Abstract
Polymeric nanoparticles-based therapeutics show great promise in the treatment of a wide range of diseases, due to the flexibility in which their structures can be modified, with intricate definition over their compositions, structures and properties. Advances in polymerization chemistries and the application of reactive, efficient and orthogonal chemical modification reactions have enabled the engineering of multifunctional polymeric nanoparticles with precise control over the architectures of the individual polymer components, to direct their assembly and subsequent transformations into nanoparticles of selective overall shapes, sizes, internal morphologies, external surface charges and functionalizations. In addition, incorporation of certain functionalities can modulate the responsiveness of these nanostructures to specific stimuli through the use of remote activation. Furthermore, they can be equipped with smart components to allow their delivery beyond certain biological barriers, such as, skin, mucus, blood, extracellular matrix, cellular and subcellular organelles. This tutorial review highlights the importance of well-defined chemistries, with detailed ties to specific biological hurdles and opportunities, in the design of nanostructures for various biomedical delivery applications.
Introduction
Nanomedicine can be defined as the design of diagnostics and/or therapeutics on the nanoscale, which provides advantages due to the high degree of coincident transport and delivery of the active species with mediation of their navigation within the biological systems for the treatment, prevention and diagnosis of diseases. The biological transport processes, anatomically and down to the cellular and sub-cellular levels, are affected by the physical attributes of the nanocarriers, including their size, shape, and flexibility, as well as their chemical characteristics, including for instance the incorporation of active ligands for recognition by and triggering of biological receptors. Therefore, it is of critical importance to utilize procedures that prepare nanostructures with high degrees of uniformity, and with control over their physical and chemical traits. Nanoparticles can be constructed from various materials (e.g. polymers, lipids, metals) and can host a wide range of active components, including chemotherapeutics, contrast agents, proteins and nucleic acids, for various biomedical applications. In particular, polymeric nanoparticles have received great interest due to the versatility in which their structures can be modified to package and deliver their cargoes to the desired site of action or to respond to specific physiological or external stimuli (Fig. 1 and 2). Incorporation of certain functionalities can modulate the responsiveness (assembly/disassembly) of the nanoparticles in biological environments under different pH, enzymatic, oxidative, and reductive conditions, etc., or in response to external stimuli, such as variation in temperature, irradiation with near-IR or UV-vis light, activation with magnetic fields, and application of ultrasound vibrations, etc. The chemistry of polymeric nanoparticles and their payloads affects their stability, biodegradability, biocompatibility, biodistribution and cellular and subcellular fate. The design of polymeric nanoparticles depends on the therapeutic application, target site (organs, tissues, cellular or subcellular organelles) and the route of administration. Although intravenous injection is the main route of administration for polymeric nanoparticles, there are other ways to deliver them via less invasive ways, such as, dermal/transdermal, oral and mucosal delivery. In all cases, these nanoparticles have to be equipped with smart components to allow their delivery beyond the different biological barriers, such as, skin, mucus, blood, extracellular matrix, in addition to the cellular and subcellular barriers.
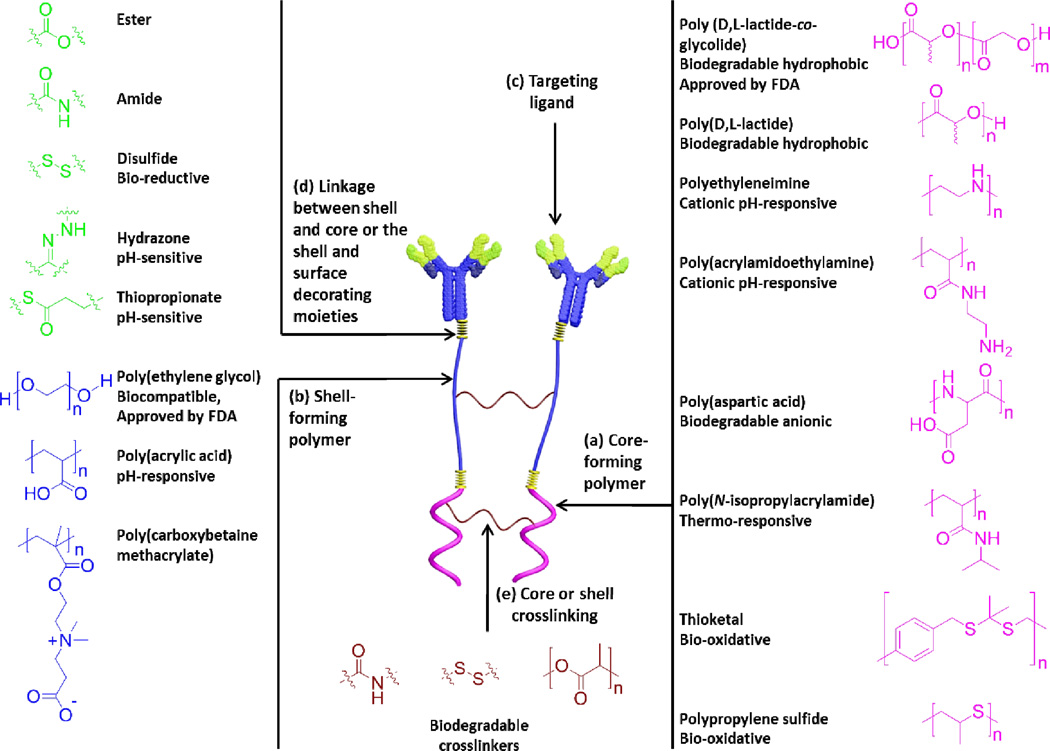
Fig. 1.
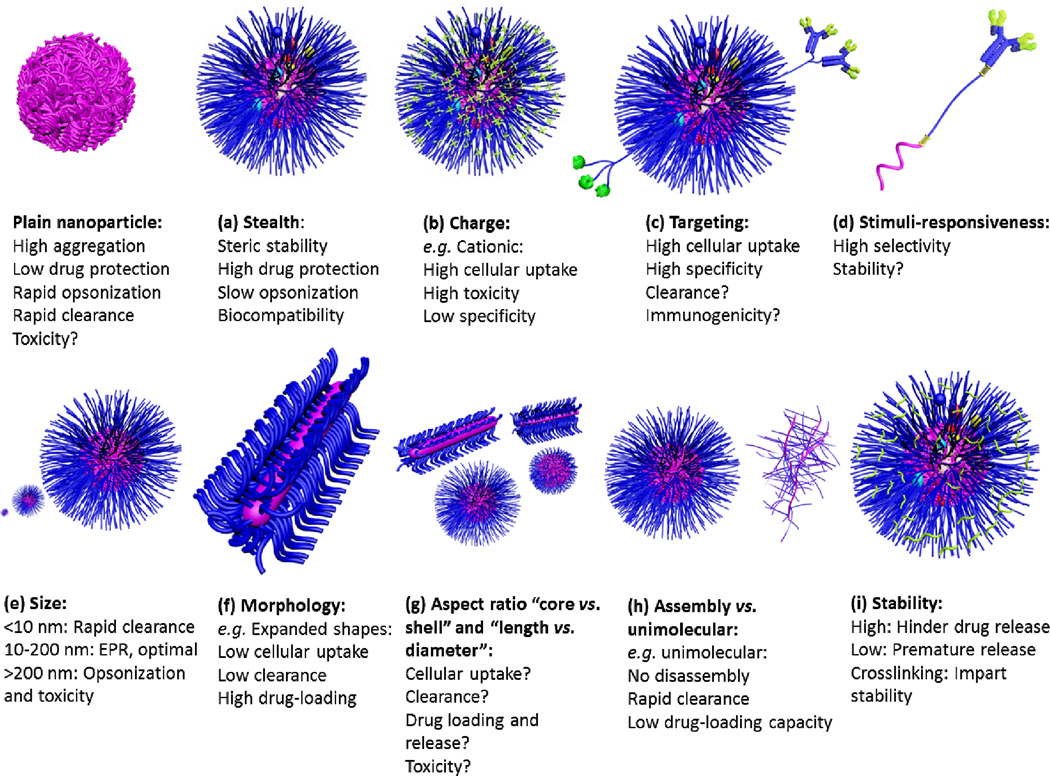
Building blocks of various types of polymeric nanoparticles with examples of some commonly used polymers and linkages. The main building blocks of polymeric nanoparticles are usually comprised of core-forming polymer; hydrophobic or charged (a), shell-forming polymer; neutral, hydrophilic and flexible properties are important for stealth nanoparticles (b), targeting ligand for selective cellular uptake and accumulation at target sites (c), and linkages between the shell and core and/or targeting moieties (d). Stimuli-responsiveness (pH, temperature, enzymatic, reductive or oxidative, etc.) can be imparted into the core, shell and/or the linkages. Shell or core-crosslinking can be also utilized to enhance the stability of nanoparticles (e).
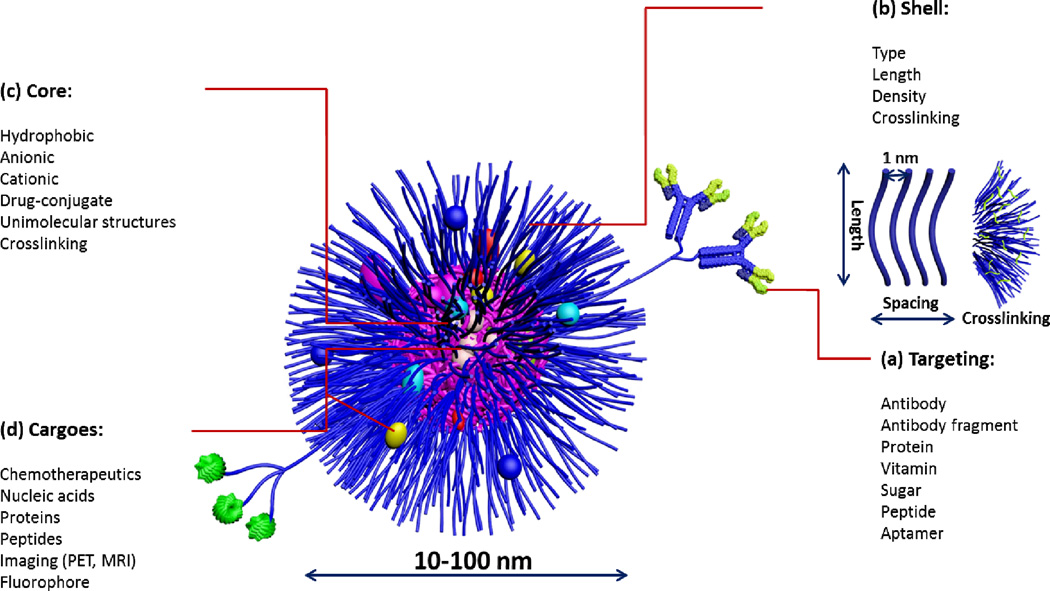
Fig. 2.
Composition of multifunctional nanoparticles for biomedical delivery applications: (a) clusters of targeting moieties have been shown to be important for multivalent binding to receptors for enhanced cellular uptake; the use of various ligands (antibody, antibody fragment, peptide) depends on the therapeutic application and disease type, (b) shell: length, spacing and crosslinking of the shell are critical parameters that dictate the blood cir culation time and stability of nanoparticles with ~1 nm spacing found to be efficient in preventing protein adsorption, (c) core: nature of the core dictates the type of the drug to be encapsulated. Crosslinking and conjugation of drugs to the core-forming polymer are common strategies for enhancing the stability of nanoparticles and drug-encapsulation efficiency, respectively. (d) drug: a wide range of therapeutics can be used ranging from small molecules to macromolecular cargoes.
