Abstract
We report a cross-sectional study of olfactory impairment with age based on both odorant-stimulated responses of human olfactory sensory neurons (OSNs) and tests of olfactory threshold sensitivity. A total of 621 OSNs from 440 subjects in two age groups of younger ( 45 years) and older (≥60 years) subjects were investigated using fluorescence intensity ratio fura-2 imaging. OSNs were tested for responses to two odorant mixtures, as well as to subsets of and individual odors in those mixtures. Whereas cells from younger donors were highly selective in the odorants to which they responded, cells from older donors were more likely to respond to multiple odor stimuli, despite a loss in these subjects’ absolute olfactory sensitivity, suggesting a loss of specificity. This degradation in peripheral cellular specificity may impact odor discrimination and olfactory adaptation in the elderly. It is also possible that chronic adaptation as a result of reduced specificity contributes to observed declines in absolute sensitivity.
Keywords: Olfactory sensory neurons, odor thresholds, age-related odor impairment, intracellular calcium, olfactory epithelium
1. Introduction
Olfactory impairment places individuals at risk of poor nutritional status (Aschenbrenner, et al., 2008,Mattes and Cowart, 1994,Rolls, 1999), and exposes them to hazardous events such as failure to detect spoiled food, leaking gas or toxic vapors from household cleaning agents (Santos, et al., 2004). These dangers are magnified in the elderly population, for whom food intake, resistance to food-borne diseases and the ability to recover from toxic or caustic injuries are also reduced (Murphy, 2008,Schiffman, 1997). Age-associated changes in olfaction include deficits in sensitivity, discrimination and identification, as well as enhanced adaptation with slower recovery, which may be independent of changes in sensitivity (Cowart, 1989,Doty, et al., 1984,Hummel, et al., 2007,Kaneda, et al., 2000,Stevens and Cain, 1986). While these phenomena have been widely studied using a variety of psychophysical and physiological measures, the extent to which changes in perception are due to changes in the peripheral or the central components of the olfactory system is not known. Loss in olfaction is also one of the earliest signs of neurodegenerative diseases of the brain (Doty, et al., 1988,Graves, et al., 1999,Wilson, et al., 2007).
Studies of functional magnetic resonance imaging (fMRI) and olfactory event-related potential (OERP) responses to olfactory stimuli indicate reduced and altered activity levels in various CNS regions of hyposmic elderly compared with controls (Evans, et al., 1995,Murphy, et al., 1998). It has also been suggested that loss in olfactory discrimination may result from atrophy of the olfactory neuroepithelium, as well as a reduction in epidermal growth factor (EGF) dependent olfactory neurogenesis, revealed by a selective reduction in expression levels of EGF receptor signaling elements in the aged forebrain subventricular zone in mice (Enwere, et al.,2004). Studies of animal models and human biopsy specimens support a gradual decline in the extent of the olfactory epithelium that could account for olfactory loss (Hirai, et al., 1996,Nakashima, et al., 1984,Paik, et al., 1992). However, it is unclear whether this loss of the epithelium accounts for the olfactory decline observed in most elderly individuals.
The aim of this study was to determine whether age-related differences in the functional properties of OSNs might contribute to age-related olfactory impairment. The intracellular calcium response properties of neurons can be used to reveal neurophysiological characteristics relevant to aging. In this study we used calcium-imaging to evaluate age-related changes in OSN odor responsiveness to different odor mixtures. We test the responsiveness of OSNs from young (≤45 years old) and old donors (≥60 years old).
2. Methods
2.1 Subjects
This study was approved by the Institutional Review Board of Thomas Jefferson University (TJU) in Philadelphia, PA. Subjects were recruited through advertisements and flyers and contacted the Monell-Jefferson Taste & Smell Clinic in the Department of Otolaryngology, Head-Neck Surgery at TJU. Prescreening eliminated any subjects with a condition or receiving a medication that would preclude participation (e.g., bleeding disorder, use of anti-clotting medication). Subjects came to the Clinic in the morning and, after giving informed consent, completed a medical history questionnaire and underwent sensory testing to determine unilateral detection thresholds for two odorants, phenylethyl alcohol (PEA/rose) and lyral, using a two-alternative, forced-choice staircase procedure described previously (Rawson, et al., 1995). Each of these odorants is also included in one of the stimulus batteries used to assess cell function. One or two biopsies, at the discretion of the otorhinolaryngologist (EP), were obtained under local anesthesia from the mid-portion of the medial side of the medial turbinate and the opposed septum as described elsewhere (Lowry and Pribitkin, 1995). The Kuhn-Bolger giraffe forceps were used to take biopsies under endoscopic visualization ensuring correct targeting of the forceps and that no polyps were present. As indicated in the results only a fraction of ~1/3 of the biopsies yielded OSNs consistent with the fact that the olfactory epithelium of human is patchy (Morrison and Costanzo, 1990) and as a result not every biopsy contains olfactory epithelium (Paik et al 1992). In addition, low density of OSNs may have contributed to not obtaining OSNs from a subset of the subjects. Average age in the young group with 378 participants was 31.1 ± 15.8 (mean ± SD) years and in the group of older donors with 62 participants was 70.2 ± 13 years.
