Abstract
Telomeres, the ends of linear chromosomes, safeguard against genome instability. The enzyme responsible for extension of the telomere 3′ terminus is the ribonucleoprotein telomerase. Whereas telomerase activity can be reconstituted in vitro with only the telomerase RNA (hTR) and telomerase reverse transcriptase (TERT), additional components are required in vivo for enzyme assembly, stability and telomere extension activity. One such associated protein, dyskerin, promotes hTR stability in vivo and is the only component to co-purify with active, endogenous human telomerase. We used oligonucleotide-based affinity purification of hTR followed by native gel electrophoresis and in-gel telomerase activity detection to query the composition of telomerase at different purification stringencies. At low salt concentrations (0.1 M NaCl), affinity-purified telomerase was ‘supershifted’ with an anti-dyskerin antibody, however the association with dyskerin was lost after purification at 0.6 M NaCl, despite the retention of telomerase activity and a comparable yield of hTR. The interaction of purified hTR and dyskerin in vitro displayed a similar salt-sensitive interaction. These results demonstrate that endogenous human telomerase, once assembled and active, does not require dyskerin for catalytic activity. Native gel electrophoresis may prove useful in the characterization of telomerase complexes under various physiological conditions.
INTRODUCTION
In eukaryotic cells linear chromosome ends are protected by telomeres, a repetitive tract of G-rich DNA recognized and stabilized by protein complexes such as shelterin, which execute telomere-specific and more generalized functions in suppressing a DNA damage response (1). In all but a few eukaryotic species the enzyme responsible for the maintenance of telomere length is telomerase, a ribonucleoprotein that extends the 3′ G-rich terminal overhang co-ordinately with C-strand replication by conventional DNA polymerases (2). In vitro, it is possible to reconstitute human, yeast and ciliate telomerase activities in crude rabbit reticulocyte extracts by expressing only the telomerase RNA (TER, or hTR in humans) and the telomerase reverse transcriptase (TERT) (3–7). This crude extract supplies the chaperones essential for the assembly of these two components into an active complex (7–9). Thus, the telomerase core enzyme consists minimally of TERT and TER.
The characterization of endogenous telomerase has been hampered by its extremely low abundance, even in organisms replete with telomeres such as ciliates (10). One solution to this challenge has been the expression and purification of tagged telomerase components, which further identified several proteins important in telomerase assembly and regulation (11–16). Native human telomerase purified from cell extracts varies in mass from 550 kDa to 1 MDa, supporting the notion of telomerase subunit multimerization or the presence of additional components (17–19). One successful approach for the purification of endogenous telomerase employs oligonucleotides that bind the telomerase RNA with high affinity, first described in the characterization of the telomerase RNA itself (20) and later used to purify telomerase from ciliates and humans (18,19,21–23). For example, in Euplotes crassus the La ortholog p43 is tightly associated with affinity-purified telomerase and facilitates processive telomere extension in vitro and in vivo (24–26). In another large-scale purification, endogenous human telomerase was isolated from 109 immortalized human cells and contained just three subunits: hTERT, hTR and dyskerin (18). Thus, akin to other large DNA replication machineries there is a core enzyme consisting of relatively few components augmented by various additional components that regulate assembly, localization and activity (11–16).
Processing of the telomerase RNA is highly regulated and differs between species. In mammals, the pseudouridylase dyskerin is required for maturation of snoRNAs and for the stability of the telomerase RNA in vivo (27,28). hTR, like other members of the snoRNA family, possesses an H/ACA box, and although it has not yet been reported as a target for pseudouridylation in vivo, it binds the other pseudouridylase accessory factors NOP10, NHP2 and GAR1 (28,29). Unlike other snoRNAs that are generated from introns (30), hTR is an intron-less RNA that is transcribed by RNA pol II (31,32). hTR also contains a CAB box that is required for localization to Cajal bodies (33,34). hTR distribution is regulated among nucleoplasmic, nucleolar and Cajal body compartments, and several RNA processing pathways appear involved in its maturation into an active telomerase RNP (33–36). In Saccharomyces cerevisiae, processing of the telomerase RNA TLC1 occurs between the cytoplasm and nucleus, and is regulated by Crm1p and Mtr10p (37,38). Sm proteins, which play a critical role in the biogenesis, transport and function of snRNP particles, associate with human telomerase (39) and serve to stabilize TLC1 in S. cerevisiae (40). Despite the absence of introns in the telomerase RNA gene of S. pombe (ter1+), the spliceosome is nonetheless critical for TER1 3′ processing (41). The telomerase RNA thus employs various RNA processing pathways that facilitate its localization, stability and assembly into active telomerase.
Mutations that affect the stability, activity or telomere recruitment of telomerase have a profound impact on stem cell function and lifespan in mammals. Mutations in dyskerin (DKC1) are associated with X-linked dyskeratosis congenita (DKC); in addition to dyskerin, some patients affected with autosomal DKC carry mutations in NOP10, NHP2, Terc (hTR), TERT, the telomerase-associated protein TCAB1 (WRAP53) and shelterin components such as TIN2 (TINF2) (42–44). Telomere attrition as a result of reduced telomerase activity appears to account for many of the manifestations of this disease, and mutations in Terc and Tert, are also linked to aplastic anaemia, pulmonary lung fibrosis and cancer (45–47). In fact, the co-incidence of aplastic anaemia and pulmonary fibrosis in patient families is highly predictive of a telomerase mutation (48). The critical role of hTR in human disease is also underscored by the ability of hTR upregulation or enforced expression to rescue the proliferative and telomere maintenance defects in patient-derived cells carrying mutations in hTR or dyskerin (49–51). DKC-like phenotypes are recapitulated in mice with limiting levels of TR (52,53), especially when combined with a Pot1 deficiency (54,55). Even in a wild-type murine background, short telomeres confer a DKC-like phenotype (53,56). Likely due to profound effects on all H/ACA-containing RNAs, mice deficient in dyskerin (Dkc1) exhibit early embryonic lethality (57). Thus, genetic evidence in mice and humans demonstrates unequivocally that critically short telomeres are pathological.
Improvements in the detection of endogenous human telomerase would further illuminate the regulation of telomerase assembly and composition in normal and diseased states. Native gel electrophoresis and the electromobility shift assay (EMSA) have been used previously to detect the incorporation of exogenously synthesized hTR into partially purified human telomerase (58) and to probe the association of telomerase with the telomerase RNA-binding proteins p80 and TEP1 (59–61). EMSA has also been employed to assess the association of endogenous, purified ciliate telomerase (21) and of recombinant hTERT fragments (62) with telomeric DNA. Here, we used native gel electrophoresis to assess the mobility of endogenous human telomerase after affinity purification at different salt concentrations, and demonstrated the ability to purify endogenous telomerase that retained activity yet lacked dyskerin.
MATERIALS AND METHODS
Cell culture and lysate preparation
Raji cells, a human lymphoblastic cell line, were grown in RPMI 1640 media supplemented with 10% v/v FBS and 2 mM l-glutamine in a 100 -l reactor at Amgen, Inc. (Thousand Oaks, CA, USA), harvested in mid-log phase, washed in PBS and frozen in liquid nitrogen. For each frozen pellet of ~2 × 1010 cells, the cells were thawed and resuspended in an equal volume (100 ml) of 2.3× hypo buffer (23 mM HEPES, 7 mM KCl, 2.3 mM MgCl2) supplemented with protease inhibitor cocktail tablets (Roche), RNase inhibitor (20 U/ml; Roche) and 1 mM DTT (63). The mixture was subjected to 90 strokes in a Dounce homogenizer with a loose pestle, on ice. The lysate was adjusted to 0.1 M NaCl or 0.6 M NaCl and incubated at 4°C for 45 min. Each lysate was then centrifuged at 140 000g in a SW28 Ti rotor (Beckman) at 4°C for 1 h. The supernatant was removed, adjusted to 15% v/v glycerol and snap frozen.
