Abstract
Objectives
Serum adiponectin (APN) is associated with lower childhood obesity, and APN concentration in human milk is associated with slower growth during active breastfeeding. Here, we examine infant weight gain in the second year of life after exposure to high or low levels of mother’s milk APN.
Methods
Breastfeeding mother-infant pairs were recruited in Mexico City and followed for 2 years; 192 infants with ≥12 months’ follow-up were analyzed. Monthly milk samples were assayed for APN; mothers were classified as producing high or low levels of milk APN. Infant and maternal serum APN were assessed during year 1. Infant anthropometry was measured monthly (year 1) or bi-monthly (year 2), and WHO Z-scores calculated. Longitudinal adjusted models assessed weight-for-age (WEI) and weight-for-length (WFL) Z-score trajectories from 1 to 2 years.
Results
Maternal serum APN modestly correlated with milk APN (r=0.37, p<0.0001) and infant serum APN (r=0.29, p=0.01). Infants exposed to high milk APN experienced increasing WEI and WFL Z-scores between age 1 and 2 years in contrast to low milk APN exposure (p for group*time=0.02 and 0.054, respectively), adjusting for growth in the first 6 months and other covariates. In contrast, infant serum APN in year 1 was not associated with rate of weight gain in year 2.
Conclusions
High human milk APN exposure was associated with accelerated weight trajectory during the second year of life suggesting its role in catch up growth after slower weight gain during the first year of life.
Keywords: adiponectin, human milk, breastfeeding, weight gain, infancy
Introduction
Pediatric obesity is a critical public health problem, affecting an increasing number of children and adolescents worldwide 1, 2. Breastfeeding has been identified as protective against later obesity compared with formula-feeding, with increased duration of breastfeeding often associated with lower obesity in a dose-dependent manner 3–5. However, the mechanism of action by which breastfeeding confers a protective advantage is unclear. While the macro-nutrient composition of human milk is quite stable, human milk is actually a complex mixture of bioactive factors associated with infant growth and metabolism, including insulin6, leptin7, 8, adipocyte fatty acid binding protein (AFABP)9, 10, several growth factors and their binding proteins11, 12, and ghrelin13–15, the composition of which varies from mother to mother and over the course of lactation.
One intriguing component of human milk is adiponectin9, 10, 16–18, which is secreted by breast adipose tissue 19. Adiponectin is an insulin-sensitizing and anti-inflammatory molecule, which is typically found in circulation at higher concentrations among individuals with lower adiposity and better metabolic health20, 21. Consistent with that concept, we have previously demonstrated that higher maternal milk adiponectin is associated with lower infant weight-for-length Z-scores in the first 6 months of life in two predominantly breastfed cohorts22. However, in contrast, another study reported that higher maternal milk adiponectin concentrations were associated with an increased risk of overweight in breastfed infants by age two years 23.
The current study examines the relationship between maternal milk adiponectin, maternal serum adiponectin, infant serum adiponectin and infant growth using a well-characterized longitudinal cohort with frequent follow-up. In this same cohort, we have previously reported that milk adiponectin was associated with lower infant weight during the first 6 months of life22, but longer-term relationships with growth during the second year of life and with infant and maternal serum adiponectin have not been examined. In particular, we tested the hypothesis that concentrations of human milk adiponectin influences breastfed infants’ growth trajectory during the second year of life, beyond the period of active breastfeeding. In addition, we examined the relationships among maternal serum adiponectin, maternal milk adiponectin, and infant serum adiponectin levels to clarify the maternal-infant links with respect to adiponectin in breastfed infants.
Materials and Methods
Methods for this study have been described previously 22, thus are described only briefly here. From March 1998 to April 2003, 306 infants in San Pedro Martir, Mexico City, were enrolled and monitored prospectively from birth to 2 years of age 24. All enrolled infants were healthy, full-term infants born ≥2.2 kg without congenital defects, whose mothers intended to breastfeed. This study was approved by the IRBs of the National Institute of Medical Sciences and Nutrition (Mexico City) and Cincinnati Children’s Hospital Medical Center, and all mothers provided written informed consent.
Demographic, maternal, household and birth characteristics were ascertained by baseline questionnaire. Infant diet was ascertained by weekly 24-hour recall. Measurements of infant weight (±0.1 kg, Model MP25, CMS Weighing Equipment Ltd., London, England) and length (±0.1 cm using recumbent length board) were collected monthly between 1 and 12 months, and bi-monthly between 12 and 24 months. Milk samples (n=1074) collected at baseline (week 1) and months 1, 3, 5 and 6 were assayed after a single freeze-thaw cycle.
A maternal blood sample was collected at the baseline visit (2 to 20 days postpartum), and infant blood samples were collected at baseline, 3, 6, and 12 months of age. All blood samples were maintained on ice after collection, processed, aliquotted and stored at −70 C until assaying. Serum samples from the mother at baseline (n=274) and a subset of 92 infants at baseline (n=87), 3 months (n=84), 6 months (n=66) and 12 months of age (n=55) were included in the analysis.
Assay of Serum and Milk Adiponectin
Serum total adiponectin was measured in duplicate using radioimmunoassay (Linco Research, St. Charles, MO). Milk adiponectin was measured in skimmed milk by radioimmunoassay (Linco Research, St. Charles, MO) as described previously17
Calculated Variables
Breastfeeding durations were calculated using weekly 24-hour recalls, excluding data from the first 7 days of life. Duration of exclusive breastfeeding (EBF) was calculated based on the World Health Organization (WHO) definition as the last age in days at which the infant was reported to receive 100% of all feeds as breast milk. A second breastfeeding variable (BF85%) denoted the last age at which breastfeeding was reported to account for at least 85% of all feeds, regardless of the composition of the rest of the diet; this definition corresponds to “Full or Nearly Full Breastfeeding” 25. Age of introduction of solid food was determined as the first age (in days) at which the infant was reported to have consumed any solid or semi-solid foods (e.g., cereals, soups, yogurt, fruits or vegetables).
Infant anthropometrics were standardized to the WHO Child Growth Standards 26, and the resulting Z-scores for weight-for-age (WEI), length-for-age (LEN), and weight-for-length (WFL) were analyzed.
Longitudinal assessment of the relationship between milk adiponectin (measured only during the first 6 months) and 2-year growth required two special data manipulations. First, to avoid the use of a single proxy milk adiponectin value to represent longitudinal characterization of milk, individual-level milk adiponectin values were summarized as the median of each mother’s milk adiponectin values across her available samples. In this phase, 1 outlier sample was excluded from each of 4 individuals.
