Abstract
Fibroblast growth factors (FGFs) maintain and promote vascular integrity; however whether FGFs protect the blood-brain barrier (BBB) after intracerebral hemorrhage (ICH) remains unexplored. In this present study, we hypothesized that exogenous FGF administration attenuates brain injury after ICH, specifically by preserving endothelial adherens junctions, therefore reducing vasogenic brain edema and attenuating neurofunctional deficits in mice subjected to experimental ICH.
Acid fibroblast growth factor (FGF1) or basic fibroblast growth factor (FGF2) was administered intracerebroventricularly (ICV) at 0.5 hours after intrastriatal injection of bacterial collagenase (cICH) or autologous whole blood (bICH). Fibroblast growth factor receptor (FGFR) inhibitor PD173074 and phosphatidylinositol 3-kinase (PI3K) inhibitor LY294002 were additionally administered with FGF2. The selective Rho–associated coiled-coil forming protein serine/threonine kinase (ROCK) inhibitor Y27632 was independently administered at 0.5 hours after cICH. Brain water content and neurofunctional deficits were evaluated at 24 and 72 hours after ICH induction. Evans blue extravasation as well as Western blot analysis for the quantification of activated FGFR, Akt, Ras-related C3 botulinum toxin substrate 1 (Rac1), Ras homolog gene family member A (RhoA) and adherens junction proteins (p120-catenin, β-catenin and VE-cadherin) were conducted at 72 hours post-cICH. FGF treatment reduced perihematomal brain edema and improved neurofunctional deficits at 72 hours after experimental ICH (p<0.05, compared to vehicle); however, FGFR and PI3K inhibition reversed these neuroprotective effects. Exogenous FGF2 increased activated FGFR, Akt, and Rac1 but reduced activated RhoA protein expression at 72 hours after cICH (p<0.05, compared to vehicle), which was reversed by FGFR and PI3K inhibition. Y27632 treatment reduced brain injury at 72 hours after cICH (p<0.05, compared to vehicle) and increased the expression of catenins (p120-catenin, β-catenin). In conclusion, our findings suggest that exogenous FGF treatment reduced RhoA activity via FGFR-induced activation of the PI3K-Akt-Rac1 signaling pathway, thus preserving BBB integrity, and therefore attenuating secondary brain injury after experimental ICH in mice.
Keywords: Fibroblast growth factor, thrombin, Rac1, RhoA, intracerebral hemorrhage, brain injury, blood-brain barrier, brain edema, behavior
Introduction
Intracerebral hemorrhage (ICH) defines a potentially life-threatening neurological emergency, which accounts for 10–15% of all stroke-related hospitalizations (Broderick et al., 1999; Qureshi et al., 2009). It results in a 30-day mortality rate of approximately 50%, and often causes debilitating neurofunctional deficits in survivors (Aronowski and Hall, 2005). The development of vasogenic brain edema, as a consequence of increased blood-brain barrier (BBB) permeability, has been identified as an important contributor of secondary brain injury evoked by ICH (Batjer et al., 1990; Elliott and Smith, 2010). Furthermore, the extent of brain edema highly correlates with the severity of neurofunctional deficits in ICH-patients (Batjer et al., 1990; Elliott and Smith, 2010). Thrombin, an essential component in the coagulation cascade, induces the disintegration of endothelial adherens junctions, specifically by stimulation of proteinase-activated receptors (e.g. PAR-1), and subsequent activation of downstream effectors, such as Ras homolog gene family member A (RhoA) and Rho–associated coiled-coil forming protein serine/threonine kinase (ROCK), (Lee et al., 1995; Xi et al., 1998; Xi et al., 2006). Activated ROCK directly phosphorylates and therefore disintegrates adherens junction proteins, causing increased BBB permeability and consequent vasogenic edema formation after ICH (van Nieuw Amerongen et al., 2000). Fibroblast growth factors (FGFs) are extensively involved in wound healing processes (Ortega et al., 1998a) and play an important role in the regulation of vascular integrity (Murakami et al., 2008). Inhibition of fibroblast growth factors receptor (FGFR) signaling resulted in a decrease of cell-cell adhesions, which was primarily caused by disintegration of the p120-catenin/VE-cadherin complex (Murakami et al., 2008). Furthermore, previous studies demonstrated that FGFs activate Ras-related C3 botulinum toxin substrate 1 (Rac1), through the phosphatidylinositol 3-kinase (PI3K) - Akt signaling pathway, in human endothelial cells of the cornea (Lee and Kay, 2011), as well as in bone marrow stromal cells (Kamura et al., 2010). Activated Rac1 inhibits RhoA (Wojciak-Stothard et al., 2001), which possibly will preserve BBB integrity and therefore reduce the development of vasogenic brain edema after ICH.
In this present study we hypothesized that exogenous FGF administration attenuates secondary brain injury, specifically by preserving endothelial adherens junctions, therefore reducing vasogenic brain edema and attenuating neurofunctional deficits in mice subjected to ICH. We furthermore hypothesized that FGFR-induced activation of the PI3K-Akt-Rac1 signaling pathway and consequent inhibition of RhoA results in preservation of endothelial adherens junctions.
Materials and Methods
Animals and surgical procedures
All animal procedures were conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee at Loma Linda University. Eight week old male CD-1 mice (35–45g, Charles River, Wilmington, MA) were housed in a light and temperature controlled environment with unlimited access to food and water.
Experimental ICH was induced by intrastriatal injection of either bacterial collagenase (cICH) or autologous blood (bICH) as previously reported (Ma et al., 2011a; Rosenberg et al.,1990; Wang et al., 2008). Briefly, following an intraperitoneal co-injection of the anesthetics ketamine (100 mg/kg) and xylazine (10mg/kg), mice were positioned prone and secured onto a stereotactic head frame (Kopf Instruments, Tujunga, CA). A cranial burr hole was made close to the right coronal suture, 1.7mm lateral to the midline and 0.9mm posterior to bregma. To perform the cICH model, a 10μl Hamilton syringe (26 Gauge; Hamilton Company, Reno, NV) was filled with bacterial collagenase (VII-S, Sigma-Aldrich, St Louis, MO). The syringe was then connected onto a micro perfusion pump (Harvard Apparatus, Holliston, MA) and lowered 3.7mm ventrally through the burr hole. Bacterial collagenase (0.075U dissolved in 0.5μL of PBS) was injected into the right striatum at a rate of 2μL/min. After completed injection, the needle was left in place for an additional 10 minutes to prevent backflow of collagenase along the needle tract, before being withdrawn at a rate of 1mm/min.
