Introduction
Particulate matter (PM) has been epidemiologically associated with death and cardiopulmonary illnesses in the elderly and in those individuals with pre-existing respiratory conditions (e.g., smokers, asthmatics, etc.) (Brook et al. 2010; Krewski et al. 2009; Pope and Dockery 2006). Numerous animal studies indicate that inhalation of PM results in inflammatory toxicity in the airways and cardiovascular effects (Chen et al. 2010; deHaar et al. 2006; Maier et al. 2008; Oberdorster et al. 2000). Although the historical focus of PM toxicity has been on these cardiopulmonary targets, it is now appreciated that inhaled nano-size particulates can quickly exit the lungs and enter the circulation where they distribute (i.e., liver, kidneys, testes, lymph nodes, brain) (Kreyling et al. 2004; Oberdorster et al. 2002a; Oberdorster et al. 2002b) and these organ systems through oxidative stress (Pohjola et al. 2003; Samet et al. 2004; Schulz 2006).
Because of its high energy demands and its inherently low level of endogenous scavengers (e.g., vitamin C, catalase, superoxide dismutase etc.) the brain is extremely sensitive to free radical damage (Halliwell 1992; Tanaka 1997). In the central nervous system (CNS), oxidative stress is largely mediated by microglia, which are macrophage-like, phagocytic cells that are activated by a broad range of stimuli, including PM (Block et al. 2004; Sama et al. 2007) and nanoparticles (Long et al. 2006). Once activated, the microglia produce multiple reactive oxygen species (ROS) such as hydrogen peroxide, hydroxyl radicals and peroxy-nitrites (Block et al. 2007; Fariss et al. 2005) that can diffuse from their plasma membrane and damage nearby neurons. Dopaminergic neurons are especially vulnerable to oxidative stress damage since they support a high synaptic network within the mesencephalic brain (striatum) and because dopamine metabolism in itself generates ROS (Double et al. 2010; Gao et al. 2002; Gonzalez-Hernandez et al. 2010; Jackson-Lewis and Smeyne 2005; Mitchell et al. 1999). The striatum is a brain area, which houses large populations of dopaminergic neurons and a disproportionately large population of microglia (Hirsch 1994; Koutsilieri et al. 2002).
The major components of PM include transition metals, sulfate and nitrate ions, organics, minerals, adsorbed gases, and biocontaminants (e.g., endotoxins, mold, pollen etc.,), attached to a core of carbonaceous material. The size of the particulates found in PM has been inversely related to its inflammatory damage (deHaar et al. 2006; Oberdorster 1996; Pedata et al. 2010; Valavanidis et al. 2008), with smaller, ultrafine size particles being more toxic to the target tissue. Mechanisms to explain this phenomenon may be physiological and/or physicochemical in nature The current study examines if this phenomenon (size-dependent toxicity) can be reproduced in isolated dopaminergic neurons (N27) and cultures dissociated from the embryonic rat brain striatum sized-PM material.
Materials and Methods
Collection of size-fractionated ambient PM Samples
The PM samples were collected over a two week period in August 2005, within the Sterling Forest State Park (Tuxedo, NY). The park is a largely undeveloped woodland park located approximately 50 km north-west of Manhattan. It is bordered by a lightly traveled two-lane road and there are no large power generators or industrial operations within 20 miles of the collection site. The ambient air in Sterling Forest is considered as regional background PM and is representative of the ambient air over the Virginia-Maine megalopolis (Maciejczyk and Chen 2005). The air samples were collected using the Multi-Orifice Uniform Deposit Impactor (MOUDI) (MSP, St. Paul, MN) which size-classifies the PM material as it is collected. Sized-samples were classified as Accumulation Mode Fine (AMF) (>0.18.–1 μm) or Ultrafine (UF) (<0.18 μm). The ambient air in Sterling Forest is considered as regional background PM and is representative of the ambient air over the Virginia-Maine megalopolis. The volume-size distribution of ambient air collected in this area of the park during the spring and summer is bimodal with mean diameters in both the AMF and UF range. The volume-size distribution of ambient air collected from Sterling Forest are described in detail in (Maciejczyk et al. 2005).
Samples were collected on pre-weighed Teflon filters and then immersed in Dubelco's Minimal Essential Media to yield a concentration of 500 μg/ml. Aliquots of these suspensions were diluted to stock concentrations for in vitro exposure. Care was taken not to sonicate or vigorously vortex the suspensions to preserve their original size at the time of collection.
Analyses for 35 elements were performed New York University's A. J. Lanza Laboratory by non-destructive XRF (ModelEX-6600-AF; Jordan Valley) using five secondary fluorescers (Si, Ti, Fe, Ge, and Mo), and spectral software XRF2000v3.1 (US EPA and ManTech Environmental Technology). The major components in the test samples consisted of sulfur (most likely as secondary aerosol particles) and elements associated with soil, (e.g., Si, Al, Ca, and Fe) (data not shown). A detailed composition analysis of the ambient samples collected at Sterling Forest has been reported previously (Maciejczyk and Chen 2005).
