Abstract
Changes in step width (SW), step length (SL), and/or the variability of these parameters have been prospectively related to risk of falling. However, it is unknown how voluntary changes in SW and SL directly alter variability and/or dynamic stability of walking. Here, we quantified how variability and dynamic stability of human walking changed when individuals voluntarily manipulated SW and SL. Fourteen unimpaired, young adults walked on a treadmill at their preferred walking speed with normal gait, with a metronome and with narrower, wider, shorter and longer steps than normal. Taking narrower steps caused increased SL variability while mediolateral (ML) movements of the C7 vertebra (i.e., trunk) became locally more stable (p < 0.05) and anterior-posterior (AP) C7 movements became locally less stable (p < 0.05). Taking wider steps caused increased SW and SL variability, while ML C7 movements became both locally and orbitally less stable (p < 0.05). Any change in SL caused increased SW, SL, and stride time variability. When taking shorter steps, ML C7 movements exhibited greater short-term local and orbital instability, while AP C7 movements exhibited decreased short-term and long-term local instability (p < 0.05). When taking longer steps, AP, ML, and vertical C7 movements all exhibited increased long-term local instability and increased orbital instability (p < 0.05). Correlations between mean SW, SL and dynamic stability of C7 marker motions were weak. However, short-term voluntary changes in SW and SL did significantly alter local and orbital stability of trunk motions.
Keywords: Dynamic stability, walking, step width, step length
INTRODUCTION
In clinical studies, gait parameters are frequently used to indicate patients’ dynamic stability. However, the correct interpretation of these results is not clear. Walking with wider and shorter steps than normal is often termed “cautious”. However, retrospective studies indicate that individuals who exhibit increased fall risk sometimes walk with shorter [1, 2], longer [3], narrower [1], wider and/or faster [3] steps than normal. Slower walking speeds alone lead to decreased local instability [4-6] yet are also associated with history of falling [7]. Slower walking speeds also increase motion variability [5, 6], which may or may not also indicate increased risk of falling. In one study, too much or too little step width variability was associated with fall history in older adults who walked at normal speeds (> 1 m/s) [8]. Results of these and other retrospective studies do not reveal a clear understanding of the relationship between gait characteristics and fall risk.
Prospective studies on gait characteristics and falling are similarly mixed. Maki [9] found that while shorter and slower steps predicted increased fear of falling, increased variability of stride length and speed doubled an individual’s actual likelihood of falling. Additionally, individuals who fell while walking took wider steps with less step width (SW) variability [9]. Contrary to Maki [9], Hausdorff and colleagues [10] found that increased stride time (ST) variability over a 6 minute walk predicted falls in older adults. Verghese and colleagues [11] found that slow gait speeds and increases in swing time and stride length variability all predicted increased fall risk in older adults. However, in both studies [10, 11], the greater variability observed could have been due simply to slower walking speeds [5, 6]. DeMott et al. [12] determined that falls in older individuals with peripheral neuropathy were predicted by greater step time variability when walking on irregular surfaces but not on smooth surfaces. Recently, others found that no primary gait variables (means nor variability) predicted falls, but subtle left-right asymmetries in statistical persistence of stride time did [13]. Thus, all five of these prospective studies reached different findings with similar methods and measures.
The fact that shorter, wider and/or more variable steps in patients are associated with increased fall risk might suggest that people who exhibit these patterns are more unstable. However, when external lateral stabilization was applied, young and older subjects both took narrower steps, without changing their mean step length (SL), SW variability, or SL variability [14]. Likewise, when we destabilized healthy young subjects by applying continuous perturbations, they took shorter, wider and faster steps and exhibited greater variability both of stepping parameters and trunk kinematics [15]. These gait changes were accompanied by specific increases in measures of local dynamic instability [16], a measure of within-step dynamic stability. If individuals adopted these gait characteristics to increase their stability, then voluntarily taking shorter or wider steps during unperturbed walking should lead specifically to decreased variability and local instability of stepping parameters and/or trunk movements.
