Abstract
Objectives
To report long-term HIV treatment outcomes in 7 Caribbean countries.
Design
Observational cohort study.
Methods
We report outcomes for all antiretroviral therapy (ART) naïve adult patients enrolled on ART from program inception until study closing for cohorts in Barbados, the Dominican Republic, Haiti, Jamaica, Martinique, Trinidad, and Puerto Rico. Incidence and predictors of mortality were analyzed by time-to-event approaches.
Results
8,203 patients started ART from 1998 to 2008. Median follow-up time was 31 months (interquartile range: 14 to 50 months). Mortality was 13% overall: 6% in Martinique, 8% in Jamaica, 11% in Trinidad, 13% in Haiti, 15% in the Dominican Republic, 15% in Barbados, and 24% in Puerto Rico. Mortality was associated with male gender (HR 1.58; 95% CI: 1.33 – 1.87), body weight (HR 0.85 per 10 pounds; 95% CI: 0.82 – 0.89), hemoglobin (HR 0.84 per g/dl; 95% CI: 0.80 – 0.88), CD4 cell count (0.90 per 50 CD4 cells; 95% CI: 0.86 – 0.93), concurrent TB (HR 1.58; 95% CI: 1.25 – 2.01) and age (HR 1.19 per 10 years; 95% CI: 1.11 – 1.28). After controlling for these variables, mortality in Martinique, Jamaica, Trinidad and Haiti was not significantly different. A total of 75% of patients remained alive and in-care at the end of the study period.
Conclusions
Long-term mortality rates vary widely across the Caribbean. Much of the difference can be explained by disease severity at ART initiation, nutritional status, and concurrent TB. Earlier ART initiation will be critical to improve outcomes.
Keywords: HIV/AIDS, HIV, antiretroviral therapy, tuberculosis, low and middle-income countries, Caribbean
INTRODUCTION
The Caribbean is the region most heavily affected by HIV outside of sub-Saharan Africa, with an overall adult HIV prevalence of 1.0%, and an estimated 240,000 patients living with HIV [1]. An estimated 12,000 persons die annually of AIDS-related illnesses [1], and AIDS is the leading cause of death in the Caribbean among men and women from 20 to 59 years of age [2]. As in the United States, the predominant HIV-1 subtype in the Caribbean is subtype B [3]. Due to its close proximity, the HIV epidemic in the Caribbean is also a major public health issue for the United States. Twenty-seven percent of new HIV diagnoses in New York City are among foreign-born persons [4]. The Caribbean accounts for more HIV diagnoses in New York City than any other region (38%), and if Puerto Rico is added, the region accounts for 56% of all new HIV diagnoses [4]. With frequent travel and migration between the Caribbean and the United States and Europe, the importance of HIV/AIDS in the Caribbean transcends regional boundaries.
With the global expansion of antiretroviral treatment (ART), an increasing number of studies have documented positive short-term outcomes of ART for early cohorts of patients in low and middle-income countries, including high adherence rates and favorable virological, immunological, and clinical responses [5–10]. In recent years, data on long-term outcomes of ART programs have begun to emerge, demonstrating that earlier successes can be maintained even as programs undergo rapid scale-up [11–30]. However, mortality rates in the first 6 months of ART are disproportionately higher in low and middle-income countries, compared to higher-income countries [8, 9, 18, 26], and there have been reports of high rates of program attrition after two years on therapy [31–33].
Published data on long-term HIV treatment outcomes in the Caribbean are limited [18, 34, 35], though ART coverage in the region has increased dramatically in the last decade. By the end of 2009, 48% of patients with a CD4 cell count < 350 cells/mm3 in the region were receiving ART, compared to 1% of those eligible in 2004 [36, 37]. Countries in the Caribbean region vary widely in economic status, health infrastructure, culture, and language. Long-term data on HIV treatment outcomes across the region are critically needed.
In 2006, the Trans-Caribbean HIV/AIDS Research Initiative (TCHARI) was launched to develop a cohesive HIV/AIDS research agenda that would address the specific issues relating to HIV/AIDS in the Caribbean. This is the first study to be conducted in the TCHARI network and includes data from seven countries: Barbados, Dominican Republic, Haiti, Jamaica, Martinique (France), Puerto Rico (United States), and Trinidad. This is the first multi-cohort study to present regional outcomes for ART across countries in the Caribbean, as well as the first regional outcomes paper on health in the Caribbean.
METHODS
Participants and Settings
TCHARI is a collaboration among several of the largest HIV/AIDS clinical and research centers in the Caribbean and the National Institutes of Health Office of AIDS Research. The TCHARI sites include: 1) Ladymeade Reference Unit, St. Michael’s, Barbados; 2) Comprehensive Care Units of Centro Sanitario de Santo Domingo and Hospital Luis E. Aybar, Santo Domingo, Dominican Republic; 3) Haitian Study Group for Kaposi’s Sarcoma and Opportunistic Infections (GHESKIO), Port-au-Prince, Haiti; 4) The University Hospital Centre for HIV/AIDS Research, Education and Services and the Comprehensive Health Centre, Kingston, Jamaica; 5) Infectious Diseases Unit/Inserm CIE 802, Centre Hospitalier Universitaire, Fort-de-France, Martinique; 6) the Medical Research Centre, Port of Spain, Trinidad and Tobago; and 7) Retrovirus Research Center of the Universidad Central del Caribe, Ramon Ruiz Arnau University Hospital, Bayamon, Puerto Rico. Institutional Review Board approval was obtained by all local and partner academic sites.
This study includes all ART-naive HIV-infected patients of age 13 years or older who were consecutively enrolled on ART at these 7 sites during the study period, beginning at program inception at each site. The study dates vary among sites, but all patients initiated ART between January 1, 1998 and December 31, 2008. Table 1 describes the country and program characteristics of sites participating in TCHARI. Across the Caribbean region, HIV-infected patients consistently tend to be members of marginalized populations that experience economic disparity.
Table 1.
