SUMMARY
Microsomal triglyceride transfer protein (MTP), essential for apoB-lipoprotein biosynthesis, evolved as a phospholipid transfer protein and acquired triglyceride transfer activity during a transition from invertebrates to vertebrates. But it is unknown whether MTP directly transfers lipids onto apoB in vivo and, if it does, whether both neutral and polar lipid transfer activities of MTP are critical for lipoprotein assembly. The molecular bases for differences in lipid transfer activities with respect to distinct domains in dMTP and hMTP are not obvious because both proteins have very similar primary, secondary and tertiary structures. We used an in vivo approach to delineate physiological significance of these distinct lipid transfer activities by expressing Drosophila (transfers phospholipids) and human MTP (transfers phospholipids and triglycerides) orthologs using adenoviruses in liver-specific MTP deficient (L-MTP−/−) mice that have low plasma and high hepatic lipids. Both orthologs improved plasma lipids but plasma triglycerides were lower in dMTP mice due to lower hepatic triglyceride and apoB production. Hepatosteatosis in L-MTP−/− mice was ameliorated to similar levels by both. Attenuation of hepatosteatosis upon dMTP expression pertained to enhanced β-oxidation with no changes in lipogenesis. Phospholipid transfer activity of MTP promoted biogenesis of both apoB48 and apoB100-containing VLDL in addition to a phospholipid-rich apoB48-containing HDL-size particle. Triglyceride transfer activity augmented the biosynthesis of triglyceride-rich lipoproteins by increasing the formation of these particles in the lumen of the endoplasmic reticulum. Based on these findings, we posit that the selective inhibition of MTP triglyceride transfer activity might reduce hyperlipidemia while protecting liver from excess lipid accumulation.
INTRODUCTION
High plasma concentrations of apolipoprotein B (apoB)-containing lipoproteins are a risk factor for various cardiovascular and metabolic disorders such as atherosclerosis, obesity, diabetes, and metabolic syndrome (1). Both overproduction and decreased catabolism of apoB-lipoproteins contribute to hyperlipidemic states. Microsomal triglyceride transfer protein (MTP) is the essential chaperone for the biosynthesis of apoB-lipoproteins as evidenced by the absence of these lipoproteins in the plasma of abetalipoproteinemia subjects that have mutations in the MTTP gene (2, 3). Although the exact mechanism of apoB-lipoprotein assembly is still undefined, it is believed that MTP transfers neutral lipids onto nascent apoB while it is co-translationally translocated into the endoplasmic reticulum (ER) to form primordial lipoproteins (4–6). These are subsequently lipidated by a second step ‘core-expansion’ involving fusion of primordial lipoproteins with apoB-free triglyceride-rich droplets to form mature lipoproteins (7–9). MTP is also implied to be essential for the partitioning of triglyceride into the ER lumen (10–13).
In vitro studies indicate that MTP transfers lipids by a ping-pong bi-bi shuttle mechanism wherein MTP interacts with donor membranes and extracts lipids, and then associates with acceptor membranes to deposit them (14, 15). A breakthrough in understanding of the lipid transfer properties of MTP came with the identification of a Drosophila MTP (dMTP) ortholog that transfers both fluorescent and radiolabeled phospholipids but does not transfer triglycerides (16, 17). Evolutionary studies showed that MTP evolved as a phospholipid transfer protein and acquired triglyceride transfer activity during a transition from invertebrates to vertebrates (18). Identification of distinct domains with respect to differences in lipid transfer activities in dMTP and hMTP have been challenging due to very similar primary, secondary and tertiary structures (18). It has been previously reported that helix A might be critical for triglyceride transfer activity (19, 20) but we did not observe conservation of this helix in dMTP (16). Importantly, all these characterization studies were performed in reconstituted systems in vitro, in COS7 cells. Here we explored the role of distinct lipid transfer activities of MTP in lipoprotein assembly in vivo using these orthologs. Furthermore, we dissected physiological mechanisms that lead to reduced hepatic and plasma lipids in mice expressing dMTP that lack triglyceride transfer activity.
MATERIALS AND METHODS
A detailed description of methods used is provided in the supplementary information.
Animals
To obtain liver specific Mttp gene deletion (Alb-cre-Mttpfl/fl, L-MTP−/−), Mttpfl/fl mice with floxed exon 1 (21) were crossed with Alb-cre (The Jackson Laboratories) mice and maintained in 7:00–19:00 h lighting schedule with free access to water and standard laboratory chow or western-diet. We used 8–12 week old male mice. All animal experiments were approved by the institutional animal care and use committee of SUNY Downstate Medical Center.
Generation and in vivo delivery of recombinant adenoviral vectors
FLAG tagged Ad-hMTP and Ad-dMTP were generated, amplified (supplementary methods), and injected into the tail veins of male mice immobilized in a restrainer (BrainTree Scientific). All experiments were performed in 6 h fasted mice. Blood was collected in EDTA after cardiac puncture to obtain plasma. Liver and intestine were collected, washed with ice-cold PBS and used for lipid extraction (22) or snap frozen in liquid nitrogen for storage at −80°C.
Hepatic and plasma lipid analyses
MTP activity in tissues was determined (23, 24) using triglyceride and phospholipid transfer assay kits (Chylos, Inc.). Tissues were also used for immunoblotting and histology. Plasma and tissue triglyceride, total and free cholesterol, and phospholipid were determined using kits, and concentrations were deduced from standard curves (25).
Fatty acid oxidation and hepatic de novo lipogenesis
Lipid synthesis was measured by incubating primary hepatocytes with [3H]-oleate for 2 h. Lipids were extracted from cells (22) and separated by thin layer chromatography (TLC). Bands were scraped and counted to measure label incorporation in lipids. Hepatic de novo lipogenesis was determined by measuring [14C]-acetate incorporation in fatty acids (26). Fatty acid oxidation in primary hepatocytes was determined (27) by incubating with [14C]-oleate (0.3 µCi/ml).
Metabolic studies in mice and isolated primary hepatocytes
Mice were intravenously administered with [35S]-Promix (Perkin Elmer) for in vivo studies. Primary hepatocytes were isolated (10) three days after the transduction of L-MTP−/− mice with 1010 pfu of Ad-hMTP or Ad-dMTP and incubated with [3H]-oleate (10 µCi/ml) for 30 min. Microsomal lumenal contents and membranes were isolated and apoB-containing lipoproteins were immunoprecipitated in the absence of detergent under non-denaturing conditions (10, 13). Lipids were extracted (22), separated by TLC (13), visualized by iodine vapor, scraped and counted.
To study apoB synthesis, primary hepatocytes were labeled with [35S]-Promix (150 µCi/ml) in the presence or absence of MG132 (Sigma) in methionine/cysteine free media for the indicated times. Intracellular and secreted apoB was immunoprecipitated from cell lysates and media, separated on gels, dried and fluorographed.
Statistical analyses
Three different comparisons were made using One-way ANOVA unless notified in the figure legend. First, WT and all knockout groups were compared and significant differences were depicted by###. Second, significant differences between PBS injected L-MTP−/− and L-MTP−/− mice transduced with different viruses are designated ***. Third, differences between hMTP and dMTP groups were evaluated using t-test and are shown as @. Data are representative of two to three experiments. All data sets were performed in triplicates and represented as, Mean ± SEM.
