Abstract
Generation of the 3′ overhang is a critical event during homologous recombination (HR) repair of DNA double strand breaks. A 5′–3′ nuclease, NurA, plays an important role in generating 3′ single-stranded DNA during archaeal HR, together with Mre11–Rad50 and HerA. We have determined the crystal structures of apo- and dAMP-Mn2+-bound NurA from Pyrococcus furiousus (Pf NurA) to provide the basis for its cleavage mechanism. Pf NurA forms a pyramid-shaped dimer containing a large central channel on one side, which becomes narrower towards the peak of the pyramid. The structure contains a PIWI domain with high similarity to argonaute, endoV nuclease and RNase H. The two active sites, each of which contains Mn2+ ion(s) and dAMP, are at the corners of the elliptical channel near the flat face of the dimer. The 3′ OH group of the ribose ring is directed toward the channel entrance, explaining the 5′–3′ nuclease activity of Pf NurA. We provide a DNA binding and cleavage model for Pf NurA.
INTRODUCTION
DNA double-stranded breaks (DSB) are generated by genotoxic stresses such as reactive oxygen species, ionizing radiation, chemical agents and by normal cellular processes such as V(D)J recombination and DNA replication. If not properly repaired, DSBs cause chromosome losses or deletions, translocations and genomic instability. Cells primarily employ non-homologous end joining (NHEJ) and homologous recombination (HR) mechanisms to repair DSBs (1). While the NHEJ process involves processing and direct ligation of the DNA ends, the HR pathway uses a homologous template to repair DNA breaks.
HR processing is believed to be preferred route in bacteria (2). RecBCD, a helicase-nuclease in Escherichia coli, initiates HR repair by binding and degrading both ends of DNA as it translocates (3). When RecBCD binds to a cis-acting Chi sequence, its nuclease activity becomes attenuated and the polarity of the nuclease activity switches to 3′ strand nicking at Chi, which leads to 5′ DNA strand resection. The RecBCD-Chi interaction also facilitates loading of RecA recombinase onto 3′ single-stranded (ss) DNA, which catalyzes strand exchange. Unlike bacterial HR, no homologous RecBCD proteins have been identified in archaea and eukaryotes. Instead, the Mre11–Rad50 complex starts the archaeal and eukaryotic HR processes (4).
In eukaryotes, Mre11–Rad50–Nbs1 (human) or Mre11–Rad50-Xrs2 (budding yeast) in conjunction with Ctp1/CtIP (or Sae2) initially recognizes DNA DSBs and removes small nucleotides to form an early intermediate during HR (5–7). Exo1 nuclease, Sgs1 helicase and Dna2 nuclease bind to this intermediate and generate 3′ ssDNA (8,9). Exo1 is a member of the Rad2 family of structure-specific nucleases which possess 5′–3′ exonuclease and 5′ flap endonuclease activities in vitro, generating mononucleotide products (10). The 3′ ss tails are Rad51 recombinase substrates that initiate the homology search and strand invasion for recombination (11).
In archaea, the Mre11–Rad50 complex also senses DNA DSB ends, and processes DSB ends through 3′–5′ exonuclease and endonuclease activities to form a short 3′ overhang (12–14). However, NurA and HerA are believed to replace the role of Exo1 and Sgs1 to generate 3′ ssDNA, in which the RadA recombinase subsequently binds and catalyzes strand exchange (15). NurA, a highly conserved protein in archaea, exhibits a 5′–3′ ss- and dsDNA exonuclease and ssDNA endonuclease activities and functions together with HerA helicase to unwind dsDNA in both the 5′–3′ and 3′–5′ directions in an ATP-dependent manner (15–18). In all archaea genomes, the NurA and HerA genes are present in one operon along with genes encoding Mre11 and Rad50 (17). An analysis of transcriptional responses to induction by ultraviolet radiation revealed that the nurA, mre11, rad50 and herA genes are upregulated 2- to 3-fold in some strains of Sulfolobus solfataricus (19) and about 10-fold in other Sulfolobus strains (20), suggesting that the four proteins are associated and involved in DNA repair by recombination.
NurA physically interacts with HerA and functions cooperatively with the Mre11–Rad50 complex in Pyrococcus furiosus (15). However, the NurA–HerA complex does not interact directly with the PfMre11–Rad50 complex. In contrast, Mre11 directly interacts and stimulates HerA helicase activity in Sulfolobous tokodaii and Sulfolobous acidocaldarius (21,22). NurA from S. tokodaii physically interacts with SSB to inhibit nuclease activity, implicating a possible regulatory role in Mre11–Rad50-mediated DSB repair (23).
Genetic analysis using Thermococcus kodakaraensis showed that cells lacking HerA or NurA are difficult to grow if intra-chromosomal recombination occurs to delete these genes, which illustrates the significance of these genes in cell proliferation (24). Secondary structure predictions of NurA suggest that NurA forms a novel pattern that does not bear any resemblance to the previously characterized folds found in nucleases or other proteins (25). The NurA group appears to be a rapidly diverging group, with a low level of sequence similarity between paralogous and even within orthologous groups (25). While Exo1, the eukaryotic functional homolog of NurA, has been extensively studied both in vitro and in vivo, not much information is available on NurA from the biochemical and structural aspects, despite its importance in archaeal HR.
To understand the functional and structural conservation of NurA and Exo1, and to provide the basis for 5′ to 3′ processing, we determined the structures of apo- and dAMP–Mn2+-bound Pf NurA and characterized its biochemical properties. We show that Pf NurA alone cleaved the 5′-end of DNA substrates, whereas Pf NurA exhibited both 5′ and 3′ exonuclease activities in the presence of PfHerA. Pf NurA possesses a PIWI domain with an RNase H fold, and forms a pyramid-shaped dimer through two interfaces and contains a wide channel on one face, which becomes narrower the other end. The surface near the channel contains two active sites at its corners, where Mn2+ ion(s) and a dAMP molecule bind to each active site. We show the active site architecture, which explains the basis for its nuclease activity. Finally, we provide a model for the NurA–DNA and HerA-NurA complexes.
MATERIALS AND METHODS
Protein expression and purification
All constructs were generated using a standard polymerase chain reaction (PCR)-based strategy. Genes encoding residues 1–451 of Pf NurA were inserted into the pET28a vector. Escherichia coli Rosetta (DE3) containing the vector was cultured in LB broth medium at 37°C. When the absorbance at 600 nm reached 0.6, the culture was induced with 1 mM isopropyl-β-d-thiogalactopyranoside for 4 h. The cells were harvested by centrifugation, and cell pellets containing the Pf NurA were resuspended in 25 mM Tris–HCl (pH 7.4), 300 mM NaCl, 5% glycerol, 5 mM β-mercaptoethanol and 1 mM phenylmethanesulfonylfluoride, and lysed by sonication. Pf NurA was initially purified by Ni–NTA affinity chromatography using a His-tag at the N-terminus of Pf NurA, and subsequently purified using cation exchange (HiTrap-SP) and gel-filtration chromatography (Superdex 200), concentrated to 10 mg/ml by ultrafiltration, and stored at −80°C. To facilitate structure determination, we made selenomethionine (Se-Met)-substituted Pf NurA by growing the E. coli strain B834 (DE3) harboring the pET28a-Pf NurA plasmid in M9 minimal medium. The Se-Met-Pf NurA was purified by the same method used to purify the native protein.
Crystallization and data collection
Crystals of the apo-Pf NurA were grown at 22°C by the hanging drop vapor diffusion method. The crystallization buffer contained 21–25% PEG400, 0.1 M Tris–HCl pH 8.5 and 5 mM dithiothreitol. Crystals were soaked in a solution containing crystallization buffer, 5 mM MnCl2 and 10 mM dAMP for 8 days to obtain dAMP and Mn2+-bound crystals before data collection. The Mn2+-bound crystals were soaked in a crystallization buffer containing 100 mM MnCl2 for 4 h before data collection. Diffraction data were collected at −170°C using crystals flash-frozen in crystallization buffer containing 25% (v/v) glycerol. Diffraction data were collected from apo- and Mn2+-bound crystals at 0.9795 Å and 1.0000 Å, respectively, on beamline 4A at the Pohang Advanced Light Source. dAMP–Mn2+-bound Pf NurA crystals were collected at 1.5418 Å using a home source. The apo-Pf NurA crystals form in space group P212121 with a = 65.0 Å, b = 114.2 Å and c = 122.3 Å and have one dimer molecule in the asymmetric unit (Table S1). Data integration, scaling and merging were performed using the HKL2000 package (26).
