Abstract
This short review aims at presenting some recent illustrative examples of spontaneous nucleolipids self-assembly. High-resolution structural investigations reveal the diversity and complexity of assemblies formed by these bioinspired amphiphiles, resulting from the interplay between aggregation of the lipid chains and base–base interactions. Nucleolipids supramolecular assemblies are promising soft drug delivery systems, particularly for nucleic acids. Regarding prodrugs, squalenoylation is an innovative concept for improving efficacy and delivery of nucleosidic drugs.
INTRODUCTION
Hybrid molecules composed of a lipid covalently linked to a nucleoside have been identified in both eukaryotic and prokaryotic cells. The most common of these nucleolipids, cytidine diphosphate diacylglycerol, is a key intermediate of the biosynthesis of glycolipids and lipoproteins. It may also be involved in the synthesis of phosphatidylinositol and cardiolipin, two components of mammalian cell membranes. Other naturally occurring nucleolipids, isolated from bacteria (tunicamycins, liposidomycins and septacidin) or marine sponges (agelasines), which elicit biological responses such as antimicrobial, antifungal, antiviral or antitumor activities, have also been reviewed by Rosemeyer (1).
Many molecules currently used in clinic against cancer or viral infections such as AIDS are nucleoside analogues. For example, Gemcitabine and Zalcitabine are derived from cytidine, while Didanosine is derived from adenosine and Zidovudine (AZT) from thymidine. Their main mechanism of action is a potent inhibition of DNA or RNA synthesis, which may lead to cell cycle blockage and eventually apoptosis. However, their therapeutic potential is often restricted by a poor stability in vivo, the induction of severe side effects and a limited passive intracellular diffusion due to their hydrophilicity. Nucleoside analogues enter cells mainly through specific membrane transporters, whose inactivation can result in resistance. Various lipophilic derivatives of nucleoside analogues have thus been designed in an attempt to circumvent these drawbacks. Site-specific bioconjugation enables to protect sensitive groups from enzymatic degradation and the coupling of hydrophobic moieties may yield amphiphilic conjugates able to cross the plasma membrane by passive diffusion.
Beyond prodrugs, the interest in nucleolipids was further stimulated by the self-organization properties of these amphiphilic molecules combining the aggregation characteristics of lipids and the specific functionalities of nucleosides. Self-assembly in an aqueous medium of amphiphiles possessing both a polar head derived from a nucleobase and a lipidic moiety offers the possibility to obtain new supramolecular systems with original biophysical properties for numerous applications ranging from biomaterials to drug delivery systems. Supramolecular devices formed by nucleolipids are promising carriers for nucleic acids delivery. Indeed, nucleotides being the constituting monomers of nucleic acids, it is expected that base–base interactions between nucleic acids and nucleolipids can ensure the entrapment of DNA, RNA or oligonucleotides in these systems. In an effort to expand the field of this family of lipids, numerous molecules have thus been recently synthesized and their supramolecular organization–properties relationship investigated.
The purpose of this short review is to present recent examples in the field of spontaneous nucleolipids self-assembly, focussing on the structures formed and their possible therapeutic applications. The interactions between nucleolipids are first presented, with emphasis on molecular recognition properties between complementary bases. The methods most often used to study the structure of self-assembled nucleolipids are recalled. In the following part, examples of hierarchical architectures formed by nucleolipids reveal how aggregation of the lipid moieties interferes with interactions involving nucleobases. The use of nucleolipid-based devices for nucleic acids transfection is then presented. Finally, we describe lipidic derivatives of nucleosidic drugs that self-organize as nanoparticles. A new strategy, known as ‘squalenoylation’, has been recently conceived in our laboratory. It involves the coupling of squalene, a natural precursor in the biosynthesis of sterols, to nucleoside analogues such as Gemcitabine or Zalcitabine. These derivatives exhibited impressive higher anti-cancer or antiviral activity than the parent drugs. Remarkably, the amphiphilic molecules obtained self-organize into nanoassemblies in aqueous medium, whatever the nucleoside analogue. Squalenoylation provides an original platform for improving efficacy and delivery of nucleosidic drugs, which could be extended to other polar therapeutic compounds.
INTERACTIONS BETWEEN NUCLEOLIPIDS: MOLECULAR RECOGNITION AND SELF-ASSEMBLY
The self-assembly of complex structures, from similar or different components interacting by multiple non-covalent intermolecular forces, is ubiquitous in biology, soft condensed matter, and nanoscience. The resulting supramolecular ordered assemblies usually exhibit a hierarchy of structural levels. Weak dynamic bonds may impart stimuli-responsive and time-dependent properties to the aggregates. For instance, nanoparticles could alter their structure in response to an external stimulus such as pH or temperature change (2).
The self-assemblies of lipid-nucleoside (or nucleotide) conjugates are typical examples of such ordered stimuli responsive structures. These nucleolipids possess a polar head derived from either a purine base (adenine or guanine) or a pyrimidine base (cytosine, thymine or uracil). These bases can interact through π-stacking and hydrogen bonding, each base–base motif displaying different binding characteristics (3). The base pairs present in nucleic acids are adenine–thymine and guanine–cytosine in DNA double strands or adenine–uracil and guanine–cytosine in RNA double strands. Each strand of DNA (or RNA) is coupled through H-bonding to the other strand formed by the complementary bases sequence. These interactions being weak, base pairing is a cooperative process assisted by the macromolecular nature of nucleic acids. It has been evidenced that simple nucleolipids in micellar or vesicular aggregates, or adsorbed on a surface, could display similar molecular recognition properties between complementary bases (4–10). For example, in mixed micelles formed at physiological pH from the equimolar mixture of two phospholipids functionalized with either uridine or adenosine, i.e. dioctanoylphosphatidyl-adenosine (diC8PA) and dioctanoylphosphatidyl-uridine (diC8PU), a specific molecular recognition process was identified at the micellar surface. Both stacking and H-bonding between adenosine and uridine bases were evidenced by UV and circular dichroism (CD) spectra and 1H NMR measurements. These interactions induced a decrease of the mean area per polar headgroup, compared to that of the pure components in individual micelles (5,6).
It should be emphasized that H-bonding is not a sufficient driving force for molecular recognition between complementary bases in water. Below the critical micellar concentration (cmc), molecular recognition is absent in monomeric solutions of nucleolipids bearing complementary headgroups. Likewise, stacked structures of isolated nucleosides are more stable than hydrogen-bonded ones in water. Anchoring of at least one of the bases to an organized interface seems to be a prerequisite for molecular recognition, highlighting the importance of the cooperative effect.
Identical nucleolipids can also interact. The interplay between supramolecular organization of nucleolipids and base–base interactions has been underlined. Stacking and H-bonding of the headgroups are expected to depend on the structure of the aggregates through the confinement induced by aggregation. The interfacial curvature of aggregates may further influence the efficiency of the interactions. In turn, base–base interactions can themselves modulate the supramolecular structure of nucleolipids. Small differences between polar headgroups may cause variation in the phase behaviour. Regarding the lipid moiety, beside the number, length and degree of unsaturation of hydrophobic chains, their position relative to the base can have an impact on the formed structure. As a result, self-assembled nucleolipids may exhibit a variety of structures. Vesicules, lamellar phases, ribbons, helical strands, spherical or wormlike micelles, hexosomes or cubosomes, etc., have been identified (9,11–15).
METHODOLOGIES USED FOR INVESTIGATING NUCLEOLIPIDS SUPRAMOLECULAR ASSEMBLIES
Self-assembled nucleolipids are organized at different length scales. Their morphology and structure have been investigated by transmission electron microscopy (TEM), X-rays, neutrons and light scattering while spectroscopic methods, mainly UV and CD spectroscopy and 1H NMR measurements, gave an insight into base–base interactions. Whereas TEM allowed a direct imaging of nucleolipids-based nanoassemblies, the characteristic lengths of the supramolecular structures (ranging from atomic distances up to hundreds of nanometres) may be probed by X-ray (small- and wide-angle X-ray scattering, SAXS–WAXS) and small-angle neutron scattering (SANS). WAXS describes the structure at an atomic scale (from ~1 to 10 Å), whereas SAS is related to the supramolecular organization. SAXS allows measuring characteristic dimensions between ~10 Å and 1000 Å, whereas larger length scales can be reached with SANS. Generally, the symmetry of ordered phases (lamellar, cubic, hexagonal, etc.) results in Bragg reflections whose positions are in characteristic ratios. In the case of a micellar phase, without long-range order, the SAS pattern contains information on the size and shape of the micelles and on their mutual interactions within the solution. Noteworthy, the availability of Synchrotron radiation (SR) sources with high brightness and high collimation enlarges the possible uses of X-ray scattering for the investigation of large structural features and dilute, or weakly scattering, samples. In view of the lability of the interactions between the molecular components of a supramolecular entity, the structure of nanoparticles could change as a function of temperature. Microcalorimetry, able to determine with high sensitivity the thermal exchanges involved in modifications of the nanoassemblies, can indicate phase transitions. When DSC is coupled with SAXS, a detailed phase diagram can be established. The UV and CD spectra of bases, in the 180–300 nm range, are sensitive to base–base interactions. Stacking of bases gives rise to a hypochromic effect accompanied by an increase of the molar ellipticities. Hydrogen bonding between bases can be assessed by FTIR spectroscopy and 1H NMR. H-bonding causes a downfield shift of the resonances of the base protons, while stacking induces an upfield shift. The use of these techniques to elucidate the supramolecular structures of self-assembled nucleolipids is illustrated by examples below.