To perform the bICH model we utilized a modified double injection method of autologous blood, as described by Wang et al. (Wang et al., 2008). Briefly, 30μL of the rodent’s central tail artery blood was collected in a capillary tube and quickly transferred into a 250μL Hamilton syringe (26 Gauge; Hamilton Company, Reno, NV). A burr hole was made 1.7mm lateral to the midline and 0.9mm posterior to bregma. The needle was connected to the micro perfusion pump and lowered 3.0mm ventrally through the burr hole. Next, 5μL of autologous blood were injected at a rate of 2μL/min. Following that, the needle was advanced 0.7mm in depth and after a waiting period of 5 minutes, 25μL of autologous blood was injected into the right striatum. The needle was left in place for an additional 10 minutes after completed injection, before being withdrawn at a rate of 1mm/min. In both models, the burr hole was sealed with bone wax and the scalp was sutured. All mice were allowed to fully recover under observation. Sham operated animals received needle insertion only.
Drugs and experimental groups
Recombinant human acid fibroblast growth factor (FGF1), recombinant human basic fibroblast growth factor (FGF2), (R&D Systems, Minneapolis, MN), and ROCK inhibitor Y27632 (Ascent Technology, Inc. Cambridge, MA) were dissolved in sterile PBS. FGFR inhibitor PD173074 and PI3K inhibitor LY294002 (Sigma-Aldrich, St Louis, MO) were dissolved in 25% dimethylsulfoxide solution (DMSO). All drugs were administered at 0.5 hours after hemorrhage induction via intracerebroventricular (ICV) injection. For this purpose a cranial burr hole was made close to the left coronal suture, 1.1mm lateral to bregma. A total volume of 2μL of the respective solutions was injected into the lateral ventricle (2.5mm ventrally) at a rate of 0.25μL/min. After completed injection, the needle was left in place for an additional 10 minutes, before being withdrawn at a rate of 1mm/min.
CD-1 mice (n=222) were either subjected to ICH (cICH, n=172; bICH, n=16) or sham surgery (n=34). cICH mice were randomly selected and treated with either 30ng/kg (FGF2-30ng, n=8) or 100ng/kg of FGF2 (FGF2-100ng, n=34), 100ng/kg of FGF1 (FGF1-100ng, n=8) or with 20nmol/kg of ROCK inhibitor Y27632 (Y27632-20nmol, n=20). Additionally, FGF2 (100ng/kg) was combined with FGFR inhibitor PD173074 in concentrations of 0.1 or 0.5nmol/kg (FGF2+PD-0.1nmol, n=8; FGF2+PD-0.5nmol, n=20). FGF2 (100ng/kg) was also co-injected with 50nmol of PI3K inhibitor LY294002 (FGF2+LY-50nmol, n=20) or with DMSO (FGF2+DMSO, n=20). bICH mice also received 100ng/kg of FGF2 (FGF2-100ng, n=8). Vehicle animals (n=42) received same volumes of PBS.
Behavior assessments
The sensorimotor Garcia (Garcia et al., 1995) and Cylinder Test (Li et al., 2004) were conducted in a blinded fashion, and used to assess neurofunctional deficits in mice at 24 and 72 hours after surgery. The Garcia Test has been modified and consisted of 7 individual tests, examining spontaneous activity (1), axial sensation (2), vibrissae proprioception (3), limb symmetry (4) as well as the animal’s ability of lateral turning (5), forelimb outstretching (6) and climbing (7). A score of 0 (worst performance) to 3 (best performance) was given for each sub-test and a total Garcia score was calculated as the sum of all sub-tests (maximum score of 21).
To conduct the Cylinder test the mouse was placed into a Plexiglas cylinder (diameter: 9cm, height: 15cm) and forepaw placements along the cylinder wall were recorded over a time period of 5 minutes. A score was calculated as number of non-impaired (right) forelimb contacts minus the number of impaired (left) forelimb contacts divided by the number of all wall contacts and multiplied by 100.
Measurement of brain water content
Brain water content (brain edema) was evaluated via the wet-weight/dry-weight method, as previously reported (Ma et al., 2011a; Tang et al., 2004). Briefly, animals were sacrificed at 24 or 72 hours after surgery, brains were quickly removed and divided into 4mm thick sections. These brain sections were further divided into ipsilateral cortex (Ipsi-CX) and basal ganglia (Ipsi-BG) and contralateral cortex (Cont-CX) and basal ganglia (Cont-BS). All specimens were weighed using an analytical microbalance (APX-60, Denver Instrument, Bohemia, NY) before, as well as after drying (100°C for 24 hours). Brain water content was calculated as percentage of (wet weight – dry weight)/ wet weight × 100.
Evans Blue extravasation assay
Evans Blue extravasation assays were conducted at 72 hours after surgery as previously reported (Ma et al., 2011b). Briefly, mice received an intraperitoneal injection of 0.25ml Evans Blue dye (4%), 3 hours prior to sacrifice. Following that, deeply anesthetized animals were transcardially perfused with ice-cold PBS (50ml) and brains were removed, divided into right and left hemispheres, snap-frozen in liquid nitrogen, and stored at −80ºC. Right hemispheres were then homogenized in 1100μl of PBS, sonicated and then centrifuged for 30 minutes with a relative centrifugal force (rcf) of 15000. An equal amount of trichloroacetic acid (50%) was added to 500μl of the supernatant and allowed to incubate over night at 4ºC before being re-centrifuged (30min, 15000 rcf). The quantity of extravasated Evans Blue dye was detected by spectrophotometer (Thermo Fisher Scientific Inc. Waltham, MA) at 610nm and quantified according to a standard curve. Results were presented as μg of Evans Blue dye per g of brain tissue.
Hemoglobin Assay
The hemoglobin assay was performed using an established protocol (Ma et al., 2011a; Manaenko et al., 2011). Ipsilateral hemispheres were homogenized for 60 seconds in 3ml of distilled water. The homogenized specimens were centrifuged (30min, 12000 rcf) and 400μl of Drabkin’s reagent (Sigma-Aldrich, St Louis, MO) was added into 100μl of supernatant. After 15 minutes the hemoglobin absorbance was measured via spectrophotometer (540nm), and quantified using a standard curve. Results were presented as μl of blood.
Western Blotting
Mice were sacrificed at 72 hours after surgery and right brain hemispheres were isolated and processed as previously described (Wu et al., 2010). Equal amounts of protein (50μg) were loaded onto SDS-PAGE gels, before being separated and transferred onto a nitrocellulose membrane. The membrane was blocked and incubated overnight at 4ºC with the following antibodies: anti-p-FGFR, anti-p-Akt (Cell Signaling Technology), anti-β-catenin and anti-p120-catenin (Abcam, Cambridge, MA) and anti-VE-cadherin, (Santa Cruz Biotechnology, Santa Cruz, CA). GTP-Rac1, total-Rac1, GTP-RhoA and total-RhoA were detected using Rac1/Cdc42 and Rho Activation Assay Kits (Millipore, Temecula, CA). The appropriate secondary antibodies were obtained from Santa Cruz Biotechnology. Immunoblots were visualized with ECL Plus chemiluminescence kit (Amersham Bioscience, Arlington Heights, IL) and semi-quantitavely analyzed using the software Image J (4.0, Media Cybernetics, Silver Springs, MD). Results were expressed as a relative density ratio, normalized to the mean value of the sham group.
