Abstract
Substantial evidence supports dysregulated B cell immune responses in patients with primary biliary cirrhosis (PBC), including the presence of serum anti-mitochondrial antibodies (AMAs). However, recent reports from murine models of PBC suggest that B cells may also provide regulatory function and indeed the absence of B cells in such models leads to exacerbation of disease. The vast majority of patients with PBC have readily detectable antimitochondrial antibodies, but a minority (<5%), are AMA negative (AMA−) even with recombinant diagnostic technology. This issue prompted us to examine the nature of B cell infiltrates surrounding the portal areas in AMA positive (AMA+) and AMA− patients since they display indistinguishable clinical features. Of importance was the finding that the degree of bile duct damage around the portal areas was significantly milder in AMA+ PBC than those seen in AMA− PBC patients. The portal areas from AMA− patients had a significant increase of CD5+ cells infiltrating the ductal regions and the levels of B cell infiltrates were worse in the early phase of bile duct damage. The frequency of positive portal areas and the magnitude of CD5+ and CD20+ cellular infiltrates within areas of ductal invasion is associated with the first evidence of damage of biliary duct epithelia, but becomes reduced in the ductopenia stage, with the exception of CD5+ cells which remain sustained and predominate over CD20+ cells. In conclusion, our data suggest a putative role of B cell autoimmunity in regulating the portal destruction characteristic of PBC.
Keywords: B cells, CD20, CD5, Primary biliary cirrhosis
The pathognomic destruction of biliary epithelial cells (BEC) in primary biliary cirrhosis (PBC) is primarily attributed to autoreactive T cells (1–9). In contrast, the contribution of B cells to PBC immunopathology remains in need of further clarification (10), despite the nearly universal presence of anti-mitochondrial antibodies (AMA). The cellular infiltrates of PBC include foci of B cells within portal areas of the liver (11). Autoantibodies to the E2 subunit of the PDC enzymes inhibit the catalytic activity of PDC-E2 and such anti-PDC-E2 specific antibodies are reasoned to facilitate the transcytosis of IgA-AMA through BEC in the form of dimeric IgA-AMA complexes, leading to the induction of apoptosis of these cells (12–14). Sera from patients with PBC react with apoptotic blebs formed on the epithelial cell surface of human intrahepatic bile ducts not control cells (15), and induce an innate immune response (16). Moreover, autoantibodies to PDC-E2 markedly enhanced cross presentation as well as generation of PDC-E2-specific cytotoxic T cell responses in the presence of PDC-E2-pulsed antigen presenting cells (17). However, neither the presence nor the levels of AMA correlate with the recurrence of PBC in patients following orthotopic liver transplantation (18). Thus, although there is evidence for a profound loss of both B- and T- cell tolerance to the autoantigenic epitope(s) of PDC-E2, the degree to which B cells or autoantibodies are involved as effector elements in the pathogenesis of BEC damage in PBC remains unclear.
The autoimmune cholangitis that develops spontaneously in the TGF-β receptor II dominant negative (dnTGF-βRII) mouse is associated with a readily detectable inflammatory lymphocytic infiltrate in liver that closely simulates the chronic non-suppurative destructive cholangitis (CNSDC) of human PBC (19, 20). In this murine model of human PBC, therapeutic in vivo B cell depletion from 4 weeks of age using anti-CD20 monoclonal antibody (mAb) markedly attenuates the PBC-like liver disease but exacerbated the colitis which also spontaneously develops in these transgenic mice (21). In contrast, the same treatment in 20 week-old mice induced less effective changes on either cholangitis and/or colitis. Thus, anti-CD20 therapy may be potentially efficacious, and the results of these murine studies suggests that similar B cell depletion studies could have therapeutic benefit in PBC patients particularly if initiated during early stage PBC. Given the paucity of data on the role of B cells in PBC, and the potential for therapeutic relevance, we set out to compare the degree and frequency of bile duct damage in portal areas of liver tissues from AMA positive (AMA+) and AMA negative (AMA−) PBC patients. We report herein that portal areas from AMA− patients manifest more severe damage of bile ducts.
METHODS
PBC Patients and Liver biopsy samples
Patients who presented with the clinical manifestations of fatigue, pruritus and/or jaundice and elevation of serum ALP and/or gamma-GT were examined for AMA using both the anti-M2 ELISA kit (Euroimmun AG, Lǘbeck, Germany) and our well defined triple hybrid MIT3(22, 23); these detect AMA with 93.6% and 98.8% sensitivity, respectively (24). All patients were examined by liver biopsy and the criteria for the diagnosis of PBC was defined using recent AASLD guidelines (25).
Liver biopsy specimens were obtained from all patients including PBC (n=42) and chronic hepatitis C (CHC) controls (n=17), at the University of Jilin and Toyama University Hospital. The PBC cohort included 28 consecutive patients with AMA+ PBC and 14 patients with AMA− PBC (Table 1). The average age was 51.3±9.9 years and included 36 women and 6 men. Importantly, only 3 of these 42 patients were treated with UDCA. The PBC patients included in this study were histologically characterized as belonging to stage I (n=13), stage II (n=15), stage III (n=12) and stage IV (n=2). In contrast, of the 17 controls with CHC, 6 were considered stage I, 5 stage II, 6 stage III and none at stage IV. The mean age of the CHC patients was 55.4 ± 9.9 years which included 9 men and 8 women; none of the CHC patients were receiving steroids or immunosuppressive drugs at the time of this study. After approval from our institutional review board, all subjects provided written informed consent prior to enrollment in the study.
Table 1.
