Abstract
Recently, we proposed an oxygen radical absorbance capacity method that directly quantifies the antioxidant’s scavenging capacity against free radicals and evaluated the radical scavenging abilities for water soluble antioxidant compounds. In this study, we determined the radical scavenging abilities of lipophilic antioxidants which were solubilized by cyclodextrin in water. Commonly employed fluorescence-based method measures the antioxidant’s protection capability for the fluorescent probe, while we directly quantify free-radical level using electron paramagnetic resonance spin trapping technique. In addition, the spin trapping-based method adopted controlled UV-photolysis of azo-initiator for free radical generation, but in fluorescence-based method, thermal decomposition of azo-initiator was utilized. We determined the radical scavenging abilities of seven well-known lipophilic antioxidants (five flavonoids, resveratrol and astaxanthin), using methylated β-cyclodextrin as a solubilizer. The results indicated that the agreement between spin trapping-based and fluorescence-based values was only fair partly because of a large variation in the previous fluorescence-based data. Typical radical scavenging abilities in trolox equivalent unit are: catechin 0.96; epicatechin 0.94; epigallocatechin gallate 1.3; kaempferol 0.37; myricetin 3.2; resveratrol 0.64; and astaxanthin 0.28, indicating that myricetin possesses the highest antioxidant capacity among the compounds tested. We sorted out the possible causes of the deviation between the two methods.
Keywords: oxygen radical absorbance capacity, spin trapping, lipophilic antioxidant, cyclodextrin, inclusion complex
Introduction
The health benefit of antioxidant is believed to be based on its scavenging capability against free radical species.(1–3) In recent years, antioxidant-capacity evaluation for pure antioxidant compounds or plant/food extracts using oxygen radical absorbance capacity (ORAC) methods has attracted considerable attention.(4–6) ORAC values for nearly 300 selected foods are listed in the home page of the US Department of Agriculture (http://www.ars.usda.gov/). These values were obtained using the fluorescence-based ORAC method (hereafter abbreviated as ORAC-FL) that was originated from Glazer’s laboratory.(7) Its principle was to measure the antioxidant-mediated protection of the fluorescent protein β-phycoerythrin from free radical damage. Later, confirming studies named this method as ORAC(2,8,9); however, ’oxygen radical’ that was mentioned in ORAC has never been identified. More recently, the low molecular weight fluorescent-probe fluorescein was adopted instead of the protein probe.(10–12)
In ORAC-FL, free radicals are produced with the thermal decomposition of a water-soluble azo-radical initiator, 2,2-azobis(2-amidinopropane) dihydrochloride (AAPH). Antioxidants added to this system protect the fluorescence probe from AAPH-derived free radicals, and the extent of protection is quantified. The time course of the fluorescence-loss during free radical production is recorded and converted into ORAC values with the computer-aided analysis. We proposed a new method of ORAC evaluation, ORAC-EPR based on the direct quantification of free radical level with the electron paramagnetic resonance (EPR or ESR) spin trapping technique.(13,14) In contrast to ORAC-FL where free radicals are thermally produced, ORAC-EPR employs a short UV-photolysis of AAPH to generate a constant amount of free radicals. In the presence of antioxidant, the free radical level is decreased, from which ORAC-EPR values are calculated using a simple formula.