Country and Program Characteristics of the TCHARI sites
| Country | Barbados | DR | Haiti | Jamaica | Martinique | Trinidad | PR |
|---|---|---|---|---|---|---|---|
| GDP per Capita ($US)* | 13,849 | 5078 | 785 | 4705 | 39,922 | 19,076 | 17,100 |
| Funding sources for ART | Ministry of Health with World Bank loan | Ministry of Public Health and Welfare with World Bank loan | GFATM and PEPFAR** | GFATM** | Ministry of Health | Governme nt of Trinidad | Ryan White |
| HIV prevalence (%) *** | 1.4 | 0.9 | 2.2 | 1.7 | 0.3 | 2.0 | 0.2 |
| % HIV-infected patients HBsAg-positive** | Not known in HIV+; felt to be low | Not known in HIV+; felt to be low | 4% of HIV+ | Not known in HIV+; felt to be low | 2.5% of HIV+ | 4.5% of HIV+ | 6% of HIV+ without IVDU; 7% of HIV+ with IVDU |
| % HIV-infected patients HCV Ab positive** | Not known in HIV+; felt to be low | Not known in HIV+; felt to be low | <1% of HIV+ | Not known in HIV+; felt to be low | 5.5% of HIV+ without IVDU; 96% of HIV+ with IVDU | Not known in HIV+; felt to be low | 54% of HIV+ without IVDU; 96% HIV+ with IVDU |
| Year of expanded ART access | 2002 | 2003 | 2003 | 2004 | 1996 | 2003 | 1998 |
| Total number of patients in the study | 560 | 1207 | 4717 | 476 | 325 | 725 | 193 |
| Total number of adults on ART at the of end 2009 | 793 | 14,000 | 25,673 | 6549 | 750 | 2335 | Not calculated |
| Percentage of patients receiving free ART | 100% | 100% | 100% | 100% | 100% | 100% | 85% |
| ART initiation criteria during the study period | WHO criteria**** | WHO criteria**** | WHO criteria**** | WHO criteria**** | CD4<350 cells/mm3 or symptomatic HIV disease | CD4<250 cells/mm3 or AIDS-defining condition | WHO criteria or clinical judgment of physician |
| Virologic monitoring during study period | Conducted routinely | Suspected treatment failure | Suspected treatment failure | Suspected treatment failure | Conducted routinely | Conducted routinely | Conducted routinely |
| Method of tracking patients that miss visits (contact is done with phone calls and/or home visits) | Community nurse contacts patient if they miss 2 visits or are 6 months late | Peer counselors contact patient after one missed visit | Field workers contact patients within one week of each missed visit | Adherence counselors contact patients after 2 missed visits (visits usually every 3 months) | Phone and mail after one late visit. | Adherence nurse calls or visits patient at home after one late visit. | Phone call after 2 missed visits (visits usually every 6 months) |
GDP (Gross Domestic Product). International Monetary Fund. Economic Outlook Database available at: http://imf.org/external/pubs/ft/weo/2009/01/weodata/index.aspx [60]
GFATM (Global Fund to Fight AIDS, Tuberculosis and Malaria); PEPFAR (U.S. President’s Emergency Plan for AIDS Relief); HBsAg (hepatitis B surface antigen); HCV Ab (hepatitis C antibody)
UNAIDS database, 2010 [61]; for Haiti, Republique d’Haiti Programme National de Lutte contre le Sida, 2010 [62]
At the time of the study, the World Health Organization (WHO) recommended ART for all patients with a CD4 cell count <200 cells/ml or an AIDS-defining illness
Data Collection and Measurement
At each site, de-identified data were entered into a Microsoft Access database (Microsoft, Redmond, Washington) and sent to GHESKIO, where they were checked for errors, inconsistencies, and missing data. These were compiled into queries which were addressed by each site. Baseline weight, hemoglobin, and CD4 cell count were defined as the measurement closest to the date of ART initiation, but not more than 2 weeks after ART initiation. ART was defined as a combination of two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitor (NNRTI) or protease inhibitor (PI), or three NRTIs. Clinical stage of disease was defined according to World Health Organization guidelines [38]. Tuberculosis (TB) and ART co-treatment was defined as any overlap of treatment with these two therapies.
Outcomes
The primary outcomes were all-cause mortality and retention in care over the duration of the study. Time was measured from the date of ART initiation to the date of death, loss to follow-up (LTFU), or closing date of the study. Death was ascertained through several strategies. In Barbados, deaths were determined by chart review. In Haiti, deaths were ascertained by chart review, phone calls to next of kin, and home visits. In Jamaica and Martinique, deaths were ascertained by chart review, ART treatment database, phone calls to next of kin, and a centralized death registry. In Trinidad, Dominican Republic and Puerto Rico, deaths were ascertained by reviewing clinical and hospital records, tracking patients that were LTFU, taking reports from family members, and reviewing death certificates, as necessary. The study closed on May 3, 2008 in Puerto Rico, February 28, 2009 in Barbados, May 15, 2009 in the Dominican Republic, June 18, 2009 in Trinidad, December 15, 2009 in Martinique, and December 31, 2009 in Haiti and Jamaica. Patients were considered alive and in-care if they were not known to be dead and had at least one visit within 6 months of the closing date of the database. The data were analyzed by an intention-to-treat approach.
Statistical Analysis
Data were entered into the Access database described above. All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC). Kaplan-Meier survival analyses were used to estimate the time from initiation of ART to death, and results were plotted at the country cohort level. Patients who were transferred to other clinics were censored at their last visit. Cox proportional-hazards models were used to assess the relationship between demographic and clinical variables, treatment site, and time to death.
We conducted univariate and multivariate analyses using the following variables: year of ART initiation, ART regimen, and country as categorical variables; gender, history of intravenous drug use, and TB and ART co-treatment as binary variables; and age, baseline weight, hemoglobin, and CD4 cell count as continuous variables. WHO stage, income, and education were not included in the analyses, because they were not collected at all sites. We analyzed all variables with Cox proportional hazards models to determine predictors of time to death. In the multivariate model, we included all variables significant at the 0.05 level in univariate analyses. No sets of variables in the reported model showed signs of unacceptable colinearity.
We performed sensitivity analyses to assess the effect of LTFU on mortality rates. We reviewed the literature from tracking studies among ART patients presumed LTFU in low and middle-income countries. We considered a scenario with the lowest and highest mortality rates (27% and 87%, respectively) among patients presumed LTFU in the published literature [39, 40]. We also used the combined mortality from the meta-analysis of 17 studies reported by Brinkhof et al (combined mortality of 40% among patients presumed LTFU) [41]. Finally we considered the scenario where all patients that were LTFU with a CD4 cell count < 50 cells/mm3 and < 100 cells/mm3 had died.
RESULTS
A total of 8,203 ART-naive HIV-infected patients of age 13 years or older that were consecutively enrolled on ART were included in this study: 560 (7%) from Barbados, 1207 (15%) from the Dominican Republic, 4717 (58%) from Haiti, 476 (6%) from Jamaica, 325 (4%) from Martinique, 725 (9%) from Trinidad and 193 (2%) from Puerto Rico. Patient characteristics are summarized in Table 2 (note that percentages were computed using the number of patients with non-missing values). Across all countries, 51% were female and the median age at ART initiation was 38 years. Education was measured in all sites except Jamaica; overall 42% of patients attended no school or primary school only. Income was measured in Barbados, Haiti, Trinidad, and Puerto Rico, and 57% of patients in these cohorts lived on less than $US 1 per day.
Table 2.
