Abstract
Critically ill patients frequently experience poor sleep, characterized by frequent disruptions, loss of circadian rhythms, and a paucity of time spent in restorative sleep stages. Factors that are associated with sleep disruption in the intensive care unit (ICU) include patient-ventilator dysynchrony, medications, patient care interactions, and environmental noise and light. As the field of critical care increasingly focuses on patients' physical and psychological outcomes following critical illness, understanding the potential contribution of ICU-related sleep disruption on patient recovery is an important area of investigation. This review article summarizes the literature regarding sleep architecture and measurement in the critically ill, causes of ICU sleep fragmentation, and potential implications of ICU-related sleep disruption on patients' recovery from critical illness. With this background information, strategies to optimize sleep in the ICU are also discussed.
Keywords: sleep, sleep deprivation, intensive care unit, mental health, outcomes
Introduction
Poor sleep is a frequent occurrence in the intensive care unit (ICU) setting. Despite decades of research describing sleep loss in ICU patients, most ICUs have made few changes to improve patient sleep. This inertia to change clinical practice may be largely due to a lack of research on the negative effects of sleep loss on critical care outcomes, and minimal research regarding the efficacy of sleep-promoting interventions in the ICU. However, such research is beginning to emerge and have growing importance in the ICU. This review provides a broad overview of normal sleep and of sleep in critically ill patients, factors impacting sleep in the ICU, and the consequences of ICU-related sleep disruption on physical and psychological recovery from critical illness.
Normal Sleep
Sleep is a complex physiologic and behavioral process essential for rest, repair, well-being, and survival.1,2 Sleep is defined as a periodic, reversible state of cognitive and sensory disengagement from the external environment.3 While sleep quality and characteristics vary markedly in critically ill patients, an understanding of normal sleep is necessary to fully appreciate sleep abnormalities in the ICU.
Sleep Architecture
Sleep is divided into nonrapid eye movement (NREM) and rapid eye movement (REM), each defined by unique physiologic, electroencephalographic (EEG), and behavioral properties (Figure 1). The normal human sleep period consists of four to six 90- to 100-minute periods during which NREM and REM alternate in a cyclical fashion.3,4 Nonrapid eye movement sleep is divided into three stages—N1, N2 and N3—which account for 2% to 5%, 45% to 55%, and 15% to 20% of the total sleep period, respectively. Stage N1, or “light sleep”, marks the entry into sleep from the waking state and is characterized by low-voltage theta waves (4–8 Hz) on EEG. Compared to N1, stage N2 is characterized by slower, higher amplitude waves with K-complexes and sleep-spindles on EEG. During stage N3, there is a particularly high threshold for arousal with high amplitude delta waves (0.5–2 Hz) on EEG. For this reason, stage N3 is referred to as “slow wave” or “deep” sleep (and formerly known as stages 3 and 4 under the Rechtschaffen and Kales system).4 Stage N3 is significant for its role in restorative processes, such as memory consolidation.5
Figure 1.
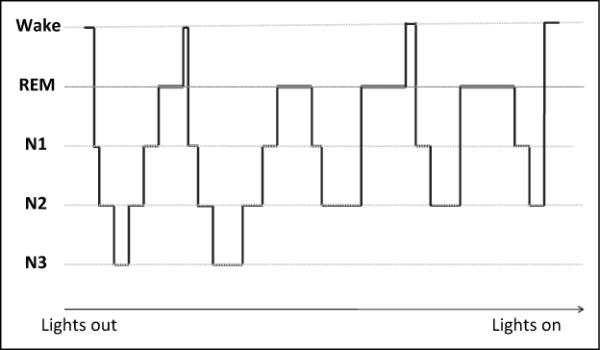
Normal adult hypnogram demonstrating usual sleep stage transitions. REM indicates rapid eye movement sleep.
Rapid eye movement sleep occupies 20% to 25% of the total sleep period and is composed of tonic REM, which occurs throughout the REM period, and intermittent bursts of phasic REM. Tonic REM is characterized by skeletal muscle atonia and low voltage, high amplitude, mixed frequency beta and “saw-tooth” theta waves on EEG. Phasic REM is characterized by rapid eye movements, along with autonomic variability and somatic muscle twitches. The brain is highly active during REM sleep and is associated with dreaming and perceptual learning.3,5
Circadian Rhythms
The sleep-wake cycle is regulated by two finely balanced, opposing processes. The drive for sleep, which includes sleepiness, sleep onset, and sleep promotion is modulated by the sleep homeostat (process S). Process S is primarily driven by the neurotransmitter adenosine, the end-product of ATP (energy) metabolism which increases as a function of wakefulness.4 The transition from wake to sleep occurs at a certain homeostatic threshold. Diurnal secretion of melatonin by the pineal gland also plays a role in sleep promotion.
Wakefulness, on the other hand, is driven by the circadian pacemaker (process C). Located in the suprachiasmatic nucleus, process C is modulated by neural pathways that inhibit melatonin release via exposure to bright light. Several other clinically relevant neurotransmitters that promote wakefulness include orexin (also known as hypocretin), acetylcholine, serotonin, norepinephrine, dopamine, and histamine.
Physiology During Sleep
The body undergoes a constellation of physiologic changes during sleep that play an important role in growth and homeostasis. These alterations are particularly significant in patients with unstable hemodynamics, impaired defense mechanisms, and limited physiologic reserve; hence, these alterations may be particularly important in critically ill patients who may suffer severe consequences from abrupt physiologic fluctuations.
Thermoregulation
Body temperature and thermoregulation are regulated, in part, by sleep and circadian rhythms. In normal subjects, core body temperature peaks late in the day and declines before sleep onset. Temperature sensitivity decreases during NREM, and REM is characterized by poikilothermia (variation in body temperature based on surroundings) and total loss of compensatory responses, such as shivering and sweating. Body temperature reaches a nadir during the latter part of sleep, following a temperature rise preceding awakening.
Respiratory physiology
During sleep, voluntary control of respiration is lost and hypoxic and hypercapnic ventilatory drives are reduced.6 Responsiveness to low oxygen and high carbon dioxide levels is lowest during REM compared to NREM sleep.7–10
Respiration varies markedly during each stage of sleep. The transition from wakefulness to N1 is marked by a decrease in minute ventilation due to variations in tidal volume and respiratory rate.11 As NREM sleep progresses, hypoventilation and a 3 to 7 mm Hg increase in arterial PCO2 levels occurs as a result of several factors, including relaxation of upper respiratory muscles, increased airway resistance, and diminished central respiratory drive.12 Minute ventilation also decreases across N2 and N3 sleep. During REM sleep, both respiratory rate and tidal volume undergo wide variations, with increased variability during bursts of phasic REM.
Cardiovascular physiology
The cardiovascular system undergoes dramatic alterations during sleep. Dynamic fluctuations in blood flow and electrical activity occur and have been associated with life-threatening arrhythmias and ischemic events in patients with underlying heart disease.13 Autonomic stability characterizes NREM sleep, where increased parasympathetic tone results in decreased blood pressure, heart rate, and systemic vascular resistance. During NREM, heart rate rises with increased venous return during inspiration, and falls with decreased venous return during expiration.4
Rapid eye movement sleep is characterized by autonomic variability. During tonic REM, bursts of vagal activity on the background of decreased sympathetic tone lead to bradyarrhythmias and sinus pauses. In contrast, increased sympathetic activity during phasic REM results in transient increases of up to 35% in heart rate and blood pressure.14
Gastrointestinal physiology
During sleep, esophageal motility is reduced and rectal tone is preserved, while gastrointestinal motility remains relatively unchanged. A decrease in swallowing and saliva production also occurs, and the tonic contraction of upper esophageal sphincter prevents aspiration. Gastric acid secretion follows a circadian rhythm, peaking during early sleep.
Endocrine physiology
Growth hormone and prolactin, anabolic hormones necessary for cell differentiation and proliferation, follow the sleep-wake cycle and are suppressed during sleep restriction.15 Growth hormone (GH) peaks during the early stages of N3, while prolactin (PRL) peaks during the second half of the sleep period. In contrast to GH and PRL, cortisol and thyroid hormone fluctuate with circadian rhythm. Cortisol levels rise in the early morning, peak in the late morning, and decline toward nighttime, reaching a nadir after sleep onset. Thyroid stimulating hormone (TSH) follows a similar circadian rhythm, peaking before sleep onset and declining slowly during sleep. Thyroid stimulating hormone secretion is inhibited by N3 sleep and increases with sleep deprivation.16
Sleep in the ICU
Sleep Architecture and Quality in ICU Patients
Critically ill patients experience poor sleep quality and consistently report poor perceived sleep quality in the ICU compared to home.17,18 Surveys of ICU survivors have shown that sleep deprivation and the inability to sleep rank among the top 3 major sources of anxiety and stress during the ICU stay (along with pain and intubation).19–21
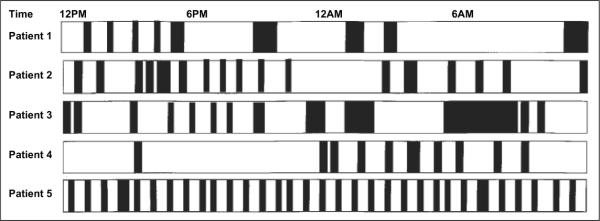
The reports of poor sleep quality by ICU patients are supported by numerous 24-hour polysomnographic (PSG) studies.18,22–28 Critically ill adult patients have markedly fragmented sleep compared to healthy adults, with approximately 50% of sleep occurring during daytime hours.18,29–31 Freedman et al demonstrated that ICU patients experience 41 ± 28 sleep periods per 24 h, with each sleep period averaging 15 ± 9 min (Figure 2).30 Polysomnography in ICU patients is characterized by increased arousals and a predominance of stages N1 and N2, with a lack or absence of N3 and REM. Mean total sleep time in critically ill patients is comparable to healthy adults but shows considerable inter-patient variation.30–33 In summary, ICU patients commonly have broken, light sleep with a lack of restorative N3 and REM sleep.
Figure 2.