Cells and Culture Maintenance
The PM samples were tested on N27 neurons, an immortalized dopaminergic immortalized neuron from the mesencephalic brain of wild type, Sprague-Dawley rats (Zhou et al. 2000). N27 neurons were grown in RPMI 1640 media, supplemented with 10% fetal calf serum and 1% penicillin-streptomycin (ATCC, Manassas, VA). The N27 neurons were plated in 96 well plates (Costar, Corning, Inc., Corning, NY) and grown to ∼85% confluency before exposure to the individual particulate samples. All cell culture reagents were purchased from InVitrogen, Carlsbad, CA.
Primary cultures of Sprague Dawley embryonic striatum were purchased from BrainBits™ (http://www.brainbitsllc.com). Upon receipt the tissues were triturated in Neurobasal™, a serum-free media supplemented with glutamine, plated on poly-lysine-coated BTM 96 well plates (Nagle Nunc International-NUNC™, Rochester, NY), and grown to 85% confluency before exposure.
Neuronal Assays
To examine if PM was neurotoxic to isolated neurons, N27 cells were exposed to non-sonicated aliquots of AMF and UF (12.5, 25, and 50 μg/ml) for 24 hr. After exposure, cells were washed multiple times to remove extraneous particles. Neuronal loss was measured with Hoechst 33258, a nuclear stain that binds to the adenine-thymine rich regions of double stranded nuclear DNA. After exposure, cells were washed with Hank's basic salt solution, and then lysed by alternating temperatures (1 hr at 37° C followed by 1 hr -80°C). Hoechst reagent (2.0 μg/ml) was added to each well for 30 min and the plates spectrophotometrically read at 346/460nm wavelengths.
Reactive nitrogen species and nitric oxide are important physiological messengers in neuronal and cardiovascular tissues (Halliwell 1992; Mohanakumar et al. 2002). Inducible nitric oxide is generated from microglia (Chandra et al., 2000; Fariss et al., 2005) and can be measured with nitrite, a primary and stable breakdown product of nitrous oxide (Hengartner, 2000). Nitrite was measured in complex brain (striatum) cultures using Griess reagent under acidic (pH 4.0) conditions (Miranda et al. 2001). Plates were read using chemiluminescence.
For all spectrophotometric measurements, cells were plated in either black (fluorescence) or white (chemiluminescence) 96-well culture plates (Corning Inc., Corning, NY) to minimize light scatter. Spectrophotometers (Molecular Device, Sunnyvale, CA) included the Spectramax EM (fluorescence) and Lmax II 384 (chemiluminescence) plate readers.
Immunohistochemistry
Neurons in the striatum cultures were selectively stained with (mouse, rat, human) neuron specific enolase (NSE) (Dako Inc., Glostrup, Denmark, www.dako.com) at a 1:200 concentration according to protocol. For visualization, cells were biotinylated, linked to Streptavidin and counter-stained with a chromagen substrate according to directions given in the LSAB 2 System-HRP (Dako Inc). Support reagents were purchased from Sigma (St. Louis, MO).
Morphometry
Semi-confluent cultures (n=3 wells) of embryonic brain striatum were exposed to AMF (80 μg/ml) or UF (8 μg/ml) for 24 hr. After exposure, cultures were fixed in 3.7% formalin, immunocytochemically stained, and analyzed morphometrically for neuronal loss. For this, six photographs were taken of each well (10× apocromatic lens) using a Nikon TE300 inverted microscope fitted with a cooled-frame CCD camera (Orca I, Hamamatsu, Inc.). Each digitized image was analyzed using Metamorph 7.0 software (Molecular Device, Sunnyvale, CA). Threshold levels of pixel stain were used to quantitate the Total Area (i.e., neuronal cell bodies and axonal plexus) of NSE staining and the values binned according to area. Shape and size parameters were also defined to describe the neuronal cell body alone and applied to the Total Area data set using the Integrated Morphometric Analysis mode. Values for the Total Area and those measuring the neuronal cell bodies were collected in AMF (80 μg/ml and UF (8 μg/ml) or) treated cultures.
Statistical Analysis
All data were collected using SoftMax Pro 4.8 software (Molecular Devices, Sunnyvale, CA). Graphing was performed with GraphPad Prism 4.02. Data were analyzed using a one-way ANOVA with Dunnett's post-testing to determine the lowest statistically significant treatment relative to media control. Statistically significant difference is indicated (#) on the graph (p ≤ 0.05) and described in the figure legends.
Results
Direct neurotoxicity
To determine if PM was neurotoxic in the absence of other cell types, dopaminergic neurons (N27) were exposed to AMF or UF (12.5, 25, 50 μg/ml) samples for 24 hr. Significant neuronal loss (as measured by Hoechst nuclear stain) occurred in response to all tested concentrations of UF (≥12.5 μg/ml) but only in response to the highest concentration of AMF (50 μg/ml), suggesting a size-dependent neurotoxicity in isolated neurons (Figure 1).
Figure 1.
