Abstract
Lipid rafts are microdomains of the plasma membrane enriched in cholesterol and sphingolipids, and play an important role in the initiation of many pharmacological agent-induced signaling pathways and toxicological effects. The structure of lipid rafts is dynamic, resulting in an ever-changing content of both lipids and proteins. Cholesterol, as a major component of lipid rafts, is critical for the formation and configuration of lipid rafts microdomains, which provide signaling platforms capable of activating both pro-apoptotic and anti-apoptotic signaling pathways. A change of cholesterol level can result in lipid rafts disruption and activate or deactivate raft-associated proteins, such as death receptor proteins, protein kinases, and calcium channels. Several anti-cancer drugs are able to suppress growth and induce apoptosis of tumor cells through alteration of lipid raft contents via disrupting lipid raft integrity.
Keywords: Lipid raft, cholesterol, death receptor, kinase, calcium channel, apoptosis
Introduction
Lipid rafts are microdomains of the plasma membrane enriched in cholesterol and sphingolipids, and play many important roles in cell signal transduction (Simons and Toomre, 2000). Lipid rafts are heterogeneous in both protein and lipid content, and coexist within a cell (Pike, 2005). The structure of lipid rafts is dynamic, resulting in an ever-changing content of both lipids and proteins. The heterogeneous and dynamic makeup of these lipid raft domains contributes to the large number of signals capable of being transduced from the outer membrane of the cell to cellular organelles of the cytoplasm or the nucleus of the cell, through the lipid rafts. In many cases, the function of proteins depends greatly on their association with lipid rafts. The inclusion of proteins into lipid raft domains is dependent on the makeup of lipids and other proteins of each lipid raft. Cholesterol is a main lipid component of lipid rafts. The presence of cholesterol is critical for membrane raft formation; however, its absolute configuration is not, as cholesterol's interaction with other lipids is not chiral in nature (Westover and Covey, 2003; Westover et al., 2003). Some rafts require less cholesterol than others in order to maintain their integrity. Ceramide is one of the other main lipid components of lipid rafts. Acid sphingomyelinase converts sphingolipid to ceramide, and released ceramide self-aggregates into lipid raft domains, reordering the lipid raft domain to form a signaling structure that produces raft fusion resulting in signaling platforms (Grassme et al., 2001). In accordance with “The Induced-Fit Model of Raft Heterogeneity”, the association of proteins with lipid rafts can affect the lipid content of the lipid rafts and vice versa (Abrami et al., 2001; Pike, 2004). Lipid raft microdomains provide signaling platforms capable of activating various cellular signaling pathways, both pro-apoptotic and anti-apoptotic, which may be inhibited upon lipid raft disruption (Algeciras-Schimnich et al., 2002; Li et al., 2003b; Bang et al., 2005). In this review, the role of lipid rafts in mediating apoptosis and as a therapeutic target for cancer treatment will be discussed.
Lipid Rafts Cholesterol and Apoptosis
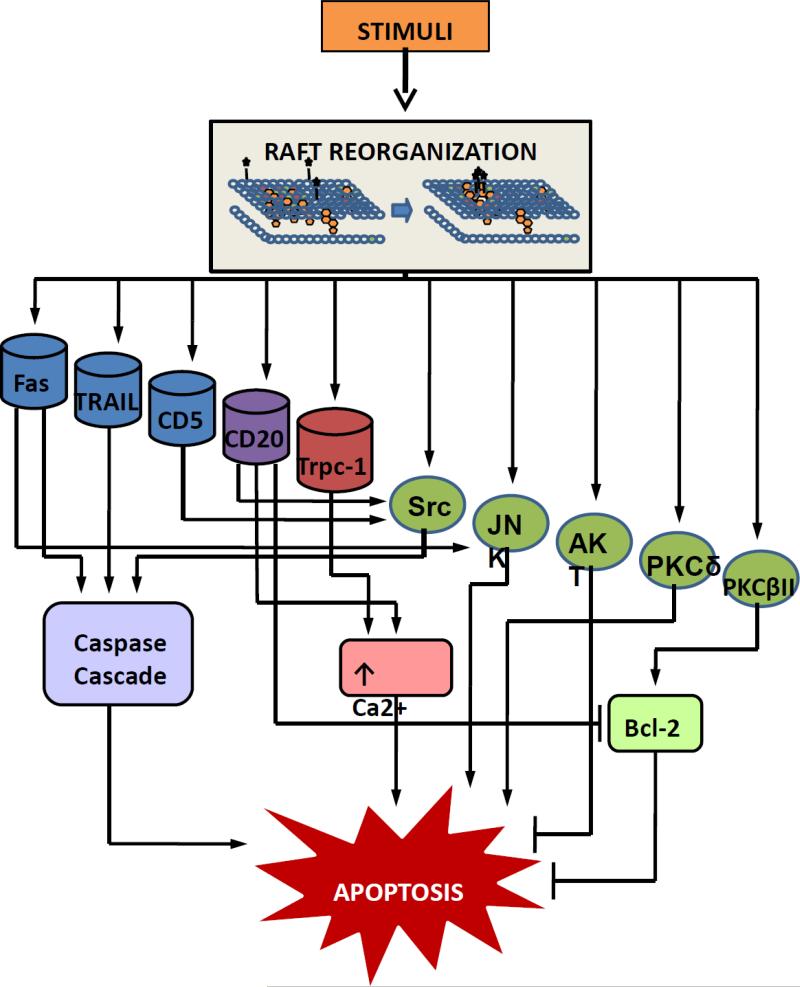
While many regulatory mechanisms of apoptosis are well elucidated (Tournier et al., 2000; Elmore, 2007; Bialik et al., 2010; Benbrook and Masamha, 2011; Indran et al., 2011; Takekawa et al., 2011), the role of lipid rafts in programmed cell death has only recently begun to be explored. Alteration of ceramide has been shown to attribute to the process of apoptosis (Obeid et al., 1993; Colombini; Gorgoglione et al., 2010). However, although ceramide is present in the lipid rafts of cells, the most commonly documented apoptotic functions of ceramide take place on the mitochondria of the cell and in formation of ceramide channels on the cellular membrane (Ion et al., 2006; Woodcock, 2006; Colombini). Lipid rafts may play a role in these apoptotic functions by in- or exclusion of ceramide into raft domains, allowing for ceramide shuttling or channel formation (Colombini). On other hand, it has been documented that alterations of raft cholesterol levels can directly lead to activation of multiple pathways: including but not limited to protein kinase activation or deactivation, apoptotic membrane receptor activation, and through an increase in intracellular calcium levels (Fig. 1).
Figure 1.
Apoptotic pathways mediated by lipid rafts involving protein kinase activation or deactivation, apoptotic membrane receptor activation, or increased intracellular calcium levels
Lipid raft analysis
The roles of raft cholesterol and lipid rafts in apoptosis are often examined by analysis of the alteration of apoptotic events following lipid raft disruption, which is achieved by lipid raft component alteration, such as cholesterol depletion. A variety of techniques have been developed to analyze the changes of lipid raft-associated lipids and proteins during lipid raft disruption. Traditionally, lipid rafts have been isolated from cells for analysis based on the fact that they are insoluble in Triton X-100 at 4˚C and have a light buoyant density on iodoxinal gradients (Jacobson et al., 2007). Although it has been shown that this method of raft isolation is plagued by the ability of detergent to promote fusion of heterogeneous rafts, isolating different subsets of rafts together in the preparation, it remains one of the most popular forms of lipid raft isolation for analysis (Shogomori and Brown, 2003). Non-detergent isolation methods have been explored, in which the isolation is based only on the low buoyant density of the lipid raft domains, resulting in non-raft contamination during isolation.
