Summary
The connection between cancer and inflammation is widely recognized; yet the underlying molecular mechanisms are poorly understood. We report here that TIPE2 provides a molecular bridge from inflammation to cancer by targeting the Ras signaling pathway. TIPE2 binds the Ras-interacting domain of the RalGDS family of proteins, which are essential effectors of activated Ras. This binding prevented Ras from forming an active complex, thereby inhibiting the activation of the downstream signaling molecules Ral and AKT. Consequently, TIPE2 deficiency led to heightened activation of Ral and AKT, resistance to cell death, increased migration, and dysregulation of exocyst complex formation. Conversely, TIPE2 overexpression induced cell death and significantly inhibited Ras-induced tumorigenesis in mice. Importantly, TIPE2 expression was either completely lost or significantly down-regulated in human hepatic cancer. Thus, TIPE2 is an inhibitor of both inflammation and cancer, and potential drug target for inflammatory and neoplastic diseases.
Keywords: Ras, apoptosis, oncogenesis, inflammation, cytoskeleton
Introduction
The TNFAIP8 (tumor necrosis factor-α-induced protein-8, also known as TIPE) family consists of four members, TNFAIP8, and TIPEs 1–3. Recently, we found that germ line deletion of TIPE2 (a.k.a. TNFAIP8L2) results in fatal inflammation and hypersensitivity to Toll-like receptor and T cell receptor stimulation (Sun et al., 2008). TIPE2 possesses a unique structural fold, shared by all members of the TNFAIP8 family, with no known function or significant homology to other known proteins (Zhang et al., 2009). TIPE2 is down-regulated in patients with chronic inflammatory diseases such as systemic lupus erythematosus and hepatitis, and its expression inversely correlates with disease progression (Li et al., 2009)(unpublished data). Although TIPE2 has emerged as a molecule critical for preventing inflammatory diseases, the mechanisms of its action remained unclear.
Ras is a major regulator of cell survival, proliferation, migration, and transformation. Alongside phosphatidylinositol (PI) 3 kinase and Raf1, Ral guanine nucleotide dissociation stimulator (RalGDS) family makes up the third arm of Ras effectors. The RalGDS family members are Guanine nucleotide Exchange Factors (GEFs) for the small GTPases RalA and RalB, switching GDP-bound inactive to GTP-bound active form of Ral (Ferro and Trabalzini, 2010; Spaargaren and Bischoff, 1994). A subset of the RalGEFs, including RalGDS, RGL and RGL2/Rlf, are direct effectors for activated Ras, which binds their C-termini and enhances their GEF activity towards Ral (White et al., 1996; Wolthuis et al., 1996). The RalGEF pathway plays a prominent role in mediating Ras-induced oncogenic transformation in humans. RalGDS deficiency suppresses Ras-mediated tumor formation (Gonzalez-Garcia et al., 2005). In rodent fibroblasts, the RalGEF effector pathway cooperates with the MAPK pathway to promote transformation and metastasis (Ward et al., 2001; White et al., 1996). In humans, the activation of this pathway is essential for transformation in a variety of cell types (Hamad et al., 2002; Rangarajan et al., 2004).
The RalGDS transforming ability is mediated by the active RalA and RalB. Active Rals regulate various biological processes including cell proliferation, motility, endo- and exocytosis, and cellular architecture (Feig, 2003). RalA and RalB have distinct and sometimes conflicting roles during oncogenic transformation despite their high sequence identity (over 80%). RalA, but not RalB, is critical for RalGDS-mediated transformation, and RalA is more potent than RalB in promoting anchorage-independent growth and targeted delivery of proteins to basolateral membrane in epithelial cells. RalB is more effective than RalA in promoting cell migration and activation of the TBK1 kinase, and in suppressing apoptosis and promoting metastasis (Chien et al., 2006; Chien and White, 2003; Lim et al., 2005; Lim et al., 2006). Constitutively active forms of RalA and RalB can transform human cell lines (Lim et al., 2005). Both RalA and RalB are activated in human malignancies such as bladder, pancreatic and colon cancers, and they collaborate to promote and maintain oncogenic transformation (Lim et al., 2006; Martin et al., 2011; Smith et al., 2007).
Recent studies suggest that the Ral effects on motility, secretion, and cell proliferation are largely mediated through the regulation of the exocyst complex. This octameric complex regulates targeting and tethering of secretory vesicles to specific plasma membrane domains, such as the leading edge of migrating cells (Rosse et al., 2006; Spiczka and Yeaman, 2008). Two exocyst subunits, Sec5 and Exo84, are bona fide Ral effectors (Moskalenko et al., 2002), each of which belongs to a different sub-complex. One sub-complex contains Exo84 and Sec10, and is localized on the plasma membrane, whereas the other contains Sec 5, 6, 8 subunits, and is located on secretory vesicles (He and Guo, 2009). Active Ral promotes assembly of these two sub-complexes through dual subunit interaction, leading to vesicular tethering to the plasma membrane (Jin et al., 2005; Moskalenko et al., 2003). As the exocyst complex is becoming better understood, its involvement in carcinogenesis has been brought to the forefront. Exocyst subunit interaction with active Ral is required for tumorigenesis of colorectal carcinoma, progression of skin cancer, motility, anchorage-dependence, and survival of transformed cells (Chien et al., 2006; Martin et al., 2011; Sowalsky et al., 2011).
An important function of the RalGDS family is to promote cell survival. This may be mediated through both Ral GTPases (Chien and White, 2003) and non-canonical activation of AKT (Hao et al., 2008). Canonical AKT activation requires the generation of PIP3 by PI3K at the membrane. The PH domain-containing proteins AKT and PDK1 bind this phosphoinositide, allowing AKT to be phosphorylated by PDK1 (T308) and mTOR (S473). By contrast, in the non-canonical AKT activation pathway, RalGDS acts as a scaffold for PDK1 and enhances its kinase activity, resulting in increased phosphorylation of AKT. Active AKT phosphorylates a large number of substrates thereby protecting cells from death (Sale and Sale, 2008). RalGDS-mediated AKT activation is responsible for the proliferative effect of RalGDS in NIH3T3 cells (Hao et al., 2008). In vivo, RalGDS regulates tumor growth by providing survival signals to tumor cells, and consequently, in Ralgds−/− mice, apoptosis of carcinogen-induced papillomas is enhanced (Gonzalez-Garcia et al., 2005). Thus, the regulation of the RalGEF effector pathway is key to Ras-mediated transformation. In this report, we describe an unexpected and previously unknown connection between TIPE2 and the RalGDS family, and demonstrate its relevance to cell survival, motility, and Ras-induced oncogenesis.
Results and Discussion
Inflammatory factors significantly down-regulate TIPE2 expression
Our previous work indicates that TIPE2 prevents inflammation and maintains immune homeostasis. To test whether TIPE2 itself is regulated by inflammatory signals, we examined TIPE2 mRNA levels in lymphoid and myeloid cells after stimulation with ligands for Toll-like receptors (TLRs) 3, 4, 7, and 9, and mitogenic activators of immune cells (Figure S1, A and D, and unpublished data). We found that TIPE2 expression was significantly down-regulated by all these ligands. Blocking NF-κB activation in cells treated with lipopolysacchride (LPS), a TLR4 ligand, completely rescued the defect in TIPE2 expression (Figure S1B), indicating that LPS down-regulates TIPE2 by NF-κB activation. These results indicate that TIPE2 expression can be shut down by inflammatory signals, which may in turn contribute to inflammation-induced pathologies.