Structure determination and refinement
Pf NurA structure was determined by single-wavelength anomalous scattering dispersion (SAD) method using Se-Met-derivatized crystals. Fourteen selenium sites were identified in the asymmetric unit, and the apo Pf NurA structure was determined with the PHENIX program (27). After flattening the solvent, a high-quality electron density map with a resolution of 2.82 Å was obtained. Successive rounds of model building using COOT (28) and refinement using CNS (29) and PHENIX were performed to build the complete model. Restrained NCS was applied throughout the refinement. The final apo- model consists of 860 residues, seven glycerol molecules and 19 water molecules. The structure of Mn2+-bound Pf NurA and dAMP–Mn2+-bound Pf NurA were determined by difference Fourier methods using the apo–Pf NurA structure. The Mn2+-bound Pf NurA consists of 845 residues, four Mn2+ ions, one glycerol molecule and 20 water molecules. The dAMP and Mn2+-bound model consists of 865 residue, two Mn2+ ions, one dAMP molecule, one glycerol molecule and nine water molecules. The N-terminal His-tagged residues were not visible and were presumably disordered. The statistics are summarized in Supplementary Table S1. Missing residues in each structure are described in the Supplementary Data (Information on substrates, nuclease assays, analytical ultracentrifugation and mutant protein structural analysis is described in the Supplementary Data).
RESULTS
Nuclease activities of Pf NurA and the PfHerA–NurA complex
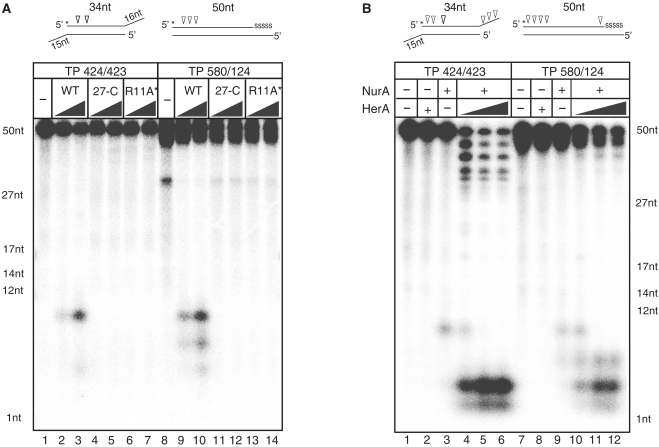
Previous studies have reported that NurA from Sulfolobous acidocaldarius (SaNurA) exhibits 5–3′ exonuclease activity which produced ~5 nt from the 5′-end of a substrate (16). Pf NurA also displays exonuclease activity (15). However, it is unclear if Pf NurA produces any specific cleavage products like SaNurA. To understand if the products of the archaeal NurA nucleases are conserved, we further examined the nuclease activities of Pf NurA using a 5′[32P]-labeled dsDNA oligonucleotide substrate with a 16-nt 3′-overhang (TP424/423) and a 50-nt duplex DNA containing five phosphorothioate bonds at the 3′-end of the top strand (TP580/124). Pf NurA (350 nM) in the presence of Mn2+ exhibited weak endonuclease activity on 20 nM of each substrate, and cleaved primarily ~10 nt from the 5′-end (Figure 1A, lanes 2 and 9). In the presence of a higher amount of Pf NurA (1750 nM), smaller amounts of 6- and 8-nt products were generated from the 5′-end of these substrates (Figure 1A, lanes 3 and 10).
Figure 1.
Biochemical properties of PfNurA and PfHerA (A) Comparison of nuclease activities of PfNurA and dimeric interface mutants. TP 424/423 (lanes 1–7) or TP 580/124 (lanes 8–14) DNA substrates (20 nM) were incubated for 120 min at 65°C with increasing amounts of PfNurA (350 and 1750 nM) and 5 mM MnCl2. 27-C (Δ1–26) and R11A/I12E/S60Y (R11A*) are the PfNurA dimeric interface mutant proteins. Reaction mixtures were analyzed on 15% denaturing polyacrylamide gels containing 7 M urea in TBE buffer. Schematic diagrams of each substrate with a 32P-labeled 5′-end (asterisk) and cleavage sites (arrows) are shown on the top. Five nucleotides in one 3′-end are connected through phosphorothioate bonds, which are shown as ‘SSSSS’. ssDNA markers are indicated. (B) Nuclease activities of PfNurA in the presence of PfHerA. DNA substrates (20 nM) were incubated for 120 min at 65°C with 350 nM of PfNurA, increasing amounts of PfHerA (35, 70 and 115 nM), 5 mM MnCl2 and 1 mM ATP. Lanes 2 and 8 contain 115 nM PfHerA.
Because NurA functions together with HerA, we examined the effect of PfHerA on Pf NurA nuclease activity (Figure 1B) using various ratios of PfHerA and Pf NurA of; 1:3, 1:5 and 1:10. PfHerA (115 nM) alone did not exhibit any nuclease activity. We have used 350 nM of Pf NurA which generated a 10-nt product from the 5′-end. Interestingly, we found that adding various amounts of PfHerA altered the Pf NurA products by cleaving ~1 or 2 nt from both ends of the TP424/423 substrate in an exonucleolytic manner (Figure 1B, lanes 4–6). A small amount of 10-nt product was formed in the presence of a reduced amount of PfHerA (35 nM), which was no longer visible when PfHerA was increased to 70 nM. Pf NurA also exhibited 5′-strand exonucleolytic activity towards the TP580/124 substrate in the presence of PfHerA (Figure 1B, lanes 10–12). Thus, our data show that Pf NurA exhibits 5′-endonucleolytic activity alone and together with PfHerA, and that Pf NurA has exonucleolytic activity on both 5′ and 3′ of a substrate.We presumed that PfHerA unwinds the DNA substrates in both directions and allows them to be more susceptible to degradation, which resulted in the generation of altered products by Pf NurA in the presence of PfHerA.
Overall structure of Pf NurA
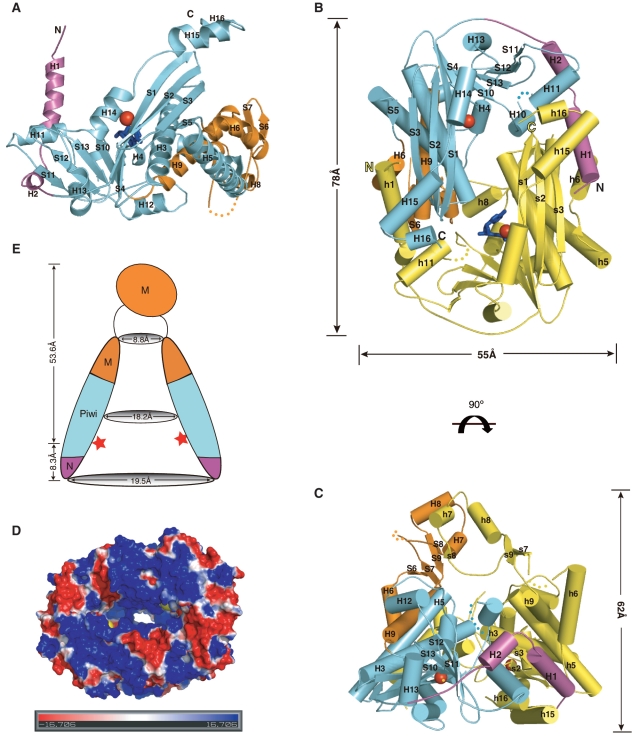
Analytical ultracentrifuge and gel filtration analyses suggest that Pf NurA forms a dimer, consistent with the biochemical studies of StoNurA (30) and Pf NurA (15) (Supplementary Figure S1). The structure of the full-length Pf NurA was determined at 2.82 Å resolution using single-wavelength anomalous dispersion method (Supplementary Table S1).