Anti-sense affinity purification of telomerase
Three hundred microlitres of Ultralink Immobilized Neutravidin Protein Plus (Pierce) was washed with 2.3× hypo buffer containing 0.1 M or 0.6 M NaCl and 0.5% v/v Triton X-100. Thirty nanomoles of affinity oligonucleotide 5′-biotin-CTAGACCTGTCACCUUCUCAGUUAGG-3′ (19,23,63) was coupled to the resin and washed in 2.3× hypo buffer containing 0.1 M or 0.6 M NaCl. A 15-ml aliquot of cell extract (corresponding to 1.5 ×109 cells) was thawed and pre-cleared by centrifugation at 14 000g for 10 min, mixed with 300 µl of coupled AAS resin and incubated for 10 min, with rocking, at 30°C followed by 2 h at 4°C. The resin was then collected by brief centrifugation (700 ×g for 2 min) and transferred to a 5-ml disposable Bio-spin Column (Bio-Rad, Hercules, CA, USA) and washed at 4°C with 2.3× hypo buffer containing 0.5% v/v Triton X-100 in 0.6 M NaCl or 0.1 M NaCl. The last wash, irrespective of initial salt concentration, was performed with 1 ml of 2.3× hypo buffer containing 0.1 M NaCl and 10% v/v glycerol. Elution was performed by addition of the displacement oligonucleotide 5′-CCTAACTGAGAAGGTGACAGGTCTAG-3′ at a ratio of 3 nmol oligonucleotide/nmol of biotinylated affinity oligonucleotide (19,23,63) in 1 ml of 2.3× hypo buffer containing 0.1 M NaCl and 10% v/v glycerol. The elution step was repeated again for a total of 2 × 1 ml eluate fractions that were subsequently pooled, adjusted to 20% v/v glycerol and stored at −70°C.
In vitro transcription of hTR and hvg1
Human telomerase RNA (hTR) and the human vault RNA (hvg1) RNAs were synthesized with the Ribomax Large-Scale RNA Production System-T7 (Promega, UK) as per the manufacturer's instructions. Telomerase RNA (hTR) was transcribed from pUC-hTR (1–451) digested with EcoRI or the same plasmid digested with StuI to generate a truncated hTR lacking the H/ACA box (nt 1–352) (3), and hvg1 RNA was transcribed from the HindIII-linearized pUC118-hvg1 (1–98) plasmid (64). Transcription products were purified using MEGAclear (Ambion, Austin, TX, USA), according to manufacturer's instructions.
Semi-quantitative RT-PCR of hTR or hvg1
RNA abundance was assessed by semi-quantitative RT-PCR using the Qiagen OneStep RT-PCR kit according to manufacturer's instructions in a 20 -µl reaction volume (Qiagen, UK). Amplification of hTR employed primers 5′-GGTGGTGGCCATTTTTTGTC-3′ (forward) and 5′-CTAGAATGAACGGTGGAAGGC-3′ (reverse; at 0.6 µM each). In experiments where purified hvg1 was added as an amplification control (Figures 3 and 4), 0.1 µM of hvg1 forward primer 5′-GGCTTTAGCTCAGCGGTTACTTCG-3′, and 0.1 µM hvg1 reverse primer 5′-GCGCCCGCGGGTCTCGAAC-3′ were included in the reaction. Approximately one-third volume of each reaction was resolved by electrophoresis on a 2% w/v agarose/TAE (Tris–Acetate–EDTA) gel and stained with ethidium bromide. Gel images were acquired on a Bio-Rad Geldoc XR using Quantity One 4.6.1 1D Analysis software.
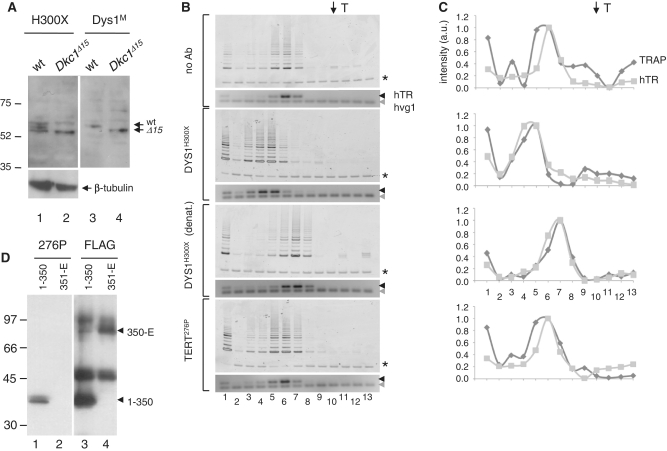
Figure 3.
Supershift analysis of telomerase after purification at low stringency. (A) Specificity of the commercial anti-dyskerin rabbit antibody. Fifty micrograms of total lysate from MEFs containing wild-type (wt) dyskerin or a C-terminal 21 amino acids truncation (DkcΔ15), probed with H300X (Dys1H300X, Santa Cruz) (lane 1 and 2) or another dyskerin antibody (Dys1M) (lanes 3 and 4) (65,66). Arrows indicate predicted molecular mass of wt and DkcΔ15 (57 and 55 kDa, respectively). Protein markers indicated at left. (B) Telomerase activity and hTR distribution profiles after incubation with no antibody (top panel), dyskerin antibody (DysH300X, Santa Cruz), the same dyskerin antibody denatured prior to addition to cell extract (see ‘Materials and Methods’ section) or TERT anti-peptide antibody (276P). Asterisk indicates internal control for TRAP PCR reactions; black arrow indicates hTR; grey arrow indicates exogenous hvg1 RNA added to the RT–PCR reaction mixture as an internal control. T (top arrow) indicates mobility of thyroglobulin. (C) The same profiles as in (B) represented graphically after normalization to the respective internal controls (see ‘Materials and Methods’ section). Dark grey; TRAP profile, light grey; hTR profile. Lane numbers correspond to eluate fractions. (D) The specificity of a rabbit antibody generated against hTERT amino acids 276–294 (276P) (18) was demonstrated by western blot analysis of FLAG-tagged hTERT, amino acids 1–350 (lane 1) and FLAG-tagged hTERT, amino acids 351–1132 (351-E, lane 2) after translation in rabbit reticulocyte lysate and immunoprecipitation with anti-FLAG antibody. As a control, equivalent samples were probed with anti-FLAG (lanes 3 and 4).
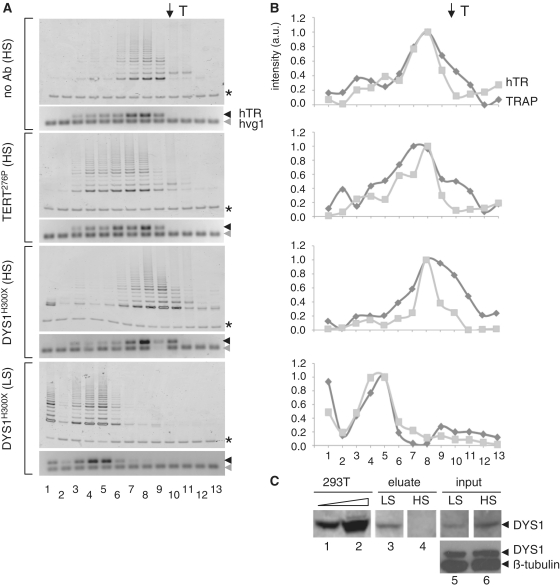
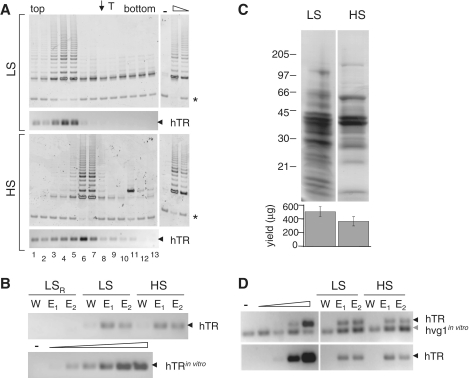
Figure 4.