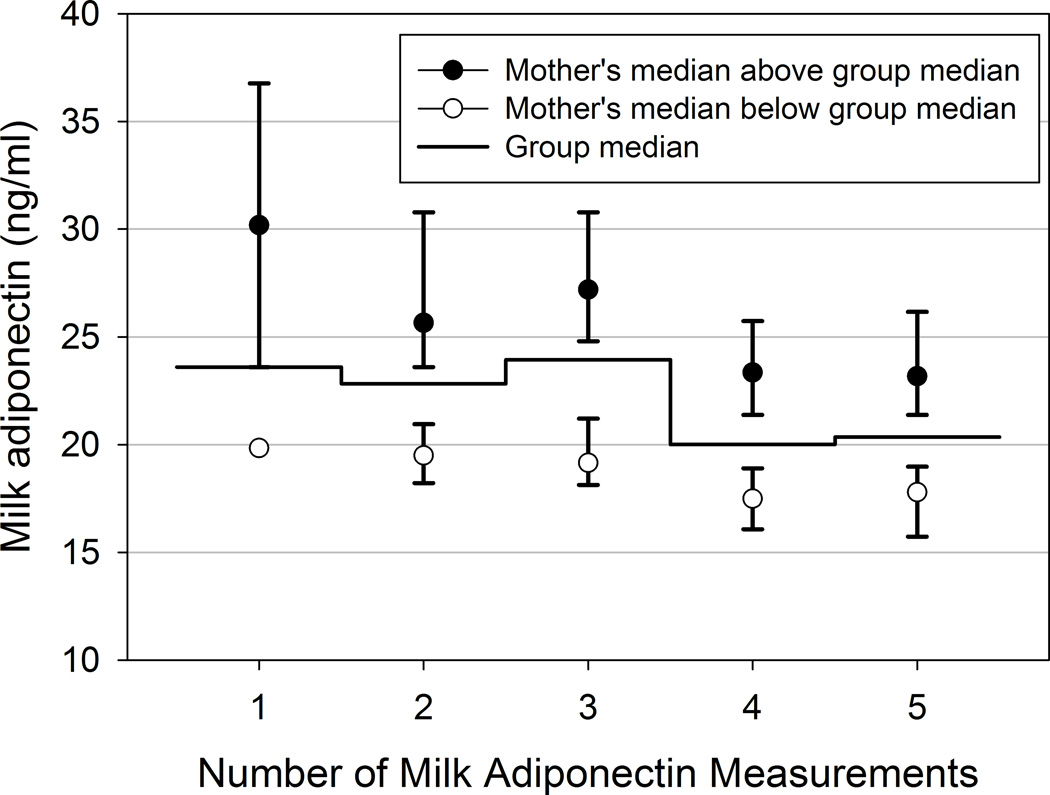
Second, to avoid confounding introduced by the decline in milk adiponectin through lactation, which was reported for this cohort previously22 (e.g., with more or later samples resulting in lower median values), the individual’s median was determined to be either above or below the median of the group of mothers with the same number of milk measurements. Group sizes were as follows: 3 (1%) mothers had a single milk measurement, 29 (10%) women had two measurements, 38 (14%) had 3 measurements, 140 (51%) had 4 measurements and 67 (24%) had all five measurements of milk adiponectin. Figure 1 shows the median and interquartile range (IQR) of the milk adiponectin values for the “above median” and “below median” groups of women within each measurement stratum. This manipulation resulted in a designation of “above median” or “below median” milk APN across the cohort that is not confounded by the duration of breastfeeding or number of samples available. Infant serum adiponectin measurements from months 0, 3, 6, and 12 were treated in the same manner, as 21 infants (23%) had 2 serum adiponectin measurements, 33 (36%) had 3 measurements and 38 (41%) had all 4 measurements.
Figure 1. Maternal median milk adiponectin in above- versus below-median groups.
N per group: n=3, 29, 38, 140, and 67 with 1, 2, 3, 4, or 5 measurements, respectively. Median and interquartile range (IQR) presented.
Statistical Analysis
All analyses were conducted using SAS v.9.1. (SAS Institute, Cary, NC). Descriptive statistics were calculated for the entire cohort (n=277), and were also compared between participants with less than 12 months’ follow-up (n=85) or ≥12 months’ follow-up (n=192), as the latter group was the subset used for the longitudinal anthropometric models. Differences between these follow-up groupings were determined using Student’s t-test or χ2 tests, as appropriate.
Infant serum adiponectin values were normally distributed at each time point, so were analyzed in original units (µg/ml). Analysis of longitudinal patterns of infant serum adiponectin and comparisons between time points were conducted using repeated measures modeling (PROC MIXED), which allows for missing data and accounts for both the intra- and inter-individual variability present in this data, resulting in larger, and more valid, estimates of standard error (SE) 27. Differences between infant and maternal serum adiponectin concentrations were evaluated using Student’s t-test. For visual presentation only, serum adiponectin values were plotted using medians and interquartile ranges (IQR), because the standard errors on the estimates were not distinguishable from the plotted symbol. Cross-sectional Spearman correlations among infant serum, maternal serum, maternal milk and infant anthropometry Z-scores during the first year of life were calculated in the entire cohort.
Longitudinal analysis of WFL, WEI and LEN Z-scores were conducted using data from months 12 through 24 to examine growth trajectories during the second year of life. The analysis set for this model thus included only those with at least 12 months of follow-up (n=192). Modeling used repeated measures models, as above, with the intercept and infant’s growth trajectory across time (slopes) allowed to vary by individual. Other covariates considered for model inclusion were: infant sex, birthweight, duration of exclusive breastfeeding, age at introduction of solid food, change in WFL Z-score or WEI Z-score between birth and 6 months, maternal age at delivery, parity, type of delivery, maternal education, and maternal marital status. Changes in WFL and WEI Z-scores during the first 6 months were specifically included to account for the previous finding in this cohort that milk adiponectin was negatively associated with these parameters during this time period22, and could potentially confound the relationships between 1 and 2 years of age. Covariates were tested in multivariate models if bivariate p ≤0.10, and retained if p≤0.05. Interaction terms between month and indicators for “above median” or “below median” adiponectin were specifically tested to determine whether high or low milk or serum adiponectin groups demonstrated different weight trajectories in year 2.