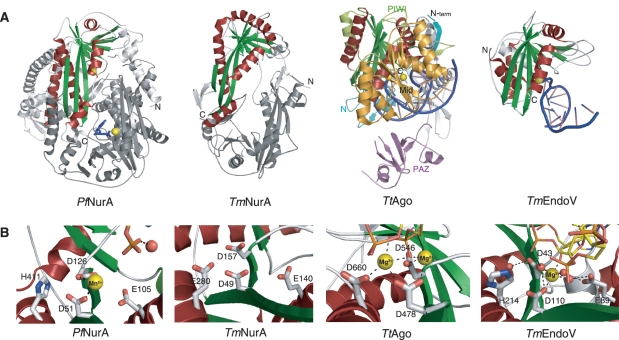
The crystal structure of Pf NurA revealed that the monomer forms an α/β fold that consists of 16 α-helices and 13 β-strands (Figure 2A and Supplementary Figure S2). Each subunit consists of two extended N-terminal helices and a compact body that assembles into a pyramid-shaped dimer with dimensions of 78 × 55 × 62 Å (Figure 2B and C). A large central channel is formed at the base of the pyramid and becomes narrower toward a peak that is formed by the two-helices (H7 and H8) from each subunit.
Figure 2.
Overall structure of Pf NurA. (A) Structure of the Pf NurA monomer. The N-(magenta), PIWI (aqua), and M (orange) domains are shown with Mn2+ ions (red spheres). (B) The flat side of the Pf NurA dimer is viewed from the front and (C) from a 90° rotated view (along the horizontal axis of Figure 2B). Three domains of one monomer are shown in the same color scheme as in 2A (magenta, aqua and orange) and another monomer is shown in yellow for clarity. Secondary structures are labeled. Red spheres represent Mn2+ ions, and the two dAMP molecules are colored in blue. The disordered region is shown as dots. Dimension of the central hole is 29 × 19 Å2. (D) Electrostatic potential of the Pf NurA dimer mapped onto the solvent-accessible protein surface (blue indicates positive regions; red indicates negative regions; the metal ion is in yellow). The figure is shown as a 90° rotated view of 2B along a 2-fold axis. (E) A schematic drawing of the Pf NurA central channel in the same view as that of 2C.The Mn2+ ions are represented as red stars.
The overall structure of the Pf NurA monomer is highlighted by a twisted central eight-stranded sheet that is packed by several helices on both sides (Figure 2A). This part of the structure strongly resembles the PIWI domain with an RNase H fold, which is found in argonaute proteins (31–33). We divided the Pf NurA subunit into three domains (Figure 2A and B): the N-domain (magenta color, residues 3–43), the PIWI domain (aqua color, residues 44–188 and 287–441), and the M domain (orange colored middle domain, residues 189–286). In Figure 2C, the PIWI and N-domains form the base and the M-domain is on the top of the base of the pyramid to form a peak.
The N domain is composed of two perpendicularly located helices (H1 and H2), which play an important role in dimerization. The PIWI domain of Pf NurA, an α/β structure with an RNase H fold, is dominated by a central mixed β-sheet consisting of eight β-strands (11, 12, 13, 10, 4, 1, 2 and 3 order). The β-sheet can be divided into three-long strands (S1–S3) and five short strands (S4, S10–S13), with a deep cleft between them. The three long strands are flanked by three continuous helices (H14 to H16) on one face and helices H3 and H5 on the opposite face. The five short strands are packed by helix H13 on one side and helices H4 and H10 on another side (Figure 2A).
The PIWI domain is divided into two regions by the M domain; one part of the PIWI domain is formed with five strands (S1–S3, S4, S5) and three helices (H3–H5), which is followed by the M domain (orange color) with two helices (H6 and H9), a hairpin (S6 and S7), two strands (S8 and S9) and two additional helices (H7 and H8). Subsequently, another part of the PIWI domain with four strands (S10–S13), four helices (H10–H13) and three extended helices (H14–H16) that form the base of the pyramid and boundary of the channel follows.
Overall, the two Pf NurA subunits are similar with a r.m.s.d. value of 0.72 Å for all Cα atoms. However, the two subunits show significant differences in the region M domain region (residues 216–257) (Supplementary Figure S3). Different parts of the M domain contain similar secondary structures with three strands (S7–S9) and two helices (H7 and H8). However, they are separated by >20 Å and to approximately superimpose, one subunit has to be rotated by 42° and translated by 22 Å onto another Pf NurA.
Pf NurA active sites are located at the two corners of an elliptical hole, surrounded by helices H3 and H14 and strands S1 and S4. The active site is ~8 Å deep from the surface of the outer channel, and the two active sites in the Pf NurA dimer are ~29 Å apart. The dAMP-binding site is further away from the surface of the channel compared to that of the Mn2+ site (Figure 2B). The highly conserved C-terminal region of another subunit is directed toward the active site cleft of the Pf NurA subunit.
Dimerization of Pf NurA creates a large central channel
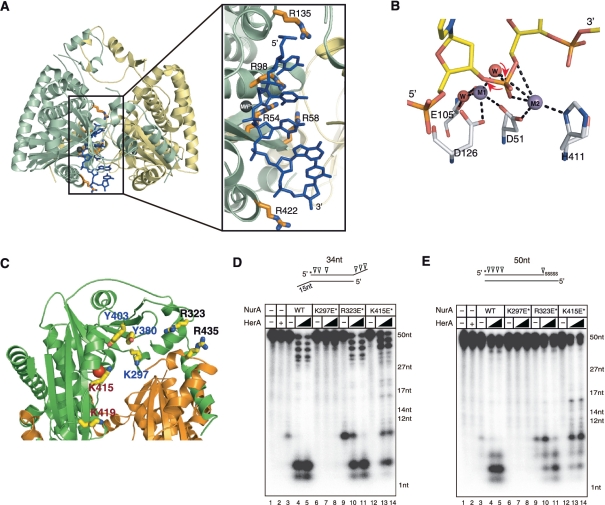
The base of the pyramid (front view of the dimer in Figure 2B and D) is formed with an elliptical channel with three helices (H14–H16) from each subunit, which is followed by strands S1, S4 and S10, and helices H3, H4 and H5 in the next layer. As the channel directed toward the peak, the width of the channel becomes narrower and helices H7 and H8 form the last layer (Figure 2E). Although the channel that has a positive electrostatic potential becomes narrower, it is large enough to hold ssDNA (Figure 2D). In particular, several conserved residues including Arg54, Arg58 and Arg135 are aligned along the channel.
Dimeric interface of Pf NurA
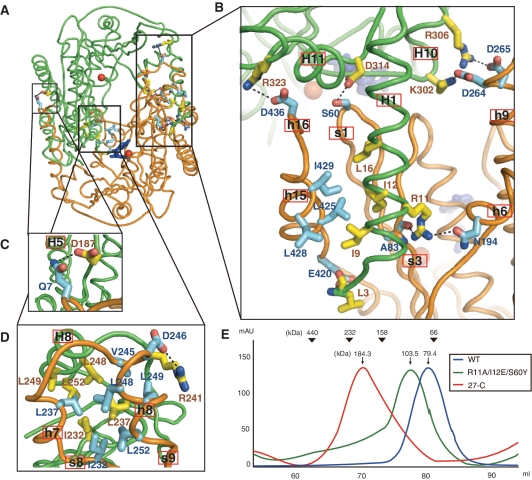
Pf NurA forms a tightly packed dimer with a buried surface area of 7481 Å2. These two interfaces play a major role in dimerization (Figure 3A–D). In the first interface which is formed with the N and PIWI domains, helix H1 from one Pf NurA protrudes into the pocket formed by strand s3 and helices h6 and h15 from another Pf NurA, and embraces another Pf NurA (Figures 2B, 2C and 3A–C). Here, helix H11 also interacts with helix h16 from another Pf NurA. In the second interface which is formed with the M domain, helices H7 and H8 from one Pf NurA interact with the equivalent helices from another Pf NurA (Figures 2C and 3D). In addition, strand S8 from a subunit interacts with strands s8 and s9 from another Pf NurA. The first interface is observed in many archaeal NurA proteins, whereas the second interface is likely to be unique to a few NurA proteins that only contain the M domain region (Supplementary Figure S2). Interestingly, although Thermotoga maritima NurA (TmNurA) shares part of the conserved region in the first interface, it exhibits a different dimeric interface mode (see below), which suggests that even the first dimeric interface may not be structurally conserved in various NurA family members.
