Abstract
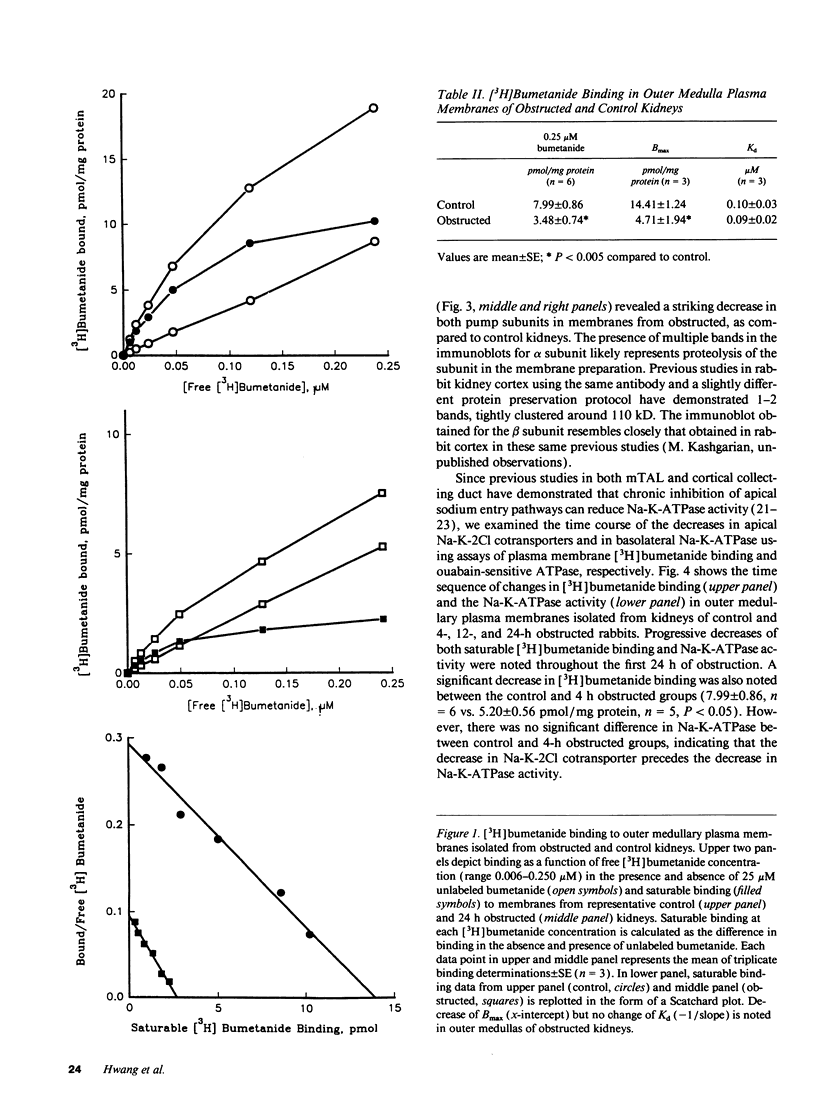
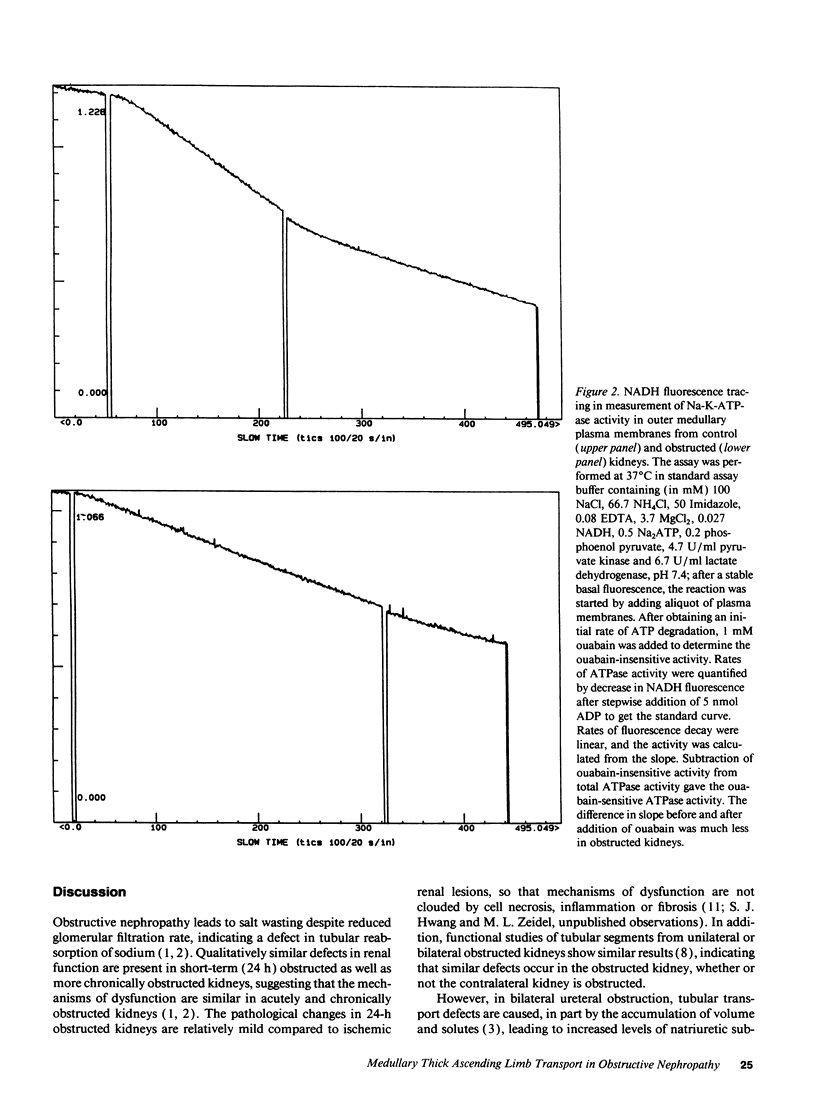
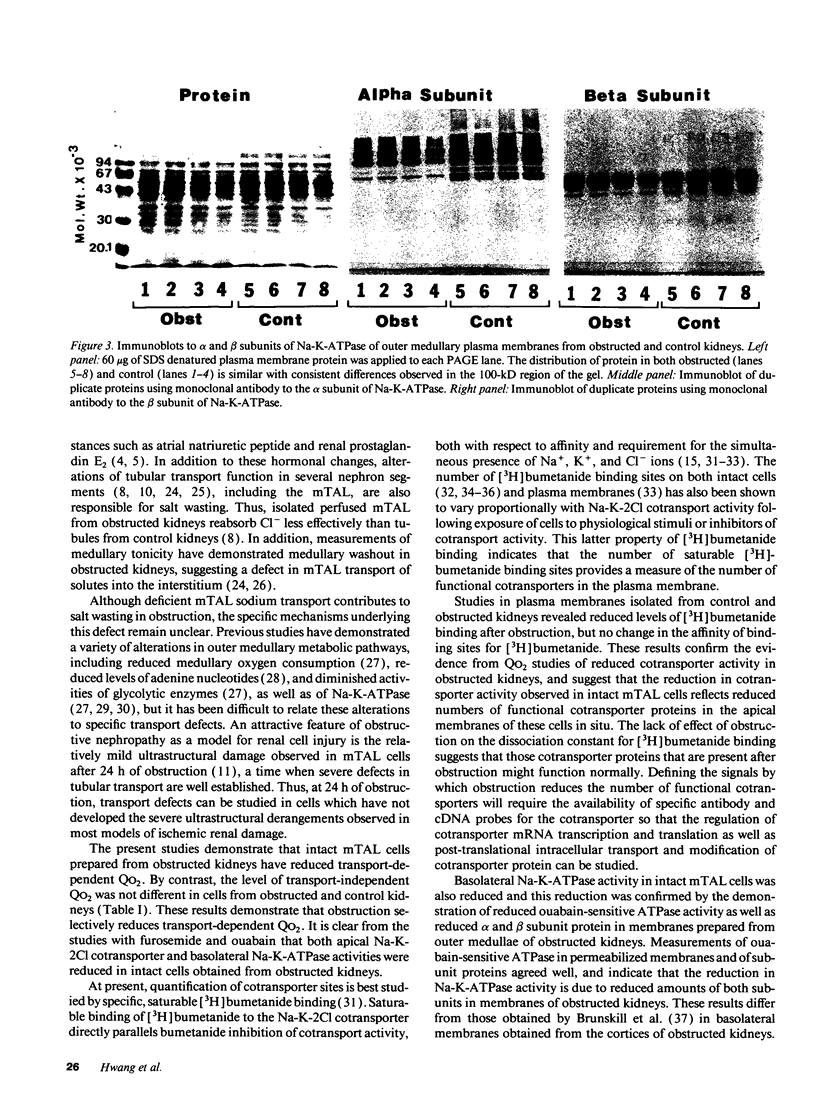
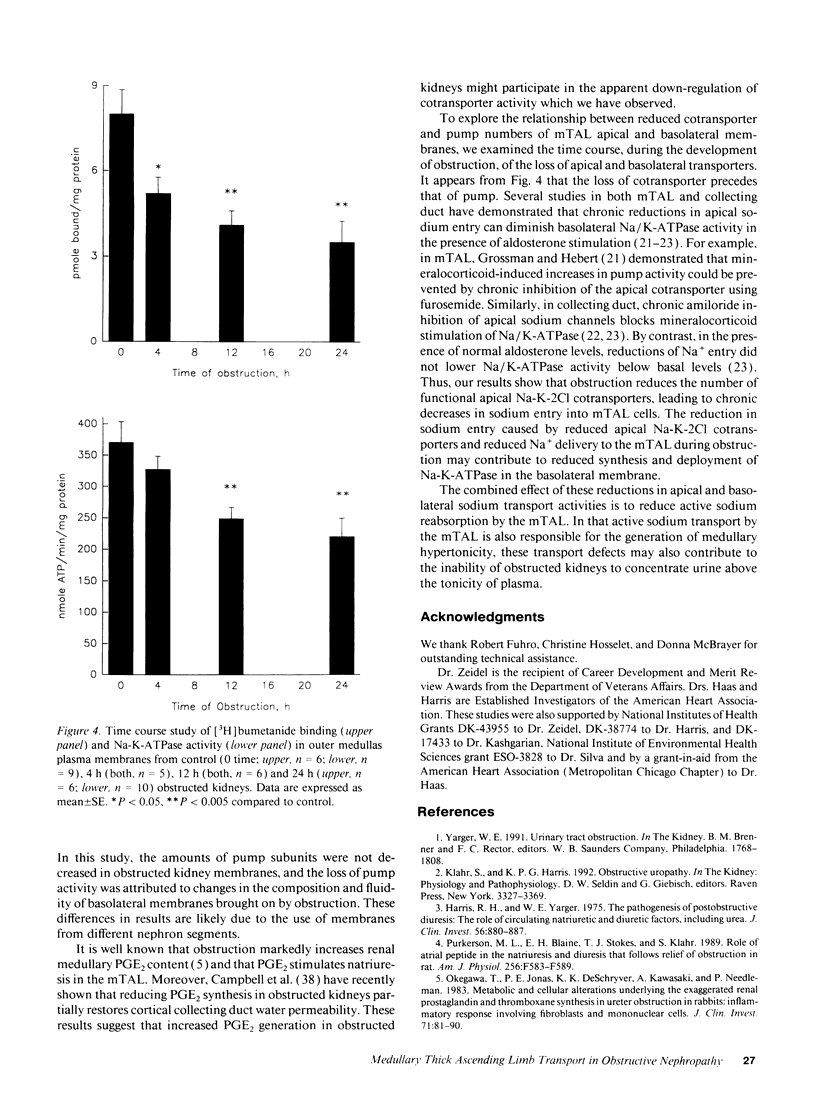
To characterize the sodium transport defect responsible for salt wasting in obstructive nephropathy, the major sodium transporters in the medullary thick ascending limb (mTAL), the apical Na-K-2Cl cotransporter and the basolateral Na-K-ATPase, were studied in fresh suspensions of mTAL cells and outer medulla plasma membranes prepared from obstructed and untreated kidneys. Oxygen consumption (QO2) studies in intact cells revealed marked reductions in the inhibitory effects of both furosemide and ouabain on QO2 in cells from obstructed, as compared with control animals, indicating a reduction in activities of both the Na-K-2Cl cotransporter and the Na-K-ATPase. Saturable [3H]bumetanide binding was reduced in membranes isolated from obstructed kidneys, but the Kd for [3H]bumetanide was unchanged, indicating a decrease in the number of functional luminal Na-K-2Cl cotransporters in obstructed mTAL. Ouabain sensitive Na-K-ATPase activity in plasma membranes was also reduced, and immunoblots using specific monoclonal antibodies directed against the alpha and beta subunits of rabbit Na-K-ATPase showed decreased amounts of both subunits in outer medullas of obstructed kidney. A significant decrease in [3H]bumetanide binding was detected after 4 h of ureteral obstruction, whereas Na-K-ATPase activity at this time was still not different from control. We conclude that ureteral obstruction reduces the amounts of both luminal Na-K-2Cl cotransporter and basolateral Na-K-ATPase in mTAL of obstructed kidney and that