Sleep fragmentation in 5 critically ill patients. Black areas represent sleep and white areas represent wakefulness. Reprinted with permission of the American Thoracic Society. Copyright © American Thoracic Society. Freedman NS, Gazendam J, Levan L, et al.30
Measurement of Sleep in the ICU
Sleep measurement in critically ill patients remains an important barrier to large studies on ICU-related sleep disturbances.34 Polysomnography, the gold standard and widely used mode of sleep measurement, involves simultaneous electroencephalogram (EEG), electromyogram (EMG), and electrooculogram (EOG) recordings, which require cumbersome equipment, skilled technicians, and interpretation by a sleep expert. Consequently, PSG is costly and logistically challenging. In the ICU, interpretation of PSG is especially difficult since common ICU medications and illnesses, such as sepsis, shock, hepatic encephalopathy, and renal failure, are associated with altered EEG patterns.35,36
Compared to PSG, actigraphy and the BIS have been investigated as more feasible tools for objective sleep measurement in ICU patients. Actigraphy involves an automated wristwatch device that evaluates patient motion to measure sleep-wake periods and sleep efficiency.37 Actigraphy is widely regarded as a low-cost, minimally invasive alternative for sleep-wake measurement. However, 24-hour actigraphy in ICU patients has been shown to consistently overestimate total sleep time compared to PSG.33,37 This discrepancy is likely due to inability of actigraphy to decipher between sleep and motionless wakefulness in mostly inactive, bedridden ICU patients.33,37.
Bispectral index integrates EEG data to provide a scaled numerical value from 0 to 100, with a larger value representing a higher degree of consciousness. Bispectral index utilizes a single foam sensor containing several EEG electrodes and is sometimes used in monitoring the depth of anesthesia in the operating room. Unlike actigraphy, BIS has the potential to estimate sleep depth, although significant overlap and variability in inter-rater cutoffs for sleep stages can lead to inaccurate characterization of sleep architecture.38–41 Further-more, studies employing BIS in the ICU have been complicated by detachment of electrodes and artifact due to patient movement.38 Bispectral index has not yet shown clinical benefit in ICU care.42,43
Subjective survey instruments also may be used for sleep measurement.33 Probably the most widely used instrument44–47 is the Richards-Campbell Sleep Questionnaire (RCSQ), a 5-question survey validated against PSG in 70 critically ill patients.44 The RCSQ uses a visual analog scale to rate perceived sleep depth, efficiency, and quality.48 As a patient self-report measure, use of RCSQ in ICU patients may be limited by cognitive impairment, such as delirium.46 However, the survey does allow for nurse-reported sleep quality ratings on behalf of patients unable to complete the survey, with high rates of agreement between nurses and patients (r = .869; P < .001).44,46,47 The RCSQ may be a feasible option for large scale, ICU-wide perceived sleep measurement as part of routine clinical care and quality improvement efforts.
Causes of Sleep Disruption in the ICU
There are several common causes of disrupted sleep in hospitalized patients, including underlying sleep disorders, medical conditions, and psychological problems. There are also several modifiable factors causing sleep disruption in critically ill patients, such as noise, light, patient care interactions, and medications (Figure 3).49 Other factors such as severity of illness and mechanical ventilation also contribute to sleep disruption.
Figure 3.
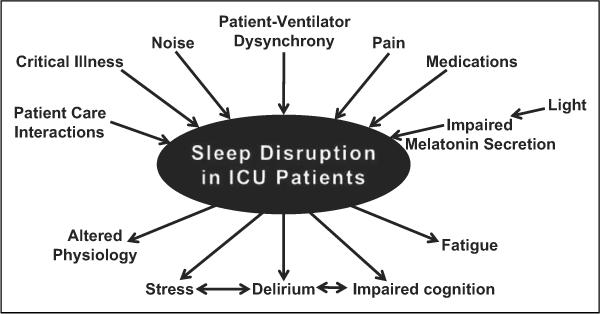
Etiology and potential outcomes of sleep disruption in the intensive care unit (ICU).
Noise
High levels of noise are common in ICUs. Noise is commonly reported by ICU patients as a significant disruptor of sleep and most often is due to staff conversations, alarms, overhead pages, telephones, and televisions.26,50,51 The Environmental Protection Agency recommends maximum hospital noise levels of 45 decibels (dB) during the day and 35 dB at night.52In the ICU setting, peak daytime and nighttime noise levels routinely exceed 80 dB, the threshold associated with sleep disruption in critically ill patients.22,30,32,53–56 However, 18 to 24 hour PSG recordings in ICU patients have attributed only 11% to 18% of arousals and 17% to 24% of awakenings to environmental noise.30,32,55 Interestingly, healthy participants experienced more arousals due to noise when exposed to an ICU setting, suggesting that critically ill patients may be more sensitive to environmental noise as they are recovering.32,57
Patient care activities
ICU patients may experience 40 to 60 interruptions each night due to patient care activities.32,58 These activities include patient assessments, vital sign measurement, equipment adjustment, medication administration, phlebotomy, radiographs, wound care, transportation, and bathing. In 1 study, patient care interactions accounted for 7% of sleep arousals in the ICU, while 18% of all interactions resulted in sleep disruption.32 This data is supported by a survey of 203 patients awaiting discharge from 4 ICUs revealing that 2 patient care activities—vital signs and phlebotomy—were more disruptive to sleep than noise.51
Indeed, frequent patient interactions are necessary for physiologically unstable ICU patients. However, nurses have reported that modifiable factors, such as visitation times, bathing, patient turning, and linen changes, often prevent ICU patients from obtaining consolidated sleep.59
Light
Light plays a vital role in synchronization of the circadian rhythm. Light levels of 1500 lux are necessary to disrupt sleep and 100 to 500 lux are needed to suppress melatonin release (normal indoor light ~180 lux).60,61 While light may disrupt sleep in ICU patients, ICU survivors have reported that light is less disruptive to sleep than noise and patient care activities.51 These reports are supported by continuous light measurements in 4 ICUs, where the mean maximum nocturnal level of 128 to 1445 lux is high enough to suppress melatonin, but below the threshold necessary to disturb sleep.61 Independent of light levels, nocturnal melatonin secretion in ICU patients is perturbed or suppressed, suggesting that other factors in addition to light and dark affect circadian rhythms in this population.62,63
Mechanical ventilation
Multiple studies have demonstrated that mechanical ventilation contributes to sleep disruption.30,64–66 Compared to their nonventilated counterparts, mechanically ventilated patients exhibit more fragmented sleep, reduced sleep efficiency, and increased sleep during daytime hours (up to 50% of total sleep time).64 Patients receiving mechanical ventilation report significantly increased levels of perceived daytime sleepiness.51
The aspects of mechanical ventilation that contribute to sleep fragmentation include increased ventilatory effort, abnormal gas exchange, and patient-ventilator dysynchrony.67–71 Noxious factors associated with mechanical ventilation, such as endotracheal tube discomfort, ventilator alarms, suctioning, positioning, and frequent assessments likely contribute to sleep disruption as well; however, these associations have not been studied.
Over the past decade, the impact of ventilation mode on sleep has received particular attention. Parthasarathy and Tobin were among the first to study the association between sleep architecture and ventilator mode.65 Their study was based on the theory that pressure support ventilation (PSV) leads to hyperventilation and decreased PCO2 levels, thus potentiating central apneas and sleep arousals. The investigators performed overnight PSG on 11 critically ill patients receiving mechanical ventilation, alternating 2 hours each of PSV, assist control ventilation (ACV), and PSV plus dead space ventilation. As predicted, PSV resulted in significantly more arousals and awakenings per hour than ACV (79 ± 7 vs. 54 ± 7, P < .05). The addition of dead space (maintaining higher levels of PCO2) significantly reduced sleep disruptions (P < .01) and central apneas (P < .01). Toublanc et al added to these findings, showing that patients receiving PSV at lower levels of pressure (6 cm H2O) also experienced significantly disrupted sleep compared to those receiving ACV.72 However, sleep quality did not differ when Cabello and colleagues compared clinician-adjusted PSV, automatically adjusted PSV, and ACV, likely due to appropriate matching of ventilator settings with patient mechanics.55 Hence, adjusting a ventilator to maximize patient comfort may depend more on ventilator settings and not ventilator mode, but the final answer remains uncertain.
More recent research has focused on sleep disruption and patient-ventilator dysynchrony with studies of proportional-assist ventilation (PAV).66,73 Unlike PSV, where the same pressure is applied with each breath, PAV adjusts flow and volume based on respiratory resistance, elastance, and inspiratory effort. In a crossover study comparing PSV and PAV, PAV was associated with improved patient-ventilator synchrony, tidal volume, and minute ventilation, resulting in decreased sleep arousals and increased REM and N3 sleep.66 However, when applied only to those with good patient-ventilator synchrony, PAV did not result in significant improvements in sleep quality compared to PSV.73
There is little data describing sleep in ICU patients receiving non-invasive positive pressure ventilation (NPPV). Preliminary 24-hour PSG in 4 patients receiving NPPV demonstrated frequent arousals and nearly absent REM and N3 sleep.74 However, in patients with hypoventilation (eg obesity hypoventilation syndrome, neuromuscular disease, COPD), nocturnal NPPV may improve sleep quality while preventing worsening hypoventilation.75
In summary, evaluation of ventilation mode and sleep quality is still an emerging field, and decisions should be individualized to optimize patient-ventilator mechanics.
Medications
Several commonly used ICU medications have profound effects on sleep quantity and quality (Table 1).76,77 These agents act through a variety of neurotransmitter pathways, receptors, and modulators. Although the interplay of these medications with sleep is difficult to study in ICU patients, their effects in normal participants has been well described. Drug withdrawal can also alter sleep architecture and precipitate delirium and must be appreciated in any patient using long-term medications that influence sleep.
Table 1.