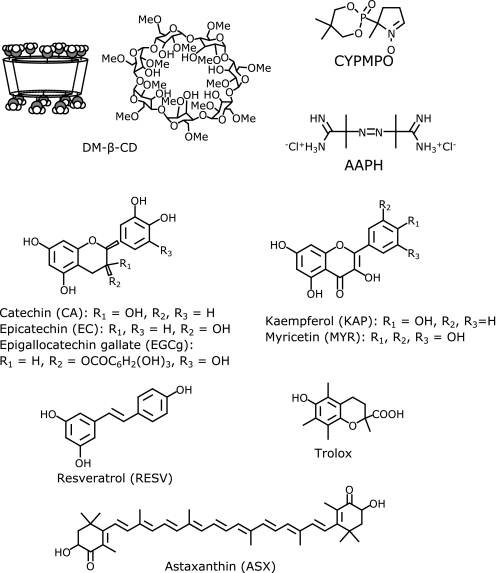
Both ORAC-FL and ORAC-EPR are water-based methods; however, a majority of antioxidants are classified as lipophilic compounds such as flavonoids. The poor solubilization or dispersion of antioxidants could lead to poor assay validity.(15,16) In ORAC-FL, Huang et al.(17) proposed the use of modified β-cyclodextrin to solubilize lipophilic compounds. β-Cyclodextrin (β-CD) is a cyclic hexamer of glucose units having a molecular cavity (see structure in Fig. 1), wherein a lipophilic molecule is encapsulated or included to form water-soluble inclusion complex. Such peculiar properties of β-CD have been found to be useful in industrial and domestic applications, such as drug-solubilizers and deodorizers.(18–20)
Fig. 1.
Structures of 2,6-di-O-methylated β-CD (DM-β-CD), CYPMPO, AAPH, and antioxidants.
Hydroxypropyl-β-cyclodextrin (HP-β-CD) has been customarily used in the ORAC-FL measurements. However, it has been suggested that HP-β-CD is not a suitable solubilizer because the reactive site of the antioxidant may be included and protected from free radical attack.(21) Recently, Folch-Cano et al.(22) made it clear the effects of cyclodextrin-inclusion on the ORAC assays based on the stoichiometry of inclusion of some catechin. In this study, 2,6-di-O-methylated β-CD (DM-β-CD, Fig. 1) was employed as a solubilizer. The inclusion modes of flavonoids in the DM-β-CD are well studied.(22–24) We conducted ORAC-EPR measurement for seven pure lipophilic antioxidants (five flavonoids, resveratrol and astaxanthin) in the aqueous solution containing DM-β-CD as a solubilizer and compared with the ORAC-FL values.
Experimental
Materials and reagents
Chemical formulas of a majority of compounds used in this study are shown in Fig. 1. Antioxidants studied are catechin (CA), epicatechin (EC), epigallocatechin gallate (EGCg), kaempferol (KAP), myricetin (MYR), resveratrol (RESV), and astaxanthin (ASX) and those were purchased from Tokyo Kasei Co. (Tokyo, Japan) and Nakalai Tesque (Kyoto, Japan). For the sake of comparison, we used trolox (6-hydroxy-2,5,7,8-tetra-methylchroman-2-carboxylic acid), a water-soluble analog of vitamin E, as a standard material. A newly developed spin-trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoranyl)-5-methyl-1-pyrroline N-oxide (CYPMPO) were obtained from Radical Research Inc. (Hino, Japan).(25) Free radical precursor 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH) was purchased from Wako Pure Chem. Ind. (Osaka, Japan). Heptakis(2,6-di-O-methyl)-β-cyclodextrin (DM-β-CD) was purchased from Tokyo Kasei Chem. (Tokyo, Japan). Water was purified by distillation and passing through a Mili-Q system (Milipore Corp. Billerica MA) and used as a solvent.
Sample preparation and EPR measurements
EPR spin trapping is a widely used technique in free radical biology.(26) This technique is based on the following reaction:
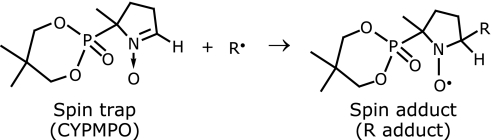 |
Chemical compound called spin trap (CYPMPO is shown as an example in the above scheme) reacts with free radical R to form stable compound called spin adduct, that is also free radical. The spin adduct is relatively stable and can be readily identified and quantified with EPR spectrometer. In this study, free radicals were generated from AAPH with UV irradiation (5 s irradiation, 200 W mercury arc RUF-203s, Radical Research Inc.) to the sample solution that was set in the EPR resonant cavity. The sample solution contained AAPH and CYPMPO in sodium phosphate buffer (0.1 M, pH = 7.4). As a result, EPR spectra shown in Fig. 2 were obtained. The analysis of the EPR spectral pattern provides the identification of the free radical, and EPR-signal height is proportional to the free radical concentration. The spin trap and the antioxidant compete to react with AAPH radicals, from which the ORAC value of the antioxidant is calculated.(13) EPR signal intensity of the spin adduct in the presence and absence of antioxidant was measured to calculate ORAC-EPR values. The calculation method is described in the following section.