Collectively, these earlier findings suggest two opposing ideas: people increase their risk of falling because they take shorter and/or wider steps, or they take wider and/or shorter steps because they are at greater risk of falling. We wanted to test the latter idea: i.e., that adopting wider and/or shorter steps would decrease individuals’ instability thereby decreasing their risk of falling. We hypothesized that individuals could alter their orbital (i.e., step-to-step) and local dynamic (i.e., within-step) trunk stability by voluntarily changing their SW and SL. We further hypothesized that individuals would exhibit decreased orbital and local instability when walking with wider steps or shorter steps than when walking normally. Our findings would indicate whether and how voluntarily changing gait characteristics contributes to local and orbital stability during walking, the latter of which, in particular, has been linked to fall-risk status [17].
METHODS
Fourteen young healthy adults (7 male, 7 female; age 18 - 35) participated. Participants were excluded for any history of lower extremity injuries, surgery or neurological conditions, which could affect their gait. The University of Texas Institutional Review Board approved this study, and all participants provided written, informed consent prior to participation.
Participants walked on a motorized treadmill (Desmo ProXL model, Woodway USA, Waukesha, WI). Each subject completed a ~10 minute warm-up. The first 5 minutes were used to determine participants’ preferred walking speeds (PWS) using an established protocol [5]. During the second 5 minutes, participants walked at their PWS. Participants then completed three 3-minute walking trials for each of 6 experimental conditions. During the normal (NO) condition, participants walked normally. During the normal with metronome (NM) condition, participants walked normally with a metronome matched to their preferred cadence. During the SW manipulations, participants were instructed to walk with wider (WI) and narrower (NA) steps than normal. During the SL manipulations, participants walked with shorter (SH) and longer (LO) steps. The latter were achieved by walking in time with a metronome, set to a cadence that was 10 steps/min faster or slower, respectively, than their preferred step cadence. Participants walked at their PWS for all conditions. The NO condition was always presented first and the remaining 5 conditions were presented in a random order to minimize learning effects. Rest breaks, during which the treadmill was stopped, were provided between conditions.
Participants wore reflective markers on their trunk and feet. Ten Vicon (Oxford Metrics, Oxford, UK) MX cameras captured participants’ motion at 60 Hz. Vicon Nexus software was used to reconstruct, label and export the data for further processing in Matlab (The Mathworks, Inc.).
SL was defined as the anterior posterior distance between the heel markers at heel strike. SW was defined as the lateral distance between heel markers at heel strike. Stride time (ST) was the amount of time elapsed between two consecutive heel strikes of the same foot. Means and standard deviations of SL, SW and ST were calculated for each trial.
For stability analyses, we focused on trunk motion stability as indicated by the C7 vertebral marker motion. Trunk motions were studied because maintaining dynamic stability of the upper body is a primary objective of human locomotion [18]. Delay embedded state spaces describing trunk motion were constructed for the anterior-posterior (AP), mediolateral (ML) and vertical directions from the C7 marker velocity and time-delayed copies of the C7 marker velocity [5, 16]:
| (1) |
where S(t) is the dE-dimensional state vector, v(t) is the original 1-dimensional data (i.e. C7 velocity in the AP, ML or vertical direction), τ is the time delay and dE is the embedding dimension. Time delays were set equal to τ = 0.333, 0.250 and 0.167 (i.e. 20, 15 and 10 data samples) for the ML, AP and vertical directions, respectively. Results were not expected to be sensitive to the exact values of τ [19]. For the local stability analysis, 120 consecutive strides of data were normalized to 12,000 total data points, or approximately 100 data points per stride [4, 20], prior to defining the state space.
Floquet multipliers (FM) estimated orbital instability, which is defined as the ability of a periodic system (i.e. one’s motion) to return to a “preferred” state (i.e., a limit cycle) within one stride after being perturbed away from that preferred limit cycle state. FM are specifically defined for use with periodic systems or motions. The calculations are based on well-established techniques [16, 21-23] and are explained in detail in the Supplementary Material. When the magnitude of the largest FM (MaxFM) is < 1, this indicates orbital stability (i.e., state space trajectories converge towards the limit cycle after successive strides). Relative increases in MaxFM indicate increases in orbital instability.
Local divergence exponents (λ*) estimated local dynamic instability, which is defined as the rate at which a system responds in real time to infinitesimally small perturbations away from some nominal state. Unlike Floquet multipliers, these λ* exponents are defined to quantify stability of aperiodic systems. It is thus assumed that there is no specific “preferred” state or limit cycle [22]. Here, short-term (λ*S) and long-term (λ*L) exponents were calculated between 0 and 1 strides (λ*S) and between 4 and 10 strides (λ*L), respectively [24]. Positive λ* indicate local instability (i.e., state space trajectories diverge away from each other in real time). Smaller, positive λ* indicate less instability than larger, positive λ*.