Clinical features of AMA+ and AMA− PBC patients and CHC patients
| PBC | CHC | |||
|---|---|---|---|---|
| AMA+ | AMA− | |||
| Number of Subjects | 28 | 14 | 17 | |
| Liver Histology: PBC Scheuer’s stage Hepatitis stage (fibrosis) and grade (activity) |
I | 8 | 5 | N/A |
| II | 13 | 2 | N/A | |
| III | 6 | 6 | N/A | |
| IV | 1 | 1 | N/A | |
| F1/(A1–2) | N/A | N/A | 6 | |
| F2/(A2) | N/A | N/A | 5 | |
| F3/(A2–3) | N/A | N/A | 6 | |
| F4 | N/A | N/A | 0 | |
| Age (range) |
52.1 ± 10.3 (35 – 79) |
49.7 ± 9.4 (27 – 63) |
55.4 ± 9.9 (26 – 67) |
|
| Gender (Male/Female) | 4/24 | 2/12 | 9/8 | |
Immunohistochemistry
Murine monoclonal antibodies against human CD20 (clone L26), and CD5 (clone 4C7) (Nichirei Bioscience, Tokyo, Japan), were used for immunohistochemical staining of liver (26). Tissue sections were cut at 4µm from paraffin-embedded tissue blocks and placed on slides. After de-paraffinization, sections were soaked in target retrieval buffered saline (TRS, pH 6.1, Dako Cytomation, Carpinteria, CA) in a plastic-made pressure cooker containing no metals and irradiated in a microwave oven for 10 minutes (maximum 500W). After irradiation, sections were rinsed under running water for 2 minutes, soaked in 3% H2O2 in methanol solution for 5 minutes, and then soaked in 5% BSA for 1 minute. Primary antibodies were diluted to a previously determined optimal concentration in PBS containing 5% BSA. The diluted antibodies were applied to the tissue sections in a moist chamber and irradiated intermittently for 10 minutes (250W, 4 seconds-on, 3 seconds-off). After 3 washes with Tris-buffered saline containing 1% Tween (TBS-T) for 1 minute, peroxidase-conjugated Envision kit for mouse (Envision System, Dako Cytomation) were applied on the appropriate specimens in the moist chamber. Irradiation was then performed intermittently for 10 minutes, as described above. After washing 5 times with TBS-T, the sections were immersed in DAB solution (Sigma-Aldrich) with H2O2, counterstained with hematoxylin (Dako Cytomation), and mounted under coverslips. For the detection of double stained cells with CD20 and CD5, CD5-stained sections were applied for light microscopic shots and thereafter soaked in 56°C water for 30 minutes to denature anti-CD5 antibodies, and then applied for CD20 staining. After 3 washes with Tris-buffered saline containing 1% Tween (TBS-T) for 1 minute, alkaline phosphatase-conjugated Envision kit for mouse and rabbit (Envision-AP, Envision System, Dako Cytomation), were applied to the appropriate specimens as described above.
Liver Tissue Evaluation
The degree of CD20+ cellular infiltration was scored in immunohistochemically stained liver sections with each portal area visualized using a 400× objective and scored as follows: 1= minimal, 2= mild, 3= moderate and 4= severe pathology. This scale contains 0–15, 15–50, 51–150, and >150 CD20+ cells respectively. Regarding patterns of CD20+ cellular infiltration, each portal area was classified into four categories: 1) Follicle-like aggregation, 2) Periductal aggregation, 3), Ductal invasion, and 4) Diffuse distribution. The frequency of single positive CD5 and CD20 cells and dual positive CD5/CD20 intraepithelial lymphocytes of bile duct was also evaluated and scored as 0= nil, 1= minimal, 2= mild, 3= moderate, and 4= severe pathology concomitantly with bile duct damage evaluation as nil, CNSDC, and ductopenia.
Statistical Analysis
Values were expressed graphically as the mean ± standard deviation (SD). Statistical differences between groups were determined using a two tailed Mann-Whitney unpaired test with 95% confidence interval (CI). The frequencies of each degree and pattern of CD20+ and CD5+ cellular infiltration and bile duct damage were evaluated using Fisher’s exact test. Values having p < 0.05 were considered statistically significant.
RESULTS
Liver tissues from AMA− PBC patients exhibit more severe bile duct damage
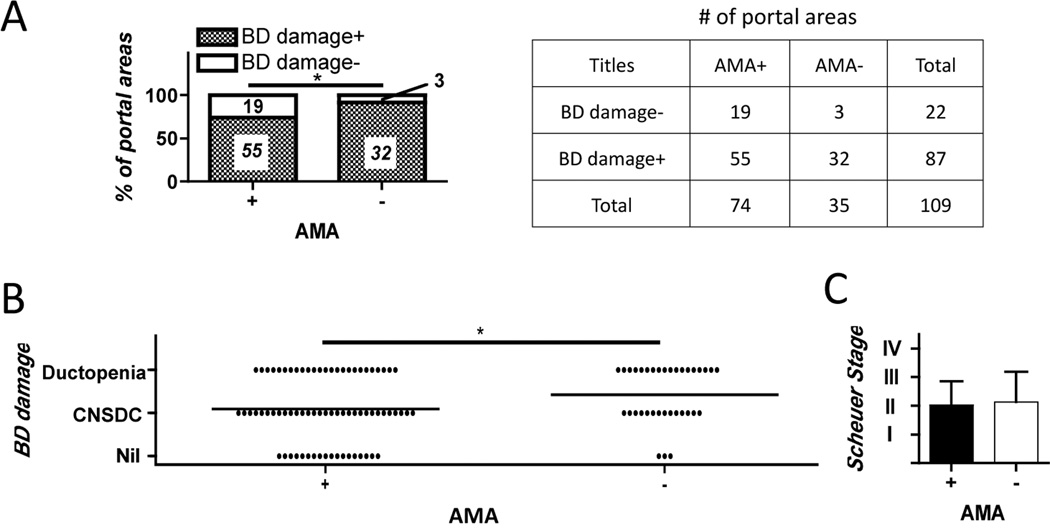
To address whether the presence of AMA was related to bile duct damage in PBC, we evaluated and compared the frequency and severity of bile duct destruction between AMA+ and AMA− patients. Liver specimens from 28 AMA+ and 14 AMA− PBC patients were stained with hematoxylin and eosin and duct damage quantified as nil, CNSDC, or ductopenia for each portal area; liver samples from patients with CHC were used as controls. Surprisingly, the portal areas of liver tissues from the AMA+ had significantly milder damage of bile ducts than similar tissues from the AMA− patients (Figure 1).
Figure 1.
