Abstract
Trigger-inducible transcription-control devices that reversibly fine-tune transgene expression in response to molecular cues have significantly advanced the rational reprogramming of mammalian cells. When designed for use in future gene- and cell-based therapies the trigger molecules have to be carefully chosen in order to provide maximum specificity, minimal side-effects and optimal pharmacokinetics in a mammalian organism. Capitalizing on control components that enable Caulobacter crescentus to metabolize vanillic acid originating from lignin degradation that occurs in its oligotrophic freshwater habitat, we have designed synthetic devices that specifically adjust transgene expression in mammalian cells when exposed to vanillic acid. Even in mice transgene expression was robust, precise and tunable in response to vanillic acid. As a licensed food additive that is regularly consumed by humans via flavoured convenience food and specific fresh vegetable and fruits, vanillic acid can be considered as a safe trigger molecule that could be used for diet-controlled transgene expression in future gene- and cell-based therapies.
INTRODUCTION
Synthetic control devices providing precise expression fine-tuning of mammalian transgenes in response to molecular cues have been fundamental for functional genomic research (1), biopharmaceutical manufacturing of difficult-to-express protein therapeutics (2–4), drug discovery (5,6), tissue engineering (7–9), the design of complex synthetic gene networks (10–13), prototypic gene therapy applications (9,14,15) and the design of functional materials (16). Most of the currently available mammalian transgene-control devices share a common design principle, which ensures that the basic transcription controllers are compatible and could be assembled to higher order control networks (10–13). The basic control switches consist of synthetic transcription factors, prokaryotic response regulators fused to either a transactivation or a transsilencing domain, which bind and induce or repress chimeric promoters containing specific operator sites (17–19). Transcription can be fine-tuned by a specific trigger compound that modulates the promoter affinity of the chimeric transcription factors in a dose-dependent manner (20–24).
Besides optimal regulation performance the characteristics of the trigger molecule is of key importance when control circuits are designed for future gene- and cell-based therapies. Therefore, the latest generation of transgene control systems considers inducer molecules with minimal potential side effects such as those derived from endogenous metabolites (15,25–27) or physiologically inert molecules, such as licensed cosmetic compounds (28).
Vanillic acid is the oxidized form of vanillin and found at high concentrations in vanilla beans (29,30) and in Angelica sinensis, a plant used in traditional Chinese medicine (31). Vanillic acid has been associated with a variety of pharmacologic activities such as inhibiting snake venom activity (32,33), carcinogenesis (34), apoptosis (35,36) and inflammation (37) but it has become most popular for its pleasant creamy odour that is widely used in fragrances and licensed as a food additive (FAO/WHO Expert Committee on Food Additives, JECFA no. 959). Vanillic acid is also one of the main metabolites found in humans after consumption of green tee infusions (38). Overall, vanillic acid has all it takes to be a safe and physiologically inert inducer for gene switches designed for future therapeutic applications.
Caulobacter crescentus is a Gram-negative, oligotrophic freshwater bacterium that plays an important role in the carbon cycle by disposing of the soluble phenolic intermediates such as vanillic acid that result from fungal oxidative cleavage of lignin originating from decaying vascular plant material (39–41). Caulobacter crescentus can utilize vanillic acid as its sole carbon source by metabolizing it to protocatechuate and then to succinyl-CoA as well as acetyl-CoA, which is converted to metabolic energy in the citric acid cycle (42,43). Unless C. crescentus comes across vanillic acid the corresponding monooxygenase encoded by the VanAB gene cluster remains shut down as the transcriptional repressor VanR binds a distinct perfect inverted repeat operator (VanO; ATTGGATCCAAT) upstream of the vanAB promoter region (43). However, vanillic acids binds VanR and derepresses the metabolic pathway (43–47). Capitalizing on the C. crescentus vanillic acid-responsive transcriptional repressor VanR, we engineered different variants of a synthetic vanillic acid-responsive mammalian expression system (VAC) that show excellent regulation performance in mammalian cells as well as in mice receiving vanillic acid.
MATERIALS AND METHODS
Plasmid construction
Table 1 lists all the plasmids used in this study and provides detailed information about their construction. Using the basic local alignment search tool (BLAST) all of our promoter and operator sequences were shown to be free of binding sites specific from mammalian proteins.
Table 1.
Expression vectors and oligonucleotides designed and used in this study
| Plasmid | Description | Reference |
|---|---|---|
| pBP99 | Vector encoding a tetracycline-responsive SAMY expression unit (PhCMV*-1-SAMY-pA). pCF59 was restricted with BprPI/EcoRV and religated. | unpublished |
| pBP100 | Vector encoding an erythromycin-responsive SAMY expression unit (PETR3-SAMY-pA). | 18 |
| pcDNA3.1 | Commercial cloning vector containing a constitutive promoter (PhCMV). | Invitrogen |
| pCF59 | Vector encoding a PPIR-driven SAMY expression unit (PhCMV*-1-pA-IRES-PPIR-SAMY-pA). SAMY was excised from pSS158 using SpeI/BglII and ligated into pMF187 (SpeI/BglII). | unpublished |
| pCK9 | Vector encoding a PUREX8-driven SEAP expression unit (PUREX8-SEAP-pA). | 15 |
| pCK73 | Vector encoding a uric acid-responsive SEAP expression unit, driven by a size-reduced PhCMV (PhCMV-8xhucO-SEAP-pA). Size reduced PhCMV was amplified using OCK72: 5′- ccgctcgaggaagatctCGCGTTGACATTGATTATTGAC-3′ and OCK74: 5′- gcgacgtcccaagcttGCTTATATAGACCTC CCACC-3′ (lower case italics, restriction sites; upper case italics, annealing), digested with XhoI/HindIII and ligated into pCK9 (XhoI/HindIII). | 15 |
| pCK188 | Constitutive VanA4 expression vector (PSV40- VanA4-pA; VanA4, VanR-KRAB). VanR was PCR-amplified from pMG250 using OCK190: 5′- ggaattccaccATGGACATGCCGCGCATAAAG-3’ and OCK191: 5′ ggcgacgcgtaGTCGGCGCGAATGCTCCAC-3′ (lower case italics, restriction sites; upper case italics, annealing), digested with EcoRI/XbaI and ligated into pWW43 (EcoRI/BssHII). | This work |
| pCK189 | Constitutive VanA4 expression vector (PhCMV-VanA4-pA). VanR was PCR-amplified from pCK188 using OCK190: 5′- ggaattccaccATGGACATGCCGCGCATAAAG-3′ and OCK192: 5′- gctctagaCTTACCAGAGATCATTCCTTGC-3′ (lower case italics, restriction sites; upper case italics, annealing), digested with EcoRI/XbaI and ligated into pcDNA3.1 (EcoRI/XbaI). | This work |
| pCK191 | Vector encoding a PvanON8-driven SEAP expression unit (PVanON8-SEAP-pA; PvanON8, PhCMV-VanO8). Oligonucleotides OCK193: 5′-agcttATTGGATCCAATGCATTGG ATCCAATGGATTGGATCCAATCGATTGGATCCAATgatatcATTGGATCCAATGCATTGGATCCAATGGATTGGATCCAATCGATTGGATCCAATg-3′ and OCK194: 5′- aattcATTGGATCCAATCGATTGGATCC AATCCATTGGATCCAATGCATTGGATCCAATgatatcATTGGATCCAATCGATTGGATCCAATCCATTGGATCCAATGCATTGGATCCAATa-3′ (lower case italics, restriction sites; upper case, 8xVanO) were annealed and ligated directly into pCK73 using HindIII/EcoRI. | This work |
| pd2EYFP | Mammalian d2EYFP expression vector | Clontech |
| pMF187 | Dual-regulated expression vector (PhCMV*-1-MCSI-IRES-MCSII-pAI-PPIR-MCSIII-pAII). | 68 |
| pMG10 | Vector encoding a PTtgR1-driven SEAP expression unit (PTtgR1-SEAP-pA; PTtgR1, OTtgR-0bp-PhCMVmin). | 28 |
| pMG18 | Constitutive TtgA2 expression vector (PSV40-TtgA2-pA). | 28 |
| pMG19 | Constitutive TtgA3 expression vector (PSV40-TtgA3-pA). | 28 |
| pMG20 | Vector encoding a PTtgR2-driven SEAP expression unit (PTtgR2-SEAP-pA; PTtgR2, OTtgR-2bp-PhCMVmin). | 28 |
| pMG21 | Vector encoding a PTtgR3-driven SEAP expression unit (PTtgR3-SEAP-pA; PTtgR3, OTtgR-4bp-PhCMVmin). | 28 |
| pMG22 | Vector encoding a PTtgR4-driven SEAP expression unit (PTtgR4-SEAP-pA; PTtgR4, OTtgR-6bp-PhCMVmin). | 28 |
| pMG23 | Vector encoding a PTtgR5-driven SEAP expression unit (PTtgR5-SEAP-pA; PTtgR5, OTtgR-8bp-PhCMVmin). | 28 |
| pMG24 | Vector encoding a PTtgR6-driven expression unit (PTtgR6-SEAP-pA; PTtgR6, OTtgR-10bp-PhCMVmin). | 28 |
| pMG250 | Constitutive VanA1 expression vector (PSV40-VanA1-pA; VanA1, VanR-VP16). VanR was PCR-amplified from C. crescentus genomic DNA using ODF150: 5′-cggaattccaccATGGACATGCCGCGCATAAAGCCGGGC-3′ and ODF151: 5′- gtacgacgcgtggctgtacgcggaGTCGGCGCGAATGCT CCACGCCGCGC-3′ (lower case italics, restriction sites; upper case italics, annealing), digested with EcoRI/MluI and ligated into pWW35 (EcoRI/BssHII). | This work |