Both sets of nonlinear stability analyses were conducted here because walking is neither purely periodic nor purely aperiodic, but lies somewhere in between. Thus, it is appropriate to calculate both orbital and local dynamic stability, as each of these measures quantifies unique aspects of how humans respond to small perturbations [22]. All stability calculations were performed separately for C7 marker movements in the ML, AP and vertical directions.
Two-factor (Condition × Subject) analyses of variance (ANOVA) were used to assess statistical differences between SW, SL, ST and SW, SL and ST variability, MaxFM, λ*S and λ*L for the SW and SL conditions separately (i.e. NO vs. WI and NA and NM vs. LO and SH). P-values < 0.05 were considered significant. All statistical analyses were conducted using PASW Statistics 18 (SPSS, Inc., Chicago, IL). Correlations were calculated using Matlab.
RESULTS
Instructing participants to walk with narrower or wider steps than normal resulted in SWs that were narrower and wider than normal (p < 0.0005; Fig. 1A), as expected. Walking with wide steps significantly increased SW variability (p < 0.0005; Fig. 1B). Narrow steps were associated with increased mean SL (p = 0.002) and SL variability (p < 0.0005) whereas wide steps decreased mean SL (p < 0.005) and increased SL variability (p < 0.0005). Both narrower and wider steps caused increases in mean ST (p < 0.0005 and p = 0.004, respectively) and decreases in ST variability (p = 0.001 and p < 0.0005, respectively). There were significant subject interactions for mean SL (p < 0.0005), SL variability (p = 0.027) and mean ST (p < 0.0005) but not mean SW (p = 0.276), SW variability (p = 0.08) or ST variability (p = 0.105).
Figure 1.
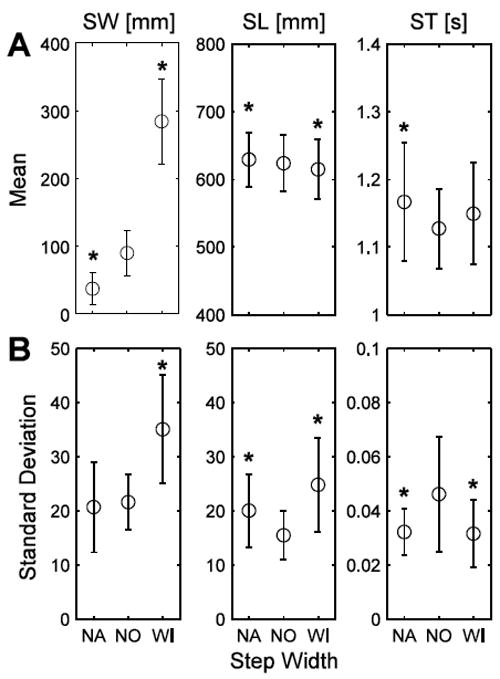
(A) SW, SL and ST and (B) step parameter variability for the SW manipulation conditions: narrow (NA), normal (NO) and wide (WI). * indicates significance at p < 0.05-level from NO. Error bars indicate +/- one standard deviation of the mean.
When walking with wide steps, participants’ C7 marker movements exhibited increased short-term local instability (λ*S) in all directions of motion (p < 0.0005; Fig. 2A). Walking with wide steps was also associated with greater long-term local instability (λ*L; Fig. 2B) and less orbital stability (i.e., larger MaxFM; Fig. 2C) of the ML C7 marker movements (p < 0.0005). AP C7 marker movements became more long-term locally stable, however, when walking with wide steps (p < 0.0005; Fig. 2B). When walking with narrow steps, ML C7 marker motions became locally more stable (i.e., decreased λ*) in both the short- (p < 0.0005) and long-term (p = 0.014) (Fig. 2A,B). Conversely, AP C7 marker motions became locally more unstable in both the short- and long-term (p < 0.0005; Fig. 2A,B). Vertical C7 marker motions exhibited greater short-term local instability (p < 0.0005; Fig. 2A,B) when walking with narrow steps. There were significant subject interactions for λ*S calculations in all directions (p < 0.05) and for λ*L and MaxFM in the AP direction (p < 0.02).