Characteristics of Patients Receiving Antiretroviral Therapy in the Caribbean
| Barbados (n=560) | DR (n=1207) | Haiti (n=4717) | Jamaica (n=476) | Martinique (n=325) | Trinidad (n=725) | PR (n=193) | Combined (n=8203) | |
|---|---|---|---|---|---|---|---|---|
| Age* | 39 (32, 46) | 38 (32, 45) | 38 (31, 45) | 38 (31, 45) | 41 (34, 49) | 35 (28, 44) | 41 (35, 47) | 38 (31, 45) |
| Female gender – no. (%) | 241 (43) | 627 (52) | 2566 (54) | 229 (48) | 120 (37) | 344 (47) | 64 (33) | 4191 (51) |
| Education – no. (%) | ||||||||
| Missing** | 26 (5) | 493 (41) | 39 (1) | *** | 10 (3) | 164 (23) | 3 (2) | 1211 (15) |
| None or primary only | 1 (0) | 312 (44) | 2366 (51) | *** | 149 (47) | 111 (20) | 31 (16) | 2970 (42) |
| At least some secondary | 487 (91) | 342 (48) | 2146 (46) | *** | 113 (36) | 427 (76) | 126 (66) | 3641 (52) |
| At least some university | 46 (9) | 60 (8) | 166 (4) | *** | 53 (17) | 23 (4) | 33 (17) | 381 (5) |
| Income – no. (%) | ||||||||
| Missing** | 23 (4) | *** | 45 (1) | *** | *** | 71 (10) | 7 (4) | 2154 (26) |
| <365 USD/year | 131 (24) | *** | 2961 (63) | *** | *** | 228 (35) | 117 (63) | 3437 (57) |
| 365 – 999 USD/year | 157 (29) | *** | 1547 (33) | *** | *** | 61 (9) | 14 (8) | 1779 (29) |
| 1000 – 2999 USD/year | 146 (27) | *** | 114 (2) | *** | *** | 137 (21) | 26 (14) | 423 (7) |
| > 3000 USD/year | 103 (19) | *** | 50 (1) | *** | *** | 228 (35) | 29 (16) | 410 (7) |
| Current or past intravenous drug use – no. (%) | ||||||||
| Missing** | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 13 (4) | 0 (0) | 0 (0) | 17 (0) |
| No intravenous drug use | 560 (100) | 1180 (98) | 4717 (100) | 453 (95) | 275 (88) | 725 (100) | 91 (47) | 7908 (97) |
| Intravenous drug use | 0 (0) | 27 (2) | 0 (0) | 23 (5) | 37 (12) | 0 (0) | 102 (53) | 278 (3) |
| Weight at ART initiation – no. (%)* | ||||||||
| Missing** | 70 (13) | 213 (18) | 187 (4) | 95 (20) | 35 (11) | 68 (9) | 24 (12) | 692 (8) |
| Median for females (pounds) | 134 (112, 161) | 121 (104, 140) | 111 (98, 127) | 131 (110, 154) | 139 (121, 163) | 128 (110, 151) | 134 (115, 165) | 116 (101, 136) |
| Median for males (pounds) | 146 (127, 166) | 136 (122, 152) | 125 (111, 140) | 141 (127, 157) | 146 (130, 164) | 139 (124, 155) | 152 (134, 168) | 131 (116, 148) |
| Baseline hemoglobin – no. (%)* | ||||||||
| Missing** | 95 (17) | 98 (8) | 498 (11) | 164 (34) | 31 (10) | 22 (3) | 37 (19) | 945 (12) |
| Median for females | 11.3 (10.3, 12.3) | 11.1 (9.9, 12.0) | 10.0 (8.9, 11.0) | 10.2 (9.1, 11.3) | 11.1 (10.1, 12.0) | 10.0 (9.0, 11.0) | 11.8 (10.2, 13.1) | 10.3 (9.1, 11.4) |
| Median for males | 12.5 (11.3, 13.7) | 12.0 (10.5, 13.2) | 11.0 (9.6, 12.2) | 11.8 (10.4, 13.0) | 12.6 (11.3, 13.7) | 11.0 (10.0, 13.0) | 13.2 (11.5, 14.9) | 11.4 (10.0, 12.9) |
| Baseline CD4 Cell Count – no. (%)* | ||||||||
| Missing** | 94 (17) | 211 (17) | 423 (9) | 124 (26) | 10 (3) | 19 (3) | 10 (5) | 891 (11) |
| Median CD4 cell count | 128 (42, 221) | 96 (33, 175) | 122 (49, 192) | 123 (47, 230) | 196 (47, 333) | 85 (32, 173) | 221 (72, 340) | 118 (44, 196) |
| Baseline CD4 Cell Count – no. (%) | ||||||||
| Missing** | 94 (17) | 211 (17) | 423 (9) | 124 (26) | 10 (3) | 19 (3) | 10 (5) | 891 (11) |
| < 50 cells/mm3 | 133 (29) | 321 (32) | 1074 (25) | 92 (26) | 79 (25) | 255 (36) | 34 (19) | 1988 (27) |
| 50 to 99 cells/mm3 | 72 (15) | 192 (19) | 776 (18) | 62 (18) | 31 (10) | 133 (19) | 19 (10) | 1285 (18) |
| 100 to 199 cells/mm3 | 109 (23) | 302 (30) | 1501 (35) | 91 (26) | 49 (16) | 201 (28) | 31 (17) | 2284 (31) |
| 200 to 349 cells/mm3 | 117 (25) | 163 (16) | 827 (19) | 85 (24) | 90 (29) | 117 (17) | 55 (30) | 1454 (20) |
| >/= 350 cells/mm3 | 35 (8) | 18 (2) | 116 (3) | 22 (6) | 66 (21) | 0 (0) | 44 (24) | 301 (4) |
| WHO Stage – no. (%) | ||||||||
| Missing** | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | *** | 0 (0) | 725 (9) |
| Stage I | 156 (28) | 223 (18) | 366 (8) | 75 (16) | 198 (61) | *** | 91 (47) | 1109 (15) |
| Stage II | 107 (19) | 439 (36) | 0 (0) | 134 (28) | 12 (4) | *** | 54 (28) | 746 (10) |
| Stage III | 99 (18) | 359 (30) | 3080 (65) | 160 (34) | 28 (9) | *** | 4 (2) | 3730 (50) |
| Stage IV | 198 (35) | 186 (15) | 1271 (27) | 107 (22) | 87 (27) | *** | 44 (23) | 1893 (25) |
| Year of ART Initiation – no. (%) | ||||||||
| Missing** | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 1998–2002 | 152 (27) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0) | 127 (66) | 280 (3) |
| 2003–2004 | 250 (45) | 76 (6) | 1414 (30) | 65 (14) | 97 (30) | 355 (49) | 39 (20) | 2296 (28) |
| 2005–2006 | 158 (28) | 562 (47) | 1128 (24) | 336 (71) | 109 (34) | 369 (51) | 24 (12) | 2686 (33) |
| 2007–2008 | 0 (0) | 569 (47) | 2175 (46) | 75 (16) | 119 (37) | 0 (0) | 3 (2) | 2941 (36) |
| First ART Regimen – no. (%) | ||||||||
| Missing** | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| NNRTI-containing regimen | 486 (87) | 1192 (99) | 4475 (95) | 458 (96) | 25 (8) | 718 (99) | 30 (16) | 7384 (90) |
| PI-containing regimen | 67 (12) | 14 (1) | 181 (4) | 16 (3) | 271 (83) | 6 (1) | 156 (81) | 711 (9) |
| Other regimen | 7 (1) | 1 (0) | 61 (1) | 2 (0) | 29 (9) | 1 (0) | 7 (4) | 108 (1) |
| Tuberculosis – no. (%)**** | ||||||||
| Missing** | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| No TB and ART Co-Treatment | 558 (100) | 1155 (96) | 4235 (90) | 469 (99) | 321 (99) | 707 (98) | 193 (100) | 7638 (93) |
| TB and ART Co-Treatment | 2 (0) | 52 (4) | 482 (10) | 7 (1) | 4 (1) | 18 (2) | 0 (0) | 565 (7) |
Continuous variables are reported as medians (interquartile range)
Percentages are computed using the number of patients with non-missing values
Data not collected at site
TB and ART co-treatment was defined as any overlap of treatment with these two therapies.
Median baseline weight varied across countries, from a low of 111 pounds for women and 125 for men in Haiti, to highs of 139 pounds for women in Martinique and 152 for men in Puerto Rico. Baseline hemoglobin values were lowest in Haiti and Trinidad (10.0 g/dl for women and 11.0 g/dl for men) and highest in Puerto Rico (11.8 g/dl for women and 13.2 g/dl for men). Intravenous drug use was common only in Puerto Rico (53%), and 534 of 565 cases of TB and ART co-treatment (95%) occurred in Haiti and the Dominican Republic. Among the 565 patients with concurrent TB and ART treatment, 266 (47%) were started on TB treatment prior to or at the same time as ART, 177 (31%) were started on TB treatment within the first 6 months after ART initiation, and 122 (22%) were started on TB treatment after at least 6 months of ART.