| pMG252 | Vector encoding a P1VanO2-driven SEAP expression unit (P1VanO2-SEAP-pA; P1VanO2, VanO2-0bp-PhCMVmin). VanO2 was created by annealing Oligos OMG65 (5′-phosphate-tcgacgtcaattcgcgaATTGGATCCAATagc gctATTGGATCCAATgatatcggacctgca-3′; lower case italics, restriction sites; upper case italics, VanO) and OMG66 (5′-phosphate-gcagttaagc gctTAACCTAGGTTAtcgcgaTAACCTAGGTTActatagcctgg-3′; lower case italics, restriction sites; upper case italics, VanO), and was directly ligated into SalI/SbfI-digested pWW1088 thereby resulting in P1VanO2. | This work |
| pMG256 | Constitutive VanA2 expression vector (PSV40-VanA2-pA; VanA2, VanR-p65). VanR was PCR-amplified from C. crescentus genomic DNA using ODF150: 5′-cggaattccaccATGGACATGCCGCGCATAAAGCCGGGC-3′ and ODF151: 5′- gtacgacgcgtggctgtacgcggaGTCGGCGCGAATGCT CCACGCCGCGC-3′ (lower case italics, restriction sites; upper case italics, annealing), digested with EcoRI/MluI and ligated into pMG18 (EcoRI/BssHII). | This work |
| pMG257 | Constitutive VanA3 expression vector (PSV40-VanA3-pA; VanA3, VanR-E2F4). VanR was PCR-amplified from C. crescentus genomic DNA using ODF150: 5′-cggaattccaccATGGACATGCCGCGCATAAAGCCGGGC-3′ and ODF151: 5′- gtacgacgcgtggctgtacgcggaGTCGGCGCGAATGCT CCACGCCGCGC-3′ (lower case italics, restriction sites; upper case italics, annealing), digested with EcoRI/MluI and ligated into pMG19 (EcoRI/BssHII). | This work |
| pMG262 | Vector encoding a P1VanO1-driven SEAP expression unit (P1VanO1-SEAP-pA; P1VanO1, VanO1-0bp-PhCMVmin). pMG252 was digested using either NruI/HindIII or Eco47III/HindIII. The resulting fragments were ligated to result in pMG262 harboring one VanO-operator element. | This work |
| pMG263 | Vector encoding a P1VanO3-driven SEAP expression unit (P1VanO3-SEAP-pA; P1VanO3, VanO3-0bp-PhCMVmin). pMG252 was digested using either EcoRV/HindIII or Eco47III/HindIII. The resulting fragments were ligated to result in pMG263 harboring three VanO-operator elements. | This work |
| pMG264 | Vector encoding a P1VanO4-driven SEAP expression unit (P1VanO4-SEAP-pA; P1VanO4, VanO4-0bp-PhCMVmin). pMG252 was digested using either EcoRV/HindIII or NruI/HindIII. The resulting fragments were ligated to result in pMG264 harboring four VanO-operator elements. | This work |
| pMG265 | Vector encoding a P2VanO2-driven SEAP expression unit (P2VanO2-SEAP-pA; P2VanO2, VanO2-2bp-PhCMVmin). 2bp-PhCMVmin-SEAP was excised from pMG20 (SbfI/XhoI) and ligated into pMG252 (SbfI/XhoI). | This work |
| pMG266 | Vector encoding a P3VanO2-driven SEAP expression unit (P3VanO2-SEAP-pA; P3VanO2, VanO2-4bp-PhCMVmin). 4bp-PhCMVmin-SEAP was excised from pMG21 (SbfI/XhoI) and ligated into pMG252 (SbfI/XhoI). | This work |
| pMG267 | Vector encoding a P4VanO2-driven SEAP expression unit (P4VanO2-SEAP-pA; P4VanO2, VanO2-6bp-PhCMVmin). 6bp-PhCMVmin-SEAP was excised from pMG22 (SbfI/XhoI) and ligated into pMG252 (SbfI/XhoI). | This work |
| pMG268 | Vector encoding a P5VanO2-driven SEAP expression unit (P5VanO2-SEAP-pA; P5VanO2, VanO2-8bp-PhCMVmin). 8bp-PhCMVmin-SEAP was excised from pMG23 (SbfI/XhoI) and ligated into pMG252 (SbfI/XhoI). | This work |
| pMG269 | Vector encoding a P6VanO2-driven SEAP expression unit (P6VanO2-SEAP-pA; P6VanO2, VanO2-10bp-PhCMVmin). 10bp-PhCMVmin-SEAP was excised from pMG24 (SbfI/XhoI) and ligated into pMG252 (SbfI/XhoI). | This work |
| pMG270 | Autoregulated vanillic acid-controlled SEAP expression vector (P1VanO2-SEAP-IRESPV-VanA1-pA). VanA1 was excised from pMG250 (SspI/NotI) and ligated into pMG252 (SspI/NotI). | This work |
| pPur | Selection vector conferring puromycin resistance. | Clontech |
| pSAM200 | Constitutive tTA expression vector (PSV40-tTA-pA). | 69 |
| pSEAP2-Control | Constitutive SEAP expression vector (PSV40-SEAP-pA). | Clontech |
| pSS158 | PhCMV-driven SAMY expression vector (PhCMV-SAMY-pA). | 50 |
| pSV2neo | Selection vector conferring neomycin resistance. | Clontech |
| pWW35 | Constitutive ET1 expression vector (PSV40-ET1-pA; ET1, E-VP16). | 18 |
| pWW43 | PSV40-driven expression vector encoding the macrolide-dependent transrepressor ET4: PSV40-ET4-pA; ET4, E-KRAB. | 18 |
d2EYFP, destabilized variant of the yellow fluorescent protein; E2F4, transactivation domain of the human E2F transcription factor 4; ET1, macrolide-dependent transactivator (E-VP16); ET4, macrolide dependent transrepressor (E-KRAB); ETR, operator specific for macrolide-dependent transactivators; IRESPV, polioviral internal ribosome entry site; KRAB, Human Kruppel-associated box protein; NF-κB, human transcription factor; OTtgR, TtgR-specific operator; p65, transactivation domain of NF-κB; pA, polyadenylation site; PETR3, macrolide-responsive promoter; PhCMV, human cytomegalovirus immediate early promoter; PhCMVmin, minimal PhCMV; PhCMV*-1, tetracycline-responsive promoter; PSV40, simian virus 40 promoter; PTtgR1-6, phloretin-responsive promoters containing different spacers between OTtgR and PhCMVmin; PUREX8, uric acid- responsive promoter containing 8 hucO-operator sites in 3′ of a PCMV promoter; P1-6VanO2, vanillic acid-responsive promoters containing different spacers between VanO and PhCMVmin; P1VanO1-4, vanillic acid-responsive promoters harboring 1, 2, 3 or 4 VanO-operator repeats in front of PhCMVmin; SEAP, human placental secreted alkaline phosphatase; SAMY, Bacillus stearothermophilus-derived secreted α-amylase; TtgR, repressor of the Pseudomonas putida DOT-T1E ABC multi-drug efflux pump; TtgA2, phloretin-dependent transactivator (TtgR-p65); TtgA3, phloretin-dependent transactivator (TtgR-E2F4); VanAB, gene cluster within C. crescentus that plays a role within the vanillic acid metabolism; VanO, VanR specific operator; VanR, repressor of the C. crescentus VanAB gene cluster; VP16, Herpes simplex virus-derived transactivation domain.
Cell culture
Wild-type Chinese hamster ovary cells (CHO-K1, ATCC: CCL-61) and their derivatives were cultivated in standard medium: ChoMaster® HTS (Cell Culture Technologies, Gravesano, Switzerland) supplemented with 5% (v/v) foetal calf serum (FCS, PAN Biotech GmbH, Aidenbach, Germany, Cat. No. 3302, Lot No. P251110) and 1% (v/v) penicillin/streptomycin solution (Biowest, Nuaillé, France, Cat. No. L0022-100, Lot No. S07497L0022). Human embryonic kidney cells (HEK293-T, ATCC: CRL-11268), human cervical carcinoma cells (HeLa, ATCC: CCL-2), African green monkey kidney cells (COS-7, ATCC: CRL-1651), baby hamster kidney cells (BHK-21, ATCC: CCL10), human fibrosarcoma cells (HT-1080, ATCC: CCL-121) and mouse fibroblasts (NIH/3T3, ATCC CRL-1658) were cultivated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FCS and 1% penicillin/streptomycin solution. All cell lines were cultivated at 37°C in a humidified atmosphere containing 5% CO2. Viable cell numbers were determined using a Casy® Cell Counter and Analyser Model TT (Roche Diagnostics GmbH, Basel, Switzerland).
Transfection
The CHO-K1 cells were transiently transfected using 0.5 μg of total plasmid DNA (equimolar plasmid ratios were used for co-transfections) according to an optimized calcium phosphate-based protocol (48). As for other cell lines the transfection efficiency was tuned to reach 35% ± 5% as determined by parallel transfections using pd2EYFP (Clontech, Mountain View, CA, USA) (15). In brief, 50 000 CHO-K1 cells were seeded into each well of a 24-well plate and cultured overnight. The plasmid DNA was then diluted to a total volume of 25 μl with 0.5 M CaCl2 solution, which was mixed with 25 μl 2 × HBS solution (50 mM HEPES/280 mM NaCl/1.5 mM Na2HPO4, pH 7.1). This mixture was incubated for 15 min at room temperature before the precipitates were directly added into the well and centrifuged onto the cells (5 min at 1200 g). After 3 h, the cells were treated with 0.5 ml glycerol solution (ChoMaster® HTS medium containing 15% glycerol) for 60 s. After washing once with phosphate-buffered saline (PBS, Dulbecco's phosphate-buffered saline; Invitrogen, Basel, Switzerland, Cat. No. 21600-0069), the cells were cultivated in 0.5 ml standard ChoMaster® HTS medium in the presence or absence of different concentrations of vanillic acid or its derivatives. For the transient transfection of BHK-21, COS-7 and HEK-293 cells, a plasmid DNA-Ca2PO4 precipitate was prepared and applied to the 50 000 cells cultivated per well of a 24-well plate, as described above. The HEK-293 and COS-7 cells were washed once with PBS after 3 h incubation with the DNA-Ca2PO4 precipitate and subsequently cultivated in standard DMEM, while BHK-21 and HeLa cells were incubated overnight with the precipitates and then cultivated in DMEM after being washed once with PBS. The HT-1080 and NIH/3T3 cell lines were transfected with Fugene™ 6 (Roche Diagnostics AG, Basel, Switzerland, Cat. No. 11814443001) according to the manufacturer's protocol and cultivated in the cell culture medium specified above. After transfection, all cells were cultivated in DMEM supplemented with different concentrations of vanillic acid and reporter protein levels were profiled 48 h after transfection, unless indicated otherwise.