2.2 Specimen
Biopsies were immediately placed into RPMI media (Sigma, Inc., St. Louis, MO) and brought to our laboratory at Monell Chemical Senses Center about 15 min away. The tissue was then minced and placed into isolation solution containing 20 – 30 mU of papain for 15 min, then triturated with a fire polished pipette. Dissociation was stopped by addition of an equal volume of stop solution containing (in mM): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 5 D-glucose, 1 Na-pyruvate, 20 HEPES, 10μg/ml leupeptin, 5 μM of fura-2/AM (Molecular Probes) and 80 μg/ml pluronic acid, and filtered with a 70 μm nylon cell strainer (BD Falcon, Bedford, MA). Aliquots of cell suspension were then placed onto coverslips (No. 0, Thomas Scientific) coated with concanavalin A and allowed to sit for one hour to attach to the coverslip and take up and de-esterify the fura-2/AM. Recordings were obtained from 442 OSNs in the group of young donors and 179 OSNs in the group of older donors.
2.3 Imaging
In order to obtain the maximum amount of information from each biopsy, two imaging systems were used concurrently. Imaging was performed by illumination with 340/360 nm or 340/380 nm light from a xenon bulb and emitted fluorescence was collected through a bandpass filter centered at 510nm. OSNs were identified and differentiated from microvillar and supporting cells because of their round cell body, long dendrite and olfactory knob (Morrison and Costanzo, 1990). We assayed OSN health by measuring intracellular fura-2 ratio and only recorded from OSNs with fluorescent ratios reflecting intracellular calcium concentrations below 200 nM (it was rare to find OSNs with such a high fura-2 ratio). Exposure times were short (typically, 50 – 200ms), and the light was shuttered between exposures to minimize photobleaching. Cells attached to coverslips were superfused with Ringer’s solution and exposed to odorant mixtures or single odorants via the superfusion buffer or via a computer-controlled Solution Changer (Bio-Logic, RSC-100, Pullman, WA), as described in Rawson et. al (1997). Cells were tested with two odorant mixtures, mix A (hedione, geraniol, phenylethyl alcohol, citralva, citronellal, eugenol, and menthone) and mix B (lyral, lilial, triethylamine, ethylvanillin, isovaleric acid, and phenylethyl amine), or with subsets of these or individual odorants dissolved in Ringer’s solution at 1 or 100 μM. Images were acquired approximately every 4 – 7 seconds via an SC-90-cooled CCD camera from Theta System Elektronik GmbH (Munich, Germany) in an imaging system from T.I.L.L. Photonics GmbH (Munich, Germany) or by an OPELCO KS-1380 image intensifier coupled with a Sanyo CCD camera by a Quantimet 570 image analysis work station (Leica, Inc). These cameras were able to image the cell body, dendrite and olfactory knob of OSNs. However, the system was not capable of imaging olfactory cilia. Images were analyzed with Merlin software (PE Systems, Inc.), which computes the ratio values for the intensities within identified regions of interest and generates a graphical output. Finally, an odor was considered to elicit a response if the fluorescence ratio increased or decreased after odor exposure by more than two standard deviations.
2.4 Statistics
Statistical analyses were performed with SAS, Vers. 7 (Cary, NC). A Generalized Estimating Equations (GEE) analysis (GENMOD procedure in SAS) was carried out to assess factors associated with olfactory performance. All chi-square comparisons were Yates’ corrected.
3. Results
3.1 Olfactory threshold
To determine whether OSN response frequency is in any way associated with perceptual sensitivity to the odorants, we performed a Mann-Whitney rank analysis to compare olfactory thresholds in the two age groups. Older subjects had significantly higher detection thresholds (reflecting poorer sensitivity) for both PEA (z=−3.31, p=.00045) and lyral (z=−2.37, p=.00878).
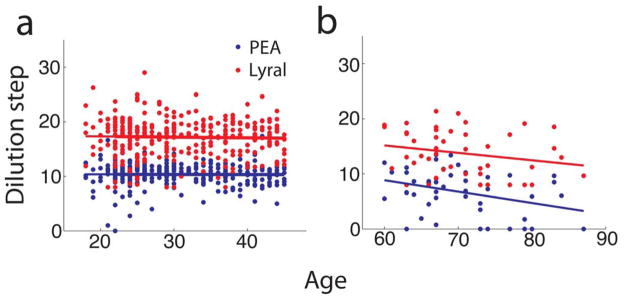
In the group of young donors (≤45) we did not observe changes in detection thresholds for PEA and lyral with age (see Fig. 1a). A regression analysis for this younger age group revealed a negligible slope for both PEA (R2=0.00005, p=0.6) and lyral (R2=0.0009, p=0.9); thus age is not predictive of odor detection performance for this group. For the group of older donors (≥60 years) a decline in olfactory sensitivity was observed with increasing age (Fig. 1b), which was significant for PEA (R2=0.12, p=0.017), but not for lyral (R2=0.049, p=0.139).
Fig. 1.