Isolation methods employing moderately selective detergents have given mixed results. In some cases, a combination of the aforementioned negative effects seen in the detergent and non-detergent preparations were observed. In other cases, these negative effects were alleviated by using low concentrations of moderately selective detergent during isolation (Prior et al., 2003; Shogomori and Brown, 2003; Pike, 2004; George et al.). Additionally, membrane protein crosslinking has been employed in order to preserve protein-protein and protein-lipid interactions across the cellular membrane for analysis (George et al.). Avoiding all plagues of isolating lipid rafts, some researchers are focusing on using microscopic techniques to observe physical lipid raft-protein interactions on the membrane of the whole cell. These techniques include atomic force microscopy (AFM) and fluorescence resonance energy transfer (FRET) (Hooper, 1999; El Kirat and Morandat, 2007; Chiantia et al., 2008).
Pro- and Anti-apoptotic Effects of Lipid Raft Disruption
Depending on the stimulation and apoptotic factors involved, disruption of lipid rafts integrity can be pro- or anti-apoptotic (Algeciras-Schimnich et al., 2002; Li et al., 2003b; Mu et al., 2003; Bang et al., 2005). Without any additional stimuli, methyl-β-cyclodextrin (MβCD), filipin III, cholesterol oxidase, and mevastatin have all shown pro-apoptotic effects upon disruption of lipid rafts of HaCaT keratinocytes (Gniadecki, 2004; Bang et al., 2005). MβCD induces cholesterol efflux from the plasma membrane by binding the molecule with high affinity, disrupting the structure of membrane lipid rafts (Ohtani et al., 1989; Bang et al., 2005). Filipin III is known to form complexes with cholesterol on the membrane of the cell, again disrupting the structure of the lipid rafts (Bang et al., 2005). Mevastatin is a 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase) inhibitor that interferes with cholesterol synthesis, affecting the cholesterol content and overall organization of the lipid rafts (Gniadecki, 2004; Bang et al., 2005). These results suggest that the apoptotic response to the aforementioned agents was not specific to a single drug treatment, but to cellular lipid raft disruption, since apoptosis was evidenced following treatment with any one of the chemicals.
While disruption of lipid rafts promotes apoptosis, it can also prevent apoptosis induced by various stimuli. MβCD is capable of inhibiting avicin D-induced apoptosis in Jurkat cells, an immortalized T lymphocyte leukemia cell line (Xu et al., 2009), completely blocking anandamide-induced apoptosis in rat pheochromocytoma (PC12) cells, rat glioma (C6) cells, mouse neuroblastoma (Neuro-2a) cells, Chinese hamster ovary (CHO) cells, human embryonic kidney (HEK) cells, smooth muscle cells (SMC), human Jurkat cells and human promyelocytic leukemia (HL-60) cells (Sarker and Maruyama, 2003) and reducing UVB-induced apoptotic death of human melanoma (M624) cells (George et al., 2011). Another cyclodextrin, 2,6-di-O-methyl-α-cyclodextrin (DM-α-CD), prevented stearlyamine-liposome-induced macrophage apoptosis, as incorporation of stearlyamine-liposomes into lipid raft domains was inhibited (Arisaka et al.). In conclusion, the integrity of lipid rafts, and raft cholesterol levels in particular, is important in maintaining cellular homeostasis and in providing signaling platforms for incorporation of both pro- and anti-apoptotic factors. This may explain why both proand anti-apoptotic effects are observed upon lipid raft disruption.
The Role of Lipid Rafts in Mediating Membrane Receptor and Channel-Induced Apoptosis
There are a number of membrane receptors and channels that are capable of promoting apoptosis when activated. Some of these membrane proteins can be activated in the absence of their ligand molecules; this type of activation often takes place through aggregation of receptor molecules in lipid raft domains. Raft-dependent membrane receptor aggregation is likely due to changes of lipid raft domains caused by changes in lipid and protein content of the membrane, which lead to the formation of signaling platforms within lipid raft domains. The functions of the following membrane receptors and channels have been shown to be lipid raft-dependent under certain circumstances.
Fas
Fas (CD95, Apo1) is a member of tumor necrosis factor (TNF)-receptor family that plays a critical role in apoptosis (Lavrik). Upon binding of the Fas ligand (FasL), Fas is trimerized and activated, which initiates apoptotic signaling cascades (Algeciras-Schimnich et al., 2002; Legler et al., 2003). However, Fas can aggregate and become activated in the absence of the Fas ligand (FasL) (Bang et al., 2003; Gajate et al., 2004). It has been proposed that redistribution of Fas into lipid rafts is important in the activation of ligand-independent Fas-induced apoptosis (Scheel-Toellner et al., 2002; Gniadecki, 2004). Fas receptor activation results in recruitment/translocation of additional pro-apoptotic proteins into the lipid raft domains, initializing the apoptotic caspase cascade and/or activating the JNK apoptotic pathway (Wu et al., 2002). It is well documented that Fas oligomerization within lipid raft microdomains results in sequential recruitment of Fas-associated death domain (FADD) and then procaspase-8, allowing formation of death-inducing signaling complex (DISC) and autocleavage of caspase 8. This results in activation of a caspase cascade which promotes apoptosis. Lipid raft-dependent Fas aggregation and apoptosis have been shown to be induced by various stimuli, including but not limited to UVB irradiation of M624 melanoma cells and HaCaT keratinocytes (Elyassaki and Wu, 2006; George et al., 2011); Fas ligand-binding of antigen-activated CD4+ T-cells (Scheel-Toellner et al., 2002; Muppidi and Siegel, 2004); and uptake of an antitumor drug Edelfosine (1-O-octadecyl-2- O-methyl-rac-glycero-3-phosphocholine) by various cancer cells, such as leukemic T cells, multiple myeloma and Jurkat cells (Gajate et al., 2004; Gajate and Mollinedo, 2007; Gajate et al., 2009).
CD5
Cluster of differentiation 5 (CD5, Leu-1, Ly-1, T1 and Tp67) is a 67 kD single chain type I glycoprotein found on the membrane of B lymphocytes and T lymphocytes. CD5 has been shown to be associated with the CD79, another cluster of differentiation protein that complexes with the B-cell receptor (Alfarano et al., 1999; Rolinski et al., 1999). Crosslinking of CD5 by antibodies leads to apoptotic death of resting B cells, but not T cells (Pers et al., 1998). A monoclonal anti-CD5 antibody (UCHT2) has been used to induce apoptosis of B-cell chronic lymphocytic leukemia (B-CLL) cells (Renaudineau et al., 2005). Upon crosslinking of CD5 on the membrane of B-CLL cells by the antibody, both the CD79 dimer (CD79a/CD79b) and CD5 are translocated to the lipid rafts of the cells. This translocation of the two cluster of differentiation proteins into the lipid raft domains results in the activation of a number of protein kinases, consequently activating the CD79 protein resulting in apoptosis. In one subgroup of BCLL cells shown to be nonresponsive to CD5-crosslinking and CD5-induced apoptosis, CD5 and CD79 were segregated to different sets of lipid rafts domains, resulting in no apoptotic signal transduction (Renaudineau et al., 2005). These results indicate that heterogeneous raft domains exist upon a cellular membrane, and that lipid rafts provide signaling platforms necessary for CD5-induced apoptosis.