Effect of Common ICU Medications on Sleep
| Drug Use/Medications | Mechanism of Action | Effect on Sleep |
|---|---|---|
| Sedation | ||
| Benzodiazepines | GABA receptor agonist | ↑TST, ↓N3, ↓ REM |
| Dexmedetomidine | α2-agonist | ↑N3, ↓SL, ↓REM |
| Propofol | GABA receptor agonist | ↑TST, ↓SL, ↓W |
| Analgesia | ||
| Opioids | CNS opioid receptor agonist | ↓TST, ↓N3, ↓REM, ↑W |
| Antipsychotic | ||
| Haloperidol | Dopamine-receptor antagonist | ↑TST, ↑N3, ↑SE, ↓SL, ↓W |
| Olanzapine | 5HT2-, D2-receptor antagonist | ↑TST, ↑N3, ↑SE, ↓SL, ↓W |
| Cardiovascular | ||
| β-blockers | CNS β-receptor antagonist | ↑W, ↓REM, nightmares |
| Dopamine | D2-, β1-, α1-receptor agonist | ↓N3, ↓REM |
| Norepinephrine/Epinephrine | α- and β-receptor agonist | ↓N3, ↓REM |
| Phenylephrine | α1-receptor agonist | ↓N3, ↓REM |
| Other | ||
| Corticosteroids | Decreases melatonin levels | ↑W, ↓N3, ↓REM |
Abbreviations: CNS, central nervous system; GABA, gamma-aminobutyric acid; N3, deep or slow wave sleep stage; REM, rapid eye movement sleep; SE, sleep efficiency; SL, sleep latency; TST, total sleep time; W, wakefulness after sleep onset
Sedation is used in many ICU patients, particularly in those requiring mechanical ventilation. Despite their sedative, anxiolytic, and analgesic properties, benzodiazepines and opiates are potentially disruptive to sleep. Benzodiazepines provide sedation through GABA-ergic pathways but increase stage N2 and reduce N3 sleep at low doses in healthy participants.78,79 Opiates such as fentanyl and morphine promote sleep onset in healthy adults, but inhibit REM, profoundly suppress N3, provoke nocturnal awakenings, and can precipitate central apneas.80–84 Both benzodiazepines and opiates are associated with delirium in critically ill patients, even at low doses.1,85.
Propofol, a GABA receptor agonist, is associated with suppressed N3 sleep in human studies.86 However, in a rat model, a continuous propofol infusion did not result in sleep deprivation87 and suggested rebound sleep (based on EEG analysis) following prolonged sleep deprivation.88 Dexmedetomidine, a newer α2-agonist with both sedative and analgesic properties, enhances N3 sleep in a rat model, and is associated with lower incidence of delirium compared to benzodiazepines in ICU patients.85,89 Further investigation is needed to examine the effects on sleep architecture in both healthy and ICU populations receiving these sedative agents.
Finally, off-label use of medications with sedating side effects to treat insomnia in ICU patients can also compromise sleep quality.90 Medications such as diphenhydramine are deliriogenic.91 Additionally, sedating antidepressants such as trazodone (the most commonly prescribed sleep aid in the United States91), amitryptyline, and mirtazapine have not been studied for use in insomnia and have important potential side effects including hypotension, arrhythmias, and anticholinergic syndrome. Use of these medications to promote sleep has been discouraged by an NIH consensus panel on chronic insomnia.91 Given the common use of sedatives in the ICU, little data exists regarding the use of medications to treat insomnia. However, given the effect of medications commonly used in the ICU (eg, benzodiazepines, narcotics, and propofol) on delirium, these medications should not be used to treat insomnia. Further studies are needed to understand the potential risks and benefits of newer sleep-promoting medications in the ICU setting.
Other causes of ICU-related sleep disruption
Sleep deprivation in the ICU can be exacerbated by factors inherent to critical illness. Pain, a very common symptom in critically ill patients, contributes to awakenings during sleep.92–95 Anxiety and stress due to unfamiliarity with the ICU environment, inability to speak, or illness can also contribute to sleep loss.93,94,96
Data on the association of severity of illness and sleep quality are limited.97 Critically ill patients with sepsis have more pronounced perturbations in melatonin excretion than nonseptic ICU patients, suggesting worse sleep quality with sepsis.98 Sleep evaluation is challenging, however, since septic patients have slowed EEG activity resembling an altered state of consciousness different from both sleep and wakefulness.37
Preexisting disease can also contribute to poor sleep quality in the ICU. Chronic obstructive pulmonary disease (COPD), for instance, is a common comorbidity in mechanically ventilated ICU patients and associated with prolonged sleep latency and decreased total sleep time.99 Sleep-related hypoventilation and hypoxia in COPD trigger sleep arousals and disrupt REM and N3 sleep.99–105 Stroke patients and congestive heart failure (CHF) patients with depressed left ventricular systolic function often exhibit nocturnal Cheyne-Stokes respiration, which markedly disrupts sleep.106,107 Finally, sleep disorders such as obstructive sleep apnea and obesity hypoventilation syndrome are increasing in prevalence in ICU populations and can cause severe sleep fragmentation.108,109
Physical Consequences of Sleep Deprivation in Critically Ill Patients
Ventilatory Disturbances
There is little data regarding the effect of sleep deprivation on the respiratory system in the critically ill. However, studies in non-ICU patients have shown that altered respiration can occur after small amounts of sleep loss.110 For example, after 1 night without sleep, COPD patients have a significant decline in FEV1 and FVC, and a decrease in maximal inspiratory pressure.111 Additionally, healthy participants with 24 to 30 hours of sleep deprivation have shown significantly increased respiratory muscle fatigue112 and a 17% to 24% decrease in ventilatory response to hypercapnea, suggesting a potential role of sleep deprivation in ventilatory chemoreceptor mechanisms;113–116 however, these results are not universally supported.117 Finally, disrupted sleep leads to greater upper airway collapsibility, which can precipitate obstructive apnea and potentiate problems following extubation.118,119 Although not yet investigated, these data may indicate that the typical prolonged and sustained sleep disruption in ICU patients may have a detrimental effect on respiration, particularly in those patients with preexisting pulmonary morbidity and difficulty weaning from mechanical ventilation.
Cardiovascular Disturbances
A relationship between sleep deprivation and cardiovascular morbidity is well established but poorly understood.120 Studies have shown that normal participants with insufficient sleep have increased sympathetic and decreased parasympathetic tone, and elevated catecholamines, resulting in blood pressure and heart rate lability and increased risk of acute myocardial infarction.121–123 Sleep restriction, even over 1 night, triggers the release of inflammatory cytokines (see “Immunologic disturbances” below) that foster endothelial disruptions associated with atherosclerosis, hypertension, and coronary artery disease.124,125 Despite this evidence, it remains unknown whether sleep deprivation or ICU-related sleep disruption contribute to cardiovascular mortality.
Immunologic Disturbances
Common perception holds that adequate sleep is necessary to prevent and combat infection. The notion that sleep reinforces host defense mechanisms is supported by murine models demonstrating that prolonged sleep deprivation produced a catabolic state, opportunistic infection, and death from septicemia within 27 days.126 Rats undergoing prolonged sleep restriction also had decreased lymphocytes, total leukocytes, and spleen weight, possible early signs of immune compromise.127
The relationship between immunity and sleep in humans is less clear and more complex.128,129 Numerous studies have demonstrated attenuated response to vaccination130,131 and disruption in markers and modulators of immunity following sleep deprivation; however, the findings have questionable clinical relevance due to inconsistent data and/or lack of microbiological or morbidity correlates. Two nights of sleep deprivation (63 hours awake) in normal participants produces a linear decline in T-helper cells and an increase in leukocytes, monocytes, and natural killer cell activity,132 but neither 1 night of partial sleep deprivation, selective slow-wave sleep restriction, nor prolonged sleep restriction produces similar results.127,133 Similarly, natural killer cells reach a nadir after 1 night without sleep but increase substantially during a second night of sleep loss.132 While partial sleep deprivation suppresses release of IL-2,133 other pro-inflammatory cytokines such as intercellular adhesion molecule 1 (ICAM-1), E-selectin, interleukin (IL)-1β, IL-6, and TNF-α are stimulated following 1 night without sleep.125,134 These data suggest that duration of sleep deprivation affects cellular immunity and cytokine function, but the exact mechanism and clinical implications are not known. Clearly, significant knowledge gaps remain to be filled on sleep and immune physiology before similar mechanisms can be studied in the critically ill.
Hormonal and Metabolic Disturbances
Sleep loss profoundly effects metabolism and endocrine function.135 During sleep deprivation, cortisol and catecholamine levels increase136–138 along with indices of energy expenditure, such as oxygen consumption (VO2) and carbon dioxide production (VCO2).139,140 Similar hormonal and metabolic disturbances are observed in critically ill patients, especially in sepsis,141,142 which may suggest that sleep deprivation intensifies the stress response. Thyroid stimulating hormone, T3, and T4 also increase during sleep deprivation137 but are inhibited during critical illness,143 suggesting potential antagonism between the 2 processes.
There is little published data on the effect of ICU-related sleep disruption on growth hormone (GH) or prolactin (PRL) levels. Studies have shown that GH and PRL levels rise early in critical illness.144 Days after the onset of critical illness, however, both GH and PRL levels fall and may play a role in ICU muscle wasting and impaired immunity, respectively.144 The potent inhibitory effect of sleep deprivation on release of GH and PRL may influence this process, particularly during prolonged critical illness and associated sleep fragmentation, which provides an interesting avenue for future research.137
Recent research has focused on sleep deprivation and glucose metabolism. Following slow wave sleep restriction145 and 2 to 6 consecutive nights of restricted sleep,138,146 previously healthy participants had blunted insulin secretion, decreased sensitivity to insulin, and impaired glucose regulation.135 These findings are particularly relevant in the critically ill, where hyperglycemia is common and associated with adverse outcomes.147–149 Whether ICU-related sleep disruption contributes to or amplifies glucose abnormalities in critical illness is unknown, but provides another compelling area for investigation.
Physical Activity
Sleep loss leads to significant reductions in energy and activity levels,150–152 which may impact physical recovery from critically illness. A large body of research demonstrates a variety of complications from bed rest,153 and increasing research is evaluating these specific harms in critically ill patients. Emerging evidence demonstrates that early and intensive physical rehabilitation in the ICU improves physical function, ICU delirium, and ICU length of stay.154–158 As we learn more about the feasibility, efficacy, and outcomes of early physical medicine and rehabilitation for ICU patients, it is conceivable that fatigue from ICU-related sleep deprivation may impair mobilization efforts, stimulating much-needed initiatives to improve ICU sleep quality.156,159,160
Psychological Consequences of Sleep Deprivation in Critically III Patients
Delirium
The connection between sleep deprivation and delirium has particular importance in the ICU setting. The vast majority of ICU patients may experience both sleep deprivation and delirium, especially among those who are elderly and/or mechanically ventilated.161 Delirium is independently associated with patient mortality, increased cost and length of stay, and long-term cognitive impairment.161–165
Whether sleep deprivation directly contributes to ICU delirium has not been investigated. However, both conditions share a number of important mechanisms, risk factors, and symptoms.1,26,161,162,166–168 Circadian rhythm disturbance has been described in delirious ICU patients.169 Sedating medications such as benzodiazepines85,170 and opiates171 also contribute to both delirium and sleep disruption. Additionally, both inattention and hallucinations can occur with both sleep deprivation and delirium.166 Given these overlapping factors, and the deleterious short- and long-term consequences of ICU delirium, evaluation of sleep is a logical next step in ICU delirium research.