Construction and characterization of the stable cell line CHO-VAC
The CHO-VAC12 cell line, transgenic for vanillic acid-controlled SEAP expression, was constructed by sequential co-transfection and clonal selection of (i) pMG250 (PSV40-VanA1-pA) and pSV2neo (Clontech, Cat. No. 6172-1) at a ratio of 20:1 to result in the cell line CHO-VanA, (ii) CHO VanA was subsequently co-transfected with pMG252 (P1VanO2-SEAP-pA) and pPur (Clontech, Cat. No. 6156-1) (ratio of 20:1), and the resulting double-transgenic cell line CHO-VAC12, showing vanillic acid-responsive SEAP production, was chosen after clonal selection. To assess the vanillic acid-mediated transgene regulation characteristics, 100 000 cells/ml of CHO-VAC12 were cultivated for 48 h in standard ChoMaster® HTS medium supplemented with increasing concentrations of vanillic acid, ranging from 0 to 1000 μM. Reversibility of the vanillic acid-mediated SEAP production was assessed by culturing CHO-VAC12 (100 000 cells/ml) for 144 h while alternating vanillic acid concentrations from 0 to 500 μM every 48 h.
Quantification of reporter gene expression
Production of the human placental secreted alkaline phosphatase (SEAP) and the heat-stable Bacillus stearothermophilus-derived secreted α-amylase was quantified as described previously (49,50).
Inducer compounds: vanillic acid and its derivatives
The following were prepared as 50 mM stock solutions in 70% (v/v) EtOH and adjusted to pH 7 using 2.5 M NaOH when required: 2-vanillic acid (2-hydroxy-3-methoxybenzoic acid, ABCR, Karlsruhe, Germany, Cat. No. AB177480), 2-vanillin (2-hydroxy-3-methoxybenzaldehyde, ABCR, Cat. No. AB117268), acetovanillone (4′-hydroxy-3′-methoxyacetophenone, ABCR, Cat. No. AB125832), benzaldehyde (Acros, Geel, Belgium, Cat. No. 378361000), benzoic acid (ABCR, Cat. No. AB113879), benzyl acetate (ABCR, Cat. No. AB131641), benzyl alcohol (ABCR, Cat. No. AB171491), ethyl-vanillate (ABCR, Cat. No. AB178082), ethyl-vanillin (3-ethoxy-4-hydroxybenzaldehyde, ABCR, Cat. No. AB126381), eugenol (ABCR, Cat. No. AB111881), homovanillic acid (Sigma, St Louis, MO, USA, Cat. No. H1252-1G), isovanillic acid (3-hydroxy-4-methoxybenzoic acid, ABCR, Cat. No. AB117271), isovanillin (3-hydroxy-4-methoxybenzaldehyde, ABCR, Cat. No. AB117270), methyl-vanillate (ABCR, Cat. No. AB132603), protocatechualdehyde (3,4-dihydroxybenzaldehyde, ABCR, Cat. No. AB110948) and vanillin (ABCR, Cat. No. AB117415). Vanillic acid (Fluka, Buchs, Switzerland, Cat. No. 94770-10G) was prepared as a 50 mM stock solution in water and adjusted to pH 7 with 2.5 M NaOH. All solutions were used at a final concentration of 250 µM unless otherwise indicated. Tetracycline (Sigma, Cat. No. T7660) was prepared as a 1 mg/ml stock solution in H2O, and erythromycin (Fluka, Cat. No. 45673) as a stock solution of 1 mg/ml in ethanol. Both antibiotics were used at a final concentration of 2 µg/ml.
In vivo methods
The CHO-K1 cells, engineered for vanillic acid-controlled SEAP expression (CHO-VAC12) and for constitutive SEAP expression [CHO-SEAP18 (51)], were encapsulated in 400 µm alginate-poly-(l-lysine)-alginate beads (400 cells/capsule) using an Inotech Encapsulator Research IE-50R (EncapBioSystems Inc., Greifensee, Switzerland) according to the manufacturer's instructions and the following parameters: 0.2 mm nozzle, 20 ml syringe at a flow rate of 405 U, nozzle vibration frequency of 1116 Hz and 950 V for bead dispersion. Female OF1 mice (oncins France souche 1, Charles River Laboratories, France; n = 8 for all experiments) were implanted intraperitoneally with 700 µl of FCS-free ChoMaster® HTS containing 4 × 106 encapsulated CHO-VAC12 cells. Control mice were implanted with microencapsulated CHO-K1 or CHO-SEAP18. One hour after implantation, vanillic acid (50 mg/ml in PBS, pH7) was administered twice daily by injection for the next three days at doses ranging from 0 to 500 mg/kg. After 72 h, the mice were sacrificed, blood samples were collected retroorbitally and SEAP levels were quantified in the serum, which was isolated using a microtainer SST tube according to the manufacturer's instructions (Beckton Dickinson, Plymouth, UK, Cat. No. 365968). All the experiments involving mice were performed according to the directives of the European Community Council (86/609/EEC), approved by the French Republic (No. 69266310) and performed by Marie Daoud El-Baba at the Université de Lyon, F-69622 Villeurbanne, France.
Preparation of mouse organ extracts
Organs (kidney, liver, lung, muscle) of female OF1 mice were homogenized in PBS (1:2 (v/v); organ:PBS) using an IKA T18 basic ULTRA-TURRAX® disperser (IKA GmbH, Staufen, Germany), mixed with 10% (v/v) hydrochloric acid (Fluka, Cat. No. 35327) for 5 s using a vortex and incubated with 6 ml ethyl acetate (Sigma Cat. No. 270989) at 4°C while shaking overnight to extract vanillic acid from the tissue samples. As positive control a liver and a kidney sample were spiked with 100 µl of a 50 mM vanillic acid stock solution prior to tissue homogenization. The tissue homogenates were then centrifuged for 10 min at 6000 g and 4°C and the supernatants were dried using a rotary evaporator (Rotavapor R-215, Büchi Labortechnik AG, Flawil, Switzerland). Dried samples were re-suspended in 300 µl methanol (Sigma, Cat. No. 322415), dried again using an Eppendorf concentrator (Eppendorf concentrator plus, Vaudaux-Eppendorf, Schönenbuch, Switzerland, Cat. No. 5305 000.304) and were finally resuspended in 300 µl dimethyl sulfoxide (DMSO, Sigma, Cat. No. D4540). 5 µl of organ extracts were mixed with 500 µl ChoMaster® HTS and added to CHO-K1 cultures containing the VACOFF or the VACON expression systems.
RESULTS
Design of vanillic acid-responsive mammalian transcription-control devices
Using the basic parts that control vanillic acid metabolism in C. crescentus, the transcriptional repressor VanR and its target operator VanO, we have designed two different vanillic acid-responsive transcription-control devices that either induce (VACON) or repress (VACOFF) target gene transcription in the presence of the food additive vanillic acid.
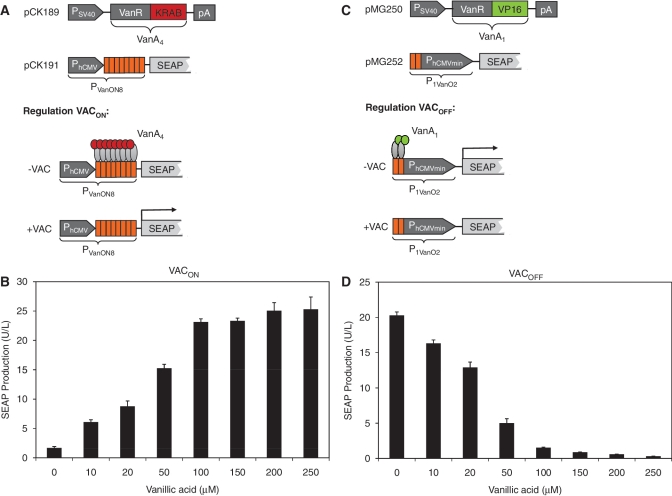
The VACON system was assembled by fusing VanR C-terminally to the human Krueppel-associated box [KRAB; (52)] domain to generate a mammalian transsilencer (VanA4) that binds and represses a chimeric promoter (PVanON8) consisting of a constitutive human cytomegalovirus immediate early promoter (PhCMV) containing an octameric VanO operator module (VanO8) immediately downstream. Vanillic acid triggers the release of VanA4, which derepresses PVanON8 and results in PhCMV-driven transgene expression (Figure 1A). When co-transfecting pCK189 (PhCMV-VanA4-pA) and pCK191 (PVanON8-SEAP-pA) into CHO-K1 expression of SEAP was insignificant (1.70 ± 0.23 U/L). However, when adding increasing concentrations of vanillic acid (0–250 μM) SEAP expression was dose-dependently induced up to a level of 25.3 ± 2.08 U/l (Figure 1B).
Figure 1.
