Abstract
Multiple protein 4.1 isoforms are expressed in a variety of tissues through complex alternative pre-mRNA splicing events, one function of which is to regulate use of two alternative translation initiation signals. Late erythroid cells express mainly the downstream initiation site for synthesis of prototypical 80-kD isoforms; nonerythroid cells in addition use an upstream site to encode higher molecular mass isoform(s). In this study, we examined the effects of a 5' gene rearrangement in a family with hereditary elliptocytosis and complete deficiency of erythrocyte 4.1 protein on 4.1 isoform expression in erythroid vs. nonerythroid cells. Patient 4.1 mRNAs from reticulocytes, fibroblasts, and B lymphocytes were amplified by reverse transcriptase/polymerase chain reaction techniques and shown to exhibit a 318-nucleotide deletion that encompasses the downstream AUG, but leaves intact the upstream AUG. Immunoblot analysis revealed a total deficiency of 4.1 in patient red cells and a selective deficiency of 80-kD isoform(s) but not high molecular weight 4.1 in patient nonerythroid cells. Thus, the 4.1 gene mutation in this family produces an isoform-specific deficiency that is manifested clinically in tissue-specific fashion, such that red cells are affected but other cell types are unaffected because of tissue-specific differences in RNA splicing and translation initiation.
Full text
PDF
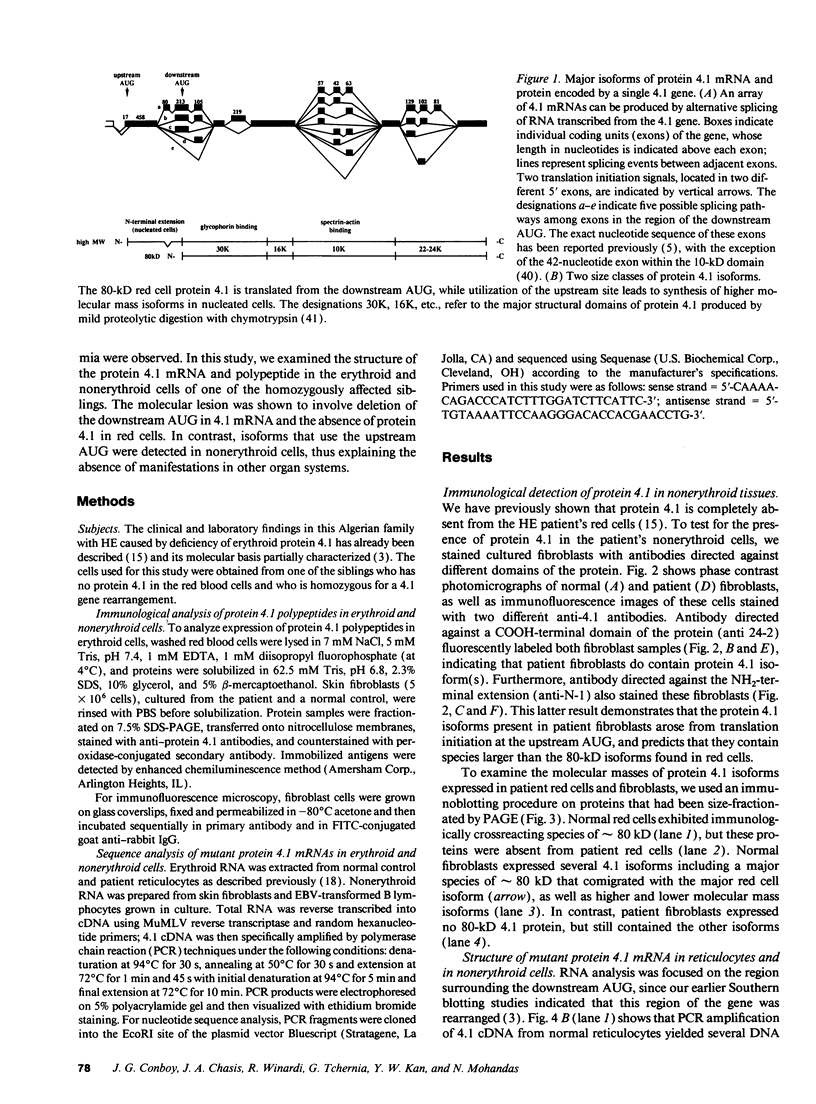
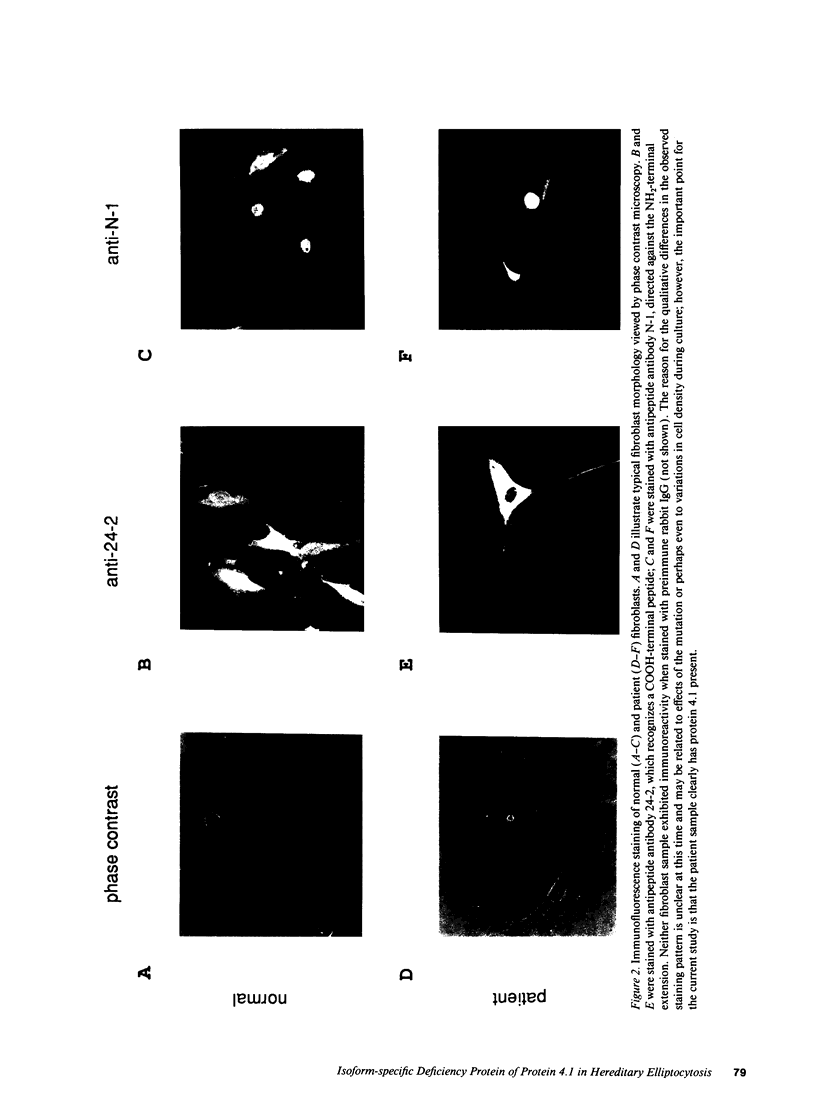
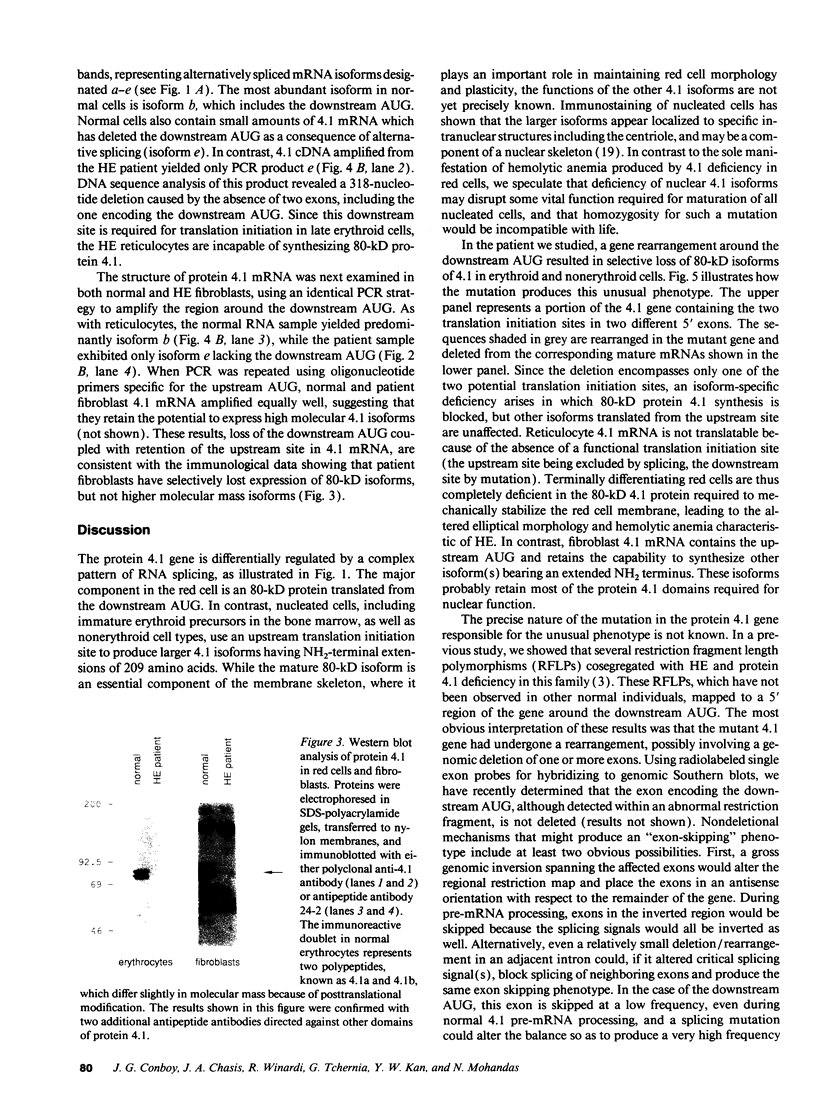


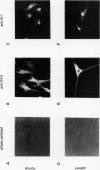
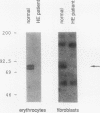
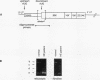
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alloisio N., Dorléac E., Delaunay J., Girot R., Galand C., Boivin P. A shortened variant of red cell membrane protein 4.1. Blood. 1982 Jul;60(1):265–267. [PubMed] [Google Scholar]
- Alloisio N., Morlé L., Dorléac E., Gentilhomme O., Bachir D., Guetarni D., Colonna P., Bost M., Zouaoui Z., Roda L. The heterozygous form of 4.1(-) hereditary elliptocytosis [the 4.1(-) trait]. Blood. 1985 Jan;65(1):46–51. [PubMed] [Google Scholar]