Statistical Analysis
Data were expressed as mean±SEM. Behavior data were statistically analyzed using Kruskal-Wallis One Way Analysis of Variance on Ranks, followed by the Student-Newman-Keuls Method. All other data were statistically analyzed using One Way Analysis of Variance followed by Tukey post hoc test. A P value <0.05 was considered statistically significant. All statistical analyses were performed using the software SigmaPlot version 10.0 for Windows.
Results
FGF treatment reduced neurofunctional deficits and brain edema at 72 hours after intrastriatal collagenase injection
All mice (n=222) subjected to cICH, bICH or sham surgery survived until day of sacrifice (mortality=0%). Neurofunctional deficits and brain water content were evaluated at 24 and 72 hours after intrastriatal collagenase injection (cICH). At 24 hours after surgery vehicle mice showed significantly worse performances during the Garcia and Cylinder Test as well as increased perihematomal brain water content, than sham operated animals (p<0.05); however, no significant differences were found between the vehicle and the FGF2–100ng group (n=6 per group; data not shown).
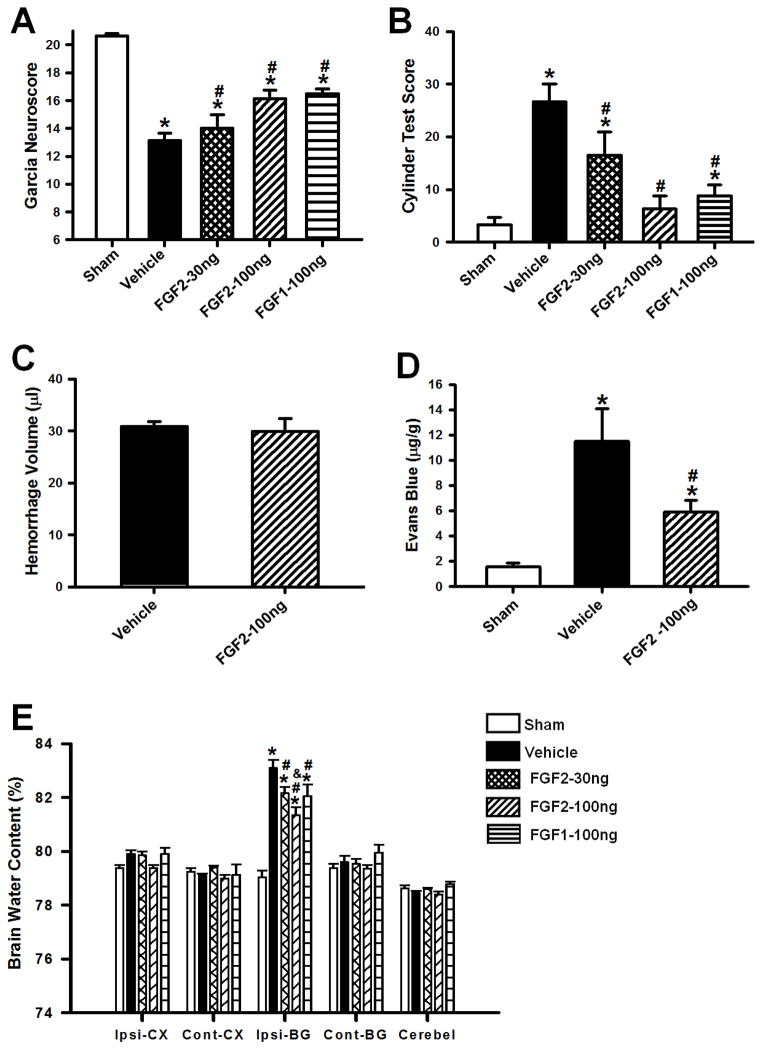
At 72 hours after surgery, FGF2 (FGF2–30ng and FGF2–100ng) and FGF1 (FGF1-100ng) treatments ameliorated neurofunctional deficits and reduced perihematomal brain water content (p<0.05 compared to vehicle; n=8 per group; Figure 1A, B, E). In order to evaluate BBB permeability, Evans Blue extravasation assays were conducted at 72 hours after cICH. Significantly, more extravasated dye was found in brain specimen of cICH mice (p<0.05 compared to sham). Treatment with FGF2–100ng preserved BBB integrity, which was shown by a significantly reduced dye extravasation (p<0.05 compared to vehicle; n=6 per group; Figure 1D). However, administration of FGF2 (100ng/kg), did not affect the hemorrhage volume at 72 hours after cICH (p=0.747; n=8 per group; Figure 1C). FGF2 (100ng/kg) conferred neuroprotection to a greater extent than the same dosage of FGF1, and was therefore used for all following experiments.
Figure 1.
Effects of exogenous FGF1 (100ng/kg) and FGF2 (30 and 100ng/kg) administration on Garcia (A) and Cylinder Test Scores (B) at 72 hours after surgery. FGF1 and FGF2, improved sensorimotor functions in mice subjected to collagenase induced ICH. Effects of FGF2 on hemorrhage volume (C), Evans blue extravasation (D) and brain water content (E) at 72 hours after surgery. Data are expressed as mean ± SEM. * p<0.05 compared to sham, # p<0.05 compared to vehicle, & p<0.05 compared to FGF2-30ng. N=6–8 per group. Ipsi-CX: ipsilateral cortex, Cont-CX: contralateral cortex, Ipsi-BG: ipsilateral basal ganglia, Cont-BG: contralateral basal ganglia, Cerebel: cerebellum.
FGF2 treatment reduced neurofunctional deficits and brain edema at 72 hours after intrastriatal injection of autologous blood
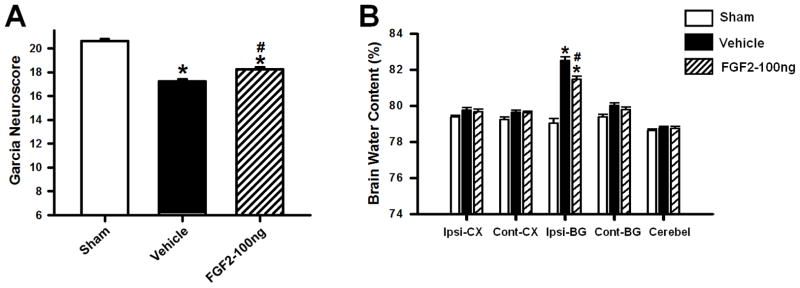
Neurofunctional deficits and brain water content were additionally evaluated in a collagenase-independent mouse model of ICH, implementing a double injection of autologous whole blood into the right striatum (b-ICH). At 72 hours after surgery, FGF2 treated (FGF2–100ng) and untreated (vehicle) animals demonstrated significantly worse performances in the Garcia Test when compared to sham operated mice (p<0.05; n=8 per group; Figure 2A). FGF2 treatment (FGF2–100ng) significantly improved the Garcia Test outcome after bICH compared to vehicle (p<0.05).