A. Representative H&E profile of liver tissues from an AMA+ and AMA− PBC patient depicting the frequency and absolute number of bile duct (BD)-damaged portal areas. B. The degree of comparative bile duct damage was evaluated using three categories; nil, chronic non-suppurative destructive cholangitis (CNSDC), and ductopenia; it was more severe in portal areas of AMA− than AMA+ PBC livers. C. Scheuer stage of PBC livers did not significantly differ regardless of AMA positivity. (*: p < 0.05 in Fisher’s Exact Test and Mann-Whitney Test in A and B respectively)
CD20+ cellular infiltration is associated with bile duct damage in liver tissues from AMA− PBC patients
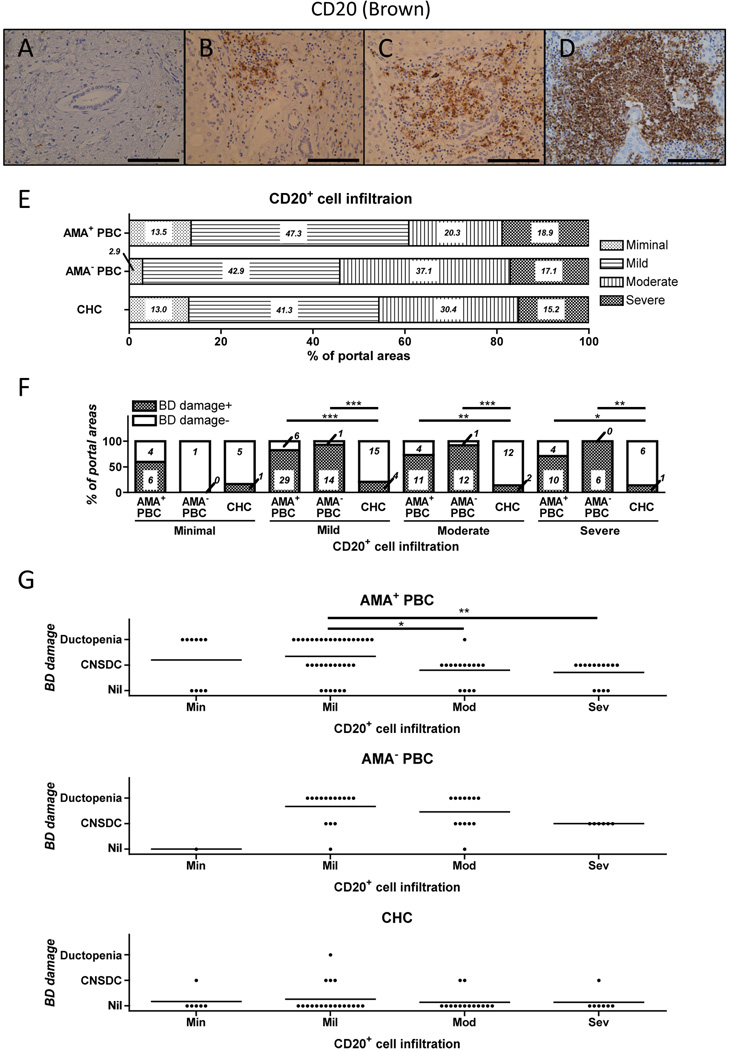
Detailed studies were performed using CD20 mAb and immunohistochemical staining techniques on liver specimens from 42 PBC patients and involved the examination of a total of 109 portal areas. In parallel, liver tissue specimens from 17 CHC patients which included the examination of a total of 46 portal spaces, were used as controls. We should note that incomplete portal areas with defects at the edges of the specimen were not included. We scored the degree of B cell infiltration around portal areas as minimal, mild, moderate, and severe, as outlined in the methods section (Figure 2A–D). No significant differences in the levels of B cell infiltration were observed (Figure 2E). However, when the degree of CD20 infiltration was evaluated in the context of the frequency and the degree of bile duct damage, we observed that the degree of CD20+ cellular infiltration was positively associated with the presence and the severity of bile duct damage in AMA− as compared with liver tissues from AMA+ PBC patients (Figure 2F, G). As expected, the frequency of bile duct damage within portal areas was significantly higher in liver tissues from PBC when compared to CHC patients (80.7 % vs 17.4 % respectively, p < 0.0001). Of note, gender difference did not influence the degree of ductal damage in liver tissues from either PBC or CHC patients (data not shown).
Figure 2.
A–D. Immunohistological evaluation of the degree of CD20+ cell infiltrates (stained brown) was conducted and profiles scored as minimal, mild, moderate and severe are shown (A to D). The frequency of each degree of CD20+ cellular infiltration was evaluated by the study of 74, 35 and 46 portal areas of liver tissues from AMA+ and AMA− PBC and CHC patients, respectively. E. CD20+ cellular infiltration was similar in the portal areas of PBC and CHC. F. Bile duct (BD) damage was more frequent in the mildly to severely CD20+ cellular infiltrated portal areas of liver tissues from PBC than CHC. Bile duct damage was more severe in liver tissues from PBC patients, but was not proportional to the degree of CD20+ cellular infiltration (G). (Scale bars indicate 100 µm in A–D. *: p < 0.05, **: p < 0.01 in Mann-Whitney Test.)
Distinct patterns of CD20+ cellular infiltrates in liver tissues from PBC and CHC patients
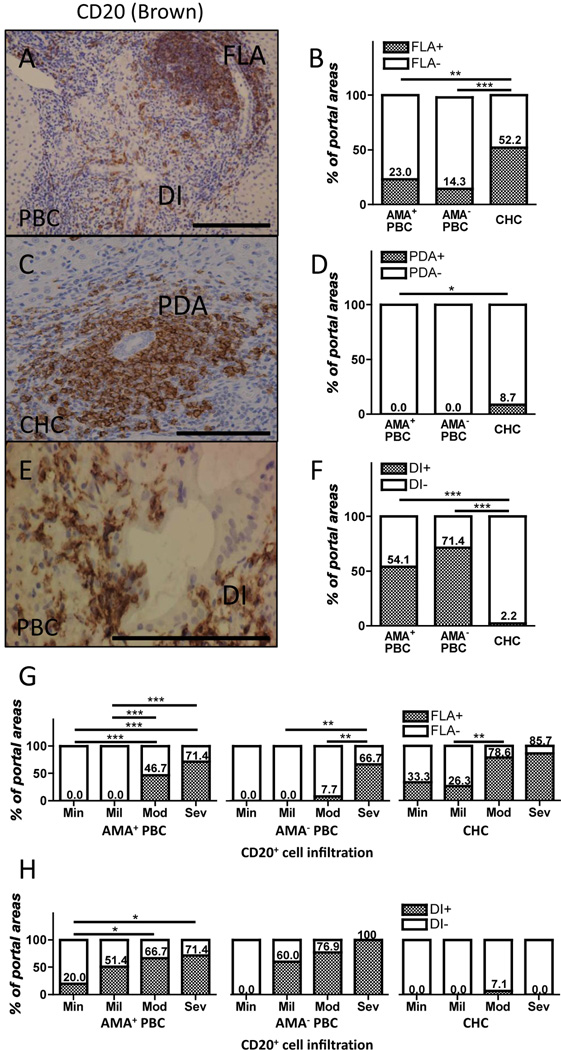
During the course of our studies we observed four distinct patterns of B cell infiltration within the portal area. These included : 1) follicle-like aggregation, 2) periductal aggregation, 3), ductal invasion, and 4) diffuse distribution (Figure 3). While no significant difference in the percentage of portal spaces with follicle-like aggregation was observed between AMA+ and AMA− PBC patients, follicle-like aggregation of CD20+ cells was more frequently observed in liver tissues from CHC patients (Figure 3A, B). Moderate to severe CD20+ cellular infiltrates more commonly formed follicle-like aggregation within portal areas (Figure 3G). While liver tissues from PBC patients did not demonstrate periductal aggregation of B cells, it was clearly observed in select portal areas of liver tissues from CHC patients (Figure 3C, D). Of note, ductal invasion of CD20+ cells was significantly and most commonly observed within the portal areas of liver tissues from PBC patients and its presence was associated with a higher degree of B cell infiltration in portal areas of AMA+ PBC livers, but rarely observed in liver tissues from CHC patients (Figure 3E, F, H). Both follicle-like aggregation and ductal invasion of CD20+ cells were occasionally observed in a moderately to severely CD20+ cellular infiltrated single portal area of PBC liver (Figure 3A). It is important to note that there was no sex difference in the degree of follicle-like aggregation, periductal aggregation, and ductal invasion in portal areas in liver tissues from both PBC and CHC patients.
