Abstract
Alpha catenin is a cytoskeleton protein that acts as a regulator of actin rearrangement by forming an E-cadherin adhesion complex. In Dermacentor variabilis, a putative α-catenin (Dvα-catenin) was previously identified as differentially regulated in ovaries of ticks chronically infected with Rickettsia montanensis. To begin characterizing the role(s) of Dvα-catenin during rickettsial infection, the full-length Dvα-catenin cDNA was cloned and analyzed. Comparative sequence analysis demonstrates a 3069 basepair cDNA with a 2718 bp open reading frame with a sequence similar to Ixodes scapularis α-catenin. A portion of Dvα-catenin is homologous to the vinculin-conserved domain containing a putative actin binding region and β-catenin binding and dimerization regions. Quantitative RT-PCR analysis demonstrated that Dvα-catenin is predominantly expressed in tick ovaries and is responsive to tick feeding. The tissue-specific gene expression analysis of ticks exposed to Rickettsia demonstrates that Dvα-catenin expression was significantly downregulated at 12 hours post-exposure to R. montanensis, but not in R. amblyommii-exposed ovaries, compared to Rickettsia-unexposed ticks. Studying tick-derived molecules associated with rickettsial infection will provide a better understanding of the transmission dynamics of tick-borne rickettsial diseases.
Keywords: Dermacentor variabilis, Rickettsia montanensis, R. amblyommii, α-catenin
Introduction
Ticks are known as arthropod vectors for many pathogenic and nonpathogenic organisms of the genera Anaplasma, Babesia, Borrelia, Ehrlichia and Rickettsia (Sonenshine, 1993). In the United States, ticks are responsible for the transmission of more vector-borne diseases than any other group of arthropods, with recent reports of tick-borne rickettsioses, such as Rocky Mountain spotted fever (RMSF), human monocytic ehrlichiosis, and human granulocytic anaplasmosis increasing extensively over the last ten years (Dumler, 2010). Unique among the tick-borne rickettsial pathogens are the spotted fever group (SFG) Rickettsia, which in addition to potentially being transmitted horizontally via vertebrate hosts, are maintained by vertical transmission in tick populations. Despite sympatric distribution of multiple tick and SFG Rickettsia species, stable vertical transmission has likely contributed to the establishment of specific relationships between ticks and species of Rickettsia. The American dog tick, Dermacentor variabilis, is a vector and host for Rickettsia rickettsii, the causative agent of RMSF and Rickettsia montanensis, a Rickettsia considered nonpathogenic to humans (Mcdade & Newhouse, 1986). Despite the apparently transient infection of D. variabilis with other SFG Rickettsia (Williamson et al., 2010), due to stable vertical transmission the most commonly encountered microbe is R. montanensis (Ammerman et al., 2004; Smith et al., 2010; Stromdahl et al., 2010). The contributing molecular factors that facilitate rickettsial infection, including the host specificity are not well defined.
Previous studies utilizing PCR-based transcriptional analyses in chronically SFG Rickettsia-infected ticks demonstrated differential expression of several tick-derived genes encoding ATPase, tubulin α-chain, glycine-rich protein, and α-catenin (Macaluso et al., 2003; Mulenga et al., 2003). Most molecules identified could be categorized as putative immune, stress response, or cytoskeletal associated molecules, such as α-catenin. In humans, α-catenin is able to bind various cytoskeleton proteins and regulates actin dynamics in the cells (Gates & Peifer, 2005; Drees et al., 2005). This interaction is in part due to the molecule’s two forms, one of which is a monomeric α-catenin that binds β-catenin, thereby forming an E-cadherin-dependent cell-cell adhesion complex linking to actin filaments. The other form is homodimeric α-catenin which binds actin filaments and inhibits the formation of the Arp2/3 and actin filament complex (Hartsock & Nelson, 2008). During host cell infection, many bacterial species utilize host α-catenin to mediate actin rearrangement in infected cells. For example, enterohemorrhagic and enteropathogenic Escherichia coli secrete a bacterial effector protein (EspB) into host cells that binds many host-derived proteins including α-catenin. EspB promotes α-catenin dimerization by competing with the Arp2/3 complex (Hamaguchi et al., 2008; Kodama et al., 2002). Likewise, during internalization of Listeria monocytogenes into epithelial cells, Listeria Internalin A binds to the E-cadherin-β-catenin complex on the host cell membrane, which is linked via α-catenin to actin filaments and recruits the cytoskeleton protein to the entry site (Sousa et al., 2005). It has been demonstrated that in some species of SFG Rickettsia, actin polymerization is also required for bacterial invasion and motility during infection (Martinez & Cossart, 2004; Serio et al., 2010). For example, Rickettsia conorii binds to host Ku70 and mediates actin polymerization via the Arp2/3 complex during internalization (Martinez & Cossart, 2004; Martinez et al., 2005). Also, recent studies have identified a core set of actin-associated cytoskeletal proteins coupled with motility of Rickettsia parkeri in Drosophila cells (Serio et al., 2010). In an Ixodes scapularis cell line (ISE6), Rickettsia felis, a flea-borne transitional group rickettsiosis agent interacts with tick cell surface histone H2B and depletion of histone H2B by RNAi and enzymatic treatment decreased rickettsial infection in the tick cells, suggesting a role of histone H2B in R. felis internalization into tick cells (Thepparit et al., 2010). Despite progress in understanding rickettsial entry into host cells, the molecules and mechanisms of invasion of tick cells by SFG Rickettsia in ticks have not been identified.
