Abstract
Thrombin has been implicated in the stimulation of smooth muscle cell (SMC) proliferation that contributes to post angioplasty restenosis. The present studies demonstrated that human alpha-thrombin was a potent and efficacious mitogen for cultured rat aortic SMC, stimulating an increase in 3H-thymidine incorporation, as well as an increase in cell number at 1 to 10 nM concentration. gamma-Thrombin, which is enzymatically active but lacks fibrinogen clotting activity, stimulated SMC mitogenesis but was approximately 10-fold less potent than alpha-thrombin. In contrast, D-phenylalanyl-L-propyl-L-arginyl-chloromethyl ketone-alpha-thrombin, which lacked enzymatic activity, had no mitogenic effect. Diisopropylfluorophosphate-alpha-thrombin failed to stimulate mitogenesis except at concentrations having equivalent enzymatic activity as that of alpha-thrombin at its threshold for mitogenesis. Thus, thrombin-induced proliferation was dependent on enzymatic activity. A 14-residue peptide (SFLLRNPNDKYEPF) corresponding to amino acids 42 through 55 of the human thrombin receptor (Vu, T. K., D. T. Hung, V. I. Wheaton, and S. R. Coughlin, 1991. Cell. 64:1057-1068) had full efficacy in stimulating SMC proliferation. Reversing the first two amino acids of this peptide abolished mitogenic activity. Northern analysis demonstrated that SMC expressed a single mRNA species that hybridized to a labeled thrombin receptor cDNA probe. These findings indicate that alpha-thrombin stimulates SMC proliferation via the proteolytic activation of a receptor very similar or identical to that previously identified.
Full text
PDF
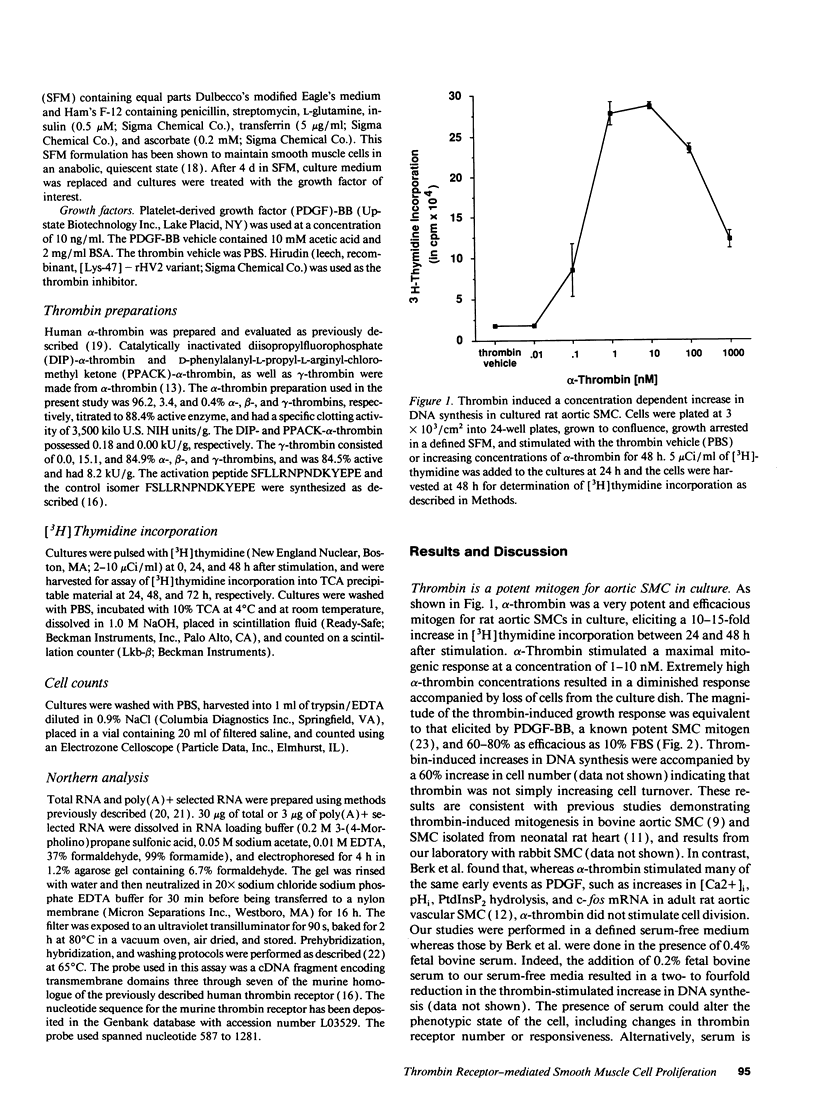
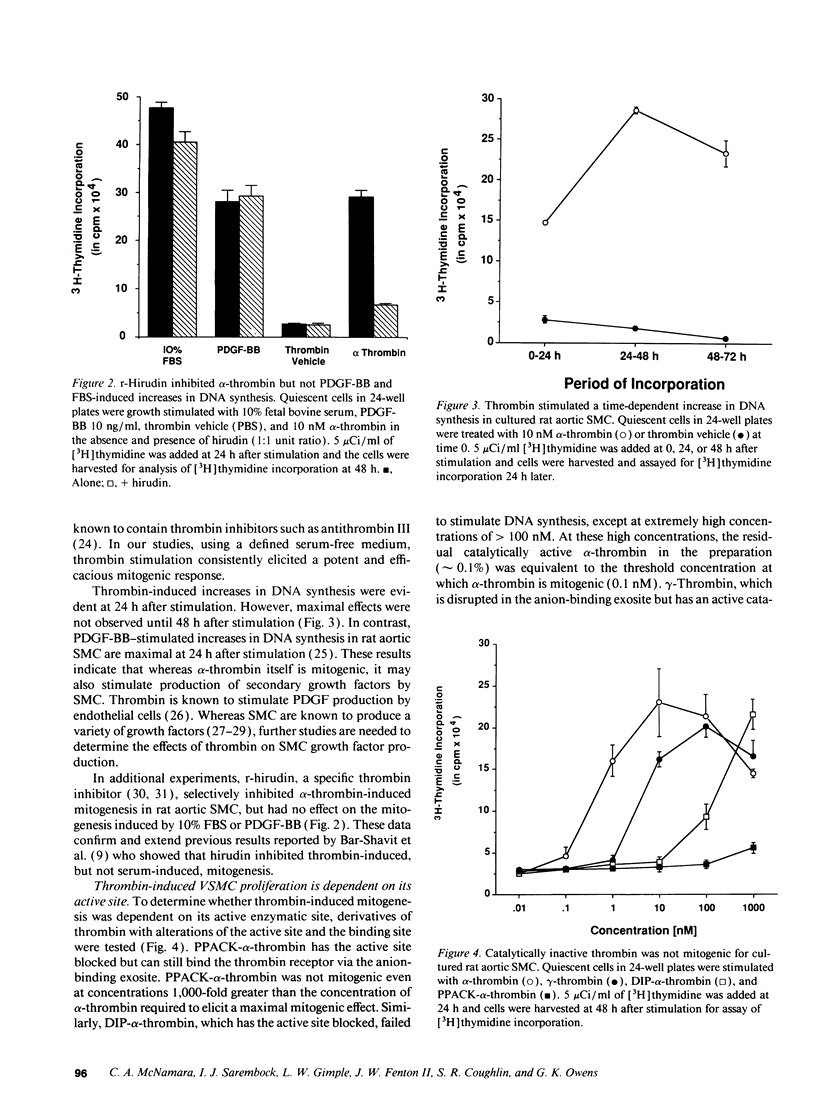
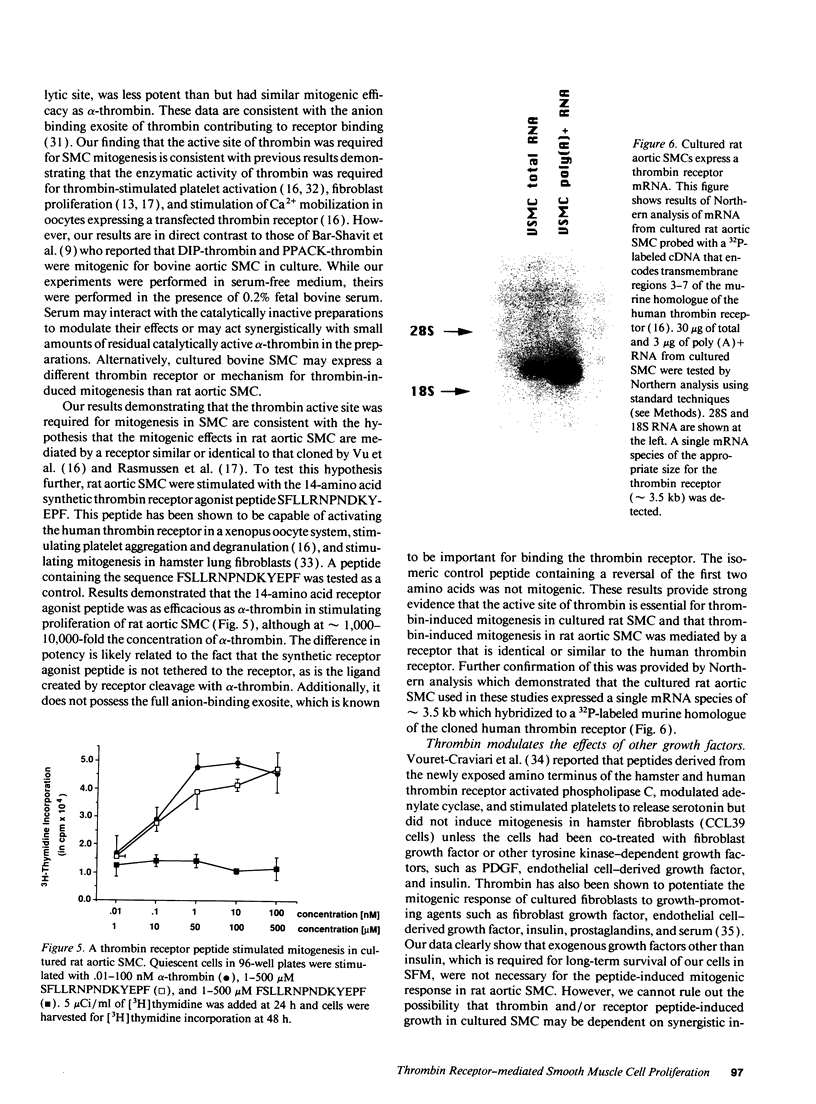

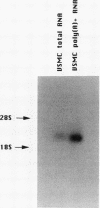
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit R., Benezra M., Eldor A., Hy-Am E., Fenton J. W., 2nd, Wilner G. D., Vlodavsky I. Thrombin immobilized to extracellular matrix is a potent mitogen for vascular smooth muscle cells: nonenzymatic mode of action. Cell Regul. 1990 May;1(6):453–463. doi: 10.1091/mbc.1.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk B. C., Taubman M. B., Griendling K. K., Cragoe E. J., Jr, Fenton J. W., Brock T. A. Thrombin-stimulated events in cultured vascular smooth-muscle cells. Biochem J. 1991 Mar 15;274(Pt 3):799–805. doi: 10.1042/bj2740799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank R. S., Owens G. K. Platelet-derived growth factor regulates actin isoform expression and growth state in cultured rat aortic smooth muscle cells. J Cell Physiol. 1990 Mar;142(3):635–642. doi: 10.1002/jcp.1041420325. [DOI] [PubMed] [Google Scholar]