Figure 2.
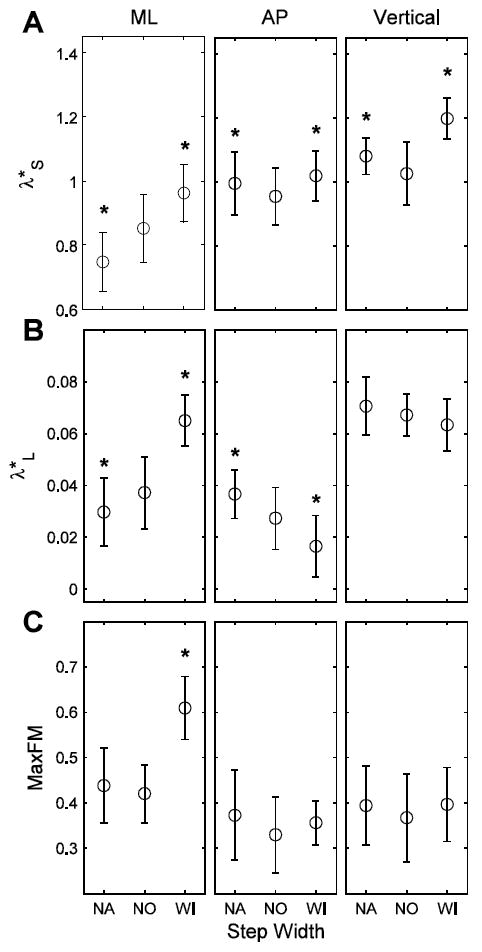
(A) λ*S, (B) λ*L and (C) MaxFM, indicating short-term, long-term and orbital stability of the C7 marker movements, in the ML, AP and vertical directions for the SW manipulation conditions (NO, WI, NA). * indicates significance at the p < 0.05-level from NO. Note: Increases in λ*S, λ*L and MaxFM indicate greater instability of the motion of the C7 vertebral marker velocity profile.
Walking with longer steps significantly increased mean SW and SW variability (p < 0.0005; Fig. 3A). However, walking with short steps only increased SW variability (p < 0.0005; Fig. 3B). As expected, walking with longer steps increased mean SL (p < 0.0005) and mean ST (p < 0.0005) and walking with shorter steps decreased mean SL (p < 0.0005) and mean ST (p = 0.002) (Fig. 3A). SL variability and ST variability (Fig. 3B) increased with both longer (p < 0.0005 and 0.001, respectively) and shorter (p < 0.0005) steps. Again, there were significant subject interactions for mean SW and SW variability (p < 0.0005), mean SL (p < 0.0005) and SL variability (p = 0.043) and mean ST (p < 0.0005) and ST variability (p = 0.014).
Figure 3.
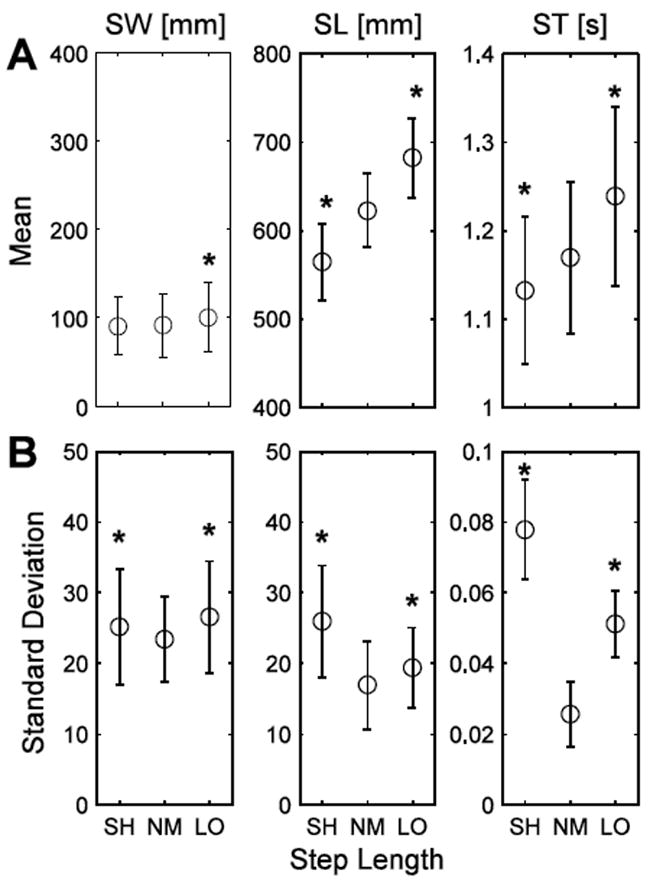
(A) SW, SL and ST and (B) step parameter variability for the SL manipulation conditions: short (SH), normal with metronome (NM) and long (LO). * indicates significance at p < 0.05-level from NM. Error bars indicate +/- one standard deviation of the mean.