Based on the previous identification of a partial transcript for putative host cytoskeletal components, the objectives of the current study were to characterize the α-catenin gene from D. variabilis and examine its association with rickettsial infection. Using functional bioassays, we tested the hypothesis that differential regulation of tick α-catenin during rickettsial infection is tissue-specific. Additionally, to begin to examine the basis of Rickettsia/tick pairing specificity, tick tissues were exposed to typical Dermacentor-associated Rickettsia (R. montanensis) or atypical non-Dermacentor associated Rickettsia (Rickettsia amblyommii) infection and expression of Dvα-catenin in response to each species was assessed. Understanding the tick-derived molecular response to rickettsial infection will provide insight into the specificity, transmission, and the ecology of tick-borne rickettsial infections.
Experimental procedures
Tick dissection
Rickettsia-free D. variabilis colonies were routinely maintained as described previously (Macaluso et al., 2001). Unmated female ticks partially fed for 3 to 5 days were forcibly detached from host animals, washed twice in 70% ethanol, and rinsed with distilled water. Selected tick tissues (salivary glands, midgut, and ovary) were dissected out of the ticks, washed in sterile diethyl pyrocarbonate-treated water or fresh phosphate buffer saline (PBS, pH 7.4), and placed in RNAlater (Ambion) until processed for nucleic acid extraction.
Nucleic acid extraction from tick tissues and cloning of Dvα-catenin full-length cDNA
As previously described (Mulenga et al., 2004), ovaries from at least five D. variabilis were pooled, total RNA and subsequently mRNA were extracted using the NucleoSpin RNAII and NucleoTrap mRNA Mini kit (Clontech) according to the manufacturer’s protocol. All RNA was stored at −80 °C until used. Cloning of full-length cDNA for Dvα-catenin was carried out using rapid amplification of cDNA ends (RACE) as previously described (Mulenga et al., 2004). Briefly, 1 µg of mRNA extracted from ovaries was used to generate templates for 3’ and 5’ RACE using the SMART RACE cDNA synthesis kit (Clontech) according to the manufacturer’s protocol. Following DNA sequencing of the putative Dvα-catenin fragment obtained by differential display PCR (Macaluso et al., 2003), gene specific primers were designed to amplify the 5’ [5’-GAAAGGTCGCTCAAAGCGGGC-3’] and 3’ [5’-GCCTTCATCTTCCACACAACAATCGG-3’] ends. PCR products were routinely cloned into TOPO TA cloning vectors (Invitrogen) and sequenced (Macaluso et al., 2003). MacVector software program (Accelrys) was used for DNA sequence analysis. Similarity comparisons to known proteins in the database were made by scanning DNA sequences against the GenBank database using tblastx.
Rickettsial culture
Two Rickettsia species, R. montanensis strain M5/6 and R. amblyommii strain Darkwater were routinely maintained and propagated in an African green monkey kidney cell line (Vero E6) in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% FBS (Hyclone) in a humidified 5% CO2 incubator at 34°C. For infection bioassays, when more than 80% of cells were infected, rickettsiae were semi-purified from Rickettsia-infected Vero E6 cells by detachment from the tissue culture flask, transferred to an Erlenmeyer flask containing sterile 3-mm borosilicate glass beads (Sigma), and vortexed at high speed for 5 min. The cell lysate was filtered through a 2-µm-pore size syringe filter (Millipore). The rickettsiae in the filtrate were collected by centrifugation at 16,000 × g at 4°C for 10 min. Rickettsial viability and enumeration were assessed as previously described by Sunyakumthorn et al. (2008). Briefly, purified rickettsiae were stained with a BacLight viability stain kit (Invitrogen) and counted in a Petroff-Hausser bacteria counting chamber using a Leica microscope.
Quantitative Reverse Transcription-PCR (qRT-PCR)
The PCR reaction reagents were mixed in 96-well plates containing 2 µl of cDNA, 2X iTaq SYBR Green Supermix with ROX (Bio-Rad), 100 mM each of forward and reverse primers in a total volume of 35 µl per reaction. The qRT-PCR primers used for catenin and actin were DvCat2555F (5’-CACCGATTGTTGTGTGGAAG-3’) and DvCat2661R (5’-CTTTTTCTGTGAGCCCTTGC-3’)] and [DvAct1424F (5’-CTTTGTTTTCCCGAGCAGAG-3’) and DvAct1572R (5’-CCAGGGCAGTAGAAGACGAG-3’)], respectively. A no-RT reaction (water was added instead of reverse transcriptase) was performed to verify the absence of genomic DNA. Ten microlitres of each reaction mixture were transferred into three wells of a 384-well plate and reacted in an ABI 7900HT unit (Applied Biosystems) at Louisiana State University, School of Veterinary Medicine using system software (SDS v2.3). Data for each sample was initially calculated as the percent difference in threshold cycle (CT) value (ΔCT = CT Actin − CT α-catenin).
Tissue-specific transcription of Dvα-catenin during bloodfeeding and rickettsial infection bioassay
To determine the specific expression of Dvα-catenin in tick tissues and response of Dvα-catenin expression during blood feeding, unfed (3 ticks) and 5-day fed female ticks (3 ticks) were dissected. Tick tissues (salivary glands, midgut, and ovary) were collected and stored in RLT buffer (QIAGEN) for RNA extraction. Total RNA was isolated from tick tissues using the RNeasy Mini kit (QIAGEN) according to the manufacturer’s instruction. RNA was then treated with Dnase (Ambion) and purified using an RNA cleanup kit (Zymo Research). The synthesis of cDNA with random hexamers was carried out using 200 ng total RNA in 25 µl reaction volumes of an iScript reverse transcription kit (Bio-Rad).