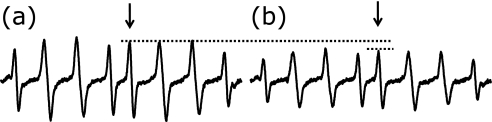
Fig. 2.
EPR spectra of AAPH radical adduct (assigned to RO• radical adduct) of CYPMPO that was recorded after the UV-photolysis of phosphate buffer solution, containing AAPH (5 mM), CYPMPO (5 mM) and DM-β-CD solubilized CA: (a) [DM-β-CD] = 0.94 mM and (b) [DM-β-CD] = 0.94 mM plus [CA] = 22.0 µM. Horizontal broken lines in the spectra demonstrate the change in EPR signal height of the selected peak by the addition of the antioxidant CA.
Except RESV and ASX, the antioxidant compounds were first dissolved in sodium hydroxide solutions (pH = 11) and diluted with phosphate buffer (pH = 7.4) solution of DM-β-CD. The antioxidants were precipitated if diluted without the solubilizer. Typical concentrations of the reagents were: [CYPMPO] = 5 mM, [AAPH] = 5 mM, [DM-β-CD] = 1 mM, and [AOx] = 20 µM at pH = 7.4, where AOx is the abbreviation for antioxidant. Because RESV and ASX were insoluble even in basic aqueous solution, acetonitrile was used as a solvent for stock solution. Acetonitrile has been shown to have negligible antioxidant capacity.(13) The acetonitrile solution was diluted with phosphate buffer containing DM-β-CD; and in final solutions, the acetonitrile content was less than 5%. Blank control solution contained CYPMPO (5 mM), AAPH (5 mM) and DM-β-CD (1 mM).
The sample was loaded in an EPR flat cell and set inside the EPR resonant cavity, and EPR signals were recorded immediately after in situ UV irradiation. Half life of the AAPH radical adduct was longer than 1 h. The same measurement was repeated for five times and the result was presented as average ± SD. The sample temperature was maintained at 298 ± 0.1 K and EPR signals were recorded in a JEOL FE3XG X-band spectrometer (Akishima, Japan). The spectrometer settings for EPR measurements were as follows: microwave power, 6 mW; field modulation amplitude, 0.1 mT at 100 kHz; time constant, 0.1 s; field scan rate, 5 mT min−1.
Calculation of ORAC-EPR values
The competitive spin-trapping method was applied to evaluate ORAC-EPR values.(13) The competitive reaction was taken place between the spin trap and the solubilized antioxidant against free radical R• as follows:
| AAPH → R• |
| ST + R• → ST-R (EPR active) ……… rate constant kST |
| AOx (solubilized) + R• → Product (EPR silent) …. rate constant kAOx, |
where ST and ST-R denote the spin trap and the radical adduct, respectively. The third reaction expresses the scavenging reaction by AOx against R• radical.
A simple formulation for the ORAC-EPR calculation can be derived from the above reactions and has been reported elsewhere(13):
 |
(1) |
where I and I0 are EPR signal heights of the spin adduct ST-R in the presence and absence of AOx, and [ ]0 denotes initial concentration of the component. EPR signal height is proportional to the concentration of the EPR active species. We assume that AOx is completely solubilized so that [AOx] is equal to [AOx (solubilized)]. A linear plot of (I0–I)/I against [AOx]0/[ST]0 provides the slope kAOx/kST which is equal to the ORAC value for AOx relative to that of ST. The hydrophilic antioxidant trolox has been conventionally adopted as a standard.(10–12) Thus, the ORAC value of trolox with respect to CYPMPO (ktrolox/kCYPMPO) was used to express ORAC value in trolox equivalent unit.