Results
Infant Feeding and Anthropometric Characteristics
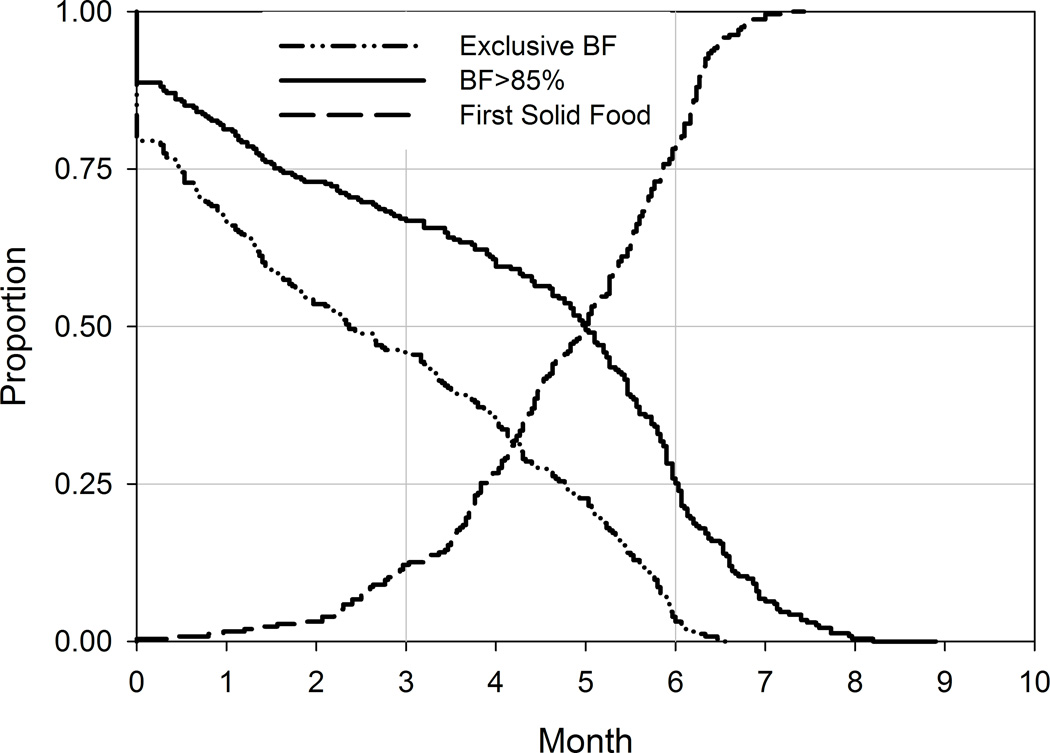
The patterns of breastfeeding in the present cohort are indicative of a highly breastfed cohort (Figure 2A). Median duration of EBF was 68 days (interquartile range [IQR]: 19 to 130 days), and duration of breastfeeding comprising at least 85% of feeds (BF85%) was 151 days (IQR: 49 to 182 days). Solid food was also introduced at a median of 150 days (IQR: 118 to 177 days). Other characteristics of the infants in this study, previously reported 22, are included in Table 1.
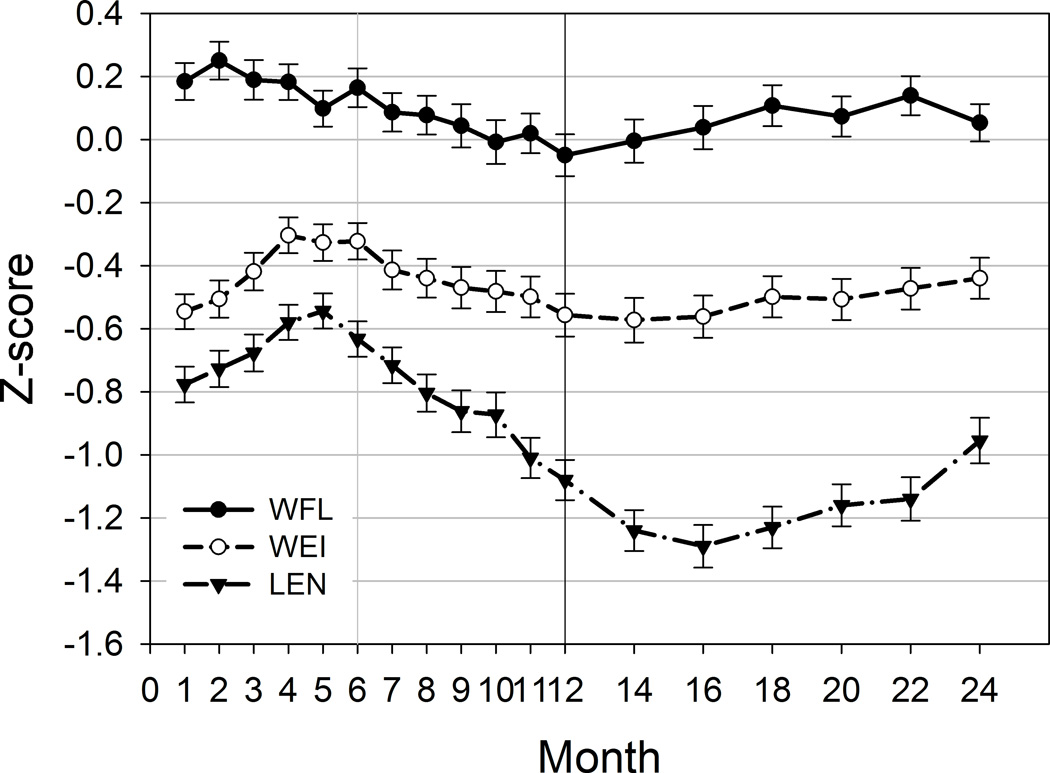
Figure 2. Descriptive presentation of breastfeeding and anthropometry.
A. Infant feeding patterns for exclusive breastfeeding (EBF), BF >85% and introduction of solid foods. B. Unadjusted means (± standard error of mean [SE]) of WEI, WFL and LEN Z-scores by month. Vertical solid lines indicate 3, 6 and 12 months of age for visual reference.
Table 1.
Baseline characteristics of cohort
| Entire cohort | ≥12 months’ followup |
<12 months’ followup |
|
|---|---|---|---|
| N | 277 | 192 | 85 |
| Maternal Characteristics | |||
| Age at delivery | 25.0 ± 5.7 | 25.4 ± 5.7 | 24.0 ± 5.5* |
| Employed (n [%] yes) | 39 (14%) | 31 (16%) | 8 (9%) |
| Education | |||
| ≤Elementary | 104 (38%) | 68 (35%) | 34 (40%) |
| ≤High school | 133 (48%) | 95 (49%) | 38 (45%) |
| ≤College | 42 (15%) | 29 (15%) | 13 (15%) |
| Marital status (% married) | 261 (94%) | 183 (95%) | 78 (92%) |
| Delivery type (% vaginal) | 183 (66%) | 121 (63%) | 62 (73%) |
| Parity | 1.2 ± 1.2 | 1.3 ± 1.2 | 1.1 ± 1.2 |
| Infant Characteristics | |||
| Sex (% male) | 125 (45%) | 90 (47%) | 35 (41%) |
| Birth weight (kg) | 3.2 ± 0.4 | 3.2 ± 0.4 | 3.2 ± 0.4 |
| Follow-up duration (months) | 17.2 ± 8.5 | 22.4 ± 3.5 | 5.4 ± 3.2* |
| Infant Feeding | |||
| Exclusive BF duration (days) | 78.1 ± 62.8 | 83.8 ± 63.6 | 65.0 ± 59.2* |
| Age at food introduction (days) | 143.7 ± 41.0 | 146.3 ± 38.5 | 134.1 ± 48.2 |
| Adiponectin | |||
| Maternal serum (µg/ml) | 8.37 ± 3.36 | 8.49 ± 3.43 | 8.09 ± 3.19 |
| Infant Serum (µg/ml) | 31.08 ± 5.49 | 30.69 ± 5.79 | 32.37 ± 4.25 |
| Milk adiponectin (ng/ml) | 21.57 ± 5.12 | 21.11 ± 5.13 | 22.63 ± 4.95* |
Unadjusted mean ± standard deviation (SD) or frequency (percent) presented.
p≤0.05 for difference by duration of follow-up, by Student’s t-test or χ2 test, as appropriate.