- Brown A., Colen A. H., Fisher H. F. Effect of ammonia on the glutamate dehydrogenase catalyzed oxidative deamination of L-glutamate. The steady state. Biochemistry. 1979 Dec 25;18(26):5924–5928. doi: 10.1021/bi00593a025. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey M. G., Lüscher E. F. Actions of thrombin and other coagulant and proteolytic enzymes on blood platelets. Nature. 1967 Dec 2;216(5118):857–858. doi: 10.1038/216857a0. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Fenton J. W., 2nd Thrombin interactions with hirudin. Semin Thromb Hemost. 1989 Jul;15(3):265–268. doi: 10.1055/s-2007-1002718. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Villanueva G. B., Ofosu F. A., Maraganore J. M. Thrombin inhibition by hirudin: how hirudin inhibits thrombin. Haemostasis. 1991;21 (Suppl 1):27–31. doi: 10.1159/000216259. [DOI] [PubMed] [Google Scholar]
- Glenn K. C., Carney D. H., Fenton J. W., 2nd, Cunningham D. D. Thrombin active site regions required for fibroblast receptor binding and initiation of cell division. J Biol Chem. 1980 Jul 25;255(14):6609–6616. [PubMed] [Google Scholar]
- Graham D. J., Alexander J. J. The effects of thrombin on bovine aortic endothelial and smooth muscle cells. J Vasc Surg. 1990 Feb;11(2):307–313. doi: 10.1067/mva.1990.17098. [DOI] [PubMed] [Google Scholar]
- Gravanis M. B., Roubin G. S. Histopathologic phenomena at the site of percutaneous transluminal coronary angioplasty: the problem of restenosis. Hum Pathol. 1989 May;20(5):477–485. doi: 10.1016/0046-8177(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Hanke H., Strohschneider T., Oberhoff M., Betz E., Karsch K. R. Time course of smooth muscle cell proliferation in the intima and media of arteries following experimental angioplasty. Circ Res. 1990 Sep;67(3):651–659. doi: 10.1161/01.res.67.3.651. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Thompson P. J., Ross R. R., Bowen-Pope D. F. Alpha-thrombin induces release of platelet-derived growth factor-like molecule(s) by cultured human endothelial cells. J Cell Biol. 1986 Sep;103(3):1129–1133. doi: 10.1083/jcb.103.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. L., Ives H. E. Growth inhibition by protein kinase C late in mitogenesis. 1987 Oct 29-Nov 4Nature. 329(6142):849–850. doi: 10.1038/329849a0. [DOI] [PubMed] [Google Scholar]