Supershift analysis of native telomerase after purification at high stringency. (A) Telomerase activity and hTR distribution profiles of telomerase purified at high stringency (HS; 0.6 M NaCl) after incubation with no antibody (top panel), TERT anti-peptide antibody (276P) (second panel) or dyskerin antibody (DysH300X) (last panel), each incubated with extracts prior to native gel electrophoresis (see ‘Materials and Methods’ section). For comparison, the incubation of DysH300X with telomerase purified at 0.1 M NaCl (LS; third panel, as in Figure 3) is shown. Asterisk indicates internal control for TRAP PCR reactions; black arrow indicates hTR; grey arrow indicates exogenous hvg1 RNA added to the RT–PCR reaction mixture as an internal control. T indicates mobility of thyroglobulin (top arrow). (B) The same profiles as in (A) represented graphically, after normalization to the respective internal PCR controls. Dark grey; TRAP, light grey; hTR. Lane numbers correspond to eluate fractions. (C) 293T cell extract, 24 and 240 µg, respectively, LS and HS eluates and input material (5%) were resolved by SDS–PAGE (on the same gel, but lanes are separated to omit irrelevant material) and analysed by western blotting with the anti-dyskerin antibody. Bottom panel, 40 µg of the same input extracts (LS, HS) were probed simultaneously with anti-dyskerin and anti-β-tubulin as a loading control.
Native gel electrophoresis
Non-denaturing gels contained 3.5% w/v polyacrylamide (acrylamide:bisacrylamide, 60:1) and 0.4% w/v agarose, and samples were subjected to electrophoresis in 75 mM Tris–glycine buffer (pre-chilled). Approximately 75 µl affinity purified sample (corresponding to ~10–45 µg of total protein, or the amount of material purified from the equivalent of 107 cells) was loaded per well. Immediately following electrophoresis, the gel was sliced into 13 equal sections. Each lane was placed in a 1.5-ml microcentrifuge tube with 200 µl of elution buffer (10 mM Tris–HCl pH 8.3, 1.5 mM MgCl2, 10 mM KCl) and eluted overnight at 4°C. Eluates were stored at −70°C, and were used subsequently for TRAP and RT–PCR analysis (below). For each native gel, one outside lane was loaded with 20 µg of thyroglobulin gel filtration marker (AP Biosciences, UK).
Mobility shift experiments
Seventy-five microlitres of purified sample after affinity purification (corresponding to ~10–45 µg of total protein) was incubated with 1 µl (1 U) RNase-free DNaseI (Roche, Mannehim, Germany) and 10 µg of the indicated antibody for 75 min at 4°C with gentle agitation. As a negative control, antibody was boiled at 95°C for 5 min prior to mixing. Samples were then loaded onto native gels and processed as described earlier. Antibodies employed were anti-hTERT (276–294), generated and purified according to Cohen et al., 2007 (18), and anti-dyskerin, H300 or H300X (10-fold further concentrated) (Santa Cruz).
TRAP assay and RT–PCR
For each of the 13 native gel slice eluates, 15 µl was assayed for telomerase activity by the Trapeze Telomerase Detection kit (Millipore, Temecula, CA, USA) according to the manufacturer's instructions, and 10 µl was assayed for hTR in RT–PCR reactions as described earlier. In Figures 3 and 4, all RT–PCR reactions were ‘spiked’ with purified hvg1 RNA as an internal PCR control. hTR and hvg1 signals were quantified from gel images using ImageQuant 5.2 software (Molecular Dynamics) and ImageJ (NIH). Each hTR signal was normalized to its respective hvg1 signal. TRAP versus hTR signals were plotted across all 13 fractions using Image J, by subtracting a background lane (i.e. containing no sample) from the same-sized object encompassing TRAP or hTR signal, and dividing each by the 36-bp internal control (for TRAP) or hvg1 (for hTR), respectively. Fractions containing the peak hTR (and TRAP) signal were assigned in at least five independent experimental replicates, and the difference between the mean peak fractions assessed using a Student's t-test. Assessment of hTR yield was carried out using Image J quantification of purified hTR titrations and fitting individual samples to the linear response curve.
Recombinant dyskerin purification from Escherichia coli
The human dyskerin gene (Full-length Mammalian gene collection ID 4303933-Invitrogen, Oslo, Norway) was cloned into a vector containing HIS-tag, pET30 (Novagen), to enable purification from E. coli. BL21 Codon Plus RIL cells (Stratagene, La Jolla, CA, USA) were transformed and grown at 37°C. The liquid culture was induced with 1 mM IPTG for 4 h at 30°C, and lysed with Tris 50 mM, 10 mM imidazole and 300 mM NaCl containing protease inhibitors (Roche, Mannheim Germany) and lysozyme (Sigma, UK) (0.25 mg/ml). Sonication was performed with five cycles of 30 s at 30% amplitude on a sonicator Vibra Cell (Jencons). The bacterial lysate was clarified by centrifugation at 15 000g and loaded on a 1-ml Hitrap IMAC charged with Ni2+ (GE healthcare, UK) using an AKTA Explorer (GE Healthcare, UK). The column was washed with 20 volumes at 50 mM imidazole, and the protein was eluted with linear gradient (50–500 mM imidazole, 20 column volumes). The purity and concentration of dyskerin was evaluated by the Bradford assay (Bio-Rad, Munchen, Germany) and via Coomassie staining of the sample after resolution by SDS–PAGE. The purity of dyskerin ranged between 80% and 85%. The eluate was adjusted to 50% glycerol and stored at −80°C. Western blot analysis of recombinant or endogenous dyskerin employed H300X, C15 (Santa Cruz) or anti-Dys1 provided by P. Mason (65,66).
hTR labelling and immunoprecipitation
Full-length hTR (1–451) or hTRΔ (1–352) was produced by in vitro transcription in the presence of Cy5-UTP (Perkin Elmer, Waltham, USA) using Ribomax Large-Scale RNA Production System-T7 (Promega, Madison, USA) according to the manufacturer's instructions (the rNTP mixture contained 1 mM each of CTP, GTP, ATP and 0.64 mM UTP with 0.56 mM Cy5-UTP). The reaction was terminated by treatment with DNAse for 15 min and RNA was extracted using RNeasy minelute (Qiagen, UK). The RNA was denatured and refolded using a thermocycler (Bio-Rad, UK) by decreasing the temperature from 95°C to 23°C via 0.1°C increments per second. For immunoprecipitation, 2 µg of recombinant dyskerin was incubated on ice with 20 ng of Cy5-hTR in 300 µl of 1× hypo buffer, adjusted to 0.1 or 0.6 M NaCl, for 1 h. Four micrograms of anti-dyskerin antibody (H300X, Santa Cruz) was added and incubated for a further hour. Five microlitres of protein A-conjugated Dynabead solution (Invitrogen), previously equilibrated in 1× hypobuffer at 0.1 M NaCl or 0.6 M NaCl, was incubated with the mixture for 30 min. After four washes in 1× hypobuffer adjusted to 0.1 M NaCl, or three washes at 0.6 M NaCl followed by one wash at 0.1 M NaCl, the beads were resuspended in 10 µl 1× hypobuffer, and resolved via 6% w/v SDS–PAGE. The gel was subsequently scanned with a Li-Cor scanner (Li-Cor Biosciences, UK) to detect Cy5-hTR and transferred onto PVDF membrane and probed with goat dyskerin antibody (C-15, Santa Cruz). The secondary antibody, IRDye 800 donkey anti-goat (Li-Cor Biosciences), permitted quantification using an Odyssey scanner (Odyssey Imaging system, Li-Cor Biosciences). Briefly, rectangular boxes of equal size were marked around hTR and dyskerin on the respective images. Background was subtracted based on average pixel intensity surrounding the rectangle, and a ratio of hTR/dyskerin calculated for each sample. The experiment in Figure 5C is representative of four independent experiments; the graph in Figure 5D represents triplicate samples for the same experiment in Figure 5C. P-values for differences in association at 0.1 M versus 0.6 M NaCl were calculated using a two-tailed t-test, assuming unequal variance.
