Abstract
Ginkgo biloba/EGb 761® (EGb 761) is a popular and standardized natural extract used worldwide for the treatment of many ailments. Although EGb 761 is purported to have a plethora of benefits, here, we were interested to study the neuroprotective properties of EGb 761 and its components and determine whether nuclear factor E2 (Nrf2)/heme oxygenase 1 (HO1) induction of the collapsin response mediator protein 2 (CRMP2) pathway contributes to neuroprotection. Mice were pretreated with EGb 761 or one of its constituents (bilobalide, ginkgolide A, ginkgolide B, and terpene free material [TFM]) for 7 days and then subjected to transient middle cerebral artery occlusion (tMCAO) and 48 h of reperfusion. All components except TFM significantly reduced infarct volumes and neurologic deficits. Next, we examined the antioxidant and neuritogenic properties of EGb 761 in primary neurons. Compared with vehicle-treated cells, pretreatment with EGb 761 significantly enhanced the survival of neurons exposed to tertiary butylhydroperoxide (t-BuOOH), hydrogen peroxide (H2O2), and N-methyl-D-aspartate (NMDA). Bilobalide and ginkgolide A also protected cells against NMDA-induced excitotoxicity. Immunofluorescence and Western blot analysis showed that EGb 761 pretreatment significantly increased the protein expression levels of Nrf2, HO1, GAPDH, β-actin, CRMP2, and histone H3 during t-BuOOH-induced oxidative stress. These findings suggest that EGb 761 not only has antioxidant activity but also neuritogenic potential. Demonstrating such effects for possible drug discovery may prove beneficial in stroke and ischemic brain injury.
Keywords: Collapsin response mediator protein 2, Ginkgo biloba, Heme oxygenase 1, Middle cerebral artery occlusion, Neuroprotection, Neurite growth
Introduction
Used as an over-the-counter drug to enhance energy and memory, the standardized Ginkgo biloba leaf extract, or EGb 761® (EGb 761), is one of the most effective and commonly used nutraceuticals in the market. In Europe, EGb 761 is prescribed for multiple problems related to mental health and overall wellbeing. Several studies have shown EGb 761 to have neuroprotective properties, but the mechanism(s) underlying this effect has not been fully studied and requires further evaluation. Previously, our group has shown that heme oxygenase 1 (HO1) is essential for EGb 761 neuroprotection against ischemia in rodent models of transient middle cerebral artery (MCA) occlusion (tMCAO) (Saleem et al., 2008) and permanent distal MCA occlusion (pMCAO) (Shah et al., 2011). Furthermore, EGb 761 was shown to upregulate endothelial nitric oxide synthase and vascular endothelial growth factor in ipsilateral cortices of mice that have undergone experimental stroke, suggesting the possible contribution of these vasodilators (Shah, Nada et al. 2011).
Ischemic stroke is a leading cause of disability in the United States and worldwide (Elkins and Johnston, 2003). Insufficient oxygen and nutrients during cerebral ischemia triggers multiple biochemical cascades that result in axonal injury, breakdown of neuronal cytoskeleton, and neuronal degeneration and death (Won et al., 2002). Collapsin response mediator protein 2 (CRMP2) is crucial for axon outgrowth and determines the fate of axons and dendrites. It was originally identified as a signaling molecule required for growth cone collapse of dorsal root ganglion neurons in response to a repulsive guidance cue (Goshima et al., 1995). Overexpression of CRMP2 induces the formation of multiple axons, whereas knockdown of CRMP2 suppresses axon formation (Inagaki et al., 2001, Suzuki et al., 2003, Yoshimura et al., 2005), indicating that CRMP2 has a positive effect on axonal extension and plays a key role in dendrite specification and axon regeneration. CRMP2 colocalizes with F-actin in the growth cones of different types of neurons (Goshima et al., 1995, Minturn et al., 1995, Yuasa-Kawada et al., 2003) and also binds to actin, but its binding is not affected by phosphorylation (Arimura et al., 2005).
With the backdrop of failed neuroprotective agents in phase III clinical trials, there is an urgent prerequisite to develop neuroprotective agents that have few side effects and multiple mechanism(s) of action. In this study, we investigated the neuroprotective properties of EGb 761 and its components (bilobalide [BB], ginkgolide A [GA], ginkgolide B [GB], and terpene free material [TFM]) in a mouse tMCAO model with 48 h reperfusion. In addition, we explored the neuritogenic properties of EGb 761 in primary cultured neuronal cells by studying the roles of CRMP2, GAPDH, and β-actin and confirming Nrf2/HO1 involvement. We found that EGb 761 has the potential to reduce infarct volume in vivo, reduce neuronal apoptosis in vitro, and reverse excitotoxicity-induced cytoskeletal collapse.
Materials and methods
Animals
All animal protocols were approved by the University of Toledo Health Science Campus Institutional Animal Care and Utilization Committee. Guidelines of the National Institutes of Health were followed throughout the study. Male C57BL/6 mice (20-30 g) and timed 14-day pregnant female C57BL/6 mice were procured from Charles River Laboratories, Wilmington, MA.
Drug treatment
EGb 761, BB, GA, GB, and TFM were kindly provided by Dr. Willmar Schwabe Pharmaceuticals, Germany. Test drugs and vehicle (polyethylene glycol) were orally administered to mice daily for 7 days before ischemia at the following dosages: EGb 761, 100 mg/kg; BB, 6 mg/kg; GA, 6 mg/kg; GB, 6 mg/kg; and TFM, 10 mg/kg. Dosages were selected based on the amount of each constituent present in the EGb 761.