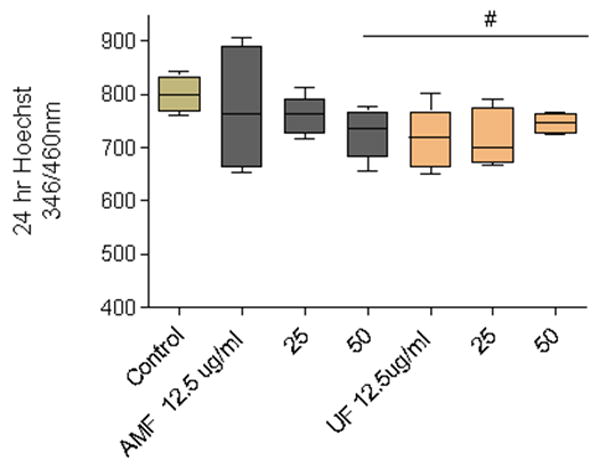
Loss of nuclear material in N27 isolated dopaminergic neurons was measured using the Hoechst fluorescent probe which binds nuclear adenine-thymine rich regions of double stranded DNA. Cells were exposed to AMF or UF (12.5 μg/ml - 50 μg/ml) for 24 hr. Significant cellular decreases were observed in N27 cultures exposed to AMF (50 μg/mL) and UF (≥12.5 μg/ml). (#, p<0.05, Dunnett's correction).
Microglia-mediated neurotoxicity
To examine if this relationship was altered in the presence of other brain cell types (e.g., microglia), primary cultures of embryonic rat striatum were exposed to either AMF (80 μg/ml) or UF (8.0 μg/ml). Equivalent increases of nitrite were measured by chemiluminescence after 24 hr exposure (Figure 2A). Microscopic examination indicated that the larger neuronal cell bodies in both the AMF and UF - treated neurons showed an apoptotic morphology (i.e., swollen cell body, fragmented nuclear material, centrally located nuclei, blebbing etc.) (Chandra et al., 2000; Fariss et al., 2005; Hengartner, 2000) (Figure 2B-D). A histogram of Total Area (neurons and axons) indicated that NSE-staining was most reduced in the larger size bins 110-130 (Figure 3A). When size and shape parameters specific for the larger neurons were applied to the analysis, a significant reduction of NSE staining occurred only in cultures treated with UF, relative to controls (Figure 3B).
Figure 2.
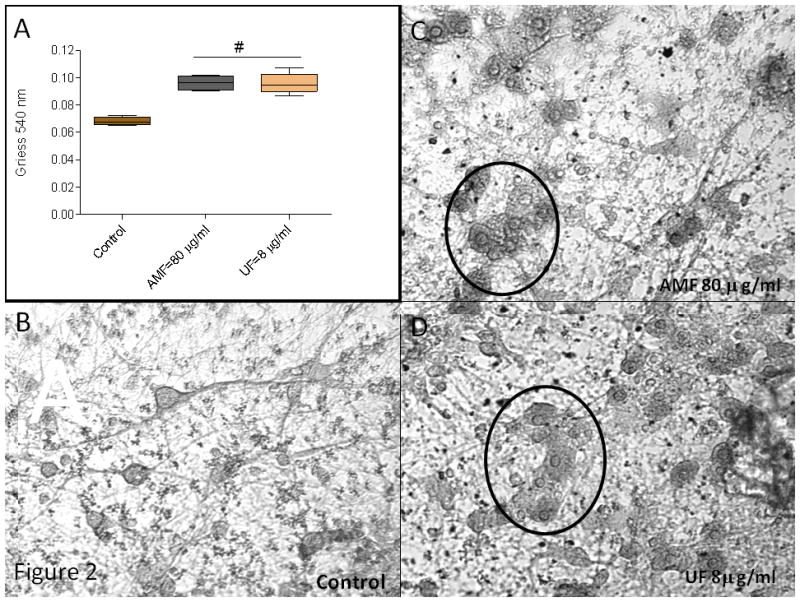
(A) Microglia-generated nitrite was measured in the supernatant of media or PM exposed striatal cultures using the Griess Reagent. Significant increases over controls were measured in both AMF and UF supernatants (#, p<0.01, one way ANOVA, Dunnett's correction). (B-D) Primary cultures of embryonic rat striatum were immunohistochemically stained with NSE. Microscopic examination indicated that the majority of AMF and UF exposed larger diameter neurons were apoptotic (circles) after 24hr exposure.
Figure 3.
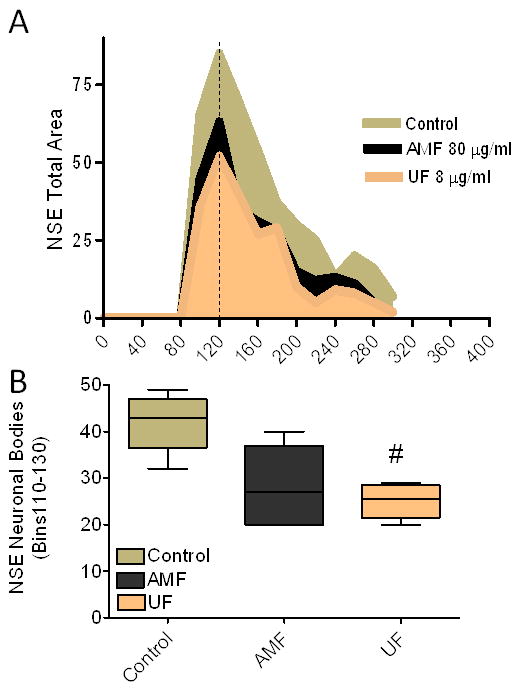
(A) Morphometric analysis of total NSE stain area (neuron plus axonal plexi) indicated peak reductions in bins 110-130 in cultures treated with either AMF or UF particles, relative to controls after 24 hr. (B) Parameters which described the neuronal (cell body alone) population were used to measure NSE staining in bins of peak reduction. Only the UF treated cultures showed significant reduction of stain (#, p<0.05, One way ANOVA, Dunnett's p corrected) relative to controls. Photos were shot using a 10× apocromatic lens on a Nikon TE300 inverted microscope fitted with a cooled-frame CCD camera (Orca I, Hamamatsu, Inc.). Each digitized image was analyzed using Metamorph 7.0 software (Molecular Device, Sunnyvale, CA).