Figure 3.
Structural analyses of the dimeric interface. (A) Overall view of the Pf NurA dimeric interface. Two different Pf NurA subunits are colored in green and orange.(B) A close-up view of the first interface of Pf NurA dimer. In the first interface, one subunit (green) and another Pf NurA (orange) interact primarily through the N- and PIWI domains as described in the text. (C) In addition to these interactions, an ion pair Gln7–Asp187 is present. (D) In the second interface, helices H7 and H8 of one Pf NurA interact with helices h7 and h8 of another Pf NurA. Strands s8 and s9 are also involved in dimer formation. Oxygen and nitrogen atoms are shown in red and blue, respectively. (E) Comparison of the oligomeric states of the wild-type and dimeric interface Pf NurA mutants using gel filtration chromatography. Standard molecular weights are shown at the top. Gel filtration analysis (Superdex 200) was performed using a buffer containing 25 mM Tris–HCl (pH 7.4), 200 mM NaCl, 5% glycerol and 5 mM DTT.
In the first interface, the H1-s3 interaction is largely formed by hydrogen (H)-bonds and hydrophobic interactions. Here, four H-bonds including Arg11–Ala83 (main chain) are observed. In addition, three ion-pairs including Asp264–Lys302, Asp265–Arg306 and Arg323–Arg436 occur (Figure 3B). Hydrophobic interactions further stabilize this interface; Ile9, Ile12 and Leu16 from a subunit and are tightly packed against Leu425, Leu428 and Ile429 from another Pf NurA (Figures 2C and 3B).
To examine the importance of the N-terminal helix H1 in the dimer interface, we generated two Pf NurA mutant proteins by deleting the first 26 residues or by simultaneously replacing Arg11 with Ala, Ile12 with Glu, and Ser60 with Tyr. Both of these Pf NurA mutant proteins exhibited abrogated nuclease activities on the two different DNA substrates (Figure 1A). Gel filtration analysis revealed that a triple (R11A/I12E/S60Y) mutant eluted earlier than that of the wild-type Pf NurA at about a dimer size, and that the ΔN26 mutant protein eluted at a tetramer size (Figure 3E), suggesting that the mutations on helix H1 affect the dimeric interface and alter the overall oligomer shape of Pf NurA.
In the second interface, hydrophobic interactions are highlighted by Ile232 (S8) and Leu237 (H7), which bind to the pocket formed by Ile232, Leu237, Val245, Leu248, Leu249 and Leu252 (Figure 3D). An ion-pair between Arg241 and Asp246 further stabilizes this interface.
Active site of Pf NurA
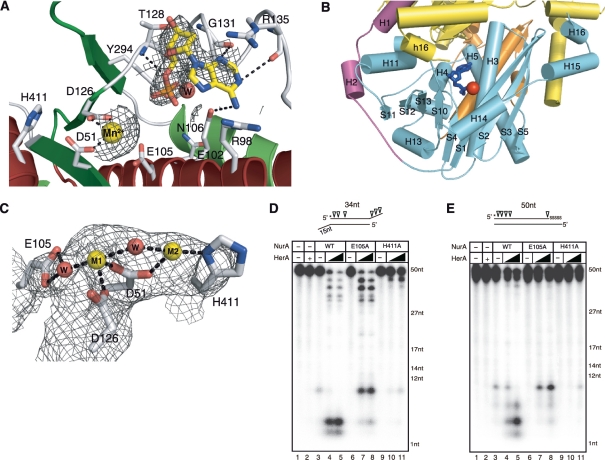
To identify the metal and substrate binding sites, we added 5 mM MnCl2 and 10 mM dAMP to the Pf NurA crystal, which clearly revealed a Mn2+ ion site in each subunit, coordinated by Asp51 and Asp126, and lying close to Glu105 (Figure 4A). The distance between Mn2+ and the side chain of Asp51 or Asp126 is between 2.2 to 2.5 Å, whereas Glu105 is further from the Mn2+ ion. In the Pf NurA subunit, the active site is positioned in a cleft formed between the long (S1) and short (S4) strands. Figure 4B shows that the bottom of the cleft is formed by loop S4–H4, the right wall is formed by strands S1 and S4 and helix H3, the left wall is formed by helix H4 and strand S10. Helix H5 forms the back of the cleft. The C-terminus (yellow) of another subunit, formed with several highly conserved residues, is directed toward the active site cleft of the Pf NurA subunit (Figure 4B).
Figure 4.
Active site of PfNurA. (A) 2FO–FC electron density map (1.2σ) showing dAMP, Mn2+ and the interacting residues. H-bonds and ion pairs are shown as dotted lines. (B) An active site cleft. An active site cleft with a Mn2+ ion (red sphere) and dAMP (blue stick). The C-terminal end of another PfNurA monomer (yellow) is directed toward the active site cleft of the PfNurA monomer. The three domains are colored as in Figure 2B. (C) 2FO–FC electron density map (1.5σ) of two Mn2+ ions. Active site residues and water molecules are shown. H-bonds and ion pairs are shown as dotted lines. (D) Nuclease activity analysis of the two active site mutant proteins. TP 424/423 or (E) TP 580/124 was used as the substrate. Assay conditions were the same as those used in Figure 1B.
We observed another strong density near the metal-binding site where we added dAMP. The density was clear in one subunit (subunit B), while it is ambiguous in another subunit and we did not build dAMP. A phosphate group is directed to helix H3 and strand S4 and the base is exposed to the surface of the open channel, interacting with helix H4. The base interacts with the guanidinium group of Gly131(O) and Arg135(O) and is close to Arg98 and Glu102. The ribose ring is packed against the ring of Tyr90. A phosphate is located near Glu102, Glu105 and Asn106 on one side, the backbone of Asp126–Thr128, and the side chain of Tyr294. A Mn2+ ion is 6.5 Å separated from the phosphate of dAMP. The 3′ OH group points toward the metal and the entrance of the channel. Highly, but not absolutely, conserved Arg residues are lined along the open channel to the narrow end. It is likely that these Arg residues coordinate with phosphate backbones of a DNA substrate (see below).
To examine the possibility that additional Mn2+-binding site may be present at the active site, we added an excess amount (100 mM) of MnCl2. In addition to the Mn2+ I site (M1), we did observe additional density (at the opposite side of the dAMP-binding site from the M1 site), raising the possibility that a second Mn2+ ion may bind to the active site (Figure 4C). The added Mn2+ (M2) coordinated with His411 and Asp51, and a water molecule which also interacted with M1. The distance between the two Mn2+ ions is 4.2 Å. It is possible that the water molecule that interacts with both M1 and M2 ions functions as a nucleophile for the nuclease reaction.