Compared with the WHO growth standard, WFL Z-scores peaked between 1 and 2 months of age, and WEI and LEN Z-scores peaked between 3 and 6 months of age (Figure 2B). During the second year of life, both WEI and WFL Z-scores demonstrate an upward trend relative to WHO growth curves, while LEN Z-scores declined steadily between 5 and 16 months of age.
Associations among infant serum, maternal serum, and maternal milk adiponectin during the first year of life
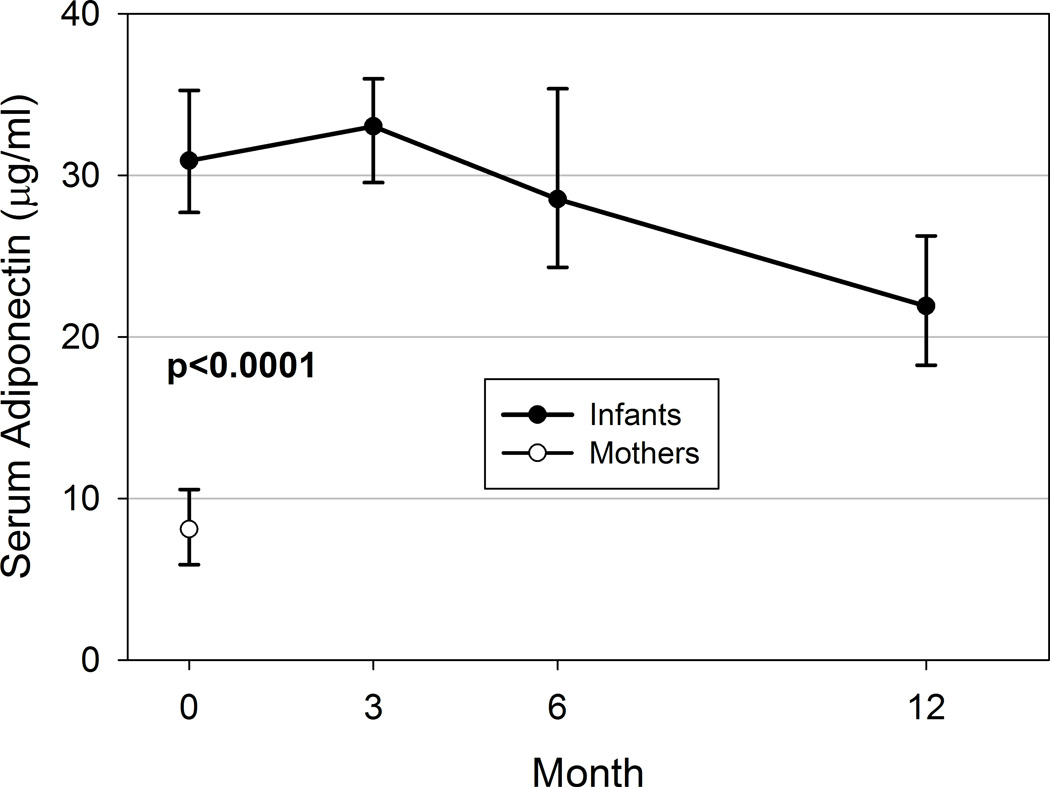
Serum adiponectin concentrations were 22.5 ± 0.6 µg/ml higher in infants than their mothers at baseline (p<0.0001; Figure 3). Infants’ serum adiponectin concentrations significantly increased between baseline (mean ± SE: 31.1 ± 0.6 µg/ml) and 3 months (33.2 ± 0.5 µg/ml, p=0.002 vs. baseline), then significantly declined by 6 months (28.6 ± 0.6 µg/ml, p<0.0001 vs. 3 months) and continued to decline to 12 months of age (23.5 ± 0.8 µg/ml, p<0.0001 vs. 6 months; Figure 3 presents medians and interquartile ranges [IQR] of data for clarity). Infant serum adiponectin was not associated with median milk adiponectin concentrations at baseline (Table 2), but by 3, 6, and 12 months of age, higher infant serum adiponectin was associated with higher exposure to milk adiponectin. Infant serum adiponectin at 12 months, but not at earlier ages, was also associated with lower concurrent WEI and LEN Z-scores (both p<0.01).
Figure 3. Infant and mothers’ serum adiponectin concentrations during first 12 months.
Analysis conducted using means and standard errors; however, unadjusted median and IQR are presented for graphical clarity. p≤0.002 for all pairwise comparisons of means among infant serum values; p<0.0001 for cross-sectional comparison of mean maternal and infant serum values at baseline.
Table 2.
Correlations among infant serum adiponectin, maternal serum adiponectin, milk adiponectin and infant anthropometry in the first year of life
| Adiponectin | Anthropometry | |||||
|---|---|---|---|---|---|---|
| N | Maternal baseline serum APN |
Median milk APN |
Infant WFL Z-score |
Infant WEI Z-score |
Infant LEN Z-score |
|
| Infant serum APN at: | ||||||
| Baseline | 87 | 0.29** | 0.04 | 0.02 | 0.11 | 0.002 |
| 3 months | 84 | 0.20 | 0.22* | −0.02 | −0.20 | −0.23* |
| 6 months | 66 | 0.32** | 0.38** | 0.07 | −0.06 | −0.09* |
| 12 months | 55 | 0.18 | 0.28* | −0.26 | −0.34** | −0.40** |
| Median infant serum APN | 92 | 0.28** | 0.25** | −0.08 0.03 0.01 −0.16 |
−0.16 −0.06 −0.12 −0.19 |
−0.003 −0.11 −0.17 −0.24* |
| Maternal baseline serum APN | 274 | -- | 0.37*** | −0.11 −0.07 −0.17** −0.09 |
−0.15** −0.15** −0.14* −0.12 |
−0.11 −0.10 0.01 −0.08 |
| Median milk APN | 276 | -- | -- | −0.11 −0.15* −0.14* −0.07 |
−0.17** −0.20** −0.14* −0.04 |
−0.13* −0.10 −0.09 −0.02 |
Unadjusted Spearman rank correlations presented. The 4 values in each box in the lower right panel correspond to correlations of adiponectin values with indicated z-scores at 0, 3, 6 and 12 months, respectively.
p≤0.05
p≤0.01
p≤0.0001
Despite differences in mean levels, maternal and infant serum adiponectin were directly correlated with each other at baseline (Table 2, r=0.29, p=0.007) and 6 months of age (r=0.32, p<0.01). Maternal serum adiponectin was also significantly correlated with her own median milk adiponectin concentration (r=0.37, p<0.0001). Both maternal baseline serum adiponectin and median milk adiponectin were associated with lower infant WEI Z-scores at 0, 3, and 6 months, as previously reported.