TIPE2 prevents Ras from binding the Ras-interacting domain of RGL
The mechanisms of TIPE2 function are not clear. To address this issue, we searched for binding partners of TIPE2 using a two-pronged approach. Firstly, we conducted a yeast two-hybrid screen of a mouse splenic cDNA library using TIPE2 as the bait, and secondly, we performed a large-scale coimmunoprecipitation of TIPE2-binding proteins followed by mass spectrometry. Among several clones isolated in the yeast-two hybrid screen, two were found to encode the C-terminus region of the RGL. Consistent with this finding, mass spectrometry results showed that TIPE2 pulled down with proteins of the RGL-Ral pathway. Together, these results suggested a role for TIPE2 in Ras-mediated signaling.
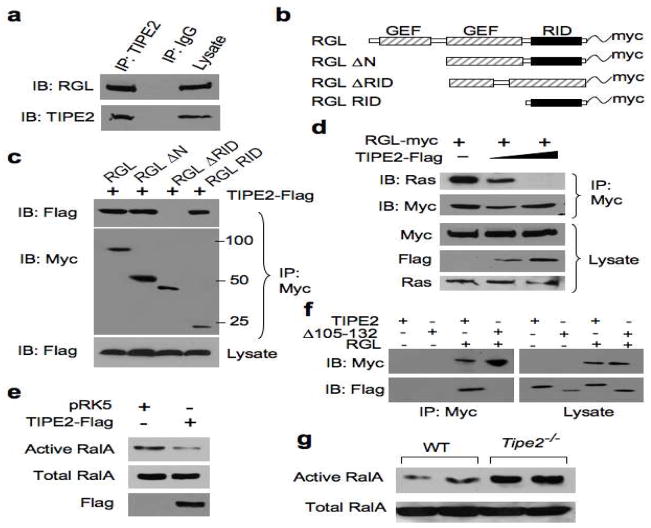
To establish whether endogenous TIPE2 interacts with RGL in mammalian cells, we immunoprecipitated TIPE2 from the murine macrophage cell line, Raw 264.7. We found that endogenous RGL co-precipitated with endogenous TIPE2 (Figure 1A), as did two other RalGEF family members, RalGDS and RGL2 (Figure S2 and data not shown). To map the region within RGL that is responsible for TIPE2 interaction, we cloned the murine full-length RGL (amino acids 1–768) or truncated RGL (Figure 1B), in frame with a myc tag and co-transfected the full-length or truncated RGL constructs into cells together with the TIPE2-Flag plasmid. TIPE2 pulled down with full-length RGL and the C-terminus of RGL. However, TIPE2 did not pull down with a truncated RGL that lacked the C-terminal region (Figure 1C). These data indicate that TIPE2 binds to the C-terminus of RGL, which contains the Ras Interacting Domain (RID) (Murai et al., 1997).
Figure 1. TIPE2 binds the Ras-interacting domain of RGL, and inhibits Ral activation.
(a) Interaction between endogenous RGL and TIPE2. Raw 264.7 cell lysates were immunoprecipitated (IP) with mouse anti-TIPE2 or control IgG, and subjected to SDS-PAGE and immunoblotting (IB). (b) Schematics of the RGL constructs used in this study. GEF, guanine nucleotide exchange factor; RID, Ras-interacting domain. (c) Interaction of TIPE2 with RGL Ras-interacting region. Lysates of 293T cells transiently transfected with RGL constructs and a TIPE2-Flag construct were immunoprecipitated with anti-Myc, and subjected to SDS-PAGE and IB. (d) TIPE2 prevents Ras from binding to RGL. Lysates of 293T cells transiently transfected with RGL-Myc (2 μg/10-cm plate) and increasing amounts of TIPE2-Flag constructs (5 μg to 10 μg/10-cm plate) were immunoprecipitated with anti-Myc, and subjected to SDS-PAGE and IB. (e) 293T cells were transiently transfected with TIPE2-Flag or pRK5 plasmids. 18 hours later, cell lysates were subjected to pull-down using GST-RalBP1 RBD. RalA from the pull-down and total RalA in the lysates were determined by IB. (f) TIPE2 Δ105–132 does not bind RGL. 293T cells were transielntly transfected with either TIPE2-Flag or TIPE2 Δ105–132 constructs, with or without RGL plasmid. 18 hours later, cell lysates were immunoprecipitated with anti-Myc antibody, and subjected to SDS-PAGE and IB. (g) Active and total RalA levels in wild type and Tipe2−/− bone marrow-derived murine macrophages were determined as in panel (e). Data shown are representative of 3 independent experiments.
Active Ras binds the RID of RalGEFs and activates their GEF activity (Murai et al., 1997; Urano et al., 1996). Our finding that TIPE2 binds the RID of RGL (Figure 1C) suggests that in the presence of TIPE2, Ras would be unable to bind RGL. We examined the presence of Ras in complex with RGL in 293T cells transiently expressing full-length RGL and increasing amounts of TIPE2. TIPE2 inhibited endogenous Ras from forming a complex with RGL, in a dose-dependent manner (Figure 1D). This indicates that TIPE2 can exclude active Ras from binding to RGL.
TIPE2 inhibits RGL-induced activation of Ral and AKT, thereby promoting cell death
We then asked whether the outcome of TIPE2 binding to RGL could be inhibition of RGL GEF activity towards its substrate Ral. In 293T cells transiently overexpressing TIPE2, we detected more than 60 percent decrease in Ral GTP level compared to the control (Figure 1E) Similar results were obtained in Raw 264.7 macrophages (data not shown). Active Ras levels were not affected by overexpression of TIPE2 (Figure S2). TIPE2 protein and mRNA levels were downregulated in Raw 264.7 cells treated with LPS (Figures S1 and S5), and Ral activity was elevated as a result of the treatment (Figure S1). Moreover, TIPE2-deficient bone marrow-derived macrophages showed a three-fold increase in active Ral level over wild type control cells (Figure 1G). These results indicate that TIPE2 serves as an inhibitor of RGL activity by blocking active Ras binding to RGL.
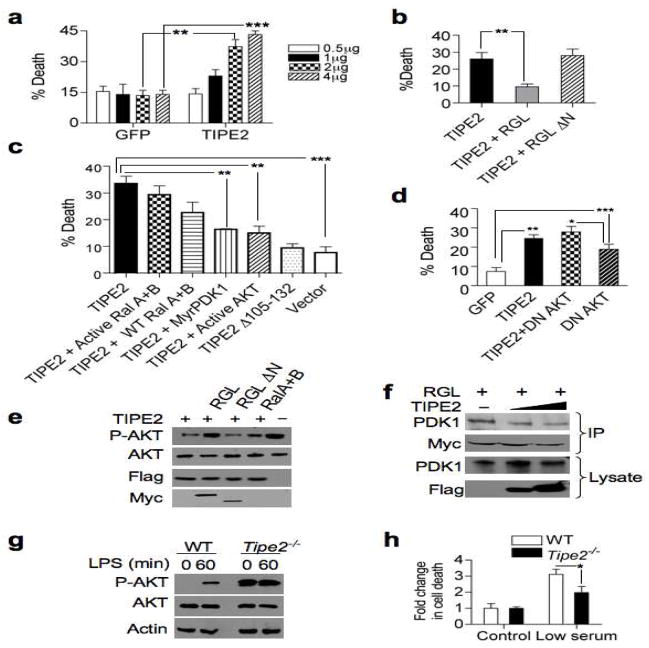
Knockdown of TIPE2 in T cells confers resistance to Fas ligand-induced apoptosis, indicating that TIPE2 may promote cell death. Tipe2−/− mice suffer from splenomegaly and leukocytosis, caused by increased numbers of myeloid and lymphoid cells (Sun et al., 2008). These abnormalities could be rooted in a decrease of apoptosis in TIPE2-deficient cells. We observed in Raw 264.7 cells that TIPE2 expression was induced upon cell death (Figure S1). We therefore hypothesized that increased expression of TIPE2 might lead to cell death. To test this, we transfected 293T cells with a TIPE2-expressing plasmid. Within 24 hrs, TIPE2 expression in these cells induced significant cell death in a dose-dependent manner (Figure 2A).