Figure 2.
Effects of exogenous FGF2 (100ng/kg) administration on Garcia Test Score (A) and brain water content (B) at 72 hours after intrastriatal injection of autologous blood in mice. Data are expressed as mean ± SEM. * p<0.05 compared to sham, # p<0.05 compared to vehicle. N=8 per group. Ipsi-CX: ipsilateral cortex, Cont-CX: contralateral cortex, Ipsi-BG: ipsilateral basal ganglia, Cont-BG: contralateral basal ganglia, Cerebel: cerebellum.
At 72 hours after surgery, perihematomal brain water content was found significantly higher in mice subjected to bICH than in sham-operated animals (p<0.05; n=8 per group; Figure 2B); however, FGF2 treatment (FGF2–100ng) caused a significant reduction of brain water content in the ipsilateral basal ganglia (p<0.05, compared to vehicle).
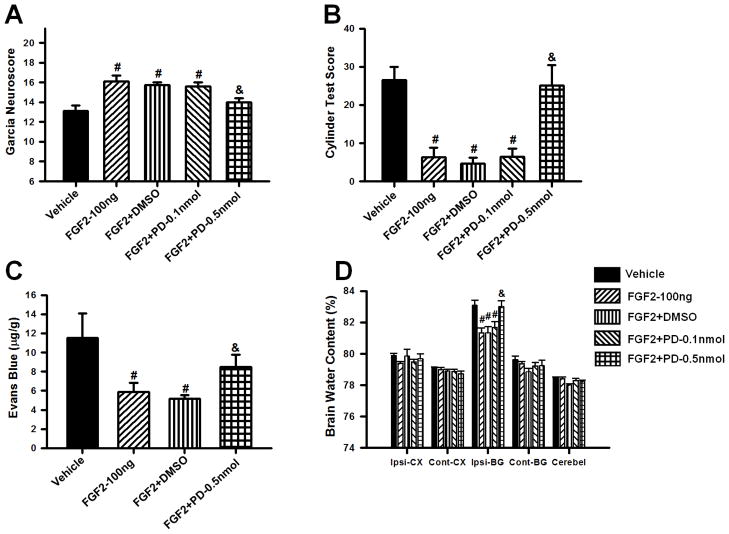
FGFR inhibition reversed the neuroprotective effect of FGF2
In order to confirm that the previously observed neuroprotection was caused by FGF2-induced activation of the FGFR, 0.1 or 0.5nmol of the FGFR inhibitor PD173074 was co-injected with 100ng/kg of FGF2 (FGF2+PD–0.1nmol, FGF2+PD–0.5nmol). Neurofunctional deficits, Evans Blue extravasation, and brain water content were evaluated at 72 hours after cICH. Administration of 0.5nmol/kg PD173074 (FGF2+PD–0.5nmol) but not 0.1nmol/kg PD173074 entirely reversed the protective effect of the treatment as demonstrated by significantly worse performances in both the Garcia and Cylinder Tests. PD173074 (0.5nmol/kg) administration also increased dye extravasation as demonstrated in the Evans Blue assay, and also resulted in higher perihematomal brain water content compared to FGF2-100ng (p<0.05). Since PD173074 was dissolved in DMSO, an additional control group receiving the vehicle (FGF2+DMSO) was included. The FGF2+DMSO outcomes were comparable to the FGF2-100 ng group (p>0.05, n=8 per group, Figure 3A–D).
Figure 3.
Effects of FGFR inhibitor PD173074 (0.1 and 0.5 nmol/kg) and DMSO on FGF2 (100ng/kg) induced attenuation of brain injury at 72 hours after surgery. Evaluation of Garcia (A) and Cylinder Test Scores (B), Evans blue extravasation (C) and brain water content (D) in mice subjected to collagenase induced ICH. Data are expressed as mean ± SEM. * p<0.05 compared to sham, # p<0.05 compared to vehicle, & p<0.05 compared to FGF2-30ng. N=6–8 per group. Ipsi-CX: ipsilateral cortex, Cont-CX: contralateral cortex, Ipsi-BG: ipsilateral basal ganglia, Cont-BG: contralateral basal ganglia, Cerebel: cerebellum.
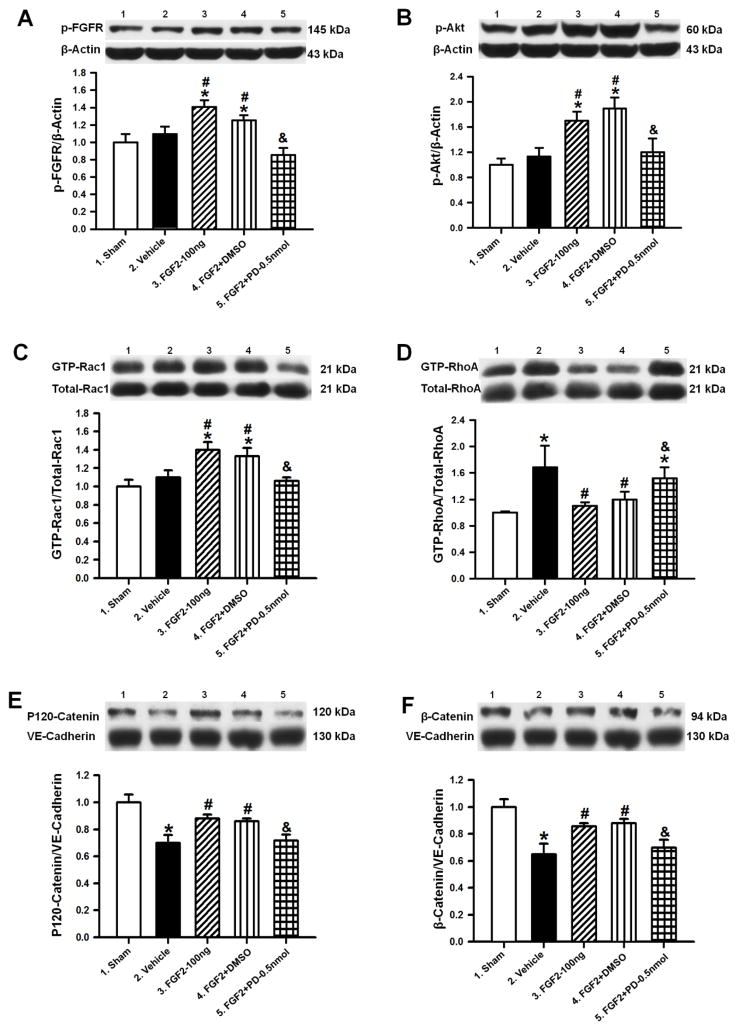
FGF2 treatment inhibited RhoA activity via the PI3K-Akt-Rac1 signaling pathway and thereby stabilized adherens junction proteins
To further confirm that FGF2-induced activation of the FGFR protects the BBB via PI3K-Akt-Rac1 dependent inhibition of RhoA, Western blot analyses of the ipsilateral hemispheres were conducted, and changes in the protein levels of phosphorylated FGFR (p-FGFR), phosphorylated Akt (p-Akt, Ser473), activated and total Rac1 (GTP-Rac1, Total-Rac1) and RhoA (GTP-RhoA, Total-RhoA) as well as adherens junction proteins (p120-catenin, β-catenin, and VE-cadherin) were quantified at 72 hours after cICH or sham surgery.