(a) Threshold tests performed at different dilutions of PEA and lyral did not show age-related changes in the group of younger subjects. (b) Consistent with published studies, there was an age-associated decline in sensitivity for PEA in the group of older donors (the slope of the linear regression was different from zero with a p-value of 0.018). Lines are linear regressions. For young subjects in panel a (n=316) the slope, intercept and p-value for the difference for the slope from zero were −0.00195, 10.4, 0.9 (PEA) and −0.013, 17.6, 0.6 (Lyral). For old subjects in panel b (n=46) the slope, intercept and p-value were −0.21, 21.2, 0.018 (PEA) and −0.13, 23.3, 0.14 (lyral).
3.2 Cellular responses of OSNs
Human olfactory epithelium is patchy (Morrison and Costanzo, 1990) and as a result not every biopsy contains olfactory epithelium (Paik, et al., 1992). Thus, as expected, tissue biopsies from only 129 of the 378 younger subjects (≤45 years old) and 28 of the 62 older donors (≥60 years old) yielded OSNs. From these subjects we obtained 442 viable OSNs from younger subject biopsies and 179 viable OSNs from the elderly. Finally, the percentage of biopsies that yielded viable cells was similar in younger (34%) and older subjects (45%).
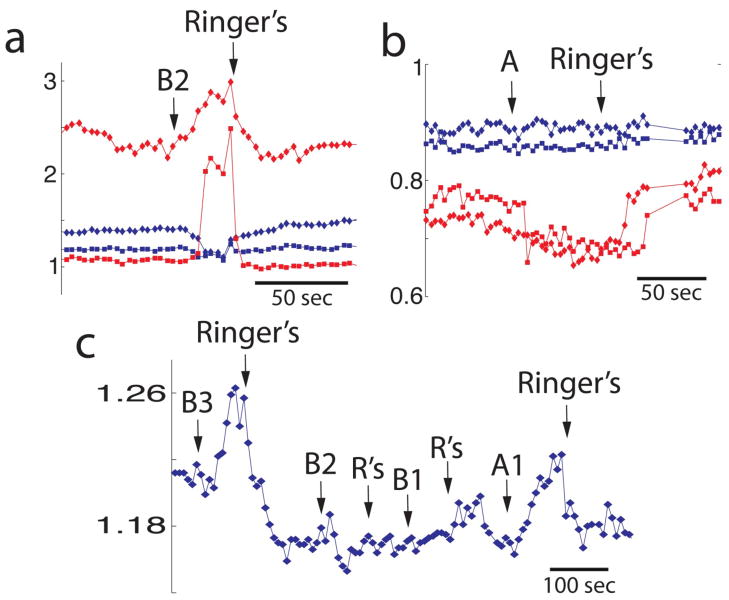
Fig. 2 shows changes in calcium elicited by stimulation with odors that returned to basal calcium after removal of the odor (addition of Ringer’s). Odorant-stimulated changes in calcium were primarily apical increases in intracellular calcium (Fig. 2a, red trace, Fig. 2c and Fig. 3), as reported previously (Rawson, et al., 1997). However, as was found in our previous study, odorant-stimulated decreases in calcium were also observed (Fig. 2b, red trace). To further investigate the degree of odorant selectivity of individual OSNs, we carried out experiments using subsets of the odorant mixtures. When tested with subsets or individual odors from the mixtures, some cells from older subjects responded to two or more different stimuli with increases (Fig. 2c) or decreases in calcium; in a few cases, one stimulus elicited an increase in [Ca2+]i while another elicited a decrease (the data in Figs. 2a and b are from the same cells).
Fig. 2.
Odor-induced changes in calcium in fura-2-loaded human OSNs isolated from older subjects (>65 years old). (a and b) show odor responses in two cells. Red and blue points display fura-2 ratio in different cells. Fura-2 ratio was measured in the apical end of the dendrite (squares) and the cell body (diamonds). In panel a the cell whose fura-2 ratio is displayed in red showed increases in intracellular calcium when stimulated with odor mixture B2. The same cell responded to A with a decrease in calcium (panel b). (c) Example of an OSN that responded with increases in calcium when stimulated with different odor mixtures. A1=PEA, hedione, geraniol; A2= citralva, citronellal, A3= eugenol, menthone, B1= lyral, lillial, B2= triethylamine, isovaleric acid, phenylethyl-amine, B3= ethyl-vanillin, R’s= Ringer’s solution. The criterion for judging whether the cell responded to an odor was a change in the ratio more than two standard deviations.
Fig. 3.
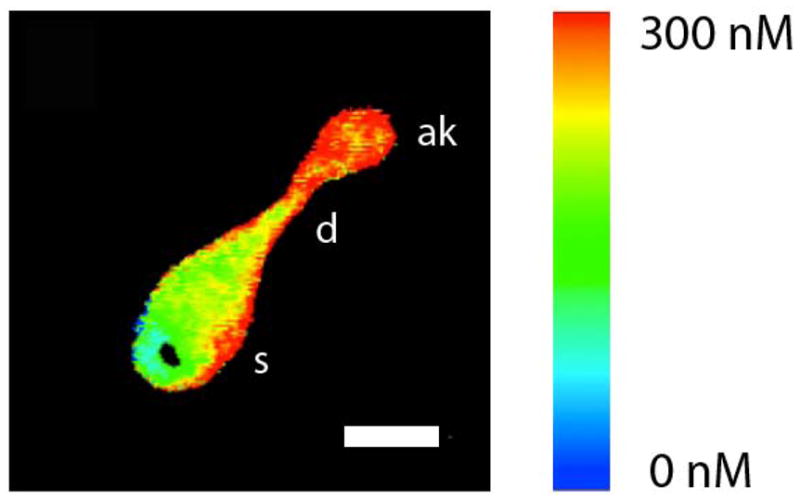
Image of an isolated OSN at the peak of the response to mix B. Pseudocolor shows the estimated intracellular calcium calculated from the fura-2 fluorescence ratio. Calcium increases in apical knob (ak) and dendrite (d). s stands for soma. The white bar is 5 μm long.