EXAMPLES OF HIERARCHICAL ARCHITECTURES FORMED BY NUCLEOLIPIDS
Advantage may be taken from the interactions between nucleolipids to construct original materials. In this view, non-ionic, zwitterionic, anionic and cationic nucleolipids have been synthesized. Examples of chemical structures of nucleolipids belonging to the main groups of synthetic bioconjugates are given in Figure 1. Their self-organization gave rise to a diversity of aggregate morphologies.
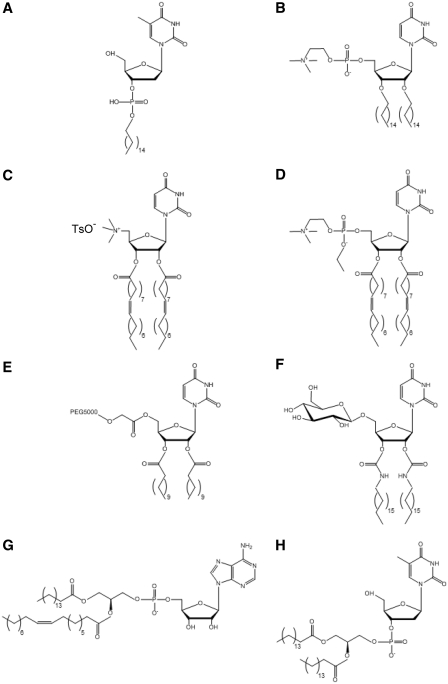
Figure 1.
Examples of chemical structures of main synthesized nucleolipids (except prodrugs). (A) Deoxythymidine 3′-palmitoylphosphate (C16-3′ TMP) (21); (B) 1,2-dipalmitoyluridinophosphocholine (DPUPC) (20); (C) 2′,3′-dioleoyl)uridine-trimethylammonium tosylate (DOTAU) (28); (D) O-ethyl-dioleyl-phosphatidylcholinium-uridine (O-ethyl-DOUPC) (27); (E) 5′-PEG5000-2′,3′-dilauroyl-sn-glycero-3-uridine (DLU-PEG5000) (31). (F) 1-(2′,3′-dioleylcarbamoyl-uridine-5′)-β-D-glucopyranoside (DOUGluc) (32). (G) 1-palmitoyl-2-oleoyl-sn-glycero-3-adenosine (POPA) (16); (H) deoxythymidine-3′-(1,2-dipalmitoyl-sn-glycero-3-phosphate) (diC16-3′dT) (38).
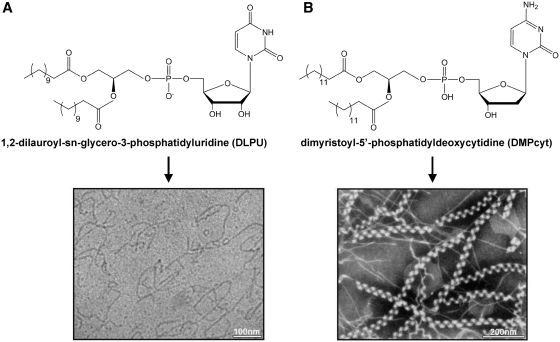
Baglioni and co-workers have synthesized nucleolipids by exchanging the choline headgroup of phosphatidylcholines with a nucleoside being either uridine or adenosine. The lipidic moiety was attached on 5′-position of the nucleoside. The obtained molecules had a nucleotide group, bearing a negative charge, as polar heads. Globular micelles, flexible cylindrical aggregates and bilayers were observed upon self-assembly of these nucleolipids, depending on the length of the alkyl chains used. Short-chain derivatives (C6–C9) formed quasi-spherical micelles whereas long-chain derivatives adopted locally flat topologies, consistent with the increase in the packing parameter with longer chains. 1-palmitoyl-2-oleoyl-phosphatidyl-uridine (POPU) and 1-palmitoyl-2-oleoyl-phosphatidyl-adenosine (POPA) (Figure 1G) formed bilayers (16,17). Remarkably, 1,2-dilauroyl-phosphatidyl-uridine (DLPU) was found to behave as an ‘associative polynucleotide’. At physiological pH DLPU spontaneously self-assembled into cylindrical aggregates whose length could be tuned by the nucleolipid concentration and by the ionic strength of the aqueous medium (Figure 2A). Increase in concentration in the presence of added salt or increase in ionic strength at sufficient concentration entailed micellar uni-dimensional growth, as shown by a combination of scattering techniques and cryo-TEM. The cylindrical local structure, with cross-sectional radius of 20 Å, remained unchanged during micellar growth. Giant wormlike micelles, characterized by an apparent persistence length of 230 Å (in the 0.1 M PBS buffer), entangled to form a transient network with properties comparable to those of semi-dilute polymer solutions. However, unlike polymers, flexible micelles could break and recombine (18).
Figure 2.
Spontaneous formation of wormlike and helical micelles from phospholipid-nucleoside conjugates. (A) Cryo-TEM image of 10 mM DLPU micellar solution in 0.1 M PBS at pH 7.5 showing wormlike micelles polydisperse in length [reprinted with permission from (19). Copyright (2006) American Chemical Society]. (B) Electron micrograph of superhelical strands formed from dimyristoyl-5′-phosphatidyldeoxycytidine (DMPcyt) after ageing at 25°C for 1 day [redrawn from Ref. (12)].
In general, the dominant factor determining these supramolecular structures was the hydrophobic part of the nucleolipids but, as emphasized, the nature of the nucleobase modulated their aggregation behaviour. The impact of the nucleobase was anticipated to be more evident for DLP-nucleoside and POP-nucleoside derivatives than for diC8P-nucleoside derivatives because the lower interfacial curvature of cylindrical or planar nanoassemblies was expected to favour base interactions, especially base stacking, in comparison to the high interfacial curvature exhibited by spherical micelles. Meaningful differences between adenosine and uridine long-chain derivatives have, indeed, been stressed. The 1,2-dilauroyl-phosphatidyl-adenosine (DLPA) bioconjugate showed a more complex self-assembly pattern than DLPU. DLPA displayed a time-dependent evolution leading to the hierarchical aggregation of wormlike micelles initially formed. The helical superstructures obtained were promoted by stacking and H-bonding of adenosine moieties (19). Likewise, the lamellar phase of POPA was characterized by an higher main transition temperature Tm, a lower hydration and a less ordered stacking of bilayers, compared to the lamellar phase of POPU. The above differences have been explained by the different stacking and H-bonding characteristics of adenosine and uridine. In particular, purines, like adenine, have higher staking constants than pyrimidines, like uracil. Molecular modelling further suggested that POPA molecules arranged preferentially in pairs. Tighter arrangement of adenosine groups at the interface could result in the lower cross-sectional area found for adenosine, although this derivative is bulkier, and in partial water exclusion from the interfacial region of bilayers (17).
Noteworthy, spontaneous formation of helical structures was evidenced by Yanagawa et al. (12) upon aging at room temperature of dimyristoyl-5′-phosphatidyl-deoxycytidine conjugate solution (Figure 2B). This conjugate first self-assembled as double helical strands with a diameter of ~110 Å and a helical pitch of ~240 Å. Superhelical structures with a helical pitch of ~1000 Å further appeared. Yanagawa et al. have then attempted to clarify the conditions of helical structure formation by varying the alkyl chains length and the nature of the nucleoside. They concluded that helical strands were stabilized by the balance between hydrophobic chains interactions, depending on their length, and bases stacking (13).
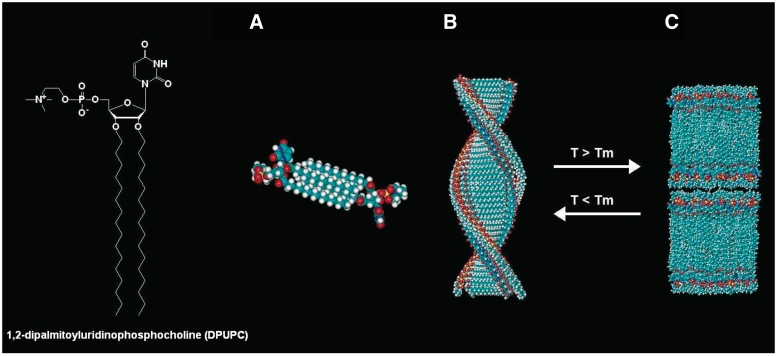
Barthelemy and co-workers have chosen to attach two lipophilic chains to the 2′- and 3′-positions of the nucleoside or one chain to its 3′-position. In the first study, they have synthesized a series of uridine-based zwitterionic nucleolipids possessing two acyl chains on the 2′- and 3′-positions of the nucleoside and a phosphocholine group on the 5′-position (20). The 1,2-dipalmitoyluridinophosphocholine (DPUPC) compound (Figure 1B), displaying two saturated chains of 16 C, illustrates the interplay between the organization of the lipid chains and base–base interactions. In aqueous medium, DPUPC self-assembled as bilayers when the lipid chains were in the fluid phase above the main transition temperature Tm and formed fibres when they were in the gel state, below Tm. As shown by SAXS and TEM, the fibres resulted from hexagonal packing of helical strands (Figure 3). The UV and CD spectra of DPUPC aggregates as a function of temperature evidenced a different organization of bases in the fibres and bilayers. The transition between the two supramolecular structures was reversible. Above a threshold concentration (6% w/w), a hydrogel was stabilized by a network of fibres, at temperatures below Tm.