Figure 2. TIPE2 promotes cell death by inhibiting RGL-induced AKT activation.
(a) TIPE2 promotes cell death in 293T cells. Cells were transfected with the indicated amounts of TIPE2-Flag construct (TIPE2) or control GFP vector (GFP). After 24 hrs, cell death was assessed by trypan blue staining. Data shown are means ± S.E.M (n=3), and are representative of 5 independent experiments. ** p < 0.01; *** p < 0.001. (b) RGL rescues TIPE2-induced cell death. 293T cells were transfected with TIPE2-Flag together with RGL or RGL ΔN plasmids for 24 hrs, and cell death was assessed as in (a). Data shown are means ± S.E.M (n=5), and are representative of 5 independent experiments. ** p < 0.02. (c) TIPE2-induced death is mediated by the PDK1-AKT axis. 293T cells were transfected with TIPE2-Flag or TIPE2 Δ3105-132 plasmids, with or without activated RalA and RalB, wild type RalA and RalB, activated AKT, or myristoylated PDK1 plasmids for 24 hrs. Cell death was assessed as in (a). Data shown are means ± S.E.M (n=3). *** p< 0.001, ** p< 0.005. (d) AKT inhibition is responsible for TIPE2-induced cell death. 293T cells were transiently transfected with either GFP, TIPE2, dominant negative (DN) AKT, GFP plus TIPE2, or DN AKT plus TIPE2 plasmids. Cell death was assessed as in (a). Data shown are means ± S.E.M (n=5) of the cell death rates, and are pooled from 2 independent experiments. *** p < 0.001, ** p < 0.01, * p < 0.05. (e) TIPE2 reduces phospho (P)-AKT (S473) levels. 293T cells were transfected with the indicated constructs for 24 hours, and protein levels were determined by IB. (f) TIPE2 decreases RGL interaction with PDK1. Lysates of 293T cells transiently transfected with RGL-Myc (2 μg/10-cm plate) and increasing amounts of TIPE2-Flag constructs (5 μg to 10 μg/10-cm plate) were immunoprecipitated with anti-Myc, and subjected to SDS-PAGE and IB. (g) Increased phosphorylation of AKT in Tipe2−/− splenocytes. Wild type and Tipe2−/− splenocytes were treated with LPS (200 ng/ml) for the indicated times. Cell lysates were subjected to SDS-PAGE and IB. Data shown are representative of three independent experiments. (h) Tipe2−/− splenocytes are resistant to serum deprivation-induced death. Tipe2−/− or wild type splenocytes were incubated in DMEM containing 10% (control) or 0.2% (low serum) FBS for 4 hrs, and cell death was assessed as in (a). Data shown are means ± S.E.M (n=3), and are representative of three independent experiments. * p < 0.05.
Next, we examined the consequence of TIPE2-RGL interaction in cell death. In 293T cells, we found that ectopic TIPE2 expression caused a 2-fold increase in cell death, as assessed by trypan blue staining (Figures 2A and 2B) and cleavage of PARP (data not shown). A truncated TIPE2 that lacked amino acids 105–132 was not able to bind to RGL (Figure 1F) or induce cell death when expressed in 293T cells (Figure 2C). Importantly, co-transfection of RGL and TIPE2, rescued the death phenotype (Figure 2B). However, wild type RalA and RalB, or activated mutants of RalA and RalB, had little protective effect against TIPE2-induced death (Figure 2C), suggesting that additional components downstream of RGL could be involved in the rescue. Previous work showed that RalGDS binds PDK1 and enhances AKT activity. We found that RGL bound to PDK1 through the N-terminal region of the RGL (Figure S3). Expression of TIPE2 together with RGL ΔN, which did not bind PDK1 could not rescue TIPE2-induced death (Figure 2B). Furthermore, TIPE2 caused a reduction in phosphorylated AKT level, which was rescued by RGL (Figure 2E), but not by RGL ΔN, or RalA and RalB, even though RGL ΔN could induce Ral activity (Figure S3).
The RGL-PDK1 complex is induced by growth stimuli, and activated Ras plays an important role in its formation. PDK1 relieves the intra-molecular inhibition of the catalytic domain of RalGEFs by binding to its N-terminus, and RalGEFs enhance PDK1 kinase activity preferentially towards AKT (Hao et al., 2008; Tian et al., 2002). Ras binding to RGL seems to be a necessary but insufficient step in promoting RGL-PDK1 interaction, since a Ras mutant that preferentially binds RalGEFs could not activate AKT under serum starvation conditions (data not shown)(Tian et al., 2002). Expression of TIPE2 reduced PDK1 binding to RGL by ~44% compared to the control (Figure 2F), implying that the disruption of Ras binding to RGL may prevent RGL-PDK1 complex formation. PDK1 binding to RGL and the subsequent inhibition of AKT could be responsible for TIPE2-induced cell death. To test this hypothesis, we co-expressed TIPE2 with a constitutively active form of AKT (AKT T308D, S473D) and found that activated AKT could rescue from TIPE2-induced cell death (Figure 2C). Dominant negative AKT (DN-AKT) increased the basal level of cell death, but when co-expressed with TIPE2 did not induce death beyond that of TIPE2 alone (Figure 2D, Figure S4A). Furthermore, co-expression of TIPE2 with myristoylated PDK1 also rescued cells from death (Figure 2C). These results indicate that TIPE2 promotes cell death primarily through the inhibition of AKT (Figure S4A). Enhanced susceptibility to serum starvation was observed in Ralgds−/− cells and RGL2 overexpression rendered resistance to serum withdrawal (Gonzalez-Garcia et al., 2005; Wolthuis et al., 1997). In Tipe2−/− cells, AKT was constitutively phosphorylated, and was unable to be further induced by lipopolysacchride (LPS) (Figure 2G). We therefore examined the impact of serum deprivation on Tipe2−/− cells (Figure 2H). We found that Tipe2−/− cells were resistant to serum deprivation-induced death (Figure 2H). Taken together, these results indicate that TIPE2 promotes cell death by inhibiting the RGL-PDK1-AKT axis.