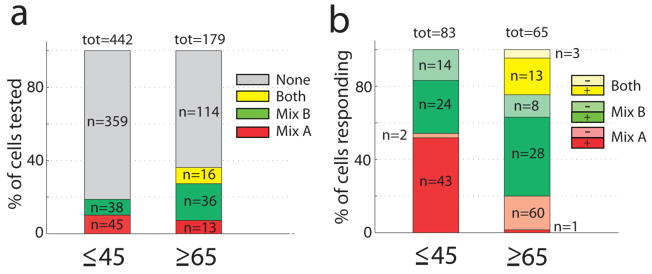
Experiments from 50% (young) and 81% (old) of the subjects yielded responsive OSNs (these percentages are not statistically significantly different, Yates’ χ2=2, df=1, p=0.15). The percentage of cells responding to mix A and B was higher in the older group (36.3%, n=65) than in the young group (18.7%, n=83), with the percentages of non-responding cells being 63.7% (n=114) and 81.3% (n=359), respectively (Fig. 4a; Yates’ χ2=20.6, df=1, p=5.6 × 10−6). All odorant-responsive cells from younger subjects responded only to one odorant mixture. In sharp contrast, 24.6% of the cells in older subjects responded to both odor mixtures with increases or decreases in calcium (Yates’ χ2=20.4, df=1, p=6.2 × 10−6). Moreover, odor-elicited decreases in [Ca2+]i (see Fig. 4b), were more frequent in the cells from older subjects (Yates’ χ2= 4.08 df=1,p=0.043). Therefore OSNs from younger subjects are more selective.
Fig. 4.
Selectivity and polarity of recorded OSN calcium responses to odor mixtures A and B. (a) The percentage of responsive cells was higher in OSNs from older donors, and a subset of cells from the older subjects responded to both odor mixtures. (b) This panel shows the percent of cells responding with different polarities to odors A and B (or to both odors). “+” denotes an odor-induced increase in calcium while “−“ denotes an odor-induced decrease in calcium. The percentage of odor-induced decreases in calcium in responses to mix A was higher in cells from older donors.
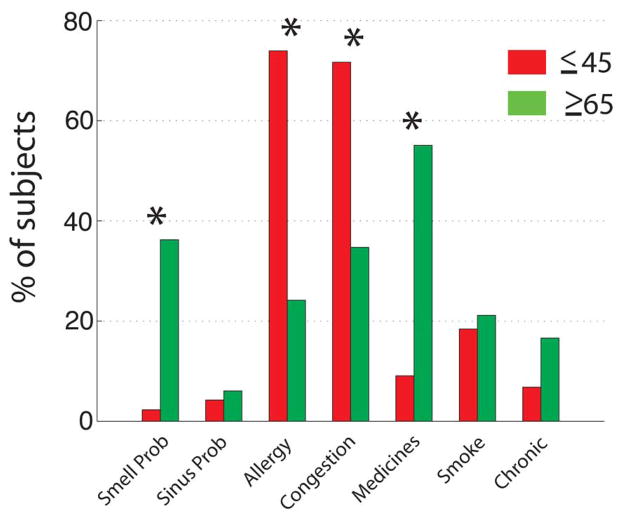
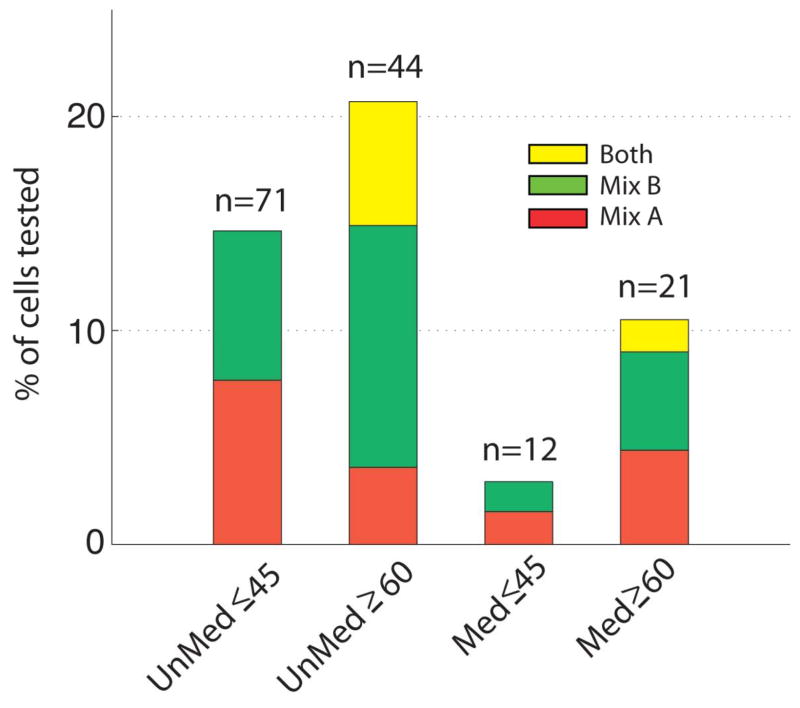
While every effort was made to recruit healthy subjects, a number of subjects reported health symptoms and medication usage, and differences between the two groups were determined by Wilcoxon test at the 0.05 level of significance (Fig. 5). Younger subjects were significantly less likely to report medication usage than older subjects, and primarily reported agents used to treat allergy or sinus symptoms, although some other types of medications were also reported. Importantly, however, while medicated and unmedicated younger subjects were equally likely to contribute responsive OSNs (Yates’ χ2=1.386, df=1, p=0.239), older subjects who contributed responsive OSNs were significantly more likely to be unmedicated than medicated (Yates’ χ2=5.653, df=1, p=0.017). In addition, while neither medicated nor unmedicated younger subjects contributed cells responding to multiple stimuli, both medicated and unmedicated older subjects did (Fig. 6). There is a tendency for medicated older subjects to contribute cells more like those of younger subjects in their relatively low responsiveness to multiple stimuli than did unmedicated older subjects (Yates’ χ2=5.869, df=2, p=0.053), but this simply points to medication status not being a primary driving factor in the differences we observed between younger and older subjects.