Figure 3.
Illustration of the thermoreversible helical nanofibres, lamellar phase transition undergone by DPUPC in water. Proposed model for the helical nanofibres (A and B): (A) two molecules of DPUPC forming the basic repeat unit of the helical structure shown in (B). (B) Drawing of one helical strand (helical pitch 7.0 ± 0.5 nm, diameter 4.2 ± 0.5 nm). (C) Drawing of the multilamellar organization [reprinted with permission from (20). Copyright (2004) American Chemical Society].
Later investigations have assessed the impact of the nature of the nucleoside on the supramolecular structure of nucleolipids bearing a single monophosphate palmitoyl or eicosyl chain on the nucleoside 3′-position (Figure 1A) (21). These nucleotide-based amphiphiles formed colloidal dispersions upon sonication in an aqueous medium. They self-organized into ribbon-like nanoassemblies for the thymidine derivatives and in aggregates of small crystals for the adenine derivatives, as revealed by TEM. Wide angle X-ray diffraction patterns evidenced crystal-like 3D structural ordering of the dried aggregates. FTIR spectra were indicative of extended alkyl chains arranged in an orthorhombic lattice for the thymidine derivatives and displaying a triclinic packing for the adenine derivatives. The structural ordering also arose from hydrogen bonding between opposite bases and adjacent bases stacking in the 3D lattices, as shown by FTIR spectra.
Self-assembly of nucleotide-terminated bolaamphiphiles has also been used to obtain hydrogels through spontaneous formation of a network of intertwined fibres. The chain-end functionalization of long oligomethylene spacers (C18 or C20) with 3′-phosphorylated thymidine moieties yielded a hydrogel at concentration as low as 0.2 wt%. Both hydrophobic interactions between the spacer chains and interactions involving the nucleotide moieties were responsible for the formation of nanofibres (22).
TOWARDS GENE THERAPY: NANOSTRUCTURED NUCLEOLIPID ASSEMBLIES FOR NUCLEIC ACIDS TRANSFECTION
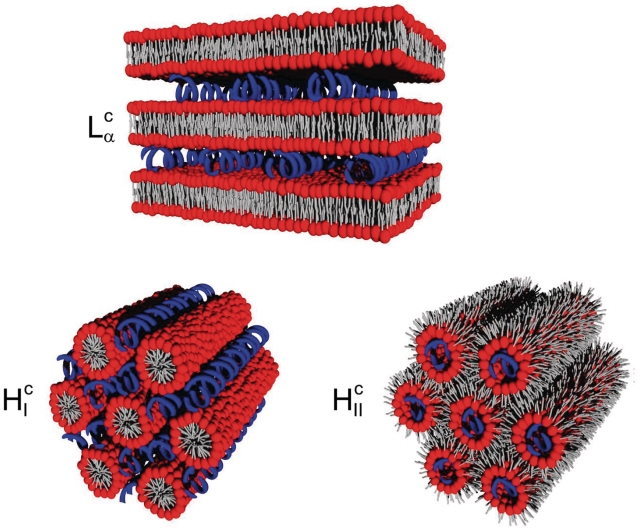
The aim of gene therapy is to cure inherited or acquired genetic disorder or diseases, including cancer, by replacing or inhibiting the faulting gene in cells, using natural or synthetic nucleic acids. The delivery to cells of DNA, antisense oligonucleotides or si-RNA, requires a vector such as lipoplexes. The majority of lipoplexes consists of nucleic acids mixed with cationic lipids in the presence of a ‘helper’ lipid, usually dioleoylphosphatidylethanolamine (DOPE) or dioleoylphosphatidylcholine (DOPC). In this approach, the negatively charged DNA interacts through electrostatic forces with the positively charged lipid. Structural studies have shown that DNA can be complexed into lamellar or hexagonal lipid phases (Figure 4). The addition of the ‘helper’ lipid favours the inverse hexagonal phase, which has been suggested to enhance transfection. The transfection efficiency of lamellar cationic lipoplexes depends on the charge density of lipid membranes, probably because of their electrostatic interactions with cells. In the regime of optimal membrane charge density, maximum in transfection efficiency was obtained when bilayers were made of a multicomponent lipid mixture, highlighting the importance of carrier lipidic composition (23). Unfortunately, after administration, the positive charges of the cationic lipids were demonstrated to dramatically interact with serum proteins, resulting in their fast elimination from the blood flow. Another drawback is the common cytotoxicity of cationic formulations due to cell membrane destabilization. Alternative delivery systems, bearing fewer charges, are thus considered in an attempt to overcome these problems. For instance, new lipids containing in their polar head thiourea functions, known as strong hydrogen bond donors, have been developed. Lipopolythioureas were capable of compacting DNA thanks to multiple hydrogen bonds involving the DNA phosphate groups. Moreover, the presence of the three forms of the thiourea function (i.e. thiourea, iminothiol and charged thiourea), afforded in vitro transfection of the lipids/ DNA complexes (24–26).
Figure 4.
Schematic representation of the structure of lamellar and hexagonal lipid–DNA complexes. The lamellar phase Lαc exhibits DNA rods intercalated between lipid bilayers. The hexagonal phase HIc consists of DNA rods between rodlike lipid micelles arranged on a hexagonal lattice. The inverse hexagonal HIIc phase consists of DNA rods coated with a lipid monolayer arranged on a hexagonal lattice. The lipids are in red (headgroup) and grey (chain) and the DNA rods are in blue [redrawn from Ref. (61)].
A new strategy, initiated by the research groups of Barthelemy and Baglioni, relies on self-assembled nucleolipids to complex and transfect nucleic acids, taking advantage of base–base interactions between nucleolipids and nucleic acids. In this approach, a series of cationic nucleolipids derived either from uridine, like O-ethyl-dioleyl-phosphatidylcholinium-uridine (O-ethyl-DOUPC) (Figure 1D) and 2′,3′-dioleoyluridine-trimethylammonium tosylate (DOTAU) (Figure 1C), or from a 3-nitropyrrole universal base analogue were synthesized by Barthelemy and co-workers. O-Ethyl-DOUPC and DOTAU interacted with both DNA double helix and polyadenylic acid (polyA) single strand. TEM and SAXS revealed multilamellar systems with a lattice of DNA rods intercalated between lipid bilayers (27,28). O-Ethyl-DOUPC at high nucleolipid/DNA ratios (18/1 and 36/1 w/w) displayed a good transfection efficacy in CHO cells, comparable to that of the commercially available Transfast, without cytotoxicity or inhibition of cell proliferation. Vesicles formed by cationic nucleolipid based on a 3-nitropyrrole universal base analogue were shown to efficiently transfect siRNA in three different cell lines (29). The weakly hydrogen bonding 3-nitropyrrole is known to form base pairs indiscriminately with the natural bases when opposite them in an oligonucleotide duplex, thanks to stacking interactions with adjacent bases (30).
Beside cationic nucleolipids, neutral amphiphiles derived from uridine and possessing one or two alkyl chains and either a glucose (Figure 1F) or a PEG (Figure 1E) moiety have been shown to complex nucleic acids (31,32). The rationale behind these chemical modifications of the headgroups was to impact the aggregation of the nucleolipids and to reinforce the interactions between their supramolecular assembly and nucleic acids, by introducing additional interactions, such as phosphate–sugar interactions. In particular, the compound displaying an oleyl chain, a nucleoside and a glucose moiety linked together by 1,2,3-triazole bridges (GNL) was found to be a highly effective hydrogelator at concentrations >2.5% w/w. The resulting gel was thermoreversible, melting upon heating and turning back to gel on cooling. It was formed by an entangled network of fibres of 20–30 nm in diameter, as evidenced by phase contrast microscopy and TEM. SAXS experiments, performed to unravel the structure of these fibres, strongly suggested that interdigitated GNL molecules assembled as bilayers forming hollow nanotubes. Base-stacking interactions within the bilayers were supported by UV measurements. The gel network was able to entrap small condensed particles of oligonucleotides, aggregated on the fibre surface. This nanostructured material favoured the internalization of oligonucleotides into cells, without significant toxicity (33). Recently, fluorinated analogues of these glycosyl-nucleolipids have been synthesized and their gelation properties studied (34).