FGF2 treatment (100ng/kg) significantly increased phosphorylation and therefore activation of FGFR and Akt, compared to both the sham and the vehicle group (p<0.05; n=6 per group; Figure 4A–B). The FGFR inhibitor PD173074 (FGF2+PD–0.5nmol) reversed this treatment effect entirely (p<0.05 compared to FGF2–100ng). Furthermore, GTP-Rac1/Total-Rac1-ratio was significantly increased in the FGF2–100ng group compared to the sham and vehicle group (p<0.05), but decreased in the FGF2+PD–0.5nmol group (p<0.05 compared to FGF2-100ng, Figure 4C). Both the vehicle and FGF2+PD–0.5nmol group demonstrated significantly higher protein levels of activated RhoA (GTP–RhoA) than the sham and treatment groups (p<0.05, Figure 4D). Untreated cICH-mice (vehicle) showed significantly reduced expressions of catenins (p<0.05 compared to sham, Figure 5E–F). Specifically, the ratio of p120-catenin and VE-cadherin as well as the ratio between β-catenin and VE-cadherin were reduced in vehicle animals. FGF2 treatment significantly increased these ratios compared to the vehicle group, which was reversed by PD173074 (FGF2+PD–0.5nmol), (p<0.05 compared to FGF2–100ng). The DMSO–control group (FGF2+DMSO) showed results comparable with the FGF2–100ng group (p>0.05, Figures 4A–F).
Figure 4.
Representative Western blots and densitometric quantification of p-FGFR/β-Actin (A), p-Akt/β-Actin (B), GTP-Rac1/Total-Rac1 (C), GTP-RhoA/Total-RhoA (D), P120-Catenin/VE-Cadherin (E) and β-Catenin/VE-Cadherin (F) at 72 hours after collagenase induced ICH or sham surgery. Data are expressed as mean ± SEM. * p<0.05 compared to sham, # p<0.05 compared to vehicle, & p<0.05 compared to FGF2-30ng. N=6 per group.
Figure 5.
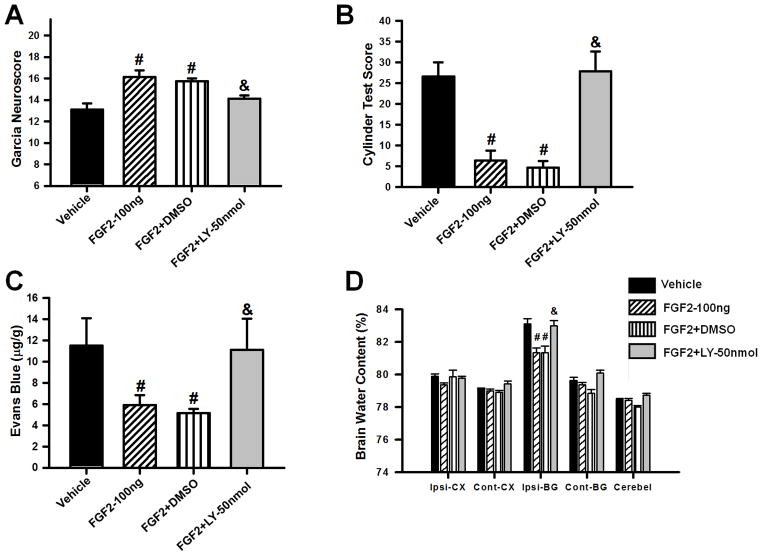
Effects of PI3K inhibitor LY294002 (50nmol/kg) on FGF2 (100ng/kg) induced attenuation of brain injury at 72 hours after surgery. Evaluation of Garcia (A) and Cylinder Test Scores (B), Evans blue extravasation (C) and brain water content (D) in mice subjected to collagenase induced ICH. Data are expressed as mean ± SEM. * p<0.05 compared to sham, # p<0.05 compared to vehicle, & p<0.05 compared to FGF2-30ng. N=6–8 per group. Ipsi-CX: ipsilateral cortex, Cont-CX: contralateral cortex, Ipsi-BG: ipsilateral basal ganglia, Cont-BG: contralateral basal ganglia, Cerebel: cerebellum.
PI3K inhibition reversed the neuroprotective effect of FGF2
In order to evaluate whether PI3K is involved in the neuroprotection by FGFR activation, 50nmol/kg of the PI3K inhibitor LY294002 was co-administered with 100ng/kg of FGF2 (FGF2+LY–50nmol). Neurofunctional deficits, Evans Blue dye extravasation, and brain water content were evaluated at 72 hours after cICH. The results of the FGF2+LY–50nmol group were compared to vehicle, FGF2–100ng, and FGF2+DMSO group, respectively. LY294002 co-administration (FGF2+LY–50nmol) reversed the neuroprotection caused by FGF2 as shown by the results in the Garcia and Cylinder Test, Evans Blue extravasation assay, and evaluations of brain water content. FGF2+LY–50nmol animals performed significantly worse in both neurofunctional assessments than treated animals (p<0.05; n=8 per group: Figure 5A–B). Furthermore, mice receiving LY294002 in combination with FGF2 (FGF2+LY–50nmol) showed significantly more dye extravasation and significantly higher perihematomal brain edema than animals that received FGF2 treatment only (p<0.05; n=6–8 per group; Figure 5C–D). The DMSO-control group (FGF2–100ng + 25% DMSO) showed results comparable to the FGF2–100ng group (p>0.05, Figures 5A–D).