these reductions contribute to the salt wasting observed after release of obstruction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunskill N., Hayes C., Morrissey J., Klahr S. Changes in lipid environment decrease Na, K-ATPase activity in obstructive nephropathy. Kidney Int. 1991 May;39(5):843–849. doi: 10.1038/ki.1991.106. [DOI] [PubMed] [Google Scholar]
- Buerkert J., Martin D., Head M., Prasad J., Klahr S. Deep nephron function after release of acute unilateral ureteral obstruction in the young rat. J Clin Invest. 1978 Dec;62(6):1228–1239. doi: 10.1172/JCI109243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell H. T., Bello-Reuss E., Klahr S. Hydraulic water permeability and transepithelial voltage in the isolated perfused rabbit cortical collecting tubule following acute unilateral ureteral obstruction. J Clin Invest. 1985 Jan;75(1):219–225. doi: 10.1172/JCI111677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveloff J., Bayerdörffer E., Silva P., Kinne R. Sodium-chloride transport in the thick ascending limb of Henle's loop. Oxygen consumption studies in isolated cells. Pflugers Arch. 1981 Mar;389(3):263–270. doi: 10.1007/BF00584788. [DOI] [PubMed] [Google Scholar]
- Forbush B., 3rd, Palfrey H. C. [3H]bumetanide binding to membranes isolated from dog kidney outer medulla. Relationship to the Na,K,Cl co-transport system. J Biol Chem. 1983 Oct 10;258(19):11787–11792. [PubMed] [Google Scholar]
- Franklin C. C., Turner J. T., Kim H. D. Regulation of Na+/K+/Cl- cotransport and [3H]bumetanide binding site density by phorbol esters in HT29 cells. J Biol Chem. 1989 Apr 25;264(12):6667–6673. [PubMed] [Google Scholar]