Polymeric nanoparticles-based therapeutics are being developed to improve the diagnosis and treatment of a wide range of diseases, ranging from cancer, viral infections, cardiovascular diseases to pulmonary and urinary tract infections. Advances in controlled polymerization have enabled the engineering of advanced multifunctional polymeric nanoparticles with precise control over architecture, shape, size, surface charge and functionalization. The careful design and control over the targeting properties of these nanoparticles will secure their future development and versatility. The proper selection of the chemistry of the building blocks of these nanocarriers and their payload can drastically impact their safety, pharmacokinetics and intracellular fate. Our group has recently reviewed the application of orthogonal chemistry in the synthesis, preparation and functionalization of polymeric nanostructures of different architectures1 and the available technologies to precisely control the self assembly and stability of these nanostructures.2 This review highlights the importance of well-defined chemistries in the design of nanostructures to surmount certain biological barriers for various biomedical applications. In particular, the underlying potential, current challenges, and future directions of polymeric micelles, crosslinked knedel-like nanoparticles and unimolecular/hyperbranched nanostructures will be discussed.
Biological barriers
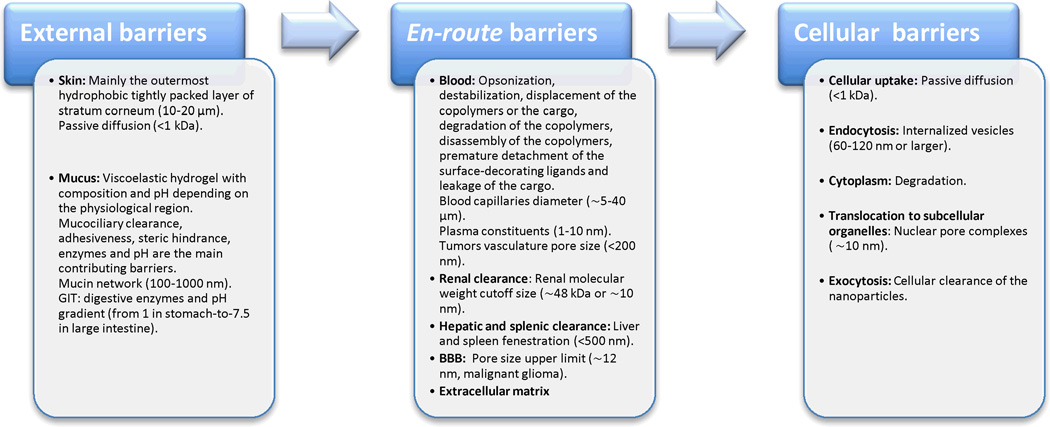
For therapeutic benefits, the drugs, either free or loaded into nanoparticles, have to reach to their destinations (i.e. sites of action), which are on the cellular or molecular levels. The barriers towards the delivery of these nanoparticles can be classified into external barriers (skin and mucosa), en-route (blood and extracellular matrix) and cellular barriers (the limited cellular uptake, endosomal/lysosomal degradation and the inefficient translocation to the targeted subcellular organelles) (Fig. 3).
Fig. 3.
Barriers towards the delivery of polymeric nanoparticles can be classified into external barriers (skin and mucosa), en-route barriers (mainly destabilization and clearance in the blood and the extracellular matrix) and cellular and subcellular barriers.
External barriers
The body surface is covered and protected with either skin or mucus. Both skin and mucus hinder polymeric nanoparticles from reaching to their target sites that are located either locally in the underlying tissues or systemically in the blood. Although skin and mucus have different structures, both of them can prevent the uptake of the polymeric nanoparticles through different mechanisms. In addition to hindering the uptake of polymeric nanoparticles, they may alter the surface characteristics and stability of the nanoparticles before they are able to reach the surface of the underlying tissues.
Skin consists of several layers of different thicknesses and structures, stratum corneum (SC), epidermis, dermis and subcutaneous tissues.3 The outermost layer, SC, is a highly-organized and hydrophobic structure consisting of several layers (10–20 µm) of terminally-differentiated nonliving corneocytes embedded in intercellular lipid matrix-forming bilayers. Corneocytes contain intracellular crosslinked macrofibrillar bundles of keratin giving the SC a rigid and hydrophobic structure. The SC is considered as the major permeability barrier and the rate-limiting step for the delivery through the skin. The viable epidermis (50–100 µm) and dermis are immunologically-active sites consisting mainly of keratinocytes and fibroblasts, respectively, and rich in immune cells, such as, Langerhans cells in the epidermis and dendritic cells in the dermis. The dermis and subcutaneous tissues (1–2 mm) are rich in blood vessels that can be used for the systemic (i.e. transdermal) delivery. Disrupting the lipid bilayers of the SC is utilized commonly to enhance the permeation through the skin.
Mucus coats regions that are not covered by the skin, including the gastrointestinal tract, eyes, lung airways, nasal, rectal and vaginal cavities. Mucus is a viscoelastic hydrogel secreted by the mucosal glands to protect the cells below. The composition, vascularity, surface area, thickness, permeability, enzymatic activity and pH of mucus vary from one region to another (e.g. respiratory vs. vaginal) and depend on the disease status (e.g. mucus becomes thick and sticky in patients with cystic fibrosis).4 The mucus gel is mainly comprised of a network of crosslinked mucin fibers. These fibers are composed of alternating glycosylated hydrophilic regions and relatively hydrophobic regions. Mucin is negatively-charged due to the presence of N-acetylneuramic acids (sialic acids) and sulfated monosaccharides in the sugar chains. Mucus gels are also loaded with cells, bacteria, lipids, salts, proteins, macromolecules, and cellular debris. The various components work together to form a nanoscopic network that hinders nanoparticle transport. Mucociliary clearance is a major reason for the clearance of nanoparticles. Other contributing factors include the adhesiveness and enzymatic activity of the mucus gel and steric hindrance by the nanoscopic layer. In addition, binding of mucus ingredients to the surface of nanoparticles may result in their destabilization and aggregation or charge neutralization and displacement of their cargoes. Other factors can also complicate mucosal delivery, depending on the delivery route. For instance, oral delivery is challenging due to the harsh conditions that nanoparticles experience as they transit from the stomach to the intestine, such as, the pH gradient from 1–3.5 in the stomach, 5–7 in the small intestine to 6–7.5 in the large intestine and digestive enzymes, which can destabilize or degrade a wide range of polymers and therapeutics.
En-route barriers
Injection of polymeric nanoparticles into the systemic circulation circumvents the skin and mucosal barriers. However, injection is an invasive and unfavorable way of administration because it raises the requirements of quality control, such as sterility, increases the cost, lowers the patient compliance and places a burden on both the patients and caregivers. In addition, nanoparticles in the blood are subject to renal and hepatic clearance, destabilization, aggregation, opsonization and clearance by the mononuclear phagocytic system (MPS) (Fig. 4). During circulation, nanoparticles can extravasate or distribute into the various body tissues and organs. During the filtration of the blood in the kidney, some of the blood is reabsorbed into the circulation, whereas a fraction is processed to secrete components into the urine. The excretion of nanoparticles into the urine depends on the characteristics of nanoparticles. Hepatic clearance of nanoparticles, into bile, and then into feces is another route of excretion from the body. The adsorption of plasma proteins and interactions with other components in the blood dictate the fate of the nanoparticles. Opsonization is considered as one of the major barriers to nanoparticle stability and delivery in vivo. The most recognized opsonins are immunoglobulins, complement proteins, albumin, apolipoprotein and fibrinogen.5 These proteins of different sizes adsorb on the surface of nanoparticles and tag them for attack by the MPS. MPS is a part of the immune system that consists of phagocytic cells, such as blood monocytes and macrophages accumulated in lymph nodes, spleen and other tissues (e.g. Kupffer cells in the liver). These cells roam through the body and act as scavengers to attack and engulf foreign particles, when they are tagged by the appropriate opsonin. The opsonization of nanoparticles and subsequent clearance by MPS could also initiate severe immunological reactions. In addition, the adsorption of these proteins can potentially destabilize nanoparticles and lead to the premature release of their payloads. The released drug loses the favorable pharmacokinetics of the nanocarrier and becomes then available to induce toxicity. Plasma proteins can also bind to or displace the encapsulated drug. The abilities of proteins to destabilize nanostructures are often measured, for instance a series of proteins (e.g. albumin, α- and β-globulins, γ-globulins) were tested with polymeric micelles (e.g. poly(ethylene glycol) (PEG)-b-poly(propyl methacrylate-co-methacrylic acid)/poly(amidoamine) and PEG-b-poly(D,L-lactide)),6–8 for which it was observed that, although most proteins were found to contribute to micelle destabilization, a more significant effect was observed for α- and β-globulins. However, destabilization of nanoparticles may also result from interactions with other molecules in blood (e.g. blood cells) and the degradation of the polymeric constituents of the nanoparticle. In tissues, binding and interactions with extracellular matrix and immune cells can also hinder nanoparticles from reaching their sites of action. The extent of opsonization, destabilization and clearance depends mainly on the nanoparticle characteristics and will be discussed in detail in the following sections.
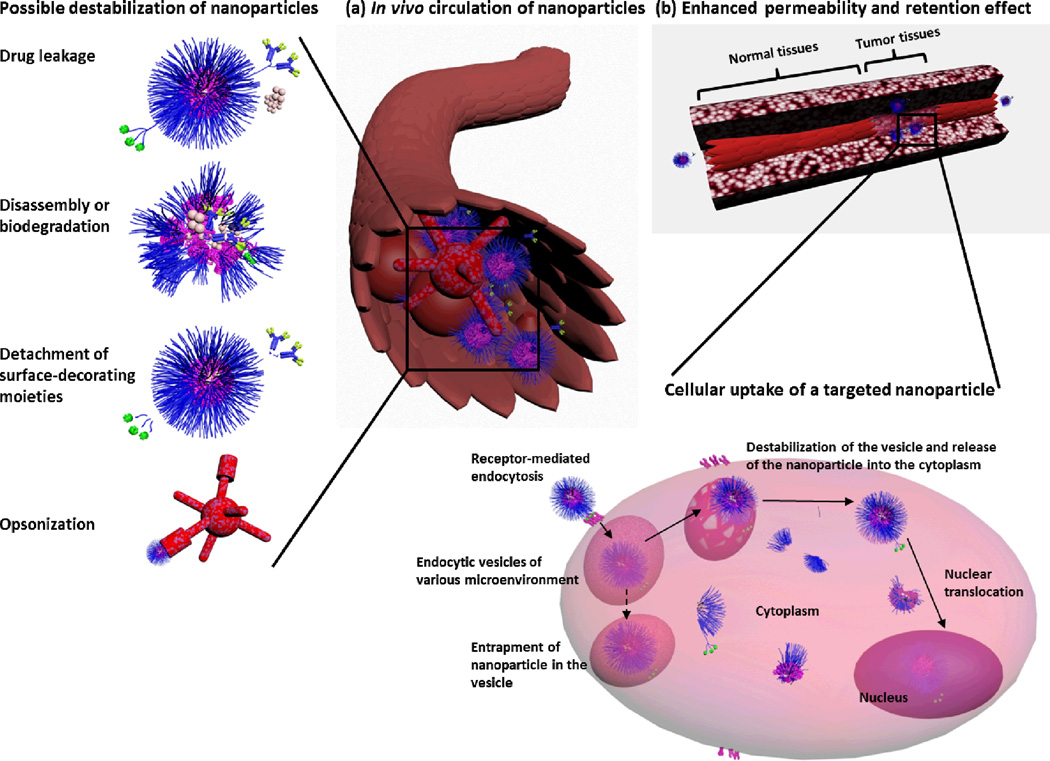
Fig. 4.