RESULTS
dMTP with phospholipid lipid transfer activity alleviates hepatosteatosis and partially restore plasma lipids in L-MTP−/− mice
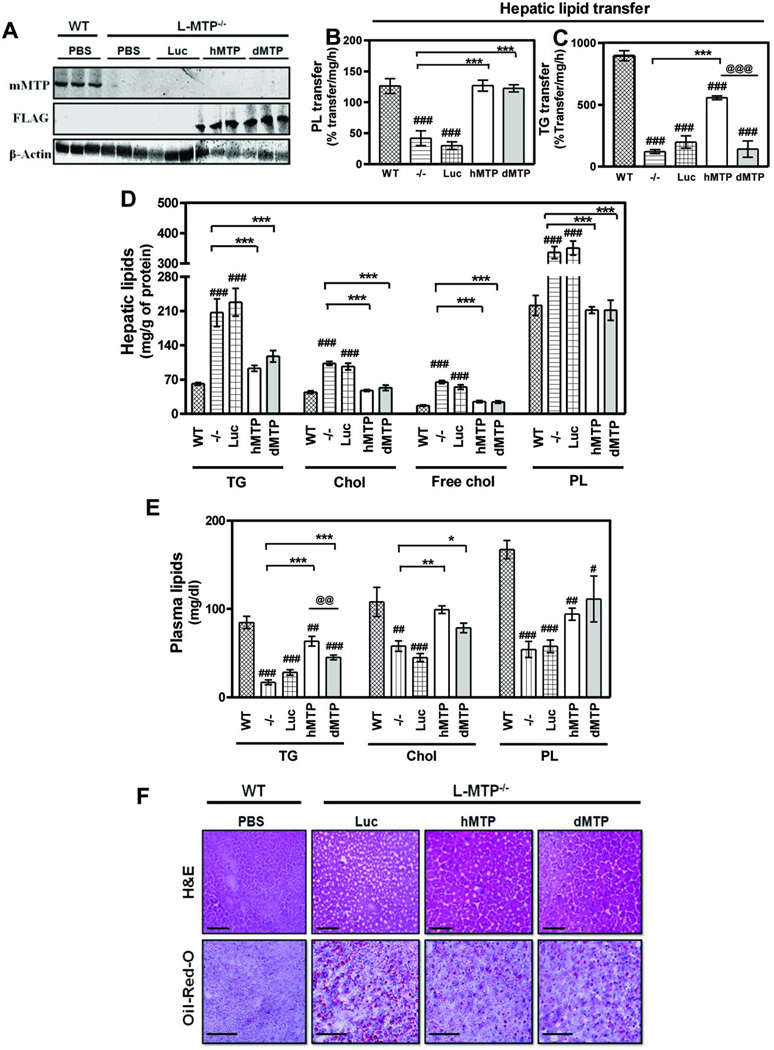
To understand the physiologic dichotomy manifested by the different lipid transfer properties of MTP, FLAG-tagged adenoviruses expressing hMTP (Ad-hMTP) and dMTP (Ad-dMTP) were prepared. Their abilities to promote lipid transfer and apoB48-lipoprotein secretion were established in transduced COS7 cells (Fig S1). Next, these adenoviruses were transduced in liver-specific Mttp ablated (L-MTP−/−) mice. Mouse MTP was barely detectable in the liver of L-MTP−/− mice (Fig 1A, top), consistent with other conditional MTP deletion studies (28, 29), indicating successful disruption of the endogenous Mttp gene. FLAG-tagged proteins were detected in Ad-hMTP and Ad-dMTP transduced livers (Fig 1A, middle). Next, hepatic lipid transfer activities were examined. Phospholipid transfer activity was significantly reduced in PBS or Ad-luciferase (Ad-Luc) injected L-MTP−/− mice compared to Mttpfl/fl (WT); hMTP and dMTP expression restored this activity to WT levels (Fig 1B). Mttp gene deletion reduced triglyceride transfer activity by ~80–85% consistent with other reports (28, 29) and expression of luciferase had no effect on this activity (Fig 1C). However, robust triglyceride transfer activity was present in hMTP, but not in dMTP-expressing livers (Fig 1C). No changes were observed in MTP mediated lipid transfer activities in the intestines of different groups indicating a liver specific effect (Fig S2A–B). These studies show that hMTP but not dMTP restores triglyceride transfer activity in L-MTP−/− mice whereas both reconstitute phospholipid transfer activity.
Figure 1. hMTP and dMTP expression alters hepatic and plasma lipids in L-MTP−/− mice.
Adenoviruses (1010 pfu) were transduced into L-MTP−/− mice via tail vein. Additionally, L-MTP−/− (−/−) and WT (MTPfl/fl) mice received PBS. After 3 days, mice were fasted (6 h) before collecting plasma and tissues. Mean ± SEM, n=3–6. ***p < 0. 001, **p < 0. 01, *p < 0. 05. (A) Top panel: Immunoblotting showing expression of endogenous mouse MTP (mMTP). Middle panel: FLAG expression in the liver of mice transduced with FLAG-tagged, Ad-hMTP (hMTP) and Ad-dMTP (dMTP). Bottom panel: β-actin shown as loading control. (B–C) Hepatic phospholipid (PL) (B), and triglyceride (TG) (C) transfer activities, (D) Hepatic triglyceride, total cholesterol (Chol), free cholesterol (Free Chol), and phospholipids (E) Plasma triglycerides, cholesterol and phospholipids are shown. (F) Liver sections were stained with hematoxylin and eosin (H&E) or with Oil-Red-O and counterstained with hematoxylin for nucleus. Bars = 50 µm.
Analysis of hepatic lipids (Fig 1D) showed that relative to WT mice, livers of L-MTP−/− mice (PBS or Ad-Luc) accumulated higher amounts of triglyceride (~2 –3 fold), total cholesterol (~2 fold) primarily due to elevated free cholesterol (~ 2 fold), and phospholipids (~1.5 fold). Expression of hMTP significantly lowered hepatic triglyceride, total and free cholesterol and phospholipid (Fig 1D). Surprisingly, expression of dMTP also reduced hepatic lipids to similar extents in L-MTP−/− mice (Fig 1D). Intestinal lipids did not show any changes between groups suggesting that changes were specific to hepatic lipids (Fig S2C–E). These studies demonstrate that both hMTP and dMTP ameliorate hepatic steatosis associated with Mttp gene ablation.
Plasma lipid analyses (Fig 1E) revealed that L-MTP−/− mice (PBS or Ad-Luc) were hypolipidemic due to significant reductions in plasma triglyceride, cholesterol and phospholipid compared to WT. hMTP restored plasma triglycerides; dMTP also raised plasma triglycerides in L-MTP−/− mice but levels were significantly lower than hMTP. Expression of both MTP orthologs rescued cholesterol and phospholipid to similar levels (Fig 1E). These studies demonstrate that dMTP, relative to hMTP, expression results in lower plasma triglyceride.
Hematoxylin/eosin staining (Fig 1F) showed numerous vacuoles indicating a fatty liver in Ad-Luc injected L-MTP−/− mice compared to WT. hMTP and dMTP expression reduced the number of vacuoles, consistent with the reduced triglyceride content (Fig 1D). Oil-Red-O staining also showed significant lipid accumulation in L-MTP−/− livers compared to WT which were reduced upon hMTP and dMTP expression (Fig 1F). Therefore, expression of both hMTP and dMTP reduces hepatic lipids in L-MTP−/− mice.
Physiologic effects of different MTP orthologs are not limited by lipid availability
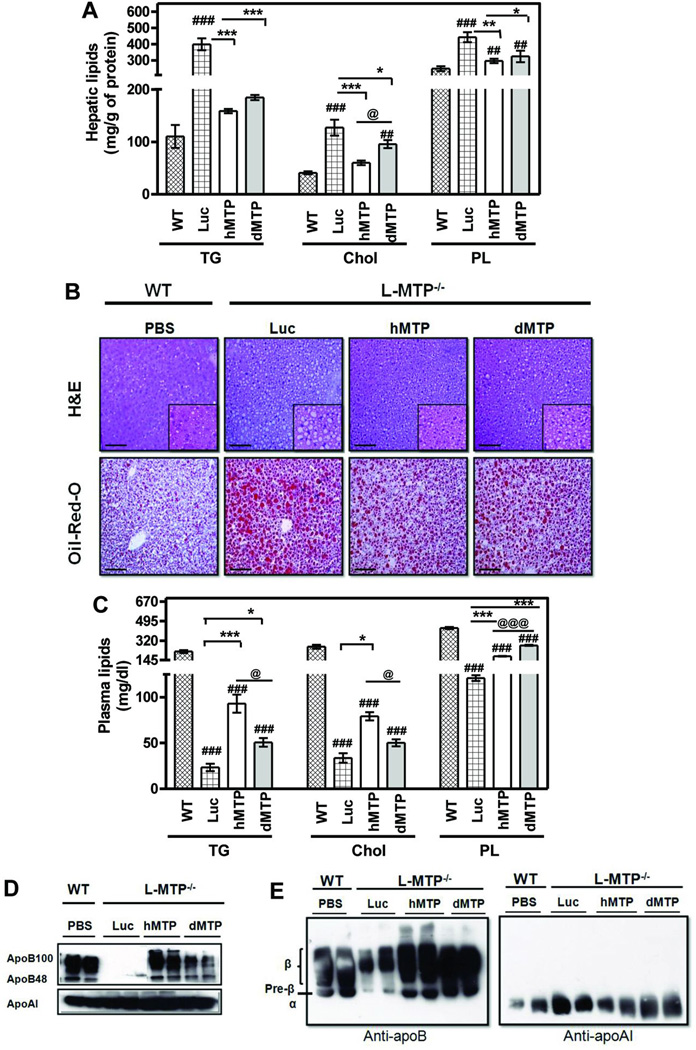
Consideration was given to the possibility that low plasma and hepatic lipids in dMTP mice might be secondary to the feeding of a low-fat chow-diet. Therefore, we assessed the efficacy of hMTP and dMTP in reducing hepatic lipids in western-diet fed L-MTP−/− mice. Immunoblotting and phospholipid transfer activity indicated that hepatic expression of dMTP was ~40% higher than hMTP (Fig S3A–B). Hepatic triglyceride (~ 4 fold;); total cholesterol (~ 2–2.5 fold;); and phospholipids (~1.5 fold;) were higher in L-MTP−/− mice (Ad-Luc) compared to WT (Fig 2A). hMTP and dMTP expression significantly lowered hepatic lipids (Fig 2A). Hematoxylin/eosin and Oil-Red-O staining showed steatotic liver with significant accumulation of lipids in L-MTP−/− livers compared to WT and these lipids were reduced after hMTP and dMTP expression (Fig 2B). These studies indicate that both hMTP and dMTP attenuate steatosis in western-diet fed L-MTP−/− mice to similar levels.