Seventy-six percent of patients initiated ART with a CD4 cell count < 200 cells/mm3: 83% in Trinidad, 81% in the Dominican Republic, 78% in Haiti, 70% in Jamaica, 67% in Barbados, 51% in Martinique, and 46% in Puerto Rico. The median baseline CD4 cell count for the combined overall cohort was 118 cells/mm3. Baseline CD4 cell count increased only slightly with expansion of access to ART, from 114 cells/mm3 for those initiating ART in 2003–2004, to 137 cells/mm3 for those initiating ART in 2007–2008 (see Web Appendix). Ninety percent of patients overall (n=7384) were treated with a first-line regimen containing a NNRTI; of these 4382 (59%) were treated with an efavirenz-based regimen and 3002 (41%) were treated with a nevirapine-based regimen. Martinique and Puerto Rico were the exceptions, treating over 80% of patients with a PI in the first-line regimen. Among the 271 patients in Martinique that received a PI-based regimen, 158 (58%) were treated with lopinavir/ritonavir, 79 (29%) with atazanavir, 23 (8%) with fosamprenavir, 8 (3%) with indinavir, and 3 (1%) with nelfinavir. In contrast, of 156 patients treated with a PI-based regimen in PR, 113 (72%) were treated with nelfinavir, 27 (17%) were treated with indinavir, 9 (6%) were treated with atazanavir, 6 (4%) were treated with other PI’s, and only one patient was treated with lopinavir/ritonavir.
The median follow-up time across sites was 31 months (interquartile range [IQR]: 14 to 50 months), ranging from 20 months (IQR: 9 to 33 months) in the Dominican Republic to 52 months (IQR: 33 to 70 months) in Barbados (Table 3). A total of 1048 patients (13%) were known to have died during the study period. Mortality rates varied widely by country, as follows: 6% in Martinique, 8% in Jamaica, 11% in Trinidad, 13% in Haiti, 15% in the Dominican Republic, 15% in Barbados, and 24% in Puerto Rico.
Table 3.
Mortality and Loss to Follow-up during the Study Period (per 100 person-years in care)
| Barbados (n=560) | DR (n=1207) | Haiti (n=4717) | Jamaica (n=476) | Martinique (n=325) | Trinidad (n=725) | Puerto Rico (n=193) | Combined (n=8203) | |
|---|---|---|---|---|---|---|---|---|
| Median Follow-up Time and Patient Status | ||||||||
| Median Follow-up (Months) | 52 (33, 70) | 20 (9, 33) | 27 (13, 49) | 40 (28, 47) | 39 (21, 60) | 48 (37, 59) | 35 (10, 63) | 31 (14, 50) |
| Alive or Transferred | 434 (78) | 871 (72) | 3517 (75) | 361 (76) | 290 (89) | 593 (82) | 56 (29) | 6122 (75) |
| Deceased | 85 (15) | 181 (15) | 598 (13) | 38 (8) | 21 (6) | 78 (11) | 47 (24) | 1048 (13) |
| Lost to Follow-up | 41 (7) | 155 (13) | 602 (13) | 77 (16) | 14 (4) | 54 (7) | 90 (47) | 1033 (13) |
| Mortality | ||||||||
| 3 months | 13.9 | 34.2 | 21.8 | 10.8 | 3.8 | 17.2 | 6.8 | 20.9 |
| 6 months | 10.8 | 23.8 | 15.7 | 6.4 | 5.1 | 10.8 | 8.3 | 14.8 |
| 1 year | 8.1 | 15.5 | 10.1 | 3.8 | 3.6 | 6.5 | 7.0 | 9.6 |
| 3 years | 4.6 | 8.9 | 5.9 | 3.2 | 2.3 | 3.2 | 6.7 | 5.5 |
| 5 years | 3.8 | 8.4 | 5.0 | 2.7 | 2.1 | 2.8 | 6.9 | 4.7 |
| Loss to Follow-up | ||||||||
| 6 months | 1.1 | 15.1 | 16.7 | 16.1 | 3.8 | 3.5 | 39.1 | 14.0 |
| 1 year | 1.3 | 10.5 | 10.7 | 10.6 | 2.6 | 3.7 | 26.8 | 9.3 |
| 3 years | 2.1 | 7.2 | 6.1 | 6.5 | 1.6 | 2.1 | 15.7 | 5.5 |
| 5 years | 1.7 | 7.2 | 5.1 | 5.5 | 1.3 | 2.0 | 14.6 | 4.7 |
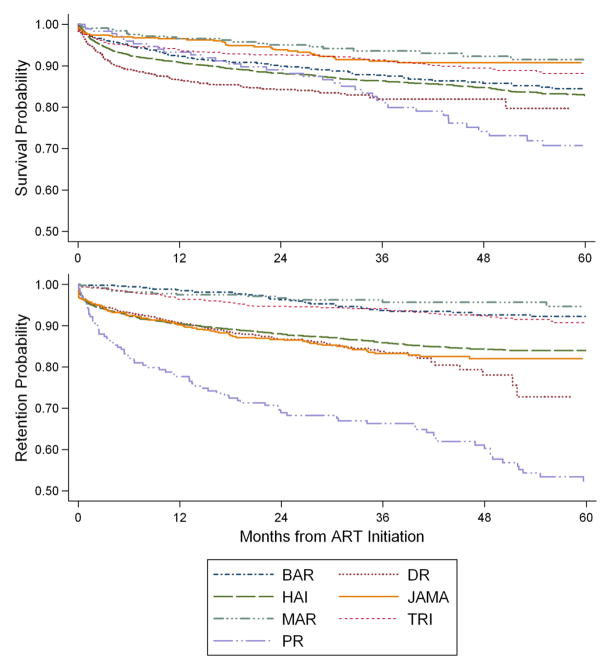
The overall mortality rate was highest in the first 3 months (20.9 deaths/100 person-years [PY]), and it progressively declined to 4.7 deaths/100 PY at 5 years. Figure 1A shows the Kaplan-Meier plots of mortality over the study duration. The high early mortality was most pronounced in the Dominican Republic (34.2 deaths/100 PY) and Haiti (21.8 deaths/100 PY), the countries with the lowest baseline body weight and nearly all of the cases of TB and ART co-treatment, and Trinidad (17.2 deaths/100 PY), which had the lowest median CD4 cell count at ART initiation.
Figure 1.
Cumulative estimates of survival and retention in care by treatment site
Table 4 shows the univariate and adjusted hazard ratios (HRs) for mortality for the combined cohort. In the univariate analyses, older age, male gender, TB and ART co-treatment, intravenous drug use, and lower baseline weight, hemoglobin, and CD4 cell count were associated with mortality. ART regimen was not associated with mortality (p-value=0.3164). In the multivariate analyses, higher body weight (HR 0.85 per 10 pounds; 95% CI: 0.82 – 0.89; p<0.0001), higher hemoglobin (HR 0.84 per g/dl; 95% CI: 0.80 – 0.88; p<0.0001), and higher CD4 cell count (0.90 per 50 CD4 cells; 95% CI: 0.86 – 0.93; p<0.0001) at ART initiation were associated with a lower hazard of death. Male gender (HR 1.58; 95% CI: 1.33 – 1.87; p<0.0001), TB and ART co-treatment (HR 1.58; 95% CI: 1.25 – 2.01; p=0.0002) and increased age (HR 1.19 per 10 years; 95% CI: 1.11 – 1.28; p<0.0001) were associated with an increased hazard of death. In the multivariate analysis, year of ART initiation and intravenous drug use were not associated with mortality.
Table 4.