Design and validation of the VACON and VACOFF systems. (A and B) Diagram and functionality of the VACON system. (A) VanR was fused to the KRAB transrepressor domain, a human Krueppel-associated box protein, resulting in VanA4 (VanR-KRAB), which was expressed by the constitutive human cytomegalovirus immediate early promoter (PhCMV) (pCK189). The vanillic acid-inducible promoter PVanON8 harbors eight VanO operator sites immediately 3′ of a constitutive Simian virus 40 promoter (PSV40) that was set to drive the human placental secreted alkaline phosphatase (SEAP) (pCK191). OFF status: VanA4 is constitutively expressed and, in the absence of vanillic acid (–VAC), binds to PVanON8 and represses SEAP expression. ON status: in the presence of vanillic acid (+VAC), VanA4 is released from PVanON8 which fully induces SEAP expression. (B) CHO-K1 cells were transiently transfected with pCK189 (PSV40-VanA4-pA) and pCK191 (PVanON8-SEAP-pA) and SEAP-expression profiles were assessed 48 h after cultivation of the cells in medium containing different concentrations of vanillic acid (0–250 μM). (C and D) Diagram and functionality of the VACOFF system. (C) VanR was fused to the VP16 transactivation domain of the Herpes simplex virus, resulting in VanA1 (VanR-VP16), which was expressed by the constitutive Simian virus 40 promoter (PSV40) (pMG250). The vanillic acid-responsive promoter P1VanO2 contains two VanO operator sites (ATTGGATCCAATAGCGCTATTGGATCCAAT; VanR binding sites in italics) immediately 5′ of a minimal human cytomegalovirus immediate-early promoter (PhCMVmin), which was set to drive the human placental secreted alkaline phosphatase (SEAP) (pMG252). ON status: VanA1 is constitutively expressed and, in the absence of vanillic acid (–VAC), binds to P1VanO2 and activates SEAP expression. OFF status: in the presence of vanillic acid (+VAC), VanA1 is released from P1VanO2 which shuts down SEAP expression. (D) CHO-K1 cells were transiently transfected with pMG250 (PSV40-VanA1-pA) and pMG252 (P1VanO2-SEAP-pA) and SEAP-expression profiles were assessed 48 h after cultivation of the cells in medium containing different concentrations of vanillic acid (0–250 μM).
The VACOFF system was designed by fusing VanR C-terminally to the Herpes simplex virus transactivation domain VP16 to generate a chimeric transcription factor (VanA1) that binds to two VanO-operator sequences separated only by an Eco47III restriction site (VanO2, 5′-ATTGGATCCAATagcgctATTGGATCCAAT-3′, VanO operator upper case) and activates a 3′-placed minimal version of PhCMV (PhCMVmin) (P1VanO2). Vanillic acid triggers the release of VanA1, which inactivates P1VanO2 and shuts transgene expression down (Figure 1C). Co-transfection of pMG250 (PSV40-VanA1-pA), and pMG252 (P1VanO2-SEAP-pA) into CHO-K1 resulted in high SEAP expression levels (20.3 ± 0.5 U/l) comparable to an isogenic constitutive SEAP control vector (pSEAP2-Control; PSV40-SEAP-pA) (33.9 ± 0.2 U/l). However, when adding increasing concentrations of vanillic acid (0–250 μM) SEAP was dose-dependently shut down to almost complete repression (0.31 ± 0.01 U/l) (Figure 1D).
In order to assess the impact of vanillic acid on mammalian cell cultures, we exposed CHO-K1 cells, transiently transfected with pSEAP2-control (PSV40-SEAP-pA), to increasing concentrations of vanillic acid (0–1000 μM) and profiled SEAP production as well as viable cell numbers for 48 h. The observation that SEAP levels and cell numbers remained equally high at all vanillic acid concentrations (0 μM: 33.9 ± 0.17 U/l, 9.08 ± 0.84 (106 cells/ml); 1000 μM: 35.4 ± 1.74 U/l, 9.22 ± 0.9 (106 cells/ml)) indicates that the licensed food additive has no obvious impact on cell physiology below a concentration of 1 mM. This observation was confirmed by scoring the viability of CHO-K1 and HEK-293 exposed to regulation-effective vanillate concentrations (Supplementary Figure S1). Since the basic VACOFF system shows tighter regulation performance, no epigenetic imprinting compared to the KRAB-containing VACON design (53,54) and is the configuration of choice for the assembly of advanced synthetic multi-control gene networks (13), we chose to use VACOFF in all follow-up studies.
VACOFF-controlled transgene expression in various mammalian cell lines
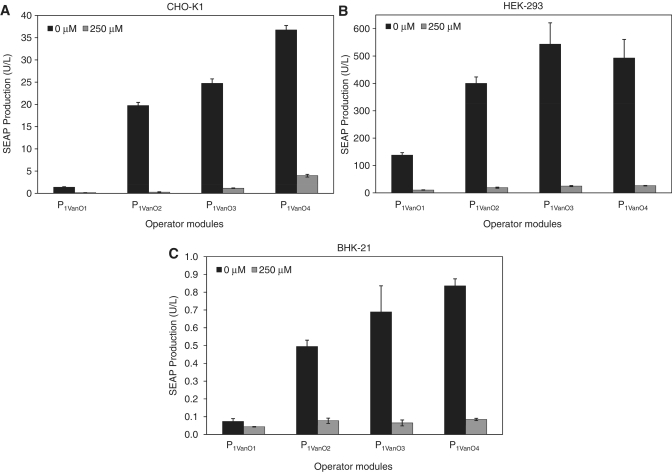
Versatility of the VACOFF-system was assessed by co-transfection of pMG250 (PSV40-VanA1-pA) and pMG252 (P1VanO2-SEAP-pA) into different rodent, monkey and human cell lines followed by cultivation for 48 h in the presence (+) and absence (–) of 250 μM vanillic acid (BHK-21: –, 0.52 ± 0.02 U/l; + , 0.05 ± 0.01 U/l; COS-7: –, 17.95 ± 0.55 U/l; +, 0.45 ± 0.02 U/l; HEK-293: –, 484.96 ± 54.24 U/l; +, 20.85 ± 1.24 U/l; HeLa: –, 7.89 ± 0.99 U/l; +, 1.38 ± 0.11 U/l; HT-1080: –, 0.65 ± 0.11 U/l; +, 0.07 ± 0.01 U/l; and NIH/3T3: –, 7.41 ± 0.16 U/l; +, 0.28 ± 0.12 U/l). SEAP production levels indicated that VACOFF-controlled transgene regulation was functional in all tested cell lines, suggesting a broad applicability of this control technology.
Optimizing the VACOFF system I—assessment of promoter variants containing varying numbers of VanO operator modules
Altering the number of operator modules in front of an inducible minimal promoter impacts the regulation performance regarding (i) maximal expression levels, as an increasing number of operator sites can recruit more transactivators and (ii) basal expression of the system's repressed state, as more transactivators have to be released from an increasing number of operator sites (27,55). To evaluate the optimal number of VanO-operator sites for VACOFF-controlled gene regulation, we constructed VanA1-responsive promoter variants harbouring either one (pMG262, P1VanO1-SEAP-pA; P1VanO1, NruI-VanO-0bp-PhCMVmin), two (pMG252, P1VanO2-SEAP-pA; P1VanO2, NruI-VanO-Eco47III-VanO-0bp-PhCMVmin), three (pMG263, P1VanO3-SEAP-pA; P1VanO3, NruI-VanO-Eco47III-VanO-6bp-VanO-0bp-PhCMVmin) or four (pMG264, P1VanO4-SEAP-pA; P1VanO4, NruI-VanO-Eco47III-VanO-6bp-VanO-Eco47III-VanO-0bp-PhCMVmin) operators immediately 5′ of the minimal promoter. Co-transfection of corresponding SEAP expression vectors harbouring the different promoter variants with pMG250 (PSV40-VanA1-pA) into CHO-K1, HEK-293 and BHK-21 cells and scoring of SEAP levels 48 h after cultivation in the presence and absence of 250 μM vanillic acid revealed their transcriptional performances. In all cell lines, it was observed that an increasing number of operator modules resulted in higher maximum expression levels but also in higher basal expression of the repressed status. The dimeric promoter configuration (pMG252, P1VanO2-SEAP-pA) resulted in the best regulation performance with a 72-fold induction factor in CHO-KI cells and 23-fold in HEK-293 cells, while the trimeric promoter set-up showed the best performance in BHK-21 cells with a ON/OFF control factor of 11 (Figure 2A–C).
Figure 2.
Validation of vanillic acid-responsive promoter variants containing different numbers of VanO operator modules. Vectors encoding SEAP expression driven by a vanillic acid-responsive promoter harbouring monomeric (pMG262), dimeric (pMG252), trimeric (pMG263) or tetrameric (pMG264) operator modules were co-transfected with pMG250 (PSV40-VanA1-pA) into (A) CHO-K1, (B) HEK-293 and (C) BHK-21 cells and SEAP production was scored after cultivation for 48 h in the presence and absence 250 µM vanillic acid.
Optimizing the VACOFF system II—engineering of different vanillic acid-dependent transactivator variants
Basal and maximum expression of synthetic mammalian gene regulation systems can be influenced by the kind of mammalian transactivation domain, which is fused to the bacterial DNA-binding protein (19,56). We have therefore evaluated different transactivation domains by designing vectors containing VanR fused to the Herpes simplex-derived VP16 domain (pMG250, PSV40-VanA1-pA; VanA1,VanR-VP16), the human nuclear factor kappa B (NF-κB) -derived transactivation domain p65 (pMG256, PSV40-VanA2-pA; VanA2, VanR-p65) or the transactivation domain of the human E2F transcription factor 4 (E2F4) (pMG257, PSV40-VanA3-pA; VanA3, VanR-E2F4). Co-transfection of corresponding transactivator-encoding expression vectors with pMG252 (P1VanO2-SEAP-pA) into CHO-K1, HEK-293, BHK-21, HT-1080 and HeLa cells and scoring of SEAP levels 48 h after cultivation in the presence and absence of 250 μM vanillic acid revealed the performances of the individual transactivators. The basal as well as maximum expression levels varied considerably amongst the different transactivators. In general, E2F4 showed the weakest maximum expression levels in all tested cell lines and could therefore not compete with the best-in-class regulation performance of VP16 and p65. In CHO-K1, VP16 exhibited the highest maximum expression paired with the lowest leakiness, making it the transactivator of choice for this cell line (Table 2). Peak expression in HEK-293 cells was achieved by p65, although the high basal expression again rendered VP16 the best choice in terms of the regulation factor. In HeLa cells, only VP16 provided significant transactivation, while in BHK-21 and HT-1080 cells p65 offered the best performance (Table 2). Due to their cell-type specificity, their graded maximum transcription initiation capacities and their basal expressions in the repressed status, the three transactivators offered a selection of different expression windows and regulation factors depending on the cell line and application chosen.