Disturbances to Mental Health and Quality of Life
A growing body of literature has highlighted a variety of disabling psychiatric and neurocognitive impairments affecting survivors of critical illness. Several similar mood and cognitive derangements are observed following inadequate sleep, suggesting a potential influence of ICU-related sleep disruption on post-ICU mental health.
Psychiatric disturbances
Intensive care is associated with many traumatic stressors, such as respiratory distress, endotracheal intubation, pain, inability to speak, feelings of helplessness, and confusion and hallucinations associated with delirium. As a result, survivors of critical illness often experience frightening flashbacks, nightmares, anxiety, and mood disturbances related to their ICU stay.172,173
Posttraumatic stress disorder (PTSD), one of the most prevalent psychiatric disorders following critical illness, is characterized by disabling symptoms of re-experiencing, avoidance, numbing, and hyperarousability regarding a traumatic event.174 A systematic review of 12 studies of ICU-associated PTSD confirmed that 10% to 39% (median 19%) of 1104 ICU survivors suffered from clinically significant PTSD symptoms during their first year after the ICU.175 The prevalence of ICU-related PTSD symptoms is up to 45% at ICU discharge and 24% at 8 years after ICU discharge.176 Predictors of PTSD include ICU length of stay, duration of mechanical ventilation, preexisting psychiatric disorders, delirium, neuromuscular blockade, and the use of sedation, particularly benzodiazepines.175
Depression is also common following critical illness and associated with impaired quality of life and delay in returning to work.177 A systematic review of 14 studies of post-ICU depression revealed clinically significant depression in 28% of patients within the first year of ICU discharge.177 In ARDS survivors, the prevalence of depression is as high as 46% at 1 year178 and 23% at 2 years179 after ICU discharge. Preliminary studies suggest that preexisting depression180 and poor physical functioning,180 along with ICU-related hypoglycemia,181 excessive sedation,182 and delirium,183 may be associated with depression following ICU discharge.
Nonspecific anxiety symptoms have been reported in 23% to 48% of ARDS survivors up to 28 months following ICU discharge.184 While post-ICU anxiety shares many risk factors with depression, the inability of a patient to recall their ICU experience is an additional potentially important risk factor that commonly occurs in the setting of delirium.185
The potent effect of sleep quantity and quality on mood is supported by decades of research.186 Multiple studies have demonstrated depressive symptoms120 and increased levels of fatigue, anxiety, and stress187 in healthy participants undergoing total or partial sleep restriction. The underlying mechanism between sleep loss and depression is not well understood; however, it is theorized that sympathetic activation during sleep arousals potentiates stress responses underlying PTSD and nonspecific anxiety disorders.188 Likewise, sleep disruptions in the critically ill may contribute to post-ICU psychiatric disorders, but the exact association may be challenging to understand in patients with multiple comorbidities and ICU-related stressors. Research may instead focus on the effects of ICU sleep promotion on post-ICU psychological outcomes.
Cognitive dysfunction
Numerous investigations describe a variety of short- and long-term neurocognitive deficits following critical illness, including impairment of memory, attention, concentration, language, mental processing speed, visuospatial abilities, and executive function (eg, decision making, organization, and planning).189 The pathophysiology of these deficits is not fully elucidated, but several mechanisms have been proposed, including hypoxemia, hypotension, delirium, sedating medications, and hyperglycemia.173
An early study of post-ICU neurocognitive function examined 55 survivors of ARDS. At hospital discharge, 100% of these patients exhibited some level of cognitive impairment; 1-year later, 78% had impaired memory, attention, and/or concentration, 48% had decreased processing speed, and 30% had global cognitive decline, compared to population norms.190 Two-year follow-up revealed persistent neurocognitive deficits in 46% of this study population, with minimal improvement between years 1 and 2 posthospitalization.179 Even 6 years after ICU discharge, 24% of patients in a separate ARDS cohort had persistent problems with attention.191 At least 10 studies have demonstrated similar neurocognitive impairments, both in ARDS and general ICU populations.192,193
Sleep deprivation elicits neurocognitive impairments, some of which have similarity to critical illness. Such cognitive impairments have been described since 1896, when deficits in memory, attention, and reaction time were described in 3 healthy participants following 90 hours of sustained wakefulness.194 Since then, hundreds of studies have detailed a myriad of neurocognitive impairments following short-term, long-term, and partial sleep deprivation, including inattention, short-term memory loss, decreased reaction time, and altered executive function.186,195
Based on the current knowledge—that sleep fragmentation is common in the ICU, and that sleep loss and critical illness both impair cognition—ICU-related sleep disruption may causally influence post-ICU neurocognitive impairment. However, sleep loss has yet to receive significant attention as a potential contributor to cognitive outcomes in ICU survivors,173 since the effects of sleep deprivation in healthy participants are considered short-lived and reversed with adequate recovery sleep. However, whether this positive prognosis can be extrapolated to patients who are critically ill, with concomitant neurological insults, is unclear. Given that the consequences of post-ICU neurocognitive dysfunction pose a significant burden to ICU survivors, who experience challenges with daily functioning, social isolation, and difficulties returning to work,173,179,191 further investigation of the role of improving sleep in the ICU is a novel potential intervention.
Quality of life measures
Health-related quality of life (HRQOL) broadly captures one's perception of their overall well-being and incorporates measures of physical, mental, emotional, and social functioning.196 Not surprisingly, critical illness is associated with long-term impairments in HRQOL, regardless of pre-ICU functional capacity.197,198 Survivors of critical illness have statistically significant decrements in the Medical Outcome Study 36-Item Short Form Health Survey (SF-36) HRQOL instrument for years after ICU discharge.197,198 Moreover, post-ICU depression and PTSD are associated with substantial reductions in HRQOL.173 Chronically reduced sleep also leads to substantial decrements in HRQOL,199,200 suggesting that ICU-related sleep disruption may contribute to post-ICU quality of life measures.
Promotion of Sleep in the ICU
Developing a strategy to improve ICU sleep quality is a challenging proposition. Existing evidence supports ICU sleep promotion via multi-faceted interventions focused on minimization of nighttime sleep disruptions and maintenance of the homeostatic sleep-wake cycle. While specific interventions will differ based on ICU staffing, equipment, and facilities, any effective sleep intervention will require elimination of unnecessary noise and light, consolidation of patient care interactions, and consideration of nonpharmacologic sleep aids such as earplugs, eye masks, white noise, and relaxation techniques (eg, calming music, biofeedback, and massage).57,201–203 A guideline for judicious use of medications that disrupt sleep (eg, benzodiazepines and opiates) and use, when necessary, of medications that promote restorative sleep (eg, zolpidem) should also be considered. Finally, daytime wakefulness should be promoted with ambient daylight and regular mobilization.
Effective implementation of any ICU sleep guideline will involve a significant change in culture. Education and engagement of all involved ICU physicians, nurses, and staff will be paramount to achieve buy-in, and frequent performance measurement will be a vital tool for feedback and motivation.204 Monitored outcomes will vary based on available resources but may include objective sleep measurement such as polysomnography and validated sleep quality instruments such as the Richards-Campbell Sleep Questionnaire. Success and sustainability of sleep interventions also will be dictated by secondary outcomes, such as ICU length of stay and post-ICU psychological and cognitive functioning.
Conclusion
Critical illness is characterized by markedly abnormal sleep, with frequent disruptions, altered circadian rhythms, and reduction in restorative N3 and REM sleep. These perturbations are caused by factors inherent to critical illness, such as disease severity and mechanical ventilation, sedating medications, and environmental factors such as noise, light, and patient care interactions. Despite decades of research in healthy participants demonstrating numerous physiologic and psychological derangements following sleep loss, data remain scarce regarding the relative contribution of ICU-related sleep disruption on recovery from critical illness, including weaning from mechanical ventilation, cardiovascular disturbances, host-defense mechanisms, and post-ICU cognition and physical and mental health. Given our current knowledge regarding poor sleep quality in the critically ill, and its potential implications, optimization of sleep in the ICU is a logical next step in critical care outcomes research.
Acknowledgments
Funding Dr. Kamdar is a recipient of a Ruth L. Kirschstein NRSA award from the National Institutes of Health (F32 HL104901).