Risk Factors for Mortality by Cox Proportional Hazards Regression
| Variable | Reference Group | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-value | p-value for Variable | Hazard Ratio | p-value | p-value for Variable | ||
| Year of ART initiation | |||||||
| 1998–2000 | 2007–2008 | 3.21 (2.17 – 4.73) | <0.0001 | <0.0001 | 2.17 (0.92 – 5.15) | 0.0781 | 0.1794 |
| 2001–2002 | 2007–2008 | 1.27 (0.88 – 1.84 | 0.1942 | 0.87 (0.47 – 1.62) | 0.6634 | ||
| 2003–2004 | 2007–2008 | 1.54 (1.30 – 1.82) | <0.0001 | 0.94 (0.75 – 1.18) | 0.6013 | ||
| 2005–2006 | 2007–2008 | 1.24 (1.04 – 1.47) | 0.0151 | 1.05 (0.85 – 1.31) | 0.6240 | ||
| ART regimen | |||||||
| PI regimen | NNRTI | 0.88 (0.71 – 1.10) | 0.2675 | 0.3164 | N/A | N/A | N/A |
| Other regimen | NNRTI | 1.24 (0.81 – 1.92) | 0.3259 | N/A | N/A | N/A | |
| Site | |||||||
| Barbados | Haiti | 0.91 (0.72 – 1.14) | 0.4209 | <0.0001 | 1.95 (1.37 – 2.76) | 0.0002 | <0.0001 |
| Dominican Republic | Haiti | 1.40 (1.18 – 1.66) | <0.0001 | 1.72 (1.34 – 2.21) | <0.0001 | ||
| Jamaica | Haiti | 0.58 (0.42 – 0.80) | 0.0010 | 0.88 (0.52 – 1.47) | 0.6183 | ||
| Martinique | Haiti | 0.43 (0.28 – 0.67) | 0.0002 | 1.03 (0.63 – 1.70) | 0.8936 | ||
| Puerto Rico | Haiti | 1.59 (1.17 – 2.18) | 0.0033 | 2.25 (1.04 – 4.87) | 0.0389 | ||
| Trinidad | Haiti | 0.68 (0.53 – 0.86) | 0.0013 | 0.82 (0.60 – 1.12) | 0.2060 | ||
| Age at ART initiation | Unit = 10 years | 1.13 (1.07 – 1.20) | <0.0001 | 1.19 (1.11 – 1.28) | <0.0001 | ||
| Gender | Female | 1.22 (1.08 – 1.38) | 0.0014 | 1.58 (1.33 – 1.87) | <0.0001 | ||
| Intravenous drug use | None | 1.40 (1.05 – 1.86) | 0.0220 | 1.32 (0.85 – 2.05) | 0.2119 | ||
| Baseline weight | Unit = 10 pounds | 0.84 (0.82 – 0.87) | <0.0001 | 0.85 (0.82 – 0.89) | <0.0001 | ||
| Baseline hemoglobin | Unit = 1 g/dl | 0.82 (0.79 – 0.85) | <0.0001 | 0.84 (0.80 – 0.88) | <0.0001 | ||
| Baseline CD4 cell count | Unit = 50 cells/ml | 0.88 (0.85 – 0.91) | <0.0001 | 0.90 (0.86 – 0.93) | <0.0001 | ||
| TB and ART Co-Treatment | No TB | 1.84 (1.53 – 2.21) | <0.0001 | 1.58 (1.25 – 2.01) | 0.0002 | ||
In the univariate analysis, mortality varied by country, with HR 0.43 (95% CI: 0.28 – 0.67; p=0.0002) for Martinique, 0.58 (95% CI: 0.42 – 0.80; p=0.0010) for Jamaica, 0.68 (95% CI: 0.53 – 0.86; p=0.0013) for Trinidad, 0.91 (95% CI: 0.72 – 1.14; p=0.4209) for Barbardos, 1.40 (95% CI: 1.18 – 1.66; p<0.0001) for the Dominican Republic, and 1.59 (95% CI: 1.17 – 2.18; p=0.0033) for Puerto Rico, compared to Haiti (reference group). These country-level differences decreased after adjusting for other variables. In the multivariate analysis (with Haiti as reference), there was no association between site and mortality for Jamaica, Martinique, and Trinidad. Higher hazard ratios persisted in the multivariate analysis for the Dominican Republic (HR 1.72: 95% CI: 1.34 – 2.21; p<0.0001), Barbados (HR 1.95: 95% CI: 1.37 – 2.76; p=0.0002), and Puerto Rico (HR 2.25; 95% CI: 1.04 – 4.87; p=0.0389).
Seventy-five percent of patients in the overall cohort were alive and in-care at the end of the study. Figure 1B shows the Kaplan-Meier plots of retention in care over the study period. The relative positions of the countries on the retention plots are similar to those for survival, with the exception of Jamaica, which had the second-lowest mortality rate (8%) but the second-highest LTFU rate (16%) out of the 7 countries in the study. Long-term retention in care was 89% in Martinique, 82% in Trinidad, 78% in Barbados, 76% in Jamaica, 75% in Haiti, 72% in the Dominican Republic, and 29% in Puerto Rico. A total of 13% of patients were LTFU during the study period (see Table 3). The rate of LTFU was highest in the first 6 months (14 patients LTFU/100 PY), and it progressively declined to 4.7 patients LTFU/100 PY at 5 years.
In sensitivity analyses (see Table 5), overall mortality increased from 13% to 16% with the assumption that 27% of patients presumed LTFU had died. Overall mortality increased to 18% and 24% with the assumption that 40% and 87% of patients presumed LTFU had died, respectively. With the assumption that all patients presumed LTFU with a CD4 cell count < 50 cells/mm3 were dead, overall mortality increased from 13% to 16%; mortality increased to 18% with the assumption that those LTFU with CD4 cell count < 100 cells/mm3 were dead.
Table 5.
Sensitivity Analyses Examining the Effect of Loss to Follow-up on Mortality Rates at each Site
| Barbados (n=560) | DR (n=1207) | Haiti (n=4717) | Jamaica (n=476) | Martinique (n=325) | Trinidad (n=725) | Puerto Rico (n=193) | Combined (n=8203) | |
|---|---|---|---|---|---|---|---|---|
| Total Patient Months of Follow-up | 27951 | 25843 | 150722 | 17037 | 13078 | 32918 | 7759 | 275308 |
| Loss to Follow-up – Number (%) | 41 (7) | 155 (13) | 602 (13) | 77 (16) | 14 (4) | 54 (7) | 90 (47) | 1033 (13) |
| Unadjusted Deaths – Number (%) | 85 (15) | 181 (15) | 598 (13) | 38 (8) | 21 (6) | 78 (11) | 47 (24) | 1048 (13) |
| LTFU Per 100 Person-Years of Follow-up | 1.8 | 7.2 | 4.8 | 5.4 | 1.3 | 2.0 | 13.9 | 4.5 |
| Unadjusted Mortality Per 100 Person-Years | 3.6 | 8.4 | 4.8 | 2.7 | 1.9 | 2.8 | 7.3 | 4.6 |
| Sensitivity Analyses – Effect of LTFU on Mortality – Number (Percentage) of Deaths | ||||||||
| Assume Death in 27% of Patients LTFU – No. (%)* | 96 (17) | 223 (18) | 761 (16) | 59 (12) | 25 (8) | 93 (13) | 71 (37) | 1327 (16) |
| Assume Death in 40% of Patients LTFU – No. (%)** | 101 (18) | 243 (20) | 839 (18) | 69 (14) | 27 (8) | 100 (14) | 83 (43) | 1461 (18) |
| Assume Death in 87% of Patients LTFU – No. (%)* | 121 (22) | 316 (26) | 1122 (24) | 105 (22) | 33 (10) | 125 (17) | 125 (65) | 1947 (24) |
| Assume Death if LTFU and CD4 <50 Cells/mm3 | 95 (17) | 230 (19) | 734 (16) | 57 (12) | 24 (7) | 90 (12) | 59 (31) | 1289 (16) |
| Assume Death if LTFU and CD4 <100 Cells/mm3 | 99 (18) | 253 (21) | 834 (18) | 66 (14) | 24 (7) | 100 (14) | 70 (36) | 1446 (18) |
| Sensitivity Analyses – Effect of LTFU on Mortality – Mortality Rates Per 100 Person-Years of Follow-up | ||||||||
| Assume Death in 27% of Patients LTFU – No. (%)* | 4.1 | 10.3 | 6.1 | 4.1 | 2.3 | 3.4 | 11.0 | 5.8 |
| Assume Death in 40% of Patients LTFU – No. (%)** | 4.4 | 11.3 | 6.7 | 4.8 | 2.4 | 3.6 | 12.8 | 6.4 |
| Assume Death in 87% of Patients LTFU – No. (%)* | 5.2 | 14.7 | 8.9 | 7.4 | 3.0 | 4.6 | 19.4 | 8.5 |
| Assume Death if LTFU and CD4 <50 Cells/mm3 | 4.1 | 10.7 | 5.8 | 4.0 | 2.2 | 3.3 | 9.1 | 5.6 |
| Assume Death if LTFU and CD4 <100 Cells/mm3 | 4.3 | 11.7 | 6.6 | 4.6 | 2.2 | 3.6 | 10.8 | 6.3 |
The lowest mortality rate in the published literature from low and middle-income countries from tracking studies of ART patients presumed LTFU is 27% and the highest mortality is 87% [39, 40].