Mutational analysis of the active site
The Asp51 mutation (or corresponding residues from StoNurA) completely abolished nuclease activity, consistent with structural observations (15,30). We also observed that mutation of Glu105 to Ala abolished Pf NurA endonuclease activity on two different substrates (TP424/423 and TP580/124), suggesting that this residue is important for catalysis (Figure 4D and E, lane 6). Additional mutation studies showed that His411 is also important for nuclease activity, as the His411 mutation or equivalent residue abrogated Pf NurA and StoNurA nuclease activity (Figure 4D, lanes 10 and 11; 30). The H411A Pf NurA mutant exhibited weak nuclease activity in the presence of PfHerA (Figure 4D and E, lane 11). Since this residue is exposed, its effect on nuclease activity is unlikely due to the structural perturbation. The TmNurA structure does not contain any metal ion in its active site. Three residues (Asp49, Asp157 and Glu140) are conserved, His411 is not present in TmNurA, and this residue is replaced with an acidic residue (Glu280) in TmNurA (Figure 5A, B and Supplementary Figure S2).
Figure 5.
Structural comparison of RNaseH-like fold proteins. (A) Comparison of the Pf NurA, TmNurA (1zup), TtAgo (3hvr) and TmEndo (2w35) structures. Conserved α-helices (red) and β-sheets (green) form a PIWI domain. Pf NurA and TmNurA show a dimeric structure, whose interfaces significantly differ from each other. The TmNurA structure does not contain any metal ions in its active site. The monomer structures of TtAgo and TmEndoV are shown and their metal ions are denoted as yellow spheres. DNA is shown in blue. (B) Comparison of active site in Pf NurA, TmNurA, TtAgo and TmEndoV. His411 of Pf NurA is only conserved in TmEndoV and replaced to Asp in other structures. Oxygen and nitrogen atoms are shown in red and blue, respectively. A water molecule is in red sphere.
Interestingly, the E105A Pf NurA mutant showed efficient 3′ exo- and 5′-endonuclase activities (10-nt product) in the presence of PfHerA (Figure 4D, lanes 7 and 8). However, this mutant showed very weak 5′ exonuclease activity. Efficient 5′-endonuclease activity of the E105A Pf NurA mutant in the presence of PfHerA was further confirmed with the TP580/124 substrate (Figure 4E, lanes 7 and 8). Taken together, our mutational analysis demonstrates the significance of Glu105 and His411 of Pf NurA for nuclease activity, and that Pf NurA nuclease activity can be changed in the presence of PfHerA.
Structural comparison with TmNurA
The crystal structure of the TmNurA homolog (PDB ID; 1zup) has been deposited in the PDB data base (unpublished). TmNurA consists of 330 residues which is significantly shorter than that of Pf NurA (451 residues), and shares 12% sequence identity. TmNurA also forms a dimer and the r.m.s.d. between Pf NurA and TmNurA is 3.3 Å for 183 Cα atoms. Although the overall core structure containing the eight stranded-sheet (S1–S3, S4, S10–S13, green) and flanking helices (helices H3, H13–H15, red) in the PIWI domain is similar between Pf NurA and TmNurA, significant differences are observed in the dimeric architecture and dimeric interface.
First, in contrast to Pf NurA, the two N-terminal strands of TmNurA protrude into the central channel and divide the channel (Figure 5A). Second, the dimeric interface of TmNurA is not as tight as that of Pf NurA: the dimeric interface of TmNurA is formed through the interaction between N-terminal S1 and s2, between loops S1–S2 and s1–s2, and between loops S1–S2 and s2–s3. Third, although both N- and C-terminal regions are involved in dimerization in Pf NurA and TmNurA, the contents and modes of interactions between these domains are totally different: the N-terminal helix H1 of Pf NurA is not present in TmNurA, suggesting that the dimeric interaction of TmNurA might be less tight than that of Pf NurA. In addition, the M domain containing several helices (H4 and H6–H8) and strands (S5–S9) of Pf NurA (residues 130–278) is not present in TmNurA. Consequently, the central channel in the TmNurA dimer is wide-open at both the front and back sides, whereas the channel becomes narrower toward one side, and is partly blocked by helices H7 and H8 in Pf NurA (Figure 5A).
Structural comparison with other nucleases
When compared with its functional homolog, human Exo1, strands S10–S13 of Pf NurA can be superimposed with the corresponding part of the structure, whereas other regions are completely different (34). The PDB data base search using the DALI server revealed that Pf NurA is most similar to the structures of argonaute (3hvr, z score 8.1, rmsd 3.5 Å for 182 Cα; 35) and endoV nuclease (2w35, 7.7, 3.4 Å for 152 Cα; 36), in addition to the TmNurA homolog (10.1, 3.8 Å for 194 Cα). The core of these structures shares high similarity, with the RNase HII fold, in which the five strands (3, 2, 1, 4, 10) are packed against helix H14 on one face and helices H3, H4 and H9 on another face (35, 36, Supplementary Figure S5). However, large differences are observed in the overall monomeric structure and dimeric arrangement.
Figure 5A shows the structural comparison between Pf NurA and the proteins with structural homology. The red colored helices and green colored strands of Pf NurA denote high similarity with the PIWI domain of Thermus thermophilus Ago (TtAgo; 35). However, the lengths of several secondary elements (S1, S2, S3, H3 and loops S1–S2 and S3–H3) are much longer than the equivalent parts of TtAgo. Unlike the PIWI domain, an equivalent region to the Mid domain (orange, residues 328–546) of TtAgo is not present in the Pf NurA dimer. The PAZ domain (magenta) of TtAgo can overlay portions including strands S1, S2, S3, helix H4 and loop H13–H14 from another Pf NurA subunit. Furthermore, the N-domain of TtAgo (blue) can be superimposed on a part of the PIWI domain and parts of the M domain (residues 144–154, 215–224 and 252–261) of Pf NurA.
EndoV is present as a monomer; thus, DNA binds to the more open interface of Endo V compared to TtAgo or Pf NurA (37). Here, a central β-sheet comprising S1, S2, S3, S4, S10, S12 and S13 (green), which is flanked by helices H13 and H14, and H3, H9 and H12 (red), which shares structural conservation with Pf NurA.
The two Asp (Asp51 and Asp126) are conserved in RNase H, Ago, endoV and TmNurA (Figure 5B). The Glu residue (Glu105) is also conserved in all RNase H and NurA family members. In TtAgo, Asp660 (His411 of Pf NurA) coordinated the Mg2+ ion; thus, three catalytic acidic residues (Asp478, Asp546 and Asp660) coordinated the two Mg2+ ions. It has been suggested that an OH group bound to the Mg2+ ion in TtAgo, which is bound to Asp660, plays a role as a nucleophile (38). TmEndoV exhibits high similarity to the active site of Pf NurA–dAMP–Mn2+ as it contains one Mg2+ ion and a conserved His, two Asp and a Glu residue in its active site (Figure 5B).
Pf NurA–DNA model
Based on the Pf NurA–dAMP–Mn2+, TtAgo–DNA and EndoV–DNA crystal structures, we modeled both dsDNA and ssDNA on an active site of Pf NurA (Figure 6A and Supplementary Figure S5). DNA primarily binds to PIWI and PAZ in TtAgo, and the 3′-end is recognized by the N domain and the 5′-end is recognized by the Mid domain (38). When the PIWI domain of TtAgo is superimposed on the equivalent domain of Pf NurA, dsDNA bound to a sheet comprised of strands (S3, S2, S1, S4, S10, H13, S12 and S11), and regions, including helices H11 and H12 and strands S11 and S12 from another Pf NurA, encircled the DNA completely. However, helix H3 from one monomer and strands S1–S3 from another Pf NurA collided with the modeled dsDNA, implying that a conformational change in this region or dsDNA is necessary to accommodate the dsDNA (Supplementary Figure S5).
Figure 6.