2nd year weight trajectories differ by milk adiponectin concentration, but not infant or maternal serum adiponectin
Final longitudinal models for year 2 ZWFL and ZWEI were adjusted for infant sex, birthweight, change in ZWFL or ZWEI between birth and 6 months (as appropriate), and marital status (married vs. not); ZWFL models were further adjusted for delivery type (vaginal vs. C-section). Other covariates were not significantly associated with infant anthropometry in the second year of life.
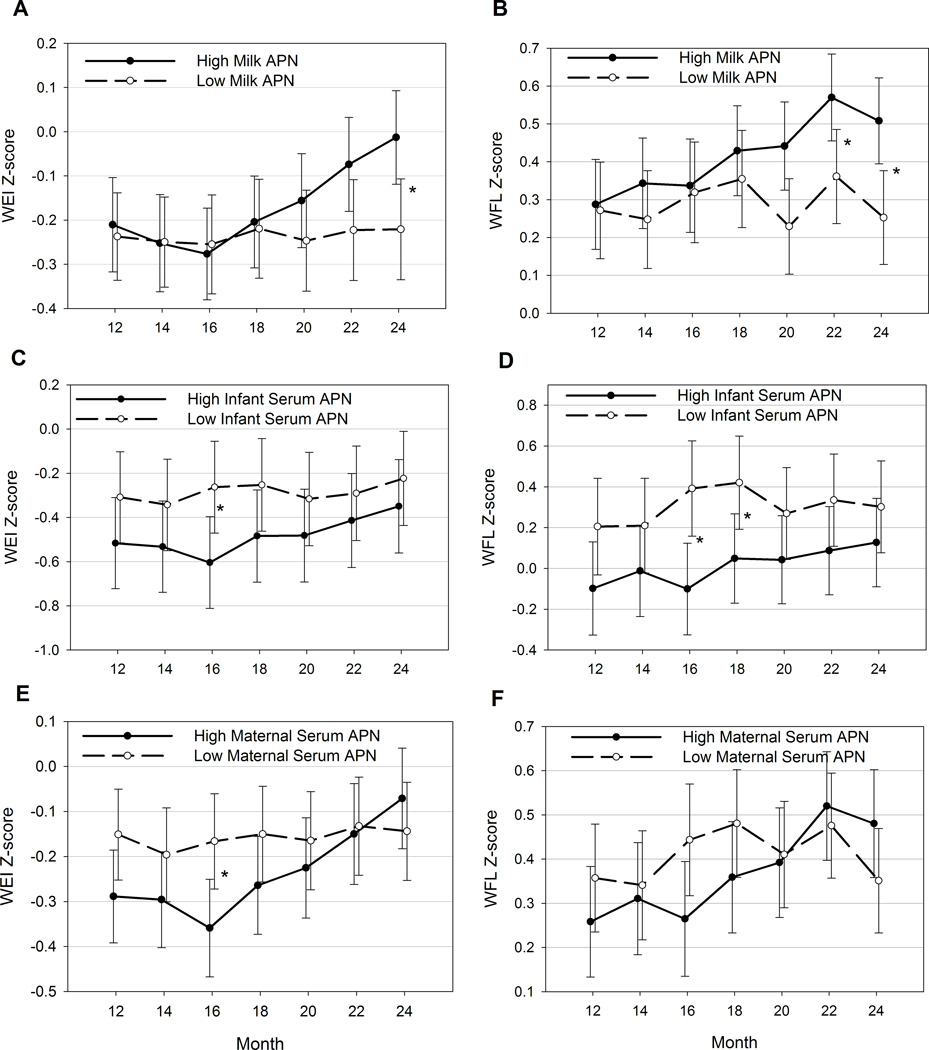
Infants exposed to high milk adiponectin experienced increasing WEI Z-score during the second year of life, while those exposed to lower milk adiponectin experienced little change in WEI Z-scores between 12 and 24 months of age (Figure 4A). These differences in trajectory over time are significant, even after adjusting for covariates (p for interaction of group by time=0.02). By 24 months of age, WEI Z-scores in infants exposed to high milk adiponectin are 0.21 ± 0.10 units higher than those in the lower milk adiponectin group, after adjusting for covariates (p=0.04). Similar patterns are evident for WFL Z-score trajectories (p for interaction=0.054, Figure 4B); however, milk adiponectin concentrations did not affect LEN Z-score trajectories (data not shown).
Figure 4. Year 2 infant growth trajectories by adiponectin concentrations.
Least square means ± SE presented from fully adjusted models. A. WEI Z-score, Milk adiponectin; B. WFL Z-score, Milk adiponectin; C. WEI Z-score, Infant serum adiponectin; D. WFL Z-score, Infant serum adiponectin; E. WEI Z-score, Maternal serum adiponectin; F. WFL Z-score, Maternal serum adiponectin. APN: Adiponectin. *p≤0.05 for adjusted pairwise comparison between high and low groups.
In the subset of infants with serum adiponectin and at least 12 months’ follow-up (n=71), above-median infant serum adiponectin during the first 12 months was modestly associated with lower mean WFL (adjusted β±SE: −0.28 ± 0.14 Z-units, p=0.05; Figure 4C) but not significantly lower mean WEI Z-scores (adjusted β±SE: −0.22 ± 0.13 Z-units, p=0.10; Figure 4D) during the second year of life. However, infant serum adiponectin did not alter the infant’s growth trajectory between 12 and 24 months (both p for interaction of group by time>0.4). Maternal serum adiponectin at baseline was not significantly associated with either mean ZWFL or ZWEI or growth trajectories during the second year of life (Figures 4E and 4F).
DISCUSSION
This study provides evidence that high exposure to milk adiponectin is part of a complex series of factors associated with increasing weight gain in the second year of life. Using a cohort of breastfed infants and their mothers, we explored the complex relationships among milk adiponectin, maternal serum adiponectin, infant serum adiponectin and changes in infant weight in the second year of life. We found that breastfed infants’ weight trajectories during the second year of life are associated with their exposure to human milk adiponectin during breastfeeding, independent of birthweight or growth during the first six months of life.
Previous studies of human milk adiponectin and infant growth have been conflicting. Our previous work in this and a second birth cohort demonstrated that high milk adiponectin concentrations were associated with lower infant weight and weight-for-length Z-scores over the first 6 months of life22. However, the present analysis demonstrated that, even adjusting for this early growth pattern, by 24 months of age these same infants exposed to high levels of human milk adiponectin were significantly heavier and had higher WEI Z-scores than those exposed to low levels, indicating a reversal of effect. A recent study also found that higher human milk adiponectin at 6 weeks postpartum was associated with a greater odds of overweight at age 223. By confirming the previous counterintuitive finding, the present study suggests that human milk adiponectin may have different effects during versus after the period of active breastfeeding. This relationship does not appear to be associated with duration of breastfeeding or timing of introduction of solid foods, and appears to be independent of several other covariates in our study.