Transient occlusion of the middle cerebral artery (tMCAO)
The middle cerebral artery occlusion procedure was carried out as published previously (Shah et al., 2006). Mice were anesthetized with halothane (Nicholas Piramal, India; 3% initial, 1 to 1.5% maintenance) in O2 and air (80%:20%). A 0.5-mm diameter microfiber was glued over the area of parietal cortex with cyanoacrylate glue (Super Glue Gel, Ross Products, Inc.) and connected to a laser-Doppler flowmeter (DRT4, Moor Instruments Ltd, Devon, England), which was used to confirm successful occlusion. Mice were turned to the supine position, and a midline incision was made in the neck to clear and expose the right common carotid artery (CCA), external carotid artery, and internal carotid artery; care was taken not to disturb the vagus nerve. A 7-0 Ethilon nylon filament (Ethicon, Inc., Somerville, NJ, USA), which had 5 mm of the tip coated with silicone (Cutter Sil Light and Universal Hardener, Heraeus Kulzer, GmbH, Hanau, Germany), was slipped into the internal carotid artery through the external carotid artery stump to block blood circulation to the MCA territory. The filament was carefully advanced up to 11 mm from the carotid artery bifurcation or until resistance was felt. Mice that did not attain at least an 80% decrease in cerebral blood flow were terminated from the study. Animals were kept in a humidity/temperature-controlled chamber at 32 °C to maintain their body temperature at 37 °C during the 90 min of MCA occlusion. For reperfusion, mice were briefly anesthetized, and the filament was withdrawn carefully without rupturing the arteries; open ends of arteries were cauterized to prevent bleeding. After the neck incision was sutured, mice were again placed in a humidity/temperature-controlled chamber for 2 h and then returned to their respective home cages.
Blood Gas Measurements
Briefly, under an operating microscope mice (another cohort of animals) were placed in a porcine position and an incision was given on the limb and femoral artery was exposed. A PE-10 femoral artery catheter (Intramedic; BD Diagnostic Systems, Sparks, MD) attached to 1 ml syringe on one side was introduced in to it and fixed/secured with a silk suture. Blood was drawn intermittently at different intervals of time; 30 minutes before MCAO, 1 h after the initiation of MCAO, and 1 h after reperfusion. Blood samples were analyzed by blood gas analysis instrument (Rapidlab 248; Chiron Diagnostic Corporation, Norwood, MA) for pH, PaO2, and PaCO2 parameters.
Neurologic deficit score (NDS)
Forty-eight hours after tMCAO, neurologic deficits were evaluated by an investigator blinded to treatment group using a previously modified 28-point scoring system (Saleem et al., 2009, Zeynalov et al., 2009). Motor deficits were evaluated by tests for body symmetry, gait, climbing, circling behavior, front limb symmetry, and compulsory circling; sensory deficits were evaluated by a whisker response test. Each test was graded from 0 (no deficit) to 4 (greatest deficit), establishing a maximum NDS of 28. After assessing weight loss, mice were sacrificed for measurement of infarct volume.
Infarct size and infarct volume analysis
After 48 h of reperfusion, mice were anesthetized, and their brains dissected out. Coronal brain slices (2 mm) were stained with 1% 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St. Louis, MO, USA) and fixed in 10% buffered normal saline for 24 h. The slices were scanned individually by a video image analyzing system, and the infarct lesions were measured and analyzed by image analysis software SigmaScan pro 4 and 5 (Systat Inc., San Jose, CA, USA).
Neuronal Cell Culture
On gestational day 17, pregnant mice were sacrificed by CO2 overdose, and fetuses were collected in cold HBSS medium (Fisher Scientific, Hanover Park, IL. USA). The fetuses were decapitated and cortex was collected from each brain. The meninges were rapidly removed, dissected into 5 ml of cold HBSS medium by trituration, and centrifuged at 18 °C for 3 min at 1000 × g. After the supernatant was removed, the cells were resuspended in 5 ml of 1X DMEM (Fisher) containing 0.25% trypsin (Fisher) and incubated at 37 °C under 5% CO2 for 15 min. DNase solution (Roche Diagnostics, Indianapolis, IN, USA) was added to a final concentration of 0.02 mg/ml, and the suspension was incubated under the same conditions for another 5 min. After 5 ml of 1X DMEM containing 10% FBS was added to the trypsinized cells, the suspension was passed twice through a 70 μm nylon cell strainer (BD Falcon, Sparks, MD, USA) to remove the cell debris. Cells were spun down at 1000 × g for 3 min at 18 C, the supernatant was removed, and cells were resuspended in 5 ml of neurobasal medium (Invitrogen, Carlsbad, CA, USA) containing penicillin and streptomycin (50 U/ml of medium), glutamine (2 mM), and B27 serum-free supplement (Invitrogen). This step was repeated twice, and then the cells were counted in trypan blue and plated in poly–L-lysine (50 μg/ml)-coated plates. Cultures were maintained at 37 C in 95% air and 5% CO2. All experiments were performed on day 14 of plating. Medium was changed every third day by replacing half of the old medium with fresh.
Cell viability assays
Neurons at a population of 0.5 × 106 were plated in poly-L-lysine pre-coated 24-well dishes and pretreated with EGb 761 (0.1 mg/ml), BB (12 g/ml), GA (12 g/ml), GB (12 g/ml), or TFM (20 g/ml). After 6 h, one of the following stressors was added: tertiary butylhydroperoxide (t-BuOOH, 60 M), hydrogen peroxide (H2O2, 100 M), or N-Methyl-D-aspartate (NMDA, 100 M). After 18 h of exposure, cell viability assayed with the Promega cell proliferation assay kit (Promega, Madison, WI, USA). Optical density was measured at 570 nm. All experiments were repeated in triplicate with four separate batches of culture.