Figure 3.
A, C, E. Representative profiles of typical follicle-like aggregation (FLA), periductal aggregation (PDA), and ductal invasion (DI) of CD20+ cells. B, D, F. Frequency of FLA, PDA, and DI of CD20+ B cells within portal areas of liver tissues of PBC (n= 109 portal areas) and CHC patients (46 portal areas) was significantly different. G. Moderate to severe CD20+ cellular infiltration was frequently accompanied with FLA in liver tissues from both AMA+ and AMA− PBC and CHC patients. H. DI by CD20+ cells was more common in portal areas with a higher degree of CD20+ cell infiltration in liver tissues from PBC patients regardless of AMA positivity, but not in CHC patients. (Scale bars indicate 100 µm in A, C, E. *: p < 0.05, **: p < 0.01, ***: p < 0.001 in Fisher’s Exact Test.)
Ductal invasion of CD5+ cells was more frequently observed in portal areas of liver tissues from AMA− PBC patients
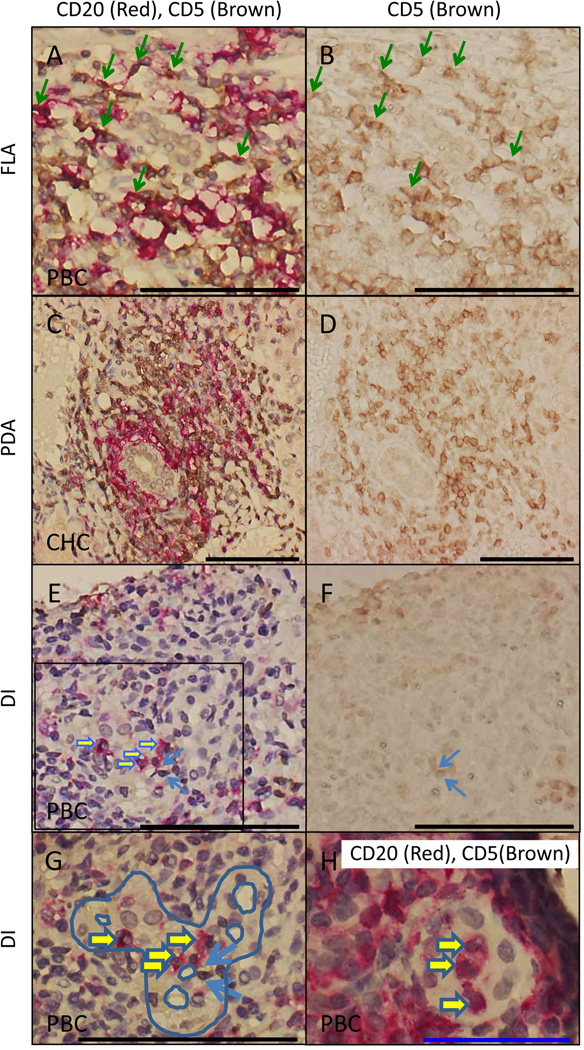
To analyze the characteristics and distribution of B cell phenotypes present within the infiltrate of portal areas, we performed CD20 and CD5 double staining by immunohistochemistry (Figure 4). The data obtained demonstrate that CD20 and CD5 double positive B cells are present in follicle-like aggregation (Figure 4A) but absent from periductal aggregation and ductal invasion (Figure 4C, E, G, H). Double positive cells were occasionally observed in follicle-like aggregation from livers of both PBC and CHC patients. Of note, CD20+ cells were most commonly distributed around bile ducts.
Figure 4.
Representative profiles of portal areas in liver tissues from PBC and CHC patients. Double staining for CD20 (red) and CD5 (brown) within follicle-like aggregates (A), periductal aggregates (C), and ductal invasive CD20+ and CD5+ cells (E). Single staining for the same aggregates as in A, C and E for CD5 (brown) expressing tissues (B, D and F). Damaged bile ducts were marginally highlighted with blue-color trace (G) in a magnified view of panel E. The ductal invasion of CD20 single positive cells was observed in a small bile duct (H). Double positive cells for CD20 and CD5, single positive cells either for CD20 or CD5 are highlighted with green, yellow, or blue arrows respectively. (Blue and black scale bars indicate 50 and 100 µm respectively in A–H.)