The most challenging approach was to induce nucleic acids complexation using anionic nucleolipids. In the formulation of anionic lipoplexes, consisting in the mixture of anionic and zwitterionic phosphoplipids with DNA, the presence of divalent cations, usually Ca2+, is critical to achieve the complexation between DNA and the phospholipids (35). The addition of Ca2+ allows overcoming the electrostatic repulsion between negatively charged lipids and DNA. Divalent cations have also been shown to mediate interactions between zwitterionic phospholipids and DNA, bridging the phospholipid headgroups and the DNA rods (36,37). Barthelemy et al. have described a formulation involving a new anionic nucleolipid possessing thymidine-3′-monophosphate as polar head and 1,2-diacyl-sn-glycerol as lipid moiety, diC16-3′-dT (Figure 1H). Mixtures of DOPE and diC16dT complexed DNA in the presence of Ca+2, which resulted in the formation of both hexagonal and lamellar phases. This formulation was non-toxic and revealed a good in vitro transfection efficacy, likely owing to the presence of the inverted hexagonal phase (38).
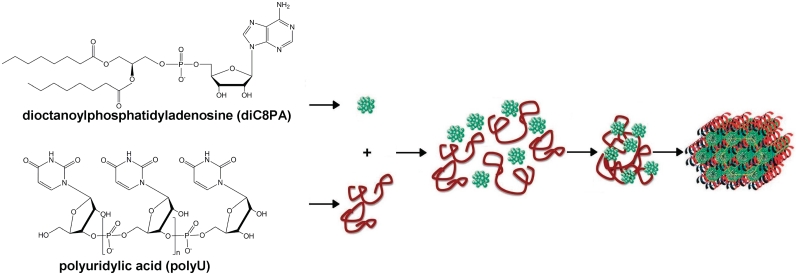
Further, Baglioni and co-workers have provided the first proof of concept that complexes of nucleic acids and anionic nucleolipids can be stabilized via base pairing only, without the presence of cations (39,40). A polyuridilic acid chain (polyU) was chosen as model of a single strand of nucleotide homopolymer. This polynucleotide was shown to interact with either POPA or diC8PA, two negatively charged nucleolipids bearing the complementary adenine base. SAXS experiments have evidenced well-ordered POPA/polyU complexes. PolyU chains were intercalated between POPA fluid bilayers, inducing a lamellar spacing increase. They formed a 1D lattice, with a characteristic spacing depending on the POPA/polyU molar ratio. PolyU also bound to diC8PA spherical micelles, mediating the formation of clusters involving several polyU chains and micelles, as shown by dynamic light scattering, SANS and SAXS. Interestingly, these clusters showed a time-dependent evolution leading to the formation of a well-defined hexagonal phase with a lattice parameter of 98 Å. This supramolecular assembly could be modelled as a hexagonal array of diC8PA cylindrical micelles templated by single strands of the complementary polynucleotide (Figure 5). For both POPA/ polyU and diC8PA/polyU systems, the selective interactions between the complementary bases of the polynucleotide and the nucleolipids, confirmed by spectroscopic measurements, were the driving force for their supramolecular association. Thus, molecular recognition between complementary bases is able to overcome electrostatic repulsion between like-charged polynucleotide and nucleolipids and to trigger structural re-organization of the mixed system. Table 1 gathers nucleolipid/nucleic acid complex devices displaying transfection efficacy in vitro on various cell lines.
Figure 5.
Proposed model for the supramolecular structures formed by polyuridilic acid (polyU) mixed with dioctanoyl-phosphatidyl-uridine (diC8PA) in 0.05 M Tris buffer (pH 7.5). Clusters of polyU chains and diC8PA micelles formed first. After storage at 4°C for one week a well defined hexagonal phase was obtained, suggesting a hexagonal arrangement of diC8PA cylindrical micelles templated by strands of the complementary polynucleotide [reproduced with permission from Ref. (39)].
Table 1.
Nucleolipid/ nucleic acid complexes displaying transfection (or internalisation) efficacy in vitro
| Nucleolipids | Nucleic acids | Expression vectors | Nucleolipid/nucleic acid ratios | Cell lines | References |
|---|---|---|---|---|---|
| O-Ethyl-DOUPC | Plasmid DNA | pUC18 encoding β-galactosidase | 18/1, 36/1 (w/w) | CHO | (27) |
| DOTAU | Plasmid DNA | pEGFP-N3 encoding GFP | 2.4/1, 7.2/1 (w/w) | HeLa MCF-7 | (28) |
| Nitropyrrole-based nucleolipid | siRNA | GAPDH siRNA | 1/1, 5/1, 10/1, 15/1, 20/1 (cation/anion mole ratio) | CHO HepG2 NIH3T3 | (29) |
| Glycosyl nucleoside lipid (GNL) | Fluorescein-labelled oligonucleotide (ON17-mer) | Huh-7 | (33) | ||
| diC16dT/DOPE (10/90 mole ratio) + 15 mM Ca2+ | Plasmid DNA | pEGFP encoding GFP | 4/1 (w/w) | Hek 293 | (38) |
Cell lines: CHO, Chinese hamster ovarian cells; HepG2, human liver cells; NIH3T3, mouse fibroblast; HeLa, human epitheloid carcinoma cells; MCF-7, human breast adenocarcinoma cells; Huh-7, human hepatoma cells; Hek 293, human embryo kidney cells. Genes: GFP, green fluorescent protein; GAPDH, glyceraldehyde-3- phosphate dehydrogenase.
It should be mentioned that, beside lipoplexes, lipid-conjugated oligonucleotides were also considered as an alternative strategy for the delivery of nucleic acids (41–43).
TOWARDS THE CONCEPTION OF NANOMEDICINES: THE SELF-ASSEMBLED NUCLEOSIDIC PRODRUGS
The prodrug strategy has been successfully explored to improve the anti-cancer or antiviral efficacy of nucleosidic analogues. In this approach, a hydrophobic moiety is usually linked to the nucleoside analogue to obtain an amphiphilic bioconjugate. After cellular uptake, the active compound must be cleaved from the promoiety to achieve its therapeutic effect. Covalent coupling to a lipidic molecule may alter the biological activity of the parent drug in a number of ways (45,46). A lipidic prodrug may improve the drug oral bioavailability by enhancing its absorption and/or by decreasing its metabolism. For example, glycerolipidic prodrugs were conceived with the aim to enhance lymphatic absorption of polar drugs by mimicking triglycerides metabolism (47). Prodrug may also be designed to modify the cellular uptake pathway of the active compound. Amphiphilic conjugates are expected to enter cells by non-endocytotic mechanism, without the help of membrane transporters. The pharmacological activity of the drug may also be prolonged by slow release. Finally, the prodrug may also decrease toxicity by achieving the selective delivery or activation of the drug molecule at the target site.
It must be emphasized that almost all lipophilic prodrugs developed up to now face poor solubility and have therefore to be encapsulated into a nanocarrier, most often liposomes, for parenteral administration. Noteworthy, it is of prime importance that the carrier can retain the prodrug until reaching its biological target. However, even a lipophilic anchor does not always ensure the stable incorporation of the prodrug into phospholipid bilayers. The size and shape of the molecule and the relative strengths of interactions between components can also influence their insertion into the liposome membranes. Low drug loading, poor stability in vivo and leakage may occur. In contrast, nanosized self-assembled devices formed by amphiphilic prodrugs have outstanding advantages including ease of processing, absence of excipients and high drug loading. Furthermore, they carry prospects of improved stability and pharmacokinetics. Supramolecular organization of prodrug nanoassemblies is expected to play a key role for the final efficacy of the drug-delivery system because stability of nanoparticles in aqueous medium is sensitive to their structure. Moreover, the ability of these nanoparticles to diffuse into tissues and to be internalized by cells, their haemolytic activity, and hence toxicity may be affected by their size, shape and structure. Two research groups have pioneered the design of self-assemblies of prodrugs in a systematic way.
Jin and co-workers (48) have synthesized cholesteryl derivatives of antiviral acyclovir, didanosine and zidovudine nucleoside analogues, involving succinyl, adipoyl or phosphoryl acyl linkers. As shown by TEM, these derivatives formed nanosized vesicles in water. As far as we know, the activity of these prodrugs has not yet been evaluated.
The research group of Couvreur (49) has evidenced that the linkage of squalene, a natural acyclic isoprenoid chain precursor in the biosynthesis of sterols, to nucleoside analogues led to amphiphilic molecules, which self-organized in aqueous medium as nanoassemblies of 100–300 nm, irrespective of the nucleoside analogue. Two of these squalenoyl derivatives, i.e. squalenoyldideoxycytidine (SQddC) and squalenoylgemcitabine (SQdFdC), have been studied in detail.