TIPE2 inhibits cell motility and exocyst complex assembly
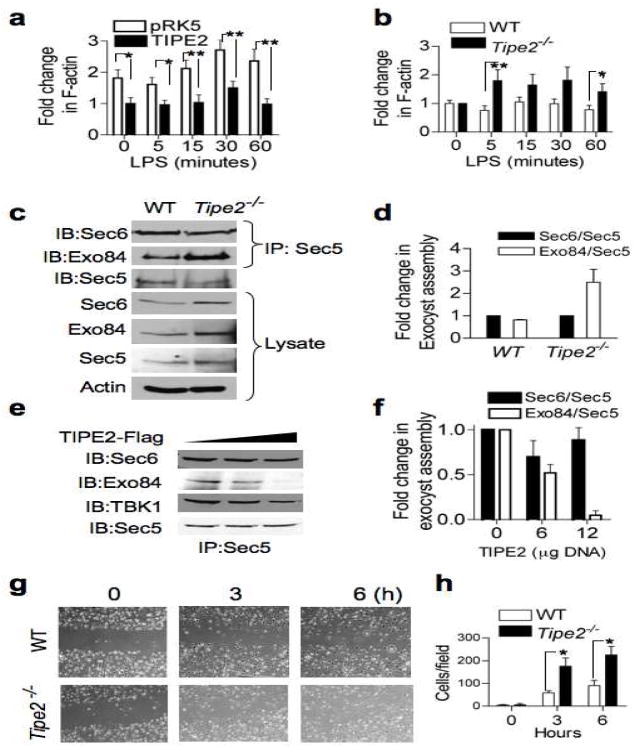
A hallmark of Ral function is its regulation of cell motility. Ral activation promotes cellular protrusions and is essential for directional movement of cells (Rosse et al., 2006; Sugihara et al., 2002). Active Ral mediates chemotaxis in lymphocytes, plays a critical role in tumor metastasis, and contributes to cytokinesis progression (Oxford et al., 2005). Ral mediates these effects by regulating both actin dynamics and exocyst complex assembly. Actin polymerization is essential for maintaining cell shape, internalization processes (endocytosis and phagocytosis), and cell motility. We therefore examined the effects of TIPE2 expression on actin polymerization. Polymerization of F-actin can be induced by LPS stimulation in monocytes (Kong and Ge, 2008). Expression of TIPE2 in Raw 264.7 cells resulted in a significant decrease in the total level of F-actin (Figure 3A). The F-actin polymerization rate in TIPE2-transfected cells was also reduced. Conversely, in Tipe2−/− splenocytes, the rate of F-actin polymerization was significantly enhanced compared to wild type cells (Figure 3B). These results suggest that TIPE2 can impact both the rate of actin polymerization and the total levels of F-actin in immune cells.
Figure 3. TIPE2 regulates cell migration, exocyst assembly, and actin dynamics.
(a) Decreased rate of F-actin polymerization in TIPE2-expressing Raw 264.7 macrophages. Cells were transfected with either TIPE2 or empty plasmid (pRK5). One day later, the cells were treated with LPS (200 ng/ml) for the indicated times, and F-actin level was measured as described in Methods. The value of the TIPE2 group at time zero was set as 1. Results are means ± S.E.M, and were pooled from three independent experiments (n=12). * p < 0.05; ** p<0.01. (b) Increased polymerization of F-actin in Tipe2−/− splenocytes. Wild type and Tipe2−/− splenocytes were treated with LPS (200 ng/ml) for the indicated times and the F-actin level was measured. The value at time zero was set as 1. Results are means ± S.E.M, and were pooled from three independent experiments (n=6). * p < 0.05; ** p < 0.02. (c–d) Increased exocyst complex assembly in Tipe2−/− bone marrow-derived macrophages. Lysates of wild type and Tipe2−/− cells were immunoprecipitated with anti-Sec5 and subjected to SDS-PAGE and IB (c). Quantitation of the exocyst assembly was performed by densitometry (d). Results are means ± S.E.M of Sec6/Sec5 and Exo84/Sec5 ratios pooled from two independent experiments. The values of Sec6/Sec5 ratio for each group were set as 1. (e–f) Decreased exocyst complexes in TIPE2-expressing cells. Lysates from 293T cells transfected with increasing amounts of TIPE2 plasmid (0, 6, and 12 μg/10-cm plate) for 18 hours were immunoprecipitated and tested as in panel c (e). Quantitation of the exocyst assembly was performed by densitometry (f). Results are means ± S.E.M pooled from two independent experiments. The values of Sec6/Sec5 ratio for each group were set as 1. (g–h) Increased migration of Tipe2−/− macrophages. Confluent monolayers of wild type and Tipe2−/− bone marrow-derived macrophages were subjected to the wound-healing assay as described in Methods. Images were then taken immediately after applying the wound (time 0), and 3 and 6 hours later (g). Cells that migrated into the wounded areas were counted using the ImageJ software. Results are means ± S.E.M of 10 fields from each group, and are representative of three independent experiments. *p < 0.005.
We next examined the exocyst subunit levels in Tipe2−/− splenocytes and bone marrow-derived macrophages, and detected a significant increase in exocyst subunits Sec 5, 6, 8 and 84 (Figure 3C). These results are consistent with previous reports showing decreased absolute amounts of Sec5 and Sec6 in RalA- or RalB-depleted rat kidney cells (Rosse et al., 2006). Next, we examined whether TIPE2 impacts the formation of the exocyst complex, by measuring its assembly from its two sub-complexes in wild type (WT) and Tipe2−/− cells. While there was no difference in Sec5/Sec6 sub-complex assembly between Tipe2−/− and WT cells, the association between Sec5 and Exo84 increased by about 3-fold in Tipe2−/− cells (Figure 3D). Therefore, the Ral-regulated step of exocyst assembly is defective in Tipe2−/− cells. Consistent with this observation, TIPE2 overexpression resulted in destabilization of the exocyst complex. In 293T cells expressing TIPE2, the assembly of the sub-complex Sec5/Sec6 was unchanged, while the assembly of Exo84 and Sec5 was markedly decreased (Figure 3E, 3F).
Ral depletion blocks exocyst complex formation at the leading edge of migrating cells, and inhibits cell migration (Rosse et al., 2006; Spiczka and Yeaman, 2008). We tested directional cell migration of Tipe2−/− macrophages in a wound-healing assay (Figure 3G). The “wound” was created in confluent Tipe2−/− and wild type cultures (time zero), and migration of cells into the gap was monitored after 3 and 6 hrs. Wild type macrophages started moving into the wound after 3 hrs, and by 6 hrs the wound was still visible. However, Tipe2−/− macrophages moved into the wound faster, and completely closed the gap by 6 hrs. Quantification of the number of cells that moved into the gap showed that the rate of Tipe2−/− cell migration was 3-fold higher than that of the wild type (Figure 3H). Moreover, Tipe2−/− macrophages that had moved into the wound were elongated, and had increased number of cellular extensions, generally assuming a “migratory” form. In contrast, wild type cells looked round, with smaller number of extensions (Figure S4B). Consistent with this finding, in vivo migration of TIPE2 knockout leukocytes into skin air-pouches injected with the chemokine KC (keratinocyte chemoattractant) was significantly enhanced as compared to wild type controls (unpublished data). The enhanced migratory phenotype of Tipe2−/− cells could be mediated by irregularities of both actin dynamics and exocyst complex assembly. These abnormalities may partly explain the increased inflammation in Tipe2−/− mice.
Recently, it has been established that active RalB induces Sec5 dimerization and subsequent activation of TBK1 kinase (Chien et al., 2006; Ou et al., 2011). This pathway results in AKT activation, protects cancer cells from apoptosis, and is required for mounting host defense responses. RalB and Sec5 are required for TLR3-induced IRF3-dependent interferon-β production. We observed reduced interaction between Sec5 and TBK1 in TIPE2-overexpressing cells (Figure 3E), and a reduction in phosphorylated IRF3 (Figure S4C). These results suggest that the Ral/Sec5/TBK1 pathway is inhibited by TIPE2. It was shown previously that TIPE2-deficient cells exhibit increased NF-κB activity. Therefore, TIPE2 may regulate the NF-κB pathway through the Ral/Sec5/TBK1 axis.