Discussion
The possibility that the brain might also be targeted by PM was first raised in an editorial (Oberdorster and Utell 2002). This was followed by reports showing that nanosize particles could cross the blood brain barrier (Lockman et al. 2004) and physically enter the central nervous system of animals in small numbers (Kreyling et al. 2004; Oberdorster et al. 2002a; Oberdorster et al. 2002b; Oberdorster et al. 2004; Takenaka et al. 2001). Histological evidence of PM-related neurodegeneration was first described in the olfactory and respiratory nasal mucosa, olfactory bulb, and cortex of feral dogs living in Mexico City (Calderon-Garciduenas et al. 2003). In these tissues, alterations of the blood brain barrier, degenerated cortical neurons, apoptotic myelin producing cells (i.e., oligodendrocytes), and neurofibrillary tangles were noted. Immunohistochemical expressions of oxidative stress-mediated damage were found, including increased staining of NF-kB and inducible nitric oxide. Unfortunately, since the highly polluted Mexico City ambient air contained numerous atmospheric contaminants, the authors could only suggest a linkage of the pathology to the unspecified air pollutants.
Shortly after the above reports were published, experiments were designed by our laboratory to determine if animals exposed to PM under experimental conditions would show neuropathological damage. The Apo E -/- “knock-out” mouse strain and its normal C57/Blk6 background strain were exposed by inhalation to concentrated ambient particulate matter (CAPs) collected from the Sterling Forest State Park (Tuxedo, NY). After 6 month exposure, the brains of the oxidative stress-prone Apo E -/- were serially section and immunocytochemically stained for dopaminergic neurons. Morphometric analysis of neurons in the striatum indicated that neither air (control) nor CAPs-exposed C57/Blk6 normal mice showed significant reduction of stain. However, in the oxidative stress-prone Apo E -/- mice, CAPs exposure caused a 29% reduction in the immunocytochemically stained dopaminergic neurons of the striatum relative to the air exposed Apo E -/- mice (Veronesi et al. 2005).
Because of its critical role in oxidative stress-mediated neurodegeneration (Beal 2003; Fariss et al. 2005), immortalized microglial cells (BV2) from C57/Blk6 mice, were exposed to the same CAPs and their cellular and genomic response examined. The CAPs were first analyzed by XRP and ranked as high potency (HP-CAPs) or low potency (LP-CAPs) samples, depending on the presence of nickel and vanadium metals. Both samples reduced endogenous scavengers and both stimulated the release of pro-inflammatory cytokines TNFα and IL-6. However, the genomic response differed in magnitude and scope. Exposure of BV2 microglia to the nickel and vanadium enriched HP-CAPs activated numerous pro-apoptotic and inflammatory pathways such as Notch activating pathway for NF-kB signaling and up-regulated innate immune pathways involving the Toll-Like Receptors 2-8. In contrast, LP-CAPs stimulated pathways related to cellular maintenance and cellular division (Sama et al. 2007). These in vitro studies, coupled with the demonstration of neurodegeneration in the substantia nigra of Apo E -/- mice unequivocally defined the brain as a target of inhaled CAPs in oxidative stress-compromised mouse model, and helped to identify the vulnerable neuronal and microglia cellular targets. Numerous clinical and experimental studies have since identified the brain to be neurochemically and neuropathologically affected by various types and sizes of PM air pollution (Block et al. 2004; Calderon-Garciduenas et al. 2007; Calderon-Garciduenas et al. 2008; Campbell et al. 2005; Campbell et al. 2009; Gerlofs-Nijland et al. 2010; Sirivelu et al. 2006; Sunyer 2008; Zanchi et al. 2008).