Gemcitabine (dFdC) is a fluorinated cytidine analogue used in clinic against solid tumours such as pancreas, non-small cell lung, bladder and breast cancer cell lines. It is also active against lymphoid and myeloid cancer cell lines. However, Gemcitabine suffers from serious limitations. Gemcitabine being too hydrophilic to passively cross the plasma membrane, active transport involving hENT transporters is required for intracellular drug uptake. After conversion into its active triphosphate form by host cell kinases, Gemcitabine is incorporated into replicating DNA as a false nucleoside, which induces cell cycle blockage. Down regulation of hENT transporters or deoxycytidine kinases results in resistance to Gemcitabine at the cellular level. Moreover, Gemcitabine is rapidly inactivated in the blood stream by deoxycytidine deaminase, leading to a short plasma half-life (50). A series of lipophilic prodrugs with increasing length of the hydrophobic chain has been synthesized in order to protect Gemcitabine from deamination. Valeroyl (C5), heptanoyl (C7), lauroyl (C12) and stearoyl (C18) fatty acids were linked to the 4-amino group of Gemcitabine. The metabolic stability in plasma of the C12-dFdC and C18-dFdC derivatives was significantly enhanced, compared to that of Gemcitabine. However, these compounds precipitating in water as large aggregates, needed to be incorporated into liposomes (or surfactant micelles) for intravenous administration. The encapsulation efficiency of the prodrug markedly depended on the length of its acyl chain and on the liposomal composition. A maximum prodrug loading of 25 mol%, corresponding to ~15% w/w drug loading, was obtained for C18-dFdC. Liposomal formulations of C12-dFdC and C18-dFdC derivatives exhibited 5- and 2-fold greater cytotoxicity than free Gemcitabine against HT-29 and KB cell lines, respectively. Moreover, they improved the drug pharmacokinetics (51).
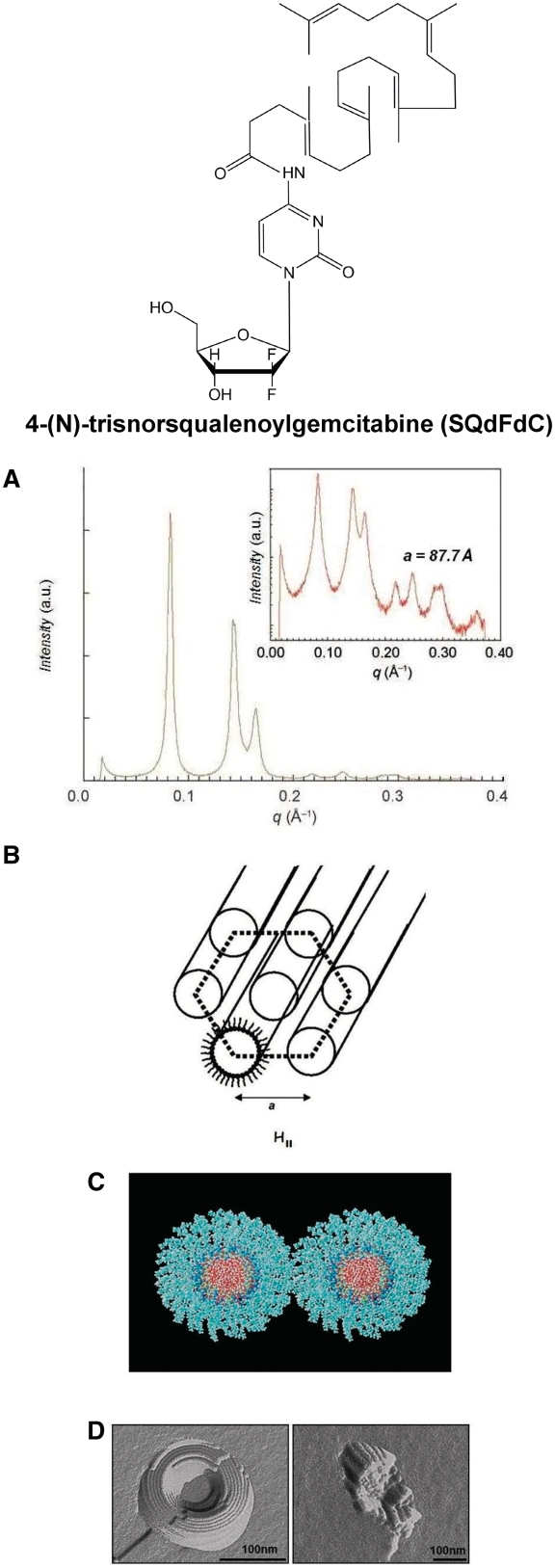
As explained before, coupling squalene to the sensitive amine function of Gemcitabine resulted, unlike the above-mentioned prodrugs, in the spontaneous formation of nanoparticles 120–140 nm in aqueous medium. As shown by cryo-TEM and high-resolution synchrotron SAXS, these nanoassemblies displayed an inverse hexagonal structure, with a lattice parameter of 87.7 Å, formed by close-packed cylinders whose aqueous cores were surrounded by hydrophilic Gemcitabine molecules linked to the squalene moieties. The formation of inverse hexagonal phase was supported by molecular modelling (Figure 6) (14). Interestingly, when Gemcitabine was phosphorylated and further conjugated with squalene, unilamellar liposomes formed (52).
Figure 6.
Self-assembly of SQdFdC. (A) SAXS pattern of the hexagonal phase shown on both linear and log (inset) scales. (B) Schematic drawing of the inverse hexagonal packing of amphiphilic SQdFdC molecules. (C) Molecular modelling: face view of two sections of column each made of six layers of a disk-like assembly composed of 20 conjugates. Molecules are arranged around the aqueous core (oxygen atoms/red), with gemcitabine moieties facing inwards (pyrimidinone nitrogen atoms/blue and fluorine atoms/yellow) and squalene chains facing outwards. (D) TEM images after freeze fracture of SQdFdC nanoassemblies [redrawn from Refs (14,15)].
The SQdFdC nanoassemblies were found impressively more active than Gemcitabine against many experimental cancer models, both in vitro and in vivo (53). In vitro, nanoassemblies displayed higher cytotoxicity than Gem in murine-resistant leukaemia L1210 10 K cells and in human leukaemia resistant cell line CEM-ARAC/8C. In vivo, the SQdFdC nanoassemblies exhibited impressively greater anticancer activity than gemcitabine against either solid subcutaneously grafted tumours (panc-1, L1210 and P388) or aggressive metastatic leukaemia (leukaemia L1210, P388 and RNK-16 LGL). The complex mechanisms resulting in enhanced efficacy of SQdFdC nanoparticles arose from the better stability of the prodrug in plasma and a modification of its uptake pathway. The nanoparticulate form of the prodrug likely contributed to shield SQdFdC from cleavage and deamination but no direct interaction was found to occur between nanoparticles and cells. Indeed, it was demonstrated that nanoparticles did not enter cells by endocytosis, thus avoiding lysosomal degradation. Mechanistic studies have evidenced an albumin-enhanced transport of SQdFdC bioconjugates from nanoparticles to cells across the aqueous medium. In fact, albumin promoted the dissociation of nanoparticles and mediated the diffusion of SQdFdC molecules towards the cell membrane where the prodrug then accumulated. Further partitioning allowed a dramatic enrichment of SQdFdC into intracellular membranes of the endoplasmic reticulum and endolysosomes (54,55). Consistently with in vitro cell uptake experiments, combined structural (SAXS–WAXS) and calorimetric studies have highlighted the ability of SQdFdC molecules to strongly interact with phospholipid model membranes (56). When fully hydrated DPPC bilayers were incubated in the presence of SQdFdC nanoparticles, a slow exchange was indeed evidenced between nanoparticles and DPPC bilayers, confirming the ability of SQdFdC molecules to be progressively released from nanoparticles before to concentrate into membranes (57). In fact, these amphiphilic bioconjugates inserted into bilayers and affected their curvature, leading to the formation of inverse bicontinuous cubic phases. This interaction may explain why SQdFdC was able to partially bypass cell resistance to dFdC treatment, when involving a decrease in the nucleoside transporters expression. Indeed, contrary to dFdC, SQdFdC was found to exhibit a significant anticancer activity in CEM/ARAC 8C cells with deficiency in hENT1 transporters.
When released into the cytoplasm from the membrane cellular reservoirs, the SQdFdC prodrug amide bond was found to be cleaved by cathepsin B, an enzyme that can be hyperexpressed in cancer cells. The progressive release of dFdC may avoid overloading of intracellular kinases, which convert Gemcitabine into its active phosphorylated counterpart. Thus, the prodrug approach could also allow us to partially overcome another factor of resistance to dFdC, when intracellular deoxycytidine kinase enzymatic activity is downregulated, for instance in resistant L1210 10 K cell line. As a whole, SQdFdC nanoparticles enabled to partially circumvent the three enzymatic or cellular mechanisms of resistance to Gemcitabine due to hENT1 downregulation, insufficient activity of deoxycytidine kinase or inactivation by deaminases. Furthermore, SQdFdC nanoparticles appeared to be an attractive delivery system because of their high deposition in spleen, liver and lungs, the major metastatic organs in leukemia.