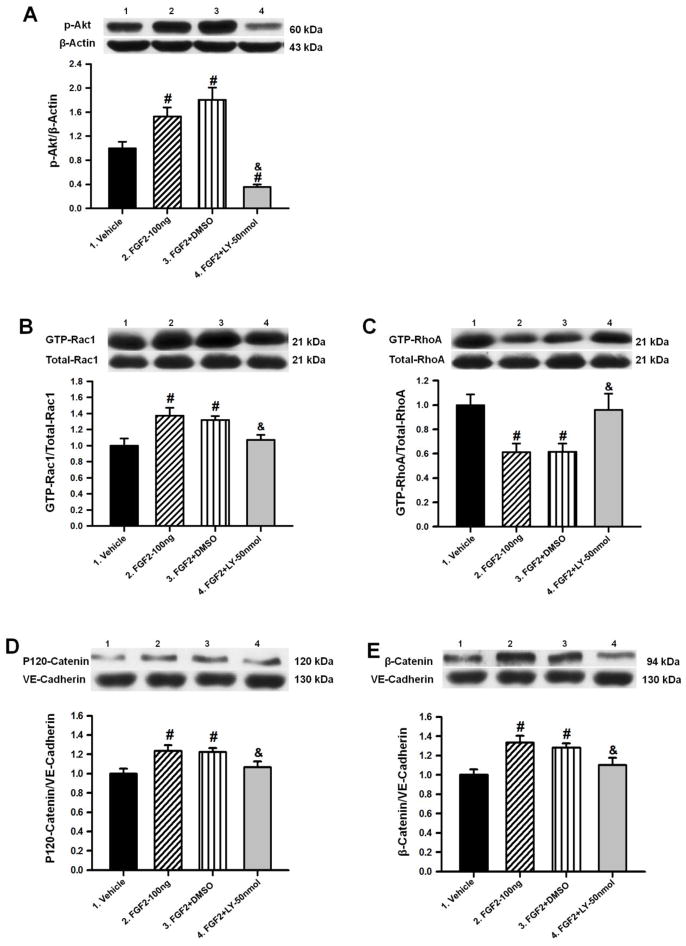
PI3K inhibition prevented FGF2-induced inhibition of RhoA
To further validate the previous results, Western blot analyses were performed using the ipsilateral hemispheres of animals at 72 hours after cICH and subsequent co-administration of FGF2 (100ng/kg) and LY294002 (50nmol/kg). The results of the quantification of all target proteins were compared to vehicle, FGF2-100ng, and FGF2+DMSO animals, respectively. LY294002 administration (FGF2+LY–50nmol) significantly decreased the protein level of p-Akt as well as the GTP-Rac1/Total-Rac1 ratio (p<0.05 compared to FGF2–100ng; n=6 per group; Figure 6A–B). The ratio of GTP-RhoA/Total-RhoA was significantly increased while the ratios of p120-catenin/VE-cadherin and β-catenin/VE-cadherin were significantly decreased in the FGF2+LY–50nmol group (p<0.05 compared to FGF2–100ng; Figure 6C–E). The DMSO-control group (FGF2+DMSO) showed protein expressions similar to the FGF2–100ng group (p>0.05, Figure 6A–E).
Figure 6.
Representative Western blots and densitometric quantification of p-Akt/β-Actin (A), GTP-Rac1/Total-Rac1 (B), GTP-RhoA/Total-RhoA (C), P120-Catenin/VE-Cadherin (D) and β-Catenin/VE-Cadherin (E) at 72 hours after collagenase induced ICH or sham surgery. Data are expressed as mean ± SEM. * p<0.05 compared to sham, # p<0.05 compared to vehicle, & p<0.05 compared to FGF2-30ng. N=6 per group.
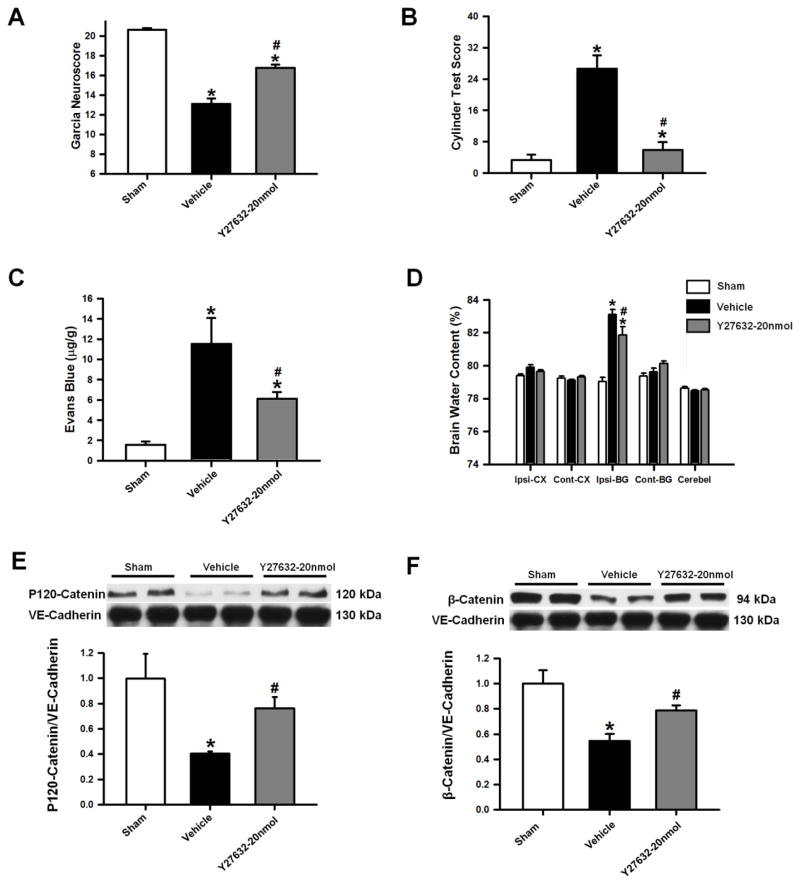
ROCK inhibition ameliorated brain injury after ICH through stabilization of adherens junction proteins
The ROCK inhibitor Y27632 (20nmol/kg) was used as an additional treatment in animals after cICH (Y27632–20ng). Neurofunctional deficits, Evans Blue extravasation assays, brain water content, and protein expressions of p120-catenin and β-catenin were evaluated at 72 hours after cICH. The outcomes of the Y27632–20ng group were compared to the sham and vehicle animals, respectively. Y27632 treatment improved neurofunctional deficits after cICH significantly, in both the Garcia and Cylinder Test (p<0.05 compared to vehicle; n=8 per group; Figure 7A–B). Furthermore, a reduction in extravasated Evans Blue dye and perihematomal brain water content was found in the Y27632–20ng group (p<0.05 compared to vehicle; n=8 per group; Figure 7C–D). Lastly, levels of adherens junction protein (ratios of p120-catenin/VE-cadherin and β-catenin/VE-cadherin) were found significantly increased compared to vehicle animals (p<0.05 n=6 per group; Figure 7E–F).
Figure 7.
Effects of ROCK inhibitor Y27632 (20nmol/kg) on Garcia (A) and Cylinder Test Scores (B) at 72 hours after surgery. Y27632 administration, improved sensorimotor functions in mice subjected to collagenase induced ICH. Effects of Y27632 on Evans blue extravasation (C), brain water content (D) and representative Western blots with densitometric quantification of P120-Catenin/VE-Cadherin (E) and β-Catenin/VE-Cadherin (F) at 72 hours after collagenase induced ICH or sham surgery. Data are expressed as mean ± SEM. * p<0.05 compared to sham, # p<0.05 compared to vehicle. N=6–8 per group. Ipsi-CX: ipsilateral cortex, Cont-CX: contralateral cortex, Ipsi-BG: ipsilateral basal ganglia, Cont-BG: contralateral basal ganglia, Cerebel: cerebellum.