Possible destabilization and degradation pathways of polymeric nanoparticles during in vivo circulation (a) and the EPR effect and intracellular fate of nanoparticles (b). Drug-leakage, disassembly or degradation, detachment of surface-decorating moieties, opsonization and clearance of nanoparticles during circulation can all be detrimental to the efficiency of nanoparticles. Tumor tissues are characterized by the leaky vasculature that allows nanoparticles to accumulate in the tumor tissues. The endocytosis of the nanoparticles can then occur via different mechanisms (e.g. via multivalent binding and receptor-mediated endocytosis), ending into endocytic vesicles of different microenvironments depending on the composition and characteristics of the nanoparticle. Entrapment of nanoparticles into the endocytic vesicles (dashed arrow) prevents them from reaching their target sites (cytoplasm, mitochondria, nucleus). The disassembly of polymeric nanoparticles and drug release can occur at various steps during the circulation and the intracellular trafficking pathway.
Cellular barriers
The previously-mentioned barriers can alter the characteristics and distribution of nanoparticles before they reach the surface of the target cells. However, these are not the only barriers towards delivery. Cellular uptake is highly challenging, especially for hydrophilic drugs and macromolecules. Interactions of nanoparticles with components of the outer surface of cells and cellular membranes, followed by internalization into vesicles (invagination of the plasma membrane surrounds the nanoparticle) are the initial steps for the endocytosis process. These vesicles are then pinched off to form membrane-bound vesicles of different sizes, compositions and internal environments, called endosomes, phagosomes or macropinosomes, depending on the internalization pathway. The endocytic pathway depends on the size, morphology and surface chemistry of the nanoparticles and varies from one cell line to another.9 Currently, there are five recognized mechanisms for the uptake of nanoparticles, phagocytosis, macropinocytosis, clathrin-mediated, caveolin-mediated, and clathrin/caveolin-independent endocytosis, depending on the proteins assisting in the endocytosis process.10 Entrapment of drugs into the vesicles (e.g. endosomes) often results in their degradation due to the acidic pH and enzymes found in the late endosomes (lysosomes). Escape of nanoparticles or their payloads from these vesicles into the cytoplasm is essential for them to reach the targeted subcellular organelles (Fig. 4). The viscosity and intracellular enzymes of the cytosol can also alter the stability and movement of nanoparticles. Translocation to subcellular organelles (e.g. nucleus or mitochondria) is challenging due to the physiological nature of these organelles and their bound membranes. Recycling (exocytosis) of the vesicle contents is also a possible pathway for the excretion of nanoparticles from cells. Direct translocation of nanoparticles across the plasma membrane is another suggested endocytic pathway that does not depend on the metabolic activity of the cells. This pathway is known as “energy independent”, “receptor-independent” uptake or “transduction”. Although still controversial, this pathway is most well studied with a unique category of peptides, called cell penetrating peptides (CPPs),11 which have been applied for the transport of CPP-functionalized nanoparticles, and in fact, this mechanism has been found recently to be involved in the uptake of nanoparticles in the absence of CPPs.12 The interesting feature of this pathway is that nanoparticles have a direct and quick access to the cytoplasm after crossing the membrane, which could simplify the design of nanoparticles by eliminating the need for additional features to be built into the nanoparticle framework for the purpose of disrupting the endocytic vesicles.
Rational design of polymeric nanoparticles
Significant efforts continue to be exerted towards the design and development of polymeric nanocarriers with tailored physical, chemical and biological properties.13 Size, shape and surface characteristics of nanoparticles dictate the blood stability, biodistribution, endocytic pathway and intracellular distribution and bioavailability of nanoparticles. In the following sections, the rational design and characteristics of polymeric nanoparticles, and their crucial constituents will be highlighted, with emphasis on polymer micelles, crosslinked knedel-like nanoparticles, and unimolecular/hyperbranched nanostructures (Table 1).
Table 1.
Summary of the important features of polymeric nanoparticles designed for biomedical delivery applications.
| Feature | Description |
|---|---|
| Stealth |
|
| Surface chemistry |
|
| Smart ingredients |
|
| Size |
|
| Shape/aspect ratio |
|
| Route of administration |
|
| Stability |
|
Stealth nanocarriers
One of the most successful strategies to avoid interaction of nanoparticles with components of biological fluids is coating with a dense brush layer of neutral hydrophilic flexible polymers to prevent adsorption of plasma proteins and avoid recognition by the MPS and subsequent clearance (Fig. 5). PEG is a biocompatible, hydrophilic, biologically-inert polymer that has been approved by the U.S. Food and Drug Administration (FDA) for internal use, for a number of applications. PEG is the most commonly utilized polymer for coating nanoparticles. PEGylation and the length and number of PEG chains per nanoparticle have been shown to produce significant differences in blood circulation times vs. elimination in clearance organs for polymeric nanoparticles.14–16 In addition, it partially prevents the aggregation of nanoparticles and hinders the non-specific interactions with cells by forming a neutral stabilizing interface, providing that the net surface charge of the nanoparticles is neutral. It could also provide steric hindrance and protection for cargoes that are sensitive towards a particular environment. For instance, incorporation of docetaxel (anticancer drug susceptible to hydrolytic degradation) and nucleic acids into PEG-decorated polymeric micelles could protect them against hydrolytic and enzymatic degradations, respectively.17, 18 Importantly, the PEG chains may also provide chemically-active centers for further functionalization or modification of nanoparticles. The usefulness of PEG shielding extends to other administration routes and not only to systemic administration. For example, PEG can reduce interactions with the extracellular matrix and mucus components and decrease the mucosal clearance.4 However, these shielded nanoparticles have limited cellular uptake and ability to escape from endosomes. Therefore, research efforts have also included the decoration of nanoparticles with cell recognition moieties to enhance their cellular uptake via receptor-mediated endocytosis. At the same time, these nanoparticles are designed to lose their PEG-shell at cell surfaces or in the acidifying endosomes to facilitate their release into the cytoplasm.13, 19, 20 Other hydrophilic polymers have also been used to coat the surface of nanoparticles, such as, poly(acrylic acid) (PAA), poly(N-vinylpyrrolidone), poly(N-isopropylacrylamide) and poly(carboxybetaine). The latter is another promising class of shell-forming polymers. The zwitterionic characteristic of poly(carboxybetaine) imparts anti-biofouling properties to the coated nanoparticles, due to the ability of the zwitterionic-based materials to electrostatically bind water molecules more tightly than the hydrogen bonding in the case of other hydrophilic polymers (e.g. PEG) and thus resulting in higher hydration of the corona.21 Effective hydration is critical in minimizing the protein adsorption and in imparting stealth properties to nanoparticles.
Fig. 5.
Characteristics of polymeric nanoparticles: (a) stealth: imparts biocompatibility, steric stability and protection of the encapsulated drug and reduces the opsonization and clearance of nanoparticles, but may also reduce the cellular uptake and endosomal escape capabilities, (b) charge: cationic character enhances cellular uptake and endosomal escape, but subject to uncontrolled tissue distribution and often associated with toxicity, (c) targeting: enhances cellular uptake and specificity, but sometimes can accelerate the clearance and/or immunogenicity, (d) stimuli-responsiveness: controls the dynamics of nanoparticles with possibility of releasing their cargoes at specific sites (selectivity). The stability and responsiveness of these materials under physiological and pathological conditions may vary and may result in premature release of the drug. (e) size: ~100 nm particles is optimal for delivery, being large enough to avoid renal clearance and small enough to reduce clearance and toxicity, (f) morphology: expanded morphology results in higher drug-loading capacity, lower clearance and cellular uptake, (g) aspect ratio: the shell vs. core volume and length vs. diameter can greatly affect the cellular uptake, clearance, drug loading and release, and toxicity, (h) assembly vs. unimolecular structures: unimolecular structures are more stable (no dissociation) but can be cleared rapidly depending on the size and usually have low drug-loading capacity, and (i) stability: intermediate stability to circumvent physiological barriers and at the same time be able to release the drug at the target sites is required and can be achieved with different methods, for instance, by crosslinking.
Smart ingredients
Incorporation of certain functionalities on the surface or into the core/shell architecture of polymeric nanoparticles can facilitate their diffusion through various biological barriers and direct their biodistribution to specific tissues, cells or organelles. In addition, they can modulate and remotely control the responsiveness of these nanostructures to specific stimuli.
Skin and mucus permeation enhancers
There are many physical and chemical methods that can hydrolyze mucin or destabilize SC, which then enhance the permeation of the delivery vehicles through the mucus and skin. However, we will focus here on the design of the polymeric nanoparticles themselves. Decorating nanocarriers with mucolytic, “mucus dissolving”, agents can reduce the mucus viscosity by degrading the biopolymers (e.g. proteins) in the mucus mesh, which in turn reduces the interactions between the nanoparticles and mucus and enhances the mobility and permeation of nanoparticles. For instance, decoration of insulin-loaded chitosan nanoparticles with the mucolytic agent acetylcysteine enhanced the nasal delivery of insulin in rats as compared to the plain nanoparticles.22 Nanoparticles, depending on their composition and delivery method, may penetrate skin via three main pathways, intercellular (lipid bilayers), transcorneocyte and the transappendageal (hair follicles) pathways. The intercellular pathway is the most characterized pathway for the delivery of nanostructured vehicles. Diffusion of nanoparticles through the skin is usually restricted and requires the incorporation of some chemical enhancers into the composition of nanoparticles to disrupt the lipid bilayers and create pathways for the penetration of nanoparticles. Lipid-based nanoparticles have been used more extensively than polymeric ones, due to their ability to destabilize the lipid channels of the SC and enhance the permeation through the skin.3
Accumulation at a specific site
Nanoparticles presenting ligands at their surfaces have been designed to enhance their selective binding to specific receptors overexpressed on the target cells. This approach is beneficial in terms of enhancing accumulation at target sites and decreasing the exposure of normal cells to the drug.23 The nanoparticles can be modified with these moieties either before or after assembly. However, it is a perquisite that they are available for receptor binding and internalization, which is usually achieved by tethering them to longer PEG chains than the ones forming the shell of the nanoparticle (Fig. 2). Nanoparticles can be decorated with a variety of targeting ligands, depending on the delivery location. For example, targeting transferrin and folate receptors is generally helpful in tumor therapy as they are overexpressed in many tumor cells. In particular, targeting asialoglycoprotein receptors and prostate specific membrane antigen is beneficial for treatment of hepatocellular carcinoma and prostate cancer, respectively.23 Targeted nanoparticles based on cyclodextrin-based polymers as the core matrix, PEG-adamantane (adamantane forms inclusion complexes with cyclodextrin) as the stealth corona, and transferrin as the tumor-targeting moieties, are currently in Phase I clinical trial for systemic delivery of small interfering RNA (siRNA).24 Davis et al. have observed a dose-dependent accumulation of these nanoparticles inside the tumor cells by examining the tumor tissues (by transmission electron microscopy and confocal microscopy) after staining them with 5 nm PEGylated gold nanoparticles decorated with adamantane at the surface. The role of chemistry here was not limited to the design and development of the nanoparticles, but was extended to also study their fate. In another study, folate moieties were tethered to the distal end of COOH-functionalized PEG-shell crosslinked knedel-like nanoparticles (SCKs). In vitro and in vivo studies demonstrated the enhanced and selective uptake of the folate-targeted polymeric nanoparticles, as well as the preferential accumulation in small-size tumors, as compared to the non-targeted ones. However, no significant accumulation in the folate-overexpressing large-tumors was observed.25 It is always controversial whether the use of targeting moieties in vivo is beneficial. The decoration of nanoparticles with targeting ligands may not significantly impact the tumor accumulation of nanoparticles, if the rate-determining step for tumor uptake is based on the enhanced permeability and retention (EPR) effect and the receptors are within the tumors. However, it enhances the cellular uptake after accumulation, thereby enhancing the therapeutic efficacy.24, 26 In addition to targeting specific cellular types, vascular targeting is also possible by recognition of specific receptors overexpressed on the endothelial cell surface.27 Targeted delivery was not only limited to cells-overexpressing recognition receptors, but also to subcellular organelles, such as, endosomes/lysosomes, cytosol, nucleus and mitochondria.28 Care should always be taken because the targeting ligand on the surface of nanoparticles may accelerate the clearance and/or increase the immunogenicity of the nanoparticles.29, 30