In a meta-analysis of 17 studies from low and middle-income countries by Brinkhof et al. [41] the combined mortality of ART patients from tracking studies of patients presumed LTFU was 40% (95% CI: 33–48%).
DISCUSSION
This is the first multi-cohort study to describe long-term HIV treatment outcomes in the Caribbean, and the only study to directly compare long-term HIV outcomes across a region. We observed excellent outcomes, with mortality rates comparable to other long-term cohorts in low and middle-income countries [11, 13–24, 30, 42, 43]. Overall mortality was 6% in Martinique, 8% in Jamaica, 11% in Trinidad, 13% in Haiti, 15% in the Dominican Republic, 15% in Barbados, and 24% in Puerto Rico. Much of the mortality difference between cohorts can be explained by severity of disease at presentation, concurrent active TB, gender, and nutritional status, with no difference in mortality between Haiti and Martinique, Jamaica, and Trinidad after controlling for these variables. There is increasing pressure to measure program effectiveness by comparing mortality across sites. Our findings demonstrate that great caution is indicated in making such comparisons.
Patient characteristics in our Caribbean cohort are more similar to those of other low and middle-income countries than those of the United States and Europe. Fifty-one percent of patients were female, and median weight and hemoglobin values were similar to those reported in African cohorts [10–16, 20, 42]. The median CD4 cell count at ART initiation was also similar to that reported in the Antiretroviral Therapy in Lower Income Countries Cohort (ART-LINC), with median CD4 cell count of 118 cells/mm3 vs. 108 cells/mm3 in ART-LINC, much lower than the 234 cells/mm3 reported in the ART Collaboration (ART-CC) groups from Europe and North America [9, 44]. Martinique and Puerto Rico are the exceptions. These two islands have among the highest incomes in the region, and the patients in these cohorts had higher CD4 cell counts at ART initiation and were more likely to receive protease inhibitors in the first-line regimen, compared with the other countries in this study [45].
Most countries in the Caribbean also had high early mortality rates, as has been reported in other low and middle-income countries [9, 12–23, 28], with overall mortality dropping from 20.9 deaths/100 PY in the first 3 months to 4.7 deaths/100 PY at 5 years. This early mortality was most pronounced in the Dominican Republic and Haiti, the two countries with the lowest baseline body weight and nearly all of the cases of TB and ART co-treatment, and Trinidad, which had the lowest median CD4 cell count at ART initiation. In comparison, Martinique and Puerto Rico had early mortality rates that were similar to industrialized countries.
Predictors of mortality in the Caribbean are similar to those reported in other studies from low and middle-income countries, and include older age, male gender, concurrent TB, and lower baseline body weight, hemoglobin, and CD4 cell count [10–12, 20–22, 30, 42, 43, 46]. Seventy-six percent of patients in these Caribbean centers initiate ART with a CD4 cell count < 200 cells/mm3. It is worrisome that CD4 cell counts at ART initiation are not improving substantially over time, even though the number of counseling and testing centers has increased throughout the region [47]. This also remains a problem in most regions of the world, including both low and middle-income countries [30, 35, 46]. Improved linkage between testing and treatment centers, streamlined strategies to increase ART enrollment, and widespread implementation of the 2010 guidelines of the WHO, which recommend earlier ART initiation are necessary [48].
Low weight and hemoglobin are due to poor nutritional status as well as advanced AIDS. Improved nutritional supplementation will be critical to lowering mortality rates in undernourished patients. Active TB infection also remains an obstacle to HIV treatment in Haiti and the Dominican Republic. Further research is needed to evaluate the impact on mortality of newly implemented guidelines for earlier ART initiation in co-infected patients [48]. More aggressive TB screening is also indicated, particularly in Haiti, as early reports suggest that TB incidence in Port-au-Prince is increasing in the aftermath of the January 12, 2010 earthquake (unpublished GHESKIO data).
The association between male gender and mortality observed in this cohort has also been reported in studies from China, Cambodia and some countries in Africa [11, 12, 21–23, 42], but not in Latin America [30, 35]. This gender difference has been attributed to lower adherence, older age, and more advanced disease in males at presentation, but two studies have found that mortality differences persist even after controlling for these variables [42, 49]. Further studies on biological, behavioral, and occupational factors that could explain the higher mortality in males are warranted to reduce this gender disparity in treatment outcomes.
Seventy-five percent of patients in the overall cohort were alive and in-care at the end of the study. Retention rates generally mirrored survival rates; Jamaica was an exception, with lower mortality but higher LTFU than the overall cohort. The proportion of patients that were alive and in-care was 89% in Martinique, 82% in Trinidad, 78% in Barbados, 76% in Jamaica, 75% in Haiti, 72% in the Dominican Republic, and 29% in Puerto Rico. The high mortality and high LTFU rate in Puerto Rico is likely due to high rates of intravenous drug use [50–52], high rates of hepatitis C even among patients that are not IVDU, and a population that is highly migratory. With the exception of Puerto Rico, the proportion of patients that were alive and in-care in the Caribbean were higher than those reported from several African countries [5, 16, 20, 21, 33, 53]. A systematic review of 32 publications from sub-Saharan Africa reported that African ART programs retain about 60% of patients in the first 24 months [31]. A follow-up meta-analysis of 39 African cohorts found a median retention rate of 70% [32]. The Caribbean sites generally retain patients at similar rates to the middle income African countries [13, 14, 20, 21, 28, 54]. This is likely because the treatment programs at these Caribbean sites are well-established, all antiretroviral therapy and HIV clinic visits are provided free of charge, all sites track patients that miss visits, and most programs subsidize transportation fees.
Our study is limited by a lack of definitive outcomes for patients who are presumed LTFU. Multiple tracking studies from low and middle-income countries have found that a significant proportion of these patients have died [32, 40, 41, 55–59]. It is possible that we may have under-estimated mortality rates, but it is noteworthy that retention in care in the Caribbean is similar or superior to reported rates from other low and middle-income countries. We also lacked data on adherence to therapy as a predictor of mortality. This and other unmeasured variables, such as incarceration or migration status, could explain the higher hazard ratios for mortality that persisted in the multivariable analysis for the Dominican Republic, Barbados, and Puerto Rico. Median follow-up times also varied between countries, with Barbados having the longest duration of follow-up. In addition, it is important to note that though we have a large sample size of patients that included from 7% to 71% of patients on ART in each country, treatment outcomes from these sites may not always be representative of whole countries, particularly as some sites are non-governmental organizations and others are public.
In summary, this study provides the first multi-cohort data on long-term HIV treatment outcomes in the Caribbean. Outcomes across the region are excellent, and similar to reports from other low and middle-income countries. Mortality rates vary widely by country, but much of the difference in mortality can be explained by disease severity at ART initiation, concurrent TB, gender, and nutritional status. Earlier ART initiation and augmented nutritional supplementation for undernourished patients will be critical to improve outcomes. Further studies are necessary to identify the reasons for the gender disparities in treatment outcomes.