CD20
B-lymphocyte antigen CD20 is expressed on the surface of B lymphocytes and functions as a store-operated calcium channel (Tedder et al., 1988; Bubien et al., 1993; Li et al., 2003a). Lipid rafts have been shown to play a critical role in the regulation of CD20-mediated calcium-dependent apoptosis (Deans et al., 1993; Deans et al., 2002; Janas et al., 2005). Like Fas and other cell membrane receptor proteins, translocation of CD20 into the lipid rafts is required for activation (Li et al., 2003a; Janas et al., 2005). Activation of CD20 results in increased calcium influx in the cell, resulting in apoptosis, suggesting an important role in Ca2+-dependent apoptosis for the integration of CD20 into cellular lipid rafts (Bubien et al., 1993; Li et al., 2003a). Rituxan/Rituximab, an anti-CD20 antibody used clinically to initiate CD20-mediated apoptosis, was used for the study of the activation mechanism of CD20 (Sacchi et al., 2001). Upon activation, CD20 translocated to the lipid rafts domains of the cell where they were activated to initiate apoptosis. Using MβCD to disrupt the lipid raft domains resulted in inhibition of Rituxan-induced translocation of CD20 to the rafts, calcium influx of the cells, and caspase-mediated apoptosis (Janas et al., 2005), which indicates that lipid rafts of B lymphocytes provide activation platforms for CD20-induced apoptosis. The integrity of lipid rafts, and specifically the proper concentration of cholesterol, is important for CD20 activation and CD20-mediated apoptosis of B lymphocytes (Janas et al., 2005).
Trpc-1
Activation of transient receptor potential calcium channel 1 (Trpc-1) occurs upon recruitment into the rafts, which results in an increase in cytosolic Ca2+ concentration, calcineurin activation, the Bcl-2 associated death promoter protein (BAD) dephosphorylation, and apoptosis (Berthier et al., 2004; Letai, 2005). Treating human acute monocytic leukemia (THP-1) cells with 7-ketocholesterol (7KC), a dietary oxysterol presented in human atherosclerotic plaque, leads to the integration of 7KC into cellular membrane lipid rafts, recruitment of Trpc-1 into these lipid raft domains and calcium-dependent apoptosis. Calcium channel blockers can inhibit 7KC-induced apoptosis of THP-1 cells (Berthier et al., 2004). The integration of 7KC into lipid raft domains indicates that an alteration in lipid content has occurred, with naturally occurring forms of cholesterol being replaced by 7-ketocholesterol. This change in cholesterol content is accompanied by changes in concentration of other lipids and proteins in the rafts, in accordance with “The Induced Fit Model of Raft Heterogeneity” (Pike, 2004). These changes in the integrity of the lipid raft domains are responsible for the recruitment of Trpc-1 into the rafts and activation of this calcium channel, ultimately resulting in apoptosis.
The Role of Lipid Rafts in Protein Kinase-Induced Apoptosis
There are a number of protein kinases that can promote or inhibit apoptosis through post-translational modification by phosphorylation of cellular proteins. The integration of certain protein kinases into lipid raft domains is a prerequisite for activation in some cases. Just as with the integration of pro-apoptotic receptor proteins into lipid raft domains, the integration of protein kinases into these domains relies on proper integrity of the rafts. Kinases are recruited into and out of the lipid rafts of a cell depending on the lipid and protein content of the raft. While protein kinase phosphorylation can occur on both proteins involved in pro-apoptotic and anti-apoptotic pathways, the phosphorylation process similarly takes place on activation platforms within the lipid rafts of the cells. Activation of the following kinases has been shown to be lipid raft-dependent.
Akt
Akt is a serine/threonine protein kinase that plays an important role in regulation of cell survival (Datta et al., 1999; Pommier et al., 2010; Benbrook and Masamha, 2011). An increase in activity of Akt protects cells from apoptotic death and is correlated to the progression of human prostate cancer cells (Coffer et al., 1998; Liao et al., 2003). Akt can be activated by stimulation of a number of cell membrane receptor proteins, which eventually results in phosphorylation of Akt at Thr308 and Ser473 (Alessi et al., 1997). This interaction between Akt and membrane receptor proteins has been suggested to take place in the lipid rafts of the cell. It has been shown that Akt is more effectively activated when located in lipid raft domains (Gao and Zhang, 2008; Lasserre et al., 2008). Disruption of lipid raft domains using MβCD destabilizes raft activation platforms of Akt, resulting in impaired Akt phosphorylation at Thr308 and Ser473, diminished Akt activity, and increased apoptosis of normal/transformed keratinocytes, rat alveolar macrophage (NR8383) cells, human lung adenocarcinoma epithelial (A549) cells and Jurkat cells (Motoyama et al., 2009; Calay et al.). In order to determine that the apoptotic response was not specific to MβCD, but to cholesterol depletion of lipid rafts, simvastatin, filipin III and 5-cholesten-5-β-ol (5-chol) were also used to deplete cholesterol, and similar pro-apoptotic effects were observed (Calay et al.). The results of these studies lead to the conclusion that lipid rafts of cells require proper concentrations of cholesterol and other lipids in order to serve as signaling platforms for Akt phosphorylation at regulatory sites Thr308 and Ser473. In the absence of lipid rafts of proper integrity, Akt activation and cell survival signaling does not take place.
JNK
c-Jun N-terminal kinase (JNK) is a MAP kinase that plays an important role in cellular apoptosis (Lin and Dibling, 2002; Liu and Lin, 2005; Dhanasekaran and Reddy, 2008). It has been shown that JNK plays a role in both death receptor-initiated extrinsic and mitochondrial intrinsic apoptotic pathways (Dhanasekaran and Reddy, 2008). Recently, it has been demonstrated that lipid rafts play a role in regulation of JNK activation via a FasL-independent Fas aggregation pathway. Edelfosine, an anti-cancer drug that is known to induce aggregation of Fas in lipid raft signaling domains and recruitment of pro-apoptotic proteins, has been shown to cause a persistent activation of JNK during edelfosine-induced apoptosis (Gajate et al., 1998; Nieto-Miguel et al., 2008; Gajate et al., 2009). Upon edelfosine treatment, JNK becomes concentrated in the lipid rafts with Heat shock protein 90 (Hsp90) in Jurkat and HL-60 cells (Nieto-Miguel et al., 2008). These results suggest a novel pro-apoptotic signaling chaperoning function for Hsp90 and a new mechanism for JNK activation occurring when JNK is located in the lipid rafts.
Src family kinases
Src kinases are a family of tyrosine protein kinases that have been shown to play a role in a number of diverse cell signaling pathways including cellular proliferation, cell cycle control and apoptosis (Aleshin and Finn). Lipid rafts provide a platform for activation of certain src kinases, and the action of these kinases is required for apoptosis. Lipid raft-mediated activation of src kinases has been shown to be associated with the activation of CD20 and CD5, which were discussed earlier. Upon activation of CD20 (using an anti-CD20 antibody), lipid raft clustering occurred which resulted in the trans-activation of the associated src kinases and initiation of apoptosis in B lymphocytes (Deans et al., 2002). Besides CD20, lipid rafts also play a role in mediating the activation of src kinase Lyn in CD5-induced apoptosis of chronic lymphocytic leukemia cells. Lyn is responsible for tyrosine phosphorylation of CD79a/CD79b following its partitioning into lipid raft domains, where Lyn resides (Renaudineau et al., 2005). During CD5-induced apoptosis, the lipid rafts provide a platform for activation of Lyn and modification of the CD79 dimer; both of these actions are necessary in the promotion of apoptosis in leukemia cells. Activation of src kinases and recruitment/modification/activation of pro-apoptotic proteins and molecules in the lipid rafts requires the formation of raft signaling activation platforms, concluding that the integrity of raft contents is important in src kinase activation.