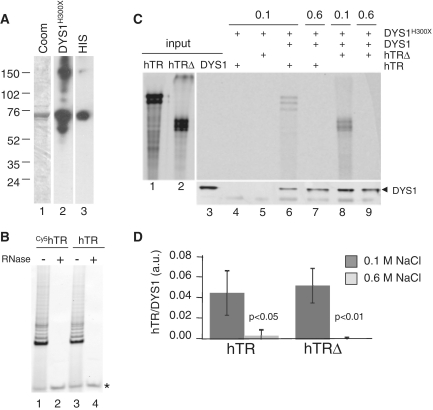
Figure 5.
Interaction of purified hTR and dyskerin in vitro. (A) Three micrograms of recombinant dyskerin was resolved by SDS–PAGE and stained with Coomassie blue (lane 1). On a separate gel, the same preparation (60 ng) was subjected to western blot analysis with anti-dyskerin (H300X, Santa Cruz) or anti-HIS (Sigma) antibody (lanes 2 and 3). (B) Telomerase activity reconstitution. Full-length, flag-tagged hTERT was expressed in rabbit reticulocyte lysates (Promega) in the presence of purified hTR that was transcribed with (lanes 1 and 2) or without (lanes 3 and 4) Cy5-UTP. Extracts were incubated with (lanes 2 and 4) or without (lanes 1 and 3) ribonuclease A (Sigma) prior to the amplification of telomerase extension products using TRAP. Asterisk, internal PCR control. (C) Immunoprecipitation of Cy5-hTR or a truncated hTR lacking the H/ACA box (Cy5-hTRΔ) with recombinant dyskerin at 0.1 and 0.6 M NaCl. Six hundred nanograms of purified hTR or hTRΔ was incubated with 1.5 µg of purified dyskerin prior to immunoprecipitation with anti-dyskerin (H300X, Santa Cruz), followed by western blot analysis with anti-dyskerin antibody (C15, Santa Cruz). Lane 1, 2; 20 ng input RNA, lane 3, 40 ng purified dyskerin. One of four independent experiments is shown. (D) Graphical representation of the data in (C).
RESULTS
Purification of telomerase by anti-sense affinity selection and native gel electrophoresis
In order to investigate the composition of endogenous telomerase, we employed a previously developed purification strategy using anti-sense affinity selection of hTR (19,22,23), with minor modifications (63). A biotinylated oligonucleotide containing a random sequence and a region of complementarity to the human telomerase RNA (hTR) (nt 50–64) was incubated with cell extracts from a human lymphoblastoma cell line (Raji). The oligonucleotide sequence differed slightly from that used in a previous study (nt 46–59) (19), and was based on optimization of the recovery of telomerase activity (63). Raji cells were chosen because of the relatively low level of hTR expression (35 copies/cell) (63) relative to other cell types (19,67,68), in order to maximize the ratio of affinity-purified hTR associated with telomerase activity. After incubation with Raji lysate, the affinity oligonucleotide was captured onto neutravidin beads, washed and eluted by virtue of a displacement oligonucleotide complementary to the affinity oligonucleotide (19,22,23,63). To further purify telomerase, this fraction was resolved on a clear, 3.5% w/v non-denaturing native acrylamide gel (Figure 1A). To locate active telomerase after electrophoresis, the native gel was separated into 1 cm slices numbered from top to bottom (e.g. Figure 1B, right), eluted into a buffered solution and subjected to the telomere repeat amplification protocol (TRAP) (Figure 1A, bottom panel). One adjacent lane of the native gel was stained with Coomassie blue dye, while another adjacent lane was probed for the presence of the telomerase RNA after transfer to nylon membrane (Figure 1A, top panels). These results demonstrated that the peak of endogenous telomerase activity and hTR co-migrated on the native gel at a position distinct from the bulk of proteins detected by Coomassie staining.
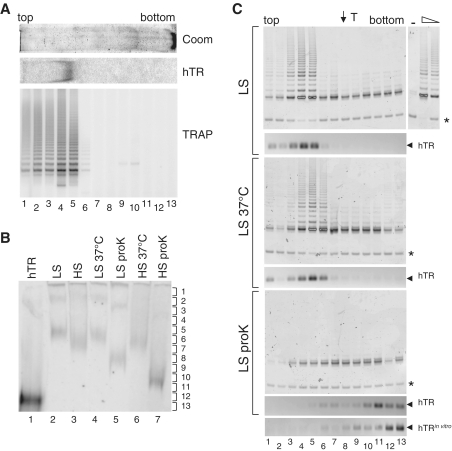
Figure 1.
Telomerase purification via affinity selection and native gel electrophoresis. (A) Co-migration of hTR and telomerase activity after native gel electrophoresis of affinity-purified Raji cell extract (at 0.1 M NaCl). After clear native gel electrophoresis, one lane was stained with Coomassie blue (top panel; gel slice oriented from top to bottom as indicated), and one lane probed with a radiolabelled probe corresponding to hTR after transfer to nylon (middle panel). A third lane was sliced into 13 fractions, eluted into buffer and subjected to the telomere repeat amplification protocol, TRAP (bottom panel). (B) Relative mobility of telomerase after native gel electrophoresis and detection with a radiolabelled probe corresponding to hTR. Lane 1, in vitro transcribed hTR alone; Raji cell extracts purified at 0.1 M NaCl (low stringency; LS) and 0.6 M NaCl (high stringency; HS) at 4°C (lanes 2 and 3, respectively), 37°C (lanes 4 and 6) and after incubation with proteinase K (lanes 5 and 7). Brackets at right indicate position of gel slices. (C) The indicated treatments as in (B) were resolved by native gel electrophoresis, sliced into 13 fractions and subjected to TRAP (upper panels) and RT–PCR analysis to detect the native mobility of hTR (lower panels). Thyroglobulin (T) was loaded onto an adjacent lane of the native gel; its position is indicated with an arrow, top. Asterisk indicates TRAP internal PCR standard; arrows at right, hTR. The bottom panel represents PCR detection of hTR after native gel electrophoresis of purified, in vitro transcribed hTR alone (no added extract). At right, 1.0 and 0.2 µl of rabbit reticulocyte lysate (RRL) containing reconstituted telomerase activity, or water alone (−) as positive and negative controls for TRAP, respectively.
We next assessed whether various treatments altered the mobility of the endogenous human telomerase complex. Similar to previously published results whereby telomerase mass was estimated by velocity sedimentation or size exclusion chromatography, we found that at low concentrations of NaCl (0.1 M = LS), telomerase activity migrated at an apparent molecular mass >670 kDa (thyroglobulin = T) (Figure 1C). Purification at 37°C (compared with 4°C) did not significantly affect the mobility of telomerase (Figure 1B and C) (63). The faster mobility of hTR after proteinase K treatment (Figure 1B and C), although variable between experiments and not coincident with in vitro purified hTR (compare lower two hTR panels in Figure 1C), nevertheless confirmed that the purified hTR complex contained protein. Upon increasing the stringency of the purification to 0.6 M NaCl the native mobility of human telomerase was increased (Figures 1B and 2A) (17–19,22). Thus, hTR mobility in native gels could be altered by salt concentration, which could reflect distinct properties including protein composition.