Footnotes
Declaration of Conflicting Interests The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Figueroa-Ramos MI, Arroyo-Novoa CM, Lee KA, Padilla G, Puntillo KA. Sleep and delirium in ICU patients: a review of mechanisms and manifestations. Intensive Care Med. 2009;35(5):781–795. doi: 10.1007/s00134-009-1397-4. [DOI] [PubMed] [Google Scholar]
- 2.Hardin KA. Sleep in the ICU: potential mechanisms and clinical implications. Chest. 2009;136(1):284–294. doi: 10.1378/chest.08-1546. [DOI] [PubMed] [Google Scholar]
- 3.Carskadon MA, Dement WC. Normal human sleep: an overview. In: Kryger M, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Elsevier/Saunders; Philadelphia, PA: 2005. [Google Scholar]
- 4.Collop NA, Salas RE, Delayo M, Gamaldo C. Normal sleep and circadian processes. Crit Care Clin. 2008;24(3):449–60. v. doi: 10.1016/j.ccc.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 2007;8(4):331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douglas NJ. Respiratory physiology: control of ventilation. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Elsevier/Saunders; Philadelphia, PA: 2005. [Google Scholar]
- 7.Douglas NJ, White DP, Weil JV, et al. Hypoxic ventilatory response decreases during sleep in normal men. Am Rev Respir Dis. 1982;125(3):286–289. doi: 10.1164/arrd.1982.125.3.286. [DOI] [PubMed] [Google Scholar]
- 8.Douglas NJ, White DP, Weil JV, Pickett CK, Zwillich CW. Hypercapnic ventilatory response in sleeping adults. Am Rev Respir Dis. 1982;126(5):758–762. doi: 10.1164/arrd.1982.126.5.758. [DOI] [PubMed] [Google Scholar]
- 9.Berthon-Jones M, Sullivan CE. Ventilatory and arousal responses to hypoxia in sleeping humans. Am Rev Respir Dis. 1982;125(6):632–639. doi: 10.1164/arrd.1982.125.6.632. [DOI] [PubMed] [Google Scholar]
- 10.Berthon-Jones M, Sullivan CE. Ventilation and arousal responses to hypercapnia in normal sleeping humans. J Appl Physiol. 1984;57(1):59–67. doi: 10.1152/jappl.1984.57.1.59. [DOI] [PubMed] [Google Scholar]
- 11.Krieger J. Breathing during sleep in normal subjects. Clin Chest Med. 1985;6(4):577–594. [PubMed] [Google Scholar]
- 12.Krieger J. Respiratory physiology: breathing in normal subjects. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Elsevier/Saunders; Philadelphia, PA: 2005. [Google Scholar]
- 13.Gillis AM. Cardiac arrhythmias. In: Dement WC, Kryger MH, Roth T, editors. Principles and Practice of Sleep Medicine. Saunders; Philadelphia, PA: 2000. pp. 1014–1029. [Google Scholar]
- 14.Dickerson LW, Huang AH, Thurnher MM, Nearing BD, Verrier RL. Relationship between coronary hemodynamic changes and the phasic events of rapid eye movement sleep. Sleep. 1993;16(6):550–557. doi: 10.1093/sleep/16.6.550. [DOI] [PubMed] [Google Scholar]
- 15.Griffin JE, Ojeda SR. Textbook of Endocrine Physiology. Oxford University Press; Oxford: 2004. [Google Scholar]
- 16.Lee-Chiong TL. Sleep Medicine Essentials and Review. Oxford University Press; Oxford: 2008. [Google Scholar]
- 17.Friese RS, Diaz-Arrastia R, McBride D, Frankel H, Gentilello LM. Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping? J Trauma. 2007;63(6):1210–1214. doi: 10.1097/TA.0b013e31815b83d7. [DOI] [PubMed] [Google Scholar]
- 18.Richards KC, Bairnsfather L. A description of night sleep patterns in the critical care unit. Heart Lung. 1988;17(1):35–42. [PubMed] [Google Scholar]
- 19.Novaes MA, Knobel E, Bork AM, Pavao OF, Nogueira-Martins LA, Ferraz MB. Stressors in ICU: perception of the patient, relatives and health care team. Intensive Care Med. 1999;25(12):1421–1426. doi: 10.1007/s001340051091. [DOI] [PubMed] [Google Scholar]
- 20.Rotondi AJ, Chelluri L, Sirio C, et al. Patients' recollections of stressful experiences while receiving prolonged mechanical ventilation in an intensive care unit. Crit Care Med. 2002;30(4):746–752. doi: 10.1097/00003246-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Simini B. Patients' perceptions of intensive care. Lancet. 1999;354(9178):571–572. doi: 10.1016/S0140-6736(99)02728-2. [DOI] [PubMed] [Google Scholar]
- 22.Aaron JN, Carlisle CC, Carskadon MA, Meyer TJ, Hill NS, Millman RP. Environmental noise as a cause of sleep disruption in an intermediate respiratory care unit. Sleep. 1996;19(9):707–710. doi: 10.1093/sleep/19.9.707. [DOI] [PubMed] [Google Scholar]
- 23.Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed) 1985;290(6474):1029–1032. doi: 10.1136/bmj.290.6474.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broughton R, Baron R. Sleep patterns in the intensive care unit and on the ward after acute myocardial infarction. Electroencephalogr Clin Neurophysiol. 1978;45(3):348–360. doi: 10.1016/0013-4694(78)90187-6. [DOI] [PubMed] [Google Scholar]
- 25.Fontaine DK. Measurement of nocturnal sleep patterns in trauma patients. Heart Lung. 1989;18(4):402–410. [PubMed] [Google Scholar]
- 26.Helton MC, Gordon SH, Nunnery SL. The correlation between sleep deprivation and the intensive care unit syndrome. Heart Lung. 1980;9(3):464–468. [PubMed] [Google Scholar]
- 27.Hilton BA. Quantity and quality of patients' sleep and sleep-disturbing factors in a respiratory intensive care unit. J Adv Nurs. 1976;1(6):453–468. doi: 10.1111/j.1365-2648.1976.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 28.Orr WC, Stahl ML. Sleep disturbances after open heart surgery. Am J Cardiol. 1977;39(2):196–201. doi: 10.1016/s0002-9149(77)80191-4. [DOI] [PubMed] [Google Scholar]
- 29.Hilton BA. Quantity and quality of patients' sleep and sleep-disturbing factors in a respiratory intensive care unit. J Adv Nurs. 1976;1(6):453–468. doi: 10.1111/j.1365-2648.1976.tb00932.x. [DOI] [PubMed] [Google Scholar]
- 30.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163(2):451–457. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 31.Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed) 1985;290(6474):1029–1032. doi: 10.1136/bmj.290.6474.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabor JY, Cooper AB, Crombach SA, et al. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167(5):708–715. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 33.Beecroft JM, Ward M, Younes M, Crombach S, Smith O, Hanly PJ. Sleep monitoring in the intensive care unit: comparison of nurse assessment, actigraphy and polysomnography. Intensive Care Med. 2008;34(11):2076–2083. doi: 10.1007/s00134-008-1180-y. [DOI] [PubMed] [Google Scholar]
- 34.Watson PL. Measuring sleep in critically ill patients: beware the pitfalls. Crit Care. 2007;11(4):159. doi: 10.1186/cc6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blume WT. Drug effects on EEG. J Clin Neurophysiol. 2006;23(4):306–311. doi: 10.1097/01.wnp.0000229137.94384.fa. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan PW. The EEG in metabolic encephalopathy and coma. J Clin Neurophysiol. 2004;21(5):307–318. [PubMed] [Google Scholar]
- 37.Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18(4):288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- 38.Bourne RS, Minelli C, Mills GH, Kandler R. Clinical review: sleep measurement in critical care patients: research and clinical implications. Crit Care. 2007;11(4):226. doi: 10.1186/cc5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nieuwenhuijs D, Coleman EL, Douglas NJ, Drummond GB, Dahan A. Bispectral index values and spectral edge frequency at different stages of physiologic sleep. Anesth Analg. 2002;94(1):125–129. doi: 10.1097/00000539-200201000-00024. table. [DOI] [PubMed] [Google Scholar]
- 40.Sleigh JW, Andrzejowski J, Steyn-Ross A, Steyn-Ross M. The bispectral index: a measure of depth of sleep? Anesth Analg. 1999;88(3):659–661. doi: 10.1097/00000539-199903000-00035. [DOI] [PubMed] [Google Scholar]
- 41.Nicholson T, Patel J, Sleigh JW. Sleep patterns in intensive care unit patients: a study using the bispectral index. Crit Care Resusc. 2001;3(2):86–91. [PubMed] [Google Scholar]
- 42.LeBlanc JM, Dasta JF, Kane-Gill SL. Role of the bispectral index in sedation monitoring in the ICU. Ann Pharmacother. 2006;40(3):490–500. doi: 10.1345/aph.1E491. [DOI] [PubMed] [Google Scholar]
- 43.Ely EW, Truman B, Manzi DJ, Sigl JC, Shintani A, Bernard GR. Consciousness monitoring in ventilated patients: bispectral EEG monitors arousal not delirium. Intensive Care Med. 2004;30(8):1537–1543. doi: 10.1007/s00134-004-2298-1. [DOI] [PubMed] [Google Scholar]
- 44.Richards KC, O'Sullivan PS, Phillips RL. Measurement of sleep in critically ill patients. J Nurs Meas. 2000;8(2):131–144. [PubMed] [Google Scholar]
- 45.Williamson JW. The effects of ocean sounds on sleep after coronary artery bypass graft surgery. Am J Crit Care. 1992;1(1):91–97. [PubMed] [Google Scholar]
- 46.Frisk U, Nordstrom G. Patients' sleep in an intensive care unit- patients' and nurses' perception. Intensive Crit Care Nurs. 2003;19(6):342–349. doi: 10.1016/s0964-3397(03)00076-4. [DOI] [PubMed] [Google Scholar]
- 47.Nicolas A, Aizpitarte E, Iruarrizaga A, Vazquez M, Margall A, Asiain C. Perception of night-time sleep by surgical patients in an intensive care unit. Nurs Crit Care. 2008;13(1):25–33. doi: 10.1111/j.1478-5153.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- 48.Richards K. Techniques for measurement of sleep in critical care. Focus Crit Care. 1987;14(4):34–40. [PubMed] [Google Scholar]
- 49.Friese RS. Sleep and recovery from critical illness and injury: a review of theory, current practice, and future directions. Crit Care Med. 2008;36(3):697–705. doi: 10.1097/CCM.0B013E3181643F29. [DOI] [PubMed] [Google Scholar]
- 50.Kahn DM, Cook TE, Carlisle CC, Nelson DL, Kramer NR, Millman RP. Identification and modification of environmental noise in an ICU setting. Chest. 1998;114(2):535–540. doi: 10.1378/chest.114.2.535. [DOI] [PubMed] [Google Scholar]
- 51.Freedman NS, Kotzer N, Schwab RJ. Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 1999;159(4):1155–1162. doi: 10.1164/ajrccm.159.4.9806141. [DOI] [PubMed] [Google Scholar]
