Abstract
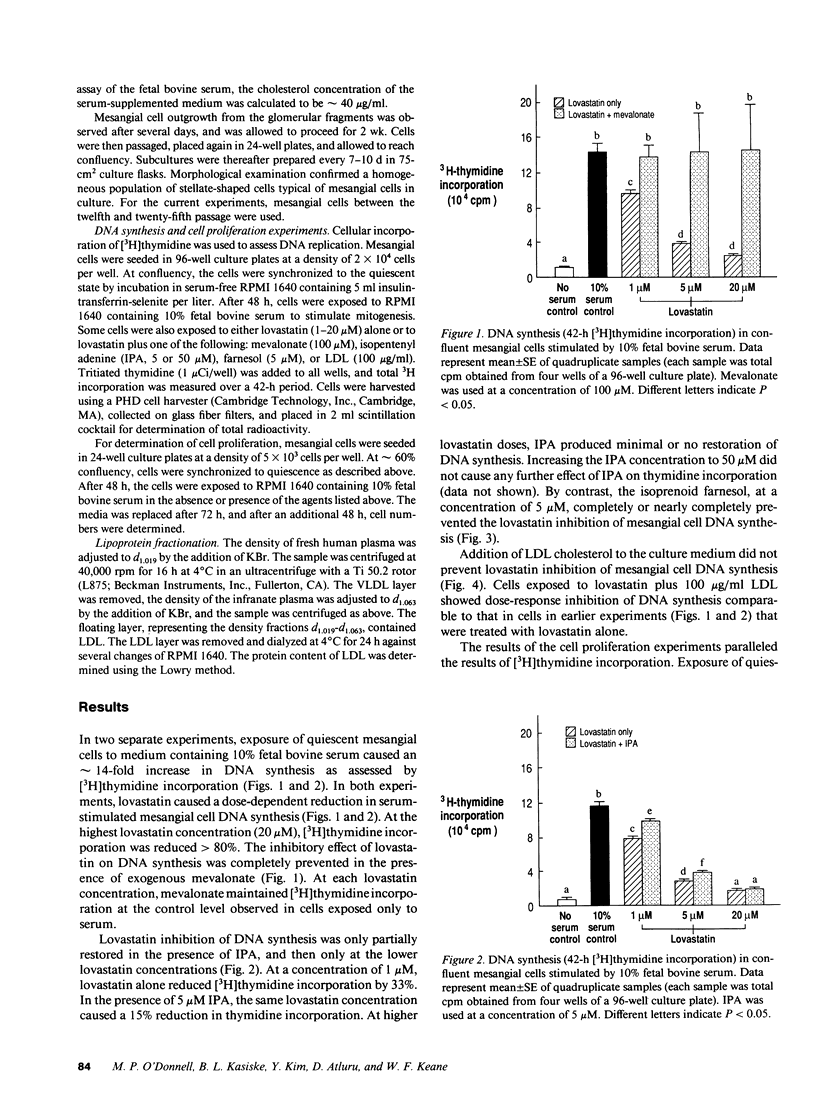
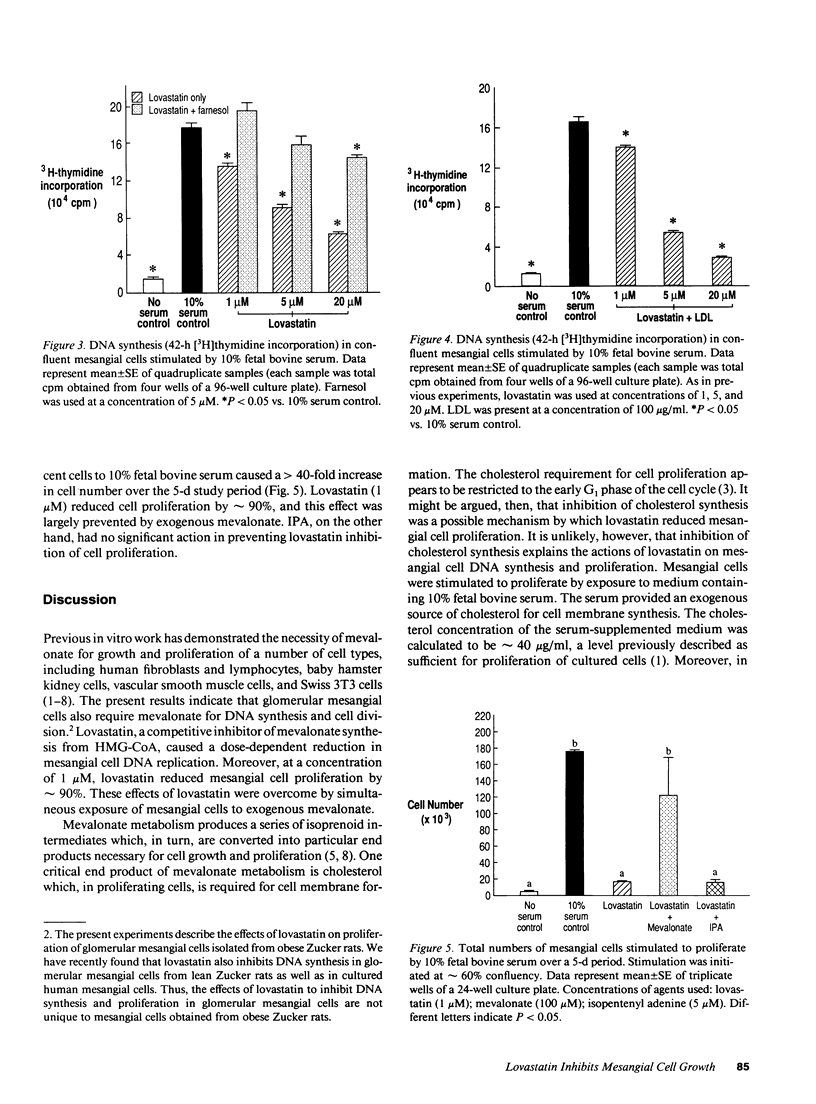
Products of intracellular mevalonate metabolism are essential for cell growth and proliferation. Lovastatin, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, blocks the formation of mevalonate and its metabolites, and has been shown to inhibit proliferation of several cell types. In vivo, lovastatin has reduced mesangial cellularity and glomerular injury in experimental renal disease. In this study, we investigated the effects of lovastatin on DNA replication and proliferation in rat glomerular mesangial cells. Growth-arrested mesangial cells were exposed to medium containing 10% fetal bovine serum to stimulate mitogenesis. Lovastatin (1-20 microM) caused a significant (P < 0.05) dose-dependent reduction in DNA synthesis ([3H]thymidine incorporation) which was completely prevented in the presence of exogenous mevalonate (100 microM). Lovastatin (1 microM) inhibited cell proliferation by 90% over a 5-d period, and this was largely overcome by added mevalonate. Exogenous low density lipoprotein (100 micrograms/ml) did not prevent lovastatin inhibition of DNA synthesis. The isoprenoid end product isopentenyl adenine (5 or 50 microM) had little effect on DNA synthesis and cell proliferation in lovastatin-blocked cells. By contrast, the isoprenoid farnesol (5 microM) largely prevented lovastatin inhibition of DNA synthesis. We conclude that mevalonate metabolism is essential for mesangial cell proliferation, possibly through the production of the isoprenoid farnesol. Moreover, the action of lovastatin to reduce experimental glomerular injury may involve a direct effect on mesangial cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casey P. J., Solski P. A., Der C. J., Buss J. E. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8323–8327. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutts J. L., Scallen T. J., Watson J., Bankhurst A. D. Role of mevalonic acid in the regulation of natural killer cell cytotoxicity. J Cell Physiol. 1989 Jun;139(3):550–557. doi: 10.1002/jcp.1041390314. [DOI] [PubMed] [Google Scholar]