Remote control of the dynamics of polymeric nanoparticles
There are many different approaches that have been developed to interplay with the dynamics of nanoparticles, for example, by controlling the dissociation of nanoparticles and the release of the encapsulated drug at specific sites or time intervals. Many pathological sites have different environments (e.g. pH, temperature, oxidative or reductive conditions) than the normal physiological ones. Change in pH is probably the most exploited stimulus to trigger drug release. Indeed, variation from physiological pH (7.4) occurs at different sites, such as tumors, endosomes and in the gastrointestinal tract. An example of how to benefit from these stimuli is the incorporation of pH-sensitive ingredients into the nanoparticles, to maintain the integrity of the carrier at one pH value while destabilizing it at a different pH.19 For instance, incorporation of endosomolytic polymers, lipids or fusogenic peptides into the composition of nanoparticles is essential for endosomal destabilization and subsequent cytoplasmic release. Most endosomolytic polymers are designed to be bi-functional, by incorporating primary and/or tertiary amines at different ratios.31–33 Some of these amine groups are involved in electrostatic complexation with negatively-charged drugs while the unbound amine groups become protonated at the acidic pH of the endosomes, which causes influx of protons together with chloride ions and induces osmotic swelling and subsequent disruption of the endosomes, which is known as the “proton sponge effect”.34 Another suggested mechanism is that these positively-charged polymers might interact with and disrupt the endosomal membrane.35 Anionic polymers have been also designed to change their hydrophobicities and/or conformations at different pH values and, thus, utilized for either endosomal destabilization or for oral delivery to remain intact at one pH while dissociating and releasing encapsulated drugs at another pH.36, 37 Alternatively, fusogenic peptides and lipids can induce endosomal destabilization via bilayer-to-micelle or lamellar-to-inverted hexagonal (HII) transitions.38 The presence of oxygen-reactive species released by activated macrophages in the inflamed tissues and certain tumors has been investigated as yet another stimulus to trigger the release of drugs from polymeric nanocarriers. Thioketal nanoparticles have been developed for oral delivery of siRNA to inflammation sites in the intestine.39 These nanoparticles were built from poly(1,4-phenyleneacetone dimethylene thioketal) polymers, which contain thioketal linkages that are stable in acidic, basic and digestive environments, but degrade and release their contents in tissues with high levels of reactive oxygen species (i.e. at the inflammation sites). Another mechanism is to take the advantage of the reductive conditions met in the cytosol, which can cleave disulfide linkages used to link a drug or to stabilize nanoparticles.40 Nanoparticles were also constructed with the shell and core connected via reducible or pH-sensitive bonds, which can be degraded and release the uncoated core in the cytosolic reducing media or acidic pH of endosomes, respectively, and thus enhancing the intracellular bioavailability.41, 42 Alternatively, external stimuli can be utilized to trigger drug release from nanoparticles after they accumulate at the desired sites. Examples of external stimuli include, temperature, visible or near-infrared light, electric or magnetic fields, and ultrasound, among others.27
Recently, Bhatia and coworkers have experimentally explored in detail two important features for the rational design of smart nanoparticles. The first feature is to decorate nanoparticles with clusters of targeting ligands to encourage the uptake via multi-binding mechanisms.43 A dendritic polymer was first functionalized with variable amounts of folate groups and then mixed with non-functionalized polymers at different proportions to form mixed micelles that presented the same amount of folate moieties, but with different spatial arrangements in variable sized clusters (i.e. different numbers of folate groups per cluster). It was found that the optimal number of ca. 3 folate groups per cluster enhanced the cellular uptake due to the multivalent binding and the resultant longer residence time on the cell surface. The second feature is the design of self-communicating nanoparticles.44 In this case, nanoparticles were used for two purposes. Firstly, tumor-targeted “signaling nanoparticles” were used to create or amplify a target (coagulation cascade, a harmonized biological process) via different mechanisms in the tumor tissues. Two mechanisms were used to initiate the coagulation process, PEG-gold nanorods followed by photothermal heating or by utilizing tumor-targeted human protein tissue factor. Secondly, the “receiving nanoparticles” were then loaded with doxorubicin and tagged with peptides that recognize fibrin (product of the coagulation process) and injected in mice-bearing tumors. Hence, the receiving nanoparticles were recruited from the circulation to the clotted tumor areas. This communicating system resulted in enhanced delivery to the tumor tissues and was able to deliver more than 40 times higher amounts of doxorubicin than the non-communicating (without target amplification) controls. Meijer and coworkers have conducted another intricate strategy for enhancing the selective cellular uptake of dendritic nanoparticles by combining both multivalent binding and natural system-mimicking.45 The dendrimer-based nanoparticles decorated with phage peptides (phage mimicking) in a multivalent platform (multivalency or cluster binding) had a higher receptor-binding affinity than did the non-mimicking or monovalent species.
Route of administration
Although injection is the most common way of delivering nanoparticles, nanoparticles can be also delivered through the skin, oral, nasal, pulmonary, vaginal, rectal, ocular and buccal routes. Selecting the appropriate route of administration is as important as the nanoparticle design. Awareness of the different barriers and challenges in every route of administration is imperative for the proper design of nanocarriers. For instance, the pH of lung and nasal mucus is neutral, whereas it is slightly basic and acidic in the eye mucus (~7.8) and vaginal secretions (3.5–4.5), respectively.4 Many nanoparticles are designed to dissociate at acidic pH, for example, to destabilize endosomes. The use of these polymeric nanoparticles for vaginal administration can be detrimental due to the premature release of their payloads. The use of mucoadhesive nanoparticles can be also useful in the case of mucosal delivery. For instance, thiolated crosslinked chitosan nanocomplexes were used for intranasal DNA delivery and showed high transfection due to their mucoadhesiveness, high stability and sustained release of the complexed DNA.46 Generally, the use of semisolid matrices or scaffolds (e.g. hydrogels or creams) is preferred for topical and mucosal delivery to allow prolonged contact with these tissues and sustained release of the encapsulated drugs. Polymeric nanoparticles can be modified, for instance, via crosslinking, to form semi-solid matrices.47 Auxiliary devices can be also useful to introduce the drug directly to the target organ (e.g. catheter for delivery to the bladder, aerosols for pulmonary delivery and microneedles for topical delivery). These devices localize the nanoparticles at the target organ, which together with targeted delivery, maximize the therapeutic benefits and minimize the toxicity to healthy tissues.
Characteristics of polymeric nanoparticles
Size
Physiological barriers are the main limiting factors for the efficacy of nanoparticles (Fig. 3). The accepted size limit of molecules for passive delivery through the skin is below 500 Da. The mesh spacing between mucin fibers of mucus ranges from 100 to 1000 nm. The blood capillaries are ~5–40 µm in diameter. The mammalian vasculature has an average pore size of ~5 nm and below this size, nanoparticles can transverse the endothelium and equilibrate with the extracellular space. Plasma constituents have diameters <4 nm, whereas most plasma proteins are >7 nm (e.g. the hydrodynamic diameter of human immunoglobulin is 11 nm). The fenestrations in the liver sinusoids and spleen are less than 500 nm in width. The renal molecular weight cutoff size is ~48 kDa (for some polymers such as PEG and dextran) and ~10 nm in diameter. In many tumors, the openings of blood vessels are less than 200 nm in width. For brain tumor (e.g. malignant glioma), the pore size upper limit of the blood brain barrier (BBB) is ~12 nm and smaller for healthy brain tissues. On the cellular level, the cell membrane blocks diffusion of complexes larger than 1 kDa and the nuclear pore complexes, which regulate the nuclear entry of materials, is around 10–25 nm in diameter. The size of internalized vesicles (endosomes) ranges from 60–120 nm, depending on the type of endocytosis and is usually much larger (micrometer range) for macropinocytosis and phagocytosis. It is important to note that these size limits are approximate and depend on the physiological condition (e.g. disease status, location, cell type), and the nanoparticle composition, size, morphology, geometry, charge and surface chemistry. For example, small and hydrophobic molecules permeate through skin or plasma membranes much easier and faster than do hydrophilic macromolecules.
It is clear now how designing nanoparticles of definite size can greatly influence the circulation time, clearance, selective tissue distribution and intracellular fate. Large particles (>1 µm) are usually opsonized and accumulate in the liver and spleen, with possibility of aggregation and capillary occlusion. Small nanoparticles (<5 nm) are cleared rapidly from the blood via extravasation or renal clearance. Rapid renal clearance is expected for nanoparticles smaller than 10 nm in diameter or for their dissociated polymer chains, if they are below the renal molecular weight cutoff size. The renal filtration is dependent also on the charge, with negatively-charged large particles being excluded from excretion. Sometimes, the smaller size can be useful to allow rapid renal clearance (e.g. contrast agents) or to help crossing the BBB, although they may not have enough circulation time for brain accumulation. Smaller nanoparticles are transported more easily to lymph nodes via lymphatic drainage. Polymeric nanoparticles of intermediate size (20–100 nm) have the highest potential for in vivo applications, due to their ability to circulate in the blood for long periods of time, when they are designed appropriately. These nanoparticles are large enough to avoid renal and lymphatic clearance and small enough to avoid opsonization. In addition, nanoparticles within this size range are believed to be internalized easily by cells, in comparison to smaller or larger particles.9 Furthermore, they may enter certain tissues with leaky vasculature or high vascular permeability such as tumors and sites of inflammation, or in other tissues, such as the liver and spleen. Accumulation in tumor tissues is particularly useful for maintaining high concentrations of therapeutics and is augmented by the impaired lymphatic drainage at these areas. This phenomenon is known as the “EPR” effect. The EPR effect depends also on the charge and shape of nanoparticles and physiological conditions of the tumor (e.g. tumor perfusion and permeability).