- Garg L. C., Narang N., Wingo C. S. Glucocorticoid effects on Na-K-ATPase in rabbit nephron segments. Am J Physiol. 1985 Apr;248(4 Pt 2):F487–F491. doi: 10.1152/ajprenal.1985.248.4.F487. [DOI] [PubMed] [Google Scholar]
- Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev. 1985 Jul;65(3):760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- Grossman E. B., Hebert S. C. Modulation of Na-K-ATPase activity in the mouse medullary thick ascending limb of Henle. Effects of mineralocorticoids and sodium. J Clin Invest. 1988 Mar;81(3):885–892. doi: 10.1172/JCI113399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M., Dunham P. B., Forbush B., 3rd [3H]bumetanide binding to mouse kidney membranes: identification of corresponding membrane proteins. Am J Physiol. 1991 Apr;260(4 Pt 1):C791–C804. doi: 10.1152/ajpcell.1991.260.4.C791. [DOI] [PubMed] [Google Scholar]
- Haas M., Forbush B., 3rd [3H]bumetanide binding to duck red cells. Correlation with inhibition of (Na + K + 2Cl) co-transport. J Biol Chem. 1986 Jun 25;261(18):8434–8441. [PubMed] [Google Scholar]
- Haas M. Properties and diversity of (Na-K-Cl) cotransporters. Annu Rev Physiol. 1989;51:443–457. doi: 10.1146/annurev.ph.51.030189.002303. [DOI] [PubMed] [Google Scholar]
- Hanley M. J., Davidson K. Isolated nephron segments from rabbit models of obstructive nephropathy. J Clin Invest. 1982 Jan;69(1):165–174. doi: 10.1172/JCI110427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. H., Yarger W. E. The pathogenesis of post-obstructive diuresis. The role of circulating natriuretic and diuretic factors, including urea. J Clin Invest. 1975 Oct;56(4):880–887. doi: 10.1172/JCI108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs K., Zeidel M. L., Silva P. Prostaglandin E2 inhibits Na+-K+-ATPase activity in the inner medullary collecting duct. Am J Physiol. 1989 Sep;257(3 Pt 2):F424–F430. doi: 10.1152/ajprenal.1989.257.3.F424. [DOI] [PubMed] [Google Scholar]