Figure 2. Effects of hMTP and dMTP expression on tissue and plasma lipids in L-MTP−/− mice fed western-diet.
WT mice and L-MTP−/− were fed a western-diet (Harlan Teklad, 42% fat) for 15 days ad libitum. Different adenoviruses (1010 pfu) were transduced into L-MTP−/− mice. WT mice received sterile PBS. Diet was continued for an additional 5 days, fasted (6 h), and plasma and tissues were collected. Mean ± SEM, n=5. ***p < 0. 001, **p < 0. 01, *p < 0. 05. (A) Hepatic lipids showing triglycerides, total cholesterol and phospholipids. (B) Representative images of liver sections from each group stained with H&E showing histology and Oil-Red-O showing lipid content. Bars = 50 µm. (C) Plasma triglyceride, cholesterol, and phospholipids. (D) Plasma samples were separated by SDS-PAGE and immunoblotted for apoB (top) and apoAI (bottom). (E) Agarose gel electrophoresis of plasma samples followed by immunoblot analysis for mouse apoB (left) and apoAI (right).
We then studied the effect of the expression of these orthologs on plasma lipids (Fig 2C). Increases in plasma lipids were seen in WT mice due to high-fat diet as expected from earlier reports (30). Lower plasma triglycerides seen in L-MTP−/− mice (Ad-Luc) compared to WT were raised upon dMTP-expression but to a lesser extent than hMTP. Plasma cholesterol levels in the L-MTP−/− mice were ~4-fold lower than WT and expression of hMTP and dMTP slightly increased these levels. Plasma phospholipids were reduced by ~3–4 folds in the L-MTP−/− mice and expression of both MTP orthologs increased their levels; however, phospholipids were higher in dMTP mice. Significant reductions in apoB100 and apoB48 in (Fig 2D) L-MTP−/− mice (Ad-Luc) compared to WT were increased upon hMTP and dMTP-expression with higher recovery by hMTP. Agarose gel electrophoresis of plasma lipoproteins (Fig 2E) showed significant reductions in pre-β and β-lipoproteins, but not in α-lipoproteins, in L-MTP−/− mice (Ad-Luc) compared to WT. Expression of hMTP and dMTP increased both pre-β and β-lipoproteins; no such changes in α-lipoproteins were observed. Hence, both hMTP and dMTP increase apoB-lipoproteins and raise plasma lipids; however, increases in plasma triglyceride in dMTP mice were lower than hMTP mice.
Increased β-oxidation and decreased triglyceride secretion in dMTP mice
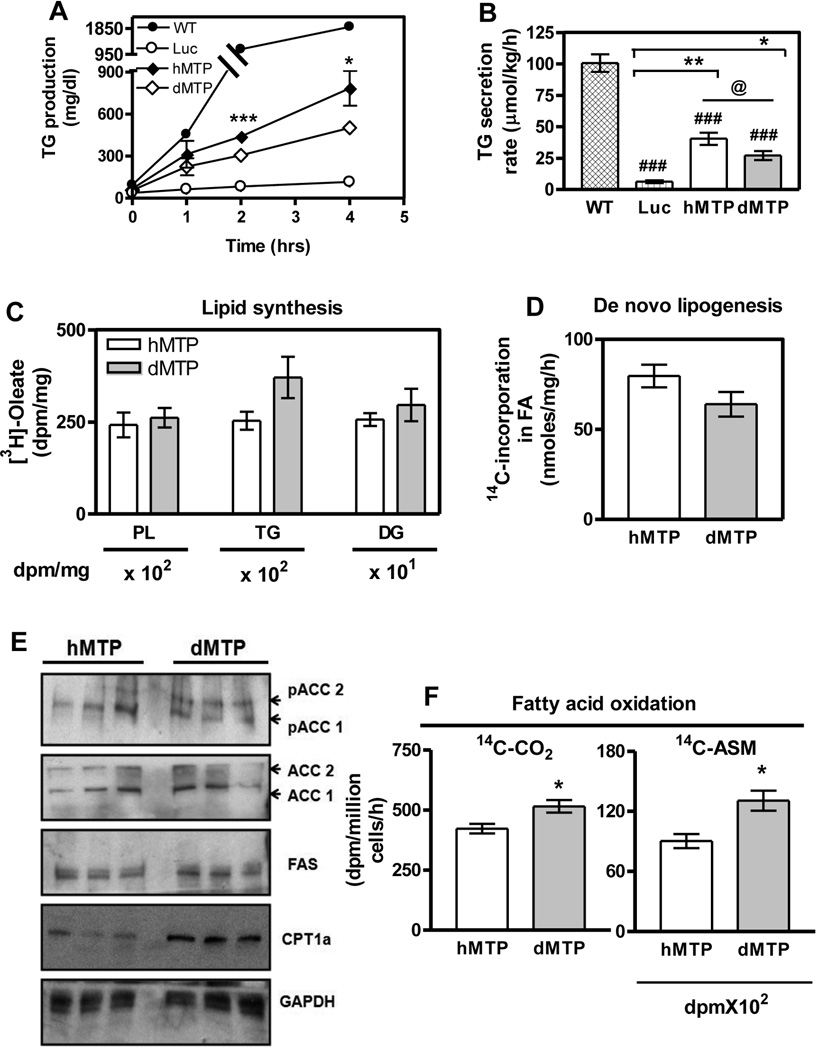
The above studies showed that dMTP expression in L-MTP−/− mice attenuates hepatic steatosis similar to hMTP while maintaining lower plasma lipids. To understand reasons for these differences, we studied triglyceride production, de novo lipogenesis and β-oxidation. First, we studied triglyceride production in lipase inhibited animals (31). Total plasma (Fig 3A) and non-HDL (Fig S4A) triglycerides increased in a time-dependent fashion in both mice, but these increases were lower in dMTP-expressing mice. Additionally, triglyceride secretion rates were lower in dMTP mice (Fig 3B, S4B). Second, we analyzed the incorporation of [3H]-oleate into phospholipids, triglyceride, and diacylglycerols (Fig 3C). There were no differences in the biosynthesis of these lipids in dMTP and hMTP-expressing hepatocytes. Also, incorporation of [14C]-acetate into fatty acids (Fig 3D) was similar in dMTP and hMTP liver indicating no differences in de novo lipogenesis. This was substantiated by measuring protein levels of acetyl-CoA carboxylase 1 (ACC1) and fatty acid synthase (FAS), two critical enzymes involved in de novo lipogenesis (32, 33) (Fig 3E). No significant differences in protein levels of total ACC1, phosphorylated ACC1 and FAS were observed corroborating similar lipogenesis rates in hMTP and dMTP mice (Fig 3D).
Figure 3. Higher fatty acid oxidation and lower triglyceride production in dMTP mice.
(A) L-MTP−/− mice were transduced (1010 pfu) with indicated viruses. After 72 h mice were fasted (4 h) and blood was sampled. Next, P407 (30 mg/mice, i.p.) was injected. Blood was sampled at indicated times, total plasma and VLDL triglyceride were measured. Increases in triglyceride were calculated after subtracting basal values (B) and triglyceride secretion rates were calculated using 2 and 4 h time points. (C) Lipid synthesis in primary hepatocytes shown as [3H]-oleate incorporation in newly synthesized phospholipids, triglycerides, and diglycerides (DG). (D) Hepatic de novo lipogenesis rates represent incorporation of [14C]-acetate in fatty acids. (E) Western blot analysis of ACC isoforms (ACC1 andACC2) and their phosphorylated forms (pACC1 and pACC2), FAS, CPT1a and GAPDH. (F) Fatty acid oxidation rates in primary hepatocytes were determined by measuring [14C]-CO2 released and [14C]-acid soluble metabolites (ASM) remained in cells. Mean ± SEM, n=3. Student’s t-test was performed. ***p < 0. 001, **p < 0. 01, *p < 0. 05.