TIPE2 inhibits tumorigenesis in vivo
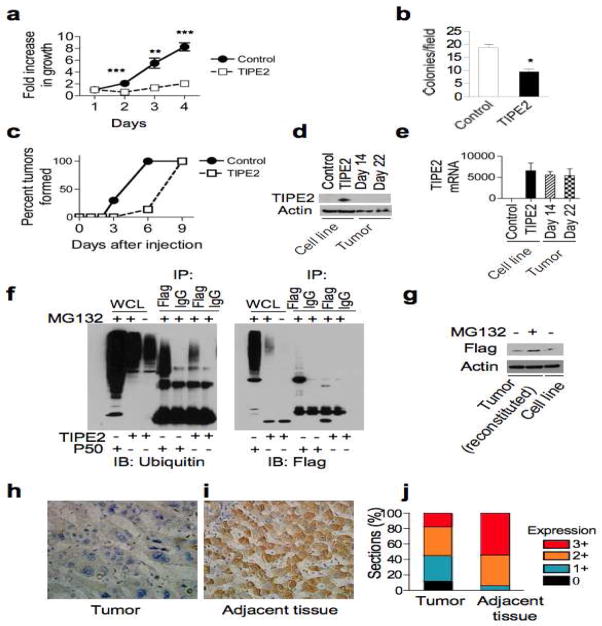
Activating mutations of Ras are found in ~30% of all malignant tumors. The best-characterized Ras effector pathways are the Raf-MAPK and PI3K pathways, and their importance in Ras-mediated oncogenesis has been extensively studied. However, a growing body of evidence supports an important role for the RalGDS family in Ras-induced growth and transformation of human cells. To determine the potential roles of TIPE2 in tumorigenesis, the Ras-transformed NIH 3T3 fibroblasts (Ras G12V) were used to stably express Flag-tagged TIPE2. Expression of TIPE2 significantly reduced the growth of Ras 3T3 cells (Figure 4A). The effect was the most dramatic under low serum conditions, where cells were more dependent on Ras for survival. Consistent with these results, overexpression of TIPE2 in Ras 3T3 cells reduced colony formation in soft agar (Figure 4B). Expression of TIPE2 in NIH 3T3 alone did not result in colony formation. To test the effect of TIPE2 on tumor formation in vivo, Ras 3T3 cell line stably expressing TIPE2 was injected into nude mice. TIPE2 significantly delayed tumor onset in two independent experiments, in comparison to control injections (Figure 4C). NIH 3T3 or NIH 3T3 stably expressing TIPE2 did not give rise to tumors. TIPE2 tumors, once formed, could grow to the same weight as control (Figure S5), suggesting that TIPE2 tumors did not have a growth disadvantage as compared to Ras 3T3 tumors. In addition, while Ral activity was inhibited in the pre-injected cell lines, it was restored in isolated tumor cells (Figure S5). This paradox could be explained if somehow in mice TIPE2 expression was lost. Indeed, upon examining the tumors 14 and 22 days after tumor cell inoculation, we could not detect any TIPE2 protein by immunoblotting (Figure 4D). However, by quantitative RT-PCR, we could clearly show that TIPE2 tumors expressed similar amounts of TIPE2 transcript compared to TIPE2-expressing Ras 3T3 cells before injection (Figure 4E). Therefore, it appears that TIPE2 downregulation in the tumor occurred at the protein level. The half-life of TIPE2 protein is rather short, around 4 hrs (Figure S5), and the TIPE2 protein is heavily ubiquitinated in cells (Figure 4F). These findings indicate that TIPE2 protein is regulated by ubiquitination and proteasomal degradation. Indeed, the reduced TIPE2 level in tumor cells could be restored to that of pre-injected cells after treatment with the proteasome inhibitor MG132 (Figure 4G). This indicates that TIPE2 degradation is enhanced in the tumor cells. Therefore, cells that formed tumors were those that had TIPE2 protein actively suppressed. These cells were likely present in the pre-injected pool but were outnumbered by those that did express TIPE2. However, once injected into the animal, cells that suppressed TIPE2 protein had a significant survival advantage and were therefore positively selected. Although TIPE2 tumors might have originated from less cells, as the delay in tumor onset suggests, they eventually reached the same size as the control tumors. This unexpected result suggests that mechanisms responsible for TIPE2 elimination may also result in acquisition of a growth advantage over Ras3T3 control cells. These results point to a role for TIPE2 as a tumor suppressor involved in carcinogenesis.
Figure 4. TIPE2 over-expression suppresses Ras-induced transformation whereas TIPE2 down-regulation is associated with human hepatocellular carcinogenesis.
(a) TIPE2 inhibits the growth of Ras-transformed cells. Ras-transformed NIH3T3 cells that did or did not express TIPE2 were cultured in DMEM containing 0.5% FBS. Cells were fixed at 24 hr intervals and stained with methylene blue. Results are means ± S.E.M (n=5) of relative absorbance with the value at day one set as 1, and are representative of three independent experiments. ** p<0.001; *** p < 0.0001. (b) TIPE2 inhibits soft-agar colony formation of Ras-transformed cells. Ras-transformed NIH3T3 cells that did or did not express TIPE2 were cultured in duplicates in soft-agar plates as described in Methods, and colonies were counted in 20 random fields for each culture under 40X magnification. Results are means ± S.E.M (n=20) and are representative of three independent experiments. * p < 0.0001. (c) TIPE2 delays tumorigenesis in nude mice. Ras-transformed NIH3T3 cells that did or did not express TIPE2 were injected subcutaneously into the rear flanks of nude mice (2×106 cells/injection, n=3), and tumor formation was monitored daily. All sites injected with Ras-transformed NIH3T3 cells eventually developed tumors, whereas control NIH3T3 or NIH3T3-TIPE2 cell lines did not give rise to tumors during the course of this study. Data shown are percent of tumors formed, and are representative of two independent experiments. The differences between the two groups are statistically significant (p = 0.014). (d–e) Loss of TIPE2 protein expression in Ras-transformed NIH3T3 tumors. Tumors from two separate experiments were excised at day 14 or day 22. Expression of TIPE2-Flag protein (d) and mRNA (e) was examined by SDS-PAGE and IB, and qRT-PCR, respectively. Cell lines used in these assays were Ras-transformed NIH3T3 cells that did (TIPE2) or did not (control) express TIPE2-Flag. qRT-PCR data are shown as means ± S.E.M (n=3) with the value of the control group set as 1. (f) Ubiquitination of TIPE2. 293T cells were transfected with either TIPE2-Flag, or p50-Flag as a positive control. The cells were treated with MG132 (10μM), the lysates were immunoprecipitated with an antibody against Flag, and the ubiquitination status was examined as we previously described (Carmody et al., 2007). (g) TIPE2 protein is degraded in Ras-transformed NIH3T3 tumors. Reconstituted cells from Ras3T3-TIPE2 tumors were treated with MG132 for 24 hrs and TIPE2 protein levels were examined by Western blot and compared to untreated pre-injected cells. (h–j) Loss of, or decreased, TIPE2 expression in human hepatocellular carcinoma. TIPE2 expression in tumor tissue (h) and control hepatic tissue adjacent to the tumor (i) from the same patient was determined by immunohistochemistry as described in Methods. TIPE2-positive cells are shown in brown. Original magnification, x400. Quantitation of the TIPE2 signal for 116 patients was performed as described in Methods (j). The differences between the two groups are statistically significant (p < 0.001).