In order to determine tissue-specific responses of ticks during rickettsial infection, backless ticks were generated according to a modified protocol of Bell (Bell, 1980) and used for rickettsial infection. In a laminar flow hood, forty-five unfed female D. variabilis ticks were cleaned with 70% ethanol for 2 min and 10% benzalkonium chloride solution for 5 min, and then rinsed with sterile water three times. The ticks were air-dried on sterile filter paper. Mouthparts and legs were excised to minimize contamination. Cleaned ticks were transversely cut along the perimeter of the alloscutum with a scalpel and the dorsal cuticle was taken off. The backless ticks were submerged in individual wells on a 96-well plate containing 200 µl of complete L15B medium and incubated at 34°C. After 24 h, the backless ticks were divided into three groups of 15 ticks per group. The unexposed group was incubated in L15B medium, while the second and third groups were exposed to R. amblyommii or R. montanensis (2.4 × 108 rickettsiae per tick per well), respectively. After 1 and 12 hours-post inoculation (hpi), the tick tissues (5 ticks per time time point) were dissected out, pooled, and kept in 100 µl RNALater at −20°C. Total RNA (40 ng) was used for cDNA synthesis with an iScript reverse transcription kit (Bio-Rad). cDNA template (2 µl) was subjected to qPCR as described above.
Statistical analysis
Analysis of variance was conducted using the SAS statistical package (version 9.2) GLM procedure. The relative gene expression of α–catenin from unfed and 5 day fed tick tissues was examined for potential difference. For the backless tick bioassay, the relative gene expression was analyzed after rank transformation and a two-way interaction was examined (rickettsial infection and tick tissues) analysis. When overall significance was found, Tukey’s honestly significant difference (HSD) post hoc test was performed to determine the pairwise difference of means of main effects. Pairwise t-tests of least-squares means were performed to determine any interaction effects of relative expression of Dvα-catenin between unfed and 5-day fed ticks, and unexposed and Rickettsia-exposed backless ticks. P-values of < 0.05 were considered significant.
Results
Full-length Dvα-catenin cDNA and sequence analysis
Gene specific primers designed from a Dvα-catenin fragment obtained by differential display PCR (Macaluso et al., 2003) were used to clone the full-length α-catenin cDNA. After sequence analysis (blastx), the 3069 bp full-length cDNA was designated Dvα-catenin (Genbank accession number HM755938). A putative 2718 bp ORF encodes an expected 905 amino acid protein with a calculated molecular weight of 96 kDa. The deduced amino acid sequence is shown in Figure 1. A multiple sequence alignment of Dvα-catenin amino acids showed the highest similarity to tick and insect α-catenin with 94.7% identity to Ixodes scapularis α-catenin (Genbank accession number XP002413819), 87.7% to Pediculus humanus corporis α-catenin (Genbank accession number XP002429770) and Aedes aegypti mosquito α-catenin (Genbank accession number XP001657216), and 85.6% to Drosophila melanogaster α-catenin (Genbank accession number NP524219), compared to Homo sapiens α-catenin (82.7% similarity, Genbank accession number NP004380) (Figure 1).
Figure 1. Multiple sequence comparison of α-catenin amino acid sequences.

The Dvα-catenin deduced amino acid sequence was aligned with Ixodes scapularis α-catenin (IsCatenin, accession No. XP002413819), Pediculus humanus corporis α-catenin (PhCatenin, accession No. XP002429770) Aedes aegypti α-catenin (AaCatenin, accession No. XP001657216), Drosophila melanogaster α-catenin (DmCatenin, accession No. NP524219), and Homo sapiens α-catenin (HsCatenin, accession No. NP004380). Alignment was performed using MacVector software. Shaded gray indicates conserved amino acid residues. The identity scores to Dvα-catenin were derived from pairwise alignment using ClustalW 1.83 software.
Conserved domains were identified using NCBI Conserved Domain Search Service (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=cdd). The Dvα-catenin amino acid sequence is homologous to a vinculin conserved domain at amino acid positions 19–865 and contains a putative F-actin binding region at the C-terminus as well as β-catenin binding and α-catenin dimerization regions at the N-terminus (Figure 2).
Figure 2. Putative protein binding sites of Dvα-catenin.
Numbers correspond to amino acids of the protein sequence. Shaded gray region is vinculin conserved domain containing β-catenin, α-catenin, and F-actin binding regions.