- Jaenike J. R. The renal functional defect of postobstructive nephyropathy. The effects of bilateral ureteral obstruction in the rat. J Clin Invest. 1972 Dec;51(12):2999–3006. doi: 10.1172/JCI107127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashgarian M., Biemesderfer D., Caplan M., Forbush B., 3rd Monoclonal antibody to Na,K-ATPase: immunocytochemical localization along nephron segments. Kidney Int. 1985 Dec;28(6):899–913. doi: 10.1038/ki.1985.216. [DOI] [PubMed] [Google Scholar]
- Klahr S., Schwab S. J., Stokes T. J. Metabolic adaptations of the nephron in renal disease. Kidney Int. 1986 Jan;29(1):80–89. doi: 10.1038/ki.1986.10. [DOI] [PubMed] [Google Scholar]
- Knox W. H., Sax J. A., Wilson D. R., Sen A. K. Effect of osmolality on renal medullary Na-K-ATPase activity in the postobstructive kidney. Can J Physiol Pharmacol. 1977 Oct;55(5):1112–1115. doi: 10.1139/y77-153. [DOI] [PubMed] [Google Scholar]
- Lear S., Silva P., Kelley V. E., Epstein F. H. Prostaglandin E2 inhibits oxygen consumption in rabbit medullary thick ascending limb. Am J Physiol. 1990 May;258(5 Pt 2):F1372–F1378. doi: 10.1152/ajprenal.1990.258.5.F1372. [DOI] [PubMed] [Google Scholar]
- Nagle R. B., Bulger R. E., Cutler R. E., Jervis H. R., Benditt E. P. Unilateral obstructive nephropathy in the rabbit. I. Early morphologic, physiologic, and histochemical changes. Lab Invest. 1973 Apr;28(4):456–467. [PubMed] [Google Scholar]