- 52.US Environmental Protection Agency . Information on levels of environmental noise requisite to protect public health and welfare with an adequate margin of safety. Government Printing Office; Washington. Washington DC: 1974. [Google Scholar]
- 53.Topf M. Effects of personal control over hospital noise on sleep. Res Nurs Health. 1992;15(1):19–28. doi: 10.1002/nur.4770150105. [DOI] [PubMed] [Google Scholar]
- 54.Topf M, Davis JE. Critical care unit noise and rapid eye movement (REM) sleep. Heart Lung. 1993;22(3):252–258. [PubMed] [Google Scholar]
- 55.Cabello B, Thille AW, Drouot X, et al. Sleep quality in mechanically ventilated patients: comparison of three ventilatory modes. Crit Care Med. 2008;36(6):1749–1755. doi: 10.1097/CCM.0b013e3181743f41. [DOI] [PubMed] [Google Scholar]
- 56.Elliott RM, McKinley SM, Eager D. A pilot study of sound levels in an Australian adult general intensive care unit. Noise Health. 2010;12(46):26–36. doi: 10.4103/1463-1741.59997. [DOI] [PubMed] [Google Scholar]
- 57.Stanchina ML, Abu-Hijleh M, Chaudhry BK, Carlisle CC, Millman RP. The influence of white noise on sleep in subjects exposed to ICU noise. Sleep Med. 2005;6(5):423–428. doi: 10.1016/j.sleep.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Tamburri LM, DiBrienza R, Zozula R, Redeker NS. Nocturnal care interactions with patients in critical care units. Am J Crit Care. 2004;13(2):102–112. [PubMed] [Google Scholar]
- 59.Olson DM, Borel CO, Laskowitz DT, Moore DT, McConnell ES. Quiet time: a nursing intervention to promote sleep in neurocritical care units. Am J Crit Care. 2001;10(2):74–78. [PubMed] [Google Scholar]
- 60.Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. Dose-response relationships for resetting of human circadian clock by light. Nature. 1996;379(6565):540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 61.Meyer TJ, Eveloff SE, Bauer MS, Schwartz WA, Hill NS, Millman RP. Adverse environmental-conditions in the respiratory and medical ICU settings. Chest. 1994;105(4):1211–1216. doi: 10.1378/chest.105.4.1211. [DOI] [PubMed] [Google Scholar]
- 62.Shilo L, Dagan Y, Smorjik Y, et al. Patients in the intensive care unit suffer from severe lack of sleep associated with loss of normal melatonin secretion pattern. Am J Med Sci. 1999;317(5):278–281. doi: 10.1097/00000441-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Perras B, Meier M, Dodt C. Light and darkness fail to regulate melatonin release in critically ill humans. Intensive Care Med. 2007;33(11):1954–1958. doi: 10.1007/s00134-007-0769-x. [DOI] [PubMed] [Google Scholar]
- 64.Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117(3):809–818. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 65.Parthasarathy S, Tobin MJ. Effect of ventilator mode on sleep quality in critically ill patients. Am J Respir Crit Care Med. 2002;166(11):1423–1429. doi: 10.1164/rccm.200209-999OC. [DOI] [PubMed] [Google Scholar]
- 66.Bosma K, Ferreyra G, Ambrogio C, et al. Patient-ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med. 2007;35(4):1048–1054. doi: 10.1097/01.CCM.0000260055.64235.7C. [DOI] [PubMed] [Google Scholar]
- 67.Berry RB, Mahutte CK, Light RW. Effect of hypercapnia on the arousal response to airway occlusion during sleep in normal subjects. J Appl Physiol. 1993;74(5):2269–2275. doi: 10.1152/jappl.1993.74.5.2269. [DOI] [PubMed] [Google Scholar]
- 68.Issa FG, Sullivan CE. Arousal and breathing responses to airway occlusion in healthy sleeping adults. J Appl Physiol. 1983;55(4):1113–1119. doi: 10.1152/jappl.1983.55.4.1113. [DOI] [PubMed] [Google Scholar]
- 69.Berthon-Jones M, Sullivan CE. Ventilation and arousal responses to hypercapnia in normal sleeping humans. J Appl Physiol. 1984;57(1):59–67. doi: 10.1152/jappl.1984.57.1.59. [DOI] [PubMed] [Google Scholar]
- 70.Weinhouse GL, Schwab RJ. Sleep in the critically ill patient. Sleep. 2006;29(5):707–716. doi: 10.1093/sleep/29.5.707. [DOI] [PubMed] [Google Scholar]
- 71.Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990;142(2):295–300. doi: 10.1164/ajrccm/142.2.295. [DOI] [PubMed] [Google Scholar]
- 72.Toublanc B, Rose D, Glerant JC, et al. Assist-control ventilation vs. low levels of pressure support ventilation on sleep quality in intubated ICU patients. Intensive Care Med. 2007;33(7):1148–1154. doi: 10.1007/s00134-007-0659-2. [DOI] [PubMed] [Google Scholar]
- 73.Alexopoulou C, Kondili E, Vakouti E, Klimathianaki M, Prinianakis G, Georgopoulos D. Sleep during proportional-assist ventilation with load-adjustable gain factors in critically ill patients. Intensive Care Med. 2007;33(7):1139–1147. doi: 10.1007/s00134-007-0630-2. [DOI] [PubMed] [Google Scholar]
- 74.Ozsancak A, D'Ambrosio C, Garpestad E, Schumaker G, Hill NS. Sleep and mechanical ventilation. Critical Care Clinics. 2008;24(3):517–531. doi: 10.1016/j.ccc.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 75.Ozsancak A, D'Ambrosio C, Hill NS. Nocturnal noninvasive ventilation. Chest. 2008;133(5):1275–1286. doi: 10.1378/chest.07-1527. [DOI] [PubMed] [Google Scholar]
- 76.Schweitzer PK. Drugs that disturb sleep and wakefulness. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Elsevier/Saunders; Philadelphia, PA: 2005. pp. 499–518. [Google Scholar]
- 77.Bourne RS, Mills GH. Sleep disruption in critically ill patients- pharmacological considerations. Anaesthesia. 2004;59(4):374–384. doi: 10.1111/j.1365-2044.2004.03664.x. [DOI] [PubMed] [Google Scholar]
- 78.Achermann P, Borbely AA. Dynamics of EEG slow wave activity during physiological sleep and after administration of benzodiazepine hypnotics. Hum Neurobiol. 1987;6(3):203–210. [PubMed] [Google Scholar]
- 79.Borbely AA, Mattmann P, Loepfe M, Strauch I, Lehmann D. Effect of benzodiazepine hypnotics on all-night sleep EEG spectra. Hum Neurobiol. 1985;4(3):189–194. [PubMed] [Google Scholar]
- 80.Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO. Postoperative sleep disturbance: influences of opioids and pain in humans. Sleep. 2001;24(1):39–44. doi: 10.1093/sleep/24.1.39. [DOI] [PubMed] [Google Scholar]
- 81.Kay DC, Eisenstein RB, Jasinski DR. Morphine effects on human REM state, waking state and NREM sleep. Psychopharmacologia. 1969;14(5):404–416. doi: 10.1007/BF00403581. [DOI] [PubMed] [Google Scholar]
- 82.Shaw IR, Lavigne G, Mayer P, Choiniere M. Acute intravenous administration of morphine perturbs sleep architecture in healthy pain-free young adults: a preliminary study. Sleep. 2005;28(6):677–682. doi: 10.1093/sleep/28.6.677. [DOI] [PubMed] [Google Scholar]
- 83.Dimsdale JE, Norman D, DeJardin D, Wallace MS. The effect of opioids on sleep architecture. J Clin Sleep Med. 2007;3(1):33–36. [PubMed] [Google Scholar]
- 84.Wang D, Teichtahl H. Opioids, sleep architecture and sleep-disordered breathing. Sleep Med Rev. 2007;11(1):35–46. doi: 10.1016/j.smrv.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 85.Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298(22):2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 86.Herregods L, Rolly G, Mortier E, Bogaert M, Mergaert C. EEG and SEMG monitoring during induction and maintenance of anesthesia with propofol. Int J Clin Monit Comput. 1989;6(2):67–73. doi: 10.1007/BF01720415. [DOI] [PubMed] [Google Scholar]
- 87.Tung A, Lynch JP, Mendelson WB. Prolonged sedation with propofol in the rat does not result in sleep deprivation. Anesth Analg. 2001;92(5):1232–1236. doi: 10.1097/00000539-200105000-00028. [DOI] [PubMed] [Google Scholar]
- 88.Tung A, Bergmann BM, Herrera S, Cao D, Mendelson WB. Recovery from sleep deprivation occurs during propofol anesthesia. Anesthesiology. 2004;100(6):1419–1426. doi: 10.1097/00000542-200406000-00014. [DOI] [PubMed] [Google Scholar]
- 89.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 90.Ahmed QA. Effects of common medications used for sleep disorders. Crit Care Clin. 2008;24(3):493–515. vi. doi: 10.1016/j.ccc.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 91.NIH State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults statement. J Clin Sleep Med. 2005;1:412–421. [PubMed] [Google Scholar]
- 92.Lavigne GJ, McMillan D, Zucconi M. Pain and sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Elsevier/Saunders; Philadelphia, PA: 2005. pp. 1246–1255. [Google Scholar]
- 93.Novaes MA, Aronovich A, Ferraz MB, Knobel E. Stressors in ICU: patients' evaluation. Intensive Care Med. 1997;23(12):1282–1285. doi: 10.1007/s001340050500. [DOI] [PubMed] [Google Scholar]
- 94.Nelson JE, Meier DE, Oei EJ, et al. Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med. 2001;29(2):277–282. doi: 10.1097/00003246-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 95.Hofhuis J, Bakker J. Experiences of critically ill patients in the ICU: what do they think of us? Int J Intensive Care. 1998;5(2):114–117. doi: 10.1016/j.iccn.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 96.Wong FY, Arthur DG. Hong Kong patients' experiences of intensive care after surgery: nurses' and patients' views. Intensive Crit Care Nurs. 2000;16(5):290–303. doi: 10.1054/iccn.2000.1515. [DOI] [PubMed] [Google Scholar]
- 97.Parthasarathy S, Tobin MJ. Is sleep disruption related to severity of critical illness? Abstract. Presented at the American Thoracic Society 99th International Conference; Seattle, WA. May 21, 2003; p. A968. 2003. [Google Scholar]
- 98.Mundigler G, Delle-Karth G, Koreny M, et al. Impaired circadian rhythm of melatonin secretion in sedated critically ill patients with severe sepsis. Crit Care Med. 2002;30(3):536–540. doi: 10.1097/00003246-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 99.Stege G, Vos PJ, van den Elshout FJ, Richard Dekhuijzen PN, van de Ven MJ, Heijdra YF. Sleep, hypnotics and chronic obstructive pulmonary disease. Respir Med. 2008;102(6):801–814. doi: 10.1016/j.rmed.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 100.Krachman SL, Quaranta AJ, Berger TJ, Criner GJ. Effects of noninvasive positive pressure ventilation on gas exchange and sleep in COPD patients. Chest. 1997;112(3):623–628. doi: 10.1378/chest.112.3.623. [DOI] [PubMed] [Google Scholar]