Results
EPR spin trapping of AAPH radicals
In ORAC-FL, peroxyl radical (ROO• radical) is assumed to be produced from AAPH(6,10); however, recent spin-trapping studies revealed that alkoxyl radical (RO•) but not peroxyl radical is produced after thermal or photolytic decomposition of AAPH.(27–29) The production of RO• radical was confirmed in this study, too. Fig. 2a shows the EPR spectra obtained from the UV-irradiated solution of AAPH in the presence of the spin trap CYPMPO and the solubilizer DM-β-CD. A single spin adduct species is visible in each spectrum and its hyperfine splitting constant (hfsc) was obtained with computer spectrum simulation: AP = 4.77 mT, AN = 1.36 mT, and AH = 1.23 mT. Because reported hfsc’s of the HO• adduct of CYPMPO (CYPMPO-OH) (Isomer 1: AP = 4.88 mT, AN = 1.37 mT, and AH = 1.37 mT; Isomer 2: AP = 4.70 mT, AN = 1.35 mT, and AH = 1.23 mT) are very similar to the present results,(25) we assigned AAPH radical adduct to RO• radical adduct.(27–29)
As shown in Fig. 2b, EPR signal height decreased in the presence of the antioxidant catechin (CA). This is because part of AAPH radical was scavenged by CA. The decrease of the EPR signal height (free radical concentration) is converted into ORAC-EPR value using Eq. 1.
ORAC-EPR of the ORAC-standard reagent trolox
The ORAC standard trolox (structure in Fig. 1) is water soluble and we measured the ORAC-EPR value ktrolox/kCYPMPO in the absence of the solubilizer as 114 ± 3 (cited in the comment line of Table 1). Trolox’s ORAC-EPR value was slightly modified by the presence of DM-β-CD: i.e., 95.9 with the solubilizer as compared with 114 without the solubilizer (footnote of Table 1). These values were utilized to express ORAC values relative to trolox (trolox equivalent unit).
Table 1.
ORAC-EPR values of DM-β-CD solubilized antioxidants with relative rate constants (kAOx/kST (AOx/CYPMPO))
| Antioxidants solubilized with DM-β-CD | kAOx/kST | ORAC-EPR | ORAC-FL (in trolox equivalent unit) | TEAC | DPPH | Haemolysis |
|---|---|---|---|---|---|---|
| Catechin (CA): DM-β-CD | 92.1 ± 4.4 | 0.96 ± 0.05 | 2.49a, 6.76b, 7.9e, 12.4c, 14.9d | 1.1e | 0.8e | 1.7e |
| Epicatechin (EC): DM-β-CD | 90.5 ± 8.1 | 0.94 ± 0.09 | 2.36a, 5.1e, 9.14c | 1.3e | 1.0e | 1.5e |
| Epigallocatechin gallate (EGCg): DM-β-CD | 124 ± 5 | 1.3 ± 0.1 | 1.87f, 3.4e, 3.51g, 4.55c | 2.0e | 3.7e | 1.0e |
| Kaempferol (KAP): DM-β-CD | 35.6 ± 1.9 | 0.37 ± 0.02 | 2.29g, 2.67a, 6.2e, 7.19c | 0.5e | 0.8e | 0.2e |
| Myricetin (MYR): DM-β-CD | 304 ± 6 | 3.2 ± 0.1 | 3.6e, 4.26g, 4.32a | 1.5e | 1.8e | 0.9e |
| Resveratrol (RESV): DM-β-CD | 61.0 ± 1.2 | 0.64 ± 0.02 | 4.98h | |||
| Astaxanthin (ASX): DM-β-CD | 26.9 ± 7.0 | 0.28 ± 0.07 | 0.05f | |||
| Trolox: DM-β-CDi | 95.9 ± 1.8 | 1 |
a The ORAC-FL values reported by Prior and Cao were shown by underlines (Ref. 30). b Ref. 10. c Ref. 32. d Ref. 31. e Ref. 33. f Ref. 21. g Ref. 35. h Ref. 34. i In Trolox, kAOx/kST = 114 ± 3 in the absence of CD (this work).