Potential reasons for why higher milk adiponectin is paradoxically associated with greater second-year weight gain suggest avenues for future analysis. Milk adiponectin has been reported to be positively associated with maternal pre-pregnancy9 or post-pregnancy BMI17, although this association is not consistent 23, 28. It is possible that higher milk adiponectin exposure is a proxy for higher maternal BMI, which may indirectly affect infant weight gain during the first two years of life 29. Alternately or additionally, milk adiponectin may be physiologically active in infants during the time of active breastfeeding, limiting early weight gain in children otherwise at risk of obesity. A limitation of this study is that maternal anthropometry was not collected, so it is not possible to test these hypotheses in the current study.
Milk adiponectin may also be acting as a proxy measurement for any one of a number of potentially biologically-relevant components of human milk, which may be contributing to increased second-year growth. For example, human milk leptin has been associated with infant weight gain and BMI23, 30, 31, is postulated to affect food intake and food preferences32, and has been positively correlated with milk adiponectin17, 18. Leptin and other components of human milk were not assessed in the present study, but future research is clearly needed to elucidate the relative roles of the several components of human milk.
Recent studies have pointed to low birth weight and increased growth rates during infancy as important determinants of obesity during childhood, adolescence and even adulthood 33. Although the birth weights of this cohort are not low by design, the WEI and LEN Z-scores are consistently below the median, and overweight by age 2 is rare. Thus, it is possible that the weight gain seen in this cohort during the second year of life represents not pathology (early obesity) but rather positive adaptation (catch-up growth). In this light, higher exposure to milk adiponectin may be delaying catch-up growth that might otherwise occur in the first 6 months. Previous studies have noted that weight gain in the first 6 months is preferentially fat mass, while weight gain thereafter is associated with gain in lean mass 34–36. Following this reasoning, a delay in catch-up growth in infants exposed to high milk adiponectin may also be associated with less accrual of fat mass during the first 6 months and greater accrual of lean mass later in infancy. While this is speculative, and extends far beyond the scope of the present study, our hypothesis may provide a structure for future investigations on this question.
Interestingly, this study also demonstrates that human milk adiponectin is associated with both the mother’s and infant’s circulating adiponectin within the first year of life. Weyermann37 also found positive correlations between maternal milk and maternal serum adiponectin, and additional studies note that adiponectin is secreted from human breast adipose tissue19. Furthermore, ingested adiponectin appears in the serum of neonatal mice shortly after administration38, indicating that milk adiponectin survives digestion in infants. These findings, taken together, suggest that human milk may provide a link between mothers and their infants with regard to this important adipokine.
The present study also extends knowledge about the patterns of infant serum adiponectin during the first year of life in relation to maternal adiponectin and infant growth. Similar to previous studies of cord blood adiponectin 39–42, we report that infants’ circulating adiponectin levels during the first month of life are much higher than their mothers’. In addition, we report that maternal and infant serum adiponectin levels are significantly correlated with each other not only at birth, but also at 6 months of age. Previous studies have not typically reported significant correlations between cord blood and maternal adiponectin 39, 41, 43, but this may be due to differences in timing of measurements, cohort composition or breastfeeding exposures. The present study suggests that, despite differences in mean levels, infant serum adiponectin levels are associated with their mothers’ levels, whether the reasons for this are genetic, environmental or the in utero or breastfeeding exposures.
Serum adiponectin levels are also dynamic during infancy and early life. We report that total circulating adiponectin significantly increases from birth to 3 months of age, then declines for the remainder of the first year of life. This is consistent with data from other studies showing that total adiponectin increases significantly during late gestation 42, 44–46, increases through the first month after birth47, then declines during the first year47 and between the first and second years of life48. Circulating high molecular weight (HMW) adiponectin also appears to follow the same pattern 47, 49, which is not surprising given that most cord blood and infant adiponectin occurs in the HMW form 47, 50, and milk adiponectin is also predominantly HMW 38. The reasons for these fluctuations in infant serum adiponectin are not clear, and future work would be required to determine whether infant serum adiponectin is associated with body composition, as it is in older children.
Infant serum adiponectin does not appear to be related to longitudinal growth patterns in this study. We found no association of infant serum adiponectin with WFL Z-score during the first year, and during the second year of life, higher serum adiponectin was associated with lower mean WFL Z-score, but no difference in growth trajectory. Other studies have also found no association between adiponectin at birth or one month of age with concurrent anthropometric parameters 46, 51, suggesting that the negative association of circulating adiponectin and obesity seen in older children and adults develops after infancy. However, the decline in serum adiponectin between 1 and 2 years of age has been correlated to greater increases in body fatness, particularly in girls 48. One study noted that high cord blood adiponectin was associated with greater birth weight, and consistent with our findings, with lower weight gain in the first 6 months of life, yet higher central adiposity by age 3 52, suggesting a complex relationship between adiponectin and adiposity in early life that is not reported at older ages.
The longitudinal nature of this study, concurrent serum and milk samples, and detailed infant feeding and anthropometric data provided a unique opportunity to study growth trajectories in breastfed infants in relation to milk composition. Despite these several strengths, some limitations of the current study should be noted. Only adiponectin was measured in the human milk samples, so the potential influence of other human milk bioactive components cannot be quantified here. Also, because the original study was designed to focus on infant infectious disease outcomes, maternal BMI was not ascertained, and only a single maternal blood sample was collected. Infant blood samples were limited to a subset of participants. These factors limited inferences about the impact of maternal adiposity, but the strength of the findings suggests that the sample size did not impede our ability to detect significant associations.
In conclusion, this study highlights the potential role of high human milk adiponectin exposure in the accelerated weight trajectory of infants during the second year of life, despite being associated with lower weight gain during the first 6 months in the same cohort. Infant serum adiponectin is also independently associated with lower weight-for-age and weight-for-length Z-scores between 12 and 24 months, and may be influenced by maternal serum and milk adiponectin. These complex relationships in infant growth and feeding in the first two years require additional study, as they may have long-lasting impacts on childhood obesity risk and metabolic adaptation in later life.
Acknowledgements
Grateful acknowledgement goes to Ms. Luz del Carmen Mendez and Ms. Rosa Maria Garcia-Loperena, and to the participants in the study.
Sources of Support/Funding Acknowledgement: This work was supported by funding from the NIH (R21-HD054029 to JGW, P01-13021 to ALM, GMR-P and MLG) and the Cincinnati Children’s Hospital Medical Center Trustee Award (to JGW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
LJM and ALM are listed on a U.S. patent application claiming human milk adiponectin as an oral treatment for adiposity and inflammatory disorders, and LJM received a portion of a licensing fee for this technology. The remaining authors report no conflicts of interest.