Third, measurement of fatty acid β-oxidation rates showed that dMTP expressing hepatocytes produced more CO2 and acid-soluble metabolites (ASM) than hMTP (Fig 3F). Furthermore, protein levels of ACC2 and carnitine palmitoyl transferase 1 (CPT1), critical enzymes that regulate fatty acid β-oxidation (34), were measured. We observed reduced total ACC2, increased phosphorylated ACC2 and enhanced CPT1a proteins in dMTP (Fig 3E) compared to hMTP. These studies indicate that β-oxidation is significantly higher in dMTP mice. Hence, attenuation of hepatosteatosis in dMTP expressing mice compared to hMTP mice could be due to higher hepatic β-oxidation despite decreased triglyceride secretion.
Phospholipid transfer activity assists in the hepatic synthesis and secretion of triglyceride-poor apoB100 and apoB48-containing VLDL and phospholipid-rich apoB48–HDL lipoprotein
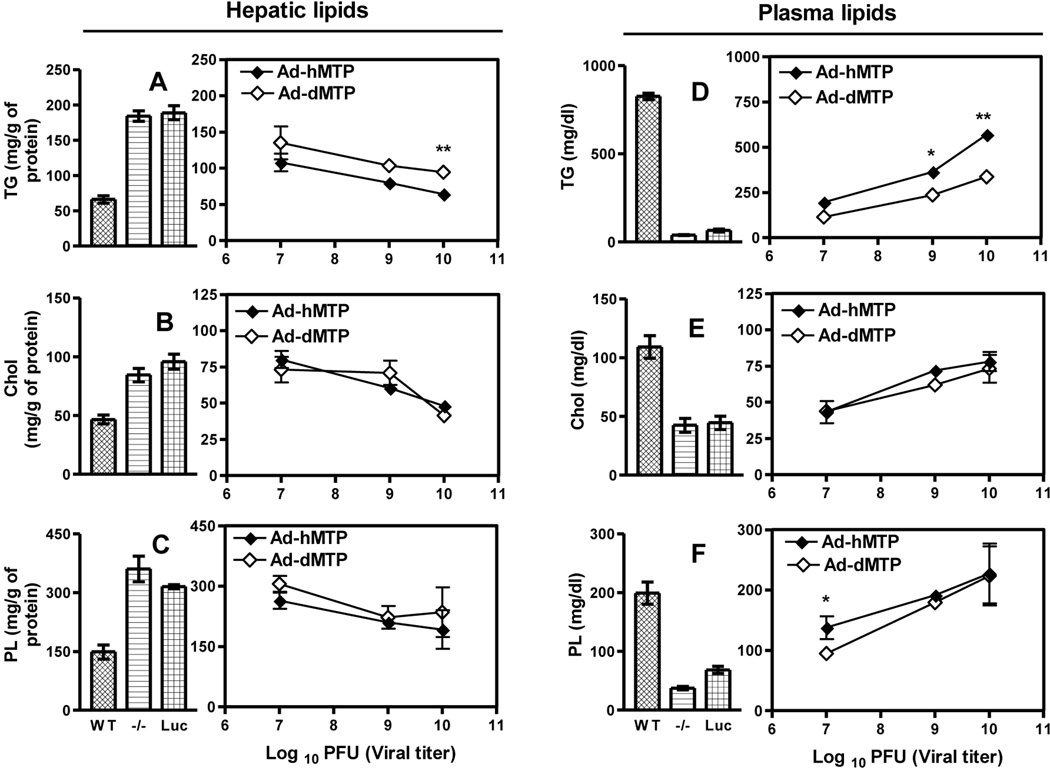
Next, we investigated whether the unique effects of dMTP on plasma and hepatic lipids seen previously would be still obtained at different hepatic expression levels of these orthologs. The relative mass of hMTP and dMTP in the liver increased with viral particle number (Fig S5A). Phospholipid transfer activity also enhanced with increasing amounts of dMTP and hMTP (Fig S5B); while triglyceride transfer activity was augmented only in the hMTP mice (Fig S5C). Both orthologs significantly reduced hepatic triglycerides in L-MTP−/− mice in a dose-dependent manner (Fig 4A); however, reductions in dMTP-expressing mice were lower than hMTP mice. Hepatic cholesterol (Fig 4B) and phospholipids (Fig 4C) were similarly reduced by both orthologs. These studies illustrate dose-dependent reductions in hepatic lipids by both orthologs in L-MTP−/− mice.
Figure 4. Effect of different levels of MTP ortholog expression on hepatic and plasma lipids.
L-MTP−/− mice were transduced with 107, 109, and1010 pfu of Ad-Luc, Ad-hMTP or Ad-dMTP. After 3 days, mice were fasted (4 h), injected with P407, and plasma was collected after 2 h. Data from WT, L-MTP−/− (−/−) and Ad-Luc (1010 pfu) are shown as bar graphs. Effects of expression of different levels of hMTP and dMTP are shown as line graphs. (A–C) Hepatic triglyceride (A), cholesterol (B), and phospholipid (C). (D–F) Plasma total triglyceride (A), cholesterol (B) and phospholipid (C). Mean ± SEM, n=3. Student’s t-test was performed between hMTP and dMTP groups for each concentration. ***p < 0. 001, **p < 0. 01, *p < 0. 05.
Plasma triglycerides progressively increased (Fig 4D) with corresponding augmentations in the expression of both MTP orthologs in L-MTP−/− mice mainly due to enhancements in plasma non-HDL, apoB-lipoproteins (Fig S5D) with no changes in HDL (Fig S5E); albeit, increments were higher in hMTP mice. Plasma cholesterol levels increased to similar extents in both orthologs (Fig 4E), again due to a greater increase in non-HDL cholesterol (Fig S5F) with no change in HDL-cholesterol compared to Ad-Luc (Fig S5G). Finally, both total (Fig 4F) and non-HDL phospholipids (Fig S5H) showed dose-dependent increase after hMTP and dMTP expression in L-MTP−/− mice, although increases in HDL-phospholipids were higher in dMTP (Fig S5I) and less in non-HDL (Fig S5H) compared to hMTP. These studies indicate that both MTP orthologs augment plasma lipids in non-HDL lipoproteins, whereas dMTP also enhances HDL phospholipids in a dose-dependent manner.
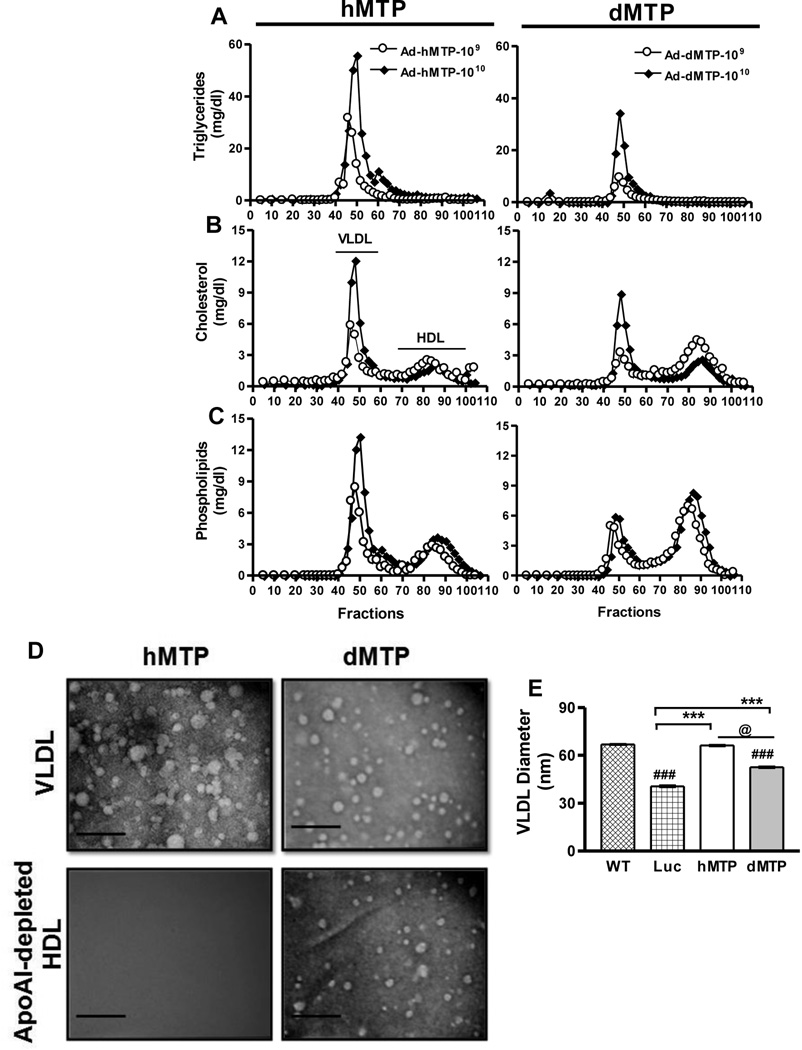
To understand the basis for greater increases in plasma VLDL-triglycerides in hMTP and plasma HDL-phospholipids in dMTP mice, plasma lipid distribution in lipoproteins were further characterized by FPLC and electron microscopy. Consistent with the lipid analyses in Fig 4, mice transduced with 109 and 1010 pfu of hMTP and dMTP displayed a dose-dependent increase in plasma triglyceride, cholesterol, and phospholipids in the VLDL fractions separated by FPLC in P407 injected animals (Fig 5A–C); however, these levels were lower in dMTP-expressing mice. Amounts of cholesterol in HDL were not affected by increasing amounts of hMTP and were similar in dMTP and hMTP mice (Fig 5B), but phospholipids were higher in the HDL fractions in dMTP mice (Fig 5C). Further characterization of lipoproteins fractionated by FPLC was performed by negative staining and electron microscopy (Fig 5D, S6A). VLDL/LDL particle diameter analysis (Fig 5E) showed that dMTP makes a smaller, homogeneous VLDL particle compared to hMTP. To understand reasons for higher levels of phospholipids in HDL fractions, plasma was subjected to FPLC and HDL fractions were pooled (Fig S6A), apoAI-containing particles were pulled-down from total HDL (Fig S6A, S6D–E). The apoAI-free HDL supernatants contained particles of 17.1±3.00 nm in the dMTP mouse plasma (Fig 5D, S6A). These data demonstrate the presence of a novel population of apoAI-free HDL particles in dMTP mice.