Acknowledgments
We acknowledge Luis Miguel Abreu, Brendan Bain, Heejung Bang, Songee Beckles, Gisella Cestero, Graeme Crookendale, Bright Dgndy, Andrew Foster, Celia Graham, Tina Hylton-Kong, Sherry-Ann Lashley, Paul Leger, Rosmund Lovell, Abdias Marcelin, Megan McLaughlin, Oris Nero-Jarvis, Glenda Ortiz, Heidy Ortiz, Charlene Sealy, Sharon Soyer-Labastide, Basil Thorpe, and Dwayne Wiltshire for their generous assistance in study development, statistical analyses, manuscript editing, and data retrieval.
Sources of Funding: The project was supported in part by the National Institutes of Health Fogarty International Center International Clinical, Operational, and Health Services Research and Training Award (ICORTHA) Grant Number 3 U2R TW006896-04S1, the Fogarty International Center Grant Number K01 TW007142, and the National Center for Research Resources Grant Number G12RR-03035.
Footnotes
Description of the role of each of the authors:
Conceptualization of the study and manuscript: All authors were involved in the conceptualization of the study and the manuscript.
Patient care: All Caribbean authors cared for the patients at their respective sites.
Data collection and management: All authors were involved in data collection or management.
Analysis: Edwards, Koenig, Pape
Manuscript writing and revision: Koenig wrote the first draft and all authors reviewed and edited the manuscript.
Funding: Pape obtained funding for this study.
Please note: We feel that an extended author list should be justified because this study reports on long-term HIV treatment outcomes from 7 countries.
Potential conflicts of interest: All authors report no conflicts.
References
- 1.UNAIDS, World Health Organization. AIDS Epidemic Update. Geneva: UNAIDS; 2009. [Google Scholar]
- 2.Pan American Health Organization, World Health Organization. Health in the Americas, 2007. Volume I - Regional. Washington, DC: PAHO; 2007. Health Conditions and Trends; pp. 58–207. [Google Scholar]
- 3.Nadai Y, Eyzaguirre LM, Sill A, Cleghorn F, Nolte C, Charurat M, et al. HIV-1 epidemic in the Caribbean is dominated by subtype B. PLoS One. 2009;4:e4814. doi: 10.1371/journal.pone.0004814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HIV Epidemiology and Field Services Program. HIV/AIDS in New York City, 2001–2007. New York City Department of Health and Mental Hygiene; 2009. [Google Scholar]
- 5.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 6.Ivers LC, Kendrick D, Doucette K. Efficacy of antiretroviral therapy programs in resource-poor settings: a meta-analysis of the published literature. Clin Infect Dis. 2005;41:217–224. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 7.Jahn A, Floyd S, Crampin AC, Mwaungulu F, Mvula H, Munthali F, et al. Population-level effect of HIV on adult mortality and early evidence of reversal after introduction of antiretroviral therapy in Malawi. Lancet. 2008;371:1603–1611. doi: 10.1016/S0140-6736(08)60693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Severe P, Leger P, Charles M, Noel F, Bonhomme G, Bois G, et al. Antiretroviral therapy in a thousand patients with AIDS in Haiti. N Engl J Med. 2005;353:2325–2334. doi: 10.1056/NEJMoa051908. [DOI] [PubMed] [Google Scholar]
- 9.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 10.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 11.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10,000 adults in Cote d’Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–882. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madec Y, Laureillard D, Pinoges L, Fernandez M, Prak N, Ngeth C, et al. Response to highly active antiretroviral therapy among severely immuno-compromised HIV-infected patients in Cambodia. AIDS. 2007;21:351–359. doi: 10.1097/QAD.0b013e328012c54f. [DOI] [PubMed] [Google Scholar]
- 13.Bussmann H, Wester CW, Ndwapi N, Grundmann N, Gaolathe T, Puvimanasinghe J, et al. Five-year outcomes of initial patients treated in Botswana’s National Antiretroviral Treatment Program. AIDS. 2008;22:2303–2311. doi: 10.1097/QAD.0b013e3283129db0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etard JF, Ndiaye I, Thierry-Mieg M, Gueye NF, Gueye PM, Laniece I, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20:1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 15.Laurent C, Ngom Gueye NF, Ndour CT, Gueye PM, Diouf M, Diakhate N, et al. Long-term benefits of highly active antiretroviral therapy in Senegalese HIV-1-infected adults. J Acquir Immune Defic Syndr. 2005;38:14–17. doi: 10.1097/00126334-200501010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Auld AF, Mbofana F, Shiraishi RW, Sanchez M, Alfredo C, Nelson LJ, et al. Four-Year Treatment Outcomes of Adult Patients Enrolled in Mozambique’s Rapidly Expanding Antiretroviral Therapy Program. PLoS One. 2011;6:e18453. doi: 10.1371/journal.pone.0018453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanne IM, Westreich D, Macphail AP, Rubel D, Majuba P, Van Rie A. Long term outcomes of antiretroviral therapy in a large HIV/AIDS care clinic in urban South Africa: a prospective cohort study. J Int AIDS Soc. 2009;12:38. doi: 10.1186/1758-2652-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leger P, Charles M, Severe P, Riviere C, Pape JW, Fitzgerald DW. 5-year survival of patients with AIDS receiving antiretroviral therapy in Haiti. N Engl J Med. 2009;361:828–829. doi: 10.1056/NEJMc0809485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;96:315–320. [PubMed] [Google Scholar]
- 20.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–572. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 21.Nglazi MD, Lawn SD, Kaplan R, Kranzer K, Orrell C, Wood R, et al. Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. J Acquir Immune Defic Syndr. 2011;56:e1–8. doi: 10.1097/QAI.0b013e3181ff0bdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thai S, Koole O, Un P, Ros S, De Munter P, Van Damme W, et al. Five-year experience with scaling-up access to antiretroviral treatment in an HIV care programme in Cambodia. Trop Med Int Health. 2009;14:1048–1058. doi: 10.1111/j.1365-3156.2009.02334.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F, Dou Z, Ma Y, Zhao Y, Liu Z, Bulterys M, et al. Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med. 2009;151:241–251. W-252. doi: 10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 24.Ferradini L, Laureillard D, Prak N, Ngeth C, Fernandez M, Pinoges L, et al. Positive outcomes of HAART at 24 months in HIV-infected patients in Cambodia. AIDS. 2007;21:2293–2301. doi: 10.1097/QAD.0b013e32828cc8b7. [DOI] [PubMed] [Google Scholar]
- 25.Van der Borght SF, Clevenbergh P, Rijckborst H, Nsalou P, Onyia N, Lange JM, et al. Mortality and morbidity among HIV type-1-infected patients during the first 5 years of a multicountry HIV workplace programme in Africa. Antivir Ther. 2009;14:63–74. [PubMed] [Google Scholar]
- 26.Nash D, Katyal M, Brinkhof MW, Keiser O, May M, Hughes R, et al. Long-term immunologic response to antiretroviral therapy in low-income countries: a collaborative analysis of prospective studies. AIDS. 2008;22:2291–2302. doi: 10.1097/QAD.0b013e3283121ca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corey DM, Kim HW, Salazar R, Illescas R, Villena J, Gutierrez L, et al. Brief report: effectiveness of combination antiretroviral therapy on survival and opportunistic infections in a developing world setting: an observational cohort study. J Acquir Immune Defic Syndr. 2007;44:451–455. doi: 10.1097/QAI.0b013e31802f8512. [DOI] [PubMed] [Google Scholar]