TIPE2 is markedly down-regulated in human hepatocellular carcinoma
It was recently published that TIPE2 plays an important role in HBV-induced hepatitis (Xi et al., 2011). Chronic HBV infection is a major cause of HCC and is prevalent among a large world population. Interestingly, RalGEF plays a more prominent role in transforming human cells than murine cells (Hamad et al., 2002). To test the possibility that TIPE2 regulates carcinogenesis in humans, we examined the level of TIPE2 expression in the livers of 116 patients suffering from hepatocellular carcinoma. We found that TIPE2 was expressed in normal hepatocytes adjacent to carcinoma cells. Remarkably, ~20% of carcinoma expressed little or no TIPE2 and the rest expressed significantly lower levels as compared to adjacent hepatocytes (Figure 4, H–J). TIPE2 re-expression in three cultured human HCC cell lines (HepG2, BEL7402, and SMMC-7721) significantly reduced their growth and viability as measured by flow cytometry and MTT [(3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (unpublished data). Consistent with the murine tumor data, down-regulation of TIPE2 occurred at the protein but not at mRNA level, because RT-PCR revealed no significant difference in TIPE2 mRNA between hepatocellular carcinoma and its adjacent tissues (unpublished data). Thus, development of human hepatocellular carcinoma is associated with the down-regulation of TIPE2 protein.
In summary, we have discovered a mode of Ras regulation that is carried out by TIPE2, a recently described anti-inflammatory protein containing a unique fold. This mode of regulation is essential for maintaining an organism’s homeostasis, because its defect leads to severe inflammation and cancer progression. This finding provides a molecular bridge between inflammation and cancer, a connection widely recognized, but poorly understood (Karin and Greten, 2005). Thus, inflammation may cause cancer by inhibiting the expression of the tumor suppressor TIPE2, in addition to activating the oncogenic NF-κB (Karin and Greten, 2005). Due to its diverse effects on cell survival and motility, the Ras inhibitor TIPE2 represents an attractive drug target for neoplastic and inflammatory diseases.
Experimental Procedures
Animals and human subjects
C57BL/6J (B6) mice that carry a Tipe2 gene null mutation were generated by backcrossing Tipe2−/− 129 mice (Sun et al., 2008) to B6 mice for 12 generations. Male nude mice (nu/nu) were purchased from Jackson Laboratories. Mice were housed in the University of Pennsylvania Animal Care Facilities under pathogen-free conditions. All animal procedures used were pre-approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
A total of 116 heptocellular carcinoma specimens and 111 normal adjacent hepatic tissue specimens were obtained from 116 patients aged between 30 and 82 years who underwent operations at the Qilu Hospital of Shandong University from January 2005 to October 2006. The pathological diagnosis was made according to the current World Health Organization (WHO) criteria for heptocellular carcinoma. None of the patients studied had received radiotherapy, chemotherapy, or adjuvant immunotherapy prior to surgery in order to eliminate their effects on gene expression. All human procedures used were pre-approved by the Institutional Review Board of the Shandong University.
Immunohistochemistry
Paraffin sections (4μm) were stained with rabbit anti-TIPE2 antibody (IgG) overnight at 4°C. Secondary staining was performed with HRP-conjugated anti-rabbit IgG using a MaxVision™ Kit and a DAB Peroxidase Substrate kit (Maixin Co., Fuzhou, China). The sections were counterstained with hematoxylin. Unrelated rabbit IgG was used as a control for the primary antibody. All slides were independently analyzed by two pathologists in a blinded manner, and scored based on both staining intensity and the percentage of positive cells as follows. Staining intensity: 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining. The percentage of positive cells: 0, <1%; 1, 1–33%; 2, 34–66%; 3, 67–100%. The two scores for each slide were then combined to produce a final grade of TIPE2 expression: 0, total score = 0; 1+, total score = 1 to 2; 2+, total score = 3 to 4; 3+, total score = 5 to 6. When there were discrepancies between the two pathologists, the average score was used.
Cell lines and plasmids
The 293T, Raw 264.7, NIH 3T3, and Ras V12 NIH 3T3 cells were grown in DMEM supplemented with 10% FBS, penicillin and streptomycin. To generate stable cell lines, NIH 3T3 or Ras NIH3T3 cell lines (gift from Dr. Rotem Karni, Hebrew University of Jerusalem) were infected with pBABE-puro retroviral vector expressing TIPE2-Flag. Culture medium was replaced 24 hrs after infection, and after an additional 24 hrs, infected cells were selected with puromycin (1–1.5 μg/ml) for 3 days. Expression of TIPE2-Flag was verified by Western blotting. pRK5 and TIPE2-Flag-pRK5 were described previously (Sun et al., 2008). TIPE2 Δ105–132 was generated from TIPE2 cDNA by PCR and cloned in-frame with a C-terminal Flag tag into vector pRK5. pcDNA3-HA-AKT AAA was a gift from Dr. Morris Birenbaum (University of Pennsylvania). Active AKT (pCDNA3-AKT T308D, S473D), myr-PDK1 (pWZL Myr Flag PDK1), Active RalA (pBABE-RalAV23), Active RalB (pBABE-RalBQ72L) were purchased from Addgene GFP-WT-RalA and GFP-WT-RalB plasmids were a gift from Dr. Wei Guo (University of Pennsylvania). Murine RGL cDNA (cDNA clone MGC:18430, IMAGE:4241244, RGL-1 complete CDS) was obtained from ATCC. Full length RGL (amino acids 1–768) was generated from the cDNA clone by PCR, and cloned in-frame with a C-terminal myc tag into vector pRK5 using BamHI-XhoI sites. ΔN RGL (amino acids 300–768), Δ RID RGL (amino acids 86–496), and RID RGL (amino acids 599–768) were generated from the cDNA clone by PCR, and cloned in-frame with a C-terminal myc tag into vector pRK5 using BamHI-XhoI sites. TIPE2-Flag-pBABE was generated by cloning PCR-amplified TIPE2-Flag fragment into vector pBABE using BamHI/EcoRI sites.
Cell death assays
The 293T cells, 1×106/dish, were plated in 6-cm dishes and transfected with the following plasmids: pRK5, TIPE2-Flag-pRK5, TIPE2 Δ3105-132-Flag-pRK5, pcDNA3-HA-AKT AAA, RGL-myc-pRK5, ΔN RGL-myc-pRK5, GFP-RalA, GFP-RalB, RalAV23, RalBQ72, AKT (Thr308D, Ser473D), myr-PDK1 or pEGFP-N3 (Clontech). All transfections were carried out using FugeneHD reagent (Roche) according to the manufacturer’s instructions. 24 hrs later, supernatant was collected, and adherent cells were trypsinized and mixed with the supernatant. Cells were centrifuged (1200 rpm, 10 minutes), resuspeneded in equal volume of media, and stained with trypan blue. Dead and live cells were counted on a hemocytometer with a light microscope. The average of four fields was then calculated for each sample. For splenocytes, freshly isolated Tipe2−/− and wild type cells, 4×106/well, were cultured in 24-well plates in triplicates, under low (0.2% FBS) or regular serum condition. 4 hrs later, cells were stained with trypan blue and counted as described above.