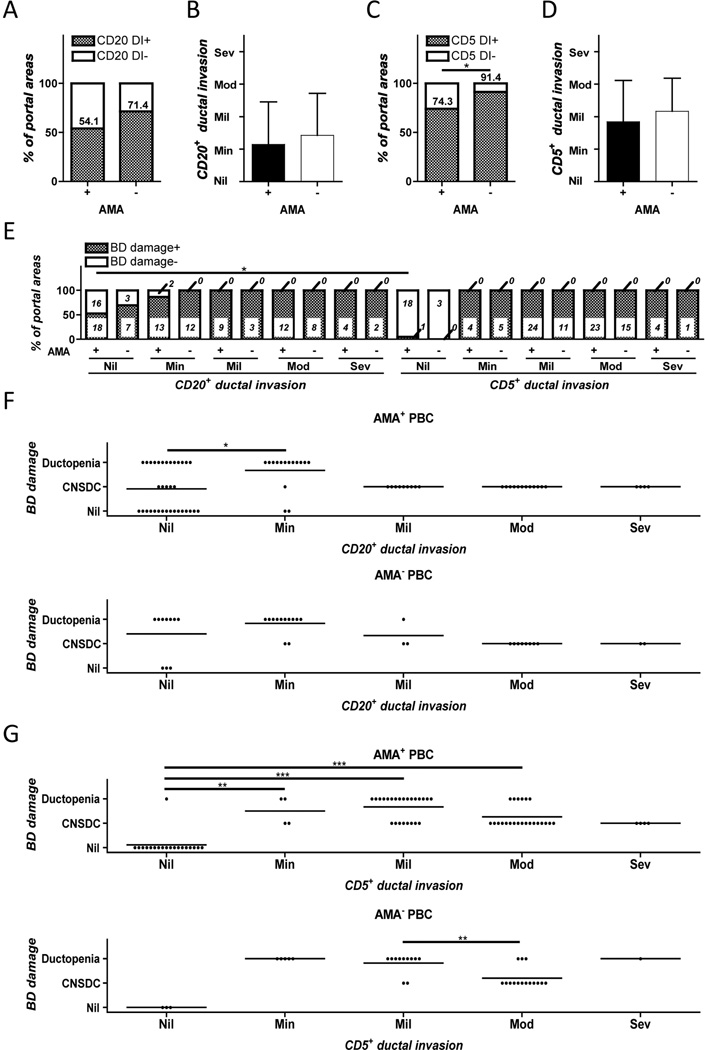
Since CD20 and CD5 double positive B cells were not observed within areas of ductal invasion, we evaluated CD20 or CD5 single positive cells respectively. While no difference was observed in the frequency of CD20+ infiltration within ductal invasion in liver tissues from AMA+ and AMA− patients (Figure 5A, B, D), the degree of CD5+ cellular infiltration was significantly increased in liver tissues from the AMA− compared to AMA+ PBC patients (Figure 5C). Bile duct damaged portal areas included mild to severe ductal invasion of both CD20+ and CD5+ cells. CD5+ cellular ductal invasion appears essential for bile duct damage.
Figure 5.
Frequency and severity of CD20+ and CD5+ cellular ductal invasion in AMA+ and AMA− PBC patients (A–D). Ductal invasion of CD5 single positive cells was found more in the portal areas of liver tissues in AMA− compared to those of AMA+ patients (C). There was no difference in the degree of CD20+ and CD5+ cellular ductal invasion between AMA− and AMA+ livers (B and D). Although both CD5+ and CD20+ cellular ductal invasion was observed in bile duct damaged portal areas, the frequency was significantly less in the absence of CD5+ than CD20+ cellular ductal invasion in AMA+ patients (E). Moderate to severe ductal invasion of CD20+ and CD5+ cells were primarily accompanied by bile duct (BD) destruction in the liver of PBC patients whereas the degree of CD20+ and CD5+cellular ductal invasion shifted to mild at the ductopenic stage but was not proportional to the bile duct damage (F and G). (Error bars stand for standard deviation in B and D. *: p < 0.05 in Fisher’s Exact Test in C. *: p < 0.05, **: p < 0.01, ***: p < 0.001 in Mann-Whitney Test in E and F.)
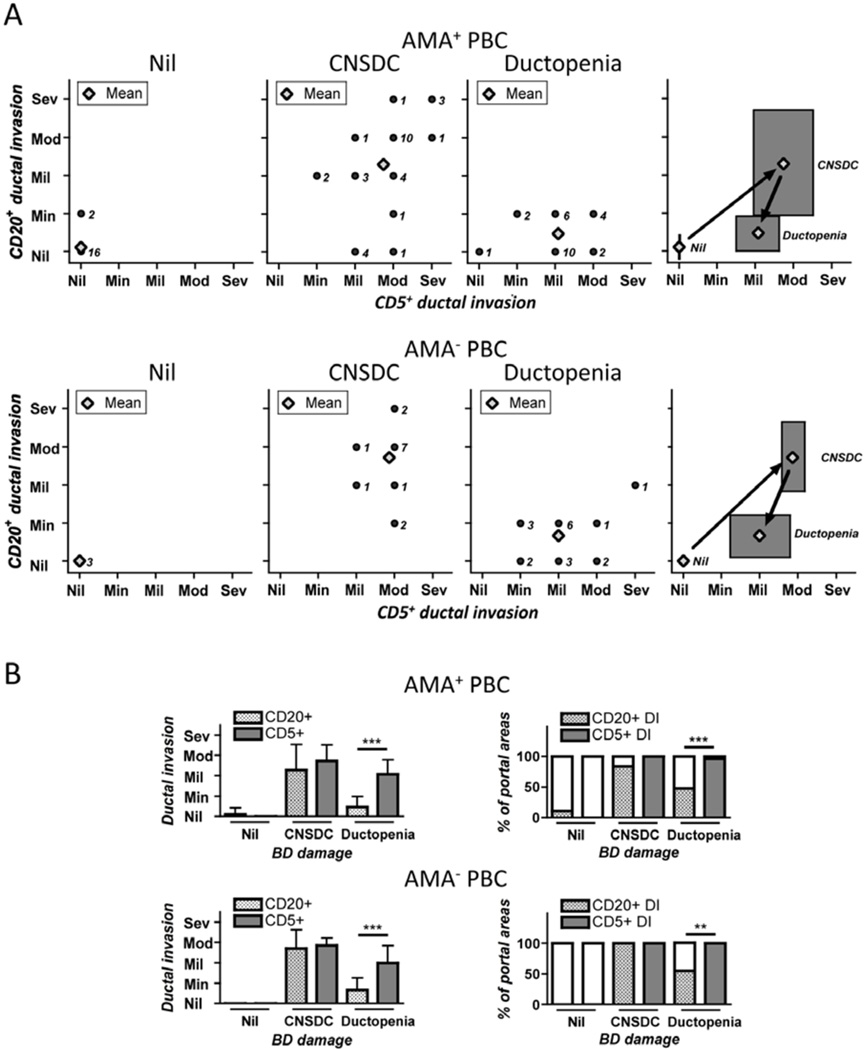
The degree of CD5+ and CD20+ cellular ductal invasion was also evaluated for each portal area (Figure 6A). While tissues scored as those with nil or no bile duct damage demonstrated no CD5+ cells infiltration accompanied by minimal if any CD20+ cellular ductal invasion, tissues scored as CNSDC primarily had mild to severe ductal invasion with either CD5+ or CD20+ cells, the degree of which diminished in the ductopenic stage. Of note, whereas CD20+ cellular ductal invasion was not observed in 5 of the 31 portal areas with CNSDC in liver tissues from AMA+ PBC, the CD5+ cellular ductal invasion was present in all portal areas with CNSDC regardless of AMA positivity. We interpret these findings as evidence for differences that occur secondary to disease progression in PBC patients. Thus the frequency of positive portal areas and the degree of CD5+ and CD20+ cells in ductal invasion significantly increases at first, to cause damage to bile duct epithelia, and later reduces at the later stage of bile duct damage (ductopenia) with the exception of CD5+ cells, which is sustained and significantly predominates over CD20+ cells (Figure 6B).