The formation of non-lamellar structures in model membranes suggested that the amphiphilic properties of the SQdFdC prodrug could be the source of side effects in vivo. Rapid hemolysis was indeed evidenced in vitro at high SQdFdC concentration (57). However, it must be underlined that preclinical studies did not show such toxicity in mice. A toxicological profile similar to that of dFdC was found for SQdFdC (58).
Zalcitabine, formerly called 2′, 3′-dideoxycytidine (ddC), was approved by the Food and Drug Administration in 1992 for the treatment of the human immunodeficiency virus (HIV) infection in combination with azidovudine (AZT). ddC is a synthetic nucleoside analogue of the naturally occurring nucleoside deoxycytidine in which the 3′-hydroxyl group is replaced by hydrogen. Within cells, ddC is phosphorylated by the sequential action of cellular kinases. Triphosphorylation allows obtaining dideoxycytidine 5′-triphosphate (ddCTP), the metabolite active against the virus. ddCTP acts as an inhibitor of HIV-reverse transcriptase by competing for utilization of the natural substrate and a chain terminator by its incorporation into viral DNA. Zalcitabine is well absorbed showing a bioavailability of ~90 % after oral administration. It is weakly metabolized to dideoxyuridine (ddU) by cytidine deaminase and has a moderate half-life (t1/2 = 2 h). This low-molecular weight, highly soluble, nucleoside analogue enters cells mainly via nucleoside carrier-mediated (rCNT1 transporter) pathways. However, it suffers from limited uptake due to its hydrophilicity and low affinity for nucleoside transporters.
In order to eradicate HIV from infected patients, ddC should reach latent HIV reservoirs, able to cause reinfection. Effectiveness of ddC is also reduced by emergence of clinical adverse events including neuropathy, lipodystrophy and pancreatitis. To avoid side effects and improve ddC efficacy, some attempts have been made to use liposomes as drug carriers for intravenous administration. Liposome loaded with ddC were prepared but encapsulation efficiency was low (26%) and ddC rapidly diffused through liposomal bilayers. Several lipophilic derivatives have also been synthesized to improve the therapeutic characteristics of ddC. Different lipophilic moieties have been attached to the 5′-O- or 4-N-position of ddC. Three principal classes of ddC prodrugs are known: ddC-dihydropyridine prodrugs, aiming at increasing the degree of penetration into the central nervous system, ddC-4-N-[(dialkylamino)methylene] prodrugs designed to increase the lipophilicity and stability of the drug, and ddC masked phosphate prodrugs, designed to deliver the monophosphate species intracellularly, thus facilitating the first phosphorylation step. Some of these prodrugs were found to increase the in vitro activity of ddC or to decrease its toxicity (46,59,60).
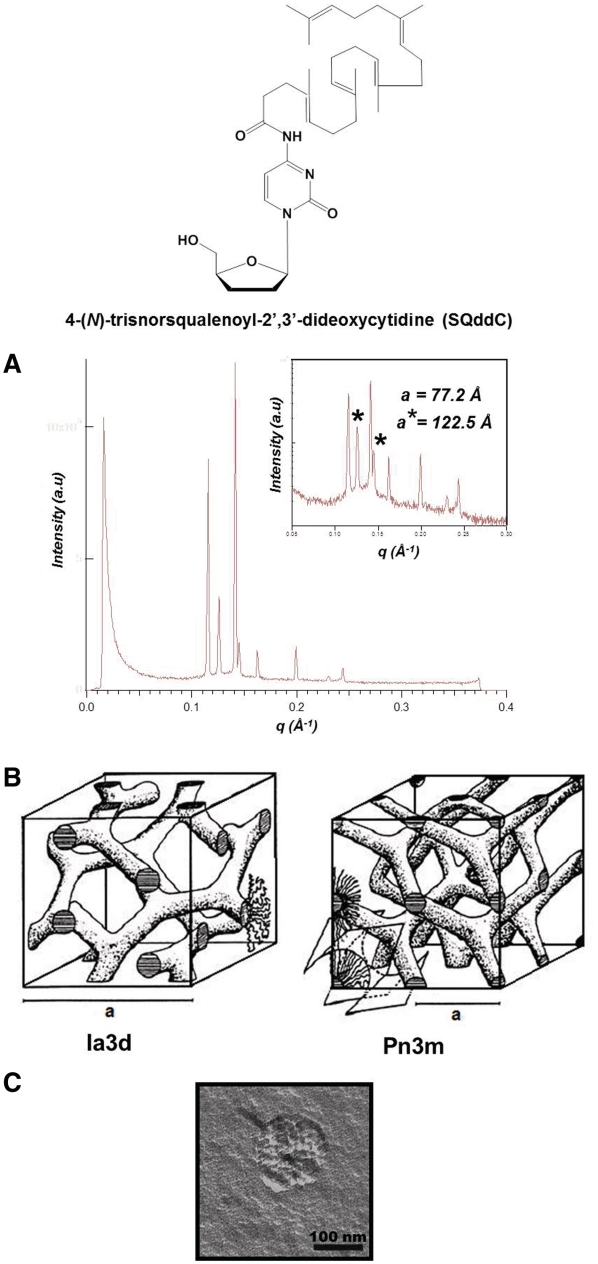
Squalenoylation of ddC (SQddC) yielded spontaneous formation of nanoassemblies with cubic inner symmetry upon nanoprecipitation of SQddC in excess water. Two lattices of space group Pn3m (lattice parameter a = 77.2 Å) and Ia3d (a = 122.5 Å) were identified using SAXS. SQddC molecules formed bilayers folded like a periodic minimal surface, either D (Diamond) or P (Primitive) surfaces (Figure 7) (15).
Figure 7.
Self-assembly of SQddC. (A) SAXS pattern of the Pn3m and Ia3d inverse bicontinuous cubic phases shown on both linear and log (inset) scales. The stars denote the reflections of the Ia3d phase. (B) Schematic drawing of the Pn3m and Ia3d inverse bicontinuous cubic phases. (C) TEM image after freeze fracture of a SQddC cubosome [redrawn from Ref. (15)].
When tested in primary cultures of HIV-1-infected lymphocytes derived from healthy human blood donors, SQddC exhibited better intracellular penetration and higher activity. Moreover, since part of the parenterally administered nanoparticles is taken up by the lymphoid system, where a significant level of HIV replication occurs, the SQddC intravenous delivery is expected to improve its efficacy.
Interestingly, SQdFdC and SQddC, which form hexosomes and cubosomes respectively, differ only by the geminal fluorines and OH group of Gemcitabine at the 2′- and 3′-positions of the nucleoside. The different supramolecular structures of these squalenoyl amphiphiles can be explained by the modulation of their spontaneous monolayer curvature, owing to variation of their interfacial area. The spontaneous monolayer curvature of SQdFdC, exhibiting a small polar headgroup interfacial area and a bulky hydrophobic SQ chain, is negative. The increase in interfacial area, due to the more hydrophilic headgroup of SQddC, promotes less curved structures like cubic phases. Similarly, the larger increase in interfacial area of the amphiphilic compound, induced by the addition of the 5′-phosphate group to SQdFdC, leads to the flattening of the monolayers and to the stabilization of liposomes.
In conclusion, it should be emphasized that, besides Gemcitabine and Zalcitabine, squalenoylation is currently extended to other nucleoside analogues such as Didanosine and to polar therapeutic compounds.
CONCLUSION
High-resolution structural investigations reveal the diversity and complexity of assemblies of bioinspired amphiphiles possessing a polar head derived from a nucleobase and linked to a lipid moiety. Self-organization of these new compounds arises from the interplay between aggregation of the lipid chains and base–base interactions. Subtle differences in molecule chemical structure may cause variations in the self-assembly pattern.
Nucleolipid supramolecular assemblies are promising biocompatible nanocarriers for therapeutic molecules. Specifically, nucleic acids can be confined into lamellar or hexagonal phases or entrapped in hydrogel networks. Neutral or anionic nucleolipids are also expected to offer an alternative to cationic lipids to improve transfection efficacy. The challenge remains to develop safe nucleolipid-based devices displaying high transfection efficiency in vivo. Noteworthy, self-assembly of oligonucleotides conjugated with non-cationic lipids could be an efficient strategy for the delivery of nucleic acids.
Concerning prodrugs, squalenoylation is an innovative nanomedicine concept, which offers new opportunities to obtain more potent therapeutic compounds, able to overcome resistance to the currently available nucleoside analogues. An attractive feature is that cubosomes or hexosomes with reproducible mean sizes formed spontaneously, without the need of high-energy dispersing methods or dispersing agents. The relationship between the chemical structure of the parent drug, the supramolecular organization of the squalenoyl prodrug and the therapeutic activity open an exciting way for future investigations.
FUNDING
The European Research Council under the European Community's Seventh Framework Programme FP7/2007-2013 (249835). Funding for open access charge: The European Research Council under the European Community's Seventh Framework Programme FP7/2007-2013 (249835).