mRNA expression of Dvα-catenin in tick tissues and response to feeding
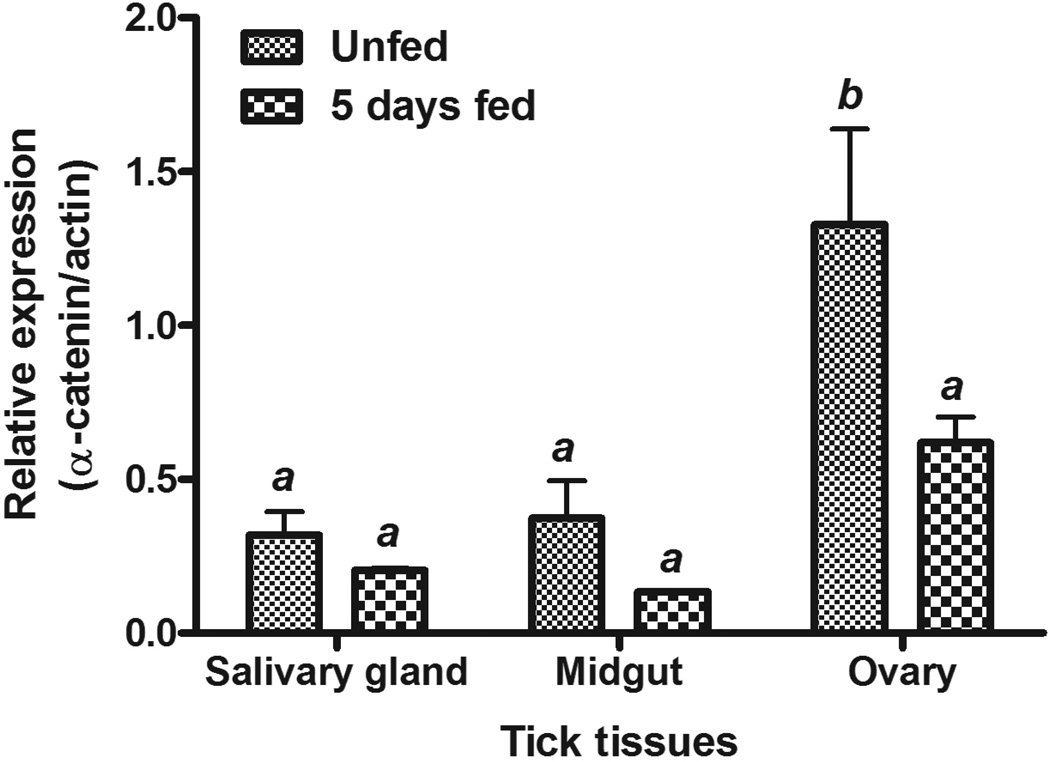
To determine the mRNA expression profile of Dvα-catenin in different tick tissues and its responses to blood feeding, total RNA samples from different tick tissues (salivary glands, midgut, and ovary) of unfed and 5-day fed ticks were subjected to qRT-PCR assay. Results show that expression of Dvα-catenin is significantly greater in tick ovary, compared to salivary gland and midgut tissues in unfed ticks. Feeding leads to a decline in the expression of this gene in all tissues, with the change in the ovary being significant (Figure 3) suggesting a response to blood feeding.
Figure 3. Tissue-specific expression of Dvα-catenin mRNA expression in unfed and 5-days fed D. variabilis.
Total RNA was extracted from tick tissues (salivary glands, midguts, and ovaries) and expression of Dvα-catenin was determined by qRT-PCR assay. Transcription level of Dvα-catenin was normalized to tick actin. Data shown are mean relative expression. Error bar represents standard error of means (SEM). The bars with same letter are not significantly different (P ≤ 0.05).
Tissue-specific gene expression of Dvα-catenin in response to rickettsial infection
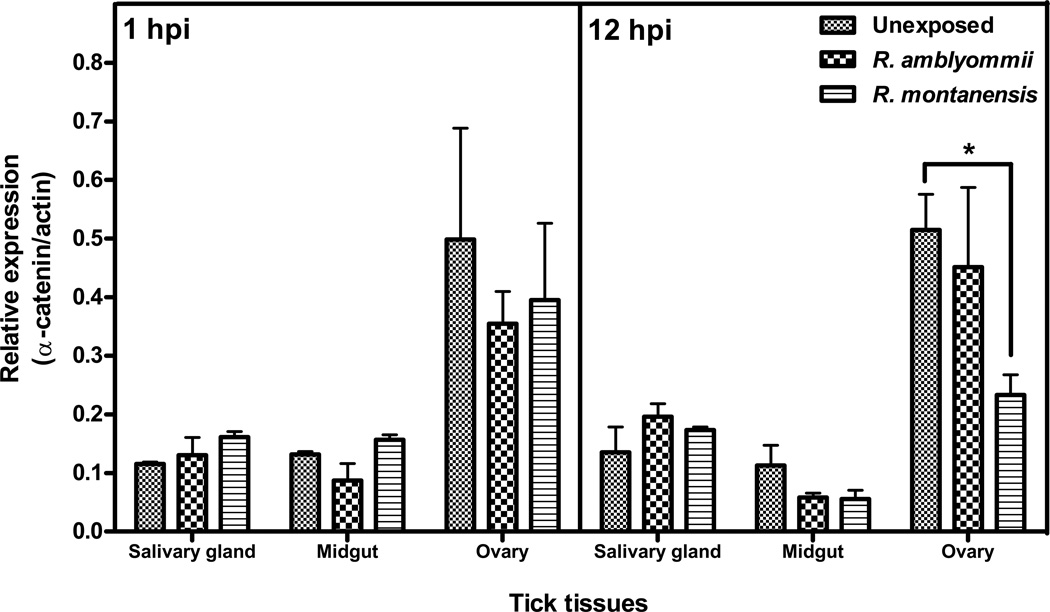
In order to assess the differential expression of Dvα-catenin in response to rickettsial infection in tick tissues and Rickettsia species-specific manner, an ex vivo study of tick tissues (backless tick bioassay) was performed. The tick dorsal integument was removed and backless ticks were exposed to equal amounts of either R. montanensis or R. amblyommii. After 1 and 12 hpi, total RNA of salivary glands, midguts, and ovaries from unexposed and Rickettsia-exposed ticks were subjected to qRT-PCR assay. Similar to the comparison between unfed and fed ticks, Dvα-catenin expression was greatest in tick ovary; no difference associated with rickettsial infection was identified at 1 hpi. A similar pattern of expression was observed at 12 hpi; however, a significant decrease of Dvα-catenin expression was observed in the R. montanensis-exposed ovary, compared to unexposed ticks (Figure 4).