PKC family proteins
Protein kinase C (PKC) are serine/threonine kinases that phosphorylate upon activation resulting from increased concentrations of diacylglycerol (DIG) or Ca2+ and play an important role in many signaling pathways, including those resulting in apoptosis (Gonelli et al., 2009). It has recently been demonstrated that lipid rafts play a role in activation of PKC in regulation of apoptosis (zum Buschenfelde et al.; Arisaka et al.). The activation of PKCδ occurs within lipid rafts during macrophage apoptosis induced by cationic liposomes (Arisaka et al.). Upon co-localization of stearylamineliposomes with lipid rafts, PKCδ is activated which leads to apoptosis. Using 2,6-di-O-methyl-α-cyclodextrin (DM-α-CD) to disrupt cellular lipid rafts led to suppression of activity of PKCδ and decreased levels of apoptosis, while lipid raft disruption using nystatin did not interrupt PKCδ activity (Arisaka et al.). DM-α-CD is known to disrupt the structure and integrity of lipid raft domains via extracting sphingolipids, while nystatin is a cholesterol-sequestering agent, indicating that the sphingolipids of lipid rafts play a more important role in stearylamine-liposome-induced macrophage apoptosis than raft cholesterol (Arisaka et al.). Besides PKCδ, expression of ZAP-70, a key factor in regulation of T-cell receptor activation in chronic lymphocytic leukemia B-cells, enhances the recruitment of PKCβII to the lipid rafts where it is activated (zum Buschenfelde et al.). Activation of PKCβII in the lipid rafts of these cells results in phosphorylation of Bcl-2 (zum Buschenfelde et al.), which is an anti-apoptotic protein that prevents apoptosis by inhibiting the activation of pro-apoptotic proteins (Lindsay et al.). ZAP-70 enhanced phosphorylation of Bcl-2 in leukemia cells leads to the development of apoptosis-resistant cells; the recruitment of PKCβII to the lipid rafts plays an important role in this process. Since lipid raft domains are demonstrated to provide an activation platform for PKCβII in the described mechanism, disruption of lipid raft domains may lead to suppression of both PKCβII activation and anti-apoptotic properties.
Lipid Raft Targeting For Cancer Treatment
The integrity of lipid raft domains, in both lipid and protein content, is important when providing signaling and activation platforms for membrane receptor proteins, protein kinases, and calcium channels. A specific or narrow concentration range of each type of lipid and protein is required for each different signaling platform, and these concentrations dictate the function of these heterogeneous microdomains. The integrity of lipid rafts is also required for cellular homeostasis. Disruption of lipid raft integrity disrupts homeostasis by altering cellular survival and apoptotic pathways. Several anti-cancer drugs have been shown to suppress growth and induce apoptosis of tumor cells through alteration of lipid raft contents.
Edelfosine
Fas-enriched lipid rafts play an important role in the pro-apoptotic response of edelfosine, which was the first drug reported to promote apoptosis through lipid rafts (Gajate and Mollinedo, 2001; Gajate et al., 2004; Gajate and Mollinedo, 2007). Edelfosine is a synthetic alkyl-lysophospholipid analogue that is capable of inducing apoptosis of human promyelocytic leukemia (HL-60) cells and human erythroblast leukemia (HEL) cells while sparing normal cells (Mollinedo et al., 1997). Using Jurkat cells, it has been show that edelfosine induces apoptosis through concentration of the drug in lipid raft domains of the cells (Gajate et al., 2009). Upon accumulation of edelfosine in lipid rafts, Fas is activated independent of the Fas ligand and pro-apoptotic proteins are recruited to the rafts to form DISC, which activates the caspase cascade resulting in apoptosis (Gajate et al., 2009). Besides edelfosine, several other antitumor drugs, including cisplatin, aplidin, and perifosine, have also been shown to induce apoptosis through Fas receptor translocation and/or activation in cellular lipid rafts in various cancer cell lines (Lacour et al., 2004; Gajate and Mollinedo, 2005; Gajate and Mollinedo, 2007). It has also been shown that edelfosine-concentrated lipid rafts are capable of altering the functional characteristics of proteins located within the raft domains. Hsp90 plays a pro-apoptotic role in edelfosine-induced apoptosis in Jurkat cells and HL-60 cells; Hsp90 inhibitors were shown to decrease edelfosine-induced apoptosis in leukemic cells (Nieto-Miguel et al., 2008). Hsp90, known as a survival signaling chaperone, was recruited into edelfosine-concentrated, Fas-enriched lipid rafts, but no other anti-apoptotic, survival signaling molecules were recruited or activated. Instead, Hsp90 acted as a stabilizer for the apoptotic JNK signaling pathway (Nieto-Miguel et al., 2008). These results suggest that a switch from an anti-apoptotic to a pro-apoptotic function for Hsp90 occurs upon integration of the protein into lipid rafts and provide newly found evidence that proteins located within lipid raft domains may be capable of new cellular functions as compared to the characteristic functions of the protein outside the rafts.
Avicin D
Avicin D is also capable of inducing apoptosis through recruitment of Fas into the lipid rafts of cells. Avicin D, a plant triterpenoid, can selectively inhibit the growth of tumor cells through activation of the pro-apoptotic caspase pathway and down regulation of anti-apoptotic proteins (Haridas et al., 2001; Mujoo et al., 2001; Gaikwad et al., 2005). Using a series of death receptor-deficient cancer cell lines, it has been shown that the avicin D-induced pro-apoptotic caspase pathway was activated through translocation of Fas into the lipid rafts and recruitment of other pro-apoptotic proteins to the lipid rafts forming DISC (Xu et al., 2009). Apoptosis, was decreased upon lipid raft disruption using MβCD, by preventing Fas clustering and DISC formation in the lipid rafts and reducing the overall sensitivity of the cells to Avicin D (Xu et al., 2009). These studies demonstrated that lipid rafts of cells provide signaling platform domains required for avicin D-induced Fas clustering and apoptosis.
Resveratrol
The mechanism of apoptosis induced by resveratrol also requires the integrity of Fas-enriched lipid rafts in human multiple myeloma cells and human T-cell leukemia cells (Reis-Sobreiro et al., 2009). Resveratrol (trans-3,4’,5-trihydroxystilbene) is a polyphenolic phytoalexin that has been shown to inhibit tumor cell growth (Jang et al., 1997; Aziz et al., 2003). Resveratrol-induced apoptosis is inhibited by raft disruption with MβCD, indicating the necessity of proper concentrations of cholesterol and other lipids and proper organization in lipid raft domains for resveratrol-induced activation of Fas death receptors (Reis-Sobreiro et al., 2009). Resveratrol-induced apoptosis occurs in much the same way as edelfosine- and avicin D-induced apoptosis, through FasL-independent activation of Fas and recruitment of pro-apoptotic proteins to the lipid raft domains of the membrane resulting in activation of the caspase cascade. However, additional pro-apoptotic membrane death receptors, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) receptors, have also been recruited to the lipid rafts of these myeloma and leukemia cells and activated in resveratrol-induced apoptosis (Reis-Sobreiro et al., 2009). These results indicate that lipid raft domains can simultaneously provide activation platforms for more than one membrane receptor protein, confirming the heterogeneous and dynamic nature of lipid raft domains.