- Anderson R. A., Correas I., Mazzucco C., Castle J. D., Marchesi V. T. Tissue-specific analogues of erythrocyte protein 4.1 retain functional domains. J Cell Biochem. 1988 Jul;37(3):269–284. doi: 10.1002/jcb.240370303. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Nguyen H. T., Medford R. M., Destree A. T., Mahdavi V., Nadal-Ginard B. Intricate combinatorial patterns of exon splicing generate multiple regulated troponin T isoforms from a single gene. Cell. 1985 May;41(1):67–82. doi: 10.1016/0092-8674(85)90062-5. [DOI] [PubMed] [Google Scholar]
- Conboy J. G., Chan J. Y., Chasis J. A., Kan Y. W., Mohandas N. Tissue- and development-specific alternative RNA splicing regulates expression of multiple isoforms of erythroid membrane protein 4.1. J Biol Chem. 1991 May 5;266(13):8273–8280. [PubMed] [Google Scholar]
- Conboy J. G., Chan J., Mohandas N., Kan Y. W. Multiple protein 4.1 isoforms produced by alternative splicing in human erythroid cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9062–9065. doi: 10.1073/pnas.85.23.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy J. G., Shitamoto R., Parra M., Winardi R., Kabra A., Smith J., Mohandas N. Hereditary elliptocytosis due to both qualitative and quantitative defects in membrane skeletal protein 4.1. Blood. 1991 Nov 1;78(9):2438–2443. [PubMed] [Google Scholar]
- Conboy J., Marchesi S., Kim R., Agre P., Kan Y. W., Mohandas N. Molecular analysis of insertion/deletion mutations in protein 4.1 in elliptocytosis. II. Determination of molecular genetic origins of rearrangements. J Clin Invest. 1990 Aug;86(2):524–530. doi: 10.1172/JCI114739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy J., Mohandas N., Tchernia G., Kan Y. W. Molecular basis of hereditary elliptocytosis due to protein 4.1 deficiency. N Engl J Med. 1986 Sep 11;315(11):680–685. doi: 10.1056/NEJM198609113151105. [DOI] [PubMed] [Google Scholar]
- Cox J. V., Lazarides E. Alternative primary structures in the transmembrane domain of the chicken erythroid anion transporter. Mol Cell Biol. 1988 Mar;8(3):1327–1335. doi: 10.1128/mcb.8.3.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta P., Reddy E. P. Identification of alternatively spliced transcripts for human c-myb: molecular cloning and sequence analysis of human c-myb exon 9A sequences. Oncogene. 1989 Dec;4(12):1419–1423. [PubMed] [Google Scholar]