TUNEL assay and immunocytochemistry
Neurons were seeded in 6-well dishes at a population of 1.2×106 on sterilized coverslips coated with poly-L-lysine. On day 14, neurons were pretreated with EGb 761 (0.1 mg) and/or tin protoporphyrin-IX (SNPPIX, 10 μM) for 6 h. Then t-BuOOH (60 μM) was added for an additional 18 h. The TUNEL assay (Promega) was carried out according the manufacturer’s protocol. Briefly, coverslips were incubated with 1% albumin bovine fraction V (RPI, Mount Prospect, IL, USA) in 1 X PBS buffer at room temperature for 1 h. After being washed, the coverslips were incubated overnight at 4 °C with primary antibody, rabbit anti-HO1 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by goat anti-rabbit secondary antibody (1:800; Jackson ImmunoResearch, West Grove, PA, USA). Coverslips were mounted with DAPI (Santa Cruz) and sealed. For CRMP2 and phalloidin staining, washed neurons were fixed with paraformaldehyde and treated with 0.3% triton X-100 for permeability. The fixed cells were blocked with 1% of BSA for 1 h at room temperature and then incubated overnight at 4 °C with rabbit anti-CRMP2 (1:8000; Millipore Corporation, Billerica, MA, USA). Slides were washed three times (10 min each) before incubation with secondary goat anti-rabbit IgG (1:800; Jackson ImmunoResearch). Slides were washed again and incubated with one unit of Phalloidin/slide (Invitrogen). Slides were washed again and mounted with DAPI and sealed. All experiments were repeated three times with three separate batches of cultures.
Western blot analysis
Nuclear and cytosolic fractions were isolated from neuronal cells according to Shah et al. (Shah et al., 2011), and protein concentration was determined by Bio-Rad Bradford reagent (Bio-Rad, Hercules, CA, USA). Equivalent amounts of total cytoplasmic or nuclear protein (25 μg) were separated by SDS polyacrylamide gel electrophoresis on 10% gels. Proteins were transferred from the gel to PVDF membrane, blocked with 5% dry nonfat milk, and incubated with the following antibodies: rabbit anti-Nfr2 (1:1000; Santa Cruz), rabbit anti-actin (1:2000; Sigma Aldrich), rabbit anti-HO2, (1:2000; Stressgen, MI, USA), rabbit anti-HO1 (1:1000; Stressgen), rabbit anti-CRMP2 (1:40,000; Millipore), rabbit anti-GAPDH (1:3000; Fisher) or rabbit anti histone H3 (1:2000; Fisher). After being washed with PBS-T buffer, membranes were incubated with secondary antibody (1:5000; Jackson ImmunoResearch). Images were analyzed by using Adobe Photoshop and ImageJ software provided by the NIH.
Statistical analysis
Data were analyzed by Student’s t-test, analysis of variance (ANOVA), or Newman-Keuls multiple range test. Comparisons of all physiological parameters and infarct volumes between treatment groups were made by one-way ANOVA with Newman Keuls post-hoc test. Neurologic deficits were analyzed by the non-parametric Kruskal-Wallis test. Data are presented as means ± SEM. A value of p < 0.05 was considered to be statistically significant.
Results
Pretreatment with EGb761 and its components improves neurologic deficit scores and reduces infarct size in mice
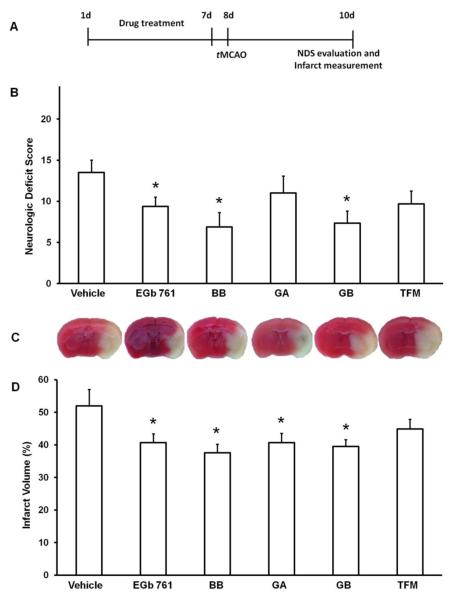
Mice randomized into different treatment groups were pretreated for 7 days with test drugs (EGb 761, BB, GA, GB, or TFM) and subjected to tMCAO. After 48 h of reperfusion, infarct volumes as analyzed by TTC staining were significantly lower in groups treated with EGb 761 (40.6 ± 2.5%; p = 0.021), BB (37.5 ± 2.4%; p < 0.005), GA (40.6 ± 2.0%; p = 0.011), and GB (39.5 ± 2.1%; p = 0.007) than in the group treated with vehicle (51.9 ± 2.9). Infarct volume in the TFM group was not significantly different from control (Fig. 1A). Similarly, the NDS was significantly reduced in the groups treated with EGb 761 (9.3 ± 0.9; p < 0.026), BB (6.8 ± 1.1; p = 0.010), or GB (7.3 ± 1.7; p = 0.030) compared to the vehicle-treated group (13.5 ± 1.1). However, GA- and TFM-treated groups did not show a statistically significant difference (Fig. 1B). Furthermore, there were no differences in brain edema, weight loss, or physiological parameters (pH, PaCO2, PaO2) in any of the treatment groups (data not shown).
Fig. 1.
Pretreatment with EGb 761 and its components reduces damage from stroke. (A) Schematic diagram of the experimental protocol. Mice were orally administered EGb 761 (100 mg/kg; n=9), BB (6 mg/kg; n=8), GA (6 mg/kg; n=6), GB (6 mg/kg; n=7), TFM (10 mg/kg; n=10), or vehicle control (n=4) once daily for 7 days; tMCAO was induced on day 8; mice were evaluated for neurologic deficit score (NDS) and sacrificed on day 10. (B) Compared with vehicle-treated controls, groups pretreated with EGb 761, BB, and GB had significantly lower NDS, (D) and those pretreated with EGb 761, BB, GA, and GB had significantly smaller infarct volumes. (C) Representative brain sections of mice showing infarct volumes of different treatment groups. Data are expressed as means ± SEM; p 0.05 vs. vehicle.