Taking longer steps resulted in increased long-term local instability (Fig. 4B) and less orbital stability (MaxFM; Fig. 4C) of the C7 marker movements in all directions and increased short-term local instability (Fig. 4A) of C7 marker movements in the AP and VT directions (p < 0.05). However, walking with longer steps reduced short-term local instability of ML C7 marker movements (p < 0.0005; Fig. 4A). Walking with shorter steps yielded inconsistent changes in both local and orbital stability of C7 marker movements. Walking with shorter steps produced increased short-term local (Fig. 4A) and orbital instability (Fig. 4C) of ML C7 marker motions (p ≤ 0.05), as well as increased short-term local instability of vertical C7 marker motions (p < 0.0005; Fig. 4A). Conversely, walking with shorter steps decreased both short-term and long-term local instability of AP C7 marker movements (p ≤ 0.001; Fig. 4A,B), as well as the long-term local instability of vertical C7 marker movements (p = 0.001; Fig. 4B). There were significant subject interactions for all SL manipulation stability results in all directions (p < 0.005) except for short-term local stability of the vertical C7 marker motion (p = 0.602).
Figure 4.
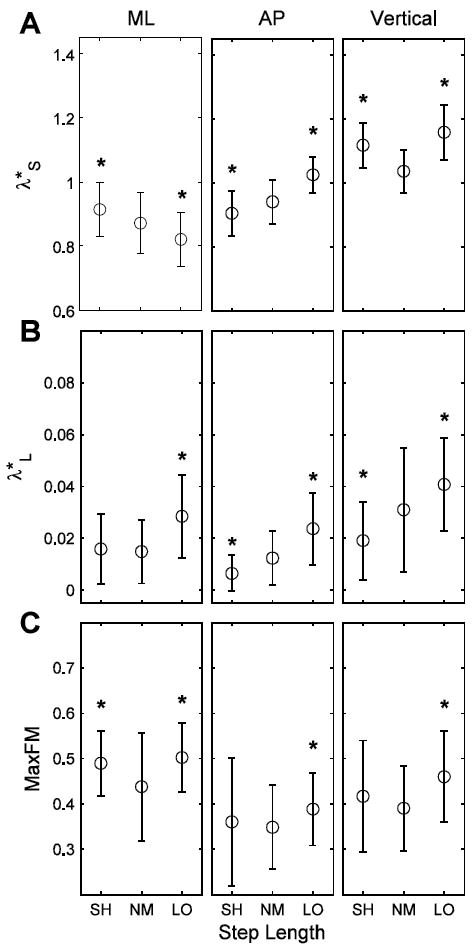
(A) λ*S, (B) λ*L and (C) MaxFM, indicating short-term, long-term and orbital stability of the C7 marker movements, in the ML, AP and vertical directions for the SL manipulation conditions (NM, LO and SH). * indicates significance at p < 0.05-level from NM. Note: Increases in λ*S, λ*L and MaxFM indicate greater instability of the motion of the C7 vertebral marker velocity profile.
Mean SW and SW variability demonstrated the strongest associations with nonlinear stability measures (Fig. 5). However, even the strongest correlations were still moderate (r2 ≈ 0.4). We have only presented results with the strongest correlations, which were between mean SW and SW variability and ML C7 movements. Correlations between stability, gait parameters and gait parameter variability were weaker for the AP and vertical C7 marker motion.
Figure 5.