Numerous studies (Araujo and Nel 2009; deHaar et al. 2006; Donaldson and Stone 2003; Henning et al. 2010; Kreyling et al. 2006; Oberdorster et al. 2002a; Oberdorster et al. 1994; Oberdorster 1996; Oberdorster et al. 2000; Pedata et al. 2010; Schmid et al. 2009; Terzano et al. 2010; Valavanidis et al. 2008) have reported that the cardiopulmonary mortality and morbidity associated with PM exposure appears to be inversely related to particle size, with ultrafine particles being more inflammatory and toxic than larger size PM. The current data, using cultures of nerve cells support this relationship. The reason for this size-dependent toxicity may be physiological and/or physicochemical. Most of the largest size PM particles are either swallowed or trapped by the nose hairs. Dosimetry studies indicate that those particles that physically enter the lungs deposit at different levels of the pulmonary lobes, depending on their size (Phalen and Mendez 2009). The inhaled ultrafine particles deposit to the lower pulmonary lobes where they have a longer retention time in the lung parenchyma and a lower clearance rate (Henning et al. 2010; Oberdorster et al. 1994; Pedata et al. 2010). The physical dimensions of the ultrafine particle gives it an exponentially higher “surface area to mass ratio” relative to larger size particles which allows them to accumulate and deliver higher “loads” of biocontaminants to target cells (Araujo and Nel 2009; Donaldson et al. 1996; Donaldson and Stone 2003; Kreyling et al. 2004; Kreyling et al. 2006; Phalen and Mendez 2009; Schmid et al. 2009).
Numerous studies have associated oxidative stress with particle toxicity (Allen 2008; Araujo and Nel 2009; Donaldson and Stone 2003; Hersoug et al. 2011; Li and Nel 2006; MohanKumar et al. 2008; Moller et al. 2010; Stone et al. 2007; Terzano et al. 2010; Xia et al. 2004; Xia et al. 2006; Zanchi et al. 2008). The current data indicate that in vitro, oxidative stress is not a prerequisite to neurotoxic damage since toxicity was produced in neurons and brain cultures dissociated from normal Sprague-Dawley rats. This study also reports that both AMF and UF samples of ambient PM produce biochemical and morphological evidence of apoptosis in the larger diameter neurons of the striatum. Evidence of apoptosis in human respiratory epithelia exposed to PM (Agopyan et al. 2003; Chin et al. 1998), in the myelin producing glia of feral dogs exposed to air pollutants in Mexico city (Calderon-Garciduenas et al. 2003), biochemical changes in the substantia nigra of animals (Villarreal-Calderon et al. 2010) and cellular and genomic expressions in BV2 microglia exposed to CAPs collected from Sterling Forest (Sama et al. 2007). Although neuronal death can occur by various morphological and biochemically distinct pathways, apoptosis results from disruption of mitochondrial bioenergetics and is largely an oxidative stress driven event (Chandra et al. 2000; Hengartner 2000). As noted in AMF and UF treated striatum, significant neuronal death was observed in the presence of both apoptosis and reactive nitrogen species. These data, coupled with the above literature suggest that microglia, which generate the nitrogen species and mediate oxidative stress in neurodegeneration, play a critical but as yet unexplored role in PM-neurotoxicity.
Highlights.
This in vitro study examines the response of isolated dopaminergic neurons and brain striatum cultures (Sprague-Dawley rat) to concentrated air pollutants (CAPs).
Evidence of particle-size dependent neurotoxicity is documented in both in vitro systems.
Evidence of oxidative stress damage is collected in both systems.
Neurotoxicity is enhanced in brain striatum cultures relative to isolated neurons exposed to CAPs.
Unlike in vivo models of CAPs Apo -/- neurodegeneration, oxidative stress is not critical to in vitro neurotoxicity.
Acknowledgments
Support for P. Gillespie and L.C. Chen provided by research Grant R01ES015495 from NIEHS, Center Grant # R827351 from the U.S.EPA, & by Center Grant #ES00260 from the NIEHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: This document has been reviewed by the EPA's National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute the endorsement of recommendation for use.
Reference List
- 1.Agopyan N, Bhatti T, Yu S, Simon SA. Vanilloid receptor activation by 2- and 10-microm particles induces responses leading to apoptosis in human airway epithelial cells. Toxicol Appl Pharmacol. 2003;192(1):21–35. doi: 10.1016/s0041-008x(03)00259-x. [DOI] [PubMed] [Google Scholar]
- 2.Allen J. Inhaled glutathione for the prevention of air pollution-related health effects: a brief review. Altern Ther Health Med. 2008;14(3):42–44. [PubMed] [Google Scholar]
- 3.Araujo JA, Nel AE. Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Part Fibre Toxicol. 2009;6:24. doi: 10.1186/1743-8977-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beal MF. Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Ann N Y Acad Sci. 2003;991:120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x. [DOI] [PubMed] [Google Scholar]