Neuroprotective effects of EGb 761 against oxidative stress and excitotoxicity in vitro
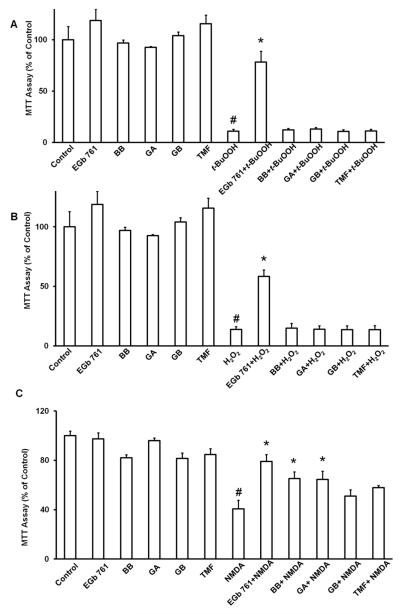
We used neuronal cell cultures to study the neuroprotective effects of EGb 761 and its components in vitro against oxidative stress induced by t-BuOOH and H2O2. Only EGb 761 (6 h pretreatment) significantly protected the neuronal cells from the effects of t-BuOOH and H2O2 (Fig. 2A and B). BB, GA, GB, and TFM failed to provide any protection in terms of neuronal survival against these two stressors. Since, BB, GA, and GB pretreatment showed significant reduction of brain infarct volume against tMCAO, we were further interested to look into the other possible mechanisms of protection, such as against excitotoxicity. Therefore, we also investigated whether each agent could protect neurons against NMDA exposure. We found that 6 h of pretreatment with EGb 761, BB, GA, prevented the NMDA-induced loss of neuronal viability (Fig. 2C).
Fig. 2.
Effects of EGb 761 and its components on neuronal survival in vitro. Cells were grown in 24-well plates for 14 days and then pretreated with EGb 761 (100 μg), BB (12 μg), GA (12 μg), GB (12 μg), TFM (20 μg), or vehicle control. Then they were exposed to (A) 60 M t-BuOOH, (B) 100 M H2O2, and (C) 100 M NMDA. Cell viability was assessed with the MTT assay and is shown as percent of control. Each experiment was conducted in triplicate and repeated four times with different primary culture batches. Data are expressed as mean ± SEM; #p 0.05 vs. control, *p 0.05 vs. stressor.
Role of HO1 in neuroprotective mechanism of EGb 761
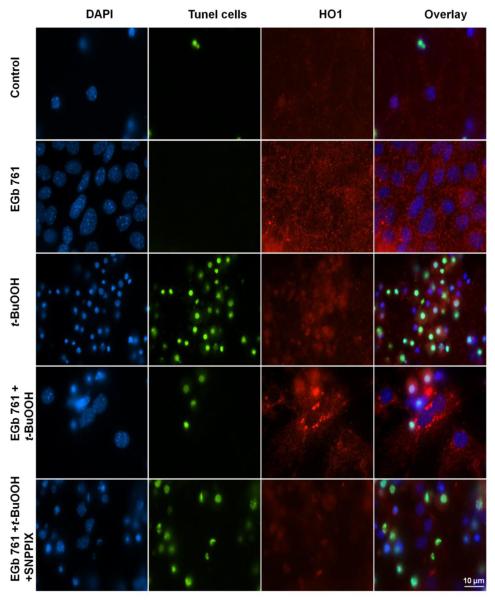
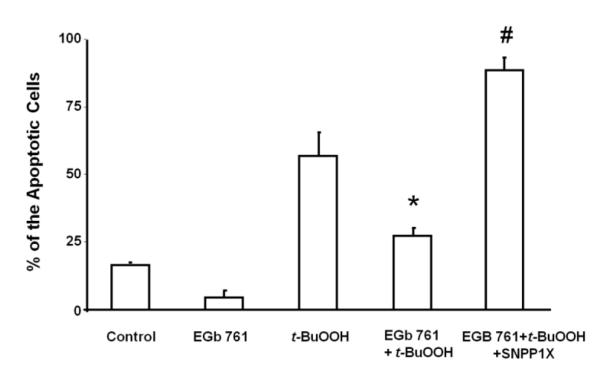
Next we investigated possible mechanism(s) by which EGb 761 might provide neuroprotection against the three different stressors. We pretreated neurons with EGb 761 in the presence and absence of t-BuOOH and/or SNPP1X (HO1 inhibitor). EGb 761 enhanced the induction of HO1 protein expression and provided significant protection to neurons from apoptosis induced by t-BuOOH-related oxidative stress (Fig. 3A and B). In the presence of the HO1 inhibitor, pretreatment with EGb 761 failed to protect neurons against the t-BuOOH-induced neuronal death, suggesting that EGb 761 might protect neurons by inducing HO1 expression.
Fig. 3.
EGb 761 protects neurons from apoptosis. Cultured cortical neurons from mice were pretreated for 6 h with vehicle or 100 μg/ml EGb 761 in the presence or absence of SNPPIX; then 60 μM t-BuOOH was added for an additional 18 h. (A) Immunocytochemical staining shows DAPI (blue), apoptotic cells (green), HO1 expression (red), and the overlay of all. Scale bar = 10 μm (B) The EGb 761-induced reduction in apoptosis was blocked by SNPPIX, indicating that the neuroprotection requires HO1. Each experiment was conducted in triplicate and repeated three times with different primary culture batches. Data are expressed as means ± SEM; p 0.05 vs. t-BuOOH; #p 0.05 vs. EGb 761+t-BuOOH.