Discussion
In this present study we demonstrated that exogenous FGF (FGF2 and FGF1) administration reduced perihematomal edema formation and attenuated neurofunctional deficits in mice at 72 hours after experimental ICH. Furthermore, FGF2 treatment induced phosphorylation and therefore activation of FGFR, Akt, and Rac1 (GTP-Rac1), which decreased the protein expression of activated RhoA (GTP-RhoA). Ultimately, FGF2 treatment led to increased ratios of p120-catenin/VE-cadherin and β-catenin/VE-cadherin at 72 hours after ICH. The functional integrity of the BBB was further evaluated via Evans blue extravasation assays. FGF2 (100ng/kg) treatment decreased BBB permeability at 72 hours after experimental ICH. However, co-administration of FGFR antagonist PD173074 or PI3K inhibitor LY294002, combined with FGF2, antagonized all treatment effects, suggesting that the observed neuroprotection was mediated by FGFR-induced activation of the PI3K-Akt-Rac1 signaling pathway. Moreover, FGF2 did not alter the hemorrhage volume at 72 hours after experimental ICH. Lastly, treatment with ROCK inhibitor Y27632 (20nmol/kg) attenuated brain injury through protection of endothelial adherens junctions at 72 hours after ICH.
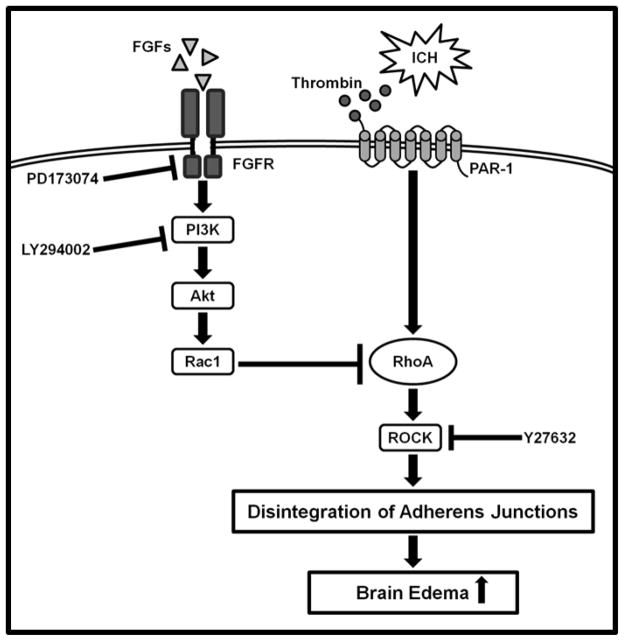
Perihematomal edema formation after ICH is initially caused by clot retraction and aggravated by coagulation factors and blood degradation products, such as thrombin, hemoglobin and iron (Suzuki and Ebina, 1980). Thrombin, specifically leads to activation of RhoA, which induces the phosphorylation of adherens junction proteins (Lee et al., 1995; Xi et al., 1998; Xi et al., 2006). Upon phosphorylation, adherens junctions are disintegrated, causing increased BBB permeability and consequent vasogenic edema formation after ICH (van Nieuw Amerongen et al., 2000), (Figure 8). Rac1 and RhoA are small guanosine triphosphates (GTPases) that belong to the Ras superfamily. It has been previously reported that Rac1 maintains and stabilizes barrier functions of microvascular endothelial cells, whereas RhoA antagonistically impairs endothelial barrier properties (Wojciak-Stothard et al., 2001). RhoA through ROCK phosphorylates and therefore disintegrates adherens junctions, and has further been identified to increase actomyosin contractility, which as well results in breakdown of intercellular junctions (Wojciak-Stothard et al., 2001). Moreover, inhibition of RhoA prevented thrombin- and histamine induced increase of endothelial permeability as well as the decrease in transendothelial resistance (Wojciak-Stothard et al., 2001).
Figure 8.
Proposed mechanism underlying FGF-mediated inhibition of RhoA after ICH. Thrombin, produced through activation of the coagulation cascade, binds on endothelial PAR-1, leading to activation of RhoA and ROCK. The latter induces phosphorylation and disintegration of adheres junctions proteins, thus facilitating the development of vasogenic brain edema after ICH. FGFs suppress RhoA via FGFR-induced activation of the PI3K-Akt-Rac1 signaling pathway. Co-administrations of FGFR inhibitor PD173074 or PI3K inhibitor LY294002, combined with FGF2 were utilized to study the suggested signaling pathway. Y27632 was additionally administered to confirm that ROCK inhibition confers neuroprotection after ICH. FGFs: Fibroblast growth factors, FGFR: Fibroblast growth factor receptor, ICH: intracerebral hemorrhage, PAR-1: Protease-activated receptor-1.
Adherens junctions provide the mechanical strength of cell-cell adhesions via VE-cadherin, a transmembrane protein that is anchored in the cytoskeleton (Wojciak-Stothard et al., 2001). Destabilization of endothelial adherens junctions, as reported by thrombin-induced activation of RhoA, resulted in endothelial cell dysfunction and increased BBB permeability (Presta et al., 2005). The interaction between p120-catenin, β-catenin and VE-cadherin is essential for adherens junction formation and stability (Hartsock and Nelson, 2008). Excessive disruption of the complexes between catenins and VE-cadherin leads to disintegration of adherens junctions and to consequent accumulation of fluids within the interstitial space. Furthermore, p120-catenin as well as β-catenin regulate the expression of VE-cadherin through inhibition of its clathrin-mediated endocytosis, which therefore prevents the subsequent proteolysis of VE-cadherin (Komarova and Malik, 2010). Prospective treatments stabilizing adherens junctions may reduce vasogenic brain edema and therefore attenuate brain injury after ICH.
Fibroblast growth factors (FGFs) are responsible for a wide variety of physiologic and pathologic processes including wound healing, angiogenesis, and embryonic development (Bottcher and Niehrs, 2005a; Bottcher and Niehrs, 2005b; Ortega et al., 1998a; Ortega et al., 1998b; Presta et al., 2005). Local administration of FGF2 protected CA1 pyramidal cells against neuronal damage caused by transient mass lesion in rats and increased angiogenesis in the injured brain region (Kawakami et al., 1995). Furthermore, FGF-18 reduced infarct volumes and behavioral deficits after transient occlusion of the middle cerebral artery in rats (Ellsworth et al., 2003). A previous study by Murakami et al. demonstrated that inhibition of FGFR signaling resulted in dissociation of p120-catenin/VE-cadherin complexes, causing disintegration of adherens junctions, which progressed to severe impairments of the endothelial barrier function (Murakami et al., 2008). Accordingly, our results showed increased protein ratios of p120-catenin/VE-cadherin and β-catenin/VE-cadherin in ICH mice subjected to FGF2 (100ng/kg) treatment.