- 28.Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 29.Laurent C, Diakhate N, Gueye NF, Toure MA, Sow PS, Faye MA, et al. The Senegalese government’s highly active antiretroviral therapy initiative: an 18-month follow-up study. AIDS. 2002;16:1363–1370. doi: 10.1097/00002030-200207050-00008. [DOI] [PubMed] [Google Scholar]
- 30.Wolff MJ, Cortes CP, Shepherd BE, Beltran CJ. Long-term outcomes of a national expanded access program to antiretroviral therapy: the Chilean AIDS cohort. J Acquir Immune Defic Syndr. 2010;55:368–374. doi: 10.1097/QAI.0b013e3181eb4fb9. [DOI] [PubMed] [Google Scholar]
- 31.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15 (Suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekouevi DK, Balestre E, Ba-Gomis FO, Eholie SP, Maiga M, Amani-Bosse C, et al. Low retention of HIV-infected patients on antiretroviral therapy in 11 clinical centres in West Africa. Trop Med Int Health. 2010;15 (Suppl 1):34–42. doi: 10.1111/j.1365-3156.2010.02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kilaru KR, Kumar A, Sippy N, Carter AO, Roach TC. Immunological and virological responses to highly active antiretroviral therapy in a non-clinical trial setting in a developing Caribbean country. HIV Med. 2006;7:99–104. doi: 10.1111/j.1468-1293.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 35.Tuboi SH, Schechter M, McGowan CC, Cesar C, Krolewiecki A, Cahn P, et al. Mortality during the first year of potent antiretroviral therapy in HIV-1-infected patients in 7 sites throughout Latin America and the Caribbean. J Acquir Immune Defic Syndr. 2009;51:615–623. doi: 10.1097/QAI.0b013e3181a44f0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.UNAIDS. Report on the Global AIDS Epidemic 2010. Geneva: UNAIDS; 2010. [Google Scholar]
- 37.UNAIDS. The Status of HIV in the Caribbean. Geneva: UNAIDS; 2010. [Google Scholar]
- 38.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. Geneva: WHO; 2006. [PubMed] [Google Scholar]
- 39.Maskew M, MacPhail P, Menezes C, Rubel D. Lost to follow up: contributing factors and challenges in South African patients on antiretroviral therapy. S Afr Med J. 2007;97:853–857. [PubMed] [Google Scholar]
- 40.Bisson GP, Gaolathe T, Gross R, Rollins C, Bellamy S, Mogorosi M, et al. Overestimates of survival after HAART: implications for global scale-up efforts. PLoS One. 2008;3:e1725. doi: 10.1371/journal.pone.0001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.May M, Boulle A, Phiri S, Messou E, Myer L, Wood R, et al. Prognosis of patients with HIV-1 infection starting antiretroviral therapy in sub-Saharan Africa: a collaborative analysis of scale-up programmes. Lancet. 2010;376:449–457. doi: 10.1016/S0140-6736(10)60666-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palombi L, Marazzi MC, Guidotti G, Germano P, Buonomo E, Scarcella P, et al. Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral- treated patients in sub-Saharan African Sites with comprehensive monitoring availability. Clin Infect Dis. 2009;48:115–122. doi: 10.1086/593312. [DOI] [PubMed] [Google Scholar]
- 44.Lanoy E, May M, Mocroft A, Phillip A, Justice A, Chene G, et al. Prognosis of patients treated with cART from 36 months after initiation, according to current and previous CD4 cell count and plasma HIV-1 RNA measurements. AIDS. 2009;23:2199–2208. doi: 10.1097/QAD.0b013e3283305a00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Washington, DC: Department of Health and Human Services; 2011. [Google Scholar]
- 46.Keiser O, Anastos K, Schechter M, Balestre E, Myer L, Boulle A, et al. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13:870–879. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bartholomew C, Boyce G, Fraser O, Sebro A, Telfer-Baptiste M, Labastide S. Late Presentation of HIV/AIDS Patients: A Caribbean Problem. AIDS Patient Care STDS. 2011 doi: 10.1089/apc.2010.0370. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization. Antiretroviral Therapy for HIV Infection in Adults and Adolescents Recommendations for a Public Health Approach 2010 Revision. Geneva: WHO; 2010. [PubMed] [Google Scholar]
- 49.Hawkins C, Chalamilla G, Okuma J, Spiegelman D, Hertzmark E, Aris E, et al. Gender Differences in Antiretroviral Treatment Outcomes among HIV-infected Adults in Dar es Salaam, Tanzania. AIDS. 2011 doi: 10.1097/QAD.0b013e3283471deb. [DOI] [PubMed] [Google Scholar]
- 50.Larsen MV, Omland LH, Gerstoft J, Larsen CS, Jensen J, Obel N, et al. Impact of injecting drug use on mortality in Danish HIV-infected patients: a nation-wide population-based cohort study. Addiction. 2010;105:529–535. doi: 10.1111/j.1360-0443.2009.02827.x. [DOI] [PubMed] [Google Scholar]
- 51.Baez-Feliciano DV, Quintana R, Gomez MA, Fernandez DM, Velazquez M, Olivares E, et al. Trends in the HIV and AIDS epidemic in a Puerto Rican cohort of patients: 1992–2005. Bol Asoc Med P R. 2006;98:174–183. [PubMed] [Google Scholar]
- 52.Malta M, Bastos FI, da Silva CM, Pereira GF, Lucena FF, Fonseca MG, et al. Differential survival benefit of universal HAART access in Brazil: a nation-wide comparison of injecting drug users versus men who have sex with men. J Acquir Immune Defic Syndr. 2009;52:629–635. doi: 10.1097/QAI.0b013e3181b31b8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brinkhof MW, Dabis F, Myer L, Bangsberg DR, Boulle A, Nash D, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–567. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Bank. [Accessed 21 Nov 2011];World Bank list of economies. 2011 Available at: http://siteresources.worldbank.org/DATASTATISTICS/Resources/CLASS.XLS.
- 55.Dalal RP, Macphail C, Mqhayi M, Wing J, Feldman C, Chersich MF, et al. Characteristics and outcomes of adult patients lost to follow-up at an antiretroviral treatment clinic in johannesburg, South Africa. J Acquir Immune Defic Syndr. 2008;47:101–107. doi: 10.1097/QAI.0b013e31815b833a. [DOI] [PubMed] [Google Scholar]
- 56.Brinkhof MW, Spycher BD, Yiannoutsos C, Weigel R, Wood R, Messou E, et al. Adjusting mortality for loss to follow-up: analysis of five ART programmes in sub-Saharan Africa. PLoS One. 2010;5:e14149. doi: 10.1371/journal.pone.0014149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fox MP, Brennan A, Maskew M, MacPhail P, Sanne I. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15:405–413. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu JK, Chen SC, Wang KY, Chang CS, Makombe SD, Schouten EJ, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85:550–554. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krebs DW, Chi BH, Mulenga Y, Morris M, Cantrell RA, Mulenga L, et al. Community-based follow-up for late patients enrolled in a district-wide programme for antiretroviral therapy in Lusaka, Zambia. AIDS Care. 2008;20:311–317. doi: 10.1080/09540120701594776. [DOI] [PubMed] [Google Scholar]
- 60.International Monetary Fund. [Accessed 13 May 2011];World Economic Outlook Database. 2011 Available at: http://imf.org/external/pubs/ft/weo/2009/01/weodata/index.aspx.
- 61.UNAIDS. [Accessed 13 May 2011];AIDSinfo. 2010 Available at: http://www.unaids.org/globalreport/AIDSinfo.htm.
- 62.Republique d’Haiti Programme National de Lutte contre le Sida. Rapport de Situation Nationale a L’intention de L’ungass; Janvier. 2008–Decembre 2009; 2010. [Google Scholar]