References
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the united states, 1999–2004. JAMA. 2006 Apr 5;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Rivera JA, Barquera S, Campirano F, et al. Epidemiological and nutritional transition in mexico: Rapid increase of non-communicable chronic diseases and obesity. Public Health Nutr. 2002 Feb;5(1A):113–122. doi: 10.1079/PHN2001282. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong J, Reilly JJ. Breastfeeding and lowering the risk of childhood obesity. Lancet. 2002 Jun 8;359(9322):2003–2004. doi: 10.1016/S0140-6736(02)08837-2. [DOI] [PubMed] [Google Scholar]
- 4.Dewey KG. Is breastfeeding protective against child obesity? J Hum Lact. 2003 Feb;19(1):9–18. doi: 10.1177/0890334402239730. [DOI] [PubMed] [Google Scholar]
- 5.Shields L, O'Callaghan M, Williams GM, et al. Breastfeeding and obesity at 14 years: A cohort study. J Paediatr Child Health. 2006 May;42(5):289–296. doi: 10.1111/j.1440-1754.2006.00864.x. [DOI] [PubMed] [Google Scholar]
- 6.Shehadeh N, Khaesh-Goldberg E, Shamir R, et al. Insulin in human milk: Postpartum changes and effect of gestational age. Arch Dis Child Fetal Neonatal Ed. 2003 May;88(3):F214–F216. doi: 10.1136/fn.88.3.F214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casabiell X, Pineiro V, Tome MA, et al. Presence of leptin in colostrum and/or breast milk from lactating mothers: A potential role in the regulation of neonatal food intake. J Clin Endocrinol Metab. 1997 Dec;82(12):4270–4273. doi: 10.1210/jcem.82.12.4590. [DOI] [PubMed] [Google Scholar]
- 8.Resto M, O'Connor D, Leef K, et al. Leptin levels in preterm human breast milk and infant formula. Pediatrics. 2001 Jul;108(1):E15. doi: 10.1542/peds.108.1.e15. [DOI] [PubMed] [Google Scholar]
- 9.Bronsky J, Karpisek M, Bronska E, et al. Adiponectin, adipocyte fatty acid binding protein, and epidermal fatty acid binding protein: Proteins newly identified in human breast milk. Clin Chem. 2006 Sep;52(9):1763–1770. doi: 10.1373/clinchem.2005.063032. [DOI] [PubMed] [Google Scholar]
- 10.Bronsky J, Mitrova K, Karpisek M, et al. Adiponectin, afabp, and leptin in human breast milk during 12 months of lactation. J Pediatr Gastroenterol Nutr. 2011 Apr;52(4):474–477. doi: 10.1097/MPG.0b013e3182062fcc. [DOI] [PubMed] [Google Scholar]
- 11.Itoh H, Itakura A, Kurauchi O, et al. Hepatocyte growth factor in human breast milk acts as a trophic factor. Horm Metab Res. 2002 Jan;34(1):16–20. doi: 10.1055/s-2002-19961. [DOI] [PubMed] [Google Scholar]
- 12.Ozgurtas T, Aydin I, Turan O, et al. Vascular endothelial growth factor, basic fibroblast growth factor, insulin-like growth factor-i and platelet-derived growth factor levels in human milk of mothers with term and preterm neonates. Cytokine. 2010 May;50(2):192–194. doi: 10.1016/j.cyto.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Savino F, Liguori SA. Update on breast milk hormones: Leptin, ghrelin and adiponectin. Clin Nutr. 2008 Feb;27(1):42–47. doi: 10.1016/j.clnu.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Aydin S, Ozkan Y, Kumru S. Ghrelin is present in human colostrum, transitional and mature milk. Peptides. 2006 Apr;27(4):878–882. doi: 10.1016/j.peptides.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Ilcol YO, Hizli B. Active and total ghrelin concentrations increase in breast milk during lactation. Acta Paediatr. 2007 Nov;96(11):1632–1639. doi: 10.1111/j.1651-2227.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- 16.Savino F, Petrucci E, Nanni G. Adiponectin: An intriguing hormone for paediatricians. Acta Paediatr. 2008 Jun;97(6):701–705. doi: 10.1111/j.1651-2227.2008.00750.x. [DOI] [PubMed] [Google Scholar]
- 17.Martin LJ, Woo JG, Geraghty SR, et al. Adiponectin is present in human milk and is associated with maternal factors. Am J Clin Nutr. 2006 May;83(5):1106–1111. doi: 10.1093/ajcn/83.5.1106. [DOI] [PubMed] [Google Scholar]
- 18.Weyermann M, Beermann C, Brenner H, et al. Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clin Chem. 2006 Nov;52(11):2095–2102. doi: 10.1373/clinchem.2006.071019. [DOI] [PubMed] [Google Scholar]
- 19.Hugo ER, Brandebourg TD, Woo JG, et al. Bisphenol a at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008 Dec;116(12):1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavrila A, Chan JL, Yiannakouris N, et al. Serum adiponectin levels are inversely associated with overall and central fat distribution but are not directly regulated by acute fasting or leptin administration in humans: Cross-sectional and interventional studies. J Clin Endocrinol Metab. 2003 Oct;88(10):4823–4831. doi: 10.1210/jc.2003-030214. [DOI] [PubMed] [Google Scholar]
- 21.Hoffstedt J, Arvidsson E, Sjolin E, et al. Adipose tissue adiponectin production and adiponectin serum concentration in human obesity and insulin resistance. J Clin Endocrinol Metab. 2004 Mar;89(3):1391–1396. doi: 10.1210/jc.2003-031458. [DOI] [PubMed] [Google Scholar]
- 22.Woo JG, Guerrero ML, Altaye M, et al. Human milk adiponectin is associated with infant growth in two independent cohorts. Breastfeed Med. 2009 Jun;4(2):101–109. doi: 10.1089/bfm.2008.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weyermann M, Brenner H, Rothenbacher D. Adipokines in human milk and risk of overweight in early childhood: A prospective cohort study. Epidemiology. 2007 Nov;18(6):722–729. doi: 10.1097/ede.0b013e3181567ed4. [DOI] [PubMed] [Google Scholar]
- 24.Guerrero ML, Morrow RC, Calva JJ, et al. Rapid ethnographic assessment of breastfeeding practices in periurban mexico city. Bull World Health Organ. 1999;77(4):323–330. [PMC free article] [PubMed] [Google Scholar]
- 25.Lung'aho M, Huffman S, Labbok M, et al. Tool kit for monitroing and evaluating breastfeeding practices and programs. Wellstart International. 1996:1–35. [Google Scholar]
- 26.WHO Multicentre Growth Reference Study Group. Who child growth standards based on length/height, weight and age. Acta Paediatrica. 2006 Suppl 450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 27.Singer JD. Using sas proc mixed to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;24(4):323–355. [Google Scholar]