Table 2.
Combinatorial profiling of different VACOFF transactivators and promoters in various cell types
| SEAP Production (U/l) |
||||||
|---|---|---|---|---|---|---|
| pMG252/pMG250 (VP16) | pMG252/pMG256 (p65) | pMG252/pMG257 (E2F4) | ||||
| Vanillic acid (250 µM) | − | + | − | − | − | + |
| BHK-21 | 0.52 ± 0.02 | 0.05 ± 0.01 | 1.22 ± 0.05 | 0.08 ± 0.01 | 0.24 ± 0.05 | 0.04 ± 0.01 |
| CHO-K1 | 27.06 ± 0.16 | 0.59 ± 0.01 | 26.31 ± 1.38 | 2.13 ± 0.17 | 15.02 ± 0.44 | 1.35 ± 0.13 |
| HEK-293 | 484.96 ± 54.24 | 20.85 ± 1.24 | 1032.46 ± 63.34 | 54.2 ± 4.00 | 203.79 ± 36.96 | 12.79 ± 2.12 |
| HeLa | 7.89 ± 0.99 | 1.38 ± 0.11 | 1.37 ± 0.06 | 1.49 ± 0.08 | 1.49 ± 0.12 | 1.44 ± 0.07 |
| HT-1080 | 0.65 ± 0.11 | 0.07 ± 0.01 | 1.28 ± 0.15 | 0.13 ± 0.02 | 0.13 ± 0.03 | 0.02 ± 0.00 |
SEAP production was quantified 48 h after transient co-transfection of pMG252 (P1VanO2-SEAP-pA) and either pMG250 (PSV40-VanA1-pA; VanR-VP16), pMG256 (PSV40-VanA2-pA; VanR-p65) or pMG257 (PSV40-VanA3-pA; VanR-E2F4).
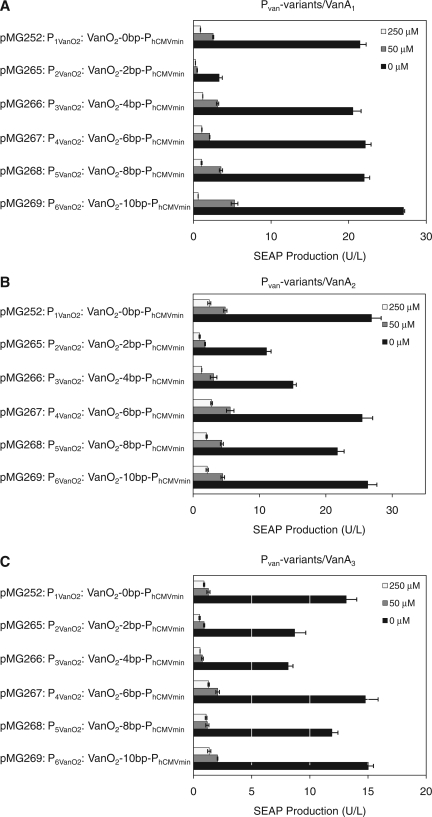
Optimizing the VACOFF system III—promoter variants that differ in the distance between VanO operator modules and the minimal promoter
Maximum transcription levels and minimum leakiness are not only influenced by the number of operator modules recruiting the transactivators, but also by the relative spacing of the operator modules to the minimal promoter and the resulting torsion angle of the operator-bound transactivator (57,58). To further assess the optimal design of PVanO configurations, we engineered spacers of 2 bp increments between the two VanO modules (VanO2) and PhCMVmin, resulting in SEAP expression vectors, isogenic to pMG252 (P1VanO2-SEAP-pA) which harbours the default 18 bp between VanO2 and PhCMVmin, while pMG265 (P2VanO2, VanO2-2bp-PhCMVmin), pMG266 (P3VanO2, VanO2-4bp-PhCMVmin), pMG267 (P4VanO2, VanO2-6bp-PhCMVmin), pMG268 (P5VanO2, VanO2-8bp-PhCMVmin) and pMG269 (P6VanO2, VanO2-10bp-PhCMVmin) contain an additional 2, 4, 6, 8 or 10 bp between VanO2 and PhCMVmin. Any of the vectors comprising the vanillic acid-responsive promoter variants were co-transfected with pMG250 (PSV40-VanA1-pA), pMG256 (PSV40-VanA2-pA) or pMG257 (PSV40-VanA3-pA) into CHO-K1 cells to evaluate the optimal promoter configuration independently of the transactivator variant. The expression of SEAP was scored 48 h after cultivation of the transfected cells in media containing 0, 50 or 250 μM of vanillic acid (Figure 3A–C). Generally, P1VanO2 exhibited the best regulation performance in terms of maximal expression and minimal leakiness. The promoter variants with 2, 4 and 10 bp spacers (pMG265, pMG266 and pMG269) showed expression performances, which were comparable to the best-in-class configuration harbouring no spacer (pMG252). However, pMG267 and pMG268 (6 and 8 bp increments) resulted in a much lower maximal expression. All promoter variants displayed similar expression profiles for all VanA transactivator variants, implying that chimeric promoters and transactivators can be independently optimized.
Figure 3.
Combinatorial validation of the VACOFF system in different transactivator and promoter configurations. VACOFF transactivators employing different transactivation domains (A: VanA1, VanR-VP16; pMG250) (B: VanA2, VanR-p65; pMG256) (C: VanA3, VanR-E2F4; pMG257) were co-transfected with different vanillic acid-responsive promoter variants containing 0 (P1VanO2; pMG252), 2 (P2VanO2; pMG265), 4 (P3VanO2; pMG266), 6 (P4VanO2; pMG267), 8 (P5VanO2; pMG268) and 10 (P6VanO2; pMG269) base-pair linkers between VanO and the minimal promoter into CHO-K1 cells. All promoter variants drove SEAP expression and the production was profiled 48 h after cultivation of the cells in media containing different concentrations of vanillic acid (0, 50 and 250 μM).
Design of an autoregulated version of the vanillic acid-responsive VACOFF expression system
Besides the conventional two-vector design, consisting of a plasmid for constitutive expression of a transactivator and a second vector encoding the responsive promoter that drives transcription of the gene of interest, we also designed an autoregulated single-vector set-up for vanillic acid-controlled transgene expression. The autoregulated design consists of P1VanO2 producing a dicistronic transcript sequentially encoding SEAP and VanA1 preceded by a polioviral internal ribosome entry site (IRESPV) (P1VanO2-SEAP-IRESPV-VanA1-pA). Whereas SEAP is translated in a classical cap-dependent manner, translation initiation of VanA1 is mediated by IRESPV. Such an autoregulated configuration represents the most compact transgene-control design, is convenient for overcoming undesired expression variations in transient transfections and is useful for the design of noise-resistant gene networks (59). Transfection of pMG270 (P1VanO2-SEAP-IRESPV-VanA1-pA) into CHO-K1 cells started leaky expression of VanA1, which then in an autoregulated feedback initiated full activation of the VanA-responsive promoter P1VanO2 to reach maximum levels of SEAP production and co-cistronic expression of VanA1. When 250 μM vanillic acid was added to the culture, the autoregulated induction was interrupted and SEAP expression remained in the fully repressed state (0 μM vanillic acid: 9.58 ± 0.29 U/l; 250 μM vanillic acid: 0.30 ± 0.04 U/l). The SEAP levels for this experiment were profiled after 48 h.
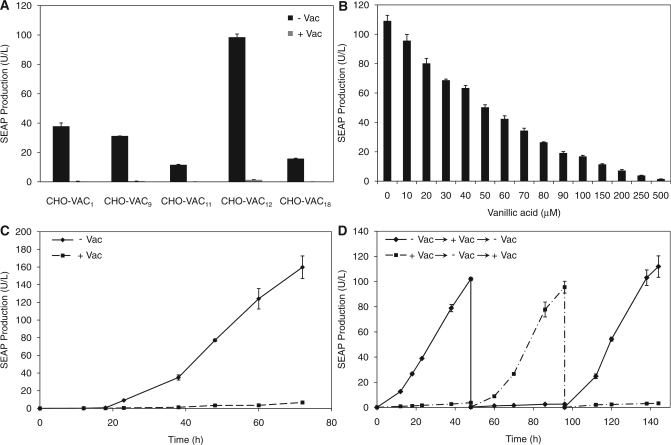
Expression kinetics, adjustability and reversibility of VACOFF-controlled transgene expression in a stably transgenic CHO-K1 cell line
Detailed characterization of long-term expression, adjustability and reversibility of the VACOFF system requires the creation of a stable cell line. We therefore established stable CHO-K1-derived VACOFF-containing cell lines (CHO-VAC) by sequential transfection of pMG250 (PSV40-VanA1-pA) and pMG252 (P1VanO2-SEAP-pA) and subsequent clonal selection. The expression of SEAP was scored for five randomly chosen single clones after cultivation for 48 h in the presence and absence of 500 μM vanillic acid. All clones showed similar basal expression, but varied substantially in their maximum SEAP expression levels (Figure 4A). With a regulation factor of 92-fold repression, CHO-VAC12 showed the best regulation performance out of the five single clones. Furthermore, CHO-VAC12 revealed precise adjustability according to the level of vanillic acid administered to the medium (Figure 4B) and displayed unchanged maximal expression and repression levels in long-term cultures of up to 90 days (Day 0, ON: 109.13 ± 3.80 U/l, OFF: 1.19 ± 0.04 U/l; Day 90, ON: 104.36 ± 5.75 U/l, OFF: 1.45 ± 0.09 U/l). Besides excellent adjustability, rapid response kinetics and reversibility are essential for high-performance mammalian gene regulation systems. When cultivating CHO-VAC12 for 72 h, the system showed exponential SEAP expression kinetics without vanillic acid in the medium, whereas upon addition of 500 μM of the trigger compound, SEAP expression levels did not significantly exceed the background levels (Figure 4C). Full reversibility of the VACOFF-system was monitored when cultivating CHO-VAC12 for 144 h while alternating the vanillic acid concentration every 48 h between 0 and 500 μM (Figure 4D).