Morphology
There is currently great effort to study the effect of shape and dimensions of nanoparticles on their behavior both in vitro and in vivo (Fig. 5). The spherical morphology is the most common shape of polymeric nanoparticles, although other morphologies are attainable depending on the polymer structure and nanoparticle composition. Elongation of spherical entities into cylindrical or vesicular architectures has the potential to display different characteristics, such as solubilization capacity, in vivo circulation time and cellular uptake. It is generally found that spherical nanoparticles enter cells to a greater extent than do elongated cylindrical ones. For instance, Discher and coworkers have prepared biodegradable PEG-b-poly(ε-caprolactone) and non-biodegradable PEG-b-polyethylethylene filamentous micelles (filomicelles) and compared them with spheres of similar chemistry.48 In rodents, filomicelles, with length of 18 µm and ~20–60 nm diameter, were reported to persist in the circulation about ten times longer than their spherical counterparts, due to the reduced rate of phagocytosis and clearance by the MPS. The clearance of filomicelles occurred upon the persistent decrease in length, which was more significant for the biodegradable poly(ε-caprolactone) than the non-degradable polyethylethylene, due to the hydrolysis of poly(ε-caprolactone) over time. However, for cylindrical nanoparticles designed to prolong the circulation (by reducing the MPS clearance), it is important to ensure enough flexibility/deformability of the nanoparticles and to maintain at least one dimension less than 200 nm to avoid entrapment in the spleen or other tissues. The Wooley group has also developed expertise in preparing polymeric nanoparticles of well-defined morphologies from block copolymers.49, 50 Conjugation of CPP onto both spherical (11 nm) and cylindrical (20 nm diameter and 200 nm length) SCKs of the same composition was carried out to study the effects of nanoparticle shapes on the cellular uptake.51 Generally, higher cellular uptake was observed with increasing levels of CPP-functionalization, and the effects of shape and size were significant, where the smaller spherical SCKs were internalized by cells more rapidly than were longer cylindrical nanoparticles. On the contrary, folate-functionalized cylinders were taken up by cells to a greater extent than were folate-functionalized spherical SCKs.52 This discrepancy was hypothesized to result from receptor clustering, where cylinders were more capable of multivalent interactions (multiple receptors binding) due to their longer dimensions, and thereby triggered more efficient cellular uptake. The particle geometry was also found to be an important parameter that affects the cellular uptake of polymer nanoparticles produced via DeSimone’s PRINT® (particle replication in non-wetting templates) technology. Nanoparticles of the same shape (i.e. cylindrical) but with aspect ratios of 3 (450 nm height × 150 nm diameter) and 1 (200 nm height × 200 nm diameter) had different cellular uptake with the nanoparticles of the higher aspect ratio being taken up more rapidly.53 This behavior is in agreement with observations made with polymer microparticles of Mitragotri et al.54, 55 There are different techniques to predict, visualize and control the morphology of nanoparticles.13, 56 However, care should be taken because sometimes these morphologies are in equilibrium and can transform from one shape to another. The transformation between different morphologies can also be controlled by adjusting polymer composition or by altering the ionic strength and pH of the solution or by addition of organic solvents.50, 56
Surface chemistry
The chemical groups that coat the surface of nanoparticles can be modified to modulate hydrophilicity, surface charge, immunogenicity, in vivo circulation, biodistribution and intracellular bioavailability (Fig. 5). The surface charge can greatly affect the fate of nanoparticles at different stages, namely, physical stability, skin and mucus penetration, interactions with extracellular matrix, blood stability, cellular uptake and endocytosis. In blood, excess positive charge results in opsonization, aggregation of particles and clearance with possible blood vessels and capillary occlusion. The types, quantities and conformations of the opsonins adsorbed on the surface of nanoparticles are dictated by the nanoparticle chemistry. Interactions of positively- or negatively-charged nanoparticles with components of the extracellular matrix hinder their penetration and may result in their clearance. At the cellular level, surface charge is directly proportional to the cellular binding and association, due to the negatively-charged proteoglycans on the cell surface. The uptake of nanoparticles usually increases with increasing the zeta potential values. However, excess positive charge can induce toxicity and initiate immunological reactions. Nanoparticles of different surface chemistries may be taken up via various endocytic mechanisms, ending up in different intracellular trafficking pathways. For instance, decorating the surface of nanoparticles with folic acid, albumin or cholesterol favor the caveolin-mediated endocytosis, which avoid the lysosomal degradation pathways. Alternatively, transferrin and CPP encourage the uptake via clathrin-mediated endocytosis and macropinocytosis, respectively. Designing nanoparticles for specific endocytic pathways remains challenging.57 The endocytosis process depends on the composition, size, charge, shape and geometry of nanoparticles and cell density and type. It is complicated to determine the main contributing factor because changing one of these factors can affect other characteristics. For instance, increasing the +/− charge ratio of nanoparticles to impart higher positive surface charge, can affect the stability, size and morphology of the formed particles. In addition, each mechanism depends on the cell type and treatment conditions. Some cells (e.g. hepatocellular carcinoma cells (HepG2), neurons and leukocytes) do not endogenously express caveolin and, hence, do not allow the cellular uptake via caveolae-mediated endocytosis.10, 58 Finally, these endocytic pathways can be interchangeable, which means blocking the uptake via a specific pathway may enhance the uptake through other endocytic pathways.
Immunological properties of polymeric nanoparticles
Although nanoparticles can stimulate or suppress the immune system and potentially induce severe toxicity, there is limited data available on the toxicological and immunological reactions induced by nanoparticles.5, 59 Nanoparticles can be specifically designed to stimulate (i.e. vaccine) or suppress (i.e. anti-inflammatory) immunity. The immune system recognizes many of the components of nanoparticles as foreign materials and initiates an immune response through a complex process. The first step usually is the adsorption of the blood proteins on the surface of nanoparticles. The type and quantity of the adsorbed proteins determine the fate of nanoparticles in terms of uptake by immune cells and interactions with other molecules. The composition of nanoparticle, size, charge, morphology, and, most importantly, surface chemistry dictate the toxicity and immune response induced by nanoparticles. Binding of nanoparticles to the cell surface can initiate signaling processes that may induce toxicity or immunogenicity. Activation of the immune system can also release cytokines that act as mediators of local and systemic inflammatory and hypersensitivity reactions. Signaling through the endosomal Toll-like receptors (TLRs) is also a well-characterized pathway for the induction of cytokines, especially with polymeric nanoparticles used for nucleic acids delivery. In this case, the immune response is usually due to the nucleic acid cargoes, which can stimulate different TLRs and initiate serious immunological reactions.
The first parameter to be considered in the design of nanoparticles is the shell thickness, density and type, and accessibility of any molecules used for surface decoration (e.g. targeting ligands, contrast agents). Cationic nanoparticles are believed to be more toxic, rapidly-cleared and induce higher inflammatory reactions than their anionic or neutral counterparts. Encapsulation of therapeutics inside the nanocarrier is expected to reduce the immune response induced by the drug. Considering the approximate size of plasma proteins and constituents (~1–10 nm), it is desirable to keep the spacing between PEG chains as small as possible to minimize the interactions between the plasma components and the core material. Crosslinking the corona by biodegradable crosslinkers is also important to retain the spacing and avoid the dissociation and segregation of the PEG chains. Shell decoration with different moieties is necessary for the preparation of multifunctional nanocarriers. Hence, it is a perquisite to keep the functionalization ratio as low as possible. In addition, the use of moieties of low immunogenicity (e.g. galactose instead of antibody for targeting) is recommended. Comprehensive studies to identify the critical parameters (e.g. PEG length and density14–16) that influence the in vivo clearance, toxicity and immunogenicity of nanoparticles are required, although it is difficult to conclude such data from one study. In vitro studies to determine which blood components are involved in the destabilization/opsonization of nanoparticles is equally important for the rational design of nanoparticles. Polymer biodegradability and biocompatibility are essential for patient safety. Biodegradability can be attained, for example, by endowing polymers with ester or disulfide linkages, providing that they will remain stable until delivering their cargoes. Careful design of the drug itself can modulate the immunotoxicity of nanoparticles. For instance, while the injection of plasmid DNA usually induces strong immune responses, designing plasmid structures that do not contain unmethylated CpG motifs (immunostimulatory sequences in the plasmid structure) did not induce immunological reactions.60 Similarly, chemical modifications of other small nucleic acids (e.g. siRNA) can modify/amend their immunogenicity.61
Polymeric nanoparticles as versatile nanomedicine platforms
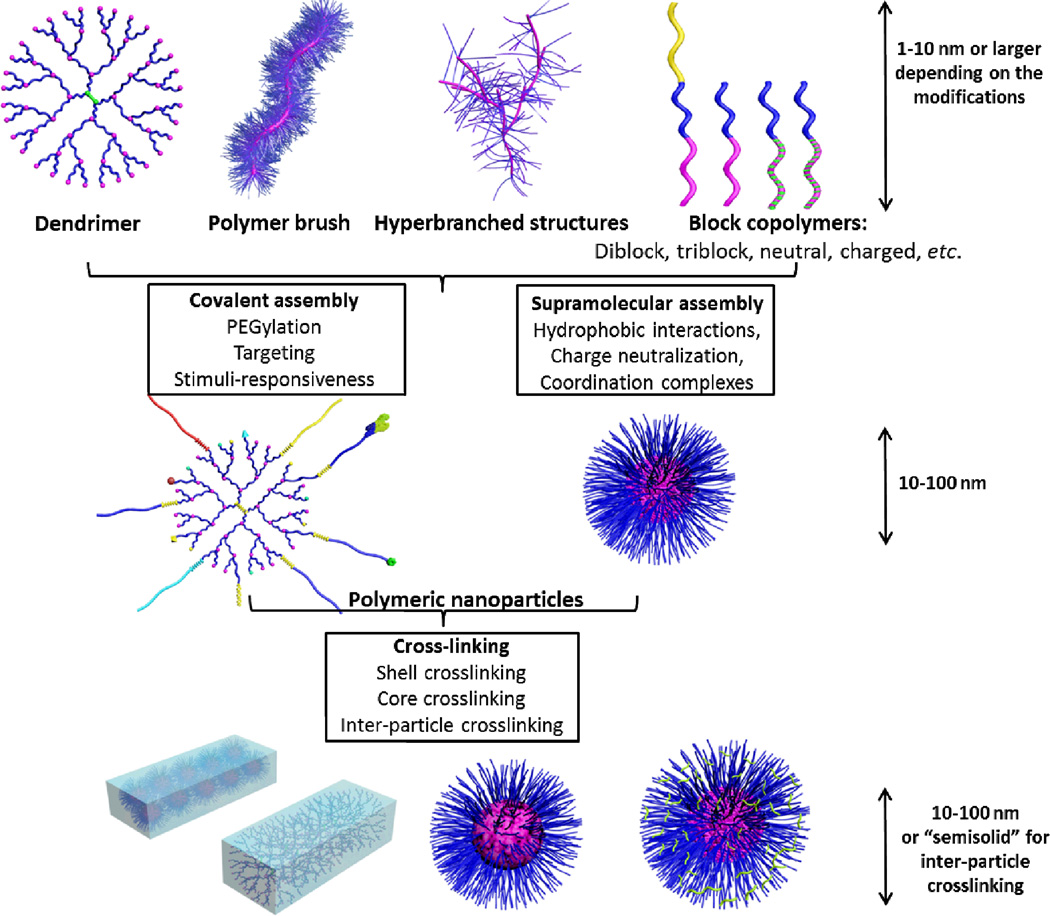
There has been an extensive research for designing polymeric nanoparticles of different types, sizes, morphologies, stabilities and biocompatibilities to surmount the various biological barriers. In this section, block copolymer micelles (self-assemblies), crosslinked knedel-like nanoparticles (stabilized self-assemblies) and branched polymeric structures (single macromolecular entities) will be briefly discussed with emphasis on their rational design (Fig. 6).
Fig. 6.
Types of polymeric nanoparticles and possible chemical modifications: polymeric blocks (dendrimer, brush, hyperbranched, block copolymer) can be either chemically modified or supramolecularly-assembled into polymeric nanoparticles. Post-modification of these nanoparticles via ligand modification, core- or shell-crosslinking or drug-loading is possible.
Block copolymer micelles
Polymeric micelles are formed via the self assembly of block- or graft-copolymer chains in aqueous milieu.56, 62, 63 They present a core/shell architecture wherein the hydrophobic core serves as a microenvironment for the incorporation of drugs while the hydrophilic corona imparts colloidal stability against aggregation. In water, hydrophobic interactions are generally the main driving force behind the micellization process. However, the self association of polymeric chains can involve additional forces. For example, electrostatic interactions were shown to induce the complexation and neutralization of oppositely-charged polymers, thereby allowing the formation of polyion complex micelles.41, 63 In addition, polymer-metal complex micelles can also be formed via substituting the ligands on a metal drug by the charged groups of the copolymer through coordination bonds. Most of these assemblies have fairly narrow size distributions with diameters ranging from 10 to 100 nm. Hydrophobic and hydrophilic blocks of different structures, charges and lengths have been utilized to prepare micelles of various size, shape and stability. PEG is probably the most commonly used hydrophilic block, although other hydrophilic segments have been also utilized, such as, poly(N-vinylpyrrolidone), poly(N-isopropylacrylamide) and PAA. The core of these polymeric micelles plays the major role in dictating the nature of the molecules to be encapsulated. Hydrophobic polymers provide good microenvironments for solubilization of poorly water-soluble drugs. Cationic or anionic polymers are often used to complex nucleic acid materials or metallic drugs by driving the formation of polyion complex micelles and polymer-metal complex micelles, respectively. Biodegradable polymers, such as, poly(ε-caprolactone), poly(D,L-lactide) and poly(amino acids) are expected to be more promising for in vivo administration than the non-degradable ones. The core-forming block can also be modified with hydrophobic moieties or conjugated with the drug to enhance the hydrophobicity and affinity to the encapsulated drug. Many polymeric micelle formulations are in different phases of clinical trials (NK-105, NK-012, Genexol and NC-6004) for the delivery of several potent anticancer drugs.64, 65 Most of these formulations could alter the pharmacokinetics and/or reduce the toxicity of drugs or the commercial surfactants used for their delivery (e.g. Cremophor® EL and Tween® 80). Even when these formulations exhibit similar pharmacokinetics and antitumor efficacy to the commercial formulations, the lower toxicity associated with polymeric micelles allows increase in the maximum tolerated dose of the encapsulated drug, thereby leading to better response among patients. Different block copolymers have been utilized for the preparation of these polymeric micelles, such as, PEG-b-polyaspartate (NK-105), where the polyaspartate is esterified with 4-phenyl-1-butanol to increase the hydrophobicity66 and PEG-b-poly(D,L-lactide) (Genexol) for the delivery of paclitaxel. PEG-b-poly(glutamic acid) copolymer was also utilized to form coordination complexes with cisplatin (NC-6004) or conjugated with 7-ethyl-10-hydroxy-irinotecan hydrochloride (NK-102) to form polymer-metal complex micelles and polymeric micelles, respectively. The average sizes of these micelles range from 20–85 nm, which is suitable for EPR targeting while minimizing the rapid clearance via the renal excretion. For instance, NK105 exhibited ~86-fold higher area under the plasma concentration time curve than the free drug at paclitaxel-equivalent dose of 50 mg/kg.66 In addition, tumors disappeared in mice treated with NK105 at a paclitaxel-equivalent dose of 100 mg/kg, and all mice remained tumor-free thereafter.