- Davies K. A., Lux S. E. Hereditary disorders of the red cell membrane skeleton. Trends Genet. 1989 Jul;5(7):222–227. doi: 10.1016/0168-9525(89)90086-3. [DOI] [PubMed] [Google Scholar]
- Feddal S., Brunet G., Roda L., Chabanis S., Alloisio N., Morlé L., Ducluzeau M. T., Maréchal J., Robert J. M., Benz E. J., Jr Molecular analysis of hereditary elliptocytosis with reduced protein 4.1 in the French Northern Alps. Blood. 1991 Oct 15;78(8):2113–2119. [PubMed] [Google Scholar]
- Grandchamp B., De Verneuil H., Beaumont C., Chretien S., Walter O., Nordmann Y. Tissue-specific expression of porphobilinogen deaminase. Two isoenzymes from a single gene. Eur J Biochem. 1987 Jan 2;162(1):105–110. doi: 10.1111/j.1432-1033.1987.tb10548.x. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., Kauppinen R., Mignotte V., Peltonen L., Mustajoki P., Roméo P. H., Goossens M., Nordmann Y. Molecular analysis of acute intermittent porphyria in a Finnish family with normal erythrocyte porphobilinogen deaminase. Eur J Clin Invest. 1989 Oct;19(5):415–418. doi: 10.1111/j.1365-2362.1989.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Kordeli E., Bennett V. Distinct ankyrin isoforms at neuron cell bodies and nodes of Ranvier resolved using erythrocyte ankyrin-deficient mice. J Cell Biol. 1991 Sep;114(6):1243–1259. doi: 10.1083/jcb.114.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert S., Conboy J., Zail S. A molecular study of heterozygous protein 4.1 deficiency in hereditary elliptocytosis. Blood. 1988 Dec;72(6):1926–1929. [PubMed] [Google Scholar]
- Lambert S., Yu H., Prchal J. T., Lawler J., Ruff P., Speicher D., Cheung M. C., Kan Y. W., Palek J. cDNA sequence for human erythrocyte ankyrin. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1730–1734. doi: 10.1073/pnas.87.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Kim C., Mitjavila M. T., Clerget M., Cartron J. P., Colin Y. An ubiquitous isoform of glycophorin C is produced by alternative splicing. Nucleic Acids Res. 1990 May 25;18(10):3076–3076. doi: 10.1093/nar/18.10.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto T. L., Marchesi V. T. A structural model of human erythrocyte protein 4.1. J Biol Chem. 1984 Apr 10;259(7):4603–4608. [PubMed] [Google Scholar]