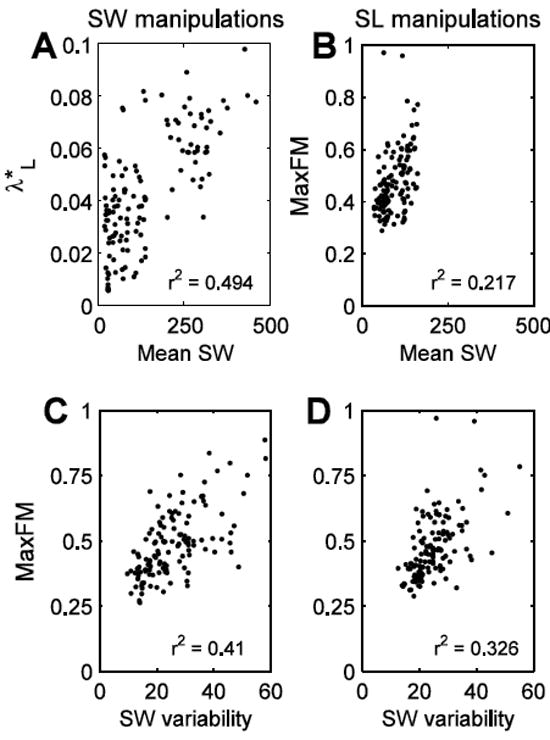
Maximum correlations between stability measures and gait characteristics or gait characteristic variability for the SW (A and C) and SL (B and D) manipulation conditions. Note that mean SW and SW variability had the greatest correlations with stability of the C7 marker movements. Though mean SW had the greatest correlation with λ*L during the SW manipulations, mean SW also had a similar correlation with MaxFM (r2 = 0.441). R2 values were significant at the p < 0.0005-level.
DISCUSSION
Simple, voluntary changes in basic gait characteristics can significantly affect an individual’s gait variability and orbital and local stability when walking at a constant speed. However, taking wider and shorter steps did not influence stability in the way we had hypothesized. Walking with wider or longer steps was associated with increased orbital and long-term local instability of ML C7 movements. Walking with shorter and wider steps was associated with increased short-term local instability of ML C7 movements. However, any change in gait characteristics from normal walking resulted in significant changes in short-term local stability.
The nonlinear measures of stability used here quantified stability of the trunk marker velocity signal. Alternative approaches that quantify the relationship between the center of mass and the boundaries of the base of support (BOS), for example [25], are based on fundamentally different definitions of what “stability” is. In these latter paradigms, one would expect walking with wider steps to make an individual more stable laterally because wider step widths increase the BOS laterally and thus increase the lateral margin of stability [25]. However, walking with wider SW also changes the velocity profile of the trunk, and thus the C7 marker movements from which our stability measures are derived, causing the trunk to move more variably between strides than during normal walking. This difference in how stability is defined may explain the weak correlations demonstrated between step characteristics and orbital and local stability of the C7 marker movements (Fig. 5).
Kuo and others [14, 23, 26, 27] suggested that control of lateral stability is more important than sagittal plane control during walking. This likely explains why we observed greater, though still low, correlations between orbital or local stability and SW than between orbital or local stability and SL (Fig. 5). It also may explain why increased SW variability while walking with wider steps (Fig. 1B) was associated with increased ML dynamic instability of the C7 marker movements (Fig. 2). Dean et al. [14] found that individuals, independent of age, preferred walking with narrower steps when external stabilization was applied. Our study indicates that voluntarily adopting narrower steps, even without external stabilization, increases ML C7 local stability (Fig. 2A,B), possibly due to the C7 marker motion being constrained to limits within which walking with narrow steps can be achieved. Meanwhile, SL variability increased when narrower steps were voluntarily adopted (Fig. 1B) which likely contributed to the loss of local stability observed in the C7 motion in the AP direction (Fig. 2A,B).