- Hung D. T., Vu T. H., Nelken N. A., Coughlin S. R. Thrombin-induced events in non-platelet cells are mediated by the unique proteolytic mechanism established for the cloned platelet thrombin receptor. J Cell Biol. 1992 Feb;116(3):827–832. doi: 10.1083/jcb.116.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent K. M., Bentivoglio L. G., Block P. C., Bourassa M. G., Cowley M. J., Dorros G., Detre K. M., Gosselin A. J., Gruentzig A. R., Kelsey S. F. Long-term efficacy of percutaneous transluminal coronary angioplasty (PTCA): report from the National Heart, Lung, and Blood Institute PTCA Registry. Am J Cardiol. 1984 Jun 15;53(12):27C–31C. doi: 10.1016/0002-9149(84)90741-0. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki N., Kanzaki T., Koshikawa T., Saito Y., Yoshida S. Secretion of a new growth factor, smooth muscle cell derived growth factor, distinct from platelet derived growth factor by cultured rabbit aortic smooth muscle cells. FEBS Lett. 1988 Mar 28;230(1-2):186–190. doi: 10.1016/0014-5793(88)80668-9. [DOI] [PubMed] [Google Scholar]
- Nobuyoshi M., Kimura T., Ohishi H., Horiuchi H., Nosaka H., Hamasaki N., Yokoi H., Kim K. Restenosis after percutaneous transluminal coronary angioplasty: pathologic observations in 20 patients. J Am Coll Cardiol. 1991 Feb;17(2):433–439. doi: 10.1016/s0735-1097(10)80111-1. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Loeb A., Gordon D., Thompson M. M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986 Feb;102(2):343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjanpelto P. Stimulation of DNA synthesis in human fibroblasts by thrombin. J Cell Physiol. 1978 May;95(2):189–194. doi: 10.1002/jcp.1040950208. [DOI] [PubMed] [Google Scholar]