Conflict of interest statement. None declared.
REFERENCES
- 1.Rosemeyer H. Nucleolipids: natural occurrence, synthesis, molecular recognition and supramolecular assemblies as potential precursors of life and bioorganic materials. Chem. Biodiv. 2005;2:977–1062. doi: 10.1002/cbdv.200590082. [DOI] [PubMed] [Google Scholar]
- 2.Lehn JM. Toward complex matter: supramolecular chemistry and self-organization. Proc. Natl Acad. Sci. 2002;99:4763–4768. doi: 10.1073/pnas.072065599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sivakova S, Rowan SJ. Nucleobases as supramoleclar motifs. Chem. Soc. Rev. 2005;34:9–21. doi: 10.1039/b304608g. [DOI] [PubMed] [Google Scholar]
- 4.Berti D, Baglioni P, Bonaccio S, Barsacchi-Bo G, Luisi PL. Base complementarity and nucleoside recognition in phosphatidylnucleoside vesicles. J. Phys. Chem. B. 1998;102:303–308. [Google Scholar]
- 5.Berti D, Pini F, Baglioni P, Teixeira J. Micellar aggregates from short-chain phospholiponucleosides: a SANS study. J. Phys. Chem. B. 1999;103:1738–1745. [Google Scholar]
- 6.Berti D, Barbaro P, Bucci I, Baglioni P. Molecular recognition through H-bonding in micelles formed by dioctylphosphatidyl nucleosides. J. Phys. Chem. B. 1999;103:4916–4922. [Google Scholar]
- 7.Berti D, Luisi PL, Baglioni P. Molecular recognition in supramolecular structures formed by phosphatidylnucleosides-based amphiphiles. Colloids Surf. A: Physicochem. Eng. Aspects. 2000;167:95–103. [Google Scholar]
- 8.Moreau L, Camplo M, Wathier M, Taieb N, Laguerre M, Bestel I, Grinstaff MW, Barthelemy P. Real time imaging of supramolecular assembly formation via programmed nucleolipid recognition. JACS. 2008;130:14454–14455. doi: 10.1021/ja805974g. [DOI] [PubMed] [Google Scholar]
- 9.Barthélémy P. Nucleoside-based lipids at work: from supramolecular assemblies to biological applications. C.R. Chimie. 2009;12:171–179. [Google Scholar]
- 10.Baglioni P, Berti D. Self-assembly in micelles combining stacking and H-bonding. Curr. Opin. Colloid Interface Sci. 2003;8:55–61. [Google Scholar]
- 11.Gissot A, Camplo M, Grinstaff MW, Barthélémy P. Nucleoside, nucleotide and oligonucleotide based amphiphiles: a successful marriage of nucleic acids with lipids. Org. Biomol. Chem. 2008;6:1324–1333. doi: 10.1039/b719280k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagawa H, Ogawa Y, Furuta H, Tsuno K. Spontaneous formation of superhelical strands. J. Am. Chem. Soc. 1989;111:4567–4570. [Google Scholar]
- 13.Itojima Y, Ogawa Y, Tsuno K, Handa N, Yanagawa H. Spontaneous formation of helical structures from phospholipid-nucleoside conjugates. Biochemistry. 1992;31:4757–4765. doi: 10.1021/bi00135a003. [DOI] [PubMed] [Google Scholar]
- 14.Couvreur P, Reddy LH, Mangenot S, Poupaert J, Desmaele D, Lepetre-Mouelhi S, Pili B, Bourgaux C, Amenitsch H, Ollivon M. Discovery of new hexagonal supramolecular nanostructures formed by squalenoylation of an anticancer nucleoside analogue. Small. 2008;4:247–253. doi: 10.1002/smll.200700731. [DOI] [PubMed] [Google Scholar]
- 15.Bekkara-Aounallah F, Gref R, Othman M, Reddy LH, Pili B, Allain V, Bourgaux C, Hillaireau H, Lepetre-Mouelhi S, Desmaele D, et al. Novel Pegylated nanoassemblies made of self-assembled squalenoyl nucleoside analogue. Adv. Funct. Mater. 2008;18:3715–3725. [Google Scholar]
- 16.Milani S, Baldelli Bombelli F, Berti D, Hauβ T, Dante S, Baglioni P. Structural investigation of bilayers formed by 1-palmitoyl-2-oleoylphosphatidylnucleosides. Biophys. J. 2006;90:1260–1269. doi: 10.1529/biophysj.105.067645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berti D, Baldelli Bombelli F, Fortini M, Baglioni P. Amphiphilic self-assemblies decorated by nucleobases. J. Phys. Chem. B. 2007;111:11734–11744. doi: 10.1021/jp0744073. [DOI] [PubMed] [Google Scholar]
- 18.Baldelli Bombelli F, Berti D, Keiderling U, Baglioni P. Giant polymer like micelles formed by nucleoside-functionalized lipids. J. Phys. Chem. B. 2002;106:11613–11621. [Google Scholar]
- 19.Baldelli Bombelli F, Berti D, Almgren M, Karlsson G, Baglioni P. Light scattering and cryo-transmission electron microscopy investigation of the self-assembling behavior of di-C12P-nucleosides in solution. J. Phys. Chem. B. 2006;110:17627–17637. doi: 10.1021/jp060594d. [DOI] [PubMed] [Google Scholar]
- 20.Moreau L, Barthélémy P, El Maataoui M, Grinstaff MW. Supramolecular assemblies of nucleoside phosphocholine amphiphiles. JACS. 2004;126:7533–7539. doi: 10.1021/ja039597j. [DOI] [PubMed] [Google Scholar]
- 21.Campins N, Dieudonné P, Grinstaff MW, Barthélémy P. Nanostructured assemblies from nucleotide-based amphiphiles. New J. Chem. 2007;31:1928–1934. [Google Scholar]
- 22.Iwaura R, Yoshida K, Masuda M, Yase K, Shimizu T. Spontaneous fiber formation and hydrogelation of nucleotide bolaamphiphiles. Chem. Mater. 2002;14:3047–3053. [Google Scholar]
- 23.Caracciolo G, Pozzi D, Caminiti R, Marchini C, Montani M, Amici A, Amenitsch H. Enhanced transfection efficiency of multicomponent lipoplexes in the regime of optimal membrane charge density. J. Phys. Chem. B. 2008;112:11298–11304. doi: 10.1021/jp803077n. [DOI] [PubMed] [Google Scholar]
- 24.Tranchant I, Mignet N, Crozat E, Leblond J, Girard C, Scherman D, Herscovici J. DNA complexing lipopolythiourea. Bioconjug. Chem. 2004;15:1342–1348. doi: 10.1021/bc049920n. [DOI] [PubMed] [Google Scholar]
- 25.Leblond J, Mignet N, Largeau C, Spanedda MV, Seguin J, Scherman D, Herscovici J. Lipopolythioureas: a new non-cationic system for gene transfer. Bioconjug. Chem. 2007;18:484–493. doi: 10.1021/bc060141b. [DOI] [PubMed] [Google Scholar]
- 26.Breton M, Leblond J, Seguin J, Midoux P, Scherman D, Herscovici J, Pichon C, Mignet N. Comparative gene transfer between cationic and thiourea lipoplexes. J. Gene Med. 2010;12:45–54. doi: 10.1002/jgm.1417. [DOI] [PubMed] [Google Scholar]
- 27.Moreau L, Barthélémy P, Li Y, Luo D, Prata CAH, Grinstaff MW. Nucleoside phosphocholine amphiphile for in vitro DNA transfection. Mol. BioSyst. 2005;1:260–264. doi: 10.1039/b503302k. [DOI] [PubMed] [Google Scholar]
- 28.Chabaud P, Camplo M, Payet D, Serin G, Moreau L, Barthélémy P, Grinstaff MW. Cationic nucleoside lipids for gene delivery. Bioconjug. Chem. 2006;17:466–472. doi: 10.1021/bc050162q. [DOI] [PubMed] [Google Scholar]
- 29.Ceballos C, Prata CAH, Giorgio S, Garzino F, Payet D, Barthélémy P, Grinstaff MW, Camplo M. Cationic Nucleoside lipids based on a 3-Nitropyrrole universal base for siRNA delivery. Bioconjug. Chem. 2009;20:193–196. doi: 10.1021/bc800432n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loakes D. The applications of universal DNA base analogues. Nucleic Acids Res. 2001;29:12, 2437–2447. doi: 10.1093/nar/29.12.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barthélémy P, Prata CAH, Filocamo SF, Immoos CE, Maynor BW, Hashmi SAN, Lee SJ, Grinstaff MW. Supramolecular assemblies of DNA with neutral nucleoside amphiphiles. Chem. Comm. 2005;10:1261–1263. doi: 10.1039/b412670j. [DOI] [PubMed] [Google Scholar]
- 32.Arigon J, Prata CAH, Grinstaff MW, Barthélémy P. Nucleic acid complexing glycosyl nucleoside-based amphiphile. Bioconjug. Chem. 2005;16:864–872. doi: 10.1021/bc050029y. [DOI] [PubMed] [Google Scholar]