- 5.Block ML, Wu X, Pei Z, Li G, Wang T, Qin L, Wilson B, Yang J, Hong JS, Veronesi B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18(13):1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- 6.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nature Reviews/Neuroscience. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 7.Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 8.Calderon-Garciduenas L, Franco-Lira M, Torres-Jardon R, Henriquez-Roldan C, Barragan-Mejia G, Valencia-Salazar G, Gonzalez-Maciel A, Reynoso-Robles R, Villarreal-Calderon R, Reed W. Pediatric respiratory and systemic effects of chronic air pollution exposure: nose, lung, heart, and brain pathology. Toxicol Pathol. 2007;35(1):154–162. doi: 10.1080/01926230601059985. [DOI] [PubMed] [Google Scholar]
- 9.Calderon-Garciduenas L, Maronpot RR, Torres-Jardon R, Henriquez-Roldan C, Schoonhoven R, Acuna-Ayala H, Villarreal-Calderon A, Nakamura J, Fernando R, Reed W, Azzarelli B, Swenberg JA. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol. 2003;31(5):524–538. doi: 10.1080/01926230390226645. [DOI] [PubMed] [Google Scholar]
- 10.Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, Villarreal-Calderon R, Osnaya N, Stone I, Garcia R, Brooks DM, Gonzalez-Maciel A, Reynoso-Robles R, Delgado-Chavez R, Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol. 2008;36(2):289–310. doi: 10.1177/0192623307313011. [DOI] [PubMed] [Google Scholar]
- 11.Campbell A, Araujo JA, Li H, Sioutas C, Kleinman M. Particulate matter induced enhancement of inflammatory markers in the brains of apolipoprotein E knockout mice. J Nanosci Nanotechnol. 2009;9(8):5099–5104. doi: 10.1166/jnn.2009.gr07. [DOI] [PubMed] [Google Scholar]
- 12.Campbell A, Oldham M, Becaria A, Bondy SC, Meacher D, Sioutas C, Misra C, Mendez LB, Kleinman M. Particulate matter in polluted air may increase biomarkers of inflammation in mouse brain. Neurotoxicology. 2005;26(1):133–140. doi: 10.1016/j.neuro.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29(3-4):323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 14.Chen LC, Hwang JS, Lall R, Thurston G, Lippmann M. Alteration of cardiac function in ApoE-/- mice by subchronic urban and regional inhalation exposure to concentrated ambient PM2.5. Inhal Toxicol. 2010;22(7):580–592. doi: 10.3109/08958371003596579. [DOI] [PubMed] [Google Scholar]
- 15.Chin BY, Choi ME, Burdick MD, Strieter RM, Risby TH, Choi AM. Induction of apoptosis by particulate matter: role of TNF-alpha and MAPK. Am J Physiol. 1998;275(5 Pt 1):L942–L949. doi: 10.1152/ajplung.1998.275.5.L942. [DOI] [PubMed] [Google Scholar]
- 16.deHaar C, Hassing I, Bol M, Bleumink R, Pieters R. Ultrafine but not fine particulate matter causes airway inflammation and allergic airway sensitization to co-administered antigen in mice. Clin Exp Allergy. 2006;36(11):1469–1479. doi: 10.1111/j.1365-2222.2006.02586.x. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson K, Beswick PH, Gilmour PS. Free radical activity associated with the surface of particles: a unifying factor in determining biological activity? Toxicol Lett. 1996;88(1-3):293–298. doi: 10.1016/0378-4274(96)03752-6. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson K, Stone V. Current hypotheses on the mechanisms of toxicity of ultrafine particles. Ann Ist Super Sanita. 2003;39(3):405–410. [PubMed] [Google Scholar]
- 19.Double KL, Reyes S, Werry EL, Halliday GM. Selective cell death in neurodegeneration: why are some neurons spared in vulnerable regions? Prog Neurobiol. 2010;92(3):316–329. doi: 10.1016/j.pneurobio.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Fariss MW, Chan CB, Patel M, Van HB, Orrenius S. Role of mitochondria in toxic oxidative stress. Mol Interv. 2005;5(2):94–111. doi: 10.1124/mi.5.2.7. [DOI] [PubMed] [Google Scholar]
- 21.Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem. 2002;81(6):1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 22.Gerlofs-Nijland ME, van BD, Cassee FR, Schins RP, Wang K, Campbell A. Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part Fibre Toxicol. 2010;7:12. doi: 10.1186/1743-8977-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Hernandez T, Cruz-Muros I, Afonso-Oramas D, Salas-Hernandez J, Castro-Hernandez J. Vulnerability of mesostriatal dopaminergic neurons in Parkinson's disease. Front Neuroanat. 2010;4:140. doi: 10.3389/fnana.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59(5):1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- 25.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 26.Henning A, Schneider M, Nafee N, Muijs L, Rytting E, Wang X, Kissel T, Grafahrend D, Klee D, Lehr CM. Influence of particle size and material properties on mucociliary clearance from the airways. J Aerosol Med Pulm Drug Deliv. 2010;23(4):233–241. doi: 10.1089/jamp.2009.0806. [DOI] [PubMed] [Google Scholar]