Fig. 5.
Health symptoms by age group. Marked with an asterisk are symptoms whose occurrence is found to be significantly different in the two age groups. Asterisks denote p<=0.05 in a Wilcoxon test.
Fig. 6.
OSNs responding to multiple stimuli were obtained from both medicated and unmedicatedolder subjects.
3.3 Cellular responses of OSNs correlate with PEA-thresholds
The average PEA-thresholds for older subjects who had cells that responded to both odor mixes (see Fig. 4A) was 7.9 (s2=14.6), and for those whose cells only responded to one mix was 6.0 (s2=12.0), this was significant at p=0.045 (one-sided t-test). Higher threshold values reflect greater sensitivity.
4. Discussion
Anatomical studies of the human olfactory system have shown that with advancing age there is a loss of OSNs (Paik, et al., 1992), with similar findings in the olfactory epithelium of rodents (Loo, et al., 1996,Weiler and Farbman, 1997). A decrease in adrenergic innervation of blood vessels vascularizing the olfactory epithelium has also been reported, which may impact on the tissue’s ability to be maintained in the face of continual cell turnover, and on the removal of volatiles or their metabolites that could affect normal function of the epithelium (Chen, et al., 1993). In view of these reports, we hypothesized that biopsies from older subjects would be less likely to yield OSNs, and that the neurons obtained would be less likely to respond to odorant stimuli. In contrast to our expectations, we were able to record from an equal number of viable OSNs per biopsy in subjects ≥60 years of age and subjects ≤45 years of age. Interestingly, our experiments showed that OSNs from elderly subjects responded to both odor mixtures A and B, in sharp contrast with OSNs from young subjects that, consistent with previous studies (Rawson, et al., 1997), responded to either A or B (Fig. 4a). In addition, OSNs from elderly subjects responded more frequently with a decrease in [Ca2+]i. Below we consider the changes that may lead to such a striking change in OSN odor selectivity in the elderly.
In spite of the fact that there are ~1000 olfactory receptor genes in mice, when a single OSN is tested it is found to express only one olfactory receptor (OR) through allelic exclusion (Chess, et al., 1994,Mombaerts, 2006). Although this principle has not been demonstrated in human OSNs (there are ~350 OR genes in the human genome), the low response frequency to single odorants and odorant subsets observed in this study and in previous publications in young adults (Rawson, et al., 1997) is consistent with that scenario. Because odor selectivity of OSNs is determined by the OR expressed by a particular cell, a loss of the tight control over OR expression leading to expression of multiple ORs per OSN could underlie the loss of odorant selectivity in the older subject group. In accord with this possibility, studies of the CNS have revealed age-related dysregulation in the expression of several molecules, including adenylate cyclase (Araki, et al., 1995,Sugawa and May, 1993) and voltage sensitive calcium channels (Herman, et al., 1998,Lee, et al., 2009,Tanaka and Ando, 2001,Thibault, et al., 2001). Additionally, dysregulation and increasing stochasticity of gene expression may occur, as shown for single cardiomyocytes in old mice (Bahar, et al., 2006).