Liver X receptors
Liver X receptors (LXRs) have been shown to change the lipid content of lipid rafts upon stimulation resulting in apoptosis in human prostate cancer cells (Pommier et al.). LXRs belong to nuclear receptor family of transcription factors and play an important role in regulation of cholesterol, fatty acids and glucose homeostasis (Ogihara et al., 2010). LXRs bind to naturally occurring oxidized forms of cholesterol upon stimulation (Tontonoz and Mangelsdorf, 2003; Volle and Lobaccaro, 2007) and affect the structural integrity of lipid raft domains in a similar way as cholesterol depletion drugs (Pike, 2004; Pommier et al.). Upon LXR stimulation (using the synthetic receptor agonist T0901317) cellular cholesterol levels decreased resulting in smaller and thinner lipid rafts in prostate cancer cells. Simultaneously, a decrease in Akt phosphorylation and subsequent increase in apoptosis occurred (Pommier et al.). When LXRs are stimulated and binding oxidized cholesterol, some of which is located in raft domains, the integrity of the lipid rafts is altered. Improper lipid concentrations of the rafts leads to their inability to provide a proper signaling platform for the process of Akt phosphorylation required for survival signaling, leading to apoptosis in the cells (Pommier et al.). Upon addition of exogenous cholesterol to the cells, a reversal of stimulated LXR effects was observed, which provides evidence that the integrity of lipid raft domains and specifically the concentration of cholesterol is very important in this raft signaling platform formation (Pommier et al.).
Conclusion
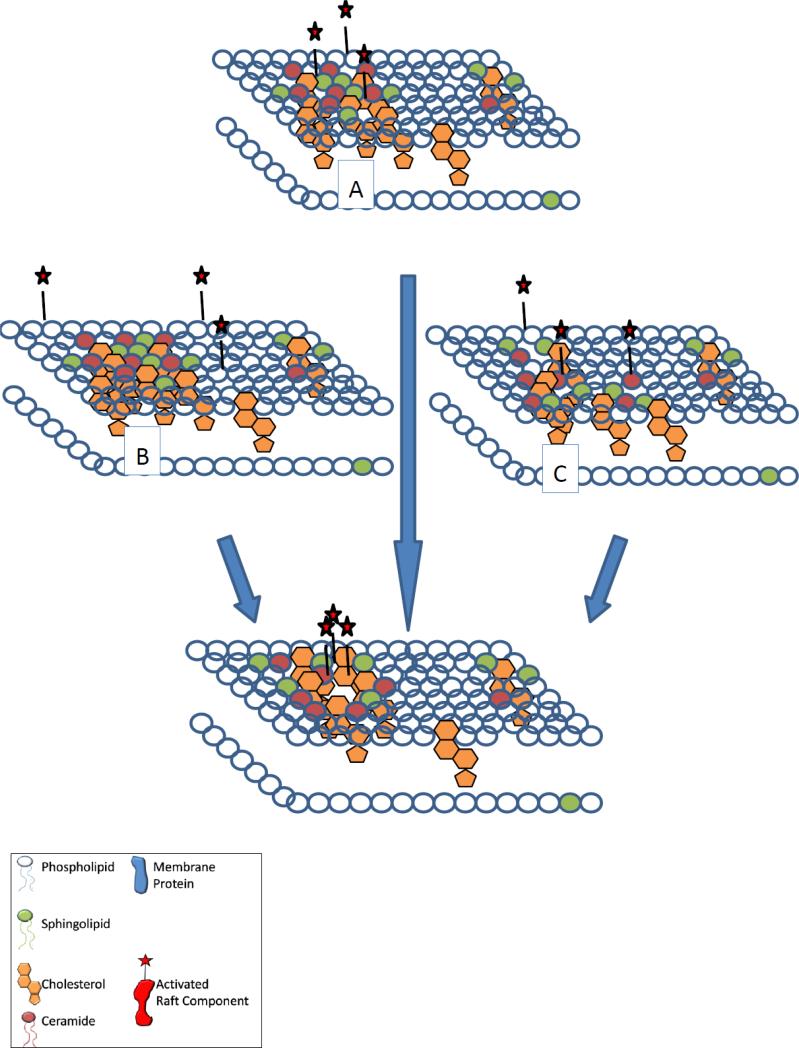
The pro- or anti-apoptotic function of each heterogeneous lipid raft microdomain depends on its lipid and protein content. Specific or narrow concentrations of lipids and proteins are necessary in order for lipid rafts to carry out each of their specific apoptotic functions. The dynamic nature of the rafts and their contents allow for various functions to be carried out on the membrane of the cell. We have highlighted the importance of lipid rafts in the use of therapeutics to initiate apoptotic pathways; these therapeutic agents and their lipid raft-associated targets are summarized in Table 1. In this review, we have documented the importance of the role of lipid rafts in the initiation of apoptosis: Lipid rafts provide signaling platforms for activation of pro-apoptotic membrane receptor molecules; this activation occurs through receptor aggregation in the lipid rafts, in the absence of receptor ligand molecules in most cases. Lipid rafts provide signaling platforms for the activation of protein kinases that occurs through integration of kinases into the rafts and produces pro- or anti-apoptotic effects. Phosphorylation action of kinase molecules often takes place in the lipid rafts, also, requiring the recruitment of the protein or molecule to be modified into the rafts. Lipid rafts provide signaling platforms for the activation of calcium channels of the cellular membrane, which causes intracellular increases in calcium and apoptosis. Figure 2 illustrates a theory for how these receptors, kinases, and calcium channels are activated within lipid rafts of cells: (a) molecules to be activated may residually be located in the lipid rafts, but become activated upon lipid raft content modification/signaling complex formation, (b) molecules to be activated may be located outside of the lipid rafts, but translocate into raft domains upon lipid raft content modification/signaling complex formation, (c) molecules to be activated may be located in smaller lipid raft domains that combine to form larger lipid raft domains/signaling complexes where molecules are activated. Lipid raft content modification may occur as a result of raft disruption caused by alteration of cholesterol levels or as a result of drug stimulation. The integrity of the lipid rafts of a cell is important in maintaining homeostasis. Alterations in lipid content of the cell can lead to changes in the functions of lipid raft domains, capable of promoting or suppressing both pro- and anti-apoptotic signaling pathways.
Table 1.
Therapeutics That Target Lipid Raft-Associated Factors
| Therapeutics | Targets | Cancer cell type | References |
|---|---|---|---|
| Edelfosine | Fas, JNK, Hsp90 | Leukemia, multiple myeloma, | Gajate and Mollinedo, 2001; Gajate et al., 2004; Gajate et al., 2009; Gajate and Mollinedo, 2007 Gajate et al., 1998; Nieto-Miguel et al., 2008 |
| Resveratrol | Fas, TRAIL | Multiple myeloma, T-cell leukemia | Reis-Sobreiro et al., 2009 |
| Avicin D | Fas | T-cell leukemia | Xu et al., 2009 |
| Cisplatin | Fas | Colon | Lacour et al., 2004 |
| Perifosine | Fas | Multiple myeloma | Gajate and Mollinedo, 2007 |
| Agonist T0901317 | Liver X receptor, AKT | Prostate | Pommier et al., 2010 |
| Rituximab/Rituxan | CD20 | Non-Hodgkin's B-cell lymphoma | Janas et al., 2005 |
| 7-ketocholesterol | Trpc-1 | Monocytic leukemia | Berthier et al., 2004 |
| Stearylamine-liposomes | PKCδ | Arisaka et al., 2011 | |
| Anti-CD5 antibody | CD5 | Chronic lymphocytic leukemia | Renaudineau et al., 2005 |
| MβCD, simvastatin, filipin III and 5-cholesten-5-β-ol | AKT | Prostate | Calay et al., 2010 |
Figure 2.