Although a great success has been accomplished to enhance the stability of polymeric micelles, these self assemblies remain prone to dissociation and are in equilibrium with non-micellized free polymer chains. Dilution of polymeric micelles upon in vivo administration can lead to premature dissociation and drug release. Micelle stabilization can be achieved via different strategies, such as, stereocomplexation, non-covalent interactions and crosslinking.67–69 Mixed polymeric micelles of PEG-b-poly(acid carbonate)/PEG-b-urea-functionalized polycarbonate have been prepared and loaded with doxorubicin. The mixed micelles have demonstrated higher kinetic stability than the PEG-b-poly(acid carbonate) micelles, due to the hydrogen bonding interactions between the carboxylate groups of the polycarbonate and the urea-derivatized polycarbonate.69 In this particular case, hydrogen bonding can occur between urea-urea, carboxylate-carboxylate, urea-carboxylate or between any of the two groups and the drug (i.e. doxorubicin). Micelle stabilization can be also achieved by crosslinking either the core or the corona of the pre-formed micelles or by designing unimolecular/hyperbranched structures that are intrinsically-stable against dissociation (Fig. 6).
Crosslinked knedel-like nanoparticles
SCKs are a member of a large family of crosslinked block copolymer micelles that have shown great potential and versatility for biotechnology and medicine, due to the ease by which the shape, composition, functionality, and stability can be tailored for a particular application (Fig. 6).70 The chemical structure of the forming copolymers, type of crosslinker and surface chemistry can affect stability, size, morphology and the type of the drug that can be encapsulated. Wooley and coworkers have focused on developing and screening SCKs as versatile carriers for a variety of biomedical applications, ranging from the delivery of large payloads of chemotherapeutics, nucleic acids, antimicrobials and diagnostic agents to the in vivo targeting of such entities to tumors, lung or bladder via the external multivalent presentation of tissue-specific ligands and/or by passive targeting. The size of SCKs is usually in the same range as polymeric micelles although crosslinking is often associated with a slight decrease in the diameter. The dynamics of these nanostructures could be remotely controlled by 1) synthesizing polymers that can respond to environmental or physiological triggers (e.g. pH, temperature, cytosolic reduction), 2) integrating additional functionalities that can stabilize these entities (e.g. crosslinking) and 3) triggering their cellular uptake by specific receptors that are overexpressed at the target sites. To prepare these nanostructures, a combination of controlled radical and ring-opening polymerizations, chemical transformations and supramolecular assembly is utilized.1, 2, 68, 71, 72 In one study, a linear triblock terpolymer of ethylene oxide, N-acryloxysuccinimide acrylate, and styrene, PEO45-b-PNAS105-b-PS45, was synthesized and used to prepare photonic multicompartmental nanostructures.73 While the PEO enhanced the water solubility and biocompatibility, the PNAS and PS segments were required for reactivity (pyrazine crosslinking) and supramolecular self-assembly, respectively. The relative molecular weight of each block was also important to form the multicompartmental micelles (i.e. longer PNAS and shorter PS). For instance, PEO45-b-PNAS95-b-PS60 formed only discrete spherical micelles. These nanocarriers exhibited different fluorescence emission intensities at different pH values. Interestingly, they could be designed to intrinsically-fluoresce or to switch their fluorescence (e.g. ON/OFF) at different physiological environments and may be also used for in vivo imaging for significant contrast enhancement. PAA-b-PS SCKs consisting of a hydrophobic core and a highly-functionalizable PAA shell have been used to encapsulate, protect and deliver silver-based antimicrobial agents for the treatment of pulmonary and urinary tract infections.74 Sustained release of the encapsulated drug from the SCKs was observed over several days and was associated with high antimicrobial activities against Gram-negative bacteria. The same copolymer has been also utilized to solubilize doxorubicin, a widely used anticancer drug, in SCK cores. Then, the effect of crosslinking and relative block length of PAA-b-PS copolymer on the characteristics of SCKs was studied.75 SCKs of different core volumes were formed depending on the ratio of acrylic acid to styrene block lengths. The nanoparticles with larger core volume (lower relative proportion of PAA to PS) were associated with higher loading capacity and higher rate and extent of drug release. Crosslinking could reduce the release rate of doxorubicin. The release rate was higher at pH 5 vs. pH 7.4 for both crosslinked and non-crosslinked nanoparticles (i.e. pH-responsive SCKs). The carboxylic acid groups of PAA could be chemically converted into primary and/or tertiary amines to invert the surface charge of SCKs from negative to positive. The micelle was then stabilized via covalent crosslinking of the shell by amide formation between chains with an activated diester to form cationic SCKs. These nanoparticles were shown to condense nucleic acid materials of different structures and chemistry, protect them against enzymatic digestion and to afford high transfection efficiency.31–33, 40 Multifunctional SCKs have been also used for theranostic applications. Theranostic nanoparticles “nanotheranostics” involve the use of multifunctional nanoparticles loaded with therapeutic drugs and imaging probes for the combined therapy and diagnosis. Advantages of combinational therapy are the ability to measure pharmacokinetics, visualize biodistribution and quantify drug release and accumulation at the target sites non-invasively in real time, which aid in predicting drug response and in the diagnosis and treatment of diseases.76 Folate-targeted SCKs were loaded with fluorescein thiosemicarbazide (for in vitro tracking), functionalized with 1,4,8,11-tetraazacyclotetradecane-N,N',N",N"'-tetraacetic acid (TETA) and used for delivering 64Cu to tumors-overexpressing folate receptors (for in vivo tracking). TETA is a macrocyclic ligand used to form stable complex with 64Cu, which is commonly used for both positron emission tomography and radiotherapy.25 The specificity of cellular uptake was confirmed both in vitro and in vivo by blocking the uptake of SCKs after the saturation of folate receptors. In mice-bearing tumors, the targeted nanoparticles were accumulated in the small-size solid tumors to a greater extent as compared to the non-targeted formulations, while no preferential accumulation was observed between both formulations in large tumors. One concern associated with such chemical stabilization of micelles is that it may impair the end elimination of the system, especially in case of non-biodegradable materials. Crosslinked micelles are often large entities that can no longer be eliminated by glomerular filtration. Likewise, crosslinking may impair the biodegradability of some polymers. To overcome these potential problems, hydrolysable-crosslinkers have recently been used.77, 78 Moreover, although the early work involving the development of SCKs for biomedical applications involved the use of non-degradable polymer components, to ensure study of the robust nanoparticle systems, current efforts focus on transformation to degradable nanoparticle structures.
Unimolecular structures
Small entities that present covalently-bound polymeric chains (single macromolecules) have been synthesized as an alternative approach to provide intrinsic stability. Examples of these structures include dendrimers, star polymers and polymer brushes (Fig. 6). This review is not detailing these structures, but highlighting their rational design. These structures were designed such that their formation and dissociation are intrinsically independent of polymer concentration and do not require a supramolecular assembly. In addition, their sizes and morphologies can be controlled precisely. Importantly, they can be decorated with different numbers and types of functional groups per molecule. Hence, different tasks can be achieved simultaneously and co-delivery of multiple drugs/probes at the desired ratio and at the same site can be ascertained.
Dendrimers are probably the most commonly utilized and characterized unimolecular structures.79, 80 Dendrimers are well-defined tree-like structures that their size is usually <5–10 nm, although decoration with different polymers or ligands on their surfaces can increase their size and alter their behavior.81 They consist of a central core, radially-branched backbone and surface functional groups. The core and backbone can be degradable or non-degradable and can have different chemical structures. Amine, carboxylic and hydroxyl-functionalized surfaces are examples of cationic, anionic and neutral dendrimers that have been studied for various biomedical applications. The surface can be also modified with polymers (e.g. PEG) or with targeting ligands for active targeting.82 Depending on the specific structure, they can solubilize or conjugate a wide range of therapeutics. The low polydispersity of dendrimers (close to 1.0) gives high control over their characteristics both in vitro and in vivo and results in less variability from batch to batch. The multivalency could be also useful to complex and condense nucleic acid materials (e.g. poly(amidoamine) dendrimers). However, the multivalency of dendrimers and their high binding affinity could adversely result in complications upon in vivo administration. The toxicity of these structures greatly depends on the surface functionality that dictates their interactions with cells and biomolecules, opsonization, immunogenicity, clearance and tissue distribution.81 Imparting biodegradability and attachment of PEG on the surface of these structures were found to significantly improve their biocompatibility and reduce their toxicity.83 The use of PEG was not only limited to the surface modification but also to the construction of the core of dendrimers. For instance, dendrimers with PEG polyester core have shown longer circulation times as compared to the same dendrimers with trisphenol core.84 The chemistry of these dendrimers could be also controlled to comply with other routes of administration. For instance, thiopyridyl functionalized poly(amidoamine) dendrimers have been crosslinked via disulfide linkages through 8-arm PEG chains bearing thiol terminations to form biodegradable hydrogels for intravaginal administration and controlled release of amoxicillin, a commonly used antibiotic.47 The gel lasted for up to 3 days in the cervicovaginal area before biodegradation occurred after intravaginal administration in guinea pigs. In this study, the hydrolysable crosslinkers were designed to degrade under reductive conditions and not at acidic pH, otherwise, they would have degraded rapidly in the acidic vaginal environment.
Polymer brushes are another promising class of unimolecular structures, consisting of a polymeric backbone with densely-grafted polymeric side chains. Polymer brushes of different structures and compositions have been synthesized and used for delivery of drugs, diagnostic agents and nucleic acid materials. The concept of stimuli-responsiveness has been also applied to molecular brushes and the use of pH-, temperature-, magnetic- and light-responsive polymer brushes has been investigated.85 Recently, unimolecular structures (e.g. polymer brush and unimolecular micelles) could be loaded with drugs, such as, paclitaxel, doxorubicin and camptothecin, which were attached to the polymers via photo-cleavable86, 87 or pH-sensitive88 linkers. The release of the drugs could be then controlled by exposure to light, or by changing the pH. For instance, doxorubicin and camptothecin-loaded polymer brushes induced ≥10-fold higher toxicity to human cancer cells after photo-initiated drug release.87 Limitations of unimolecular structures include the low loading capacity, as the solubilization of drugs into these structures is usually limited. Conjugation of drugs to the polymeric backbone or onto the surface often provides improved loading and retention of drugs, however, it also places a burden on the synthesis and preparation of the delivery vehicles. Unless the unimolecular structure is of significant size (i.e., nanoscopic dimensions) to be larger than the drug being contained within, they will provide incomplete protection of the payloads, unlike multi-polymeric nanoparticle assemblies, where the drugs are shielded in the core. Furthermore, small sizes can be rapidly cleared from the body, whereas the biodegradability and clearance of covalently-linked larger ones is questionable. Worth mentioning is that unimolecular structures can be also utilized as building blocks for polymeric nanoparticles (e.g. the use of dendrimers as core forming block of micelles19, 43 and polymer brushes to construct cylindrical nanostructures50) (Fig. 6).