Figure 4.
Characterization of stably transgenic vanillic acid-responsive CHO-K1 cell lines. CHO-K1 was stably co-transfected with pMG250 (PSV40-VanA1-pA) and pMG252 (P1VanO2-SEAP-pA) and vanillic acid-responsive SEAP expression of the resulting CHO-VAC cell lines was analysed. (A) After clonal expansion, individual clones were assessed for their vanillic acid-responsive regulation performance. SEAP levels were profiled after cultivation for 48 h in the presence and absence of vanillic acid (± VAC). (B) The dose–response profile of CHO-VAC12 was profiled after cultivation for 48 h in medium containing increasing concentrations of vanillic acid (0–500 μM). (C) SEAP expression kinetics of CHO-VAC12 cultivated for 72 h in the presence and absence of 250 μM vanillic acid (± VAC). (D) Reversibility of vanillic acid-responsive transgene expression following periodic addition and removal of the inducer. CHO-VAC12 (80 000 cells/ml) were cultivated for 144 h in the presence and absence of 250 μM vanillic acid (± VAC). Every 48 h, the cell density was re-adjusted to 80 000 cells/ml and the cells were cultivated in fresh medium with reversed vanillic acid concentrations.
Compatibility of the VACOFF-system with other transgene regulation systems
The broad applicability of mammalian transgene regulation systems within complex synthetic gene networks is also determined by their ability to function interference-free alongside other regulation systems that capitalize on different inducers (11,12,60,61). To assess this important requirement, we transiently transfected CHO-VAC12 with the established components of the tetracycline- (TETOFF) (21) or the erythromycin- (EOFF) (18) responsive expression systems. Both, the TETOFF (pSAM200, PSV40-tTA-pA; pBP99, PhCMV*-1-SAMY-pA) and the EOFF (pWW35, PSV40-ET1-pA; pBP100, PETR3-SAMY-pA) systems drove the expression of the heat-stable Bacillus stearothermophilus-derived secreted α-amylase [SAMY, (50)] under a tetracycline- or an erythromycin-responsive promoter, respectively. The levels of SEAP and SAMY were scored 48 h after transfection and cultivation of the CHO-VAC12 cell line in the presence or absence of the different inducers (500 µM vanillic acid/2 µg/ml tetracycline/2 µg/ml erythromycin) and a completely compatible, interference free and fully functional regulation performance in the same mammalian cells was demonstrated for the VACOFF, TETOFF and EOFF systems (Table 3).
Table 3.
Compatibility of vanillic acid-, erythromycin- and tetracycline-responsive transgene control systems
| Inducer | −Tet /−Vac | −Tet /+Vac | +Tet/−Vac | +Tet/+Vac |
|---|---|---|---|---|
| CHO-VAC12 transfected with the tetracycline-responsive regulation system | ||||
| Relative SEAP production (%) | 100 ± 5.62 | 2.18 ± 0.31 | 101.04 ± 6.21 | 2.07 ± 0.29 |
| Relative SAMY production (%) | 100 ± 5.03 | 99.06 ± 4.53 | 4.53 ± 0.52 | 5.01 ± 1.61 |
| Inducer | −EM/−Vac | −EM/+Vac | + EM/−Vac | +EM/+Vac |
| CHO-VAC12 transfected with macrolide-responsive regulation system | ||||
| Relative SEAP production (%) | 100 ± 6.31 | 2.56 ± 0.09 | 102.19 ± 7.08 | 1.95 ± 0.59 |
| Relative SAMY production (%) | 100 ± 5.67 | 98.97 ± 7.73 | 5.20 ± 0.68 | 4.83 ± 1.22 |
CHO-VAC12 were co-transfected with pSAM200 (PSV40-tTA-pA) and pBP99 (PhCMV*-1-SAMY-pA) (A) or pWW35 (PSV40-ET1-pA) and pBP100 (PETR3-SAMY-pA) and grown for 48 h in the presence and absence of vanillic acid (Vac, 250 µM), erythromycin (EM, 2 µg/ml) or tetracycline (Tet, 2 µg/ml) before SEAP and SAMY production was assessed.
Specificity of the VACOFF system
VanR plays a key role in controlling lignin biodegradation of C. crescentus. One of the commonly produced compounds in this pathway is vanillic acid, but closely related compounds were also suggested as being able to directly interact with VanR (43). To assess the specificity of the VAC system and the capability of isomeric compounds of vanillic acid to interact with the synthetic mammalian-adapted VanA1 transactivator, we cultivated CHO-VAC12 for 48 h in media containing 0, 250 and 500 μM of a comprehensive set of compounds closely related to vanillic acid (2-vanillic acid, 2-vanillin, acetovanillone, benzaldehyde, benzoic acid, benzyl acetate, benzyl alcohol, ethyl-vanillate, ethyl-vanillin, eugenol, homovanillic acid, isovanillic acid, isovanillin, methyl-vanillate, protocatechualdehyde and vanillin). In parallel, we assessed the toxicity of these compounds on a stable CHO-K1-derived cell line constitutively expressing SEAP [CHO-SEAP18; (51)]. Some of the compounds were toxic when administered to CHO-SEAP18 cells at concentrations of 500 μM (2-vanillin, eugenol, isovanillin, methyl-vanillate and protocatechualdehyde), but none of the 16 tested structures was able to regulate the VACOFF system. This implies an extraordinary specificity of the VACOFF system for vanillic acid (Supplementary Table S1).
Vanillic acid—transgene expression in mice is mediated by a food additive
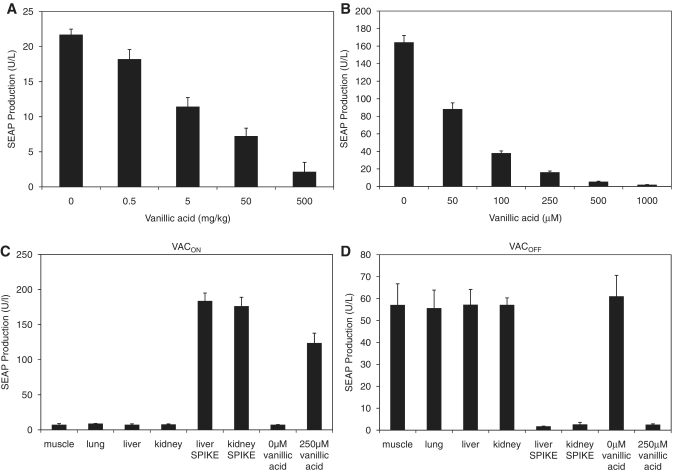
For subsequent applications in functional genomics research or future gene- and cell-based therapies, it is essential that state-of-the-art gene regulation systems are functional within entire organisms. To validate the vanillic acid-controlled gene regulation system in vivo, we implanted microencapsulated CHO-VAC12 intraperitoneally into mice. The treated mice were given a dose of vanillic acid within the range of 0–500 mg/kg twice daily. SEAP levels quantified in the blood of treated animals 72 h after implantation showed vanillic acid-dependent dose–response characteristics comparable to the control experiment using the same batch of microencapsulated CHO-VAC12 cells exposed to vanillic acid in an in vitro setting (Figure 5A and B). The serum SEAP levels of control mice, encapsulated with constitutively expressing CHO-SEAP18, were unresponsive to vanillic acid treatment of a twice-daily dose of 500 mg/kg and thus showed similar expression levels as untreated mice containing CHO-VAC12 implants (0 mg/kg vanillic acid: 15.37 ± 1.57 U/l; 500 mg/kg vanillic acid: 16.61 ± 1.33 U/l). Since vanillic acid is a standard food additive it may in principle be present in the animal and interfere with VACOFF-based fine-tuning of transgenes. We have therefore analysed whether liver, kidney, muscle and lung tissue extracts of mice kept on a standard diet could interfere with CHO-K1 cultures engineered for VACOFF- and VACON-controlled SEAP expression. Unlike positive controls consisting of organ extracts spiked with vanillic acid, none of the organ extracts produced from wild-type mice kept on a standard diet showed any interference with the VACON or VACOFF systems (Figure 5C and D).
Figure 5.
Vanillic acid-controlled SEAP expression in mice. (A) CHO-VAC12 cells were microencapsulated in alginate-poly-(l-lysine)-alginate beads and implanted intraperitoneally into female OF1 mice (4 × 106 cells per mouse). The implanted mice received different concentrations of vanillic acid twice daily. Seventy-two hours after implantation, the level of SEAP in the serum of the mice was determined. Data represent mean ± SEM of 8 mice per treatment group. (B) SEAP expression profiles of the microencapsulated CHO-VAC12 implant batch were cultivated in vitro for 72 h at different vanillic acid concentrations. (C and D) Extracts of wild-type mouse organs were assessed for their vanillic acid content based on their ability to induce the (C) VACOFF or (D) VACON systems. Vanillic acid-spiked organs were used as positive control. All samples were compared to the effect of 250 μM vanillic acid to show the fully induced state of the systems. All extracts were added to CHO-K1 cells transiently transfected with either the VACON or the VACOFF systems and SEAP expression was assessed after a cultivation period of 48 h.
DISCUSSION
Heterologous transgene expression control by non-toxic small molecule inducers remains one of the major challenges for future gene- and cell-based therapies as well as for biopharmaceutical manufacturing of difficult-to-produce protein therapeutics. The employed inducers must meet high medical standards and also need to be physiologically inert for long-term applications in humans. Antibiotics, steroid hormones, immunosuppressive drugs and a multitude of other regulating molecules fail to meet these requirements due to their high levels of side effects, particularly when given over a long period of time (62–64).
Phenolic acids are a class of compounds that are naturally produced by plants, and are therefore present in vegetables and fruits that are widely distributed throughout human dietary products, like coffee, wine, beer and vanilla (65). In general, phenolic acids are said to possess many physiological and pharmacological functions (66) and vanillic acid, in particular, was successfully evaluated as a suppressor of a potent snake venom (33), cell apoptosis in Neuro-2A cells (35,36), immune-mediated liver inflammation in mice (37) and carcinogenesis (34). Being a licensed food additive with a very agreeable smell (which also enables vanillic acid to be used in fragrances), this specific phenolic acid combines the ideal properties for functioning as a physiologically inert inducer molecule in future gene- and cell-based therapies. This judgment is supported by the reported LD50 value of 5 g/kg, which was tested intraperitoneally in rats (67).