- 101.Brezinova V, Catterall JR, Douglas NJ, Calverley PM, Flenley DC. Night sleep of patients with chronic ventilatory failure and age matched controls: number and duration of the EEG episodes of intervening wakefulness and drowsiness. Sleep. 1982;5(2):123–130. doi: 10.1093/sleep/5.2.123. [DOI] [PubMed] [Google Scholar]
- 102.Calverley PM, Brezinova V, Douglas NJ, Catterall JR, Flenley DC. The effect of oxygenation on sleep quality in chronic bronchitis and emphysema. Am Rev Respir Dis. 1982;126(2):206–210. doi: 10.1164/arrd.1982.126.2.206. [DOI] [PubMed] [Google Scholar]
- 103.Cormick W, Olson LG, Hensley MJ, Saunders NA. Nocturnal hypoxaemia and quality of sleep in patients with chronic obstructive lung disease. Thorax. 1986;41(11):846–854. doi: 10.1136/thx.41.11.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krimsky WR, Leiter JC. Physiology of breathing and respiratory control during sleep. Semin Respir Crit Care Med. 2005;26(1):5–12. doi: 10.1055/s-2005-864197. [DOI] [PubMed] [Google Scholar]
- 105.Harding SM. Sleep in patients with pulmonary disease. In: Pagel JF, Pandi-Perumal SR, editors. Primary care sleep medicine: a practical guide. Humana Press; Totowa, NJ: 2007. pp. 171–189. [Google Scholar]
- 106.Quaranta AJ, D'Alonzo GE, Krachman SL. Cheyne-Stokes respiration during sleep in congestive heart failure. Chest. 1997;111(2):467–473. doi: 10.1378/chest.111.2.467. [DOI] [PubMed] [Google Scholar]
- 107.Cherniack NS, Longobardo G, Evangelista CJ. Causes of Cheyne-Stokes respiration. Neurocrit Care. 2005;3(3):271–279. doi: 10.1385/NCC:3:3:271. [DOI] [PubMed] [Google Scholar]
- 108.Lee WY, Mokhlesi B. Diagnosis and management of obesity hypoventilation syndrome in the ICU. Crit Care Clin. 2008;24(3):533–549. vii. doi: 10.1016/j.ccc.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 109.Hiestand D, Phillips B. The overlap syndrome: chronic obstructive pulmonary disease and obstructive sleep apnea. Crit Care Clin. 2008;24(3):551–563. vii. doi: 10.1016/j.ccc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 110.Phillips B. Sleep, sleep loss, and breathing. South Med J. 1985;78(12):1483–1486. doi: 10.1097/00007611-198512000-00019. [DOI] [PubMed] [Google Scholar]
- 111.Phillips BA, Cooper KR, Burke TV. The effect of sleep loss on breathing in chronic obstructive pulmonary disease. Chest. 1987;91(1):29–32. doi: 10.1378/chest.91.1.29. [DOI] [PubMed] [Google Scholar]
- 112.Chen HI, Tang YR. Sleep loss impairs inspiratory muscle endurance. Am Rev Respir Dis. 1989;140(4):907–909. doi: 10.1164/ajrccm/140.4.907. [DOI] [PubMed] [Google Scholar]
- 113.White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128(6):984–986. doi: 10.1164/arrd.1983.128.6.984. [DOI] [PubMed] [Google Scholar]
- 114.Cooper KR, Phillips BA. Effect of short-term sleep loss on breathing. J Appl Physiol. 1982;53(4):855–858. doi: 10.1152/jappl.1982.53.4.855. [DOI] [PubMed] [Google Scholar]
- 115.Schiffman PL, Trontell MC, Mazar MF, Edelman NH. Sleep deprivation decreases ventilatory response to CO2 but not load compensation. Chest. 1983;84(6):695–698. doi: 10.1378/chest.84.6.695. [DOI] [PubMed] [Google Scholar]
- 116.Phillips B, Cooper KR, Newsome HH, Dewey WL. Effect of sleep loss on beta-endorphin activity, epinephrine levels, and ventilatory responsiveness. South Med J. 1987;80(1):16–20. doi: 10.1097/00007611-198701000-00004. [DOI] [PubMed] [Google Scholar]
- 117.Spengler CM, Shea SA. Sleep deprivation per se does not decrease the hypercapnic ventilatory response in humans. Am J Respir Crit Care Med. 2000;161(4):1124–1128. doi: 10.1164/ajrccm.161.4.9906026. [DOI] [PubMed] [Google Scholar]
- 118.Series F, Roy N, Marc I. Effects of sleep deprivation and sleep fragmentation on upper airway collapsibility in normal subjects. Am J Respir Crit Care Med. 1994;150(2):481–485. doi: 10.1164/ajrccm.150.2.8049833. [DOI] [PubMed] [Google Scholar]
- 119.Tadjalli A, Peever J. Sleep loss reduces respiratory motor plasticity. Adv Exp Med Biol. 2010;669(1):289–292. doi: 10.1007/978-1-4419-5692-7_59. [DOI] [PubMed] [Google Scholar]
- 120.Salas RE, Gamaldo CE. Adverse effects of sleep deprivation in the ICU. Critical Care Clin. 2008;24(3):461–476. doi: 10.1016/j.ccc.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 121.Zhong X, Hilton HJ, Gates GJ, et al. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J Appl Physiol. 2005;98(6):2024–2032. doi: 10.1152/japplphysiol.00620.2004. [DOI] [PubMed] [Google Scholar]
- 122.Loredo JS, Ziegler MG, Ancoli-Israel S, Clausen JL, Dimsdale JE. Relationship of arousals from sleep to sympathetic nervous system activity and BP in obstructive sleep apnea. Chest. 1999;116(3):655–659. doi: 10.1378/chest.116.3.655. [DOI] [PubMed] [Google Scholar]
- 123.Liu Y, Tanaka H. Overtime work, insufficient sleep, and risk of non-fatal acute myocardial infarction in Japanese men. Occup Environ Med. 2002;59(7):447–451. doi: 10.1136/oem.59.7.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol. 2010;108(1):68–75. doi: 10.1152/japplphysiol.00851.2009. [DOI] [PubMed] [Google Scholar]
- 125.Frey DJ, Fleshner M, Wright KP., Jr. The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007;21(8):1050–1057. doi: 10.1016/j.bbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 126.Everson CA. Sustained sleep deprivation impairs host defense. Am J Physiol. 1993;265(5):R1148–R1154. doi: 10.1152/ajpregu.1993.265.5.R1148. [DOI] [PubMed] [Google Scholar]
- 127.Zager A, Andersen ML, Ruiz FS, Antunes IB, Tufik S. Effects of acute and chronic sleep loss on immune modulation of rats. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R504–R509. doi: 10.1152/ajpregu.00105.2007. [DOI] [PubMed] [Google Scholar]
- 128.Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4(6):457–467. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- 129.Majde JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116(6):1188–1198. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 130.Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003;65(5):831–835. doi: 10.1097/01.psy.0000091382.61178.f1. [DOI] [PubMed] [Google Scholar]
- 131.Spiegel K, Sheridan JF, Van CE. Effect of sleep deprivation on response to immunization. JAMA. 2002;288(12):1471–1472. doi: 10.1001/jama.288.12.1471-a. [DOI] [PubMed] [Google Scholar]
- 132.Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93(5):1930–1939. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10(5):643–653. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 134.Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J Immunol. 1997;158(9):4454–4464. [PubMed] [Google Scholar]
- 135.Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009;5(5):253–261. doi: 10.1038/nrendo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep. 1997;20(10):865–870. [PubMed] [Google Scholar]
- 137.Van Cauter E. Endocrine physiology. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Elsevier/Saunders; Philadelphia, PA: 2005. [Google Scholar]
- 138.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 139.Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18(7):581–588. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 140.Bonnet MH, Berry RB, Arand DL. Metabolism during normal, fragmented, and recovery sleep. J Appl Physiol. 1991;71(3):1112–1118. doi: 10.1152/jappl.1991.71.3.1112. [DOI] [PubMed] [Google Scholar]
- 141.Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350(16):1629–1638. doi: 10.1056/NEJMoa020266. [DOI] [PubMed] [Google Scholar]
- 142.Moriyama S, Okamoto K, Tabira Y, et al. Evaluation of oxygen consumption and resting energy expenditure in critically ill patients with systemic inflammatory response syndrome. Crit Care Med. 1999;27(10):2133–2136. doi: 10.1097/00003246-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 143.Peeters RP, Wouters PJ, Kaptein E, van TH, Visser TJ, Van den Berghe G. Reduced activation and increased inactivation of thyroid hormone in tissues of critically ill patients. J Clin Endocrinol Metab. 2003;88(7):3202–3211. doi: 10.1210/jc.2002-022013. [DOI] [PubMed] [Google Scholar]
- 144.Vanhorebeek I, Langouche L, Van den Berghe G. Endocrine aspects of acute and prolonged critical illness. Nat Clin Pract Endocrinol Metab. 2006;2(1):20–31. doi: 10.1038/ncpendmet0071. [DOI] [PubMed] [Google Scholar]
- 145.Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A. 2008;105(3):1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99(5):2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 147.Inzucchi SE. Clinical practice. Management of hyperglycemia in the hospital setting. N Engl J Med. 2006;355(18):1903–1911. doi: 10.1056/NEJMcp060094. [DOI] [PubMed] [Google Scholar]
- 148.Mesotten D, Van den Berghe G. Clinical benefits of tight glycaemic control: focus on the intensive care unit. Best Pract Res Clin Anaesthesiol. 2009;23(4):421–429. doi: 10.1016/j.bpa.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 149.Bagshaw SM, Egi M, George C, Bellomo R. Early blood glucose control and mortality in critically ill patients in Australia. Crit Care Med. 2009;37(2):463–470. doi: 10.1097/CCM.0b013e318194b097. [DOI] [PubMed] [Google Scholar]
- 150.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–843. [PubMed] [Google Scholar]
- 151.Briones B, Adams N, Strauss M, et al. Relationship between sleepiness and general health status. Sleep. 1996;19(7):583–588. doi: 10.1093/sleep/19.7.583. [DOI] [PubMed] [Google Scholar]
- 152.Souissi N, Sesboue B, Gauthier A, Larue J, Davenne D. Effects of one night's sleep deprivation on anaerobic performance the following day. Eur J Appl Physiol. 2003;89(3–4):359–366. doi: 10.1007/s00421-003-0793-7. [DOI] [PubMed] [Google Scholar]
- 153.Brower RG. Consequences of bed rest. Crit Care Med. 2009;37(1):S422–S428. doi: 10.1097/CCM.0b013e3181b6e30a. [DOI] [PubMed] [Google Scholar]