Prospective studies indicated increased risk of falling was associated with increased SL variability and SW, decreased SW variability [9], increased ST variability [10, 12] and left-right asymmetries [13]. Here, subjects demonstrated increased SL variability regardless of the gait characteristic adopted (Fig. 1B and 3B). However, if this change indicated increased risk of falling, the increased risk was only consistently reflected in changes in λ*S of vertical C7 movements (Fig. 2A and 4A) and, to some degree, MaxFM of ML C7 movements (Fig. 2C and 4C). For the other prospective indicators of falling, no consistent response in stability of C7 marker movement occurred. For example, walking with wider steps for the WI condition versus walking with longer steps for the LO condition, during which conditions subjects exhibited increased SW (Fig. 1B), yielded different stability outcomes (Fig. 2 and 4). These discrepancies in response may be partially explained by walking speed. We controlled for speed and thus our results can only indicate that adopting these altered gait parameters without changing speed does not predictably alter stability of C7 marker motion. If speed were left unconstrained, individuals could, for example, alter their gait characteristics to slow down to try to become more stable [4, 5].
It should not be surprising that the correlations between gait parameter variability and stability measures were not stronger (Fig. 5) given the mixed findings that have been published regarding the relationship between nonlinear stability measures and spatial variability (MeanSD) [28-30]. In the present study, SW variability and SL variability quantified spatial variability of our subjects’ movement and exhibited only low to moderate correlations with measures of local and orbital stability (r2 ≈ 0.4). Likewise, correlations between step parameters and measures of local and orbital stability were low, indicating that changes in mean SW and mean SL do not, to a high degree, affect the stability of the C7 marker motion.
One potential limitation of the present study was the adaptation time for the gait patterns examined. Subjects were given 3-5 minutes to adapt to each condition. This may not have been enough time to fully optimize walking strategies with the corresponding gait modifications. This may have caused, for example, the increased gait parameter variability (Fig. 1B and 3B) or changes in C7 marker movement stability (Fig. 2 and 4). It is possible that, given additional adaptation time, subjects could have adapted their gait pattern to maximize stability while using the voluntary gait modifications. However, our results clearly show there are no short-term benefits to walking with the shorter or wider steps associated with “cautious” walking patterns.
In conclusion, we demonstrated that local and orbital stability of the C7 marker movements could be manipulated through voluntary changes in gait characteristics. Increasing SW and decreasing SL did not increase stability as we hypothesized. Rather increasing SW and decreasing SL resulted in less ML local and orbital stability of the C7 marker movement indicating more unstable, less consistent ML trunk motion. While our results cannot fully reconcile the conflicting findings of previous retrospective and prospective studies regarding step characteristics and fall-risk, we have shown that adopting altered gait characteristics in the short term (even those which can increase the base of support) can reduce stability of individuals’ trunk motions.
Supplementary Material
Highlights.
>We quantify how step characteristics influence variability and stability of walking.
>We examined voluntary changes in step width and step length.
>Any change in step characteristics was associated with increased step variability.
>Short-term, voluntary changes in step width and step length affect dynamic stability.
Acknowledgments
Support provided by American Society of Biomechanics Student Grant-in-Aid Award (to PMMY) and National Institutes of Health Grant 1-R21-EB007638-01A1 (to JBD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guimaraes RM, Isaacs B. Characteristics of the gait in old people who fall. Int Rehabil Med. 1980;2:177–80. doi: 10.3109/09638288009163984. [DOI] [PubMed] [Google Scholar]
- 2.Richardson JK, Thies SB, Demott TK, Ashton-Miller JA. Gait analysis in a challenging environment differentiates between fallers and nonfallers among older patients with peripheral neuropathy. Arch Phys Med Rehabil. 2005;86:1539–44. doi: 10.1016/j.apmr.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 3.Pavol MJ, Owings TM, Foley KT, Grabiner MD. Gait characteristics as risk factors for falling from trips induced in older adults. J Gerontol A Biol Sci Med Sci. 1999;54:M583–90. doi: 10.1093/gerona/54.11.m583. [DOI] [PubMed] [Google Scholar]