ORAC-EPR values of lipophilic antioxidants
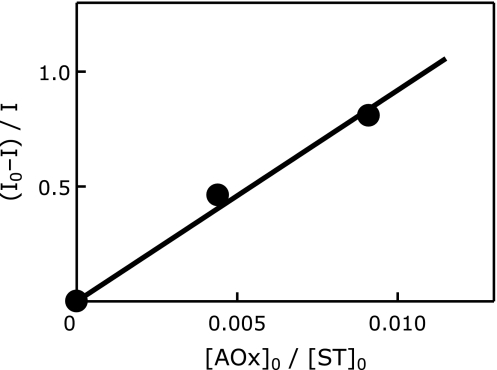
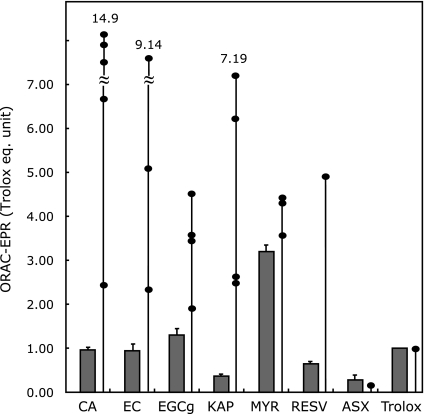
Fig. 3 shows a typical plot for Eq. 1 in CA/CYPMPO system in the presence of DM-β-CD, the slope of which demonstrates CA’s ORAC value relative to CYPMPO. The plot of the relative radical scavenging rates gives a straight line that passes through the origin, indicating that the reaction scheme and the calculation procedures of relative radical scavenging rate constants (kAOx/kST) using Eq. 1 are justified. Using similar procedures, ORAC-EPR values (kAOx/kST) relative to CYPMPO for seven lipophilic antioxidants were calculated and converted into trolox equivalent unit. Average ORAC-values after five repeated measurements are shown in Table 1 and Fig. 4 with standard deviation. Previous ORAC-FL data are also listed in Table 1 and Fig. 4.(10,30–35)
Fig. 3.
A typical plot of (I0–I)/I of catechin (CA) against [AOx]0/[ST]0 in the presence of DM-β-CD using Eq. 1 (AOx = CA and ST = CYPMPO). I0 is taken from the EPR peak height such as shown in Fig. 2a and I from Fig. 2b. The slope kAOx/kST (approximately 92 in this graph) corresponds to the ORAC-EPR value of CA relative to CYPMPO.
Fig. 4.
A bar graph for ORAC-EPR values (in trolox equivalent unit) for the seven poorly water-soluble antioxidants that were solubilized with DM-β-CD (solid bars). Published ORAC-FL data are individually illustrated with solid circle on the vertical line.
Discussion
ORAC-EPR measures the level of free radical using EPR spin trapping, while ORAC-FL indirectly measures free radical level via degree of free radical damage on the fluorescence probe. Thus, it is clear that ORAC-FL and ORAC-EPR measure the same intrinsic physical constant of the system. All seven antioxidants tested in this study showed ORAC-EPR values ranging from 0.28 to 3.2 trolox equivalent unit (teu). Solubilized myricetin (MYR) showed the largest ORAC value (3.2 ± 0.1 teu), i.e., the highest free radical scavenging activity within antioxidants tested. We speculate that three OH groups in the side phenyl ring (B-ring) in MYR may have stabilized reaction products between free radical and MYR.(36) The ORAC-EPR values fell within relatively narrow range because these compounds are all classified as phenol-type antioxidants in which the antioxidant activities depend on OH group(s) in the phenyl ring (Fig. 1). The standard deviation of the present ORAC-EPR data was less than 10% in most antioxidants (Table 1). In contrast, previous ORAC-FL data that have been published by various investigators showed large variations (Fig. 4). For example, ORAC-FL data for CA ranged from 2.49 to 14.9 teu, while its ORAC-EPR value was 0.96 ± 0.05 teu. Because CA is slightly water soluble, CA’s ORAC-EPR value in the absence of the solubilizer was evaluated to be 0.71 teu,(13) indicating that the effect of the solubilizer to the ORAC value is minor in ORAC-EPR.