- 27.Hersoug LG, Brasch-Andersen C, Husemoen LL, Sigsgaard T, Linneberg A. The relationship of glutathione-S-transferases copy number variation and indoor air pollution to symptoms and markers of respiratory disease. Clin Respir J. 2011 doi: 10.1111/j.1752-699X.2011.00258.x. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch EC. Biochemistry of Parkinson's disease with special reference to the dopaminergic systems. Mol Neurobiol. 1994;9(1-3):135–142. doi: 10.1007/BF02816113. [DOI] [PubMed] [Google Scholar]
- 29.Jackson-Lewis V, Smeyne RJ. MPTP and SNpc DA neuronal vulnerability: role of dopamine, superoxide and nitric oxide in neurotoxicity. Minireview. Neurotox Res. 2005;7(3):193–202. doi: 10.1007/BF03036449. [DOI] [PubMed] [Google Scholar]
- 30.Koutsilieri E, Scheller C, Tribl F, Riederer P. Degeneration of neuronal cells due to oxidative stress--microglial contribution. Parkinsonism Relat Disord. 2002;8(6):401–406. doi: 10.1016/s1353-8020(02)00021-4. [DOI] [PubMed] [Google Scholar]
- 31.Krewski D, Jerrett M, Burnett RT, Ma R, Hughes E, Shi Y, Turner MC, Pope CA, III, Thurston G, Calle EE, Thun MJ, Beckerman B, DeLuca P, Finkelstein N, Ito K, Moore DK, Newbold KB, Ramsay T, Ross Z, Shin H, Tempalski B. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res Rep Health Eff Inst. 2009;(140):5–114. [PubMed] [Google Scholar]
- 32.Kreyling WG, Semmler M, Möller W. Dosimetry and toxicology of ultrafine particles. J Aerosol Med. 2004;17(2):140–152. doi: 10.1089/0894268041457147. [DOI] [PubMed] [Google Scholar]
- 33.Kreyling WG, Semmler-Behnke M, Moller W. Ultrafine particle-lung interactions: does size matter? J Aerosol Med. 2006;19(1):74–83. doi: 10.1089/jam.2006.19.74. [DOI] [PubMed] [Google Scholar]
- 34.Li N, Nel AE. Role of the Nrf2-mediated signaling pathway as a negative regulator of inflammation: implications for the impact of particulate pollutants on asthma. Antioxid Redox Signal. 2006;8(1-2):88–98. doi: 10.1089/ars.2006.8.88. [DOI] [PubMed] [Google Scholar]
- 35.Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood-brain barrier integrity and permeability. J Drug Target. 2004;12(9-10):635–641. doi: 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- 36.Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006;40(14):4346–4352. doi: 10.1021/es060589n. [DOI] [PubMed] [Google Scholar]
- 37.Maciejczyk P, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. VIII. Source-related daily variations in in vitro responses to CAPs. Inhal Toxicol. 2005;17(4-5):243–253. doi: 10.1080/08958370590912914. [DOI] [PubMed] [Google Scholar]
- 38.Maciejczyk P, Zhong M, Li Q, Xiong J, Nadziejko C, Chen LC. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. II. The design of a CAPs exposure system for biometric telemetry monitoring. Inhal Toxicol. 2005;17(4-5):189–197. doi: 10.1080/08958370590912743. [DOI] [PubMed] [Google Scholar]
- 39.Maier KL, Alessandrini F, Beck-Speier I, Hofer TP, Diabate S, Bitterle E, Stoger T, Jakob T, Behrendt H, Horsch M, Beckers J, Ziesenis A, Hultner L, Frankenberger M, Krauss-Etschmann S, Schulz H. Health effects of ambient particulate matter--biological mechanisms and inflammatory responses to in vitro and in vivo particle exposures. Inhal Toxicol. 2008;20(3):319–337. doi: 10.1080/08958370701866313. [DOI] [PubMed] [Google Scholar]
- 40.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5(1):62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell IJ, Cooper AJ, Griffiths MR. The selective vulnerability of striatopallidal neurons. Prog Neurobiol. 1999;59(6):691–719. doi: 10.1016/s0301-0082(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 42.Mohanakumar KP, Thomas B, Sharma SM, Muralikrishnan D, Chowdhury R, Chiueh CC. Nitric oxide: an antioxidant and neuroprotector. Ann N Y Acad Sci. 2002;962:389–401. doi: 10.1111/j.1749-6632.2002.tb04083.x. [DOI] [PubMed] [Google Scholar]
- 43.MohanKumar SM, Campbell A, Block M, Veronesi B. Particulate matter, oxidative stress and neurotoxicity. Neurotoxicology. 2008;29(3):479–488. doi: 10.1016/j.neuro.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Moller P, Jacobsen NR, Folkmann JK, Danielsen PH, Mikkelsen L, Hemmingsen JG, Vesterdal LK, Forchhammer L, Wallin H, Loft S. Role of oxidative damage in toxicity of particulates. Free Radic Res. 2010;44(1):1–46. doi: 10.3109/10715760903300691. [DOI] [PubMed] [Google Scholar]
- 45.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002a;65(20):1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- 46.Oberdorster G. Significance of particle parameters in the evaluation of exposure-dose-response relationships of inhaled particles. Inhal Toxicol. 1996;8(Suppl):73–89. [PubMed] [Google Scholar]
- 47.Oberdorster G, Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect. 1994;102(5):173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oberdorster G, Finkelstein JN, Johnston C, Gelein R, Cox C, Baggs R, Elder AC. Acute pulmonary effects of ultrafine particles in rats and mice. Res Rep Health Eff Inst. 2000;(96):5–74. [PubMed] [Google Scholar]