- Fairbanks K. P., Witte L. D., Goodman D. S. Relationship between mevalonate and mitogenesis in human fibroblasts stimulated with platelet-derived growth factor. J Biol Chem. 1984 Feb 10;259(3):1546–1551. [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., Ross R. Relation of cholesterol and mevalonic acid to the cell cycle in smooth muscle and swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1980 Jun 10;255(11):5134–5140. [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E., Marshall C. J. All ras proteins are polyisoprenylated but only some are palmitoylated. Cell. 1989 Jun 30;57(7):1167–1177. doi: 10.1016/0092-8674(89)90054-8. [DOI] [PubMed] [Google Scholar]
- Harper P. A., Robinson J. M., Hoover R. L., Wright T. C., Karnovsky M. J. Improved methods for culturing rat glomerular cells. Kidney Int. 1984 Dec;26(6):875–880. doi: 10.1038/ki.1984.231. [DOI] [PubMed] [Google Scholar]
- Jackson J. H., Cochrane C. G., Bourne J. R., Solski P. A., Buss J. E., Der C. J. Farnesol modification of Kirsten-ras exon 4B protein is essential for transformation. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3042–3046. doi: 10.1073/pnas.87.8.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasiske B. L., O'Donnell M. P., Cleary M. P., Keane W. F. Treatment of hyperlipidemia reduces glomerular injury in obese Zucker rats. Kidney Int. 1988 Mar;33(3):667–672. doi: 10.1038/ki.1988.51. [DOI] [PubMed] [Google Scholar]
- Kasiske B. L., O'Donnell M. P., Garvis W. J., Keane W. F. Pharmacologic treatment of hyperlipidemia reduces glomerular injury in rat 5/6 nephrectomy model of chronic renal failure. Circ Res. 1988 Feb;62(2):367–374. doi: 10.1161/01.res.62.2.367. [DOI] [PubMed] [Google Scholar]
- Kita T., Brown M. S., Goldstein J. L. Feedback regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in livers of mice treated with mevinolin, a competitive inhibitor of the reductase. J Clin Invest. 1980 Nov;66(5):1094–1100. doi: 10.1172/JCI109938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltese W. A., Sheridan K. M. Differentiation of neuroblastoma cells induced by an inhibitor of mevalonate synthesis: relation of neurite outgrowth and acetylcholinesterase activity to changes in cell proliferation and blocked isoprenoid synthesis. J Cell Physiol. 1985 Dec;125(3):540–558. doi: 10.1002/jcp.1041250326. [DOI] [PubMed] [Google Scholar]