It is likely that some instrumental reasons in ORAC-FL method may have caused the large variations, i.e., 1) the difficulty in generating constant amount of AAPH free radicals by thermal decomposition (heating), 2) temperature and instrumental instability during the time course measurement (typically 30 min) of fluorescence decay, and 3) the complexity of computer-aided analysis. In ORAC-EPR, these difficulties are mostly removed, where a constant amount of AAPH radical can be readily generated with the short photolysis (typically 5.0 s) of AAPH solution, and the ORAC calculation does not require computer-aided curve-fitting analysis.
In solubilized lipophilic antioxidants, the discrepancies between ORAC-FL and ORAC-EPR data are larger than in the case of water-soluble antioxidants, partly because previous ORAC-FL values show large variations (Fig. 4). Although there have been various modified ORAC-FL methods, we selected Prior and Cao’s ORAC-FL data(30) for the comparison (Table 1). These authors have been playing a major role in improving and modifying ORAC-FL method. Table 1 shows that the agreement in absolute ORAC values was only fair, but the tendency showed a reasonable agreement, i.e., MYR > KAP and EGCg > CA ≈ EC. We speculate that the difference in free radical generation methods may be a dominant cause of the disagreement. In fact, our test by combining the heating method (40°C, 30 min) with ORAC-EPR measurement resulted in 0.7 teu for CA with 40% error.
Various free radical scavenging assays have been developed to obtain ORAC-like values of antioxidants. These include TEAC method, DPPH method, and red blood cell haemolysis method.(33) ORAC-like values for the antioxidants obtained using TEAC, DPPH, and haemolysis methods(33) are listed in Table 1. Inspection of Table 1 indicated that most ORAC-FL values are much larger than those obtained by using other methods. It is recognized that ORAC-FL assay is characteristic in the sense that it monitors both the inhibition time and the degree of inhibition, and that may explain the reason that ORAC-FL usually gave larger values than all other methods.
Finally, it should be pointed out that the ORAC-EPR values were slightly dependent on the kind of solubilizer used. We found that such difference can be explained using the NMR structure of antioxidant-cyclodextrin complex and plan to publish these results elsewhere.
Conclusions
Using ORAC-EPR method, we measured ORAC values of seven poorly water-soluble lipophilic antioxidants that are solubilized in water with DM-β-CD. The ORAC-EPR values ranged from 0.28 to 3.2 teu and the experimental errors were less than 10% in most compounds. Because of the large variation in the past ORAC-FL data, it was difficult to make the comparison of the two methods. However, the magnitude and the tendency of ORAC-FL data from Prior’s group showed reasonable agreement with the ORAC-EPR data. Judging from the size of the errors, we believe that the present ORAC-EPR values are more credible than the previous ORAC-FL values.
Acknowledgment
This work was supported in part by a research grant from the Urakami Foundation.