Figure 5. Plasma lipoprotein characterization.
L-MTP−/− mice were transduced with different titers of adenoviruses. After 3 days, mice were fasted (4 h) and injected with P407. Plasma was collected after 2 h, pooled, and subjected to FPLC. Triglyceride (A), cholesterol (B) and phospholipid (C) distribution in plasma lipoproteins of Ad-hMTP (left panel) and Ad-dMTP transduced mice (right panel). (D–E) VLDL fractions after FPLC were pooled and concentrated. Similarly, HDL fractions were pooled and apoAI-lipoproteins were removed by immunoprecipitation. VLDL and ApoAI-free HDL were subjected to negative staining and electron microscopy (D). VLDL Bars = 180 nm, HDL Bars = 72 nm. Average diameter of VLDL particles in different groups (Mean ± SEM, n=200–300) (E). ***p < 0. 001, **p < 0. 01, *p < 0. 05.
Next, we studied the kinetics of apoB secretion in hMTP and dMTP mice to gain insights into mechanisms that lead to different plasma triglyceride levels after in vivo radio-labeling. Newly synthesized albumin secretion increased with time in all animals (Fig 6A). L-MTP−/− mice (Ad-Luc) produced negligible radiolabeled apoB in the plasma compared to WT (Fig 6A–B). Both, hMTP and dMTP-expressing mice showed time-dependent increased secretion of nascent apoB48 and apoB100; however, the amounts of apoB100 in hMTP-expressing mice were higher than those in dMTP. Moreover, secretion rates of apoB48 and apoB100 in these mice were higher (Fig S7A–B). These studies indicate that triglyceride transfer activity, unique to the hMTP ortholog, facilitates biosynthesis of more apoB-lipoprotein particles.
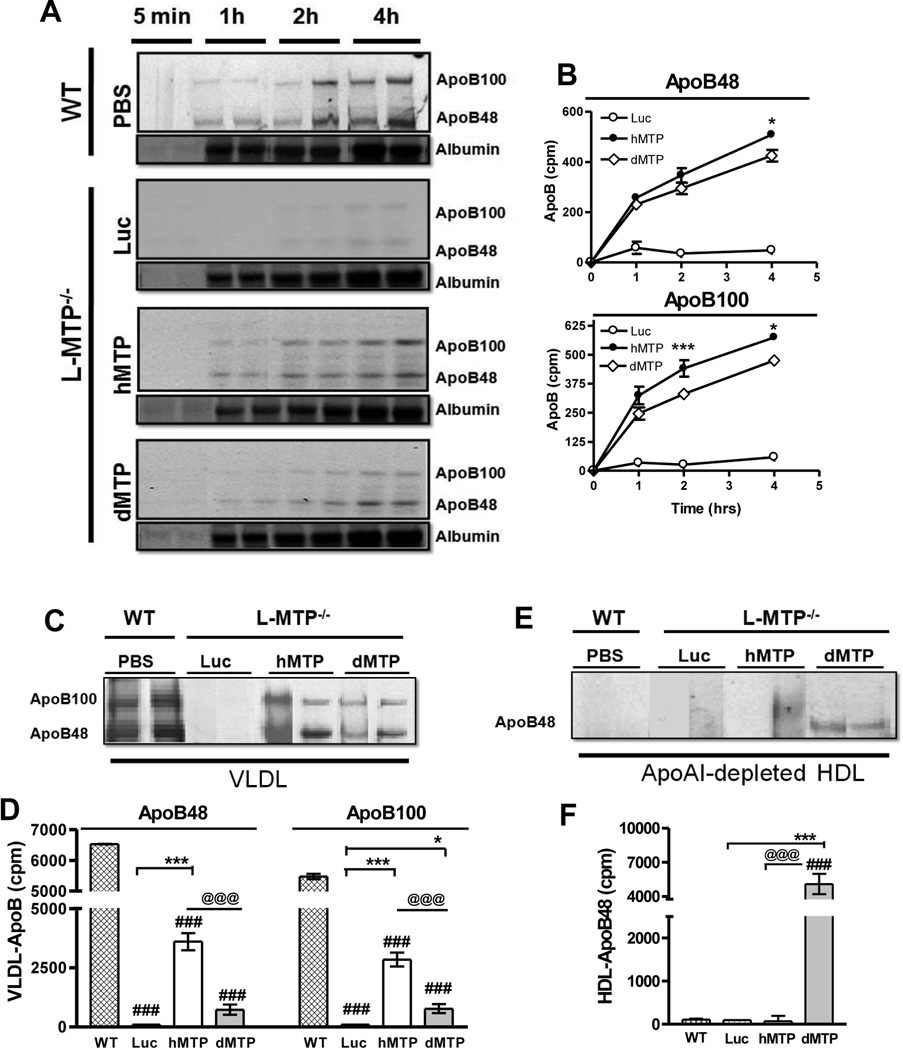
Figure 6. Hepatic total and VLDL and HDL apoB synthesis and secretion in mice expressing hMTP and dMTP.
(A–B) Synthesis and secretion of apoB-lipoproteins by the livers of dMTP and hMTP mice: 72 h after virus injection, mice were fasted (4 h) and intravenously injected with [35S]-Promix (1 mCi). After 30 min, P407 was injected intraperitoneally and blood was collected at indicated times. Plasma (15 µl) was fractionated by 5% SDS-PAGE, dried and fluorogrpahed (A). ApoB48 and apoB100 bands were dissolved in Soluene (Perkin Elmer) and counted. Counts at 5 min, were subtracted before plotting (B). (C–F) Mice were labeled and plasma samples were collected as described above. Plasma lipoproteins were separated by FPLC. VLDL were pooled, immunoprecipitated using rabbit anti-mouse apoB antibodies, separated by SDS-PAGE and fluorographed (C) and counts in apoB48 and apoB100 bands were quantified (D), Mean ± SEM, n=4. ApoAI-lipoproteins were removed from HDL by immunoprecipitation, and apoAI-free supernatants were precipitated with anti-apoB antibodies, separated on gels and fluorographed (E). Counts in HDL-apoB48 bands were plotted (F). ***p < 0. 001, **p < 0. 01, *p < 0. 05.
Furthermore, we characterized the origin and nature of VLDL and apoAI-free HDL in these mice. [35S]-labeled apoB48 and apoB100-VLDL were undetectable in L-MTP−/− mice (Ad-Luc) whereas hMTP mice secreted both apoB48 and apoB100 (Fig 6C–D); similarly, dMTP also supported the secretion of both apoB48 and apoB100-VLDL but their levels were lower compared to hMTP. Next, following pull-down of apoA1-containing particles from HDL, , apoB was immunoprecipitated with anti-apoB antibody (Fig 6E–F). We observed radiolabeled apoB48 in dMTP-expressing mice suggesting hepatic biosynthesis of a novel, phospholipid-rich apoB48-containing HDL-size particle. These data indicate that dMTP supports biosynthesis of apoB100 and apoB48-VLDL similar to hMTP; but dMTP supports synthesis of fewer numbers of particles with lesser amounts of triglycerides. Additionally, dMTP assists in the synthesis of apoB48-containing HDL lipoproteins.