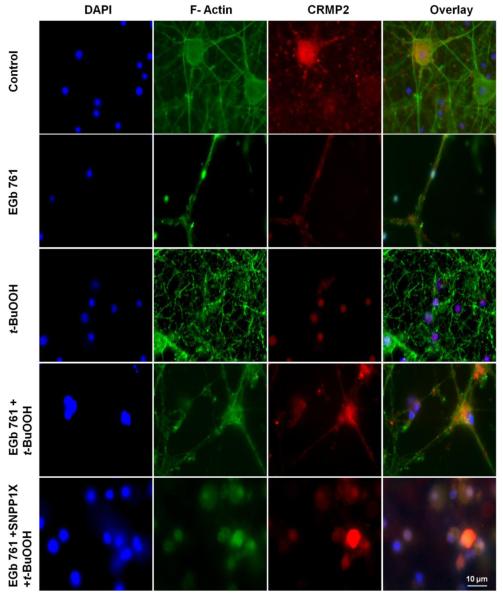
Neuritogenic properties of EGb 761 and role of CRMP2
Next, we sought to understand how EGb 761 might promote neurite growth via mechanisms in addition to or downstream of HO1 induction. We tested the hypothesis that EGb 761 can protect neurons by inducing the expression of CRMP2 proteins in the axons and dendrites. Exposure of neurons to t-BuOOH for 6 h ruptured and collapsed F-actin filaments. In addition, the CRPM2 protein became dissociated from the axons and dendrites and was observed to be localized in the nucleus. Preincubation with EGb 761 for 6 h protected the neurons against t-BuOOH-induced oxidative stress by restoring F-actin filaments and CRMP2 expression in axons and dendrites, thereby inducing and enhancing neurite growth (Fig. 4). Furthermore, EGb 761 failed to restore F-actin filaments and CRMP2 expression in axons and dendrites in the presence of the HO1 inhibitor SNPP1X. When HO1 enzymatic activity was blocked by SnPPIX, EGb 761 no longer protected neuronal cells from apoptosis or neuronal degeneration. These results suggest a possible link between HO1 and CRMP2 signaling.
Fig. 4.
EGb 761 induces neurite growth. Top panel: control neurons without any treatment. The nucleus is stained with DAPI (blue stain), F-actin (phalloidin green stain), and CRMP2 (red stain). The far right image shows the merged overlay of all the pictures. t-BuOOH treatment induced CRMP2 localization within the nucleus and the collapse of F-actin. Pretreatment with EGb 761 protected axons and dendrites from collapsing, as CRMP2 was detected in axons and dendrites. Each experiment was conducted in triplicate and repeated three times with different primary culture batches. Scale bar = 10 μm
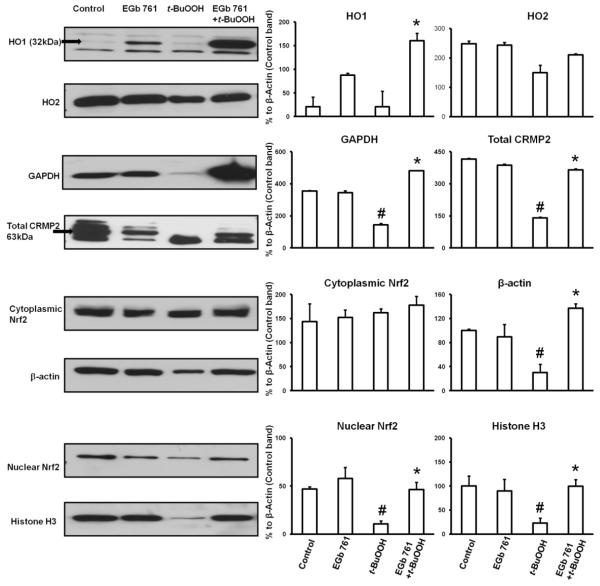
Protein expression levels induced by EGb 761
Western blot analysis showed a significant reduction in protein expression of HO1, GAPDH, β-actin, nuclear Nrf2, and histone H3 in neuronal cells exposed to t-BuOOH. Little change was observed in the expression of HO2 and cytosolic Nrf2. Pretreatment for 6 h with EGb 761 before exposure to t-BuOOH restored the expression level of all proteins. Because F-actin collapsed and dissociated in neurons exposed to t-BuOOH, β-actin and histone expression levels also decreased in response to t-BuOOH. Consequently, neither could be used as a loading control (Fig. 5). Therefore, control band of the β-actin was used to normalize different protein bands and analysis made to draw corresponding graphs.
Fig. 5.
Effect of EGb 761 on protein expression. Western blot analysis was used to measure protein expression levels of HO1, HO2, GAPDH, β-actin, total CRMP2, Nrf2, and histone H3. Each experiment was conducted in triplicate and repeated three times with different primary cultures batches. Values shown are percent of β-actin control band except for nuclear Nrf2 protein which is expressed as mean ± SEM; #p 0.05 vs. Control; *p 0.05 vs. t-BuOOH.
Discussion
We hypothesized that EGb 761 and some of its components provide protection from ischemia-reperfusion injury through the Nrf2/HO1 and CRMP2 pathways. We observed significantly lower NDS in mice that were pretreated with EGb 761, BB, and GB and significantly smaller infarct volumes in those pretreated with EGb 761, BB, GA, and GB. Only EGb 761 ameliorated neuronal death against all three stressors: t-BuOOH, H2O2, and NMDA. EGb 761 pretreatment significantly decreased the number of TUNEL-positive cells, possibly by increasing HO1 expression, as the effect was lost in the presence of the HO1 inhibitor SNPP1X. Furthermore, EGb 761 reversed the stressor-induced decrease in CRMP2 protein level, but this effect also was abolished in the presence of SNPP1X. Finally, EGb 761 prevented the decreased expression of HO1, GAPDH, β-actin, nuclear Nrf2, and histone H3 observed in primary neurons exposed to t-BuOOH. These results suggest that EGb 761 provides neuroprotection through the Nrf2/HO1-induced CRMP2 pathway.