Combining the elements of the C. crescentus VanR-regulated VanAB gene cluster and mammalian transactivation and transsilencing domains, we designed the novel mammalian heterologous transgene regulation systems VACON and VACOFF, which respond exclusively to the licensed food additive vanillic acid. Even closely related compounds with very similar structure are unable to regulate the vanillic acid control circuits. The generic design of the VACOFF system allows for several configurations using (i) diverse numbers of operators, (ii) different mammalian transactivation domains and (iii) variable distances between the specific operator site and the minimal promoter, to provide a toolbox for a wide variety of applications. Due to the high modularity of the VACOFF system, we were able to provide a specific configuration for all of the tested cell types, which exhibited an optimal regulation performance with high maximal expression and full reversibility. Furthermore, the VACOFF system demonstrated interference-free regulation characteristics when employed in parallel settings with the TETOFF (21) and the EOFF systems (18). Owing to its unprecedented specificity the presented regulation unit is likely to be an ideal building block for complex synthetic networks operating with various inducer inputs. The fact that vanillic acid is a natural plant component, which is licensed as food additive may facilitate its approval by governmental agencies for applications in future biopharmaceutical manufacturing scenarios as well as in gene- and cell-based therapies.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Table 1 and Supplementary Figure 1.
FUNDING
Swiss National Science Foundation (grant no. 31003A0-126022) and in part by the EC Framework 7 (Persist). Funding for open access charge: ETH Zurich.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ghislaine Charpin-El Hamri and Branka Roscic for technical assistance and Marcel Tigges as well as Markus Wieland for critical comments on the manuscript.
REFERENCES
- 1.Baumgartel K, Genoux D, Welzl H, Tweedie-Cullen RY, Koshibu K, Livingstone-Zatchej M, Mamie C, Mansuy IM. Control of the establishment of aversive memory by calcineurin and Zif268. Nat. Neurosci. 2008;11:572–578. doi: 10.1038/nn.2113. [DOI] [PubMed] [Google Scholar]
- 2.Gitzinger M, Parsons J, Reski R, Fussenegger M. Functional cross-kingdom conservation of mammalian and moss (Physcomitrella patens) transcription, translation and secretion machineries. Plant Biotechnol. J. 2009;7:73–86. doi: 10.1111/j.1467-7652.2008.00376.x. [DOI] [PubMed] [Google Scholar]
- 3.Ulmer JB, Valley U, Rappuoli R. Vaccine manufacturing: challenges and solutions. Nat. Biotechnol. 2006;24:1377–1383. doi: 10.1038/nbt1261. [DOI] [PubMed] [Google Scholar]
- 4.Weber W, Fussenegger M. Inducible product gene expression technology tailored to bioprocess engineering. Curr. Opin. Biotechnol. 2007;18:399–410. doi: 10.1016/j.copbio.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat. Rev. Drug Discov. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 6.Weber W, Schoenmakers R, Keller B, Gitzinger M, Grau T, Daoud-El Baba M, Sander P, Fussenegger M. A synthetic mammalian gene circuit reveals antituberculosis compounds. Proc. Natl Acad. Sci. USA. 2008;105:9994–9998. doi: 10.1073/pnas.0800663105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greber D, Fussenegger M. Mammalian synthetic biology: engineering of sophisticated gene networks. J. Biotechnol. 2007;130:329–345. doi: 10.1016/j.jbiotec.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez-Bustamante CD, Kelm JM, Mitta B, Fussenegger M. Heterologous protein production capacity of mammalian cells cultivated as monolayers and microtissues. Biotechnol. Bioeng. 2006;93:169–180. doi: 10.1002/bit.20679. [DOI] [PubMed] [Google Scholar]
- 9.Weber W, Fussenegger M. Pharmacologic transgene control systems for gene therapy. J. Gene Med. 2006;8:535–556. doi: 10.1002/jgm.903. [DOI] [PubMed] [Google Scholar]
- 10.Deans TL, Cantor CR, Collins JJ. A tunable genetic switch based on RNAi and repressor proteins for regulating gene expression in mammalian cells. Cell. 2007;130:363–372. doi: 10.1016/j.cell.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 11.Kramer BP, Fussenegger M. Hysteresis in a synthetic mammalian gene network. Proc. Natl Acad. Sci. USA. 2005;102:9517–9522. doi: 10.1073/pnas.0500345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tigges M, Denervaud N, Greber D, Stelling J, Fussenegger M. A synthetic low-frequency mammalian oscillator. Nucleic Acids Res. 2010;38:2702–2711. doi: 10.1093/nar/gkq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tigges M, Marquez-Lago TT, Stelling J, Fussenegger M. A tunable synthetic mammalian oscillator. Nature. 2009;457:309–312. doi: 10.1038/nature07616. [DOI] [PubMed] [Google Scholar]
- 14.Gersbach CA, Le Doux JM, Guldberg RE, Garcia AJ. Inducible regulation of Runx2-stimulated osteogenesis. Gene Ther. 2006;13:873–882. doi: 10.1038/sj.gt.3302725. [DOI] [PubMed] [Google Scholar]
- 15.Kemmer C, Gitzinger M, Daoud-El Baba M, Djonov V, Stelling J, Fussenegger M. Self-sufficient control of urate homeostasis in mice by a synthetic circuit. Nat. Biotechnol. 2010;28:355–360. doi: 10.1038/nbt.1617. [DOI] [PubMed] [Google Scholar]
- 16.Ehrbar M, Schoenmakers R, Christen EH, Fussenegger M, Weber W. Drug-sensing hydrogels for the inducible release of biopharmaceuticals. Nat. Mater. 2008;7:800–804. doi: 10.1038/nmat2250. [DOI] [PubMed] [Google Scholar]
- 17.Urlinger S, Helbl V, Guthmann J, Pook E, Grimm S, Hillen W. The p65 domain from NF-kappaB is an efficient human activator in the tetracycline-regulatable gene expression system. Gene. 2000;247:103–110. doi: 10.1016/s0378-1119(00)00112-8. [DOI] [PubMed] [Google Scholar]
- 18.Weber W, Fux C, Daoud-el Baba M, Keller B, Weber CC, Kramer BP, Heinzen C, Aubel D, Bailey JE, Fussenegger M. Macrolide-based transgene control in mammalian cells and mice. Nat. Biotechnol. 2002;20: 901–907. doi: 10.1038/nbt731. [DOI] [PubMed] [Google Scholar]
- 19.Weber W, Kramer BP, Fux C, Keller B, Fussenegger M. Novel promoter/transactivator configurations for macrolide- and streptogramin-responsive transgene expression in mammalian cells. J. Gene Med. 2002;4:676–686. doi: 10.1002/jgm.314. [DOI] [PubMed] [Google Scholar]
- 20.Fussenegger M, Morris RP, Fux C, Rimann M, von Stockar B, Thompson CJ, Bailey JE. Streptogramin-based gene regulation systems for mammalian cells. Nat. Biotechnol. 2000;18:1203–1208. doi: 10.1038/81208. [DOI] [PubMed] [Google Scholar]
- 21.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl Acad. Sci. USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 23.Neddermann P, Gargioli C, Muraglia E, Sambucini S, Bonelli F, De Francesco R, Cortese R. A novel, inducible, eukaryotic gene expression system based on the quorum-sensing transcription factor TraR. EMBO Rep. 2003;4:159–165. doi: 10.1038/sj.embor.embor734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber W, Rimann M, Spielmann M, Keller B, Daoud-El Baba M, Aubel D, Weber CC, Fussenegger M. Gas-inducible transgene expression in mammalian cells and mice. Nat. Biotechnol. 2004;22:1440–1444. doi: 10.1038/nbt1021. [DOI] [PubMed] [Google Scholar]
- 25.Hartenbach S, Daoud-El Baba M, Weber W, Fussenegger M. An engineered L-arginine sensor of Chlamydia pneumoniae enables arginine-adjustable transcription control in mammalian cells and mice. Nucleic Acids Res. 2007;35:e136. doi: 10.1093/nar/gkm652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber W, Bacchus W, Daoud-El Baba M, Fussenegger M. Vitamin H-regulated transgene expression in mammalian cells. Nucleic Acids Res. 2007;35:e116. doi: 10.1093/nar/gkm466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber W, Lienhart C, Daoud-El Baba M, Fussenegger M. A Biotin-triggered genetic switch in mammalian cells and mice. Metab. Eng. 2009;11:117–124. doi: 10.1016/j.ymben.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Gitzinger M, Kemmer C, El-Baba MD, Weber W, Fussenegger M. Controlling transgene expression in subcutaneous implants using a skin lotion containing the apple metabolite phloretin. Proc. Natl Acad. Sci. USA. 2009;106:10638–10643. doi: 10.1073/pnas.0901501106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha AK, Sharma UK, Sharma N. A comprehensive review on vanilla flavor: extraction, isolation and quantification of vanillin and others constituents. Int. J. Food Sci. Nutr. 2008;59:299–326. doi: 10.1080/09687630701539350. [DOI] [PubMed] [Google Scholar]
- 30.Sostaric T, Boyce MC, Spickett EE. Analysis of the volatile components in vanilla extracts and flavorings by solid-phase microextraction and gas chromatography. J. Agric. Food Chem. 2000;48:5802–5807. doi: 10.1021/jf000515+. [DOI] [PubMed] [Google Scholar]
- 31.Huang WY, Sheu SJ. Separation and identification of the organic acids in Angelicae Radix and Ligustici Rhizoma by HPLC and CE. J. Sep. Sci. 2006;29:2616–2624. doi: 10.1002/jssc.200600136. [DOI] [PubMed] [Google Scholar]