PfNurA–ssDNA-binding model. (A) A ssDNA (blue) is modeled on PfNurA based on the TtAgo–DNA and PfNurA–dAMP–Mn2+ structures. Box: a close up view of the model showing the direction of the ssDNA, where the 3′ OH of a ribose ring points toward the open channel (entrance) and the 5′-phosphate group is directed to the narrow part of the channel. (B) Schematic drawing of the PfNurA nuclease mechanism. Mn2+ (M1) is coordinated by Asp51, Asp126 and two water molecules. Mn2+ (M2) interacts with Asp51, His411 and one water molecule. In this model, a water nucleophile (red) bound to both metal ions attacks the phosphate group and M1 stabilizes the leaving 3′ oxyanion, which allows the 5′–3′ digestion.(C) Structure of the PfNurA showing the position of some residues on the surface of the elliptical channel. Residues containing K297E/Y380F/Y403F (blue label), R323E/R435E (black label) and K415E/K419E (red label) are shown. (D) Nuclease activity assay of the three DNA-binding mutant using TP 424/423 or (E) TP 580/124. Each DNA substrate (20 nM) was incubated for 120 min at 65°C with 350 nM of PfNurA, increasing amounts of PfHerA (35 and 70 nM), 5 mM MnCl2 and 1 mM ATP. In lane 2, 70 nM of PfHerA was used as a control. Abbreviations: K297E*, K297E/Y380F/Y403F; R323E*, R323E/R435E; K415E*, K415E/K419E.
In contrast, ssDNA, metal and large parts of the TtAgo structure including PIWI could be readily superimposed with the metal, dAMP and Pf NurA PIWI domain (Figure 6A). When PIWI from TtAgo–DNA or EndoV–DNA is superimposed on Pf NurA, ssDNA fits nicely into a cleft formed by strand S1, helix H4 and loops S4–H4, and S10–H10 from one Pf NurA, and strand S1 and loop H14–H15 from another Pf NurA. The modeled ssDNA threads through the channel from the 3′–5′ direction, where the 3′ OH of a ribose ring is directed to the open channel and the 5′ phosphate group is directed to the narrow part of the channel (Figure 6A, box). From the Mn2+ ion(s) to the narrow end of the channel (H7 and H8) is ~50 Å in which ~10–11 nt can be located (Figure 2E). Several conserved Arg and Lys residues (Arg54, Arg58, Arg98, Arg135, Lys297, Arg323, Lys415, Lys419 and Arg422) are positioned along the phosphate group of the modeled DNA and are likely to stabilize the substrate or reaction intermediate.
DNA binds to the surface of the open channel
Based on the structural comparison of the PIWI domains between Pf NurA and TtAgo, and the information of the metal and dAMP-binding site of Pf NurA, it is likely that DNA binds to the groove formed by strand S1, helix H4 and loop S4–H4, S10–H10 and H14–H15 of Pf NurA. These regions are rich in positively charged residues and ring-containing residues, which could interact with a DNA substrate, including Lys297, Arg323, Tyr380, Tyr403, Lys415, Lys419 and Arg435 (Figure 6C).
To examine if these residues affected DNA binding and cleavage by Pf NurA, charge-inversion mutant proteins were constructed and their nuclease activities were assessed. We made three double- or triple-mutant proteins. These mutant proteins included Lys297Glu–Tyr380Phe–Tyr403Phe, Arg323Glu–Arg435Glu and Lys415Glu–Lys419Glu. In the absence of PfHerA, the Lys297Glu–Tyr380Phe–Tyr403Phe and Lys415Glu–Lys419Glu mutants failed to show any nuclease activities on two different DNA substrates, whereas Arg323Glu–Arg435Glu showed similar (or higher) nuclease activity compared to that in wild-type Pf NurA, despite this mutant exhibited slight change in circular dichroism (CD) spectra near 210 nm (Figure 6C–E, lanes 3, 6, 9, 12 and Supplementary Figure S6). However, the Lys415Glu–Lys419Glu mutant showed strong nuclease activity in the presence of PfHerA, which was comparable to wild-type Pf NurA, and only the Lys297Glu–Tyr380Phe–Tyr403Phe mutant failed to show any nuclease activity (Figure 6C–E). The CD analysis revealed that the Lys297Glu–Tyr380Phe–Tyr403Phe triple mutation did not alter the overall Pf NurA conformation (Supplementary Figure S6). The mutational analysis suggested that Lys297, Tyr380 and/or Tyr403 on the flat face of the Pf NurA dimer are involved in DNA binding. In addition, Lys415 and Lys419 may also participate in DNA substrate binding.
DISCUSSION
In this study, we determined the crystal structure of Pf NurA and identified substrate binding sites to reveal its nuclease mechanism. NurA is a conserved nuclease in archaea and has been proposed as a functional homolog of eukaryotic Exo1 nuclease (15). Although it is only present in archaea, it has drawn substantial interest because (i) it has no reported structural Pf NurA homologs and exhibits weak sequence similarities between paralogs and within orthologs, (ii) it is likely to play an important role in archaeal HR, and (iii) it specifically processes DNA in the 5′ to 3′ direction. We have provided important insights into these questions in the present study. We have provided important insights on these questions in the present study. First, we have shown that Pf NurA contains a PIWI domain with an RNase HII fold and forms a pyramid-shape dimer with a central channel that can be used for both endo- and exonucleolytic substrate cleavage. Second, we have shown from the Pf NurA–dAMP–Mn2+ structural studies that Pf NurA resembles argonaute and provides the basis of the 5′–3′ nuclease activity of NurA.
HerA alters the Pf NurA product
We have shown that Pf NurA together with PfHerA generates 2- to 4-nt products from both ends of a substrate, displaying both 5′ and 3′ exonuclease activities, whereas Pf NurA alone produced 6–10 nt from the 5′-end of substrates in an endonucleolytic manner (Figure 1A and 1B). Although previous studies clearly showed that PfHerA facilitates Pf NurA nuclease activities in the presence of Mn2+ or Mg2+ ions, no detailed biochemical characterizations of the Pf NurA–PfHerA complex have been described (15). Previous studies showed that the Pf NurA–PfHerA complex exhibits both exo- and endonuclease activities on a 2.5-kb DNA substrate (15, Paull, personal communication). Although the same enzymes were used, we note some differences between current experiments and previous analyses by Hopkins and Paull (15). First, we used a 17.5:1 ratio of Pf NurA (350 nM) and the DNA substrate (20 nM), whereas a 1600:1 ratio of enzyme (96.2 nM) to substrate (0.06 nM) was used in previous assays (15). Second, Hopkins and Paull used a 2.5-kb substrate, whereas we used short-length DNA (50 bp) substrates. It is likely that PfHerA, a helicase that unwinds DNA in both directions, melts the DNA substrates to make them more susceptible to the Pf NurA dimer, which possesses two active sites and alters the generation of product.
Pf NurA dimer is formed by two interfaces
The dimeric form of Pf NurA appears to be essential for its nuclease activity (Figures 1A and 3A–E). Pf NurA forms a dimer through two interfaces: one is formed by the N- and PIWI domains and another by the M domain (Figure 2B and C). Our mutational analysis revealed that the N-terminal H1 helix is critical for dimer formation. Interestingly, when we removed helix H1 or mutated three residues (Arg11, Ile12 and Ser60), the Pf NurA dimer did not dissociate into a monomer. Instead, it assembled into a tetramer or formed a less compact dimer compared to that in wild-type Pf NurA (Figure 3E). These data suggest that the structural change in the N-terminal H1 helix alters the dimeric arrangement or overall Pf NurA structure.
Sequence alignment of archaeal NurA members divides NurA into those with or without the M domain (residues 190–286). NurA proteins from Pyrococcus and Methanococcus species contain the M domain, whereas those from Sulfolobous and Thermotoga do not possess the corresponding region (Supplementary Figure S2). The M domain plays an important role in Pf NurA dimerization (Figure 2B and C). Furthermore, it is located on one face of the channel (rear side), which may imply an additional role for this region such as regulation of the release of a product(s) and/or to provide additional binding surface to other partners of Pf NurA.
Interestingly, a dimeric interface formed by helices H1 and H2 from a Pf NurA subunit and strand s3, helices h6 and h15 from another Pf NurA is significantly different from that of TmNurA (Figure 5A and B). In the TmNurA dimer interface, the N-terminal S1 and S2 strands, and loops S1–S2 and S2–S3 of a subunit directly interact with its equivalent region from another TmNurA subunit. The structural divergence of the dimeric interface in NurA members may be due to the fact that NurA has very weak sequence similarity among homologous proteins. Further structural studies from another archaeal NurA are essential to understand conservation of the dimeric interface in NurA members at the structural level.