Figure 4. Dvα-catenin mRNA expression in backless ticks during R. montanensis and R. amblyommii exposure.
Unfed D. variabilis female ticks were transversely cut along the perimeter of alloscutum, and the dorsal cuticle was taken off. Then, backless ticks were exposed to R. montanensis or R. amblyommii and incubated at 34°C. After 1 and 12 hpi, tick tissues were dissected and total RNA was extracted. Total RNA from each tissue was analyzed for expression of Dvα-catenin by qRT-PCR assay. Transcription level of Dvα-catenin was normalized to tick actin. Data shown are mean relative expression from two experiments. Error bar represents standard error of means (SEM). Asterisk indicates significant difference (P ≤ 0.05).
Discussion
The present study describes the molecular and biological characterization of Dvα-catenin from the American dog tick, D. variabilis. Multiple alignments demonstrate that α-catenin is molecularly conserved among species of ticks and other arthropods, as well as in humans. Consistent with other organisms, the characteristics of the deduced amino acid sequence shows homology with vinculin protein, containing putative α-catenin dimerization, β-catenin binding domain, and the actin binding domain (Pokutta et al., 2008). The conserved nature of the molecule suggests homologous function in cell structure within ticks.
In addition to molecular characterization, this study sought to assess the activity of Dvα-catenin in ticks. Female ixodid adult ticks are known to feed for extended periods of time (1 to 2 weeks), and dynamic changes in tick gene activity are associated with tick feeding (Aljamali et al., 2009; Chalaire et al., 2011; Mulenga & Khumthong, 2010a; Mulenga & Khumthong, 2010b). Most tick genes responsive to blood feeding are upregulated in salivary glands during tick feeding and related to manipulation of blood flow, blood digestion, and host immune responses, (Aljamali et al., 2009). Dvα-catenin is constitutively expressed in the tick tissues assessed including salivary glands, midgut, and ovary, with significantly greater expression in the ovary, compared to other tissues. During the slow phase of feeding, expression is significantly downregulated. After 5-days of feeding, expression of Dvα-catenin was still greatest in the ovary, although significantly downregulated compared to unfed ticks. Downregulation of Dvα-catenin expression in individual salivary glands and midguts was not significant. While it appears Dvα-catenin is responsive to feeding, the reason for this observed regulation is unknown.
The manipulation of host cytoskeletal molecules by microorganisms is not unprecedented. In vertebrate host cells, many species of bacteria including Listeria, Shigella, Rickettsia, Burkholderia, and Mycobacterium are able to modulate rearrangement of actin cytoskeleton in order to invade host cells (Dramsi & Cossart, 1998; Gouin et al., 2004; Hamaguchi et al., 2008; Sousa et al., 2005). For SFG Rickettsia, multiple species utilize host cytoskeletal components (actin-based) for movement in the host cells (Gouin et al., 2004; Heinzen et al., 1999; Heinzen, 2003). Dvα-catenin is one of several tick-derived molecules, which were identified as differentially expressed in R. montanensis-infected tick ovary. Specifically, transcript analysis suggested that expression was greater in tick ovary constitutively infected with R. montanensis, compared to uninfected tick ovary (Macaluso et al., 2003). The association between Dvα-catenin and Rickettsia infection in the ovary is not clear. During tick feeding after attachment and ingestion of host blood, oocytes begin to further develop in tick ovary (Sonenshine, 1993). Based on the metabolic coupling of tick and rickettsiae, including the feeding responsive reactivation of rickettsiae in tick tissues (Hayes & Burgdorfer, 1982), it is possible that SFG Rickettsia utilize α-catenin to modulate actin rearrangement in order to invade neighboring host cells during this period, resulting in an increase in Dvα-catenin expression. The bioassay developed in the current study was able to capture the influence of rickettsial infection on host cell Dvα-catenin expressionat the early stages of infection. In contrast to the constitutively-infected cohort of ticks examined previously (Macaluso et al., 2003), this study demonstrated that acute infection of R. montanensis, which is a typical Rickettsia for D. variabilis, downregulated Dvα-catenin expression in the ovary at 12 hpi. The differences in tick experimental models, including chronic versus acute infection and fed versus unfed ticks, between the previous study (Macaluso et al., 2003) and the current study make comparison difficult. However, we speculate that in chronically infected ticks, R. montanensis is reactivated in response to blood feeding; resulting in upregulated Dvα-catenin expression required for invasion and movement inside the host cells. It has been demonstrated recently that the surface cell antigen 4 (Sca4) of R. prowazekii binds to and activates vinculin which, in turn, binds to host cytoskeleton (Park et al., 2011). Interestingly, Dvα-catenin also contains a vinculin-conserved domain which may also bind to rickettsial Sca4 and alter host cytoskeleton. Whether or not the rickettsiae are manipulating the host expression of α–catenin, or if we measured the host response to insult, cannot be determined from the experiments in this study. Understanding the utilization of host molecules during chronic infection may be important for identifying the mechanisms for stable vertical transmission.