EGb 761 is a multifunction antioxidant that has been shown to provide protection against cardiovascular and neurological disorders (Clark et al., 2001, Ahlemeyer and Krieglstein, 2003, Chandrasekaran et al., 2003, Shah et al., 2011). Previous studies have shown EGb 761 to be protective in tMCAO and pMCAO models of ischemia and have implicated HO1 in the underlying mechanism. Whereas previous studies have investigated the effects of EGb 761 on stroke outcome at 24 h of reperfusion, here, we extended the survival time from 24 h to 48 h and tested not only EGb 761 but also its components. We found that 7-day pretreatment with EGb 761, BB, GA, and GB provides protection against transient ischemia. These results are in consonance with our previous study in a pMCAO model (Shah et al., 2011). The fact that EGb 761 could protect even up to 48 h of tMCAO in present study, further consolidates its neuroprotective properties. Next, we sought to broaden our knowledge of the mechanism by which EGb 761 and its components provide neuroprotection. We found that only EGb 761 was able to rescue neurons in vitro against all three different types of stressors that we used—t-BuOOH, H2O2, and NMDA—highlighting its multipronged neuroprotective pathways. BB and GA also were able to rescue neurons against NMDA excitotoxicity, but no EGb 761 constituent was able to rescue neurons after exposure to t-BuOOH or H2O2. One of the possible reasons might be that BB acts through antioxidant and glutamate antagonism, while as GA acts through glutamate antagonism only. Another reason might be that in our studies, t-BuOOH and H2O2 resulted in 80% of neuronal cell death as these two stressors work through multiple cell death signalling pathways and may be one of the caveats for not observing any protection with individual components. On the contrary, NMDA works mainly through excitatory mechanism initially and resulted in only 60% of neuronal cell death, subsequently making BB and GA potent enough to provide protection. Zhang et al. (Zhang et al., 2004) also observed that neurons in cell culture are injured worse by H2O2 than by NMDA insult. BB not showing any protective effect in the present study may be attributed to higher neuronal death with H2O2 insult. Further studies may be required to evaluate this claim. The fact that the various constituents were ineffective in vitro but effective in vivo may be attributable to the absence of cell type diversity in cell culture models.
Neurons are highly polarized, and the processes that extend from their cell bodies are specialized for receiving and transmitting information. These vital functions are based on their specialized architectures, which are composed of two structurally and functionally distinct parts, axons and dendrites (Craig and Banker, 1994). After cerebral ischemia, programmed neuronal cell death plays an important role in neuronal degeneration, but the underlying mechanisms are not fully understood. To promote functional recovery after ischemic injuries, it is vital to develop strategies that enhance both neuronal survival and regeneration. To validate the potential role of EGb 761 in inducing neurite growth, we used t-BuOOH (60 μM) to induce cell death and to study its effects on axons and dendrites. Immunofluorescence revealed that t-BuOOH dissociated F-actin, β-actin, and CRMP2 from axons and dendrites. Western blot analysis confirmed these results and also showed reduced protein expression levels of GAPDH, β-actin, and histone H3. Substantial evidence in the literature supports the theory that GAPDH and β-actin filaments are broken down and protein expression levels altered in neurodegenerative diseases (Zhu et al., 2005, Butterfield et al., 2010). Targeting this novel pathway could prove beneficial in neurodegenerative diseases where functional recovery is an important aspect of therapy. Hence, it is noteworthy that, in addition to upregulating HO1 and nuclear Nrf2, EGb 761 was able to restore F-actin, β-actin, GAPDH, histone H3, and CRMP2 in axons and dendrites.
CRMP2 is a developmentally regulated protein and highly expressed in the nervous system (Bretin et al., 2005). Overexpression of CRMP2 induces the formation of multiple axons and elongation of the primary axons in hippocampal neurons (Fukata et al., 2002a, Fukata et al., 2002b). Recent evidence suggests that CRMP2 is important in calcium regulation and excitotoxicity signaling (Bretin et al., 2006, Hou et al., 2009). CRMP2 is expressed as multiple isoforms (Bretin et al., 2005). The cytosolic active isoforms of CRMP2 have apparent masses between 63 and 66 kDa and the cleaved inactive CRMP2 forms have masses that range between 54 and 58 kDa. A number of studies have shown that CRMP2 becomes proteolytically cleaved during neuronal injury (Bretin et al., 2006, Brittain et al., 2011). In the present study, the cleaved 54 kDa CRMP2 was the only form observed to be expressed in apoptotic neurons, but multiple isoforms were detected after pretreatment of neurons with EGb 761, suggesting that CRMP2 is active in the protective process. However, in the presence of HO1 inhibitor, EGb 761 failed to protect neurons from apoptosis or prevent axon and dendrite collapse. The possible link between HO1 and CRMP2 may be the equimolar quantities of carbon monoxide (CO) formed during the catabolism of pro-oxidant heme (Kirkby and Adin, 2006). Like nitric oxide, CO is known to inhibit platelet aggregation and can produce relaxation in rabbit arteries ex vivo (Brune and Ullrich, 1987, Furchgott and Jothianandan, 1991). Several studies demonstrated that CO has the ability to bind to the iron atom of the heme moiety associated with soluble guanylyl cyclase, thereby activating the enzyme and increasing intracellular cyclic guanosine monophosphate (cGMP) production (Brune and Ullrich, 1987, Furchgott and Jothianandan, 1991, Christodoulides et al., 1995). Zhao et al. (Zhao et al., 2009) were able to demonstrate that cGMP can regulate axon branching by inhibiting glycogen synthase kinase 3 β (GSK-3β) via the activation of cGMP-dependent protein kinase 1. Another study by Yoshimura et al. (Yoshimura et al., 2005) showed that GSK-3β phosphorylates CRMP2, thus inactivating it and preventing the copolymerization of CRMP2 within tubulin and dissociation of CRMP2 from microtubulin. Another possibility was highlighted by a recent study by Tsai et al. (Tsai et al., 2010) and his group that showed EGb 761 reduced calpain activity, and small interfering RNA targeting HO1 abolished this reduction. The active calpain targets CRMP2 protein and cleaves it towards the C-terminal and causes its inactivation. Alternately, calpain is also activated by calcium influx when the cells become apoptotic during stress conditions (Hou et al., 2009). Furthermore, the effect of HO1 on soluble guanylyl cyclase (sGC) resulted in a several-hundred-fold stimulation of sGC. This activation results in a pronounced accumulation of cGMP, an important second messenger involved in a variety of physiological processes. cGMP regulates axonal guidance (Song and Poo, 1999) by activating cGMP dependent protein kinases and inhibiting the GSK-3β activity. In both ways, the inactive GSK-3β regulates actin filaments (Eickholt et al., 2002), while the active GSK-3β phosphorylates and inactivates CRMP-2. Since Nrf2 has already been shown to upregulate HO1 by binding to antioxidant response elements on its promoter region (Shah et al., 2010), the pathway through which Nrf2/HO1 mediates activation of CRMP2 and other proteins may be a novel target to maintain neuronal cytoskeleton under stress conditions. This pathway needs to be evaluated further and confirmed in future in vivo studies.