Mechanism of activation of receptors, kinases, and calcium channels within lipid rafts of cells: (a) activated molecules may residually be located in lipid rafts, (b) molecules may translocate into lipid rafts upon activation, (c) smaller lipid rafts may combine to form larger lipid rafts containing activated molecules
Lipid Raft: A Floating Island Of Death or Survival.
The role of lipid rafts in apoptosis
The pro- and anti-apoptotic effects of lipid raft disruption
Cancer treatments targeting lipid rafts
Acknowledgments
This work was partially supported by NIH RO1CA086928 (to S. Wu), BMIT Graduate Assistantship from Ohio University (to K.S. George) and Graduate Research Enhancement Award from the College of Arts and Science at Ohio University (to K.S. George).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
References
- Abrami L, Fivaz M, Kobayashi T, Kinoshita T, Parton RG, van der Goot FG. Cross-talk between caveolae and glycosylphosphatidylinositol-rich domains. J Biol Chem. 2001;276:30729–30736. doi: 10.1074/jbc.M102039200. [DOI] [PubMed] [Google Scholar]
- Aleshin A, Finn RS. SRC: a century of science brought to the clinic. Neoplasia. 2010;12:599–607. doi: 10.1593/neo.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Alfarano A, Indraccolo S, Circosta P, Minuzzo S, Vallario A, Zamarchi R, Fregonese A, Calderazzo F, Faldella A, Aragno M, Camaschella C, Amadori A, Caligaris-Cappio F. An alternatively spliced form of CD79b gene may account for altered B-cell receptor expression in B-chronic lymphocytic leukemia. Blood. 1999;93:2327–2335. [PubMed] [Google Scholar]
- Algeciras-Schimnich A, Shen L, Barnhart BC, Murmann AE, Burkhardt JK, Peter ME. Molecular ordering of the initial signaling events of CD95. Mol Cell Biol. 2002;22:207–220. doi: 10.1128/MCB.22.1.207-220.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arisaka M, Takano K, Negishi Y, Arima H, Aramaki Y. Involvement of lipid rafts in macrophage apoptosis induced by cationic liposomes. Arch Biochem Biophys. 2011;508:72–77. doi: 10.1016/j.abb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: in vitro and in vivo studies and the underlying mechanisms (review). Int J Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- Bang B, Gniadecki R, Gajkowska B. Disruption of lipid rafts causes apoptotic cell death in HaCaT keratinocytes. Exp Dermatol. 2005;14:266–272. doi: 10.1111/j.0906-6705.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- Bang B, Gniadecki R, Larsen JK, Baadsgaard O, Skov L. In vivo UVB irradiation induces clustering of Fas (CD95) on human epidermal cells. Exp Dermatol. 2003;12:791–798. doi: 10.1111/j.0906-6705.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- Benbrook DM, Masamha CP. The pro-survival function of Akt kinase can be overridden or altered to contribute to induction of apoptosis. Curr Cancer Drug Targets. 2011;11:586–599. doi: 10.2174/156800911795655994. [DOI] [PubMed] [Google Scholar]
- Berthier A, Lemaire-Ewing S, Prunet C, Monier S, Athias A, Bessede G, Pais de Barros JP, Laubriet A, Gambert P, Lizard G, Neel D. Involvement of a calcium-dependent dephosphorylation of BAD associated with the localization of Trpc-1 within lipid rafts in 7-ketocholesterol-induced THP-1 cell apoptosis. Cell Death Differ. 2004;11:897–905. doi: 10.1038/sj.cdd.4401434. [DOI] [PubMed] [Google Scholar]
- Bialik S, Zalckvar E, Ber Y, Rubinstein AD, Kimchi A. Systems biology analysis of programmed cell death. Trends Biochem Sci. 2010;35:556–564. doi: 10.1016/j.tibs.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Bubien JK, Zhou LJ, Bell PD, Frizzell RA, Tedder TF. Transfection of the CD20 cell surface molecule into ectopic cell types generates a Ca2+ conductance found constitutively in B lymphocytes. J Cell Biol. 1993;121:1121–1132. doi: 10.1083/jcb.121.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calay D, Vind-Kezunovic D, Frankart A, Lambert S, Poumay Y, Gniadecki R. Inhibition of Akt signaling by exclusion from lipid rafts in normal and transformed epidermal keratinocytes. J Invest Dermatol. 2010;130:1136–1145. doi: 10.1038/jid.2009.415. [DOI] [PubMed] [Google Scholar]
- Chiantia S, Ries J, Chwastek G, Carrer D, Li Z, Bittman R, Schwille P. Role of ceramide in membrane protein organization investigated by combined AFM and FCS. Biochim Biophys Acta. 2008;1778:1356–1364. doi: 10.1016/j.bbamem.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335(Pt 1):1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombini M. Ceramide channels and their role in mitochondria-mediated apoptosis. Biochim Biophys Acta. 2010;1797:1239–1244. doi: 10.1016/j.bbabio.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- Deans JP, Li H, Polyak MJ. CD20-mediated apoptosis: signalling through lipid rafts. Immunology. 2002;107:176–182. doi: 10.1046/j.1365-2567.2002.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans JP, Schieven GL, Shu GL, Valentine MA, Gilliland LA, Aruffo A, Clark EA, Ledbetter JA. Association of tyrosine and serine kinases with the B cell surface antigen CD20. Induction via CD20 of tyrosine phosphorylation and activation of phospholipase C-gamma 1 and PLC phospholipase C-gamma 2. J Immunol. 1993;151:4494–4504. [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Kirat K, Morandat S. Cholesterol modulation of membrane resistance to Triton X-100 explored by atomic force microscopy. Biochim Biophys Acta. 2007;1768:2300–2309. doi: 10.1016/j.bbamem.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyassaki W, Wu S. Lipid rafts mediate ultraviolet light-induced Fas aggregation in M624 melanoma cells. Photochem Photobiol. 2006;82:787–792. doi: 10.1562/2005-12-09-RA-748. [DOI] [PubMed] [Google Scholar]
- Gaikwad A, Poblenz A, Haridas V, Zhang C, Duvic M, Gutterman J. Triterpenoid electrophiles (avicins) suppress heat shock protein-70 and x-linked inhibitor of apoptosis proteins in malignant cells by activation of ubiquitin machinery: implications for proapoptotic activity. Clin Cancer Res. 2005;11:1953–1962. doi: 10.1158/1078-0432.CCR-04-1704. [DOI] [PubMed] [Google Scholar]
- Gajate C, Del Canto-Janez E, Acuna AU, Amat-Guerri F, Geijo E, Santos-Beneit AM, Veldman RJ, Mollinedo F. Intracellular triggering of Fas aggregation and recruitment of apoptotic molecules into Fas-enriched rafts in selective tumor cell apoptosis. J Exp Med. 2004;200:353–365. doi: 10.1084/jem.20040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajate C, Gonzalez-Camacho F, Mollinedo F. Involvement of raft aggregates enriched in Fas/CD95 death-inducing signaling complex in the antileukemic action of edelfosine in Jurkat cells. PLoS One. 2009;4:e5044. doi: 10.1371/journal.pone.0005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajate C, Mollinedo F. The antitumor ether lipid ET-18-OCH(3) induces apoptosis through translocation and capping of Fas/CD95 into membrane rafts in human leukemic cells. Blood. 2001;98:3860–3863. doi: 10.1182/blood.v98.13.3860. [DOI] [PubMed] [Google Scholar]