The current study examined acute infection and demonstrated that after 12 hpi Dvα-catenin expression is downregulated. We suggest this is a tick response to control the level of rickettsial infection in the ovary by downregulation of the Dvα-catenin, thus preventing utilization of the cytoskeletal components necessary for cell invasion. Differential regulation of other D. variabilis-derived molecules, including a Kunitz protease inhibitor, has been described during R. montanensis challenge in tick midgut (Ceraul et al., 2011). If as described for other bacteria, and α-catenin is a component of cell entry, Rickettsia may downregulate α-catenin expression upon initial infection of the ovary in order to inhibit infection and transovarial transmission of second rickettsial species. This competitive interaction is also known as the interference phenomenon, and has been observed in SFG Rickettsia infections of ticks (Burgdorfer et al., 1981; Macaluso et al., 2002). Many studies have demonstrated a specific receptor/ligand complex facilitating rickettsial internalization in vertebrate cells (Cardwell & Martinez, 2009; Chan et al., 2009; Martinez et al., 2005; Riley et al., 2010). A role for α-catenin in receptor/ligand binding in tick ovary during rickettsial infection is not known and further characterization is needed in order to understand the molecular interaction between Rickettsia and tissues central for sustained vertical transmission.
Transmission of SFG Rickettsia among ticks is complex as the tick serves as both the vector and reservoir. However, not all rickettsial species are horizontally transmitted by ticks and vertical transmission occurs with specificity as demonstrated transovarial transmission is limited to a few pairings (Macaluso et al., 2002). Despite the overlapping distribution of several tick and rickettsial species, combined field and laboratory studies suggest that the biological association between ticks and rickettsial species is specific. In order to study these relationships and examine the tick tissue-specific response, a modified tick tissue culture of backless ticks (Bell, 1980) was used to identify the alteration of Dvα-catenin expression only during R. montanensis infection; suggesting that the tick response was specific to certain Rickettsia species. Although not a substitute for the natural route of infection, the backless tick bioassay was chosen for several reasons. First, the assay allowed for delivery of known numbers of rickettsiae with equal opportunity to expose, or infect, all organs. Several studies have examined the tick response to microbial challenge using body cavity inoculation or oral challenge via capillary feeding (Baldridge et al., 2007; Ceraul et al., 2011; Kocan et al., 2008; Mulenga & Khumthong, 2010a). While this will allow for assessment of infection in the gut, the dissemination of rickettsiae after exposure is not clear. Because this study also intended to examine Dvα-catenin in the ovary during acute infection, whole organ exposure was desired. Through normalization of dissected ticks over 24 hours, and direct comparison to uninfected controls, the backless tick model is a functional bioassay to capture organ-specific responses.
Arthropods and microbes are known to have intimate relationships and many associations between arthropods and their endosymbionts are beneficial (Werren et al., 2008). For example, a beneficial effect of the agent of human granulocytic anaplasmosis (HGA), Anaplasma phagocytophilum, in Ixodes scapularis was suggested via regulation of tick-derived molecules (Neelakanta et al., 2010). For the SFG Rickettsia, it is likely that if there is decreased fitness associated with infection by some species of Rickettsia (Niebylski et al., 1999; Burgdorfer & Brinton, 1975), and if harboring a non-lethal species is actually beneficial to the tick host (Burgdorfer et al., 1981), then a specific tick/Rickettsia relationship would develop. The intricate mechanisms that facilitate this infection are unknown. The current study provides a pathway to identifying the molecules associated with rickettsial infection of the tick vector and a bioassay to assess the tissue-specific nature of these relationships. Ultimately, studying specific interactions between tick vectors and Rickettsia will lead to a better appreciation of ecology and epidemiology of tick-borne rickettsial diseases.
Acknowledgements
We thank Christopher Paddock for R. amblyommii (Darkwater strain), Also, Chutima Thepparit, Supanee Hirunkanokpun, Britton Grasperge, and Mark Guillotte for their technical assistance. We also thank Jacqueline Macaluso for helpful comments. This research was supported by the National Institutes of Health (AI077784). This work was also part of P. Sunyakumthorn’s doctoral dissertation.