- 28.Dundar NO, Dundar B, Cesur G, et al. Ghrelin and adiponectin levels in colostrum, cord blood and maternal serum. Pediatr Int. 2010 Aug;52(4):622–625. doi: 10.1111/j.1442-200X.2010.03100.x. [DOI] [PubMed] [Google Scholar]
- 29.Deierlein AL, Siega-Riz AM, Adair LS, et al. Effects of pre-pregnancy body mass index and gestational weight gain on infant anthropometric outcomes. J Pediatr. 2011 Feb;158(2):221–226. doi: 10.1016/j.jpeds.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miralles O, Sanchez J, Palou A, et al. A physiological role of breast milk leptin in body weight control in developing infants. Obesity (Silver Spring) 2006 Aug;14(8):1371–1377. doi: 10.1038/oby.2006.155. [DOI] [PubMed] [Google Scholar]
- 31.Doneray H, Orbak Z, Yildiz L. The relationship between breast milk leptin and neonatal weight gain. Acta Paediatr. 2009 Apr;98(4):643–647. doi: 10.1111/j.1651-2227.2008.01192.x. [DOI] [PubMed] [Google Scholar]
- 32.Palou A, Pico C. Leptin intake during lactation prevents obesity and affects food intake and food preferences in later life. Appetite. 2009 Feb;52(1):249–252. doi: 10.1016/j.appet.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 33.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: Systematic reviews and hopeful suggestions. Acta Paediatr. 2006 Aug;95(8):904–908. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 34.Roggero P, Gianni ML, Orsi A, et al. Quality of growth in exclusively breast-fed infants in the first six months of life: An italian study. Pediatr Res. 2010 Aug 24; doi: 10.1203/PDR.0b013e3181f85a20. [DOI] [PubMed] [Google Scholar]
- 35.Carberry AE, Colditz PB, Lingwood BE. Body composition from birth to 4.5 months in infants born to non-obese women. Pediatr Res. 2010 Jul;68(1):84–88. doi: 10.1203/PDR.0b013e3181df5421. [DOI] [PubMed] [Google Scholar]
- 36.Veldhuis JD, Roemmich JN, Richmond EJ, et al. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005 Feb;26(1):114–146. doi: 10.1210/er.2003-0038. [DOI] [PubMed] [Google Scholar]
- 37.Weyermann M, Rothenbacher D, Brenner H. Duration of breastfeeding and risk of overweight in childhood: A prospective birth cohort study from germany. Int J Obes (Lond) 2006 Aug;30(8):1281–1287. doi: 10.1038/sj.ijo.0803260. [DOI] [PubMed] [Google Scholar]
- 38.Newburg DS, Woo JG, Morrow AL. Characteristics and potential functions of human milk adiponectin. J Pediatr. 2010 Feb;156(2 Suppl):S41–S46. doi: 10.1016/j.jpeds.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sivan E, Mazaki-Tovi S, Pariente C, et al. Adiponectin in human cord blood: Relation to fetal birth weight and gender. J Clin Endocrinol Metab. 2003 Dec;88(12):5656–5660. doi: 10.1210/jc.2003-031174. [DOI] [PubMed] [Google Scholar]
- 40.Kotani Y, Yokota I, Kitamura S, et al. Plasma adiponectin levels in newborns are higher than those in adults and positively correlated with birth weight. Clin Endocrinol (Oxf) 2004 Oct;61(4):418–423. doi: 10.1111/j.1365-2265.2004.02041.x. [DOI] [PubMed] [Google Scholar]
- 41.Chan TF, Yuan SS, Chen HS, et al. Correlations between umbilical and maternal serum adiponectin levels and neonatal birthweights. Acta Obstet Gynecol Scand. 2004 Feb;83(2):165–169. doi: 10.1111/j.0001-6349.2004.0298.x. [DOI] [PubMed] [Google Scholar]
- 42.Corbetta S, Bulfamante G, Cortelazzi D, et al. Adiponectin expression in human fetal tissues during mid- and late gestation. J Clin Endocrinol Metab. 2005 Apr;90(4):2397–2402. doi: 10.1210/jc.2004-1553. [DOI] [PubMed] [Google Scholar]
- 43.Bansal N, Charlton-Menys V, Pemberton P, et al. Adiponectin in umbilical cord blood is inversely related to low-density lipoprotein cholesterol but not ethnicity. J Clin Endocrinol Metab. 2006 Jun;91(6):2244–2249. doi: 10.1210/jc.2005-2714. [DOI] [PubMed] [Google Scholar]
- 44.Kajantie E, Hytinantti T, Hovi P, et al. Cord plasma adiponectin: A 20-fold rise between 24 weeks gestation and term. J Clin Endocrinol Metab. 2004 Aug;89(8):4031–4036. doi: 10.1210/jc.2004-0018. [DOI] [PubMed] [Google Scholar]
- 45.Pardo IM, Geloneze B, Tambascia MA, et al. Hyperadiponectinemia in newborns: Relationship with leptin levels and birth weight. Obes Res. 2004 Mar;12(3):521–524. doi: 10.1038/oby.2004.59. [DOI] [PubMed] [Google Scholar]
- 46.Mantzoros C, Petridou E, Alexe DM, et al. Serum adiponectin concentrations in relation to maternal and perinatal characteristics in newborns. Eur J Endocrinol. 2004 Dec;151(6):741–746. doi: 10.1530/eje.0.1510741. [DOI] [PubMed] [Google Scholar]
- 47.Bozzola E, Meazza C, Arvigo M, et al. Role of adiponectin and leptin on body development in infants during the first year of life. Ital J Pediatr. 2010;36:26. doi: 10.1186/1824-7288-36-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iniguez G, Soto N, Avila A, et al. Adiponectin levels in the first two years of life in a prospective cohort: Relations with weight gain, leptin levels and insulin sensitivity. J Clin Endocrinol Metab. 2004 Nov;89(11):5500–5503. doi: 10.1210/jc.2004-0792. [DOI] [PubMed] [Google Scholar]
- 49.Hibino S, Itabashi K, Nakano Y, et al. Longitudinal changes in high molecular weight serum adiponectin levels in healthy infants. Pediatr Res. 2009 Mar;65(3):363–366. doi: 10.1203/PDR.0b013e3181973b3b. [DOI] [PubMed] [Google Scholar]
- 50.Odden N, Morkrid L. High molecular weight adiponectin dominates in cord blood of newborns but is unaffected by pre-eclamptic pregnancies. Clin Endocrinol (Oxf) 2007 Dec;67(6):891–896. doi: 10.1111/j.1365-2265.2007.02981.x. [DOI] [PubMed] [Google Scholar]
- 51.Inami I, Okada T, Fujita H, et al. Impact of serum adiponectin concentration on birth size and early postnatal growth. Pediatr Res. 2007 May;61(5 Pt 1):604–606. doi: 10.1203/pdr.0b013e3180459f8a. [DOI] [PubMed] [Google Scholar]
- 52.Mantzoros CS, Rifas-Shiman SL, Williams CJ, et al. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: A prospective cohort study. Pediatrics. 2009 Feb;123(2):682–689. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]