- Rasmussen U. B., Vouret-Craviari V., Jallat S., Schlesinger Y., Pagès G., Pavirani A., Lecocq J. P., Pouysségur J., Van Obberghen-Schilling E. cDNA cloning and expression of a hamster alpha-thrombin receptor coupled to Ca2+ mobilization. FEBS Lett. 1991 Aug 19;288(1-2):123–128. doi: 10.1016/0014-5793(91)81017-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Rosenberg J. S. Natural anticoagulant mechanisms. J Clin Invest. 1984 Jul;74(1):1–6. doi: 10.1172/JCI111389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarembock I. J., Gertz S. D., Gimple L. W., Owen R. M., Powers E. R., Roberts W. C. Effectiveness of recombinant desulphatohirudin in reducing restenosis after balloon angioplasty of atherosclerotic femoral arteries in rabbits. Circulation. 1991 Jul;84(1):232–243. doi: 10.1161/01.cir.84.1.232. [DOI] [PubMed] [Google Scholar]
- Sejersen T., Betsholtz C., Sjölund M., Heldin C. H., Westermark B., Thyberg J. Rat skeletal myoblasts and arterial smooth muscle cells express the gene for the A chain but not the gene for the B chain (c-sis) of platelet-derived growth factor (PDGF) and produce a PDGF-like protein. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6844–6848. doi: 10.1073/pnas.83.18.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele P. M., Chesebro J. H., Stanson A. W., Holmes D. R., Jr, Dewanjee M. K., Badimon L., Fuster V. Balloon angioplasty. Natural history of the pathophysiological response to injury in a pig model. Circ Res. 1985 Jul;57(1):105–112. doi: 10.1161/01.res.57.1.105. [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari V., Van Obberghen-Schilling E., Rasmussen U. B., Pavirani A., Lecocq J. P., Pouysségur J. Synthetic alpha-thrombin receptor peptides activate G protein-coupled signaling pathways but are unable to induce mitogenesis. Mol Biol Cell. 1992 Jan;3(1):95–102. doi: 10.1091/mbc.3.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Walz D. A., Anderson G. F., Ciaglowski R. E., Aiken M., Fenton J. W., 2nd Thrombin-elicited contractile responses of aortic smooth muscle. Proc Soc Exp Biol Med. 1985 Dec;180(3):518–526. doi: 10.3181/00379727-180-42211. [DOI] [PubMed] [Google Scholar]
- Wilner G. D., Danitz M. P., Mudd M. S., Hsieh K. H., Fenton J. W., 2nd Selective immobilization of alpha-thrombin by surface-bound fibrin. J Lab Clin Med. 1981 Mar;97(3):403–411. [PubMed] [Google Scholar]
- Zetter B. R., Sun T. T., Chen L. B., Buchanan J. M. Thrombin potentiates the mitogenic response of cultured fibroblasts to serum and other growth promoting agents. J Cell Physiol. 1977 Aug;92(2):233–239. doi: 10.1002/jcp.1040920211. [DOI] [PubMed] [Google Scholar]