Figure 6.
Ductal invasion of CD5 but not CD20 single positive cells are the major contributors for bile duct damage in liver tissues from PBC patients. A. For each level of bile duct damage (i.e. nil, CNSDC, or ductopenia), dots corresponding to the degree of ductal invasion by both CD20+ and CD5+ cells for each portal area were plotted using a 2-D format. The number next to each dot represents the numbers of evaluated portal areas with the corresponding degree of ductal invasion by CD20+ and CD5+ cells. The average degree of ductal invasion by CD20+ and CD5+ cells was calculated as mean of the field with standard deviations. B. The degree and frequency of ductal invasion by CD20 and CD5 single positive cells within portal areas. There were significantly higher levels of CD5+ than CD20+ cells within the ductopenic portal areas. (***: p < 0.001 in Mann-Whitney Test for degree of ductal invasion in B. **: p < 0.01, ***: p < 0.001 in Fisher’s Exact Test for frequency of portal areas in B.)
DISCUSSION
A multistep etiology for PBC is likely and we believe that different stages of biliary injury are associated with differential roles for B and T cells. Unexpectedly, we found that AMA+ PBC livers had significantly milder bile duct damage than those from AMA− PBC; this point takes on significance in light of current data suggesting that AMAs contribute as effectors to the pathogenesis of PBC (11–17). Further, B cell infiltration could be classified into follicle-like aggregation, periductal aggregation and ductal invasion. Ductal invasion was the most frequently observed type of B cell infiltration in the liver tissues from PBC whereas follicle-like aggregation was the most common pattern in livers tissues from CHC. However, CD20/CD5 double positive cells were not observed in the ductal invasion of liver tissues in PBC. Studies on the evaluation of CD20 and CD5 single positive cells revealed attenuation of B cells with sustainment of T cells at later stage of bile duct damage, in keeping with the concept of T cell driven injury during chronic and late stage of the disease. Whereas both CD20+CD5+ and CD20+CD5− B cells infiltrated portal tracts, the distribution of these two cell populations differed. The purpose of our CD5 staining was to determine if the frequency of CD20+CD5+ B cells was different in various stages of bile duct injury; this was not observed as discussed herein. Thus, whereas ductal invasion comprised of CD20 and CD5 single positive cells was frequently observed in liver tissues from PBC, CD20/CD5 double positive cells were frequently observed in follicle-like aggregation of liver tissues from CHC and PBC. Of note, CD5 single positive ductal invasion was more frequently observed in portal areas of liver tissues from AMA− than AMA+ PBC patients (Figure 5C).
CD5 is primarily a pan-T cell marker. However, a unique B cell subset also expresses the CD5 molecule (27, 28). In mice, CD5+CD11b+, CD5−CD11b+, and CD11b− B cells are coined as B-1a, B-1b, and B-2 B cells respectively. CD11b+ B-1 and splenic CD5+CD1dhi B cells demonstrate regulatory functions for inflammatory responses in mouse models of autoimmune disease including inflammatory bowel disease and experimental autoimmune encephalomyelitis (29–32). B-1 cell subpopulations can be differentiated into B-1a and B-1b cells by surface and intracellular expression of CD5, which allow B cells to produce the anti-inflammatory cytokine interleukin 10 (IL-10) (33, 34).
Both PBC and CHC are associated with B cell abnormalities. While PBC is characterized by the presence of highly specific AMAs along with an elevated IgM, CHC is characterized by a wide spectrum of B cell abnormalities including mixed cryoglobulinemia and production of autoantibodies (35, 36). However, peripheral CD20+CD5+ B cells are significantly increased in patients with CHC when compared to those of healthy subjects, regardless of the presence or absence of mixed cryoglobulinemia. In contrast, peripheral CD20+ B cell frequency is comparable in both groups, and there was no correlation between the peripheral frequency of B cells and disease activity in liver tissues from CHC patients (37, 38). In addition, CD5+ B cells expand in liver tissues from CHC patients, and a higher frequency negatively correlate with liver inflammation (39, 40). Peripheral CD5+ B cells are unlikely to undergo apoptosis when compared to CD5− B cells, and the occurrence of lymphoid follicles in liver tissues from CHC patients correlate with inflammatory activity (41–43).
Our data demonstrated follicle-like aggregation of CD20+ cells frequently in liver tissues from CHC patients when compared to similar tissues from PBC patients regardless of AMA positivity, and these B cells co-expressed CD5, raising the possibility that CD20 and CD5 double positive B cells negatively regulate inflammatory responses partly by IL-10 production. In contrast, the liver tissues from PBC patients showed a significantly reduced presence of follicle-like aggregation when compared to ductal invasion by B cells. There is also no reported increase in the peripheral frequency of CD5+ B cells in patients with PBC (44).
It has been suggested that CD4+ and CD8+ T cells are major contributors to bile duct damage in liver tissues from PBC patients (2, 45). In this study, the CD5 single positive presumably represent classic T cells. Since autoreactive T cells are known to be negatively regulated in both a cell-contact dependent and an independent manner by the ligation of programmed death-1 ligands and the synthesis of prostaglandin-E2 (expressed in and secreted from biliary epithelial cells (3)), ductal invasion by T cells may be partly suppressed by biliary epithelia. Conversely, ductal invasion by T cells may be promoted by the CD5− B cell population in liver tissues of PBC patients.
In conclusion, we demonstrate that bile duct damage was attenuated in liver tissues from AMA+ PBC patients as compared with AMA− PBC patients. This study does have recognized limitations. Firstly, we have assumed that the natural history of PBC progresses through stages, but we should emphasize that the data herein reflects a cross-sectional study, rather than a prospective study and thus may not be indicative of the true natural history of disease. The ductal invasion in liver tissues from PBC patients was comprised of both CD20 and CD5 single positive cells and the degree of this invasion in ductopenic portal areas was attenuated and sustained with each cell type, respectively. In addition, CD20 and CD5 double positive cells were observed in follicle like aggregation, but this pattern of B cell distribution was less frequently observed in liver tissues from PBC patients. Taken together, our data suggest that T cells are essential for bile duct damage, but B cell immunity contributes to liver immunopathology primarily during the early phase of disease in PBC patients.
Acknowledgments
Financial Support: This study was supported by grants from National Institutes of Health grant DK39588 and from the Eleventh Five-year Plan for AIDS and Viral Hepatitis (No. 2008ZX10002-004), Ministry of Health (No. 20073531), National Natural Science Foundation of China (No. 30771912 and 30972610), Grant-in-Aid for Scientific Research (C) and Grant-in-Aid for Research Activity start-up of Japan Society of the Promotion of Science KAKENHI (No. 21590433 and 22890024) and Jilin Province Science and Technology Agency (No. 200705128).