Figure 2.
Telomerase mobility when purified at low and high stringencies. (A) Analysis of telomerase activity and hTR mobility (hTR, black arrow) by native gel electrophoresis after purification at 0.1 M NaCl (low stringency; LS), and 0.6 M NaCl (high stringency; HS), as described in Figure 1. T (arrow at top) indicates the mobility of thyroglobulin. Lane numbers correspond to gel slices, from top to bottom, as indicated. At right, a dilution of RRL-produced telomerase (1.0 and 0.2 µl, respectively) and no added extract (−) as controls. Asterisk marks the internal PCR standard for the TRAP reaction. The LS profile is the same as shown in Figure 1B. (B) Relative yield of hTR after affinity purification. Bottom panel; RT–PCR analysis using hTR-specific primers with the following amounts of input hTR: 0 (−), 0.1, 1.0, 10, 100, 1000 and 10 000 fg. Top panel: hTR recovery as assessed by RT–PCR of equal volumes of LS + RNase (LSR), LS and HS purifications from the wash (W) and first and second eluates (E1, E2). (C) The same LS and HS eluates as in (B) were resolved via denaturing SDS–PAGE and subjected to Coomassie staining. Protein markers are indicated at left, in kDa. The average protein yield of the affinity eluate (in µg) is indicated below (n = 6, LS ave. 509, SD 73, HS ave. 367, SD 67). (D) Enrichment of hTR relative to hvg1. Top panel: as a control for the ability to detect hvg1, an equivalent amount of hvg1 RNA was added to purified hTR (0, 5, 50, 500, 5000 fg, respectively) or to affinity purified fractions (right, as in B), prior to RT–PCR amplification with hTR-specific and hvg1-specific primers. Bottom panel: affinity purified samples, without the addition of any exogenous RNA, were similarly amplified with hvg1 and hTR primers. Black arrow; hTR, grey arrow; hvg1.
To further characterize the composition of telomerase purified at 0.1 M versus 0.6 M NaCl, we assessed hTR recovery and its association with telomerase activity. The peak of telomerase activity after native gel electrophoresis mirrored that of hTR regardless of salt concentration; however, when averaged across seven experiments, the hTR RT–PCR signal co-peaked with telomerase activity at fraction 5.5 ± 0.9 when purified at 0.1 M NaCl, and at fraction 7.0 ± 0.9 when purified at 0.6 M NaCl (P < 0.01) (Figure 2A; one representative experiment shown). Despite the shift in mobility, the total yield of telomerase RNA did not differ significantly between purification at 0.1 M versus 0.6 M NaCl, although, as expected, the total protein yield was lower after purification at 0.6 M NaCl (Figure 2B and C). For example, the hTR signal in the E1 eluate fraction after purification at 0.1 or 0.6 M NaCl was 33 or 37 fg, respectively (Figure 2B, quantification as described in ‘Materials and Methods’ section). Northern analysis confirmed that hTR yields in 0.1 and 0.6 M eluates were comparable and varied between 3 and 4 fmol from 5 × 109 Raji cells (data not shown) (63). To demonstrate the selectivity of the purification for hTR, the more abundant hvg1 RNA (64) did not co-precipitate with affinity-purified telomerase at 0.1 or 0.6 M NaCl (Figure 2D, bottom panel), despite the fact that hvg1 RNA was readily detected when it was ‘spiked’ into the sample prior to RT–PCR (Figure 2D, top panel). Thus, telomerase purified at 0.6 M NaCl exhibited a faster mobility upon native gel electrophoresis, yet the amount and yield of hTR was comparable to purification at 0.1 M NaCl.
The ability of dyskerin antibody to alter telomerase mobility
The migration of a protein complex on a native gel depends not only on the molecular mass but also upon its charge and hydrodynamic status (69). To test whether the difference in the migration of telomerase reflected different protein compositions, we employed a well-characterized property of antibodies to ‘supershift’ their cognate protein upon binding to the complex during electrophoresis (70). Since dyskerin is a tightly associated telomerase subunit (18), we tested the ability of an anti-dyskerin antibody (H-300X, Santa Cruz) to supershift telomerase at 0.1 M NaCl. H300X recognized endogenous murine wild-type (57 kDa) and a truncated Dkc1 variant lacking exon 15 and the C-terminal 21 amino acids, DkcΔ15 (55 kDa) (66) with a similar specificity as an independently generated dyskerin antibody (DysM) (65) (Figure 3A). In addition, H300X recognized recombinant human dyskerin and a single polypeptide of the expected molecular mass (57 kDa) in human cell lysates (Figures 4 and 5). H300X elicited a significant mobility retardation of telomerase relative to sample without added antibody (Figure 3B and C, one representative experiment shown), with the peak of RT–PCR hTR signal and telomerase activity shifting from fraction 5.5 ± 0.9 to 4.4 ± 0.2 upon incubation with H300X (n = 5, P < 0.01). This mobility shift was abolished when the H300X antibody was boiled prior to incubation with telomerase (Figure 3B and C). Thus, a significant fraction of telomerase purified at 0.1 M NaCl contained dyskerin, in accord with Cohen et al. (18) who found a stoichiometric association of dyskerin with telomerase upon purification at 0.3 M KCl.
Other antibodies directed against TERT or telomerase-associated subunits failed to supershift the telomerase complex in native gels. Two antibodies directed against the telomerase-associated subunit SMG-6/EST1A (71) and another commercial anti-dyskerin antibody (C-15, Santa Cruz) were unable to supershift telomerase (data not shown). In addition, we failed to observe a statistically significant supershift using polyclonal rabbit antibodies generated against the reverse transcriptase domain of TERT (72) or against a TERT peptide (amino acids 276–294) (18), despite their demonstrated specificity for TERT as published previously (Figure 3B–D and data not shown) (18,72). These observations may reflect the fact that it is relatively uncommon for an antibody to supershift its target in native gels (70).
The TERT and dyskerin antibodies were tested for their ability to supershift the telomerase complex after affinity purification at 0.6 M NaCl (Figure 4). The anti-hTERT peptide (amino acids 276–294) antibody elicited a slight (but not statistically significant) retardation in the mobility of active telomerase (Figure 4A and B). In contrast to the effect upon telomerase purified at 0.1 M NaCl, H300X did not retard the mobility of telomerase activity or hTR relative to no added antibody (Figure 4A and B). Western blot analysis of the 0.1- and 0.6-M eluates after affinity purification confirmed that dyskerin was lost from the complex after purification at 0.6 M NaCl (Figure 4C, lanes 3 and 4), despite a comparable level of dyskerin in lysates prior to affinity purification (Figure 4C, lanes 5 and 6). Thus, despite a comparable recovery of hTR, telomerase complexes purified at 0.6 M NaCl had lost an association with dyskerin. Immunoprecipitation using unpurified Raji cell lysates confirmed a similar ability of H300X to recover dyskerin at 0.1 and 0.6 M NaCl (data not shown). The salt sensitivity of the dyskerin–telomerase interaction was also reproduced in unpurified Raji cell lysates using the anti-TERT 276P antibody, which immunopurified dyskerin at 0.1 M NaCl but not at 0.6 M NaCl (data not shown).