A question that arises is whether the decreased odor selectivity that we find in the elderly affects axon connectivity in the olfactory bulb. Given the well known effect of OR expression on OSN axon targeting (Mombaerts, 2006), if loss of odor selectivity were due to dysregulation of OR gene expression resulting in expression of multiple ORs per OSN, we would expect changes in glomerular formation in the olfactory bulb. In addition, because axon targeting is affected by OSN activity (Kerr and Belluscio, 2006,Oliva, et al., 2008,Zou, et al., 2004), it is possible that loss of OSN selectivity in the elderly would affect glomerular structure. Indeed, studies in mice show that glomerular axon targeting by OSNs expressing the P2 olfactory receptor is increasingly diffuse with age with a subset of axons partially innervating glomeruli in elderly mice (Costanzo and Kobayashi, 2010). Interestingly, the number of glomeruli per human olfactory bulb is much larger than the number calculated using the two glomeruli per olfactory receptor gene value found in mouse (in human there are ~16 glomeruli per OR gene) (Maresh, et al., 2008,Meisami, et al., 1998), and one of two studies indicates that there is a decrease in the number of glomeruli in the elderly (Meisami, et al., 1998), consistent with findings of decreased OSN densities in aged mice (Lee, et al., 2009). Thus, there is an effect of aging on neuroanatomical features of glomeruli in the olfactory bulb that is consistent with a causal relationship with the decrease in OSN selectivity with aging found in this study.
Another possible hypothesis is that an age-related change in calcium regulation may be involved in the loss of odorant selectivity in OSNs from the aged group. It has been suggested that age-dependent changes in [Ca2+]i may involve mechanisms related to loss or change of function in several buffering systems that may modulate stimulation-evoked [Ca2+]i transients (Buchholz, et al., 2007). Interestingly, we find an increased number of decreases in calcium upon odor application (Fig. 4b) suggesting that the effect of odor transduction on calcium extrusion increases as a function of age. Changes in calcium regulation with age can also be linked to dysfunctional mitochondria, lower membrane potential and impaired calcium uptake, contributing to changes in intracellular calcium transients (Buchholz, et al., 2007,Xiong, et al., 2004,Yi, et al.,2004). Chronic or unusually frequent elevation of [Ca2+]i could shorten OSN lifespan, resulting in a higher proportion of immature or senescent OSNs that may exhibit altered functional properties. However, how a change in [Ca2+]i regulation would result in a change in odor selectivity of OSNs is not clear.
A third alternative explanation may be that the cells exhibiting nonselective responses were non-neuronal or were immature. It is possible that cells not selective to the odors used were immature and expressed multiple ORs per cell. Indeed, a recent study finds that OSNs in early postnatal life have broad odor selectivity. In contrast within two weeks they become selective, and interestingly, when OMP is ablated the OSNs of mice older than two weeks stay broadly responsive to diverse odors (Lee, et al., 2011). Another possibility is that the lack of selectivity observed was due to a difference in the integrity of the membrane such that the odors used induced leakiness. However, for a limited set of cells tested, calcium increases could be blocked by addition of the cyclic nucleotide gated channel blocker l-cis-diltiazem (data not shown), which would not be expected if a nonspecific effect of the odorant on the membrane were to occur. Further, if immature or senescent neurons are nonselective, one would predict that you would record from a few such cells in the young adults simply by chance. The complete lack of such responses argues against this possibility.
Work in the last few years has addressed the question of whether smell loss in the elderly, consisting of decreased detection threshold as well as impaired odor identification and detection, is due to central vs. peripheral changes in the olfactory system (Costanzo and Kobayashi, 2010,Lee, et al., 2009,Mirich, et al., 2002,Richard, et al., 2010). Our data provide new key information on this question by demonstrating reduced selectivity and increased excitability in the human OSN response profile. These peripheral changes might contribute to poor odor discrimination and identification in the elderly, as well as more profound and lasting olfactory adaptation that could affect odor thresholds contributing to decreased sensitivity in the elderly (Cowart, 1989,Doty, et al., 1984,Hummel, et al., 2007,Kaneda, et al., 2000,Stevens and Cain, 1986). Thus, our findings suggest that combined peripheral and central changes in the olfactory system could contribute to smell loss in the elderly.
In summary, this study indicates complex changes in peripheral olfactory function with age. We acknowledge that the data presented reflect a cross-sectional rather than a longitudinal assessment, and are based on biopsies representing a small portion of the olfactory epithelium, which may not be representative of the entire OSN population. Nonetheless, the data provide insight into the potential changes that may occur with aging in a neuronal cell population that is developmentally related to the CNS.
Acknowledgments
The authors wish to acknowledge the assistance of Jesse Chittams and David Boorman for statistical analyses, Chris Klock, Tim LaFlam and Elizabeth Varga for expert technical assistance and Kelli Mayo for support of manuscript preparation. This work was funded in part by NIH P50 DC00214 (NR), NIH P50 DC006760 (NR) and NIH R01 DC006070 (DR).