Interaction between HerA and NurA
Although NurA can function independently as a nuclease, it coordinates with HerA to generate both 5′ and 3′ ssDNA molecules; at least PfHerA and Pf NurA interact stably enough to maintain their interactions during gel filtration (15).
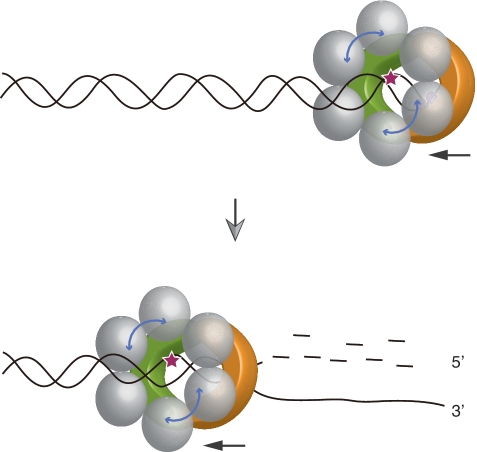
The structures of Pf NurA and MlaA/HerA determined by electron microscopy (EM) provide insights into understanding how these proteins function together (18). The flat face of the Pf NurA dimer contains an open channel (29 × 19 Å) that becomes narrower toward the opposite side (Figure 2E). EM studies of MlaA/HerA revealed that it forms a hexamer with a large hole of ~30 Å diameter, which matches the size of the channel at the flat face of Pf NurA (18). Furthermore, the metal-binding active site is located near this flat face of Pf NurA. Thus, we propose that the flat face of Pf NurA is a binding region for PfHerA.
The PfHerA hexamer interacts with one to three Pf NurA molecules (15). Based on our structural analysis, we propose that the stoichimetry of PfHerA and Pf NurA is 3:1, which is consistent with the data from the aforementioned study (Figure 7). Furthermore, the surface (helices H14–H16) in which PfHerA is proposed to bind contains several conserved residues in NurA family members. These include Pro406, Leu407, Ala410, Ile416, Ser417 and Ala427–Asn430. Taken together, we suggest that a HerA hexamer and a NurA dimer bind on the surface of the elliptical channel (Figure 7).
Figure 7.
Schematic model of NurA-HerA for 5′–3′ processing. A hexameric PfHerA (grey) and the ‘flat’ face of the Pf NurA dimer (orange and green) interact and function together to generate 5′ resected DNA. In the presence of PfHerA which unwinds dsDNA, the ssDNA molecule is expected to enter the front channel of Pf NurA to produce 3′ ssDNA for the RadA binding. An active site of Pf NurA is shown as a star.
The nuclease activity analysis revealed that the presence of PfHerA altered the Pf NurA product, from 10 to 2 nt (Figure 1B). In the presence of PfHerA, which unwinds dsDNA in both directions, the ssDNA molecule is expected to enter the front channel of Pf NurA. As we have shown in our model, dsDNA did not fit into the Pf NurA dimer and required a conformational change of a protein, whereas ssDNA which is generated by PfHerA can thread into the central channel of Pf NurA. However, we do not completely exclude the possibility that PfHerA induces the conformational change of Pf NurA for efficient binding of ssDNA with the 2-nt product generation.
DNA-binding model and nuclease mechanism of NurA
The mutational analysis revealed that replacing residues on the open elliptical channel inhibited Pf NurA nuclease activity, and that the size of this channel matched with the hole in MlaA/HerA (Figure 7; 18). Thus, we speculate that the DNA molecule passes the wide-open face of the channel and is directed toward the relatively narrow end of the Pf NurA dimer through the metal-binding site.
It is unclear if only one or two divalent metal ions are involved in the cleavage process by Pf NurA. Although we observed only one Mn2+ ion (M1) in the Pf NurA–dAMP–Mn2+ structure, adding an excess amount of MnCl2 clearly revealed additional possible Mn2+ binding (M2) at an active site, and a catalytically important residue, His411, was coordinated with this Mn2+ (M2) ion. The importance of His411 has been demonstrated by StoNurA (30) and the present Pf NurA results (Figure 4D–E). When superimposed with TtAgo, the two metal sites of Pf NurA positioned well to those of the Mg2+ ion site in TtAgo. The relatively high B value of this Mn2+ (M2) ion (B: 131.6 Å2, average protein B: 115.7 Å2) suggests that this metal weakly binds to the active site (through Asp51 and His411). A water molecule interacts with both metal ions, although this water and the M2 ion are more distantly located (3.0 Å). We presume that the M2 metal positions and activates this water molecule for nucleophilic attack on the phosphate group and M1 metal stabilizes the leaving 3′ oxyanion (Figure 6B). While the 5′ phosphate group is directed to the narrow end of the channel, the 3′ OH group points toward the open elliptical channel. This catalytic reaction may allow 5′–3′ processing of the nuclease reaction.
The distance between the putative catalytic water to end of the channel (H7 and H8) is about 50 Å, which is the length of 10–11 nt (Figure 2E). This length is consistent with the size of the dsDNA cleavage product by Pf NurA (Figure 1A and B). It is possible that helices H7 and H8 interact with dsDNA and stabilize substrate binding for efficient cleavage.
It is unresolved at this stage why two active sites are present in the Pf NurA dimer. For example, whether or not both active sites function equally is unknown. Interestingly, in the presence of PfHerA, we observed 3′-end cleavage products as well as 5′-end cleavage products (Figure 1B). Thus, it is possible that both active sites recognize each dsDNA strand (one for the 5′–3′ direction and another for the 3′–5′ direction). Since HerA has no preference in directionality, it could generate both 3′ and 5′ ssDNA ends. When the two unwound ssDNAs enter the channel of the Pf NurA dimer, one strand with the 3′-end may bind the active site, whereas another strand with the 5′-end could interact with another active site, explaining the cleavage reaction in both directions (Figure 7). Another remaining question is how Pf NurA displayed 3′–5′ exonuclease activity in the presence of PfHerA. Since our structure only provides the basis for 5′–3′ nuclease activity, further structural analyses using various substrates or ligands are required to resolve this question.
In summary, we initiated this work to understand if archaeal NurA is structurally conserved relative to its eukaryotic counterpart Exo1. Interestingly, Pf NurA does not share structural similarity with Exo1. Instead, it resembles bacterial Ago, a microRNA processing enzyme, and EndoV, both in structural (including active site) and functional aspects. The Pf NurA–dAMP–Mn2+ complex structure in conjunction with the TtAgo–DNA model and biochemical data clearly provide insight into 5′–3′ nuclease process. Further structural and functional analyses on the NurA–DNA complex should help to understand the role of this enzyme both in vivo and in vitro.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Table 1, Supplementary Figures 1–6 and Supplementary Methods.
FUNDING
Funding for open access charge: National R&D Program for Cancer Control, Ministry for Health and Welfare (1020280); National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (No. 2010-0019706 and No. 2010-0029766); a rising star program (POSTECH) and the BK21 program (Ministry of Education).
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank to Young Bong Park and Gwang Hyun Gwon for technical help and discussion. Coordinates and structure factors have been deposited with RCSB data base with 3TAI (apo Pf NurA), 3TAL (Pf NurA-Mn2+) and 3TAZ (Pf NurA-dAMP-Mn2+). Diffraction data from apo- and Mn2+-bound crystals were collected on beamline 4A at the Pohang Advanced Light Source (PAL) and Argonne National Laboratory (APS).