Lower hepatic apoB biosynthesis with phospholipid transfer activity is secondary to higher co-translational degradation of nascent apoB
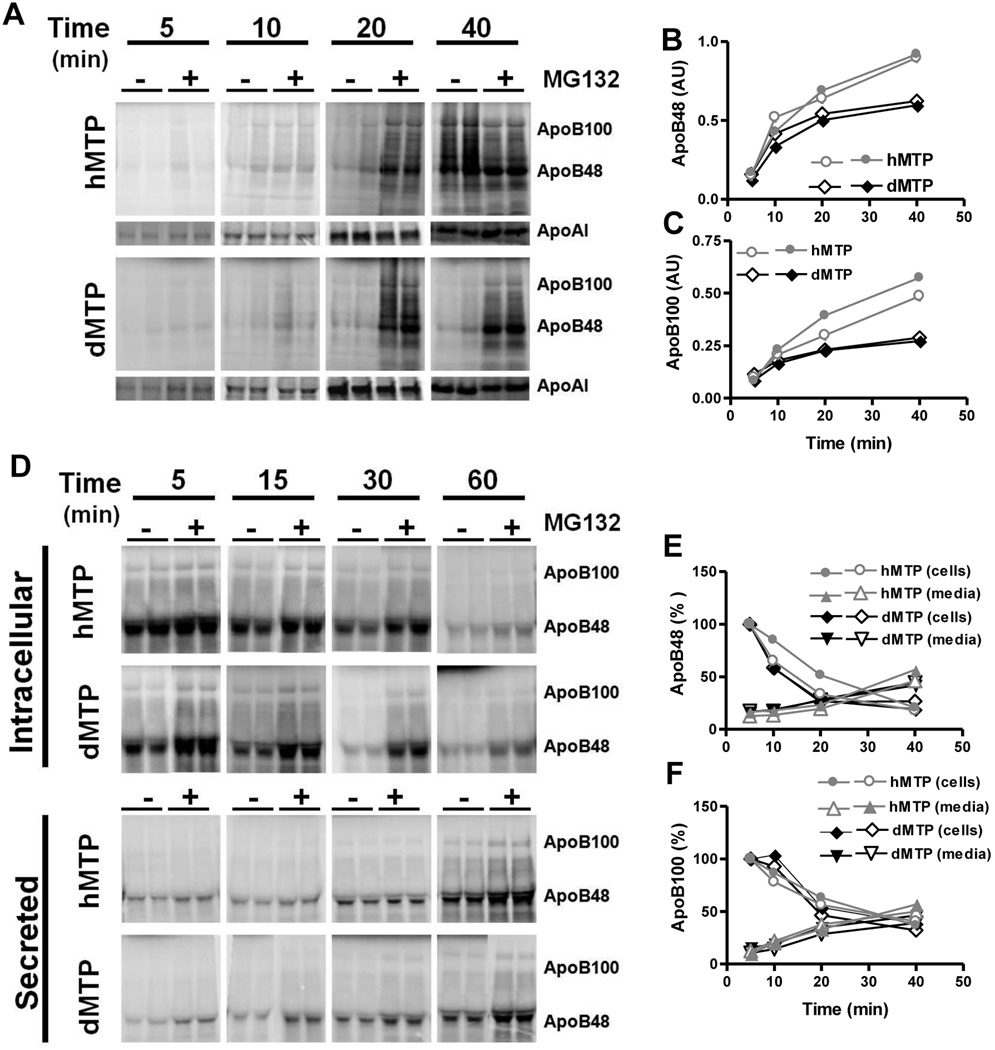
To identify mechanisms for increased apoB secretion of nascent apoB in hMTP mice, apoB synthesis was studied in primary hepatocytes in the presence and absence of proteasomal inhibitor, MG132. Amounts of apoB100 and apoB48 synthesized in the absence of MG132 were significantly higher in hMTP compared to dMTP (Fig 7A–C). These differences were not seen in the presence of MG132 (Fig S8A–B). Furthermore, no significant differences in the amounts of apoAI were found in these hepatocytes (Fig S8C). We interpret these data to suggest that nascent apoB undergoes increased co-translational degradation in dMTP mice compared to hMTP mice perhaps owing to inadequate lipidation resulting from lack of neutral lipid transfer activity.
Figure 7. Kinetics of apoB synthesis and secretion in mice expressing hMTP and dMTP.
(A–C) Decreased apoB synthesis in hepatocytes expressing dMTP: Mice were transduced with different viruses, primary hepatocytes were isolated and incubated with methionine-free medium in the absence or presence of MG132 (10 µM) for 30 min. Cells were labeled with 150 µCi/ml [35S]-Promix for the indicated times in the presence or absence of MG132. Immunoprecipitated intracellular apoB was separated by SDS-PAGE and fluorographed (A). ApoB48 (B) and apoB100 (C) bands were quantified from cells not treated with MG132. (D–F) Posttranslational fate of apoB in hepatocytes expressing different MTP orthologs: Cells were incubated with 150 µCi/ml [35S]-Promix in the presence or absence of MG132 for 1 h, washed, and chased for 5, 15, 30 and 60 min. Cells and media were collected at indicated times and intracellular and secreted apoB were fractionated by SDS-PAGE and fluorographed (D). Counts of apoB48 (E) and apoB100 (F) in cells not treated with MG132 present at 5 min were normalized to 100% and counts remaining in cells or secreted to media were plotted.
To determine further whether there were differences in post-translational degradation of apoB in dMTP and hMTP mice, we performed pulse-chase studies. In the absence of MG132, amounts of apoB100 and apoB48 at the end of the pulse were lower in dMTP-expressing hepatocytes (Fig 7D, 5 min). Importantly, when these values were normalized to 100%, the subsequent fractional disappearance from cells (Fig 7E) and appearance in the media (Fig 7F) were similar in both types of hepatocytes. Moreover, no significant differences in apoB and apoAI disappearance from cells and secretion into media were seen in MG132 treated cells (Fig S8D–F). These studies indicate that triglyceride transfer activity does not provide additional protection against post-translational degradation of nascent apoB100 and apoB48. However, it should be pointed out that the recovery of apoB counts in the cells and media of dMTP and hMTP hepatocytes at 40 min was ~30–35% (Fig 7E–F) indicating that there was a significant post-translational degradation of apoB in both. However, this rate of post-translational degradation was similar in dMTP and hMTP hepatocytes.
Enhanced association of lipids with apoB in the microsomal compartment contributes to higher triglyceride secretion in hMTP
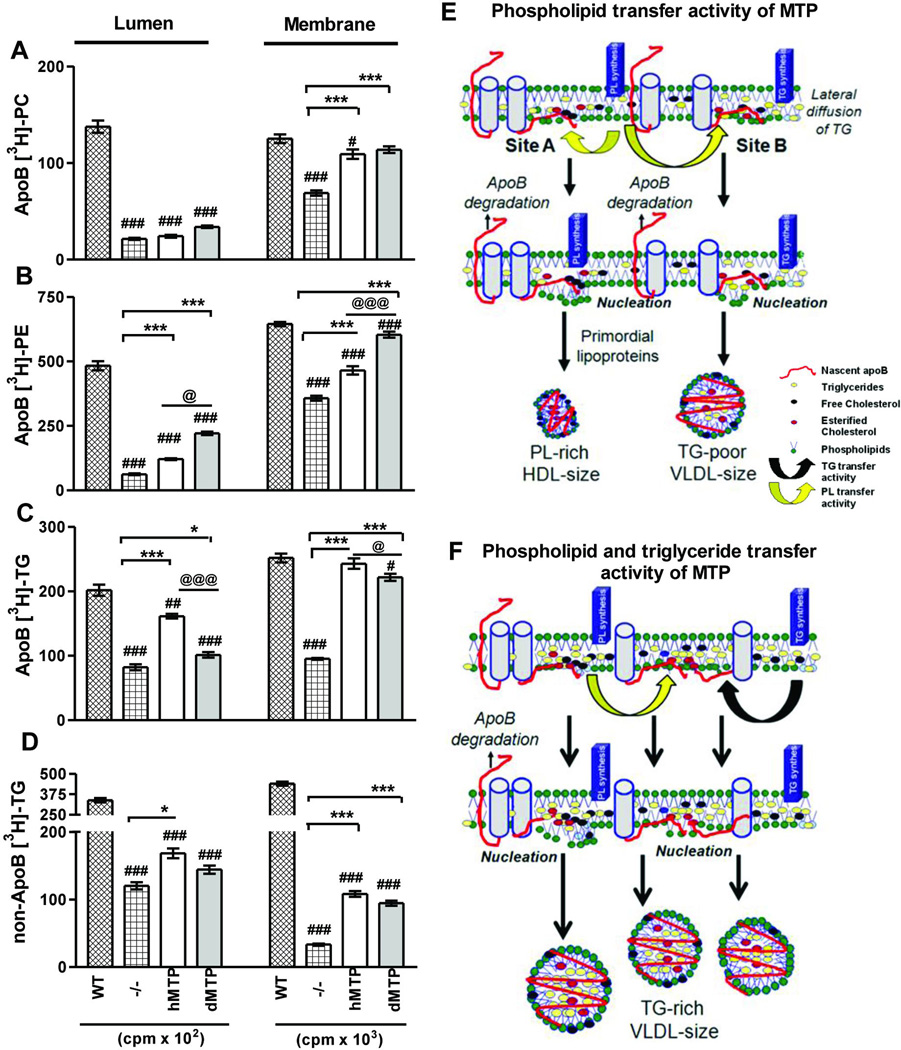
Next, we tested whether differences in plasma triglycerides were arising from association of various amounts of triglycerides with apoB in the ER membrane or lumen. We isolated microsomes to analyze subcellular partitioning of newly synthesized lipids from hMTP or dMTP-expressing hepatocytes and analyze apoB-associated membrane and lumenal lipids. L-MTP−/− hepatocytes had significantly lower amounts of membrane and lumenal phospholipids and triglycerides compared to WT (Fig 8A–D) which increased upon hMTP and dMTP expression. ApoB-associated membrane and lumenal phosphatidylcholine in both hMTP and dMTP were similar (Fig 8A). In contrast, apoB-associated phosphatidylethanolamine in the membranes and lumen were higher in dMTP-hepatocytes compared to hMTP (Fig 8B). Lower amounts of triglyceride were associated with apoB in membrane and lumen dMTP hepatocytes compared to hMTP (Fig 8C). It is possible that after initial priming of apoB with phospholipids and prevention of its degradation (Fig 7), triglycerides may be acquired by lateral diffusion within the plane of the membrane. Similarly, non-apoB triglycerides were lower in dMTP (Fig 8D). These studies indicate that triglyceride transfer activity enriches apoB with triglycerides in the microsomal membrane and lumen and contributes to higher secretion rates.