- 154.Needham DM. Mobilizing patients in the intensive care unit: improving neuromuscular weakness and physical function. JAMA. 2008;300(14):1685–1690. doi: 10.1001/jama.300.14.1685. [DOI] [PubMed] [Google Scholar]
- 155.Truong AD, Fan E, Brower RG, Needham DM. Bench-to-bedside review: mobilizing patients in the intensive care unit-from pathophysiology to clinical trials. Crit Care. 2009;13(4):216. doi: 10.1186/cc7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Korupolu R, Gifford JM, Needham DM. Early mobilization in critically ill patients: reducing neuromuscular complications after intensive care. Contemp Critical Care. 2009;6(1):1–12. [Google Scholar]
- 157.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients with acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 159.Bailey PP, Miller RR, III, Clemmer TP. Culture of early mobility in mechanically ventilated patients. Crit Care Med. 2009;37(10):S429–S435. doi: 10.1097/CCM.0b013e3181b6e227. [DOI] [PubMed] [Google Scholar]
- 160.Zanni JM, Korupolu R, Fan E, et al. Rehabilitation therapy and outcomes in acute respiratory failure: An observational pilot project. J Crit Care. 2009;25(2):254–262. doi: 10.1016/j.jcrc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 161.Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients-importance of sleep deprivation. Crit Care. 2009;13(6):234. doi: 10.1186/cc8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 163.Thomason JW, Shintani A, Peterson JF, Pun BT, Jackson JC, Ely EW. Intensive care unit delirium is an independent predictor of longer hospital stay: a prospective analysis of 261 non-ventilated patients. Crit Care. 2005;9(4):R375–R381. doi: 10.1186/cc3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 165.Girard TD, Jackson JC, Pandharipande PP, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–20. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mistraletti G, Carloni E, Cigada M, et al. Sleep and delirium in the Intensive Care Unit. Minerva Anestesiologica. 2008;74(6):329–333. [PubMed] [Google Scholar]
- 167.Trompeo AC, Vidi Y, Ranieri VM. Sleep and delirium in the critically ill: cause or effect? In: Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine. Springer; New York: 2006. pp. 719–725. [Google Scholar]
- 168.Johns MW, Large AA, Masterton JP, Dudley HA. Sleep and delirium after open heart surgery. Br J Surg. 1974;61(5):377–381. doi: 10.1002/bjs.1800610513. [DOI] [PubMed] [Google Scholar]
- 169.Miyazaki T, Kuwano H, Kato H, et al. Correlation between serum melatonin circadian rhythm and intensive care unit psychosis after thoracic esophagectomy. Surgery. 2003;133(6):662–668. doi: 10.1067/msy.2003.149. [DOI] [PubMed] [Google Scholar]
- 170.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. doi: 10.1097/00000542-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 171.Pandharipande P, Cotton BA, Shintani A, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65(1):34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jones C, Griffiths RD, Humphris G. Disturbed memory and amnesia related to intensive care. Memory. 2000;8(2):79–94. doi: 10.1080/096582100387632. [DOI] [PubMed] [Google Scholar]
- 173.Jackson JC, Mitchell N, Hopkins RO. Cognitive functioning, mental health, and quality of life in ICU survivors: an overview. Crit Care Clin. 2009;25(3):615–628. doi: 10.1016/j.ccc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 174.Kross EK, Gries CJ, Curtis JR. Posttraumatic stress disorder following critical illness. Crit Care Clin. 2008;24(4):875–887. x. doi: 10.1016/j.ccc.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 175.Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30(5):421–434. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kapfhammer HP, Rothenhausler HB, Krauseneck T, Stoll C, Schelling G. Posttraumatic stress disorder and health-related quality of life in long-term survivors of acute respiratory distress syndrome. Am J Psychiatry. 2004;161(1):45–52. doi: 10.1176/appi.ajp.161.1.45. [DOI] [PubMed] [Google Scholar]
- 177.Davydow DS, Gifford JM, Desai SV, Bienvenu OJ, Needham DM. Depression in general intensive care unit survivors: a systematic review. Intensive Care Med. 2009;35(5):796–809. doi: 10.1007/s00134-009-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Angus DC, Musthafa AA, Clermont G, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163(6):1389–1394. doi: 10.1164/ajrccm.163.6.2005123. [DOI] [PubMed] [Google Scholar]
- 179.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr. Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(4):340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 180.Weinert C, Meller W. Epidemiology of depression and antidepressant therapy after acute respiratory failure. Psychosomatics. 2006;47(5):399–407. doi: 10.1176/appi.psy.47.5.399. [DOI] [PubMed] [Google Scholar]
- 181.Dowdy DW, Dinglas V, Mendez-Tellez PA, et al. Intensive care unit hypoglycemia predicts depression during early recovery from acute lung injury. Crit Care Med. 2008;36(10):2726–2733. doi: 10.1097/CCM.0b013e31818781f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kress JP, Gehlbach B, Lacy M, Pliskin N, Pohlman AS, Hall JB. The long-term psychological effects of daily sedative interruption on critically ill patients. Am J Respir Crit Care Med. 2003;168(12):1457–1461. doi: 10.1164/rccm.200303-455OC. [DOI] [PubMed] [Google Scholar]
- 183.Davydow DS. Symptoms of depression and anxiety after delirium. Psychosomatics. 2009;50(4):309–316. doi: 10.1176/appi.psy.50.4.309. [DOI] [PubMed] [Google Scholar]
- 184.Davydow DS, Desai SV, Needham DM, Bienvenu OJ. Psychiatric morbidity in survivors of the acute respiratory distress syndrome: a systematic review. Psychosom Med. 2008;70(4):512–519. doi: 10.1097/PSY.0b013e31816aa0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Volk B, Grassi F. Treatment of the post-ICU patient in an outpatient setting. Am Fam Phys. 2009;79(6):459–464. [PubMed] [Google Scholar]
- 186.Banks S, Dinges DF. Behavioral and physiological consequences of sleep restriction. J Clin Sleep Med. 2007;3(5):519–528. [PMC free article] [PubMed] [Google Scholar]
- 187.Dinges DF, Pack F, Williams K, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. SleepM. 1997;20(4):267–277. [PubMed] [Google Scholar]
- 188.Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12(3):197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 189.Hopkins RO, Brett S. Chronic neurocognitive effects of critical illness. Curr Opin Crit Care. 2005;11(4):369–375. doi: 10.1097/01.ccx.0000166399.88635.a5. [DOI] [PubMed] [Google Scholar]
- 190.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-LOHR V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 191.Rothenhausler HB, Ehrentraut S, Stoll C, Schelling G, Kapfhammer HP. The relationship between cognitive performance and employment and health status in long-term survivors of the acute respiratory distress syndrome: results of an exploratory study. Gen Hosp Psychiatry. 2001;23(2):90–96. doi: 10.1016/s0163-8343(01)00123-2. [DOI] [PubMed] [Google Scholar]
- 192.Duning T, van dH, I, Dickmann A, et al. Hypoglycemia aggravates critical illness-induced neurocognitive dysfunction. Diabetes Care. 2010;33(3):639–644. doi: 10.2337/dc09-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hopkins RO, Ely EW, Jackson JC. The role of future longitudinal studies in ICU survivors: understanding determinants and pathophysiology of brain dysfunction. Curr Opin Crit Care. 2007;13(5):497–502. doi: 10.1097/MCC.0b013e3282efd19c. [DOI] [PubMed] [Google Scholar]
- 194.Patrick GT, Gilbert JA. On the effects of loss of sleep. Psychol Rev. 1896;3(1):469–483. [Google Scholar]
- 195.Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HP. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. 2010;33(1):47–57. doi: 10.1093/sleep/33.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Weinert CR, Gross CR, Kangas JR, Bury CL, Marinelli WA. Health-related quality of life after acute lung injury. Am J Respir Crit Care Med. 1997;156(4):1120–1128. doi: 10.1164/ajrccm.156.4.9611047. [DOI] [PubMed] [Google Scholar]
- 197.Dowdy DW, Eid MP, Dennison CR, et al. Quality of life after acute respiratory distress syndrome: a meta-analysis. Intensive Care Med. 2006;32(8):1115–1124. doi: 10.1007/s00134-006-0217-3. [DOI] [PubMed] [Google Scholar]
- 198.Dowdy DW, Eid MP, Sedrakyan A, et al. Quality of life in adult survivors of critical illness: a systematic review of the literature. Intensive Care Med. 2005;31(5):611–620. doi: 10.1007/s00134-005-2592-6. [DOI] [PubMed] [Google Scholar]
- 199.Reynolds CF, III, Serody L, Okun ML, et al. Protecting sleep, promoting health in later life: a randomized clinical trial. Psychosom Med. 2010;72(2):178–186. doi: 10.1097/PSY.0b013e3181c870a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Faubel R, Lopez-Garcia E, Guallar-Castillon P, et al. Sleep duration and health-related quality of life among older adults: a population-based cohort in Spain. Sleep. 2009;32(8):1059–1068. [PMC free article] [PubMed] [Google Scholar]
- 201.Hu RF, Jiang XY, Zeng YM, Chen XY, Zhang YH. Effects of earplugs and eye masks on nocturnal sleep, melatonin and cortisol in a simulated intensive care unit environment. Crit Care. 2010;14(2):R66. doi: 10.1186/cc8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Xie H, Kang J, Mills GH. Clinical review: the impact of noise on patients' sleep and the effectiveness of noise reduction strategies in intensive care units. Critical Care. 2009;13(3):151. doi: 10.1186/cc7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Richards KC. Effect of a back massage and relaxation intervention on sleep in critically ill patients. Am J Crit Care. 1998;7(4):288–299. [PubMed] [Google Scholar]
- 204.Pronovost PJ, Berenholtz SM, Needham DM. Translating evidence into practice: a model for large scale knowledge translation. BMJ. 2008;337(1):a1714. doi: 10.1136/bmj.a1714. [DOI] [PubMed] [Google Scholar]