Abbreviations
- AMA
anti-mitochondrial autoantibodies
- Bregs
B regulatory cells
- dnTGF-βRII mice
TGF-β receptor II dominant negative mice
- IL-10
interleukin 10
- N/A
not available
- TGF-β
transforming growth factor-β
Contributor Information
Qinglong Jin, Email: jinql@jlu.edu.cn.
Yuki Moritoki, Email: ymoritoki@hos.akita-u.ac.jp.
Ana Lleo, Email: ana.lleo@humanitas.it.
Koichi Tsuneyama, Email: ktsune@med.u-toyama.ac.jp.
Pietro Invernizzi, Email: pietro.invernizzi@humanitas.it.
Hitoshi Moritoki, Email: moritoki@coral.plala.or.jp.
Kentaro Kikuchi, Email: kentaro@med.teikyo-u.ac.jp.
Zhe-Xiong Lian, Email: zxlian1@ustc.edu.cn.
Gideon M. Hirschfield, Email: gideonhirschfield@gmail.com.
Aftab A. Ansari, Email: pathaaa@emory.edu.
Ross L. Coppel, Email: ross.coppel@monash.edu.
M. Eric Gershwin, Email: megershwin@ucdavis.edu.
Junqi Niu, Email: niq@jlu.edu.cn.
References
- 1.Gershwin ME, Ansari AA, Mackay IR, Nakanuma Y, Nishio A, Rowley MJ, Coppel RL. Primary biliary cirrhosis: an orchestrated immune response against epithelial cells. Immunol Rev. 2000;174:210–225. doi: 10.1034/j.1600-0528.2002.017402.x. [DOI] [PubMed] [Google Scholar]
- 2.Kamihira T, Shimoda S, Harada K, Kawano A, Handa M, Baba E, Tsuneyama K, et al. Distinct costimulation dependent and independent autoreactive T-cell clones in primary biliary cirrhosis. Gastroenterology. 2003;125:1379–1387. doi: 10.1016/j.gastro.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Kamihira T, Shimoda S, Nakamura M, Yokoyama T, Takii Y, Kawano A, Handa M, et al. Biliary epithelial cells regulate autoreactive T cells: implications for biliary-specific diseases. Hepatology. 2005;41:151–159. doi: 10.1002/hep.20494. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan MM, Gershwin ME. Medical progress: Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 5.Shigematsu H, Shimoda S, Nakamura M, Matsushita S, Nishimura Y, Sakamoto N, Ichiki Y, et al. Fine specificity of T cells reactive to human PDC-E2 163–176 peptide, the immunodominant autoantigen in primary biliary cirrhosis: implications for molecular mimicry and cross-recognition among mitochondrial autoantigens. Hepatology. 2000;32:901–909. doi: 10.1053/jhep.2000.18714. [DOI] [PubMed] [Google Scholar]
- 6.Shimoda S, Ishikawa F, Kamihira T, Komori A, Niiro H, Baba E, Harada K, et al. Autoreactive T-cell responses in primary biliary cirrhosis are proinflammatory whereas those of controls are regulatory. Gastroenterology. 2006;131:606–618. doi: 10.1053/j.gastro.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 7.Shimoda S, Nakamura M, Ishibashi H, Kawano A, Kamihira T, Sakamoto N, Matsushita S, et al. Molecular mimicry of mitochondrial and nuclear autoantigens in primary biliary cirrhosis. Gastroenterology. 2003;124:1915–1925. doi: 10.1016/s0016-5085(03)00387-1. [DOI] [PubMed] [Google Scholar]
- 8.Shimoda S, Nakamura M, Shigematsu H, Tanimoto H, Gushima T, Gershwin ME, Ishibashi H. Mimicry peptides of human PDC-E2 163–176 peptide, the immunodominant T-cell epitope of primary biliary cirrhosis. Hepatology. 2000;31:1212–1216. doi: 10.1053/jhep.2000.8090. [DOI] [PubMed] [Google Scholar]
- 9.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology. 2008;47:737–745. doi: 10.1002/hep.22042. [DOI] [PubMed] [Google Scholar]
- 10.He X-S, Ansari AA, Ridgway WM, Coppel RL, Gershwin ME. New insights to the immunopathology and autoimmune responses in primary biliary cirrhosis. Cellular Immunology. 2006;239:1–13. doi: 10.1016/j.cellimm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Nakanuma Y. Distribution of B lymphocytes in nonsuppurative cholangitis in primary biliary cirrhosis. Hepatology. 1993;18:570–575. [PubMed] [Google Scholar]
- 12.Fregeau DR, Prindiville T, Coppel RL, Kaplan M, Dickson ER, Gershwin ME. Inhibition of alpha-ketoglutarate dehydrogenase activity by a distinct population of autoantibodies recognizing dihydrolipoamide succinyltransferase in primary biliary cirrhosis. Hepatology. 1990;11:975–981. doi: 10.1002/hep.1840110611. [DOI] [PubMed] [Google Scholar]
- 13.Fregeau DR, Roche TE, Davis PA, Coppel R, Gershwin ME. Primary biliary cirrhosis. Inhibition of pyruvate dehydrogenase complex activity by autoantibodies specific for E1 alpha, a non-lipoic acid containing mitochondrial enzyme. J Immunol. 1990;144:1671–1676. [PubMed] [Google Scholar]
- 14.Matsumura S, Van De Water J, Leung P, Odin JA, Yamamoto K, Gores GJ, Mostov K, et al. Caspase induction by IgA antimitochondrial antibody: IgA-mediated biliary injury in primary biliary cirrhosis. Hepatology. 2004;39:1415–1422. doi: 10.1002/hep.20175. [DOI] [PubMed] [Google Scholar]
- 15.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, et al. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009 doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, Ansari AA, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987–998. doi: 10.1002/hep.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kita H, Lian ZX, Van de Water J, He XS, Matsumura S, Kaplan M, Luketic V, et al. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med. 2002;195:113–123. doi: 10.1084/jem.20010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neuberger J. Liver transplantation for primary biliary cirrhosis. Autoimmunity Reviews. 2003;2:1–7. doi: 10.1016/s1568-9972(02)00103-9. [DOI] [PubMed] [Google Scholar]
- 19.Chuang YH, Lian ZX, Yang GX, Shu SA, Moritoki Y, Ridgway WM, Ansari AA, et al. Natural killer T cells exacerbate liver injury in a transforming growth factor beta receptor II dominant-negative mouse model of primary biliary cirrhosis. Hepatology. 2008;47:571–580. doi: 10.1002/hep.22052. [DOI] [PubMed] [Google Scholar]