Footnotes
Disclosure Statement:
The authors have no actual or potential conflict of interest associated with this research. Ethical approval was obtained for data on human subjects in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Araki T, Kato H, Fujiwara T, Itoyama Y. Age-related changes in bindings of second messengers in the rat brain. Brain Res. 1995;704(2):227–32. doi: 10.1016/0006-8993(95)01117-x. [DOI] [PubMed] [Google Scholar]
- Aschenbrenner K, Hummel C, Teszmer K, Krone F, Ishimaru T, Seo HS, Hummel T. The influence of olfactory loss on dietary behaviors. Laryngoscope. 2008;118(1):135–44. doi: 10.1097/MLG.0b013e318155a4b9. [DOI] [PubMed] [Google Scholar]
- Bahar R, Hartmann CH, Rodriguez KA, Denny AD, Busuttil RA, Dolle ME, Calder RB, Chisholm GB, Pollock BH, Klein CA, Vijg J. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441(7096):1011–4. doi: 10.1038/nature04844. [DOI] [PubMed] [Google Scholar]
- Buchholz JN, Behringer EJ, Pottorf WJ, Pearce WJ, Vanterpool CK. Age-dependent changes in Ca2+ homeostasis in peripheral neurones: implications for changes in function. Aging Cell. 2007;6(3):285–96. doi: 10.1111/j.1474-9726.2007.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Getchell TV, Sparks DL, Getchell ML. Patterns of adrenergic and peptidergic innervation in human olfactory mucosa: age-related trends. J Comp Neurol. 1993;334(1):104–16. doi: 10.1002/cne.903340109. [DOI] [PubMed] [Google Scholar]
- Chess A, Simon I, Cedar H, Axel R. Allelic inactivation regulates olfactory receptor gene expression. Cell. 1994;78(5):823–34. doi: 10.1016/s0092-8674(94)90562-2. [DOI] [PubMed] [Google Scholar]
- Costanzo RM, Kobayashi M. Age-related changes in p2 odorant receptor mapping in the olfactory bulb. Chem Senses. 2010;35(5):417–26. doi: 10.1093/chemse/bjq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowart BJ. Relationships between taste and smell across the adult life span. Ann N Y Acad Sci. 1989;561:39–55. doi: 10.1111/j.1749-6632.1989.tb20968.x. [DOI] [PubMed] [Google Scholar]
- Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38(8):1237–44. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Applebaum SL, Giberson R, Siksorski L, Rosenberg L. Smell identification ability: changes with age. Science. 1984;226(4681):1441–3. doi: 10.1126/science.6505700. [DOI] [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24(38):8354–65. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WJ, Cui L, Starr A. Olfactory event-related potentials in normal human subjects: effects of age and gender. Electroencephalogr Clin Neurophysiol. 1995;95(4):293–301. doi: 10.1016/0013-4694(95)00055-4. [DOI] [PubMed] [Google Scholar]
- Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, Larson EB. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. 1999;53(7):1480–7. doi: 10.1212/wnl.53.7.1480. [DOI] [PubMed] [Google Scholar]
- Herman JP, Chen KC, Booze R, Landfield PW. Up-regulation of alpha1D Ca2+ channel subunit mRNA expression in the hippocampus of aged F344 rats. Neurobiol Aging. 1998;19(6):581–7. doi: 10.1016/s0197-4580(98)00099-2. [DOI] [PubMed] [Google Scholar]
- Hirai T, Kojima S, Shimada A, Umemura T, Sakai M, Itakura C. Age-related changes in the olfactory system of dogs. Neuropathol Appl Neurobiol. 1996;22(6):531–9. doi: 10.1111/j.1365-2990.1996.tb01132.x. [DOI] [PubMed] [Google Scholar]
- Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the “Sniffin’ Sticks” including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264(3):237–43. doi: 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- Kaneda H, Maeshima K, Goto N, Kobayakawa T, Ayabe-Kanamura S, Saito S. Decline in taste and odor discrimination abilities with age, and relationship between gustation and olfaction. Chem Senses. 2000;25(3):331–7. doi: 10.1093/chemse/25.3.331. [DOI] [PubMed] [Google Scholar]
- Kerr MA, Belluscio L. Olfactory experience accelerates glomerular refinement in the mammalian olfactory bulb. Nat Neurosci. 2006;9(4):484–6. doi: 10.1038/nn1673. [DOI] [PubMed] [Google Scholar]
- Lee AC, He J, Ma M. Olfactory marker protein is critical for functional maturation of olfactory sensory neurons and development of mother preference. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(8):2974–82. doi: 10.1523/JNEUROSCI.5067-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Tian H, Grosmaitre X, Ma M. Expression patterns of odorant receptors and response properties of olfactory sensory neurons in aged mice. Chem Senses. 2009;34(8):695–703. doi: 10.1093/chemse/bjp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo AT, Youngentob SL, Kent PF, Schwob JE. The aging olfactory epithelium: neurogenesis, response to damage, and odorant-induced activity. Int J Dev Neurosci. 1996;14(7–8):881–900. doi: 10.1016/s0736-5748(96)00046-9. [DOI] [PubMed] [Google Scholar]
- Lowry LD, Pribitkin EA. Collection of human olfactory tissue. In: Spielman A, editor. Experimental cell biology of taste and olfaction. CRC Press; Boca Raton: 1995. [Google Scholar]