In conclusion, we showed that 7 days of pretreatment with EGb 761, BB, GA, and GB protected mice from transient ischemia after 48 h of reperfusion. In vivo results also corroborated well with the in vitro results, in which EGb 761 was found to provide neuroprotection against different stressors. Finally, we showed that EGb 761 pretreatment upregulates proteins (nuclear Nrf2, HO1, CRMP2, GAPDH, β-actin, and histone H3) that were downregulated by t-BuOOH-induced oxidative stress and demonstrated a link between HO1 and CRMP2 expression and the EGb 761 neuroprotection. These findings support our hypothesis that Nrf2/HO1-mediated upregulation of CRMP2 is vital for EGb 761 neuroprotection and provides a novel pathway for drug discovery in neurodegenerative diseases.
Highlights.
>EGb 761 and its components are neuroprotective in a mouse transient ischemia model. >Putative mechanism of action is through the Nrf2/HO1-induced CRMP2 pathway. >EGb 761 can protect neurons in vitro from oxidative as well as excitatory toxicity. >EGb 761 induces neuritogenesis by upregulating CRMP2. >EGb 761 protected the filaments and restored filamental CRMP2 expression.
Acknowledgments
This work was supported by a grant from the National Institutes of Health-National Center for Complimentary and Alternative Medicine (R00AT004197) to ZAS.
Abbreviations
- BB
bilobalide
- CRMP2
collapsin response mediator protein 2
- EGb 761
Ginkgo biloba/EGb 761®
- GA
ginkgolide A
- GB
ginkgolide B
- HO1
heme oxygenase 1
- H2O2
hydrogen peroxide
- MCA
middle cerebral artery
- NDS
neurologic deficit score
- NMDA
N-Methyl-D-aspartate
- t-BuOOH
tertiary butylhydroperoxide
- TFM
terpene free material
- tMCAO
transient middle cerebral artery occlusion
- TTC
triphenyltetrazolium chloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlemeyer B, Krieglstein J. Neuroprotective effects of Ginkgo biloba extract. Cell Mol Life Sci. 2003;60:1779–1792. doi: 10.1007/s00018-003-3080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura N, Menager C, Kawano Y, Yoshimura T, Kawabata S, Hattori A, Fukata Y, Amano M, Goshima Y, Inagaki M, Morone N, Usukura J, Kaibuchi K. Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol Cell Biol. 2005;25:9973–9984. doi: 10.1128/MCB.25.22.9973-9984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretin S, Reibel S, Charrier E, Maus-Moatti M, Auvergnon N, Thevenoux A, Glowinski J, Rogemond V, Premont J, Honnorat J, Gauchy C. Differential expression of CRMP1, CRMP2A, CRMP2B, and CRMP5 in axons or dendrites of distinct neurons in the mouse brain. J Comp Neurol. 2005;486:1–17. doi: 10.1002/cne.20465. [DOI] [PubMed] [Google Scholar]
- Bretin S, Rogemond V, Marin P, Maus M, Torrens Y, Honnorat J, Glowinski J, Premont J, Gauchy C. Calpain product of WT-CRMP2 reduces the amount of surface NR2B NMDA receptor subunit. J Neurochem. 2006;98:1252–1265. doi: 10.1111/j.1471-4159.2006.03969.x. [DOI] [PubMed] [Google Scholar]
- Brittain JM, Chen L, Wilson SM, Brustovetsky T, Gao X, Ashpole NM, Molosh AI, You H, Hudmon A, Shekhar A, White FA, Zamponi GW, Brustovetsky N, Chen J, Khanna R. Neuroprotection against traumatic brain injury by a peptide derived from the collapsin response mediator protein 2 (CRMP2) J Biol Chem. 2011 doi: 10.1074/jbc.M111.255455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune B, Ullrich V. Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase. Mol Pharmacol. 1987;32:497–504. [PubMed] [Google Scholar]
- Butterfield DA, Hardas SS, Lange ML. Oxidatively modified glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and Alzheimer’s disease: many pathways to neurodegeneration. J Alzheimers Dis. 2010;20:369–393. doi: 10.3233/JAD-2010-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran K, Mehrabian Z, Spinnewyn B, Chinopoulos C, Drieu K, Fiskum G. Neuroprotective effects of bilobalide, a component of Ginkgo biloba extract (EGb 761) in global brain ischemia and in excitotoxicity-induced neuronal death. Pharmacopsychiatry. 2003;36(Suppl 1):S89–94. doi: 10.1055/s-2003-40447. [DOI] [PubMed] [Google Scholar]
- Christodoulides N, Durante W, Kroll MH, Schafer AI. Vascular smooth muscle cell heme oxygenases generate guanylyl cyclase-stimulatory carbon monoxide. Circulation. 1995;91:2306–2309. doi: 10.1161/01.cir.91.9.2306. [DOI] [PubMed] [Google Scholar]
- Clark WM, Rinker LG, Lessov NS, Lowery SL, Cipolla MJ. Efficacy of antioxidant therapies in transient focal ischemia in mice. Stroke. 2001;32:1000–1004. doi: 10.1161/01.str.32.4.1000. [DOI] [PubMed] [Google Scholar]
- Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Eickholt BJ, Walsh FS, Doherty P. An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling. J Cell Biol. 2002;157:211–217. doi: 10.1083/jcb.200201098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins JS, Johnston SC. Thirty-year projections for deaths from ischemic stroke in the United States. Stroke. 2003;34:2109–2112. doi: 10.1161/01.STR.0000085829.60324.DE. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Itoh TJ, Kimura T, Menager C, Nishimura T, Shiromizu T, Watanabe H, Inagaki N, Iwamatsu A, Hotani H, Kaibuchi K. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat Cell Biol. 2002a;4:583–591. doi: 10.1038/ncb825. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Kimura T, Kaibuchi K. Axon specification in hippocampal neurons. Neurosci Res. 2002b;43:305–315. doi: 10.1016/s0168-0102(02)00062-7. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28:52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- Goshima Y, Nakamura F, Strittmatter P, Strittmatter SM. Collapsin-induced growth cone collapse mediated by an intracellular protein related to UNC-33. Nature. 1995;376:509–514. doi: 10.1038/376509a0. [DOI] [PubMed] [Google Scholar]
- Hou ST, Jiang SX, Aylsworth A, Ferguson G, Slinn J, Hu H, Leung T, Kappler J, Kaibuchi K. CaMKII phosphorylates collapsin response mediator protein 2 and modulates axonal damage during glutamate excitotoxicity. J Neurochem. 2009;111:870–881. doi: 10.1111/j.1471-4159.2009.06375.x. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Chihara K, Arimura N, Menager C, Kawano Y, Matsuo N, Nishimura T, Amano M, Kaibuchi K. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4:781–782. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol. 2006;290:F563–571. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- Minturn JE, Fryer HJ, Geschwind DH, Hockfield S. TOAD-64, a gene expressed early in neuronal differentiation in the rat, is related to unc-33, a C. elegans gene involved in axon outgrowth. J Neurosci. 1995;15:6757–6766. doi: 10.1523/JNEUROSCI.15-10-06757.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem S, Shah ZA, Urade Y, Dore S. Lipocalin-prostaglandin D synthase is a critical beneficial factor in transient and permanent focal cerebral ischemia. Neuroscience. 2009;160:248–254. doi: 10.1016/j.neuroscience.2009.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem S, Zhuang H, Biswal S, Christen Y, Dore S. Ginkgo biloba extract neuroprotective action is dependent on heme oxygenase 1 in ischemic reperfusion brain injury. Stroke. 2008;39:3389–3396. doi: 10.1161/STROKEAHA.108.523480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, Li RC, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, Dore S. The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J Cereb Blood Flow Metab. 2010;30:1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, Nada SE, Dore S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761) neuroprotection. Neuroscience. 2011;180:248–255. doi: 10.1016/j.neuroscience.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, Namiranian K, Klaus J, Kibler K, Dore S. Use of an optimized transient occlusion of the middle cerebral artery protocol for the mouse stroke model. J Stroke Cerebrovasc Dis. 2006;15:133–138. doi: 10.1016/j.jstrokecerebrovasdis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9:355–363. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Nakagomi S, Namikawa K, Kiryu-Seo S, Inagaki N, Kaibuchi K, Aizawa H, Kikuchi K, Kiyama H. Collapsin response mediator protein-2 accelerates axon regeneration of nerve-injured motor neurons of rat. J Neurochem. 2003;86:1042–1050. doi: 10.1046/j.1471-4159.2003.01920.x. [DOI] [PubMed] [Google Scholar]
- Tsai JY, Su KH, Shyue SK, Kou YR, Yu YB, Hsiao SH, Chiang AN, Wu YL, Ching LC, Lee TS. EGb761 ameliorates the formation of foam cells by regulating the expression of SR-A and ABCA1: role of haem oxygenase-1. Cardiovasc Res. 2010;88:415–423. doi: 10.1093/cvr/cvq226. [DOI] [PubMed] [Google Scholar]
- Won SJ, Kim DY, Gwag BJ. Cellular and molecular pathways of ischemic neuronal death. J Biochem Mol Biol. 2002;35:67–86. doi: 10.5483/bmbrep.2002.35.1.067. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Kawano Y, Arimura N, Kawabata S, Kikuchi A, Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120:137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Yuasa-Kawada J, Suzuki R, Kano F, Ohkawara T, Murata M, Noda M. Axonal morphogenesis controlled by antagonistic roles of two CRMP subtypes in microtubule organization. Eur J Neurosci. 2003;17:2329–2343. doi: 10.1046/j.1460-9568.2003.02664.x. [DOI] [PubMed] [Google Scholar]
- Zeynalov E, Shah ZA, Li RC, Doré S. Heme Oxygenase 1 Is Associated with Ischemic Preconditioning-Induced Protection Against Brain Ischemia. Neurobiol Dis. 2009 doi: 10.1016/j.nbd.2009.05.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YY, Yu QH, You S, Sheng L. Ginkgolides protects the cultured rat cortical cells against metabolic, excitotoxic and oxidative insults. Journal of Health Science. 2004;50:348–355. [Google Scholar]
- Zhao Z, Wang Z, Gu Y, Feil R, Hofmann F, Ma L. Regulate axon branching by the cyclic GMP pathway via inhibition of glycogen synthase kinase 3 in dorsal root ganglion sensory neurons. J Neurosci. 2009;29:1350–1360. doi: 10.1523/JNEUROSCI.3770-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Tan KS, Zhang X, Sun AY, Sun GY, Lee JC. Hydrogen peroxide alters membrane and cytoskeleton properties and increases intercellular connections in astrocytes. J Cell Sci. 2005;118:3695–3703. doi: 10.1242/jcs.02507. [DOI] [PubMed] [Google Scholar]