Ral activity assay
The 293T cells, 2×106 per 10-cm plate, were cultured for 24 hrs, and then transfected with 10 μg/plate pRK5 or TIPE2-Flag-pRK5 plasmid. Cells were lysed at different time points after transfection with RAB buffer (Millipore) supplemented with protease inhibitor cocktail tablet (Complete, Roche) and 1mM PMSF. Protein concentration was determined by Bradford assay. 0.5 or 1 mg of lysate was mixed with GST-RalBP1 agarose beads (Millipore) for 30 minutes at 4 °C. After washing, protein on beads and in total cell lysates was subjected to Western blot to determine the level of active RalA. The levels of active RalA in Tipe2−/− or wild type macrophages were determined in the same manner.
Wound healing assay
Tipe2−/− and wild type macrophages were grown to confluence in 10-cm plates. Monolayers were wounded using a micropipette tip, and visualized using a phase-contrast microscope. Images were acquired at various time points, and the number of cells in the wounded area was counted using the ImageJ software.
Immunoprecipitation
Cells were lysed with CellLyticM buffer (Sigma) supplemented with protease inhibitor (complete, Roche) and phosphatase inhibitor (PhosStop, Roche) cocktail tablets. The lysates were cleared by centrifugation for 15 min, and protein concentration was determined by Bradford assay. 40 μl of 50% proein G-sepharose beads was incubated for 1 hour at 4 °C, with one of the following antibodies: myc (1:1000, cell signaling), Flag (2 μg, Sigma), TIPE2 (1:500, Novus biological), and Sec5 (2 μg, ProteinTech) antibodies, or IgG isotype controls (BD biosciences). The beads were incubated with 1 mg of total protein from each lysate overnight at 4 °C, washed four times with CellLyticM buffer, and boiled for 5 minutes in 40 μl 2X SDS sample buffer After SDS-PAGE and transfer, the membranes were probed with antibodies against Flag (Flag-M2-HRP, 1:1000, Sigma), Myc-HRP (1:1000, Cell signaling), Exo84 (Lifespan Biosciences), Sec6 (1:1000 Assay Designs), TBK1 (1:1000, Cell Signaling), PDK1 (1:1000, Cell Signaling), and RGL (1:500, abnova).
Immunoblotting
Cells were lysed in SDS and total protein concentration determined. 30 μg protein was loaded to each lane, and separated by SDS-PAGE. After transferring to a nitrocellulose membrane, it was blocked with 5% milk in TBST and probed with the following primary antibodies, overnight at 4 °C: Phospho–AKT (Serine 473, 1:1000, Cell signaling), total AKT (1:1000, Cell Signaling), Actin (1:1000, Sigma), RalA (1:5000, BD Transduction Laboratories), Ras (1:500, abcam), Phospho-IRF3 (1:1000, Cell Signaling), total IRF3 (1:1000, Cell Signaling) antibodies. Detection was done using enhanced chemiluminescence of HRP-conjugated secondary antibodies (anti-mouse or anti-rabbit Ig, 1:1000, GE healthcare).
Quantitative RT-PCR
Total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Reverse transcription was performed with oligo dT primers. Real-time PCR was carried out in an Applied Biosystems 7500 system with Power SYBR Green PCR Master Mix (Applied Biosystems). Relative levels of gene expression were determined with GAPDH as the control.
Anchorage-independent growth
Cells were cultured in duplicates in soft agar plates at 37 °C and 5% CO2. After 10–14 days, colonies from ten different fields in each plate were counted, and the average number of colonies per field was calculated. The colonies were photographed using a GelDock camera.
Statistical analyses
Student’s t-test was used to evaluate the statistical significance of differences in cell death, migration, and F-actin content. Mann-Whitney U test was used to evaluate tumor onset, and Wilcoxon signed-rank test was used to evaluate TIPE2 protein expression in human hepatic tissues.
Supplementary Material
Highlights.
TIPE2 links inflammation to cancer by targeting the Ras signaling pathway
TIPE2 prevents Ras from binding the Ras-interacting domain of RGL
TIPE2 suppresses Ras-induced tumorigenesis in vivo
TIPE2 expression is significantly down-regulated in human hepatic cancer
Acknowledgments
The authors thank Drs. Shunyou Gong, Wei Gou, Yongyu Shi, Zhaojun Wang, Xiaolu Yang, Warren Pear, Rotem Karni, Cheng-Jun Zhou, Jian Zhang, and Morris Birenbaum for reagents and/or valuable discussions, and Jennifer Devirgiliis, Dr. Salin Chakkalakal, and Jeanne Geskes for technical support. Supported by grants from the National Institutes of Health, USA (AI-077533, AI-050059, and GM-085112) to Y.H.C., and the National “973” Program of China (2011CB503900) to L.Z.
Footnotes
Supplementary Information is linked to the online version of the paper at http://www.cell.com/molecular-cell/.
Author contributions: Y.G. and Y.H.C. conceived the study and wrote the paper. Y.G. and D.J. designed and performed the experiments, and analyzed the data. S.Z. and P.W. were involved in the execution of several experiments. Li Z. and Lining Z. designed and performed the human hepatocellular carcinoma study. H.S. generated the Tipe2−/− mice, and Y.H.C. oversaw the project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carmody RJ, Ruan Q, Palmer S, Hilliard B, Chen YH. Negative regulation of toll-like receptor signaling by NF-kappaB p50 ubiquitination blockade. Science. 2007;317:675–678. doi: 10.1126/science.1142953. [DOI] [PubMed] [Google Scholar]
- Chien Y, Kim S, Bumeister R, Loo YM, Kwon SW, Johnson CL, Balakireva MG, Romeo Y, Kopelovich L, Gale M, Jr, et al. RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127:157–170. doi: 10.1016/j.cell.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Chien Y, White MA. RAL GTPases are linchpin modulators of human tumour-cell proliferation and survival. EMBO Rep. 2003;4:800–806. doi: 10.1038/sj.embor.embor899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig LA. Ral-GTPases: approaching their 15 minutes of fame. Trends Cell Biol. 2003;13:419–425. doi: 10.1016/s0962-8924(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Ferro E, Trabalzini L. RalGDS family members couple Ras to Ral signalling and that’s not all. Cell Signal. 2010;22:1804–1810. doi: 10.1016/j.cellsig.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia A, Pritchard CA, Paterson HF, Mavria G, Stamp G, Marshall CJ. RalGDS is required for tumor formation in a model of skin carcinogenesis. Cancer Cell. 2005;7:219–226. doi: 10.1016/j.ccr.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Hamad NM, Elconin JH, Karnoub AE, Bai W, Rich JN, Abraham RT, Der CJ, Counter CM. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 2002;16:2045–2057. doi: 10.1101/gad.993902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Wong R, Feig LA. RalGDS couples growth factor signaling to Akt activation. Mol Cell Biol. 2008;28:2851–2859. doi: 10.1128/MCB.01917-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Junutula JR, Matern HT, Ervin KE, Scheller RH, Brunger AT. Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. EMBO J. 2005;24:2064–2074. doi: 10.1038/sj.emboj.7600699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Kong L, Ge BX. MyD88-independent activation of a novel actin-Cdc42/Rac pathway is required for Toll-like receptor-stimulated phagocytosis. Cell Res. 2008;18:745–755. doi: 10.1038/cr.2008.65. [DOI] [PubMed] [Google Scholar]