- Nito H., Descoeudres C., Kurokawa K., Massry S. G. Effect of unilateral obstruction on renal cell metabolism and function. J Lab Clin Med. 1978 Jan;91(1):60–71. [PubMed] [Google Scholar]
- O'Donnell M. E. [3H]bumetanide binding in vascular endothelial cells. Quantitation of Na-K-Cl cotransporters. J Biol Chem. 1989 Dec 5;264(34):20326–20330. [PubMed] [Google Scholar]
- O'Neil R. G., Hayhurst R. A. Sodium-dependent modulation of the renal Na-K-ATPase: influence of mineralocorticoids on the cortical collecting duct. J Membr Biol. 1985;85(2):169–179. doi: 10.1007/BF01871269. [DOI] [PubMed] [Google Scholar]
- O'Neill W. C., Klein J. D. Regulation of vascular endothelial cell volume by Na-K-2Cl cotransport. Am J Physiol. 1992 Feb;262(2 Pt 1):C436–C444. doi: 10.1152/ajpcell.1992.262.2.C436. [DOI] [PubMed] [Google Scholar]
- Okegawa T., Jonas P. E., DeSchryver K., Kawasaki A., Needleman P. Metabolic and cellular alterations underlying the exaggerated renal prostaglandin and thromboxane synthesis in ureter obstruction in rabbits. Inflammatory response involving fibroblasts and mononuclear cells. J Clin Invest. 1983 Jan;71(1):81–90. doi: 10.1172/JCI110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty K. J., Kokko J. P., Marver D. Secondary effect of aldosterone on Na-KATPase activity in the rabbit cortical collecting tubule. J Clin Invest. 1981 Dec;68(6):1514–1521. doi: 10.1172/JCI110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewitt E. B., Hegde R. S., Haas M., Palfrey H. C. The regulation of Na/K/2Cl cotransport and bumetanide binding in avian erythrocytes by protein phosphorylation and dephosphorylation. Effects of kinase inhibitors and okadaic acid. J Biol Chem. 1990 Dec 5;265(34):20747–20756. [PubMed] [Google Scholar]