Limitations
Although significant progress has been made in the field of nanotechnology and in controlling the physical, chemical and biological properties of nanomaterials, expectations for their stability in the blood, preferential distribution to the target sites, and their capability of curing diseases are still far beyond being met. The development of in vivo and live-cell imaging instrumentations and the availability of various chemicals that can inhibit specific biological processes have aided in understanding the cellular and in vivo fate of the different components of nanoparticles. However, there are still many questions to be answered. Many discrepancies are found in the literature, which could be due to biological, technical and experimental complexities. Limitations are generally related to nanoparticles, instruments, biological and physiological variations (Table 2). Assembly/disassembly of nanoparticles, cellular processes and in vivo pharmacokinetics are all dynamic processes that depend on many factors, some of which are difficult to control. The size, polydispersity and charge of nanoparticles depend on the conditions, measurement techniques and hydration state, and can vary during storage, blood circulation, tissue distribution and cellular uptake. For instance, adsorption of plasma proteins and cellular binding can modify the size, geometry and surface charge of nanoparticles. Cells also behave differently depending on the cell type, cycle, density and passage number. For instance, great efforts have been focused recently to design nanoparticles for specific intracellular pathways. However, this is challenging because the endocytic pathways are interchangeable and one inhibitor for a specific pathway can inhibit other pathways. Many biological processes, either in cells (endocytosis, cytoplasmic release, nuclear uptake) or in vivo (pharmacokinetics, biodistribution), are analyzed based on tracking fluorescent probes that are either linked or encapsulated into polymeric nanoparticles. The stability and quenching of fluorophores, the leakage or dissociation from nanoparticles and the effect of the fluorophore on the stability and pharmacokinetics of nanoparticles are always questionable and need further investigations. The use of inappropriate controls, impure fluorophores, improper use of imaging instrumentations (e.g. the use of high laser power, detector saturation, spectral overlap) can all result in misunderstanding and misinterpretation of cellular and in vivo fate of nanoparticles. Besides the complexities of biological systems, the inherent heterogeneity and distribution of populations in nanoparticle samples, and difficulties with exact reproduction of comparable materials, even within a particular laboratory, also pose significant challenges to making direct comparisons within and across nanosystems under development. Furthermore, degradability of nanoparticles is considered to be of primary importance, however, release of the degradation products and the resulting compositional, structural, size, etc. changes that the nanoparticles undergo during the degradation process lead to variations in the nature of the materials and the biological responses as a function of time.
Table 2.
Limitations towards the development of polymeric nanoparticles
| Nanoparticles |
|
| Cellular |
|
| In vivo |
|
| Instrumentations |
|
Conclusions
Polymeric nanoparticles hold great promise as versatile nanocarriers for a wide range of therapeutics for various biomedical applications. The ease with which the size, shape, geometry, charge, structure and composition can be controlled will secure their future development and success. Challenges towards the development of these nanostructures include, but are not limited to, the complexity of both the nanoparticles and available technologies for physical, chemical and biological evaluations, the possible toxicity and immunological reactions, lack of scalability, batch-to-batch variation and the need for case-by-case evaluation. For toxicity and immunogenicity considerations with intravenous administration, it is attractive to have nanoparticles of sizes that can be cleared by the kidney or dissociate into individual polymer chains that have molecular weights below the renal molecular weight cutoff size, or to have the nanoparticles undergo hepatobiliary clearance. However, other routes of administration and clearance are also possible. Alternatively, biodegradable nanoparticles would be safer to use. In all cases, biocompatibility is essential for the safety of patients and should be tested both in vitro and in vivo. These restrictions are firm with systemic administration of nanoparticles, whereas they are less significant for nanoparticles designed for topical applications. The control of polymer chemistry and supramolecular assembly could be managed to design polymeric nanoparticles for imaging, pH-sensing, cancer therapy, as well as, for other applications, such as, treatment of pulmonary and urinary tract infections. The chemistry of these copolymers could be also controlled to design stimuli-responsive and self-communicating nanoparticles of different structures and morphologies, such as spherical and cylindrical nanoparticles. Robust nanoparticles (e.g. crosslinked nanoparticles) could enhance the in vitro and in vivo stability, prolong the circulation time and enhance the accumulation at the target sites. However, elimination of these entities and hindrance of the release of their cargoes should be considered. Control over the polymer chemistry and supramolecular assembly together with the availability of new targets and cell-recognition moieties will improve the stability and efficacy of polymeric nanoparticles.
Acknowledgments
We gratefully acknowledge financial support from the National Heart Lung and Blood Institute of the National Institutes of Health as a Program of Excellence in Nanotechnology (HHSN268201000046C), the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01-DK082546) and Covidien Pharmaceuticals, Inc. The Welch Foundation is gratefully acknowledged for support through the W. T. Doherty-Welch Chair in Chemistry, Grant No. A-0001.
Biographies

Mahmoud Elsabahy is Co-Assistant Director of the Laboratory for Synthetic-Biologic Interactions at the Department of Chemistry, Texas A&M University, and a Lecturer at the Department of Pharmaceutics, Faculty of Pharmacy, Assiut University, Egypt. He received a B.S. in Pharmaceutical Sciences from Assiut University, Egypt and then he finished his M.Sc. and Ph.D. degrees from the Faculty of Pharmacy, University of Montreal under supervision of Professor Jean-Christophe Leroux (currently at the Institute of Pharmaceutical Sciences, Drug Formulation & Delivery, ETH, Zürich). He spent one year as a postdoctoral fellow and another year as a Research Associate at the School of Pharmacy, University of Waterloo (Ontario, Canada), studying the application of non-viral vectors for dermal and transdermal gene therapy. Research interests include the rational design of nanomedicines for various biomedical applications. He has obtained the Academic merit award (J. A. DeSève), and has been nominated as a meritorious candidate for both a Natural Sciences and Engineering Research Council of Canada (NSERC) Visiting Fellowship and a NSERC Industrial R&D Fellowship in the cellular and molecular biology areas of expertise.

Karen L. Wooley holds the W. T. Doherty-Welch Chair in the Department of Chemistry at Texas A&M University, with a joint appointment in the Department of Chemical Engineering. She received a B.S. in chemistry from Oregon State University in 1988 and then studied under the direction of Professor Jean M. J. Fréchet at Cornell University, obtaining a Ph.D. in polymer/organic chemistry in 1993. Research areas include the synthesis and characterization of degradable polymers, unique macromolecular architectures and complex polymer assemblies, and the design and development of well-defined nanostructured materials, for which she has received several awards, including an Arthur C. Cope Scholar Award, a Herman F. Mark Scholar Award, and awards from the National Science Foundation, the Office of Naval Research, and the Army Research Office. Karen serves as an Editor for the Journal of Polymer Science, Part A: Polymer Chemistry. She directs a National Heart Lung and Blood Institute-supported Program of Excellence in Nanotechnology, serves on the National Institutes of Health NANO study section, and also serves on the Scientific Advisory Panel for the National Institutes of Health Nanomedicine Development Centers and on the International Scientific Advisory Board for the Dutch BioMedical Materials Program.
Footnotes
“Part of the nanomedicine themed issue”
References
- 1.Iha RK, Wooley KL, Nystrom AM, Burke DJ, Kade MJ, Hawker CJ. Chem. Rev. 2009;109:5620–5686. doi: 10.1021/cr900138t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Reilly RK, Hawker CJ, Wooley KL. Chem. Soc. Rev. 2006;35:1068–1083. doi: 10.1039/b514858h. [DOI] [PubMed] [Google Scholar]
- 3.Prausnitz MR, Langer R. Nat. Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai SK, Wang YY, Hanes J. Adv. Drug Deliv. Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobrovolskaia MA, McNeil SE. Nat. Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Kim S, He W, Wang H, Low PS, Park K, Cheng JX. Langmuir. 2008;24:5213–5217. doi: 10.1021/la703570m. [DOI] [PubMed] [Google Scholar]
- 7.Savic R, Azzam T, Eisenberg A, Maysinger D. Langmuir. 2006;22:3570–3578. doi: 10.1021/la0531998. [DOI] [PubMed] [Google Scholar]
- 8.Felber AE, Castagner B, Elsabahy M, Deleavey GF, Damha MJ, Leroux JC. J. Control. Release. 2011;152:159–167. doi: 10.1016/j.jconrel.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Iversena TG, Skotlanda T, Sandvig K. Nano Today. 2011;6:176–185. [Google Scholar]
- 10.Sahay G, Alakhova DY, Kabanov AV. J. Control. Release. 2010;145:182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel LN, Zaro JL, Shen WC. Pharm. Res. 2007;24:1977–1992. doi: 10.1007/s11095-007-9303-7. [DOI] [PubMed] [Google Scholar]
- 12.Verma A, Uzun O, Hu Y, Han HS, Watson N, Chen S, Irvine DJ, Stellacci F. Nat. Mater. 2008;7:588–595. doi: 10.1038/nmat2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petros RA, DeSimone JM. Nat. Rev. Drug Discov. 2010;9:615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 14.Sun G, Hagooly A, Xu J, Nystrom AM, Li Z, Rossin R, Moore DA, Wooley KL, Welch MJ. Biomacromolecules. 2008;9:1997–2006. doi: 10.1021/bm800246x. [DOI] [PubMed] [Google Scholar]
- 15.Fukukawa K, Rossin R, Hagooly A, Pressly ED, Hunt JN, Messmore BW, Wooley KL, Welch MJ, Hawker CJ. Biomacromolecules. 2008;9:1329–1339. doi: 10.1021/bm7014152. [DOI] [PubMed] [Google Scholar]