Figure 8. Subcellular partitioning of newly synthesized lipids in hMTP and dMTP expressing mice.
(A–D) Primary hepatocytes were incubated with [3H]-oleate (10 µCi/ml) for 30 minutes. ApoB-associated lumenal and membrane phosphatidylcholine (PC) (A); phosphatidylethanolamine (PE) (B); triglycerides (C) and triglycerides not associated with apoB (D) are shown. Mean ± SEM, n=3. ***p < 0. 001, **p < 0. 01, *p < 0. 05. (E–F) Model of lipoprotein biosynthesis: Nascent apoB (red) is shown to traverse the ER membrane and interact with inner leaflet to form “nucleation sites” or retro-translocated for degradation. Phospholipid transfer activity in dMTP can bring phospholipid molecules to nascent apoB (yellow arrows) and help in the formation of nucleation sites. Nucleation site A has fewer triglycerides. Desorption from this site may yield phospholipid-rich apoB48-containing HDL size particles. At site B, few triglyceride molecules are shown to arrive via lateral diffusion. Desorption from this site might result in the synthesis of triglyceride-poor apoB100 and apoB48-containing VLDL size lipoproteins (E). Triglyceride transfer activity (black arrow) can bring neutral lipids creating triglyceride enriched nucleation sites, lipidate nascent apoB, and prevent proteasomal degradation. Desorption from these sites might yield heterogeneous apoB100 and apoB48 containing VLDL-size particles (F).
To explain the role of different lipid transfer activities in the biosynthesis of these particles based on these subcellular studies and the distinct physiological effects on plasma lipids, we propose that apoB-lipoprotein biosynthesis occurs at different “nucleation sites” in the inner leaflet of the ER (Fig 8E–F). Formation of a simple nucleation site might involve interaction of a newly translated apoB peptide with the phospholipid monolayer (Fig 8E). Phospholipid transfer activity alone might bring more phospholipids to these sites helping in the phospholipidation of nascent apoB peptide and prevention of co-translational degradation of apoB as evidenced by the presence of apoB-associated phospholipids in microsomal membrane and lumen compared to the L-MTP−/− hepatocytes (Fig 8A–D). Desorption of apoB from nucleation sites that are deficient in triglycerides might lead to the synthesis of phospholipid-rich HDL size apoB48-containing lipoprotein particles (Fig 5, 6E–F). Second, these nucleation sites may acquire some triglycerides (Fig 8E) via lateral diffusion in the vicinity of triglyceride synthesizing enzyme in the membrane (Fig 8C). Desorption of lipoproteins from these nucleation sites might give rise to triglyceride-poor VLDL-size particles as seen in dMTP (Fig 5, 6C–D). Triglyceride transfer activity of hMTP might help in the transfer of triglycerides to various nucleation sites (Fig 8F) demonstrated by higher apoB-associated triglycerides in microsomal membrane. Subsequent lumenal triglyceride enrichment depicted by higher amounts of apoB-associated triglycerides in the lumen of hMTP than dMTP (Fig 8C) results in formation of increased number of triglyceride-rich VLDL (Fig 5) in vivo.
DISCUSSION
This study defines the role of polar and neutral lipid transfer activities of MTP in apoB-lipoprotein biogenesis and the unique effects they have on whole body lipid homeostasis. Our studies show that dMTP-expressing L-MTP−/− mice have low plasma triglycerides compared to hMTP. These differences persisted in mice expressing different levels of orthologs or fed different diets. Importantly, phospholipid transfer activity of dMTP is sufficient for in vivo biosynthesis of both apoB100- and apoB48-containing VLDL; although these are fewer, smaller and triglyceride-poor compared to hMTP. Hence, lower plasma triglycerides in dMTP-expressing mice are due to both reductions in size and decreases in number of apoB-lipoproteins. In contrast, dMTP mice had higher HDL-phospholipids than hMTP and mechanistic studies revealed that this was secondary to the synthesis of novel phospholipid-rich apoB48-containing HDL-sized lipoproteins. Absence of apoB100-HDL size particles could be due to an absolute need for neutral lipids for successful lipidation into secretion-competent particles.
Another surprising observation was that dMTP-expressing mice had hepatic lipids similar to WT and hMTP-expressing mice despite lower plasma lipids and the absence of neutral lipid transfer activity. Low hepatic phospholipids and cholesterol in dMTP could be attributed to the assembly of apoB48-HDL. We investigated several possible mechanisms to account for reduced hepatic triglycerides in dMTP-expressing L-MTP−/− mice. There were no significant differences in de novo lipogenesis rates, total ACC1, phosphorylated ACC1 and FAS proteins. However, we observed significant increases in fatty acid β-oxidation in dMTP, phosphorylated ACC2 and CPT1a levels. Thus, attenuation of triglyceride accumulation in dMTP mice might be in part due to increased energy expenditure via β-oxidation with no changes in lipogenesis. At this time, it is not known how dMTP affects this pathway and might be a good question to explore in the future. Second possibility for low hepatic triglycerides could be attributed to the presence of low levels of triglycerides in VLDL particles secreted by dMTP. Hence, transport of low levels of triglycerides with VLDL coupled with increased β-oxidation might be sufficient to lower hepatic triglycerides in dMTP mice.
This comparative study using dMTP as an invertebrate ortholog and hMTP as a vertebrate ortholog with distinct lipid transfer activities provides important insights into evolutionary gains made by MTP during a transition from invertebrates to vertebrates. Perhaps, acquisition of triglyceride transfer activity allowed mammals to efficiently transport neutral lipids by creating triglyceride-rich nucleation sites in the ER membrane, inhibiting proteasomal degradation of apoB and packaging more lipids in the core of these particles (Fig 7–8). This might have provided an advantage in efficient assimilation of energy when food was occasionally available. In modern era of food abundance, this activity might be superfluous. We speculate that small molecules that significantly inhibit triglyceride transfer activity while partially lowering or with no effect on phospholipid transfer activity of MTP might be useful in reducing plasma lipids while avoiding steatosis. Further, these compounds are not expected to affect CD1d biosynthesis, another molecule that is phospholipidated by MTP (21). Hence, selective rather than global inhibition of MTP lipid transfer activity might be a better therapeutic approach to combat hyperlipidemia.
Supplementary Material
Acknowledgements
We acknowledge the technical assistance of Wei Quan in negative staining and help of Dr. Jiang for introducing primary hepatocyte isolation. The aims, concepts, and experiments were conceived by MMH and conducted by IK. The paper was drafted by IK and edited by MMH. SZ and RSB generated, validated and provided the L-MTP−/− mice. IK and JI performed FAO and DNL studies. MW and DC provided protocol and training for large-scale virus amplification. GSS provided valuable suggestions and helped in critical reading of the manuscript.
Financial Support: This work was supported by NIH grants DK-46900 and HL95924 to MMH and AHA Predoctoral Fellowship 3230023 to IK.