- Lux S. E., John K. M., Bennett V. Analysis of cDNA for human erythrocyte ankyrin indicates a repeated structure with homology to tissue-differentiation and cell-cycle control proteins. Nature. 1990 Mar 1;344(6261):36–42. doi: 10.1038/344036a0. [DOI] [PubMed] [Google Scholar]
- Marchesi S. L., Conboy J., Agre P., Letsinger J. T., Marchesi V. T., Speicher D. W., Mohandas N. Molecular analysis of insertion/deletion mutations in protein 4.1 in elliptocytosis. I. Biochemical identification of rearrangements in the spectrin/actin binding domain and functional characterizations. J Clin Invest. 1990 Aug;86(2):516–523. doi: 10.1172/JCI114738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire M., Smith B. L., Agre P. Distinct variants of erythrocyte protein 4.1 inherited in linkage with elliptocytosis and Rh type in three white families. Blood. 1988 Jul;72(1):287–293. [PubMed] [Google Scholar]
- Morlé L., Garbarz M., Alloisio N., Girot R., Chaveroche I., Boivin P., Delaunay J. The characterization of protein 4.1 Presles, a shortened variant of RBC membrane protein 4.1. Blood. 1985 Jun;65(6):1511–1517. [PubMed] [Google Scholar]
- Otto E., Kunimoto M., McLaughlin T., Bennett V. Isolation and characterization of cDNAs encoding human brain ankyrins reveal a family of alternatively spliced genes. J Cell Biol. 1991 Jul;114(2):241–253. doi: 10.1083/jcb.114.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters L. L., Birkenmeier C. S., Bronson R. T., White R. A., Lux S. E., Otto E., Bennett V., Higgins A., Barker J. E. Purkinje cell degeneration associated with erythroid ankyrin deficiency in nb/nb mice. J Cell Biol. 1991 Sep;114(6):1233–1241. doi: 10.1083/jcb.114.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Skurla R. M., Jr, Owens J. D., Mushinski J. F. Alternative splicing of RNAs transcribed from the human c-myb gene. Mol Cell Biol. 1990 Jun;10(6):2715–2722. doi: 10.1128/mcb.10.6.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung L. A., Chien S., Chang L. S., Lambert K., Bliss S. A., Bouhassira E. E., Nagel R. L., Schwartz R. S., Rybicki A. C. Molecular cloning of human protein 4.2: a major component of the erythrocyte membrane. Proc Natl Acad Sci U S A. 1990 Feb;87(3):955–959. doi: 10.1073/pnas.87.