- 16.Pressly ED, Rossin R, Hagooly A, Fukukawa K, Messmore BW, Welch MJ, Wooley KL, Lamm MS, Hule RA, Pochan DJ, Hawker CJ. Biomacromolecules. 2007;8:3126–3134. doi: 10.1021/bm700541e. [DOI] [PubMed] [Google Scholar]
- 17.Elsabahy M, Zhang M, Gan SM, Waldron KC, Leroux JC. Soft Matter. 2008;4:294–302. doi: 10.1039/b714221h. [DOI] [PubMed] [Google Scholar]
- 18.Elsabahy M, Perron ME, Bertrand N, Yu GE, Leroux JC. Biomacromolecules. 2007;8:2250–2257. doi: 10.1021/bm070226v. [DOI] [PubMed] [Google Scholar]
- 19.Elsabahy M, Wazen N, Puxan N, Deleavey G, Servant M, Damha MJ, Leroux JC. Adv. Funct. Mater. 2009;19:3862–3867. [Google Scholar]
- 20.Rahbek UL, Nielsen AF, Dong M, You Y, Chauchereau A, Oupicky D, Besenbacher F, Kjems J, Howard KA. J. Drug Target. 2010;18:812–820. doi: 10.3109/1061186X.2010.527982. [DOI] [PubMed] [Google Scholar]
- 21.Jiang S, Cao Z. Adv. Mater. 2010;22:920–932. doi: 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Zheng C, Wu Z, Teng D, Zhang X, Wang Z, Li C. J. Biomed. Mater. Res. Part B. 2009;88:150–161. doi: 10.1002/jbm.b.31161. [DOI] [PubMed] [Google Scholar]
- 23.Shi J, Xiao Z, Kamaly N, Farokhzad OC. Acc. Chem. Res. 2011;44:1123–1134. doi: 10.1021/ar200054n. [DOI] [PubMed] [Google Scholar]
- 24.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossin R, Pan D, Qi K, Turner JL, Sun X, Wooley KL, Welch MJ. J. Nucl. Med. 2005;46:1210–1218. [PubMed] [Google Scholar]
- 26.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timko B, Whitehead K, Gao W, Kohane D, Farokhzad O, Anderson D, Langer R. Annu. Rev. Mater. Res. 2011;41:1–20. [Google Scholar]
- 28.Won YW, Lim KS, Kim YH. J. Control. Release. 2011;152:99–109. doi: 10.1016/j.jconrel.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Simard P, Leroux JC. Mol. Pharm. 2010;7:1098–1107. doi: 10.1021/mp900261m. [DOI] [PubMed] [Google Scholar]
- 30.Shokeen M, Pressly ED, Hagooly A, Zheleznyak A, Ramos N, Fiamengo AL, Welch MJ, Hawker CJ, Anderson CJ. ACS Nano. 2011;5:738–747. doi: 10.1021/nn102278w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang K, Fang H, Wang Z, Li Z, Taylor JS, Wooley KL. Biomaterials. 2010;31:1805–1813. doi: 10.1016/j.biomaterials.2009.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang K, Fang H, Wang Z, Taylor JS, Wooley KL. Biomaterials. 2009;30:968–977. doi: 10.1016/j.biomaterials.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 33.Zhang K, Fang H, Shen G, Taylor JS, Wooley KL. Proc. Am. Thorac. Soc. 2009;6:450–457. doi: 10.1513/pats.200902-010AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akinc A, Thomas M, Klibanov AM, Langer R. J. Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 35.Hong S, Leroueil PR, Janus EK, Peters JL, Kober MM, Islam MT, Orr BG, Baker JR, Jr, Banaszak Holl MM. Bioconjugate Chem. 2006;17:728–734. doi: 10.1021/bc060077y. [DOI] [PubMed] [Google Scholar]
- 36.Convertine AJ, Benoit DS, Duvall CL, Hoffman AS, Stayton PS. J. Control. Release. 2009;133:221–229. doi: 10.1016/j.jconrel.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Felber AE, Dufresne MH, Leroux JC. Adv. Drug Deliv. Rev. 2011 doi: 10.1016/j.addr.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Koltover I, Salditt T, Radler JO, Safinya CR. Science. 1998;281:78–81. doi: 10.1126/science.281.5373.78. [DOI] [PubMed] [Google Scholar]
- 39.Wilson DS, Dalmasso G, Wang L, Sitaraman SV, Merlin D, Murthy N. Nat. Mater. 2010;9:923–928. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang H, Zhang K, Shen G, Wooley KL, Taylor JS. Mol. Pharm. 2009;6:615–626. doi: 10.1021/mp800199w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oishi M, Nagasaki Y, Itaka K, Nishiyama N, Kataoka K. J. Am. Chem. Soc. 2005;127:1624–1625. doi: 10.1021/ja044941d. [DOI] [PubMed] [Google Scholar]
- 42.Choi SW, Lee SH, Mok H, Park TG. Biotechnol. Prog. 2010;26:57–63. doi: 10.1002/btpr.310. [DOI] [PubMed] [Google Scholar]
- 43.Poon Z, Chen S, Engler AC, Lee HI, Atas E, von Maltzahn G, Bhatia SN, Hammond PT. Angew. Chem.-Int. Edit. 2010;49:7266–7270. doi: 10.1002/anie.201003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Maltzahn G, Park JH, Lin KY, Singh N, Schwoppe C, Mesters R, Berdel WE, Ruoslahti E, Sailor MJ, Bhatia SN. Nat. Mater. 2011;10:545–552. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bastings MM, Helms BA, van Baal I, Hackeng TM, Merkx M, Meijer EW. J. Am. Chem. Soc. 2011;133:6636–6641. doi: 10.1021/ja110700x. [DOI] [PubMed] [Google Scholar]
- 46.Lee D, Zhang W, Shirley SA, Kong X, Hellermann GR, Lockey RF, Mohapatra SS. Pharm. Res. 2007;24:157–167. doi: 10.1007/s11095-006-9136-9. [DOI] [PubMed] [Google Scholar]
- 47.Navath RS, Menjoge AR, Dai H, Romero R, Kannan S, Kannan RM. Mol. Pharm. 2011;8:1209–1223. doi: 10.1021/mp200027z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE. Nat. Nanotechnol. 2007;2:249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee NS, Lin LY, Neumann WL, Freskos JN, Karwa A, Shieh JJ, Dorshow RB, Wooley KL. Small. 2011;7:1998–2003. doi: 10.1002/smll.201100567. [DOI] [PubMed] [Google Scholar]
- 50.Li Z, Ma J, Lee NS, Wooley KL. J. Am. Chem. Soc. 2011;133:1228–1231. doi: 10.1021/ja109191z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang K, Fang H, Chen Z, Taylor JS, Wooley KL. Bioconjugate Chem. 2008;19:1880–1887. doi: 10.1021/bc800160b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang K, Rossin R, Hagooly A, Chen Z, Welch MJ, Wooley KL. J. Polym. Sci. Pol. Chem. 2008;46:7578–7583. doi: 10.1002/pola.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR. Mol. Ther. 2008;16:1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Champion JA, Mitragotri S. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elsabahy M, Dufresne MH, Leroux JC. In: Materials for Nanomedicine. Torchilin V, Amiji M, editors. Hackensack: Pan Stanford Publishing; 2010. pp. 169–234. [Google Scholar]
- 57.Adler AF, Leong KW. Nano Today. 2010;5:553–569. doi: 10.1016/j.nantod.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujimoto T, Kogo H, Nomura R, Une T. J. Cell. Sci. 2000;113:3509–3517. doi: 10.1242/jcs.113.19.3509. [DOI] [PubMed] [Google Scholar]
- 59.Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, Dobrovolskaia MA. Endocrinology. 2010;151:458–465. doi: 10.1210/en.2009-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hyde SC, Pringle IA, Abdullah S, Lawton AE, Davies LA, Varathalingam A, Nunez-Alonso G, Green AM, Bazzani RP, Sumner-Jones SG, Chan M, Li H, Yew NS, Cheng SH, Boyd AC, Davies JC, Griesenbach U, Porteous DJ, Sheppard DN, Munkonge FM, Alton EW, Gill DR. Nat. Biotechnol. 2008;26:549–551. doi: 10.1038/nbt1399. [DOI] [PubMed] [Google Scholar]
- 61.Watts JK, Deleavey GF, Damha MJ. Drug Discov. Today. 2008;13:842–855. doi: 10.1016/j.drudis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Torchilin VP. Pharm. Res. 2007;24:1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 63.Nishiyama N, Kataoka K. Pharmacol. Ther. 2006;112:630–648. doi: 10.1016/j.pharmthera.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Matsumura Y, Kataoka K. Cancer Sci. 2009;100:572–579. doi: 10.1111/j.1349-7006.2009.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsumura Y. Jpn. J. Clin. Oncol. 2008;38:793–802. doi: 10.1093/jjco/hyn116. [DOI] [PubMed] [Google Scholar]
- 66.Hamaguchi T, Matsumura Y, Suzuki M, Shimizu K, Goda R, Nakamura I, Nakatomi I, Yokoyama M, Kataoka K, Kakizoe T. Br. J. Cancer. 2005;92:1240–1246. doi: 10.1038/sj.bjc.6602479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang N, Perron ME, Prud'homme RE, Zhang Y, Gaucher G, Leroux JC. Nano Lett. 2005;5:315–319. doi: 10.1021/nl048037v. [DOI] [PubMed] [Google Scholar]
- 68.Cui H, Chen Z, Zhong S, Wooley KL, Pochan DJ. Science. 2007;317:647–650. doi: 10.1126/science.1141768. [DOI] [PubMed] [Google Scholar]
- 69.Yang C, Tan JPK, Cheng W, Attia ABE, Ting CTY, Nelson A, Hedrick JL, Yang YY. Nano Today. 2010;5:515–523. [Google Scholar]
- 70.van Nostrum CF. Soft Matter. 2011;7:3246–3259. [Google Scholar]
- 71.Hales K, Chen Z, Wooley KL, Pochan DJ. Nano Lett. 2008;8:2023–2026. doi: 10.1021/nl8013082. [DOI] [PubMed] [Google Scholar]
- 72.Hawker CJ, Wooley KL. Science. 2005;309:1200–1205. doi: 10.1126/science.1109778. [DOI] [PubMed] [Google Scholar]
- 73.Sun G, Cui H, Lin LY, Lee NS, Yang C, Neumann WL, Freskos JN, Shieh JJ, Dorshow RB, Wooley KL. J. Am. Chem. Soc. 2011;133:8534–8543. doi: 10.1021/ja200182t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y, Hindi K, Watts KM, Taylor JB, Zhang K, Li Z, Hunstad DA, Cannon CL, Youngs WJ, Wooley KL. Chem. Commun. 2010;46:121–123. doi: 10.1039/b916559b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin LY, Lee NS, Zhu J, Nystrom AM, Pochan DJ, Dorshow RB, Wooley KL. J. Control. Release. 2011;152:37–48. doi: 10.1016/j.jconrel.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lammers T, Kiessling F, Hennink WE, Storm G. Mol. Pharm. 2010;7:1899–1912. doi: 10.1021/mp100228v. [DOI] [PubMed] [Google Scholar]
- 77.Rijcken CJ, Snel CJ, Schiffelers RM, van Nostrum CF, Hennink WE. Biomaterials. 2007;28:5581–5593. doi: 10.1016/j.biomaterials.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 78.Kim JO, Sahay G, Kabanov AV, Bronich TK. Biomacromolecules. 2010;11:919–926. doi: 10.1021/bm9013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee CC, MacKay JA, Frechet JM, Szoka FC. Nat. Biotechnol. 2005;23:1517–1526. doi: 10.1038/nbt1171. [DOI] [PubMed] [Google Scholar]
- 80.Svenson S, Tomalia DA. Adv. Drug Deliv. Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 81.Cheng Y, Zhao L, Li Y, Xu T. Chem. Soc. Rev. 2011;40:2673–2703. doi: 10.1039/c0cs00097c. [DOI] [PubMed] [Google Scholar]
- 82.Wood KC, Little SR, Langer R, Hammond PT. Angew. Chem.-Int. Edit. 2005;44:6704–6708. doi: 10.1002/anie.200502152. [DOI] [PubMed] [Google Scholar]
- 83.van der Poll DG, Kieler-Ferguson HM, Floyd WC, Guillaudeu SJ, Jerger K, Szoka FC, Frechet JM. Bioconjugate Chem. 2010;21:764–773. doi: 10.1021/bc900553n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Padilla De Jesus OL, Ihre HR, Gagne L, Frechet JM, Szoka FC., Jr Bioconjugate Chem. 2002;13:453–461. doi: 10.1021/bc010103m. [DOI] [PubMed] [Google Scholar]
- 85.Lee H, Pietrasik J, Sheiko S, Matyjaszewski K. Prog. Polym. Sci. 2010;35:24–44. [Google Scholar]
- 86.Johnson JA, Lu YY, Burts AO, Lim YH, Finn MG, Koberstein JT, Turro NJ, Tirrell DA, Grubbs RH. J. Am. Chem. Soc. 2011;133:559–566. doi: 10.1021/ja108441d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johnson JA, Lu YY, Burts AO, Xia Y, Durrell AC, Tirrell DA, Grubbs RH. Macromolecules. 2010;43:10326–10335. doi: 10.1021/ma1021506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zou J, Jafr G, Themistou E, Yap Y, Wintrob ZA, Alexandridis P, Ceacareanu AC, Cheng C. Chem. Commun. 2011;47:4493–4495. doi: 10.1039/c0cc05531j. [DOI] [PubMed] [Google Scholar]