- Maresh A, Rodriguez Gil D, Whitman MC, Greer CA. Principles of glomerular organization in the human olfactory bulb--implications for odor processing. PLoS One. 2008;3(7):e2640. doi: 10.1371/journal.pone.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes RD, Cowart BJ. Dietary assessment of patients with chemosensory disorders. J Am Diet Assoc. 1994;94(1):50–6. doi: 10.1016/0002-8223(94)92041-9. [DOI] [PubMed] [Google Scholar]
- Meisami E, Mikhail L, Baim D, Bhatnagar KP. Human olfactory bulb: aging of glomeruli and mitral cells and a search for the accessory olfactory bulb. Ann N Y Acad Sci. 1998;855:708–15. doi: 10.1111/j.1749-6632.1998.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Mirich JM, Williams NC, Berlau DJ, Brunjes PC. Comparative study of aging in the mouse olfactory bulb. J Comp Neurol. 2002;454(4):361–72. doi: 10.1002/cne.10426. [DOI] [PubMed] [Google Scholar]
- Mombaerts P. Axonal wiring in the mouse olfactory system. Annu Rev Cell Dev Biol. 2006;22:713–37. doi: 10.1146/annurev.cellbio.21.012804.093915. [DOI] [PubMed] [Google Scholar]
- Morrison EE, Costanzo RM. Morphology of the human olfactory epithelium. The Journal of comparative neurology. 1990;297(1):1–13. doi: 10.1002/cne.902970102. [DOI] [PubMed] [Google Scholar]
- Murphy C. The chemical senses and nutrition in older adults. J Nutr Elder. 2008;27(3–4):247–65. doi: 10.1080/01639360802261862. [DOI] [PubMed] [Google Scholar]
- Murphy C, Wetter S, Morgan CD, Ellison DW, Geisler MW. Age effects on central nervous system activity reflected in the olfactory event-related potential. Evidence for decline in middle age. Ann N Y Acad Sci. 1998;855:598–607. doi: 10.1111/j.1749-6632.1998.tb10630.x. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Kimmelman CP, Snow JB., Jr Structure of human fetal and adult olfactory neuroepithelium. Arch Otolaryngol. 1984;110(10):641–6. doi: 10.1001/archotol.1984.00800360013003. [DOI] [PubMed] [Google Scholar]
- Oliva AM, Jones KR, Restrepo D. Sensory-dependent asymmetry for a urine-responsive olfactory bulb glomerulus. J Comp Neurol. 2008;510(5):475–83. doi: 10.1002/cne.21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik SI, Lehman MN, Seiden AM, Duncan HJ, Smith DV. Human olfactory biopsy. The influence of age and receptor distribution. Arch Otolaryngol Head Neck Surg. 1992;118(7):731–8. doi: 10.1001/archotol.1992.01880070061012. [DOI] [PubMed] [Google Scholar]
- Rawson NE, Brand JG, Cowart BJ, Lowry LD, Pribitkin EA, Rao VM, Restrepo D. Functionally mature olfactory neurons from two anosmic patients with Kallmann syndrome. Brain Res. 1995;681(1–2):58–64. doi: 10.1016/0006-8993(95)00283-v. [DOI] [PubMed] [Google Scholar]
- Rawson NE, Gomez G, Cowart B, Brand JG, Lowry LD, Pribitkin EA, Restrepo D. Selectivity and response characteristics of human olfactory neurons. J Neurophysiol. 1997;77(3):1606–13. doi: 10.1152/jn.1997.77.3.1606. [DOI] [PubMed] [Google Scholar]
- Richard MB, Taylor SR, Greer CA. Age-induced disruption of selective olfactory bulb synaptic circuits. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1007931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls BJ. Do chemosensory changes influence food intake in the elderly? Physiology & behavior. 1999;66(2):193–7. doi: 10.1016/s0031-9384(98)00264-9. [DOI] [PubMed] [Google Scholar]
- Santos DV, Reiter ER, DiNardo LJ, Costanzo RM. Hazardous events associated with impaired olfactory function. Arch Otolaryngol Head Neck Surg. 2004;130(3):317–9. doi: 10.1001/archotol.130.3.317. [DOI] [PubMed] [Google Scholar]
- Schiffman SS. Taste and smell losses in normal aging and disease. JAMA. 1997;278(16):1357–62. [PubMed] [Google Scholar]
- Stevens JC, Cain WS. Aging and the perception of nasal irritation. Physiol Behav. 1986;37(2):323–8. doi: 10.1016/0031-9384(86)90241-6. [DOI] [PubMed] [Google Scholar]
- Sugawa M, May T. Age-related alteration in signal transduction: involvement of the cAMP cascade. Brain Res. 1993;618(1):57–62. doi: 10.1016/0006-8993(93)90428-p. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Ando S. Age-related changes in the subtypes of voltage-dependent calcium channels in rat brain cortical synapses. Neurosci Res. 2001;39(2):213–20. doi: 10.1016/s0168-0102(00)00212-1. [DOI] [PubMed] [Google Scholar]
- Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J Neurosci. 2001;21(24):9744–56. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler E, Farbman AI. Proliferation in the rat olfactory epithelium: age-dependent changes. J Neurosci. 1997;17(10):3610–22. doi: 10.1523/JNEUROSCI.17-10-03610.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64(7):802–8. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- Xiong J, Camello PJ, Verkhratsky A, Toescu EC. Mitochondrial polarisation status and [Ca2+]i signalling in rat cerebellar granule neurones aged in vitro. Neurobiol Aging. 2004;25(3):349–59. doi: 10.1016/S0197-4580(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167(4):661–72. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou DJ, Feinstein P, Rivers AL, Mathews GA, Kim A, Greer CA, Mombaerts P, Firestein S. Postnatal refinement of peripheral olfactory projections. Science. 2004;304(5679):1976–9. doi: 10.1126/science.1093468. [DOI] [PubMed] [Google Scholar]