- 33.Godeau G, Bernard J, Staedel C, Barthélémy P. Glycosyl-nucleoside-lipid based supramolecular assembly as a nanostructured material with nucleic acid delivery capabilities. Chem. Comm. 2009;34:5127–5129. doi: 10.1039/b906212b. [DOI] [PubMed] [Google Scholar]
- 34.Godeau G, Brun C, Arnion H, Staedel C, Barthélémy P. Glycosil-nucleoside fluorinated amphiphiles as components of nanostructured hydrogels. Tetrahedron lett. 2010;51:1012–1015. [Google Scholar]
- 35.Srinivasan C, Burgess DJ. Optimization and characterization of anionic lipoplexes for gene delivery. J. Control. Release. 2009;136:62–70. doi: 10.1016/j.jconrel.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Uhrikova D, Hanulova M, Funari SS, Khusainova RS, Sersen F, Balgavy P. The structure of DNA-DOPC aggregates formed in presence of calcium and magnesium ions: a small-angle synchrotron X-ray study. BBA. 2005;1713:15–28. doi: 10.1016/j.bbamem.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 37.Ainalem ML, Kristen N, Edler KJ, Hook F, Sparr E, Nylander T. DNA binding to zwitterionic model membranes. Langmuir. 2010;26:4965–4976. doi: 10.1021/la9036327. [DOI] [PubMed] [Google Scholar]
- 38.Khiati S, Pierre N, Andriamanarivo S, Grinstaff MW, Arazam N, Nallet F, Navailles L, Barthélémy P. Anionic nucleotide-lipids for in vitro DNA transfection. Bioconjug. Chem. 2009;20:1765–1772. doi: 10.1021/bc900163s. [DOI] [PubMed] [Google Scholar]
- 39.Banchelli M, Berti D, Baglioni P. Molecular recognition drives oligonucleotide binding to nucleolipid self-assemblies. Angew. Chem. 2007;46:3070–3073. doi: 10.1002/anie.200604826. [DOI] [PubMed] [Google Scholar]
- 40.Milani S, Baldelli Bombelli F, Berti D, Baglioni P. Nucleolipoplexes: a new paradigm for phospholipid bilayer-nucleic acid interactions. JACS. 2007;129:11664–11665. doi: 10.1021/ja0714134. [DOI] [PubMed] [Google Scholar]
- 41.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007;25:10, 1149–1157. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 42.Godeau G, Staedel C, Barthélémy P. Lipid-conjugated oligonucleotides via “Click chemistry” efficiently inhibit Hepatitis C virus translation. J. Med. Chem. 2008;51:4374–4376. doi: 10.1021/jm800518u. [DOI] [PubMed] [Google Scholar]
- 43.Wu Y, Sefah K, Liu H, Wang R, Tan W. DNA aptamer-micelle as an efficient detection/ delivery vehicle toward cancer cells. Proc. Natl Acad. Sci. 2010;107:5–10. doi: 10.1073/pnas.0909611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raouane M, Desmaele D, Gilbert-Sirieix M, Gueutin C, Zouhiri F, Bourgaux C, Lepeltier E, Gref R, Ben Salah R, Clayman G, et al. Synthesis, characterization and in vivo delivery of siRNA-squalene nanoparticles targeting fusion oncogene in papillary thyroid carcinoma. J. Med. Chem. 2011;54:4067–4076. doi: 10.1021/jm2000272. [DOI] [PubMed] [Google Scholar]
- 45.Testa B. Prodrug research: futile or fertile? Biochem. Pharmacol. 2004;68:2097–2106. doi: 10.1016/j.bcp.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Gulati M, Grover M, Singh S, Singh M. Lipophilic drug derivatives in liposomes. Int. J. Pharm. 1998;165:129–168. [Google Scholar]
- 47.Lalanne M, Khouri H, Deroussent A, Bosquet N, Benech H, Clayette P, Couvreur P, Vassal G, Paci A, Andrieux K. Metabolism evaluation of biomimetic prodrugs by in vitro models and mass spectrometry. Int. J. Pharm. 2009;379:235–243. doi: 10.1016/j.ijpharm.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 48.Jin Y, Xin R, Ai P, Chen D. Self-assembled drug delivery systems 2. Cholesteryl derivatives of antiviral nucleoside analogues: synthesis, properties and the vesicle formation. Int. J. Pharm. 2008;350:330–337. doi: 10.1016/j.ijpharm.2007.08.037. [DOI] [PubMed] [Google Scholar]
- 49.Couvreur P, Stella B, Reddy LH, Hillaireau H, Dubernet C, Desmaele D, Lepetre-Mouelhi S, Rocco F, Dereuddre-Bosquet N, Clayette P, et al. Squalenoyl nanomedicines as potential therapeutics. Nanoletters. 2006;6:2544–2548. doi: 10.1021/nl061942q. [DOI] [PubMed] [Google Scholar]
- 50.Reddy LH, Couvreur P. Novel approaches to deliver Gemcitabine to cancers. Curr. Pharm. Des. 2008;14:1124–1137. doi: 10.2174/138161208784246216. [DOI] [PubMed] [Google Scholar]
- 51.Immordino ML, Brusa P, Rocco F, Arpicco S, Ceruti M, Cattel L. Preparation, characterization, cytotoxicity and pharmacokinetics of liposomes containing lipophilic gemcitabine prodrugs. J. Controlled Release. 2004;100:331–346. doi: 10.1016/j.jconrel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Caron J, Lepeltier E, Harivardhan Reddy LH, Lepêtre-Mouelhi S, Wack S, Bourgaux C, Couvreur P, Desmaële D. Squalenoyl Gemcitabine monophosphate: synthesis, nanoassemblies characterisation and biological evaluation. Eur. J. Org. Chem. 2011;14:2615–2628. [Google Scholar]
- 53.Reddy LH, Dubernet C, Lepetre-Mouelhi S, Marque P, Desmaele D, Couvreur P. A new nanomedicine of gemcitabine displays enhanced anticancer activity in sensitive and resistant leukemia types. J. Controlled Release. 2007;124:20–27. doi: 10.1016/j.jconrel.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 54.Bildstein L, Marsaud V, Chacun H, Lepetre-Mouelhi S, Desmaele D, Couvreur P, Dubernet C. Extracellular-protein-enhanced cellular uptake of squalenoyl gemcitabine from nanoassemblies. Soft Matter. 2010;6:5570–5580. [Google Scholar]
- 55.Bildstein L, Dubernet C, Marsaud V, Chacun H, Nicolas V, Gueutin C, Sarasin A, Benech H, Lepetre-Mouelhi S, Desmaele D, et al. Transmembrane diffusion of Gemcitabine by a nanoparticulate squalenoyl prodrug: an original drug delivery pathway. J. Controlled Release. 2010;147:163–170. doi: 10.1016/j.jconrel.2010.07.120. [DOI] [PubMed] [Google Scholar]
- 56.Pili B, Bourgaux C, Amenitsch H, Keller G, Lepêtre-Mouelhi S, Desmaele D, Couvreur P, Ollivon M. Interaction of a new anticancer prodrug, gemcitabine-squalene, with a model membrane: coupled DSC and XRD study. BBA. 2010;98:19–28. doi: 10.1016/j.bbamem.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 57.Bildstein L, Pili B, Marsaud V, Wack S, Meneau F, Lepêtre-Mouelhi S, Desmaele D, Bourgaux C, Couvreur P, Dubernet C. Interaction of an amphiphilic squalenoyl prodrug of gemcitabine with cellular membranes. 2011 doi: 10.1016/j.ejpb.2011.07.003. [Epub ahead of print; doi: 10.1016/j.ejpb.2011.07.003] [DOI] [PubMed] [Google Scholar]
- 58.Reddy LH, Marque PE, Dubernet C, Lepêtre-Mouelhi S, Desmaele D, Couvreur P. Preclinical toxicology (subacute and acute) and efficacy of a new squalenoyl gemcitabine anticancer nanomedicine. J. Pharmacol. Exp. Ther. 2008;325:484–490. doi: 10.1124/jpet.107.133751. [DOI] [PubMed] [Google Scholar]
- 59.Kerr SG, Kalman TI. Highly water-soluble lipophilic prodrugs of the anti-HIV nucleoside analogue 2′,3′-dideoxycytidine and its 3′-fluoro derivative. J. Med. Chem. 1992;35:1996–2001. doi: 10.1021/jm00089a008. [DOI] [PubMed] [Google Scholar]
- 60.Tan X, Chu CK, Boudinot FD. Development and optimization of anti-HIV nucleoside analogs and prodrugs: a review of their pharmacology, structure-activity relationships and pharmacokinetics. Adv. Drug Deliv. Rev. 1999;39:117–151. doi: 10.1016/s0169-409x(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 61.Tresset G. The multiple faces of self-assembled lipidic systems. PMC Biophysics. 2009;2 doi: 10.1186/1757-5036-2-3. doi:10.1186/1757-5036-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]