- Gajate C, Mollinedo F. Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy. J Biol Chem. 2005;280:11641–11647. doi: 10.1074/jbc.M411781200. [DOI] [PubMed] [Google Scholar]
- Gajate C, Mollinedo F. Edelfosine and perifosine induce selective apoptosis in multiple myeloma by recruitment of death receptors and downstream signaling molecules into lipid rafts. Blood. 2007;109:711–719. doi: 10.1182/blood-2006-04-016824. [DOI] [PubMed] [Google Scholar]
- Gajate C, Santos-Beneit A, Modolell M, Mollinedo F. Involvement of c-Jun NH2-terminal kinase activation and c-Jun in the induction of apoptosis by the ether phospholipid 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine. Mol Pharmacol. 1998;53:602–612. doi: 10.1124/mol.53.4.602. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang J. Spatiotemporal analysis of differential Akt regulation in plasma membrane microdomains. Mol Biol Cell. 2008;19:4366–4373. doi: 10.1091/mbc.E08-05-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George KS, Elyassaki W, Wu Q, Wu S. The Role of Cholesterol In Ultraviolet Light B-Induced Apoptosis. Photochemistry and photobiology. 2011 doi: 10.1111/j.1751-1097.2011.01038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George KS, Wu Q, Wu S. Effects of freezing and protein cross-linker on isolating membrane raft-associated proteins. Biotechniques. 2010;49:837–838. doi: 10.2144/000113541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gniadecki R. Depletion of membrane cholesterol causes ligand-independent activation of Fas and apoptosis. Biochem Biophys Res Commun. 2004;320:165–169. doi: 10.1016/j.bbrc.2004.05.145. [DOI] [PubMed] [Google Scholar]
- Gonelli A, Mischiati C, Guerrini R, Voltan R, Salvadori S, Zauli G. Perspectives of protein kinase C (PKC) inhibitors as anti-cancer agents. Mini Rev Med Chem. 2009;9:498–509. doi: 10.2174/138955709787847967. [DOI] [PubMed] [Google Scholar]
- Gorgoglione V, Palmitessa V, Lofrumento DD, La Piana G, Abbrescia DI, Marzulli D, Lofrumento NE. Ceramide-induced activation of cytosolic NADH/cytochrome c electron transport pathway: An additional source of energy for apoptosis. Arch Biochem Biophys. 2010;504:210–220. doi: 10.1016/j.abb.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Grassme H, Schwarz H, Gulbins E. Molecular mechanisms of ceramide-mediated CD95 clustering. Biochem Biophys Res Commun. 2001;284:1016–1030. doi: 10.1006/bbrc.2001.5045. [DOI] [PubMed] [Google Scholar]
- Haridas V, Higuchi M, Jayatilake GS, Bailey D, Mujoo K, Blake ME, Arntzen CJ, Gutterman JU. Avicins: triterpenoid saponins from Acacia victoriae (Bentham) induce apoptosis by mitochondrial perturbation. Proc Natl Acad Sci U S A. 2001;98:5821–5826. doi: 10.1073/pnas.101619098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper NM. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae (review). Mol Membr Biol. 1999;16:145–156. doi: 10.1080/096876899294607. [DOI] [PubMed] [Google Scholar]
- Indran IR, Tufo G, Pervaiz S, Brenner C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim Biophys Acta. 2011;1807:735–745. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Ion G, Fajka-Boja R, Kovacs F, Szebeni G, Gombos I, Czibula A, Matko J, Monostori E. Acid sphingomyelinase mediated release of ceramide is essential to trigger the mitochondrial pathway of apoptosis by galectin-1. Cell Signal. 2006;18:1887–1896. doi: 10.1016/j.cellsig.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Jacobson K, Mouritsen OG, Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- Janas E, Priest R, Wilde JI, White JH, Malhotra R. Rituxan (anti-CD20 antibody)-induced translocation of CD20 into lipid rafts is crucial for calcium influx and apoptosis. Clin Exp Immunol. 2005;139:439–446. doi: 10.1111/j.1365-2249.2005.02720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Lacour S, Hammann A, Grazide S, Lagadic-Gossmann D, Athias A, Sergent O, Laurent G, Gambert P, Solary E, Dimanche-Boitrel MT. Cisplatin-induced CD95 redistribution into membrane lipid rafts of HT29 human colon cancer cells. Cancer Res. 2004;64:3593–3598. doi: 10.1158/0008-5472.CAN-03-2787. [DOI] [PubMed] [Google Scholar]
- Lasserre R, Guo XJ, Conchonaud F, Hamon Y, Hawchar O, Bernard AM, Soudja SM, Lenne PF, Rigneault H, Olive D, Bismuth G, Nunes JA, Payrastre B, Marguet D, He HT. Raft nanodomains contribute to Akt/PKB plasma membrane recruitment and activation. Nat Chem Biol. 2008;4:538–547. doi: 10.1038/nchembio.103. [DOI] [PubMed] [Google Scholar]
- Lavrik IN. Regulation of death receptor-induced apoptosis induced via CD95/FAS and other death receptors. Mol Biol (Mosk) 2011;45:173–179. [PubMed] [Google Scholar]
- Legler DF, Micheau O, Doucey MA, Tschopp J, Bron C. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity. 2003;18:655–664. doi: 10.1016/s1074-7613(03)00092-x. [DOI] [PubMed] [Google Scholar]
- Letai A. Pharmacological manipulation of Bcl-2 family members to control cell death. J Clin Invest. 2005;115:2648–2655. doi: 10.1172/JCI26250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ayer LM, Lytton J, Deans JP. Store-operated cation entry mediated by CD20 in membrane rafts. J Biol Chem. 2003a;278:42427–42434. doi: 10.1074/jbc.M308802200. [DOI] [PubMed] [Google Scholar]
- Li HY, Appelbaum FR, Willman CL, Zager RA, Banker DE. Cholesterol-modulating agents kill acute myeloid leukemia cells and sensitize them to therapeutics by blocking adaptive cholesterol responses. Blood. 2003b;101:3628–3634. doi: 10.1182/blood-2002-07-2283. [DOI] [PubMed] [Google Scholar]
- Liao Y, Grobholz R, Abel U, Trojan L, Michel MS, Angel P, Mayer D. Increase of AKT/PKB expression correlates with gleason pattern in human prostate cancer. Int J Cancer. 2003;107:676–680. doi: 10.1002/ijc.11471. [DOI] [PubMed] [Google Scholar]
- Lin A, Dibling B. The true face of JNK activation in apoptosis. Aging Cell. 2002;1:112–116. doi: 10.1046/j.1474-9728.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Esposti MD, Gilmore AP. Bcl-2 proteins and mitochondria--specificity in membrane targeting for death. Biochim Biophys Acta. 2011;1813:532–539. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- Mollinedo F, Fernandez-Luna JL, Gajate C, Martin-Martin B, Benito A, Martinez-Dalmau R, Modolell M. Selective induction of apoptosis in cancer cells by the ether lipid ET-18-OCH3 (Edelfosine): molecular structure requirements, cellular uptake, and protection by Bcl-2 and Bcl-X(L). Cancer Res. 1997;57:1320–1328. [PubMed] [Google Scholar]
- Motoyama K, Hashimoto Y, Hirayama F, Uekama K, Arima H. Inhibitory effects of 2,6-di-O-methyl-alpha-cyclodextrin on poly I:C signaling in macrophages. Eur J Pharm Sci. 2009;36:285–291. doi: 10.1016/j.ejps.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Mu Y, Lv S, Ren X, Jin G, Liu J, Yan G, Li W, Shen J, Luo G. UV-B induced keratinocyte apoptosis is blocked by 2-selenium-bridged beta-cyclodextrin, a GPX mimic. J Photochem Photobiol B. 2003;69:7–12. doi: 10.1016/s1011-1344(02)00386-x. [DOI] [PubMed] [Google Scholar]