- 49.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16(6-7):437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 50.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, Kreyling W, Cox C. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002b;65(20):1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- 51.Oberdorster G, Utell M. Ultrafine particles in the urban air: To the respiratory tract-and beyond? Environ Health Perspect. 2002;110(8):A440–A441. doi: 10.1289/ehp.110-1240959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pedata P, Garzillo EM, Sannolo N. Ultrafine particles and effects on the body: review of the literature. G Ital Med Lav Ergon. 2010;32(1):23–31. [PubMed] [Google Scholar]
- 53.Phalen RF, Mendez LB. Dosimetry considerations for animal aerosol inhalation studies. Biomarkers. 2009;14(1):63–66. doi: 10.1080/13547500902965468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pohjola SK, Lappi M, Honkanen M, Savela K. Comparison of mutagenicity and calf thymus DNA adducts formed by the particulate and semivolatile fractions of vehicle exhausts. Environ Mol Mutagen. 2003;42(1):26–36. doi: 10.1002/em.10172. [DOI] [PubMed] [Google Scholar]
- 55.Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56(6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 56.Sama P, Long TC, Hester S, Tajuba J, Parker J, Chen LC, Veronesi B. The cellular and genomic response of an immortalized microglia cell line (BV2) to concentrated ambient particulate matter. Inhal Toxicol. 2007;19(13):1079–1087. doi: 10.1080/08958370701628721. [DOI] [PubMed] [Google Scholar]
- 57.Samet J, Demarini D, Malling H. Do airborne particles induce heritable mutations? Science. 2004;304:971–972. doi: 10.1126/science.1097441. [DOI] [PubMed] [Google Scholar]
- 58.Schmid O, Moller W, Semmler-Behnke M, Ferron GA, Karg E, Lipka J, Schulz H, Kreyling WG, Stoeger T. Dosimetry and toxicology of inhaled ultrafine particles. Biomarkers. 2009;14(1):67–73. doi: 10.1080/13547500902965617. [DOI] [PubMed] [Google Scholar]
- 59.Schulz H. Fine particulate matter - a health hazard for lungs and other organs? Pneumologie. 2006;60(10):611–615. doi: 10.1055/s-2006-954969. [DOI] [PubMed] [Google Scholar]
- 60.Sirivelu MP, MohanKumar SM, Wagner JG, Harkema JR, MohanKumar PS. Activation of the stress axis and neurochemical alterations in specific brain areas by concentrated ambient particle exposure with concomitant allergic airway disease. Environ Health Perspect. 2006;114(6):870–874. doi: 10.1289/ehp.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stone V, Johnston H, Clift MJ. Air pollution, ultrafine and nanoparticle toxicology: cellular and molecular interactions. IEEE Trans Nanobioscience. 2007;6(4):331–340. doi: 10.1109/tnb.2007.909005. [DOI] [PubMed] [Google Scholar]
- 62.Sunyer J. The neurological effects of air pollution in children. Eur Respir J. 2008;32(3):535–537. doi: 10.1183/09031936.00073708. [DOI] [PubMed] [Google Scholar]
- 63.Takenaka S, Karg E, Roth C, Schulz H, Ziesenis A, Heinzmann U, Schramel P, Heyder J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ Health Perspect. 2001;109(4):547–551. doi: 10.1289/ehp.01109s4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanaka M. Oxidative stress and the brain. Nippon Ronen Igakkai Zasshi. 1997;34(9):706–710. [PubMed] [Google Scholar]
- 65.Terzano C, Di SF, Conti V, Graziani E, Petroianni A. Air pollution ultrafine particles: toxicity beyond the lung. Eur Rev Med Pharmacol Sci. 2010;14(10):809–821. [PubMed] [Google Scholar]
- 66.Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008;26(4):339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- 67.Veronesi B, Makwana O, Pooler M, Chen LC. Effects of subchronic exposures to concentrated ambient particles. VII. Degeneration of dopaminergic neurons in Apo E-/- mice. Inhal Toxicol. 2005;17(4-5):235–241. doi: 10.1080/08958370590912888. [DOI] [PubMed] [Google Scholar]
- 68.Villarreal-Calderon R, Torres-Jardon R, Palacios-Moreno J, Osnaya N, Perez-Guille B, Maronpot RR, Reed W, Zhu H, Calderon-Garciduenas L. Urban air pollution targets the dorsal vagal complex and dark chocolate offers neuroprotection. Int J Toxicol. 2010;29(6):604–615. doi: 10.1177/1091581810383587. [DOI] [PubMed] [Google Scholar]
- 69.Xia T, Korge P, Weiss JN, Li N, Venkatesen MI, Sioutas C, Nel A. Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: implications for ultrafine particle toxicity. Environ Health Perspect. 2004;112(14):1347–1358. doi: 10.1289/ehp.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xia T, Kovochich M, Nel A. The role of reactive oxygen species and oxidative stress in mediating particulate matter injury. Clin Occup Environ Med. 2006;5(4):817–836. doi: 10.1016/j.coem.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 71.Zanchi AC, Venturini CD, Saiki M, Nascimento Saldiva PH, Tannhauser Barros HM, Rhoden CR. Chronic nasal instillation of residual-oil fly ash (ROFA) induces brain lipid peroxidation and behavioral changes in rats. Inhal Toxicol. 2008;20(9):795–800. doi: 10.1080/08958370802009060. [DOI] [PubMed] [Google Scholar]
- 72.Zhou W, Hurlbert MS, Schaack J, Prasad KN, Freed CR. Overexpression of human alpha-synuclein causes dopamine neuron death in rat primary culture and immortalized mesencephalon-derived cells. Brain Res. 2000;866(1-2):33–43. doi: 10.1016/s0006-8993(00)02215-0. [DOI] [PubMed] [Google Scholar]