- 4.England SA, Granata KP. The influence of gait speed on local dynamic stability of walking. Gait & Posture. 2007;25:172–178. doi: 10.1016/j.gaitpost.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dingwell JB, Marin LC. Kinematic variability and local dynamic stability of upper body motions when walking at different speeds. Journal of Biomechanics. 2006;39:444–452. doi: 10.1016/j.jbiomech.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Kang HG, Dingwell JB. Separating the effects of age and walking speed on gait variability. Gait & Posture. 2008;27:572–577. doi: 10.1016/j.gaitpost.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Luukinen H, Koski K, Laippala P, Kivela SL. Risk factors for recurrent falls in the elderly in long-term institutional care. Public Health. 1995;109:57–65. doi: 10.1016/s0033-3506(95)80076-x. [DOI] [PubMed] [Google Scholar]
- 8.Brach JS, Berlin JE, Van Swearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehab. 2005;2:21. doi: 10.1186/1743-0003-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Amer Geriat Society. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 10.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 11.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64A:896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeMott TK, Richardson JK, Thies SB, Ashton-Miller JA. Falls and gait characteristics among older perons with peripheral neuropathy. Am J Phys Med Rehabil. 2007;86:125–132. doi: 10.1097/PHM.0b013e31802ee1d1. [DOI] [PubMed] [Google Scholar]
- 13.Paterson K, Hill K, Lythgo N. Stride dynamics, gait variability and prospective falls risk in active community dwelling older women. Gait & Posture. 2011;33:251–255. doi: 10.1016/j.gaitpost.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Dean JC, Alexander NB, Kuo AD. The effect of lateral stabilization on walking in young and old adults. IEEE Transactions on Biomedical Engineering. 2007;54:1919–1926. doi: 10.1109/TBME.2007.901031. [DOI] [PubMed] [Google Scholar]
- 15.McAndrew PM, Dingwell JB, Wilken JM. Walking variability during continuous pseudo-random oscillations of the support surface and visual field. J Biomech. 2010;43:1470–1475. doi: 10.1016/j.jbiomech.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAndrew PM, Wilken JM, Dingwell JB. Dynamic stability of human walking in visually and mechanically destabilizing environments. J Biomech. 2011;44:644–649. doi: 10.1016/j.jbiomech.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granata KP, Lockhart TE. Dynamic stability differences in fall-prone and healthy adults. J Electromyogr Kinesiol. 2008;18:172–178. doi: 10.1016/j.jelekin.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prince F, Winter DA, Stergiou P, Walt SE. Anticipatory control of upper body balance during human locomotion. Gait & Posture. 1994;2:19–25. [Google Scholar]
- 19.Kantz H, Schreiber T. Nonlinear Time Series Analysis. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 20.Bruijn SM, van Dieen JH, Meijer OG, Beek PJ. Statistical precision and sensitivity of measures of dynamic gait stability. J Neuroscience Methods. 2009;178:327–333. doi: 10.1016/j.jneumeth.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Nayfeh AH, Balachandran B. Applied nonlinear dynamics: Analytical, computational, and experimental methods. New York: New York: John Wiley & Sons; 1995. [Google Scholar]
- 22.Dingwell JB, Kang HG. Differences between local and orbital dynamic stability during human walking. J Biomech Eng. 2007;129:586–593. doi: 10.1115/1.2746383. [DOI] [PubMed] [Google Scholar]
- 23.Donelan JM, Shipman DW, Kram R, Kuo AD. Mechanical and metabolic requirements for active lateral stabilization in human walking. Journal of Biomechanics. 2004;37:827–35. doi: 10.1016/j.jbiomech.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Dingwell JB, Cusumano JP. Nonlinear time series analysis of normal and pathological human walking. Chaos. 2000;10:848–863. doi: 10.1063/1.1324008. [DOI] [PubMed] [Google Scholar]
- 25.Hof AF, Gazendam MGJ, Sinke WE. The condition for dynamic stability. J Biomech. 2005;38:1–8. doi: 10.1016/j.jbiomech.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Bauby CE, Kuo AD. Active control of lateral balance in human walking. Journal of Biomechanics. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 27.Kuo AD. Stabilization of lateral motion in passive dynamic walking. International Journal of Robotics Research. 1999;18:917–930. [Google Scholar]
- 28.Bruijn SM, van Dieen JH, Meijer OG, Beek PJ. Is slow walking more stable? J Biomech. 2009;42:1506–1512. doi: 10.1016/j.jbiomech.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 29.Su JL-S, Dingwell JB. Dynamic stability of passive dynamic walking on an irregular surface. Journal of Biomechanical Engineering. 2007;129:802–810. doi: 10.1115/1.2800760. [DOI] [PubMed] [Google Scholar]
- 30.Dingwell JB, Cusumano JP, Cavanagh PR, Sternad D. Local dynamic stability versus kinematic variability of continuous overground and treadmill walking. J Biomech Eng. 2001;123:27–32. doi: 10.1115/1.1336798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