Abbreviations
- AAPH
2,2-azobis(2-amidinopropane) dihydrochloride
- AOx
antioxidant
- ASX
astaxanthin
- CA
catechin
- β-CD
β-cyclodextrin
- CYPMPO
5-(2,2-dimethyl-1,3- propoxy cyclophosphoranyl)-5-methyl-1-pyrroline N-oxide
- DM-β-CD
heptakis(2,6-di-O-methyl)-β-cyclodextrin
- DPPH
1,1-diphenyl-2-picrylhydrazyl
- EC
epicatechin
- EGCg
epigallocatechin gallate
- EPR
electron paramagnetic resonance
- HP-β-CD
hydroxy propyl-β-cyclodextrin
- KAP
kaempferol
- MYR
myricetin
- NMR
nuclear magnetic resonance
- ORAC
oxygen radical absorbance capacity
- ORAC-EPR
EPR spin trapping-based ORAC method
- ORAC-FL
fluorescence-based ORAC method
- RESV
resveratrol
- RO•
alkoxyl radical
- ST
spin trap agent
- teu
trolox equivalent unit
- trolox
6-hydroxy-2,5,7,8-tetra-methylchroman-2-carboxylic acid
References
- 1.Jürgens G, Hoff HF, Chisolm GM, 3rd, Esterbauer H. Modification of human serum low density lipoprotein by oxidation-characterization and pathophysiological implications. Chem Phys Lipids. 1987;45:315–336. doi: 10.1016/0009-3084(87)90070-3. [DOI] [PubMed] [Google Scholar]
- 2.Cutler RG. Recent progress in testing the longevity determinant and dysdifferentiation hypotheses of aging. Arch Gerontol Geriatr. 1991;12:75–98. doi: 10.1016/0167-4943(91)90021-h. [DOI] [PubMed] [Google Scholar]
- 3.Mertz C, Gancel AL, Gunata Z, et al. Phenolic compounds, carotenoids and anitioxidant activity of three tropical fruits. J Food Comp Anal. 2009;22:381–387. [Google Scholar]
- 4.Prior RL, Cao G. Measurement of oxygen radical absorbance capacity in biological samples. Methods Enzymol. 1999;299:50–62. doi: 10.1016/s0076-6879(99)99008-0. [DOI] [PubMed] [Google Scholar]
- 5.Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byne DH. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Comp Anal. 2006;19:669–675. [Google Scholar]
- 6.Jimenez-Alvarez D, Giuffrida F, Vanrobaeys F, et al. High-throughput methods to assess lipophilic and hydrophilic antioxidant capacity of food extracts in vitro. J Agric Food Chem. 2008;56:3470–3477. doi: 10.1021/jf703723s. [DOI] [PubMed] [Google Scholar]
- 7.Glazer AN. Phycoerythrin fluorescence-based assay for reactive oxygen species. Methods Enzymol. 1990;186:161–168. doi: 10.1016/0076-6879(90)86106-6. [DOI] [PubMed] [Google Scholar]
- 8.DeLange RJ, Glazer AN. Phycoerythrin fluorescence-based assay peroxy radicals: a screen for biologically relevant protective agents. Anal Biochem. 1989;177:300–306. doi: 10.1016/0003-2697(89)90056-0. [DOI] [PubMed] [Google Scholar]
- 9.Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Rad Biol Med. 1993;14:303–311. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- 10.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–4326. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 11.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 12.Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 13.Kohri S, Fujii H, Oowada S, et al. An oxygen radical absorbance capacity-like assay that directly quantifies the antioxidant’s scavenging capacity against AAPH-derived free radicals. Anal Biochem. 2009;386:167–171. doi: 10.1016/j.ab.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Endo N, Oowada S, Sueishi Y, et al. Serum hydroxyl radical scavenging capacity as quantified with iron-free hydroxyl radical source. J Clin Biochem Nutr. 2009;45:193–201. doi: 10.3164/jcbn.08-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bangaore DV, McGlynn W, Scott DD. Effect of β-cyclodextrin in improving the correlation between lycopene concentration and ORAC values. J Agric Food Chem. 2005;53:1878–1883. doi: 10.1021/jf048258m. [DOI] [PubMed] [Google Scholar]
- 16.Mercader-Ros MT, Lucas-Abellán C, Fortea MI, Gabaldón JA, Núñez-Delicado E. Effect of HP-β-cyclodextrins complexation on the antioxidant activity of flavonols. Food Chem. 2010;118:769–773. [Google Scholar]
- 17.Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Deemer EK. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated β-cyclodextrin as the solubility enhancer. J Agric Food Chem. 2002;50:1815–1821. doi: 10.1021/jf0113732. [DOI] [PubMed] [Google Scholar]