- Maltese W. A., Sheridan K. M., Repko E. M., Erdman R. A. Post-translational modification of low molecular mass GTP-binding proteins by isoprenoid. J Biol Chem. 1990 Feb 5;265(4):2148–2155. [PubMed] [Google Scholar]
- Mendola C. E., Backer J. M. Lovastatin blocks N-ras oncogene-induced neuronal differentiation. Cell Growth Differ. 1990 Oct;1(10):499–502. [PubMed] [Google Scholar]
- Mené P., Dunn M. J. Contractile effects of TxA2 and endoperoxide analogues on cultured rat glomerular mesangial cells. Am J Physiol. 1986 Dec;251(6 Pt 2):F1029–F1035. doi: 10.1152/ajprenal.1986.251.6.F1029. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- O'Donnell M. P., Kasiske B. L., Katz S. A., Schmitz P. G., Keane W. F. Lovastatin but not enalapril reduces glomerular injury in Dahl salt-sensitive rats. Hypertension. 1992 Nov;20(5):651–658. doi: 10.1161/01.hyp.20.5.651. [DOI] [PubMed] [Google Scholar]
- Pan H. Y., DeVault A. R., Wang-Iverson D., Ivashkiv E., Swanson B. N., Sugerman A. A. Comparative pharmacokinetics and pharmacodynamics of pravastatin and lovastatin. J Clin Pharmacol. 1990 Dec;30(12):1128–1135. doi: 10.1002/j.1552-4604.1990.tb01856.x. [DOI] [PubMed] [Google Scholar]
- Quesney-Huneeus V., Galick H. A., Siperstein M. D., Erickson S. K., Spencer T. A., Nelson J. A. The dual role of mevalonate in the cell cycle. J Biol Chem. 1983 Jan 10;258(1):378–385. [PubMed] [Google Scholar]
- Quesney-Huneeus V., Wiley M. H., Siperstein M. D. Essential role for mevalonate synthesis in DNA replication. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5056–5060. doi: 10.1073/pnas.76.10.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E., Nebreda A. R. Structural and functional properties of ras proteins. FASEB J. 1989 Aug;3(10):2151–2163. doi: 10.1096/fasebj.3.10.2666231. [DOI] [PubMed] [Google Scholar]
- Schlondorff D., DeCandido S., Satriano J. A. Angiotensin II stimulates phospholipases C and A2 in cultured rat mesangial cells. Am J Physiol. 1987 Jul;253(1 Pt 1):C113–C120. doi: 10.1152/ajpcell.1987.253.1.C113. [DOI] [PubMed] [Google Scholar]
- Siperstein M. D. Role of cholesterogenesis and isoprenoid synthesis in DNA replication and cell growth. J Lipid Res. 1984 Dec 15;25(13):1462–1468. [PubMed] [Google Scholar]