- Li D, Song L, Fan Y, Li X, Li Y, Chen J, Zhu F, Guo C, Shi Y, Zhang L. Down-regulation of TIPE2 mRNA expression in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Clin Immunol. 2009;133:422–427. doi: 10.1016/j.clim.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Lim KH, Baines AT, Fiordalisi JJ, Shipitsin M, Feig LA, Cox AD, Der CJ, Counter CM. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell. 2005;7:533–545. doi: 10.1016/j.ccr.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Lim KH, O’Hayer K, Adam SJ, Kendall SD, Campbell PM, Der CJ, Counter CM. Divergent roles for RalA and RalB in malignant growth of human pancreatic carcinoma cells. Curr Biol. 2006;16:2385–2394. doi: 10.1016/j.cub.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Martin TD, Samuel JC, Routh ED, Der CJ, Yeh JJ. Activation and involvement of Ral GTPases in colorectal cancer. Cancer Res. 2011;71:206–215. doi: 10.1158/0008-5472.CAN-10-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskalenko S, Henry DO, Rosse C, Mirey G, Camonis JH, White MA. The exocyst is a Ral effector complex. Nat Cell Biol. 2002;4:66–72. doi: 10.1038/ncb728. [DOI] [PubMed] [Google Scholar]
- Moskalenko S, Tong C, Rosse C, Mirey G, Formstecher E, Daviet L, Camonis J, White MA. Ral GTPases regulate exocyst assembly through dual subunit interactions. J Biol Chem. 2003;278:51743–51748. doi: 10.1074/jbc.M308702200. [DOI] [PubMed] [Google Scholar]
- Murai H, Ikeda M, Kishida S, Ishida O, Okazaki-Kishida M, Matsuura Y, Kikuchi A. Characterization of Ral GDP dissociation stimulator-like (RGL) activities to regulate c-fos promoter and the GDP/GTP exchange of Ral. J Biol Chem. 1997;272:10483–10490. doi: 10.1074/jbc.272.16.10483. [DOI] [PubMed] [Google Scholar]
- Ou YH, Torres M, Ram R, Formstecher E, Roland C, Cheng T, Brekken R, Wurz R, Tasker A, Polverino T, et al. TBK1 directly engages Akt/PKB survival signaling to support oncogenic transformation. Mol Cell. 2011;41:458–470. doi: 10.1016/j.molcel.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford G, Owens CR, Titus BJ, Foreman TL, Herlevsen MC, Smith SC, Theodorescu D. RalA and RalB: antagonistic relatives in cancer cell migration. Cancer Res. 2005;65:7111–7120. doi: 10.1158/0008-5472.CAN-04-1957. [DOI] [PubMed] [Google Scholar]
- Rangarajan A, Hong SJ, Gifford A, Weinberg RA. Species- and cell type-specific requirements for cellular transformation. Cancer Cell. 2004;6:171–183. doi: 10.1016/j.ccr.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Rosse C, Hatzoglou A, Parrini MC, White MA, Chavrier P, Camonis J. RalB mobilizes the exocyst to drive cell migration. Mol Cell Biol. 2006;26:727–734. doi: 10.1128/MCB.26.2.727-734.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale EM, Sale GJ. Protein kinase B: signalling roles and therapeutic targeting. Cell Mol Life Sci. 2008;65:113–127. doi: 10.1007/s00018-007-7274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SC, Oxford G, Baras AS, Owens C, Havaleshko D, Brautigan DL, Safo MK, Theodorescu D. Expression of ral GTPases, their effectors, and activators in human bladder cancer. Clin Cancer Res. 2007;13:3803–3813. doi: 10.1158/1078-0432.CCR-06-2419. [DOI] [PubMed] [Google Scholar]
- Sowalsky AG, Alt-Holland A, Shamis Y, Garlick JA, Feig LA. RalA Function in Dermal Fibroblasts Is Required for the Progression of Squamous Cell Carcinoma of the Skin. Cancer Res. 2011 doi: 10.1158/0008-5472.CAN-10-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaargaren M, Bischoff JR. Identification of the guanine nucleotide dissociation stimulator for Ral as a putative effector molecule of R-ras, H-ras, K-ras, and Rap. Proc Natl Acad Sci U S A. 1994;91:12609–12613. doi: 10.1073/pnas.91.26.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiczka KS, Yeaman C. Ral-regulated interaction between Sec5 and paxillin targets Exocyst to focal complexes during cell migration. J Cell Sci. 2008;121:2880–2891. doi: 10.1242/jcs.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara K, Asano S, Tanaka K, Iwamatsu A, Okawa K, Ohta Y. The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat Cell Biol. 2002;4:73–78. doi: 10.1038/ncb720. [DOI] [PubMed] [Google Scholar]
- Sun H, Gong S, Carmody RJ, Hilliard A, Li L, Sun J, Kong L, Xu L, Hilliard B, Hu S, et al. TIPE2, a negative regulator of innate and adaptive immunity that maintains immune homeostasis. Cell. 2008;133:415–426. doi: 10.1016/j.cell.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Rusanescu G, Hou W, Schaffhausen B, Feig LA. PDK1 mediates growth factor-induced Ral-GEF activation by a kinase-independent mechanism. EMBO J. 2002;21:1327–1338. doi: 10.1093/emboj/21.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano T, Emkey R, Feig LA. Ral-GTPases mediate a distinct downstream signaling pathway from Ras that facilitates cellular transformation. EMBO J. 1996;15:810–816. [PMC free article] [PubMed] [Google Scholar]
- Ward Y, Wang W, Woodhouse E, Linnoila I, Liotta L, Kelly K. Signal pathways which promote invasion and metastasis: critical and distinct contributions of extracellular signal-regulated kinase and Ral-specific guanine exchange factor pathways. Mol Cell Biol. 2001;21:5958–5969. doi: 10.1128/MCB.21.17.5958-5969.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MA, Vale T, Camonis JH, Schaefer E, Wigler MH. A role for the Ral guanine nucleotide dissociation stimulator in mediating Ras-induced transformation. J Biol Chem. 1996;271:16439–16442. doi: 10.1074/jbc.271.28.16439. [DOI] [PubMed] [Google Scholar]
- Wolthuis RM, Bauer B, van ‘t Veer LJ, de Vries-Smits AM, Cool RH, Spaargaren M, Wittinghofer A, Burgering BM, Bos JL. RalGDS-like factor (Rlf) is a novel Ras and Rap 1A-associating protein. Oncogene. 1996;13:353–362. [PubMed] [Google Scholar]
- Wolthuis RM, de Ruiter ND, Cool RH, Bos JL. Stimulation of gene induction and cell growth by the Ras effector Rlf. EMBO J. 1997;16:6748–6761. doi: 10.1093/emboj/16.22.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W, Hu Y, Liu Y, Zhang J, Wang L, Lou Y, Qu Z, Cui J, Zhang G, Liang X, et al. Roles of TIPE2 in hepatitis B virus-induced hepatic inflammation in humans and mice. Mol Immunol. 2011;48:1203–1208. doi: 10.1016/j.molimm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang J, Fan C, Li H, Sun H, Gong S, Chen YH, Shi Y. Crystal structure of TIPE2 provides insights into immune homeostasis. Nat Struct Mol Biol. 2009;16:89–90. doi: 10.1038/nsmb.1522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.