- Purkerson M. L., Blaine E. H., Stokes T. J., Klahr S. Role of atrial peptide in the natriuresis and diuresis that follows relief of obstruction in rat. Am J Physiol. 1989 Apr;256(4 Pt 2):F583–F589. doi: 10.1152/ajprenal.1989.256.4.F583. [DOI] [PubMed] [Google Scholar]
- Sabatini S., Kurtzman N. A. Enzyme activity in obstructive uropathy: basis for salt wastage and the acidification defect. Kidney Int. 1990 Jan;37(1):79–84. doi: 10.1038/ki.1990.11. [DOI] [PubMed] [Google Scholar]
- Sonnenberg H., Wilson D. R. The role of the medullary collecting ducts in postobstructive diuresis. J Clin Invest. 1976 Jun;57(6):1564–1574. doi: 10.1172/JCI108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. D., Fanestil D. D., Blackard C. E. Etiology of postobstructive diuresis: ouabain-sensitive adenosine triphosphatase deficit and elevated solute excretion in the postobstructed dog kidney. Invest Urol. 1976 Sep;14(2):148–152. [PubMed] [Google Scholar]
- Wilson D. R. Micropuncture study of chronic obstructive nephropathy before and after release of obstruction. Kidney Int. 1972 Sep;2(3):119–130. doi: 10.1038/ki.1972.82. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Kikeri D., Silva P., Burrowes M., Brenner B. M. Atrial natriuretic peptides inhibit conductive sodium uptake by rabbit inner medullary collecting duct cells. J Clin Invest. 1988 Sep;82(3):1067–1074. doi: 10.1172/JCI113663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidel M. L., Seifter J. L., Lear S., Brenner B. M., Silva P. Atrial peptides inhibit oxygen consumption in kidney medullary collecting duct cells. Am J Physiol. 1986 Aug;251(2 Pt 2):F379–F383. doi: 10.1152/ajprenal.1986.251.2.F379. [DOI] [PubMed] [Google Scholar]