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura R. N., Cooper H. M., Collo G., Quaranta V. Cell type-specific integrin variants with alternative alpha chain cytoplasmic domains. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10183–10187. doi: 10.1073/pnas.88.22.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. K., Leto T. L., Correas I., Alonso M. A., Marchesi V. T., Benz E. J., Jr Selective expression of an erythroid-specific isoform of protein 4.1. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3713–3717. doi: 10.1073/pnas.85.11.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. K., Qin Z., Leto T., Marchesi V. T., Benz E. J., Jr Heterogeneity of mRNA and protein products arising from the protein 4.1 gene in erythroid and nonerythroid tissues. J Cell Biol. 1990 Mar;110(3):617–624. doi: 10.1083/jcb.110.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernia G., Mohandas N., Shohet S. B. Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability. J Clin Invest. 1981 Aug;68(2):454–460. doi: 10.1172/JCI110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple G. F., Chang J. C., Kan Y. W. Authentic beta-globin mRNA sequences in homozygous betaO-thalassemia. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3047–3051. doi: 10.1073/pnas.74.7.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Duan D. S., de Vries C., Peters K. G., Johnson D. E., Williams L. T. Differential splicing in the extracellular region of fibroblast growth factor receptor 1 generates receptor variants with different ligand-binding specificities. Mol Cell Biol. 1992 Jan;12(1):82–88. doi: 10.1128/mcb.12.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Gorse K. M., Clarke M. F. Alternative splicing of the human c-myb gene. Oncogene. 1990 Aug;5(8):1117–1124. [PubMed] [Google Scholar]
- Winkelmann J. C., Costa F. F., Linzie B. L., Forget B. G. Beta spectrin in human skeletal muscle. Tissue-specific differential processing of 3' beta spectrin pre-mRNA generates a beta spectrin isoform with a unique carboxyl terminus. J Biol Chem. 1990 Nov 25;265(33):20449–20454. [PubMed] [Google Scholar]