- 20.Oertelt S, Lian Z-X, Cheng C-M, Chuang Y-H, Padgett KA, He X-S, Ridgway WM, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. Journal of Immunology. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 21.Moritoki Y, Lian ZX, Lindor K, Tuscano J, Tsuneyama K, Zhang W, Ueno Y, et al. B-cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice. Hepatology. 2009;50:1893–1903. doi: 10.1002/hep.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moteki S, Leung PS, Coppel RL, Dickson ER, Kaplan MM, Munoz S, Gershwin ME. Use of a designer triple expression hybrid clone for three different lipoyl domain for the detection of antimitochondrial autoantibodies. Hepatology. 1996;24:97–103. doi: 10.1002/hep.510240117. [DOI] [PubMed] [Google Scholar]
- 23.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–3531. [PubMed] [Google Scholar]
- 24.Dahnrich C, Pares A, Caballeria L, Rosemann A, Schlumberger W, Probst C, Mytilinaiou M, et al. New ELISA for detecting primary biliary cirrhosis-specific antimitochondrial antibodies. Clin Chem. 2009;55:978–985. doi: 10.1373/clinchem.2008.118299. [DOI] [PubMed] [Google Scholar]
- 25.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 26.Kumada T, Tsuneyama K, Hatta H, Ishizawa S, Takano Y. Improved 1-h rapid immunostaining method using intermittent microwave irradiation: practicability based on 5 years application in Toyama Medical and Pharmaceutical University Hospital. Mod Pathol. 2004;17:1141–1149. doi: 10.1038/modpathol.3800165. [DOI] [PubMed] [Google Scholar]
- 27.Dalloul A. CD5: a safeguard against autoimmunity and a shield for cancer cells. Autoimmun Rev. 2009;8:349–353. doi: 10.1016/j.autrev.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006;18:547–555. doi: 10.1016/j.coi.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 30.Mizoguchi A, Bhan AK. A case for regulatory B cells. Journal of Immunology. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 31.Shimomura Y, Mizoguchi E, Sugimoto K, Kibe R, Benno Y, Mizoguchi A, Bhan AK. Regulatory role of B-1 B cells in chronic colitis. Int Immunol. 2008;20:729–737. doi: 10.1093/intimm/dxn031. [DOI] [PubMed] [Google Scholar]
- 32.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renaudineau Y, Hillion S, Saraux A, Mageed RA, Youinou P. An alternative exon 1 of the CD5 gene regulates CD5 expression in human B lymphocytes. Blood. 2005;106:2781–2789. doi: 10.1182/blood-2005-02-0597. [DOI] [PubMed] [Google Scholar]
- 34.Garaud S, Le Dantec C, de Mendoza AR, Mageed RA, Youinou P, Renaudineau Y. IL-10 production by B cells expressing CD5 with the alternative exon 1B. Ann N Y Acad Sci. 2009;1173:280–285. doi: 10.1111/j.1749-6632.2009.04616.x. [DOI] [PubMed] [Google Scholar]
- 35.Gregorio GV, Pensati P, Iorio R, Vegnente A, Mieli-Vergani G, Vergani D. Autoantibody prevalence in children with liver disease due to chronic hepatitis C virus (HCV) infection. Clin Exp Immunol. 1998;112:471–476. doi: 10.1046/j.1365-2249.1998.00574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lenzi M, Bellentani S, Saccoccio G, Muratori P, Masutti F, Muratori L, Cassani F, et al. Prevalence of non-organ-specific autoantibodies and chronic liver disease in the general population: a nested case-control study of the Dionysos cohort. Gut. 1999;45:435–441. doi: 10.1136/gut.45.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sansonno D, Lauletta G, Montrone M, Grandaliano G, Schena FP, Dammacco F. Hepatitis C virus RNA and core protein in kidney glomerular and tubular structures isolated with laser capture microdissection. Clin Exp Immunol. 2005;140:498–506. doi: 10.1111/j.1365-2249.2005.02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panasiuk A, Prokopowicz D, Zak J, Wysocka J. Peripheral blood T, B, and NK cells in relation to histological hepatitis activity and fibrosis stage in chronic hepatitis C. Hepatogastroenterology. 2003;50:178–182. [PubMed] [Google Scholar]
- 39.Curry MP, Golden-Mason L, Doherty DG, Deignan T, Norris S, Duffy M, Nolan N, et al. Expansion of innate CD5pos B cells expressing high levels of CD81 in hepatitis C virus infected liver. J Hepatol. 2003;38:642–650. doi: 10.1016/s0168-8278(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 40.Curry MP, Golden-Mason L, Nolan N, Parfrey NA, Hegarty JE, O'Farrelly C. Expansion of peripheral blood CD5+ B cells is associated with mild disease in chronic hepatitis C virus infection. J Hepatol. 2000;32:121–125. doi: 10.1016/s0168-8278(00)80198-1. [DOI] [PubMed] [Google Scholar]
- 41.Mizuochi T, Ito M, Takai K, Yamaguchi K. Differential susceptibility of peripheral blood CD5+ and CD5− B cells to apoptosis in chronic hepatitis C patients. Biochem Biophys Res Commun. 2009;389:512–515. doi: 10.1016/j.bbrc.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 42.Toubi E, Kessel A, Peri R, Shmuel Z, Bamberger E, Sabo E, Zuckerman E. Enhanced apoptosis of peripheral CD5-negative B lymphocytes from chronically hepatitis C virus-infected patients: reversal after antiviral treatment. J Virol. 2004;78:11379–11384. doi: 10.1128/JVI.78.20.11379-11384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walewska-Zielecka B, Madalinski K, Jablonska J, Godzik P, Cielecka-Kuszyk J, Litwinska B. Composition of inflammatory infiltrate and its correlation with HBV/HCV antigen expression. World J Gastroenterol. 2008;14:4040–4046. doi: 10.3748/wjg.14.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamada H, Shimizu H, Taniguchi O, Okumura K. Leu-1(CD5) B cell subpopulation in patients with various liver diseases--special reference to hepatitis B virus carrier and to changes caused by prednisolone therapy. Int Arch Allergy Appl Immunol. 1988;87:409–416. doi: 10.1159/000234711. [DOI] [PubMed] [Google Scholar]
- 45.Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, Coppel RL, et al. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109:1231–1240. doi: 10.1172/JCI14698. [DOI] [PMC free article] [PubMed] [Google Scholar]