Salt-sensitive interaction of purified hTR and dyskerin in vitro
Since dyskerin interacts directly with hTR, we tested the possibility that higher salt concentrations would dissociate the hTR–dyskerin complex. Human dyskerin was purified from E. coli (Figure 5A) and incubated with Cy5-labelled hTR. Cy5-hTR was confirmed for its competence to reconstitute active telomerase in vitro (Figure 5B). Despite the fact that hTR was produced in vitro (and thus only a subset of molecules may be in a conformation competent to interact with dyskerin), the H300X antibody immunopurified Cy5-hTR, or a Cy5-labelled hTR truncation lacking the H/ACA box (hTRΔ; nt 1–352), and this ability depended on the presence of dyskerin (Figure 5C, lanes 4–6 and 8). However, the interaction of dyskerin with hTR or hTRΔ was abolished if the immunoprepitation procedure was carried out at 0.6 M NaCl (Figure 5C, lanes 7 and 9; Figure 5D). Thus, the salt-sensitive interaction of purified dyskerin and hTR in vitro may account for the loss of dyskerin associated with active telomerase after purification at 0.6 M NaCl. The ability of dyskerin to bind hTRΔ, which lacks the H/ACA box, is in general agreement with previous studies that established that snoRNAs lacking an H/ACA box still bound Cbf5 (the dyskerin homologue in S. cerevisiae), albeit at a lower affinity than RNAs containing the H/ACA motif (73).
DISCUSSION
We utilized native gel electrophoresis to evaluate the mobility of telomerase after partial purification. This technique, combined with the ability of an anti-dyskerin antibody to supershift the telomerase complex, enabled the identification of distinct telomerase complexes after purification under low and high salt concentrations. In accord with Cohen et al. (18), dyskerin was an integral component of the telomerase complex when purified at 0.1 M NaCl, based on a significant supershift of telomerase in native gels with an anti-dyskerin antibody. However, purification at 0.6 M NaCl resulted in an active telomerase complex that lacked dyskerin. Depending on the relative stoichoimetry of TERT, hTR and dyskerin within telomerase, other proteins dispensable for catalytic activity may also have been dissociated during purification at 0.6 M NaCl. For example, although three other integral components of the H/ACA binding complex, NHP2, NOP10 and GAR1, were not identified in the large-scale affinity purification of endogenous telomerase (18), they are associated with purified, tagged TR or TERT (29,74).
Previous studies established a role for dyskerin in the stability of hTR in vivo and its ability to assemble into an active telomerase complex (49,75,76). However the necessity of its continued association with the enzyme had not been assessed. In vitro, addition of exogenous dyskerin is not required for telomerase reconstitution in crude cell extracts (3,4,8). Our results demonstrate that, once assembled, the association of dyskerin with native telomerase from cell extracts is also dispensable for catalytic activity. These results support the notion that the critical role of dyskerin in telomerase function is in holoenzyme assembly and stability (14).
This study extends the utility of native gel electrophoresis to the analysis of endogenous human telomerase complexes. Previously, native gel analysis of purified telomerase from Tetrahymena thermophila led to the identification of a ~125 kDa protein with a telomere specific and RNase-sensitive DNA binding activity (21), and native gels also established a critical role of the telomerase RNA pseudoknot in telomerase assembly (60). In humans, native gels were employed to examine the association of recombinant telomerase RNA with partially purified telomerase (58), and the association of endogenous telomerase RNA and activity with an anti-Ro60 antibody cross-reacting species hypothesized to be the telomerase RNA-associated protein TEP1 (59,61,77,78). Whereas large amounts of starting material are required to analyse endogenous complexes by mass spectrometry (18), smaller amounts can be probed by native gel electrophoresis if an antibody of sufficient specificity and avidity is available. This methodology permits analysis under various conditions where immuno-tagging would be otherwise impractical, for example, in different tissues under various physiological or diseased states. This method could also be used to assess binding of candidate small molecules or peptides, in which case the molecule could be fluorescently labelled and assessed for co-migration with the complex. This approach may therefore be of general interest to probe the composition and binding properties of telomerase and other complexes isolated from physiological sources.
FUNDING
National Cancer Institute of Canada (NCIC 15072 to L.A.H.); Canadian Institutes of Health Research (MT14340 to L.A.H.); Medical Research Council UK (G0800081 to L.A.H.); Medical Research Council of Canada (studentship award to R.O.) and Centre for Systems Biology at Edinburgh (CSBE) which is a Centre for Integrative Systems Biology (CISB) funded by BBSRC and EPSRC (reference BB/D019621/1 to T.L.B). Funding for open access charge: Medical Research Council UK (G0800081).
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
We thank Amgen, Inc. for the large-scale production of Raji cells, and Drs Valerie Kickhoefer and Leonard Rome for the hvg1-encoded plasmid. Dr Don Hunt is thanked for mass spectrometric analysis of partially purified telomerase in prior experiments that preceded this study. We thank Drs Bai-Wei Gu and Philip Mason for primary MEFs expressing DkcΔ15 and the anti-dyskerin (DysM) antibody. We thank members of the laboratory and Dr Olivier Cordin for discussion and critical comments on the manuscript.
REFERENCES
- 1.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 2.Greider CW. Telomere length regulation. Annu. Rev. Biochem. 1996;65:337–365. doi: 10.1146/annurev.bi.65.070196.002005. [DOI] [PubMed] [Google Scholar]
- 3.Beattie TL, Zhou W, Robinson MO, Harrington L. Reconstitution of human telomerase activity in vitro. Curr. Biol. 1998;8:177–180. doi: 10.1016/s0960-9822(98)70067-3. [DOI] [PubMed] [Google Scholar]
- 4.Weinrich SL, Pruzan R, Ma L, Ouellette M, Tesmer VM, Holt SE, Bodnar AG, Lichtsteiner S, Kim NW, Trager JB, et al. Reconstitution of human telomerase with the template RNA component hTR and the catalytic protein subunit hTRT. Nat. Genet. 1997;17:498–502. doi: 10.1038/ng1297-498. [DOI] [PubMed] [Google Scholar]
- 5.Zappulla DC, Goodrich K, Cech TR. A miniature yeast telomerase RNA functions in vivo and reconstitutes activity in vitro. Nat. Struct. Mol. Biol. 2005;12:1072–1077. doi: 10.1038/nsmb1019. [DOI] [PubMed] [Google Scholar]
- 6.Bryan TM, Goodrich KJ, Cech TR. A mutant of Tetrahymena telomerase reverse transcriptase with increased processivity. J. Biol. Chem. 2000;275:24199–24207. doi: 10.1074/jbc.M003246200. [DOI] [PubMed] [Google Scholar]
- 7.Licht JD, Collins K. Telomerase RNA function in recombinant Tetrahymena telomerase. Genes Dev. 1999;13:1116–1125. doi: 10.1101/gad.13.9.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holt SE, Aisner DL, Baur J, Tesmer VM, Dy M, Ouellette M, Trager JB, Morin GB, Toft DO, Shay JW, et al. Functional requirement of p23 and Hsp90 in telomerase complexes. Genes Dev. 1999;13:817–826. doi: 10.1101/gad.13.7.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forsythe HL, Jarvis JL, Turner JW, Elmore LW, Holt SE. Stable association of hsp90 and p23, but Not hsp70, with active human telomerase. J. Biol. Chem. 2001;276:15571–15574. doi: 10.1074/jbc.C100055200. [DOI] [PubMed] [Google Scholar]