- 18.Koizumi K, Okada Y, Kubota Y, Utamura T. Inclusion complexes of poorly water-soluble drugs with glucosyl-cyclodextrins. Chem Pharm Bull (Tokyo) 1987;35:3413–3418. doi: 10.1248/cpb.35.3413. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Purdy WC. Cyclodextrins and their applications in analytical chemistry. Chem Rev. 1992;92:1457–1470. [Google Scholar]
- 20.Szejtli J. The properties and potential uses of cyclodextrin derivatives. J Incl Phenom Macrocycl Chem. 1992;14:25–36. [Google Scholar]
- 21.Kim H, Choi J, Jung S. Inclusion complexes of modified cyclodextrins with some flavonols. J Incl Phenom Macrocycl Chem. 2009;64:43–47. [Google Scholar]
- 22.Folch-Cano C, Jullian C, Speisky H, Olear-Azar C. Antioxidant activity of inclusion complexes of tea catechins with β-cyclodextrins by ORAC assays. Food Res Int. 2010;43:2039–2044. [Google Scholar]
- 23.Bertacche V, Lorenzi N, Nava D, Pini E, Sinico C. Host-Guest interaction study of resveratorol with natural and modified cyclodextrins. J Incl Phenom Maclocycl Chem. 2006;55:279–287. [Google Scholar]
- 24.Jullian C, Miranda S, Zapata-Torres G, Mendizábal F, Olea-Azar C. Studies of inclusion complexes of natural and modified cyclodextrin with (+)catechin by NMR and molecular modeling. Bioorg Med Chem. 2007;15:3217–3224. doi: 10.1016/j.bmc.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 25.Kamibayashi M, Oowada S, Kameda H, et al. Synthesis and characterization of a practically better DEPMPO-type spin trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) Free Rad Res. 2006;40:1166–1172. doi: 10.1080/10715760600883254. [DOI] [PubMed] [Google Scholar]
- 26.Janzen EG. Spin trapping. Methods of Enzymol. 1984;105:188–198. doi: 10.1016/s0076-6879(84)05025-4. [DOI] [PubMed] [Google Scholar]
- 27.Krainev AG, Bigelow DJ. Comparison of 2,2'-azobis(2-amidinopropane) hydrochloride (AAPH) and 2,2'-azobis(2,4-dimethylvaleronitrile) (AMVN) as free radical initiators: a spin-trapping study. J Chem Soc Perkin Trans 2. 1996:747–754. [Google Scholar]
- 28.Rojas-Wahl RU, Zeng L, Madison SA, DePinto RL, Shay BJ. Mechanistic studies on the decomposition of water soluble azo-radical-initiators. J Chem Soc Perkin Trans 2. 1998:2009–2018. [Google Scholar]
- 29.Sueishi Y, Yoshioka D, Oowada S, et al. Is the oxygen radical absorbance capacity (ORAC) method a peroxyl-radical scavenging assay? Z Phys Chem. 2010;224:921–928. [Google Scholar]
- 30.Prior RL, Cao G. Antioxidant capacity and polyphenolic components of teas: inplications for altering in vivo antioxidant status. Proc Soc Exp Biol Med. 1999;220:255–261. doi: 10.1046/j.1525-1373.1999.d01-44.x. [DOI] [PubMed] [Google Scholar]
- 31.Davalos A, Gómez-Cordovés C, Bartolomé B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J Agric Food Chem. 2004;52:48–54. doi: 10.1021/jf0305231. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe KL, Liu RH. Structure-activity relationships of flavonoids in the celluar antioxidant activity assay. J Agric Food Chem. 2008;56:8404–8411. doi: 10.1021/jf8013074. [DOI] [PubMed] [Google Scholar]
- 33.Tabart J, Kevers C, Pincemail J, Defraigne JO, Dommes J. Comparative antioxidant capacities of phenolic compounds mearured by various tests. Food Chem. 2009;113:1226–1233. [Google Scholar]
- 34.Capitani CD, Carvalho ACL, Rivelli DP, Barros SBM. Evaluation of natural and synthetic compounds according to their antioxidant activities using multivariate approach. Eur J Lipid Sci Technol. 2009;111:1090–1099. [Google Scholar]
- 35.Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RL. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well form. J Agric Food Chem. 2002;50:4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- 36.Sadeghipour M, Terreux R, Phipps J. Flavonoids and tyrosine nitration: structure-activity relationship correlation with enthalpy of formation. Toxicol In Vitro. 2005;19:155–165. doi: 10.1016/j.tiv.2004.06.009. [DOI] [PubMed] [Google Scholar]