REFERENCES
- 1.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat. Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 2.Kowalczykowski SC, Dixon DA, Eggleston AK, Lauder SD, Rehrauer WM. Biochemistry of homologous recombination in Escherichia coli. Microbiol. Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dillingham MS, Kowalczykowski SC. RecBCD enzyme and the repair of double-stranded DNA breaks. Microbiol. Mol. Biol. Rev. 2008;72:642–671. doi: 10.1128/MMBR.00020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stracker TH, Petrini JH. The MRE11 complex: starting from the ends. Nat Rev Mol. Cell. Biol. 2011;12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lengsfeld BM, Rattray AJ, Bhaskara V, Ghirlando R, Paull TT. Sae2 is an endonuclease that processes hairpin DNA cooperatively with the Mre11/Rad50/Xrs2 complex. Mol. Cell. 2007;28:638–651. doi: 10.1016/j.molcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolette ML, Lee K, Guo Z, Rani M, Chow JM, Lee SE, Paull TT. Mre11-Rad50-Xrs2 and Sae2 promote 5' strand resection of DNA double-strand breaks. Nat. Struct. Mol. Biol. 2010;17:1478–1485. doi: 10.1038/nsmb.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mimitou EP, Symington LS. Sae2/CtIP, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cejka P, Cannavo E, Polaczek P, Masuda-Sasa T, Pokharel S, Campbell JL, Kowalczykowski SC. DNA end resection by Dna2-Sgs1-RPA and its stimulation by Top3-Rmi1 and Mre11-Rad50-Xrs2. Nature. 2010;467:112–116. doi: 10.1038/nature09355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niu H, Chung WH, Zhu Z, Kwon Y, Zhao W, Chi P, Prakash R, Seong C, Liu D, Lu L, et al. Mechanism of the ATP-dependent DNA end resection machinery from Saccharomyces cerevisiae. Nature. 2010;467:108–111. doi: 10.1038/nature09318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran PT, Erdeniz N, Symington LS, Liskay RM. EXO1-a multi-tasking eukaryotic nuclease. DNA Repair. 2004;3:1549–1559. doi: 10.1016/j.dnarep.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Mimitou EP, Symington LS. DNA end resection-unraveling the tail. DNA Repair. 2011;10:344–348. doi: 10.1016/j.dnarep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connelly JC, Kirkham LA, Leach DRF. The SbcCD nuclease of Escherichia coli is a structural maintenance of chromosomes (SMC) family protein that cleaves hairpin DNA. Proc. Natl Acad. Sci. USA. 1998;95:7969–7974. doi: 10.1073/pnas.95.14.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams RS, Williams JS, Tainer JA. Mre11-Rad50-Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template. Biochem. Cell. Biol. 2007;85:509–520. doi: 10.1139/O07-069. [DOI] [PubMed] [Google Scholar]
- 14.Lim HS, Kim JS, Park YB, Gwon GH, Cho Y. Crystal structure of the Mre11-Rad50-ATPγS complex: Understanding the interplay between Mre11 and Rad50. Genes Dev. 2011;25:1091–1104. doi: 10.1101/gad.2037811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins BB, Paull TT. The P. furiosus Mre11/Rad50 complex promotes 5′ strand resection at a DNA double-strand break. Cell. 2008;135:250–260. doi: 10.1016/j.cell.2008.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Constantinesco F, Forterre P, Elie C. NurA, a novel 5′-3′ nuclease gene linked to rad50 and mre11 homologs of thermophilic Archaea. EMBO Rep. 2002;3:537–542. doi: 10.1093/embo-reports/kvf112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Constantinesco F, Forterre P, Koonin EV, Aravind L, Elie C. A bipolar DNA helicase gene, herA, clusters with rad50, mre11 and nurA genes in thermophilic archaea. Nucleic Acids Res. 2004;32:1439–1447. doi: 10.1093/nar/gkh283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manzan A, Pfeiffer G, Hefferin ML, Lang CE, Carney JP, Hopfner KP. MlaA, a hexameric ATPase linked to the Mre11 complex in archaeal genomes. EMBO Rep. 2004;5:54–59. doi: 10.1038/sj.embor.7400037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frols S, Gordon PMK, Panlilio MA, Duggin IG, Bell SD, Sensen CW, Schleper C. Response of the hyperthermophilic archaeon Sulfolobus solfactaricus to UV damage. J. Bacterol. 2007;189:8709–8718. doi: 10.1128/JB.01016-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rolfsmeier ML, Laughery MF, Haseltine CA. Repair of DNA double-strand breaks following UV damage in three Sulfolobus solfataricus strains. J. Bacterol. 2010;192:4954–4962. doi: 10.1128/JB.00667-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quaiser A, Constantinesco F, White MF, Forterre P, Elie C. The Mre11 protein interacts with both Rad50 and the HerA bipolar helicase and is recruited to DNA following gamma irradiation in the archaeon Sulfolobus acidocaldarius. BMC Mol. Biol. 2008;9:25. doi: 10.1186/1471-2199-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, Wei T, Hou G, Zhang C, Liang P, Ni J, Sheng D, Shen Y. Archaeal DNA helicase HerA interacts with Mre11 homologue and unwinds blunt-ended double-stranded DNA and recombination intermediates. DNA Repair. 2008;7:380–391. doi: 10.1016/j.dnarep.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Wei T, Zhang S, Zhu S, Sheng D, Ni J, Shen Y. Physical and functional interaction between archaeal single-stranded DNA-binding protein and the 5′–3′ nuclease NurA. Biochem. Biophys. Res. Commun. 2008;369:523–529. doi: 10.1016/j.bbrc.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 24.Fujikane R, Ishino S, Ishino Y, Forterre P. Genetic analysis of DNA repair in the hyperthermophilic archaeon, Thermococcus kodakaraensis. Genes Genet. Sys. 2010;85:243–257. doi: 10.1266/ggs.85.243. [DOI] [PubMed] [Google Scholar]
- 25.Lyer LM, Makarova KS, Koonin EV, Aravind L. Comparative genomics of the FtsK–HerA superfamily of pumping ATPases: implications for the origins of chromosome segregation, cell division and viral capsid packaging. Nucleic Acids Res. 2004;32:5260–5279. doi: 10.1093/nar/gkh828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 27.Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. Sect. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 29.Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. Sect. D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 30.Wei T, Zhang S, Hou L, Ni J, Sheng D, Shen Y. The carboxyl terminal of the archaeal nuclease NurA is involved in the interaction with single-stranded DN A-binding protein and dimer formation. Extremophiles. 2011;15:227–234. doi: 10.1007/s00792-010-0351-2. [DOI] [PubMed] [Google Scholar]
- 31.Ma JB, Yuan YR, Meister G, Pei Y, Tuschl T, Patel DJ. Structural basis for 5'-end-specific recognition of guide RNA by the A. fulgidus Piwi protein. Nature. 2005;434:666–670. doi: 10.1038/nature03514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker JS, Roe SM, Barford D. Crystal structure of a PIWI protein suggests mechanisms for siRNA recognition and slicer activity. EMBO J. 2004;23:4727–4737. doi: 10.1038/sj.emboj.7600488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 34.Orans J, McSweeney EA, Iyer RR, Hast MA, Hellinga HW, Modrich P, Beese LS. Structures of human exonuclease 1 DNA complexes suggest a unified mechanism for nuclease family. Cell. 2011;145:212–223. doi: 10.1016/j.cell.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai L, Yokota H, Hung LW, Kim R, Kim SH. Crystal structure of archaeal RNase HII: a homologue of human major RNase H. Struct. Fold. Des. 2000;8:897–904. doi: 10.1016/s0969-2126(00)00179-9. [DOI] [PubMed] [Google Scholar]
- 36.Yang W, Steitz TA. Recombining the structures of HIV integrase, RuvC and RNase H. Structure. 1995;3:131–134. doi: 10.1016/s0969-2126(01)00142-3. [DOI] [PubMed] [Google Scholar]
- 37.Dalhus B, Arvai AS, Rosnes I, Olsen OE, Backe PH, Alseth I, Gao H, Cao W, Tainer JA, Bjoras M. Structures of endonuclease V with DNA reveal initiation of deaminated adenine repair. Nat. Struct. Mol. Biol. 2009;16:138–143. doi: 10.1038/nsmb.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Juranek S, Li H, Sheng G, Wardle GS, Tuschl T, Patel DJ. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature. 2009;461:754–761. doi: 10.1038/nature08434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.