Abbreviations used
- Ad
adenovirus
- apoAI
apolipoprotein AI
- apoB
apolipoprotein B
- Chol
cholesterol
- dMTP
Drosophila MTP
- EM
Electron microscopy
- ER
endoplasmic reticulum
- MTP
microsomal triglyceride transfer protein
- hMTP
Human MTP
- TG
triglycerides
- PFU
Plaque forming units
- PL
phospholipids
- HDL
high-density lipoprotein
- VLDL
very low density lipoprotein
Reference List
- 1.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004 Jan 27;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 2.Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, et al. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 1992 Nov 6;258(5084):999–1001. doi: 10.1126/science.1439810. [DOI] [PubMed] [Google Scholar]
- 3.Hussain MM, Rava P, Pan X, Dai K, Dougan SK, Iqbal J, et al. Microsomal triglyceride transfer protein in plasma and cellular lipid metabolism. Curr Opin Lipidol. 2008 Jun;19(3):277–284. doi: 10.1097/MOL.0b013e3282feea85. [DOI] [PubMed] [Google Scholar]
- 4.White DA, Bennett AJ, Billett MA, Salter AM. The assembly of triacylglycerol-rich lipoproteins: an essential role for the microsomal triacylglycerol transfer protein. Br J Nutr. 1998 Sep;80(3):219–229. [PubMed] [Google Scholar]
- 5.Sundaram M, Yao Z. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr Metab (Lond) 2010;7:35. doi: 10.1186/1743-7075-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res. 2003 Jan;44(1):22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 7.Rustaeus S, Lindberg K, Stillemark P, Claesson C, Asp L, Larsson T, et al. Assembly of very low density lipoprotein: a two-step process of apolipoprotein B core lipidation. J Nutr. 1999 Feb;129(2S Supp):463S–466S. doi: 10.1093/jn/129.2.463S. [DOI] [PubMed] [Google Scholar]
- 8.Gordon DA, Jamil H. Progress towards understanding the role of microsomal triglyceride transfer protein in apolipoprotein-B lipoprotein assembly. Biochim Biophys Acta. 2000 Jun 26;1486(1):72–83. doi: 10.1016/s1388-1981(00)00049-4. [DOI] [PubMed] [Google Scholar]
- 9.Alexander CA, Hamilton RL, Havel RJ. Subcellular localization of B apoprotein of plasma lipoproteins in rat liver. J Cell Biol. 1976 May;69(2):241–263. doi: 10.1083/jcb.69.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulinski A, Rustaeus S, Vance JE. Microsomal triacylglycerol transfer protein is required for lumenal accretion of triacylglycerol not associated with ApoB, as well as for ApoB lipidation. J Biol Chem. 2002 Aug 30;277(35):31516–31525. doi: 10.1074/jbc.M202015200. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton RL, Wong JS, Cham CM, Nielsen LB, Young SG. Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J Lipid Res. 1998 Aug;39(8):1543–1557. [PubMed] [Google Scholar]
- 12.Alexander CA, Hamilton RL, Havel RJ. Subcellular localization of B apoprotein of plasma lipoproteins in rat liver. J Cell Biol. 1976 May;69(2):241–263. doi: 10.1083/jcb.69.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Tran K, Yao Z. The activity of microsomal triglyceride transfer protein is essential for accumulation of triglyceride within microsomes in McA-RH7777 cells. A unified model for the assembly of very low density lipoproteins. J Biol Chem. 1999 Sep 24;274(39):27793–27800. doi: 10.1074/jbc.274.39.27793. [DOI] [PubMed] [Google Scholar]
- 14.Atzel A, Wetterau JR. Mechanism of microsomal triglyceride transfer protein catalyzed lipid transport. Biochemistry. 1993 Oct 5;32(39):10444–10450. doi: 10.1021/bi00090a021. [DOI] [PubMed] [Google Scholar]
- 15.Atzel A, Wetterau JR. Identification of two classes of lipid molecule binding sites on the microsomal triglyceride transfer protein. Biochemistry. 1994 Dec 27;33(51):15382–15388. doi: 10.1021/bi00255a019. [DOI] [PubMed] [Google Scholar]
- 16.Sellers JA, Hou L, Athar H, Hussain MM, Shelness GS. A Drosophila microsomal triglyceride transfer protein homolog promotes the assembly and secretion of human apolipoprotein B. Implications for human and insect transport and metabolism. J Biol Chem. 2003 May 30;278(22):20367–20373. doi: 10.1074/jbc.M300271200. [DOI] [PubMed] [Google Scholar]
- 17.Rava P, Ojakian GK, Shelness GS, Hussain MM. Phospholipid transfer activity of microsomal triacylglycerol transfer protein is sufficient for the assembly and secretion of apolipoprotein B lipoproteins. J Biol Chem. 2006 Apr 21;281(16):11019–11027. doi: 10.1074/jbc.M512823200. [DOI] [PubMed] [Google Scholar]
- 18.Rava P, Hussain MM. Acquisition of triacylglycerol transfer activity by microsomal triglyceride transfer protein during evolution. Biochemistry. 2007 Oct 30;46(43):12263–12274. doi: 10.1021/bi700762z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Read J, Anderson TA, Ritchie PJ, Vanloo B, Amey J, Levitt D, et al. A mechanism of membrane neutral lipid acquisition by the microsomal triglyceride transfer protein. J Biol Chem. 2000 Sep 29;275(39):30372–30377. doi: 10.1074/jbc.C000364200. [DOI] [PubMed] [Google Scholar]
- 20.Shoulders CC, Shelness GS. Current biology of MTP: implications for selective inhibition. Curr Top Med Chem. 2005;5(3):283–300. doi: 10.2174/1568026053544560. [DOI] [PubMed] [Google Scholar]
- 21.Dougan SK, Salas A, Rava P, Agyemang A, Kaser A, Morrison J, et al. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med. 2005 Aug 15;202(4):529–539. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 23.Rava P, Athar H, Johnson C, Hussain MM. Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J Lipid Res. 2005 Aug;46(8):1779–1785. doi: 10.1194/jlr.D400043-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Athar H, Iqbal J, Jiang XC, Hussain MM. A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J Lipid Res. 2004 Apr;45(4):764–772. doi: 10.1194/jlr.D300026-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Iqbal J, Dai K, Seimon T, Jungreis R, Oyadomari M, Kuriakose G, et al. IRE1beta inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 2008 May;7(5):445–455. doi: 10.1016/j.cmet.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harada N, Oda Z, Hara Y, Fujinami K, Okawa M, Ohbuchi K, et al. Hepatic de novo lipogenesis is present in liver-specific ACC1-deficient mice. Mol Cell Biol. 2007 Mar;27(5):1881–1888. doi: 10.1128/MCB.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linden D, William-Olsson L, Rhedin M, Asztely AK, Clapham JC, Schreyer S. Overexpression of mitochondrial GPAT in rat hepatocytes leads to decreased fatty acid oxidation and increased glycerolipid biosynthesis. J Lipid Res. 2004 Jul;45(7):1279–1288. doi: 10.1194/jlr.M400010-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Raabe M, Veniant MM, Sullivan MA, Zlot CH, Bjorkegren J, Nielsen LB, et al. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest. 1999 May;103(9):1287–1298. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang BH, Liao W, Li L, Nakamuta M, Mack D, Chan L. Liver-specific inactivation of the abetalipoproteinemia gene completely abrogates very low density lipoprotein/low density lipoprotein production in a viable conditional knockout mouse. J Biol Chem. 1999 Mar 5;274(10):6051–6055. doi: 10.1074/jbc.274.10.6051. [DOI] [PubMed] [Google Scholar]
- 30.Yu XX, Murray SF, Pandey SK, Booten SL, Bao D, Song XZ, et al. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology. 2005 Aug;42(2):362–371. doi: 10.1002/hep.20783. [DOI] [PubMed] [Google Scholar]
- 31.Millar JS, Cromley DA, McCoy MG, Rader DJ, Billheimer JT. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J Lipid Res. 2005 Sep;46(9):2023–2028. doi: 10.1194/jlr.D500019-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Hardie DG. Regulation of fatty acid synthesis via phosphorylation of acetyl-CoA carboxylase. Prog Lipid Res. 1989;28(2):117–146. doi: 10.1016/0163-7827(89)90010-6. [DOI] [PubMed] [Google Scholar]
- 33.bu-Elheiga L, Jayakumar A, Baldini A, Chirala SS, Wakil SJ. Human acetyl-CoA carboxylase: characterization, molecular cloning, and evidence for two isoforms. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):4011–4015. doi: 10.1073/pnas.92.9.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schreurs M, Kuipers F, van der Leij FR. Regulatory enzymes of mitochondrial beta-oxidation as targets for treatment of the metabolic syndrome. Obes Rev. 2010 May;11(5):380–388. doi: 10.1111/j.1467-789X.2009.00642.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.