Reference List
- 1.Aljamali MN, Ramakrishnan VG, Weng H, Tucker JS, Sauer JR, Essenberg RC. Microarray analysis of gene expression changes in feeding female and male lone star ticks, Amblyomma americanum (L) Arch.Insect Biochem.Physiol. 2009;71:236–253. doi: 10.1002/arch.20318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammerman NC, Swanson KI, Anderson JM, Schwartz TR, Seaberg EC, Glass GE, Norris DE. Spotted-fever group Rickettsia in Dermacentor variabilis , Maryland. Emerg.Infect.Dis. 2004;10:1478–1481. doi: 10.3201/eid1008.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldridge GD, Kurtti TJ, Burkhardt N, Baldridge AS, Nelson CM, Oliva AS, Munderloh UG. Infection of Ixodes scapularis ticks with Rickettsia monacensis expressing green fluorescent protein: a model system. J.Invertebr.Pathol. 2007;94:163–174. doi: 10.1016/j.jip.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell LJ. Organ culture of Rhipicephalus appendiculatus with maturation of Theileria parva in tick salivary glands in vitro. Acta Trop. 1980;37:319–325. [PubMed] [Google Scholar]
- 5.Burgdorfer W, Brinton LP. Mechanisms of transovarial infection of spotted fever Rickettsiae in ticks. Ann.N.Y.Acad.Sci. 1975;266:61–72. doi: 10.1111/j.1749-6632.1975.tb35088.x. [DOI] [PubMed] [Google Scholar]
- 6.Burgdorfer W, Hayes EB, Marvos AJ. Nonpathogenic rickettsiae in Dermacentor andersoni: A limiting factor for the distribution of Rickettsia rickettsii. In: Burgdorfer W, Anacker RL, editors. Rickettsiae and Rickettsial Diseases. New York: Acedemic Press, Inc.; 1981. pp. 585–594. [Google Scholar]
- 7.Cardwell MM, Martinez JJ. The Sca2 autotransporter protein from Rickettsia conorii is sufficient to mediate adherence to and invasion of cultured mammalian cells. Infect.Immun. 2009;77:5272–5280. doi: 10.1128/IAI.00201-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceraul SM, Chung A, Sears KT, Popov VL, Beier-Sexton M, Rahman MS, Azad AF. A Kunitz protease inhibitor from Dermacentor variabilis, a vector for spotted fever group rickettsiae, limits Rickettsia montanensis invasion. Infect.Immun. 2011;79:321–329. doi: 10.1128/IAI.00362-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chalaire KC, Kim TK, Garcia-Rodriguez H, Mulenga A. Amblyomma americanum (L.) (Acari: Ixodidae) tick salivary gland serine protease inhibitor (serpin) 6 is secreted into tick saliva during tick feeding. J.Exp.Biol. 2011;214:665–673. doi: 10.1242/jeb.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan YG, Cardwell MM, Hermanas TM, Uchiyama T, Martinez JJ. Rickettsial outer-membrane protein B (rOmpB) mediates bacterial invasion through Ku70 in an actin, c-Cbl, clathrin and caveolin 2-dependent manner. Cell Microbiol. 2009;11:629–644. doi: 10.1111/j.1462-5822.2008.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dramsi S, Cossart P. Intracellular pathogens and the actin cytoskeleton. Annu.Rev.Cell Dev.Biol. 1998;14:137–166. doi: 10.1146/annurev.cellbio.14.1.137. [DOI] [PubMed] [Google Scholar]
- 12.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumler JS. Fitness and freezing: vector biology and human health. J.Clin.Invest. 2010;120:3087–3090. doi: 10.1172/JCI44402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gates J, Peifer M. Can 1000 reviews be wrong? Actin, alpha-Catenin, and adherens junctions. Cell. 2005;123:769–772. doi: 10.1016/j.cell.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Gouin E, Egile C, Dehoux P, Villiers V, Adams J, Gertler F, Li R, Cossart P. The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature. 2004;427:457–461. doi: 10.1038/nature02318. [DOI] [PubMed] [Google Scholar]
- 16.Hamaguchi M, Hamada D, Suzuki KN, Sakata I, Yanagihara I. Molecular basis of actin reorganization promoted by binding of enterohaemorrhagic Escherichia coli EspB to alpha-catenin. FEBS J. 2008;275:6260–6267. doi: 10.1111/j.1742-4658.2008.06750.x. [DOI] [PubMed] [Google Scholar]
- 17.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim.Biophys.Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes SF, Burgdorfer W. Reactivation of Rickettsia rickettsii in Dermacentor andersoni ticks: an ultrastructural analysis. Infect.Immun. 1982;37:779–785. doi: 10.1128/iai.37.2.779-785.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinzen RA. Rickettsial actin-based motility: behavior and involvement of cytoskeletal regulators. Ann.N.Y.Acad.Sci. 2003;990:535–547. doi: 10.1111/j.1749-6632.2003.tb07424.x. [DOI] [PubMed] [Google Scholar]
- 20.Heinzen RA, Grieshaber SS, Van Kirk LS, Devin CJ. Dynamics of actin-based movement by Rickettsia rickettsii in vero cells. Infect.Immun. 1999;67:4201–4207. doi: 10.1128/iai.67.8.4201-4207.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kocan KM, de la Fuente J, Manzano-Roman R, Naranjo V, Hynes WL, Sonenshine DE. Silencing expression of the defensin, varisin, in male Dermacentor variabilis by RNA interference results in reduced Anaplasma marginale infections. Exp.Appl.Acarol. 2008;46:17–28. doi: 10.1007/s10493-008-9159-5. [DOI] [PubMed] [Google Scholar]
- 22.Kodama T, Akeda Y, Kono G, Takahashi A, Imura K, Iida T, Honda T. The EspB protein of enterohaemorrhagic Escherichia coli interacts directly with alpha-catenin. Cell Microbiol. 2002;4:213–222. doi: 10.1046/j.1462-5822.2002.00176.x. [DOI] [PubMed] [Google Scholar]