- 32.Dhananjaya BL, Nataraju A, Raghavendra Gowda CD, Sharath BK, D'Souza CJ. Vanillic acid as a novel specific inhibitor of snake venom 5′-nucleotidase: a pharmacological tool in evaluating the role of the enzyme in snake envenomation. Biochemistry. 2009;74:1315–1319. doi: 10.1134/s0006297909120037. [DOI] [PubMed] [Google Scholar]
- 33.Dhananjaya BL, Nataraju A, Rajesh R, Raghavendra Gowda CD, Sharath BK, Vishwanath BS, D'Souza CJ. Anticoagulant effect of Naja naja venom 5'nucleotidase: demonstration through the use of novel specific inhibitor, vanillic acid. Toxicon. 2006;48:411–421. doi: 10.1016/j.toxicon.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Vetrano AM, Heck DE, Mariano TM, Mishin V, Laskin DL, Laskin JD. Characterization of the oxidase activity in mammalian catalase. J. Biol. Chem. 2005;280:35372–35381. doi: 10.1074/jbc.M503991200. [DOI] [PubMed] [Google Scholar]
- 35.Huang SM, Chuang HC, Wu CH, Yen GC. Cytoprotective effects of phenolic acids on methylglyoxal-induced apoptosis in Neuro-2A cells. Mol. Nutr. Food Res. 2008;52:940–949. doi: 10.1002/mnfr.200700360. [DOI] [PubMed] [Google Scholar]
- 36.Huang SM, Hsu CL, Chuang HC, Shih PH, Wu CH, Yen GC. Inhibitory effect of vanillic acid on methylglyoxal-mediated glycation in apoptotic Neuro-2A cells. Neurotoxicology. 2008;29:1016–1022. doi: 10.1016/j.neuro.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Itoh A, Isoda K, Kondoh M, Kawase M, Kobayashi M, Tamesada M, Yagi K. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a-induced liver injury. Biol. Pharm. Bull. 2009;32:1215–1219. doi: 10.1248/bpb.32.1215. [DOI] [PubMed] [Google Scholar]
- 38.Pietta PG, Simonetti P, Gardana C, Brusamolino A, Morazzoni P, Bombardelli E. Catechin metabolites after intake of green tea infusions. Biofactors. 1998;8:111–118. doi: 10.1002/biof.5520080119. [DOI] [PubMed] [Google Scholar]
- 39.Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- 40.Martinez AT, Speranza M, Ruiz-Duenas FJ, Ferreira P, Camarero S, Guillen F, Martinez MJ, Gutierrez A, del Rio JC. Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 2005;8:195–204. [PubMed] [Google Scholar]
- 41.Poindexter JS. Biological properties and classification of the caulobacter group. Bacteriol. Rev. 1964;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harwood CS, Parales RE. The beta-ketoadipate pathway and the biology of self-identity. Annu. Rev. Microbiol. 1996;50:553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 43.Thanbichler M, Iniesta AA, Shapiro L. A comprehensive set of plasmids for vanillate- and xylose-inducible gene expression in Caulobacter crescentus. Nucleic Acids Res. 2007;35:e137. doi: 10.1093/nar/gkm818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunel F, Davison J. Cloning and sequencing of Pseudomonas genes encoding vanillate demethylase. J. Bacteriol. 1988;170:4924–4930. doi: 10.1128/jb.170.10.4924-4930.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merkens H, Beckers G, Wirtz A, Burkovski A. Vanillate metabolism in Corynebacterium glutamicum. Curr. Microbiol. 2005;51:59–65. doi: 10.1007/s00284-005-4531-8. [DOI] [PubMed] [Google Scholar]
- 46.Nishimura M, Ishiyama D, Davies J. Molecular cloning of streptomyces genes encoding vanillate demethylase. Biosci. Biotechnol. Biochem. 2006;70:2316–2319. doi: 10.1271/bbb.60180. [DOI] [PubMed] [Google Scholar]
- 47.Segura A, Bunz PV, D'Argenio DA, Ornston LN. Genetic analysis of a chromosomal region containing vanA and vanB, genes required for conversion of either ferulate or vanillate to protocatechuate in Acinetobacter. J. Bacteriol. 1999;181:3494–3504. doi: 10.1128/jb.181.11.3494-3504.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greber D, Fussenegger M. Multi-gene engineering: simultaneous expression and knockdown of six genes off a single platform. Biotechnol. Bioeng. 2007;96:821–834. doi: 10.1002/bit.21303. [DOI] [PubMed] [Google Scholar]
- 49.Berger J, Hauber J, Hauber R, Geiger R, Cullen BR. Secreted placental alkaline phosphatase: a powerful new quantitative indicator of gene expression in eukaryotic cells. Gene. 1988;66:1–10. doi: 10.1016/0378-1119(88)90219-3. [DOI] [PubMed] [Google Scholar]
- 50.Schlatter S, Rimann M, Kelm J, Fussenegger M. SAMY, a novel mammalian reporter gene derived from Bacillus stearothermophilus alpha-amylase. Gene. 2002;282:19–31. doi: 10.1016/s0378-1119(01)00824-1. [DOI] [PubMed] [Google Scholar]
- 51.Fluri DA, Kemmer C, Daoud-El Baba M, Fussenegger M. A novel system for trigger-controlled drug release from polymer capsules. J. Control Release. 2008;131:211–219. doi: 10.1016/j.jconrel.2008.07.036. [DOI] [PubMed] [Google Scholar]
- 52.Moosmann P, Georgiev O, Thiesen HJ, Hagmann M, Schaffner W. Silencing of RNA polymerases II and III-dependent transcription by the KRAB protein domain of KOX1, a Kruppel-type zinc finger factor. Biol. Chem. 1997;378:669–677. doi: 10.1515/bchm.1997.378.7.669. [DOI] [PubMed] [Google Scholar]
- 53.Ayyanathan K, Lechner MS, Bell P, Maul GG, Schultz DC, Yamada Y, Tanaka K, Torigoe K, Rauscher FJ., 3rd Regulated recruitment of HP1 to a euchromatic gene induces mitotically heritable, epigenetic gene silencing: a mammalian cell culture model of gene variegation. Genes Dev. 2003;17:1855–1869. doi: 10.1101/gad.1102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng H, Ivanov AV, Oh HJ, Lau YF, Rauscher FJ., 3rd Epigenetic gene silencing by the SRY protein is mediated by a KRAB-O protein that recruits the KAP1 co-repressor machinery. J. Biol. Chem. 2009;284:35670–35680. doi: 10.1074/jbc.M109.032086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malphettes L, Weber CC, El-Baba MD, Schoenmakers RG, Aubel D, Weber W, Fussenegger M. A novel mammalian expression system derived from components coordinating nicotine degradation in arthrobacter nicotinovorans pAO1. Nucleic Acids Res. 2005;33:e107. doi: 10.1093/nar/gni107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weber W, Fussenegger M. Artificial mammalian gene regulation networks-novel approaches for gene therapy and bioengineering. J. Biotechnol. 2002;98:161–187. doi: 10.1016/s0168-1656(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 57.Muller J, Oehler S, Muller-Hill B. Repression of lac promoter as a function of distance, phase and quality of an auxiliary lac operator. J. Mol. Biol. 1996;257:21–29. doi: 10.1006/jmbi.1996.0143. [DOI] [PubMed] [Google Scholar]
- 58.Sathya G, Li W, Klinge CM, Anolik JH, Hilf R, Bambara RA. Effects of multiple estrogen responsive elements, their spacing, and location on estrogen response of reporter genes. Mol. Endocrinol. 1997;11:1994–2003. doi: 10.1210/mend.11.13.0039. [DOI] [PubMed] [Google Scholar]
- 59.Becskei A, Kaufmann BB, van Oudenaarden A. Contributions of low molecule number and chromosomal positioning to stochastic gene expression. Nat. Genet. 2005;37:937–944. doi: 10.1038/ng1616. [DOI] [PubMed] [Google Scholar]
- 60.Kramer BP, Viretta AU, Daoud-El-Baba M, Aubel D, Weber W, Fussenegger M. An engineered epigenetic transgene switch in mammalian cells. Nat. Biotechnol. 2004;22:867–870. doi: 10.1038/nbt980. [DOI] [PubMed] [Google Scholar]
- 61.Tigges M, Fussenegger M. Recent advances in mammalian synthetic biology-design of synthetic transgene control networks. Curr. Opin. Biotechnol. 2009;20:449–460. doi: 10.1016/j.copbio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Kuypers DR. Benefit-risk assessment of sirolimus in renal transplantation. Drug Saf. 2005;28:153–181. doi: 10.2165/00002018-200528020-00006. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez AR, Rogers RS, 3rd, Sheridan PJ. Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity. Int. J. Dermatol. 2004;43:709–715. doi: 10.1111/j.1365-4632.2004.02108.x. [DOI] [PubMed] [Google Scholar]
- 64.Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- 65.Stalikas CD. Phenolic acids and flavonoids: occurrence and analytical methods. Methods Mol. Biol. 2008;610:65–90. doi: 10.1007/978-1-60327-029-8_5. [DOI] [PubMed] [Google Scholar]
- 66.Nardini M, Pisu P, Gentili V, Natella F, Di Felice M, Piccolella E, Scaccini C. Effect of caffeic acid on tert-butyl hydroperoxide-induced oxidative stress in U937. Free Radic. Biol. Med. 1998;25:1098–1105. doi: 10.1016/s0891-5849(98)00180-4. [DOI] [PubMed] [Google Scholar]
- 67.Comptes Rendus Hebdomadaires des Seances. Academie des Sciences. 1956. Vol. 243, 609. [Google Scholar]
- 68.Moser S, Rimann M, Fux C, Schlatter S, Bailey JE, Fussenegger M. Dual-regulated expression technology: a new era in the adjustment of heterologous gene expression in mammalian cells. J. Gene Med. 2001;3:529–549. doi: 10.1002/jgm.219. [DOI] [PubMed] [Google Scholar]
- 69.Fussenegger M, Moser S, Mazur X, Bailey JE. Autoregulated multicistronic expression vectors provide one-step cloning of regulated product gene expression in mammalian cells. Biotechnol. Prog. 1997;13:733–740. doi: 10.1021/bp970108r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.