- Mujoo K, Haridas V, Hoffmann JJ, Wachter GA, Hutter LK, Lu Y, Blake ME, Jayatilake GS, Bailey D, Mills GB, Gutterman JU. Triterpenoid saponins from Acacia victoriae (Bentham) decrease tumor cell proliferation and induce apoptosis. Cancer Res. 2001;61:5486–5490. [PubMed] [Google Scholar]
- Muppidi JR, Siegel RM. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat Immunol. 2004;5:182–189. doi: 10.1038/ni1024. [DOI] [PubMed] [Google Scholar]
- Nieto-Miguel T, Gajate C, Gonzalez-Camacho F, Mollinedo F. Proapoptotic role of Hsp90 by its interaction with c-Jun N-terminal kinase in lipid rafts in edelfosine-mediated antileukemic therapy. Oncogene. 2008;27:1779–1787. doi: 10.1038/sj.onc.1210816. [DOI] [PubMed] [Google Scholar]
- Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- Ogihara T, Chuang JC, Vestermark GL, Garmey JC, Ketchum RJ, Huang X, Brayman KL, Thorner MO, Repa JJ, Mirmira RG, Evans-Molina C. Liver X receptor agonists augment human islet function through activation of anaplerotic pathways and glycerolipid/free fatty acid cycling. J Biol Chem. 2010;285:5392–5404. doi: 10.1074/jbc.M109.064659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur J Biochem. 1989;186:17–22. doi: 10.1111/j.1432-1033.1989.tb15171.x. [DOI] [PubMed] [Google Scholar]
- Pers JO, Jamin C, Le Corre R, Lydyard PM, Youinou P. Ligation of CD5 on resting B cells, but not on resting T cells, results in apoptosis. Eur J Immunol. 1998;28:4170–4176. doi: 10.1002/(SICI)1521-4141(199812)28:12<4170::AID-IMMU4170>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Lipid rafts: heterogeneity on the high seas. Biochem J. 2004;378:281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike LJ. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta. 2005;1746:260–273. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Pommier AJ, Alves G, Viennois E, Bernard S, Communal Y, Sion B, Marceau G, Damon C, Mouzat K, Caira F, Baron S, Lobaccaro JM. Liver X Receptor activation downregulates AKT survival signaling in lipid rafts and induces apoptosis of prostate cancer cells. Oncogene. 2010;29:2712–2723. doi: 10.1038/onc.2010.30. [DOI] [PubMed] [Google Scholar]
- Prior IA, Parton RG, Hancock JF. Observing cell surface signaling domains using electron microscopy. Sci STKE. 2003;2003:PL9. doi: 10.1126/stke.2003.177.pl9. [DOI] [PubMed] [Google Scholar]
- Reis-Sobreiro M, Gajate C, Mollinedo F. Involvement of mitochondria and recruitment of Fas/CD95 signaling in lipid rafts in resveratrol-mediated antimyeloma and antileukemia actions. Oncogene. 2009;28:3221–3234. doi: 10.1038/onc.2009.183. [DOI] [PubMed] [Google Scholar]
- Renaudineau Y, Nedellec S, Berthou C, Lydyard PM, Youinou P, Pers JO. Role of B-cell antigen receptor-associated molecules and lipid rafts in CD5-induced apoptosis of B CLL cells. Leukemia. 2005;19:223–229. doi: 10.1038/sj.leu.2403601. [DOI] [PubMed] [Google Scholar]
- Rolinski J, Rupniewska ZM, Wasik-Szczepanek E. [Expression of CD5 antigen in B and T cells from umbilical cord blood, from blood of healthy adults and patients with chronic lymphocytic B-cell leukemia (PBL-B)]. Pol Arch Med Wewn. 1999;101:307–314. [PubMed] [Google Scholar]
- Sacchi S, Federico M, Dastoli G, Fiorani C, Vinci G, Clo V, Casolari B. Treatment of B-cell non-Hodgkin's lymphoma with anti CD 20 monoclonal antibody Rituximab. Crit Rev Oncol Hematol. 2001;37:13–25. doi: 10.1016/s1040-8428(00)00069-x. [DOI] [PubMed] [Google Scholar]
- Sarker KP, Maruyama I. Anandamide induces cell death independently of cannabinoid receptors or vanilloid receptor 1: possible involvement of lipid rafts. Cell Mol Life Sci. 2003;60:1200–1208. doi: 10.1007/s00018-003-3055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel-Toellner D, Wang K, Singh R, Majeed S, Raza K, Curnow SJ, Salmon M, Lord JM. The death-inducing signalling complex is recruited to lipid rafts in Fas-induced apoptosis. Biochem Biophys Res Commun. 2002;297:876–879. doi: 10.1016/s0006-291x(02)02311-2. [DOI] [PubMed] [Google Scholar]
- Shogomori H, Brown DA. Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biol Chem. 2003;384:1259–1263. doi: 10.1515/BC.2003.139. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Takekawa M, Kubota Y, Nakamura T, Ichikawa K. Regulation of stress-activated MAP kinase pathways during cell fate decisions. Nagoya J Med Sci. 2011;73:1–14. [PMC free article] [PubMed] [Google Scholar]
- Tedder TF, Streuli M, Schlossman SF, Saito H. Isolation and structure of a cDNA encoding the B1 (CD20) cell-surface antigen of human B lymphocytes. Proc Natl Acad Sci U S A. 1988;85:208–212. doi: 10.1073/pnas.85.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol Endocrinol. 2003;17:985–993. doi: 10.1210/me.2003-0061. [DOI] [PubMed] [Google Scholar]
- Tournier C, Hess P, Yang DD, Xu J, Turner TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA, Davis RJ. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- Volle DH, Lobaccaro JM. Role of the nuclear receptors for oxysterols LXRs in steroidogenic tissues: beyond the “foie gras”, the steroids and sex? Mol Cell Endocrinol. 2007;265-266:183–189. doi: 10.1016/j.mce.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Westover EJ, Covey DF. First synthesis of ent-desmosterol and its conversion to ent-deuterocholesterol. Steroids. 2003;68:159–166. doi: 10.1016/s0039-128x(02)00174-5. [DOI] [PubMed] [Google Scholar]
- Westover EJ, Covey DF, Brockman HL, Brown RE, Pike LJ. Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phosphorylation due to membrane level effects. Studies with cholesterol enantiomers. J Biol Chem. 2003;278:51125–51133. doi: 10.1074/jbc.M304332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock J. Sphingosine and ceramide signalling in apoptosis. IUBMB Life. 2006;58:462–466. doi: 10.1080/15216540600871118. [DOI] [PubMed] [Google Scholar]
- Wu S, Loke HN, Rehemtulla A. Ultraviolet radiation-induced apoptosis is mediated by Daxx. Neoplasia. 2002;4:486–492. doi: 10.1038/sj.neo.7900264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZX, Ding T, Haridas V, Connolly F, Gutterman JU. Avicin D, a plant triterpenoid, induces cell apoptosis by recruitment of Fas and downstream signaling molecules into lipid rafts. PLoS One. 2009;4:e8532. doi: 10.1371/journal.pone.0008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zum Buschenfelde CM, Wagner M, Lutzny G, Oelsner M, Feuerstacke Y, Decker T, Bogner C, Peschel C, Ringshausen I. Recruitment of PKC-betaII to lipid rafts mediates apoptosis-resistance in chronic lymphocytic leukemia expressing ZAP-70. Leukemia. 2010;24:141–152. doi: 10.1038/leu.2009.216. [DOI] [PubMed] [Google Scholar]