- 23.Macaluso KR, Mulenga A, Simser JA, Azad AF. Differential expression of genes in uninfected and rickettsia-infected Dermacentor variabilis ticks as assessed by differential-display PCR. Infect.Immun. 2003;71:6165–6170. doi: 10.1128/IAI.71.11.6165-6170.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF. Infection and transovarial transmission of rickettsiae in Dermacentor variabilis ticks acquired by artificial feeding. Vector.Borne.Zoonotic.Dis. 2001;1:45–53. doi: 10.1089/153036601750137660. [DOI] [PubMed] [Google Scholar]
- 25.Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J.Med.Entomol. 2002;39:809–813. doi: 10.1603/0022-2585-39.6.809. [DOI] [PubMed] [Google Scholar]
- 26.Martinez JJ, Cossart P. Early signaling events involved in the entry of Rickettsia conorii into mammalian cells. J.Cell Sci. 2004;117:5097–5106. doi: 10.1242/jcs.01382. [DOI] [PubMed] [Google Scholar]
- 27.Martinez JJ, Seveau S, Veiga E, Matsuyama S, Cossart P. Ku70, a component of DNA-dependent protein kinase, is a mammalian receptor for Rickettsia conorii. Cell. 2005;123:1013–1023. doi: 10.1016/j.cell.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 28.Mcdade JE, Newhouse VF. Natural History of Rickettsia rickettsii. Annual Review of Microbiology. 1986;40:287–309. doi: 10.1146/annurev.mi.40.100186.001443. [DOI] [PubMed] [Google Scholar]
- 29.Mulenga A, Khumthong R. Disrupting the Amblyomma americanum (L.) CD147 receptor homolog prevents ticks from feeding to repletion and blocks spontaneous detachment of ticks from their host. Insect Biochem.Mol.Biol. 2010a;40:524–532. doi: 10.1016/j.ibmb.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Mulenga A, Khumthong R. Silencing of three Amblyomma americanum (L.) insulin-like growth factor binding protein-related proteins prevents ticks from feeding to repletion. J.Exp.Biol. 2010b;213:1153–1161. doi: 10.1242/jeb.035204. [DOI] [PubMed] [Google Scholar]
- 31.Mulenga A, Macaluso KR, Simser JA, Azad AF. Dynamics of Rickettsia-tick interactions: identification and characterization of differentially expressed mRNAs in uninfected and infected Dermacentor variabilis. Insect Mol.Biol. 2003;12:185–193. doi: 10.1046/j.1365-2583.2003.00400.x. [DOI] [PubMed] [Google Scholar]
- 32.Mulenga A, Simser JA, Macaluso KR, Azad AF. Stress and transcriptional regulation of tick ferritin HC. Insect Mol.Biol. 2004;13:423–433. doi: 10.1111/j.0962-1075.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 33.Neelakanta G, Sultana H, Fish D, Anderson JF, Fikrig E. Anaplasma phagocytophilum induces Ixodes scapularis ticks to express an antifreeze glycoprotein gene that enhances their survival in the cold. J.Clin.Invest. 2010;120:3179–3190. doi: 10.1172/JCI42868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niebylski ML, Peacock MG, Schwan TG. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni) Appl.Environ.Microbiol. 1999;65:773–778. doi: 10.1128/aem.65.2.773-778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park H, Lee JH, Cossart P, Gouin E, Izard T. The Rickettsia surface cell antigen 4 applies mimicry to bind to and activate vinculin. J.Biol.Chem. 2011;286:35096–35103. doi: 10.1074/jbc.M111.263855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pokutta S, Drees F, Yamada S, Nelson WJ, Weis WI. Biochemical and structural analysis of alpha-catenin in cell-cell contacts. Biochem.Soc.Trans. 2008;36:141–147. doi: 10.1042/BST0360141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riley SP, Goh KC, Hermanas TM, Cardwell MM, Chan YG, Martinez JJ. The Rickettsia conorii autotransporter protein Sca1 promotes adherence to nonphagocytic mammalian cells. Infect.Immun. 2010;78:1895–1904. doi: 10.1128/IAI.01165-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serio AW, Jeng RL, Haglund CM, Reed SC, Welch MD. Defining a core set of actin cytoskeletal proteins critical for actin-based motility of Rickettsia. Cell Host.Microbe. 2010;7:388–398. doi: 10.1016/j.chom.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith MP, Ponnusamy L, Jiang J, Ayyash LA, Richards AL, Apperson CS. Bacterial pathogens in ixodid ticks from a Piedmont County in North Carolina: prevalence of rickettsial organisms. Vector.Borne.Zoonotic.Dis. 2010;10:939–952. doi: 10.1089/vbz.2009.0178. [DOI] [PubMed] [Google Scholar]
- 40.Sonenshine DE. Biology of Ticks. New York: Oxford University Press; 1993. [Google Scholar]
- 41.Sousa S, Cabanes D, Archambaud C, Colland F, Lemichez E, Popoff M, Boisson-Dupuis S, Gouin E, Lecuit M, Legrain P, Cossart P. ARHGAP10 is necessary for alpha-catenin recruitment at adherens junctions and for Listeria invasion. Nat.Cell Biol. 2005;7:954–960. doi: 10.1038/ncb1308. [DOI] [PubMed] [Google Scholar]
- 42.Stromdahl EY, Jiang J, Vince M, Richards AL. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group rickettsiae in the United States. Vector.Borne.Zoonotic.Dis. 2010;7:969–977. doi: 10.1089/vbz.2010.0099. [DOI] [PubMed] [Google Scholar]
- 43.Thepparit C, Bourchookarn A, Petchampai N, Barker SA, Macaluso KR. Interaction of Rickettsia felis with histone H2B facilitates the infection of a tick cell line. Microbiology. 2010;156:2855–2863. doi: 10.1099/mic.0.041400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat.Rev.Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- 45.Williamson PC, Billingsley M, Teltow GJ, Seals JP, Turnbough MA, Atkinson SF. Borrelia, Ehrlichia and Rickettsia spp. in ticks removed from persons, Texas, USA. Emerg.Infect.Dis. 2010;16:441–446. doi: 10.3201/eid1603.091333. [DOI] [PMC free article] [PubMed] [Google Scholar]