic priming. Journal of Experimental Psychology-Learning Memory and Cognition. 1995;21:3–23. [Google Scholar]
- Mesulam M-M. Slowly progressive aphasia without generalized dementia. Annals of Neurology. 1982;11(6):592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. Primary progressive aphasia: A language-based dementia. New England Journal of Medicine. 2003;349(16):1535–1554. doi: 10.1056/NEJMra022435. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Rogalski E, Wieneke C, Cobia D, Rademaker A, Thompson CK, Weintraub Neurology of anomia in the semantic variant of primary progressive aphasia. Brain. 2009a;132(9):2553–2365. doi: 10.1093/brain/awp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for sub typing primary progressive aphasia. Archives of Neurology. 2009b;66(12):1545–1551. doi: 10.1001/archneurol.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AS, Schriefers H. Phonological facilitation in picture-word interference experiments: Effect of stimulus onset asynchrony and types of interfering stimuli. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1991;17(6):1146–1160. [Google Scholar]
- Milberg W, Blumstein SE, Dworetzky B. Phonological processing and lexical access in aphasia. Brain and Language. 1988;34(2):279–293. doi: 10.1016/0093-934x(88)90139-3. [DOI] [PubMed] [Google Scholar]
- Miozzo M, Caramazza A. When more is less: A counterintuitive effect of distractor frequency in the picture-word interference paradigm. Journal of Experimental Psychology: General. 2003;132(2):228–252. doi: 10.1037/0096-3445.132.2.228. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Wise RJ, Vandenberghe R, Price CJ, Hodges JR. Disrupted temporal lobe connections in semantic dementia. Brain. 1999;122(1):61–73. doi: 10.1093/brain/122.1.61. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PhD, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontogemporal lobar degeneration – A consensus on clinical diagnostic criteria. Neurology. 1998;51(6):1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Osher JE, Wicklund AH, Rademaker A, Johnson N, Weintraub S. The mini-mental state examination in behavioral variant frontotemporal dementia and primary progressive aphasia. American Journal of Alzheimer's Disease and other Dementias. 2008;122(6):468–473. doi: 10.1177/1533317507307173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partington JE, Leiter RG. Partington's Pathway Test. The Psychological Service Center Bulletin. 1949;1:9–20. [Google Scholar]
- Prather PA, Zurif E, Love T, Brownell H. Speed of lexical activation in non fluent Broca’s aphasia and fluent Wernicke’s aphasia. Brain and Language. 1997;59(3):391–411. doi: 10.1006/brln.1997.1751. [DOI] [PubMed] [Google Scholar]
- Peterson SE, Fox PT, Posner ME, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;362:342–345. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The Interactive Account of ventral occipitotemporal contributions to reading. Trends in Cognitive Sciences. 2011;15(6):246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, Goldrick M. Discreteness and interactivity in spoken word production. Psychological Review. 2000;107(3):460–499. doi: 10.1037/0033-295x.107.3.460. [DOI] [PubMed] [Google Scholar]
- Rayner K, Springer CJ. Graphemic and semantic similarity effects in the picture–word interference task. British Journal of Psychology. 1986;77(2):207–222. doi: 10.1111/j.2044-8295.1986.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Roelofs A. The WEAVER model of word-form encoding in speech production. Cognition. 1997;64(3):249–284. doi: 10.1016/s0010-0277(97)00027-9. [DOI] [PubMed] [Google Scholar]
- Roelofs A. A spreading activation theory of lemma retrieval in speaking. Cognition. 1992;42(1–3):107–142. doi: 10.1016/0010-0277(92)90041-f. [DOI] [PubMed] [Google Scholar]
- Rogalski E, Rademaker A, Mesulam M-M, Weintraub S. Covert processing of words and pictures in non semantic variants of primary progressive aphasia. Alzheimers Disease and Associated Disorders. 2008;22(4):343–351. doi: 10.1097/WAD.0b013e31816c92f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HJ, Kramer JH, Gorno-Tempini ML, Schuff N, Weiner M, Miller BL. Patterns of Cerebral Atrophy in Primary Progressive Aphasia. 1. Vol. 10. American Journal of Geriatric Psychiatry; 2010. pp. 89–97. [PubMed] [Google Scholar]
- Schriefers H, Meyer AS, Levelt WJM. Exploring the time course of lexical access in language production: Picture-word interference studies. Journal of Memory and Langauge. 1990;29(1):86–102. [Google Scholar]