Our study utilized two different mouse models of ICH, a collagenase injection model (Rosenberg et al., 1990) as well as a modified double injection model of autologous blood (Wang et al., 2008), both targeting the right corpus striatum in mice. Intraparenchymally injected bacterial collagenase causes the disruption of cerebral capillaries and models a spontaneous bleed that develops over several hours, as exhibited in roughly 30% of all ICH patients (MacLellan et al., 2008). However, bacterial collagenase may cause an exaggerated inflammatory response in the brain; although in vivo studies failed to confirm this hypothesis (Matsushita et al., 2000). In order to avoid possible interference of bacterial collagenase with the inflammatory response and aggravated BBB disruption, the autologous blood injection model was utilized to verify neuroprotective effects of FGF2.
Previous studies described the incidence of acute hyperglycemia within 20 minutes after ketamine (100 mg/kg) and xylazine (10 mg/kg) anesthesia in fed rodents (Saha et al. 2005). The animals in this study were hyperglycemic throughout the duration of the anesthesia, which was maintained for 3 hours, using additional maintenance-dosages of the anesthetics. In our current study all mice received a single and identical amount of ketamine/xylazine (adjusted to bodyweight), which kept the animals anesthetized for under 1 hour. Since all animals in each group received the same amount of anesthesia, we would expect changes in blood glucose levels to a similar extent throughout all groups. Thus, the anesthesia in our experiment was a constant component of each procedure.
The development of perihematomal brain edema in murine models of ICH, has been found to peak around the third and fourth post-operative day (Xi et al., 1998). The failure of FGFs to attenuate brain injury at 24 hours after ICH induction may thus be attributable to the continuing development of brain edema at this time point.
In conclusion, our findings suggest that exogenous FGF treatment reduced RhoA activity via FGFR-induced activation of the PI3K-Akt-Rac1 signaling pathway, thus providing BBB integrity, and thereby attenuating the secondary brain injury after experimental ICH in mice (Figure 8).
Highlights.
FGFs inhibit RhoA via FGFR-induced activation of the PI3K-Akt-Rac-1 pathway.
RhoA, leads to disintegration of endothelial adherens junctions after ICH.
FGFs antagonize this effect and thereby reduce perihematomal brain edema.
Acknowledgments
Acknowledgement and sources of funding
This study was partially supported by NIH grant RO1NS053407 to J.H. Zhang.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: experience from preclinical studies. Neurol Res. 2005;27:268–79. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- Batjer HH, et al. Failure of surgery to improve outcome in hypertensive putaminal hemorrhage. A prospective randomized trial. Arch Neurol. 1990;47:1103–6. doi: 10.1001/archneur.1990.00530100071015. [DOI] [PubMed] [Google Scholar]
- Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocrine reviews. 2005a;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Bottcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005b;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Broderick JP, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30:905–15. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- Elliott J, Smith M. The acute management of intracerebral hemorrhage: a clinical review. Anesth Analg. 2010;110:1419–27. doi: 10.1213/ANE.0b013e3181d568c8. [DOI] [PubMed] [Google Scholar]
- Ellsworth JL, et al. Fibroblast growth factor-18 reduced infarct volumes and behavioral deficits after transient occlusion of the middle cerebral artery in rats. Stroke. 2003;34:1507–12. doi: 10.1161/01.STR.0000071760.66720.5F. [DOI] [PubMed] [Google Scholar]
- Garcia JH, et al. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–34. doi: 10.1161/01.str.26.4.627. discussion 635. [DOI] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–9. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamura S, et al. Basic fibroblast growth factor in the bone microenvironment enhances cell motility and invasion of Ewing’s sarcoma family of tumours by activating the FGFR1-PI3K-Rac1 pathway. British journal of cancer. 2010;103:370–81. doi: 10.1038/sj.bjc.6605775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami N, et al. Effect of local administration of basic fibroblast growth factor against neuronal damage caused by transient intracerebral mass lesion in rats. Brain Res. 1995;697:104–11. doi: 10.1016/0006-8993(95)00787-q. [DOI] [PubMed] [Google Scholar]
- Komarova Y, Malik AB. Regulation of Endothelial Permeability via Paracellular and Transcellular Transport Pathways. Annual Review of Physiology. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- Lee JG, Kay EP. PI 3-kinase/Rac1 and ERK1/2 regulate FGF-2-mediated cell proliferation through phosphorylation of p27 at Ser10 by KIS and at Thr187 by Cdc25A/Cdk2. Invest Ophthalmol Vis Sci. 2011;52:417–26. doi: 10.1167/iovs.10-6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KR, et al. Intracerebral infusion of thrombin as a cause of brain edema. J Neurosurg. 1995;83:1045–50. doi: 10.3171/jns.1995.83.6.1045. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Experimental Neurology. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Ma Q, et al. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J Cereb Blood Flow Metab. 2011a;31:881–93. doi: 10.1038/jcbfm.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, et al. PDGFR-α inhibition preserves Blood-brain barrier after intracerebral hemorrhage. Ann Neurol. 2011b doi: 10.1002/ana.22549. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan CL, et al. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab. 2008;28:516–25. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- Manaenko A, et al. Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. Journal of Neuroscience Methods. 2011;195:206–210. doi: 10.1016/j.jneumeth.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, et al. Evidence for apoptosis after intercerebral hemorrhage in rat striatum. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000;20:396–404. doi: 10.1097/00004647-200002000-00022. [DOI] [PubMed] [Google Scholar]
- Murakami M, et al. The FGF system has a key role in regulating vascular integrity. The Journal of Clinical Investigation. 2008;118:3355–3366. doi: 10.1172/JCI35298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega S, et al. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proceedings of the National Academy of Sciences of the United States of America. 1998a;95:5672–7. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega S, et al. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci U S A. 1998b;95:5672–7. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta M, et al. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine & Growth Factor Reviews. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, et al. Intracerebral haemorrhage. Lancet. 2009;373:1632–44. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA, et al. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801–7. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- Saha JK, et al. Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp Biol Med. 2005;230(10):777–84. doi: 10.1177/153537020523001012. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Ebina T. Sequential changes in tissue surrounding ICH. Spontaneous Intracerebral Hematomas. 1980:121–128. [Google Scholar]
- Tang J, et al. Mmp-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J Cereb Blood Flow Metab. 2004;24:1133–45. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, et al. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87:335–40. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- Wang J, et al. The development of an improved preclinical mouse model of intracerebral hemorrhage using double infusion of autologous whole blood. Brain Res. 2008;1222:214–21. doi: 10.1016/j.brainres.2008.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak-Stothard B, et al. Rho and Rac but not Cdc42 regulate endothelial cell permeability. Journal of cell science. 2001;114:1343–55. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- Wu B, et al. Ac-YVAD-CMK Decreases Blood-Brain Barrier Degradation by Inhibiting Caspase-1 Activation of Interleukin-1beta in Intracerebral Hemorrhage Mouse Model. Transl Stroke Res. 2010;1:57–64. doi: 10.1007/s12975-009-0002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi G, et al. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89:991–6. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- Xi G, et al. Mechanisms of brain injury after intracerebral haemorrhage. Lancet neurology. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]