- 10.Collins K. Ciliate telomerase biochemistry. Annu. Rev. Biochem. 1999;68:187–218. doi: 10.1146/annurev.biochem.68.1.187. [DOI] [PubMed] [Google Scholar]
- 11.Collins K. The biogenesis and regulation of telomerase holoenzymes. Nat. Rev. Mol. Cell. Biol. 2006;7:484–494. doi: 10.1038/nrm1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venteicher AS, Meng Z, Mason PJ, Veenstra TD, Artandi SE. Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly. Cell. 2008;132:945–957. doi: 10.1016/j.cell.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venteicher AS, Abreu EB, Meng Z, McCann KE, Terns RM, Veenstra TD, Terns MP, Artandi SE. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science. 2009;323:644–648. doi: 10.1126/science.1165357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins K. Physiological assembly and activity of human telomerase complexes. Mech. Ageing Dev. 2008;129:91–98. doi: 10.1016/j.mad.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min B, Collins K. An RPA-related sequence-specific DNA-binding subunit of telomerase holoenzyme is required for elongation processivity and telomere maintenance. Mol. Cell. 2009;36:609–619. doi: 10.1016/j.molcel.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teixeira MT, Gilson E. La sets the tone for telomerase assembly. Nat. Struct. Mol. Biol. 2007;14:261–262. doi: 10.1038/nsmb0407-261. [DOI] [PubMed] [Google Scholar]
- 17.Wenz C, Enenkel B, Amacker M, Kelleher C, Damm K, Lingner J. Human telomerase contains two cooperating telomerase RNA molecules. EMBO J. 2001;20:3526–3534. doi: 10.1093/emboj/20.13.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 19.Schnapp G, Rodi HP, Rettig WJ, Schnapp A, Damm K. One-step affinity purification protocol for human telomerase. Nucleic Acids Res. 1998;26:3311–3313. doi: 10.1093/nar/26.13.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 21.Harrington L, Hull C, Crittenden J, Greider C. Gel shift and UV cross-linking analysis of Tetrahymena telomerase. J. Biol. Chem. 1995;270:8893–8901. doi: 10.1074/jbc.270.15.8893. [DOI] [PubMed] [Google Scholar]
- 22.Lingner J, Cech TR. Purification of telomerase from Euplotes aediculatus: requirement of a primer 3′ overhang. Proc. Natl Acad. Sci. USA. 1996;93:10712–10717. doi: 10.1073/pnas.93.20.10712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurth I, Cristofari G, Lingner J. An affinity oligonucleotide displacement strategy to purify ribonucleoprotein complexes applied to human telomerase. Methods Mol. Biol. 2008;488:9–22. doi: 10.1007/978-1-60327-475-3_2. [DOI] [PubMed] [Google Scholar]
- 24.Aigner S, Cech TR. The Euplotes telomerase subunit p43 stimulates enzymatic activity and processivity in vitro. RNA. 2004;10:1108–1118. doi: 10.1261/rna.7400704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aigner S, Postberg J, Lipps HJ, Cech TR. The Euplotes La motif protein p43 has properties of a telomerase-specific subunit. Biochemistry. 2003;42:5736–5747. doi: 10.1021/bi034121y. [DOI] [PubMed] [Google Scholar]
- 26.Aigner S, Lingner J, Goodrich KJ, Grosshans CA, Shevchenko A, Mann M, Cech TR. Euplotes telomerase contains an La motif protein produced by apparent translational frameshifting. EMBO J. 2000;19:6230–6239. doi: 10.1093/emboj/19.22.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirwan M, Dokal I. Dyskeratosis congenita: a genetic disorder of many faces. Clin. Genet. 2008;73:103–112. doi: 10.1111/j.1399-0004.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 28.Meier UT. The many facets of H/ACA ribonucleoproteins. Chromosoma. 2005;114:1–14. doi: 10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu D, Collins K. Purification of human telomerase complexes identifies factors involved in telomerase biogenesis and telomere length regulation. Mol. Cell. 2007;28:773–785. doi: 10.1016/j.molcel.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiss T. Small nucleolar RNAs: an abundant group of noncoding RNAs with diverse cellular functions. Cell. 2002;109:145–148. doi: 10.1016/s0092-8674(02)00718-3. [DOI] [PubMed] [Google Scholar]
- 31.Zaug AJ, Linger J, Cech TR. Method for determining RNA 3′ ends and application to human telomerase RNA. Nucleic Acids Res. 1996;24:532–533. doi: 10.1093/nar/24.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng J, Funk WD, Wang SS, Weinrich SL, Avilion AA, Chiu CP, Adams RR, Chang E, Allsopp RC, Yu J, et al. The RNA component of human telomerase. Science. 1995;269:1236–1241. doi: 10.1126/science.7544491. [DOI] [PubMed] [Google Scholar]
- 33.Tomlinson RL, Ziegler TD, Supakorndej T, Terns RM, Terns MP. Cell cycle-regulated trafficking of human telomerase to telomeres. Mol. Biol. Cell. 2006;17:955–965. doi: 10.1091/mbc.E05-09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theimer CA, Jady BE, Chim N, Richard P, Breece KE, Kiss T, Feigon J. Structural and functional characterization of human telomerase RNA processing and cajal body localization signals. Mol. Cell. 2007;27:869–881. doi: 10.1016/j.molcel.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Theimer CA, Feigon J. Structure and function of telomerase RNA. Curr. Opin. Struct. Biol. 2006;16:307–318. doi: 10.1016/j.sbi.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Venteicher AS, Artandi SE. TCAB1: driving telomerase to Cajal bodies. Cell Cycle. 2009;8:1329–1331. doi: 10.4161/cc.8.9.8288. [DOI] [PubMed] [Google Scholar]
- 37.Ferrezuelo F, Steiner B, Aldea M, Futcher B. Biogenesis of yeast telomerase depends on the importin mtr10. Mol. Cell. Biol. 2002;22:6046–6055. doi: 10.1128/MCB.22.17.6046-6055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gallardo F, Chartrand P. Telomerase biogenesis: the long road before getting to the end. RNA Biol. 2008;5:212–215. doi: 10.4161/rna.7115. [DOI] [PubMed] [Google Scholar]
- 39.Fu D, Collins K. Human telomerase and Cajal body ribonucleoproteins share a unique specificity of Sm protein association. Genes Dev. 2006;20:531–536. doi: 10.1101/gad.1390306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seto AG, Zaug AJ, Sobel SG, Wolin SL, Cech TR. Saccharomyces cerevisiae telomerase is an Sm small nuclear ribonucleoprotein particle. Nature. 1999;401:177–180. doi: 10.1038/43694. [DOI] [PubMed] [Google Scholar]
- 41.Box JA, Bunch JT, Tang W, Baumann P. Spliceosomal cleavage generates the 3′ end of telomerase RNA. Nature. 2008;456:910–914. doi: 10.1038/nature07584. [DOI] [PubMed] [Google Scholar]
- 42.Zhong F, Savage SA, Shkreli M, Giri N, Jessop L, Myers T, Chen R, Alter BP, Artandi SE. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev. 2011;25:11–16. doi: 10.1101/gad.2006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walne AJ, Dokal I. Advances in the understanding of dyskeratosis congenita. Br. J. Haematol. 2009;145:164–172. doi: 10.1111/j.1365-2141.2009.07598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang D, He Q, Kim H, Ma W, Songyang Z. TIN2 protein dyskeratosis congenita missense mutants are defective in association with telomerase. J. Biol. Chem. 2011;286:23022–23030. doi: 10.1074/jbc.M111.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calado RT, Regal JA, Kleiner DE, Schrump DS, Peterson NR, Pons V, Chanock SJ, Lansdorp PM, Young NS. A spectrum of severe familial liver disorders associate with telomerase mutations. PLoS One. 2009;4:e7926. doi: 10.1371/journal.pone.0007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446–4455. doi: 10.1182/blood-2007-08-019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armanios M. Syndromes of telomere shortening. Annu. Rev. Genomics Hum. Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parry EM, Alder JK, Qi X, Chen JJ, Armanios M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood. 2011;117:5607–5611. doi: 10.1182/blood-2010-11-322149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wong JM, Collins K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes Dev. 2006;20:2848–2858. doi: 10.1101/gad.1476206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwal S, Loh YH, McLoughlin EM, Huang J, Park IH, Miller JD, Huo H, Okuka M, Dos Reis RM, Loewer S, et al. Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature. 464:292–296. doi: 10.1038/nature08792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gourronc FA, Robertson M, Herrig AK, Lansdorp PM, Goldman FD, Klingelhutz AJ. Proliferative defects in dyskeratosis congenita skin keratinocytes are corrected by expression of the telomerase reverse transcriptase, TERT, or by activation of endogenous telomerase through expression of papillomavirus E6/E7 or the telomerase RNA component, TERC. Exp. Dermatol. 19:279–288. doi: 10.1111/j.1600-0625.2009.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strong MA, Vidal-Cardenas SL, Karim B, Yu H, Guo N, Greider CW. Phenotypes in mTERT/ and mTERT/ mice are due to short telomeres, not telomere-independent functions of telomerase reverse transcriptase. Mol. Cell. Biol. 2011;31:2369–2379. doi: 10.1128/MCB.05312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D, Greider CW. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 54.Hockemeyer D, Palm W, Wang RC, Couto SS, de Lange T. Engineered telomere degradation models dyskeratosis congenita. Genes Dev. 2008;22:1773–1785. doi: 10.1101/gad.1679208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He H, Wang Y, Guo X, Ramchandani S, Ma J, Shen MF, Garcia DA, Deng Y, Multani AS, You MJ, et al. Pot1b deletion and telomerase haploinsufficiency in mice initiate an ATR-dependent DNA damage response and elicit phenotypes resembling dyskeratosis congenita. Mol. Cell. Biol. 2009;29:229–240. doi: 10.1128/MCB.01400-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Armanios M, Alder JK, Parry EM, Karim B, Strong MA, Greider CW. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am. J. Hum. Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He J, Navarrete S, Jasinski M, Vulliamy T, Dokal I, Bessler M, Mason PJ. Targeted disruption of Dkc1, the gene mutated in X-linked dyskeratosis congenita, causes embryonic lethality in mice. Oncogene. 2002;21:7740–7744. doi: 10.1038/sj.onc.1205969. [DOI] [PubMed] [Google Scholar]
- 58.Autexier C, Pruzan R, Funk WD, Greider CW. Reconstitution of human telomerase activity and identification of a minimal functional region of the human telomerase RNA. EMBO J. 1996;15:5928–5935. [PMC free article] [PubMed] [Google Scholar]
- 59.Ramakrishnan S, Sharma HW, Farris AD, Kaufman KM, Harley JB, Collins K, Pruijn GJ, van Venrooij WJ, Martin ML, Narayanan R. Characterization of human telomerase complex. Proc. Natl Acad. Sci. USA. 1997;94:10075–10079. doi: 10.1073/pnas.94.19.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilley D, Blackburn EH. The telomerase RNA pseudoknot is critical for the stable assembly of a catalytically active ribonucleoprotein. Proc. Natl Acad. Sci. USA. 1999;96:6621–6625. doi: 10.1073/pnas.96.12.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Poderycki MJ, Rome LH, Harrington L, Kickhoefer VA. The p80 homology region of TEP1 is sufficient for its association with the telomerase and vault RNAs, and the vault particle. Nucleic Acids Res. 2005;33:893–902. doi: 10.1093/nar/gki234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sealey DC, Zheng L, Taboski MA, Cruickshank J, Ikura M, Harrington LA. The N-terminus of hTERT contains a DNA-binding domain and is required for telomerase activity and cellular immortalization. Nucleic Acids Res. 2010;38:2019–2035. doi: 10.1093/nar/gkp1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oulton R. Ph.D. Thesis. Toronto, ON, Canada: Department of Medical Biophysics, University of Toronto; 2004. Characterization of the human telomerase complex; p. 185. [Google Scholar]
- 64.Kickhoefer VA, Poderycki MJ, Chan EK, Rome LH. The La RNA-binding protein interacts with the vault RNA and is a vault-associated protein. J. Biol. Chem. 2002;277:41282–41286. doi: 10.1074/jbc.M206980200. [DOI] [PubMed] [Google Scholar]
- 65.Mochizuki Y, He J, Kulkarni S, Bessler M, Mason PJ. Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity, and ribosomal RNA processing. Proc. Natl Acad. Sci. USA. 2004;101:10756–10761. doi: 10.1073/pnas.0402560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu BW, Bessler M, Mason PJ. A pathogenic dyskerin mutation impairs proliferation and activates a DNA damage response independent of telomere length in mice. Proc. Natl Acad. Sci. USA. 2008;105:10173–10178. doi: 10.1073/pnas.0803559105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Avilion AA. PhD Thesis. Stony Brook, NY, USA: Department of Cellular and Developmental Biology, State University of New York at Stony Brook; 1995. Characterization and expression of human telomerase; p. 234 pp. [Google Scholar]
- 68.Yi X, Shay JW, Wright WE. Quantitation of telomerase components and hTERT mRNA splicing patterns in immortal human cells. Nucleic Acids Res. 2001;29:4818–4825. doi: 10.1093/nar/29.23.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suh MH, Ye P, Datta AB, Zhang M, Fu J. An agarose-acrylamide composite native gel system suitable for separating ultra-large protein complexes. Anal. Biochem. 2005;343:166–175. doi: 10.1016/j.ab.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 70.Buratowski S, Chodosh LA. Mobility shift DNA-binding assay using gel electrophoresis. Curr. Protoc. Mol. Biol. 2001;Chapter 12:Unit 12. doi: 10.1002/0471142727.mb1202s36. [DOI] [PubMed] [Google Scholar]
- 71.Snow BE, Erdmann N, Cruickshank J, Goldman H, Gill RM, Robinson MO, Harrington L. Functional conservation of the telomerase protein Est1p in humans. Curr. Biol. 2003;13:698–704. doi: 10.1016/s0960-9822(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 72.Beattie TL, Zhou W, Robinson MO, Harrington L. Polymerization defects within human telomerase are distinct from telomerase RNA and TEP1 binding. Mol. Biol. Cell. 2000;11:3329–3340. doi: 10.1091/mbc.11.10.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Normand C, Capeyrou R, Quevillon-Cheruel S, Mougin A, Henry Y, Caizergues-Ferrer M. Analysis of the binding of the N-terminal conserved domain of yeast Cbf5p to a box H/ACA snoRNA. RNA. 2006;12:1868–1882. doi: 10.1261/rna.141206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Egan ED, Collins K. Specificity and stoichiometry of subunit interactions in the human telomerase holoenzyme assembled in vivo. Mol. Cell. Biol. 2010;30:2775–2786. doi: 10.1128/MCB.00151-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 76.Parry EM, Alder JK, Lee SS, Phillips JA, 3rd, Loyd JE, Duggal P, Armanios M. Decreased dyskerin levels as a mechanism of telomere shortening in X-linked dyskeratosis congenita. J. Med. Genet. 2011;48:327–333. doi: 10.1136/jmg.2010.085100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakayama J, Saito M, Nakamura H, Matsuura A, Ishikawa F. TLP1: a gene encoding a protein component of mammalian telomerase is a novel member of WD repeats family. Cell. 1997;88:875–884. doi: 10.1016/s0092-8674(00)81933-9. [DOI] [PubMed] [Google Scholar]
- 78.Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, Robinson MO. A mammalian telomerase-associated protein. Science. 1997;275:973–977. doi: 10.1126/science.275.5302.973. [DOI] [PubMed] [Google Scholar]