- Simons JS, Koutstaal W, Prince S, Wagner AD, Schachter DL. Neural mechanisms of visual object priming: Evidence for perceptual and semantic distinctions in fusiform cortex. NeuroImage. 2003;19:613–626. doi: 10.1016/s1053-8119(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken AU. Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience. 2008;9(102):1–11. doi: 10.1186/1471-2202-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonty SP, Mesulam M-M, Weintraub S, Johnson NA, Parrish TB, Gitelman DR. Altered effective connectivity within the language network in primary progressive aphasia. The Journal of Neuroscience. 2007;27(6):1334–1345. doi: 10.1523/JNEUROSCI.4127-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starreveld PA, La Heij W. Semantic interference, orthographic facilitation, and their interaction in naming tasks. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21(3):686–698. [Google Scholar]
- Starreveld PA, La Heij W. Time-course analysis of semantic and orthographic context effects in picture naming. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22(4):896–918. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Swinney D, Prather P, Love T. The time-course of lexical access and the role of context: Converging evidence from normal and aphasic processing. In: Grodzinsky Y, Shapiro LP, Swinney D, editors. Language and the brain: Representation and processing. New York: Academic Press; 2000. pp. 273–292. [Google Scholar]
- Swinney D, Zurif EB, Nicol J. The effects of focal brain damage on sentence processing: An examination of the neurobiological organization of a mental module. Journal of Cognitive Neuroscience. 1989;1(1):25–37. doi: 10.1162/jocn.1989.1.1.25. [DOI] [PubMed] [Google Scholar]
- Taylor JK, Burke DM. Asymmetric aging effects on semantic and phonological processes: Naming in the picture-word interference task. Psychology and Aging. 2002;17(4):662–676. doi: 10.1037//0882-7974.17.4.662. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Ballard KJ, Tait ME, Weintraub S, Mesulam M-M. Patterns of language decline in non fluent primary progressive aphasia. Aphasiology. 1997;11:297–322. [Google Scholar]
- Thompson CK, Shapiro LP, Tait ME, Jacobs B, Schneider S, Ballard K. A system for the linguistic analysis of agrammatic language production. Brain and Language. 1995;51:124–129. [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood G. Semantic interference from unattended printed words. British Journal of Psychology. 1976;67(3):327–338. [Google Scholar]
- Vandenberghe RR, Vandenbulcke M, Weintraub S, Johnson N, Porke K, Thompson CK, Mesulam M-M. Paradoxical features of word finding difficulty in primary progressive aphasia. Annals of Neurology. 2005;57(2):204–209. doi: 10.1002/ana.20362. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Paré-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: Left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Mesulam M-M, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The Northwestern Anagram Test: Measuring sentence production in Primary Progressive Aphasia. The American Journal of Alzheimer’s Disease and other Dementias. 2009;24(5):408–416. doi: 10.1177/1533317509343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler memory scale (3rd edition) San Antonio, Texas: The Psychological Corporation; 1997. [Google Scholar]
- Wicklund A, Johnson N, Weintraub S. Preservation of reasoning in primary progressive aphasia: further differentiation from Alzheimer's disease and frontal lobe dementia. Journal of Clinical and Experimental Neuropsychology. 2004;26:347–355. doi: 10.1080/13803390490510077. 2004. [DOI] [PubMed] [Google Scholar]
- Wilshire CE, Keall LM, Stuart EJ, O’Donnell DJ. Exploring the dynamics of aphasic word production using the picture–word interference task: A case study. Neuropsychologia. 2007;45(5):939–953. doi: 10.1016/j.neuropsychologia.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, Miller BL, Gorno-Tempini ML. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133(7):2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee E, Blumstein SE, Sedivy JC. Lexical-semantic activation in Broca’s and Wernicke’s aphasia: Evidence from eye movements. Journal of Cognitive Neuroscience. 2008;20(4):592–612. doi: 10.1162/jocn.2008.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]