Abstract
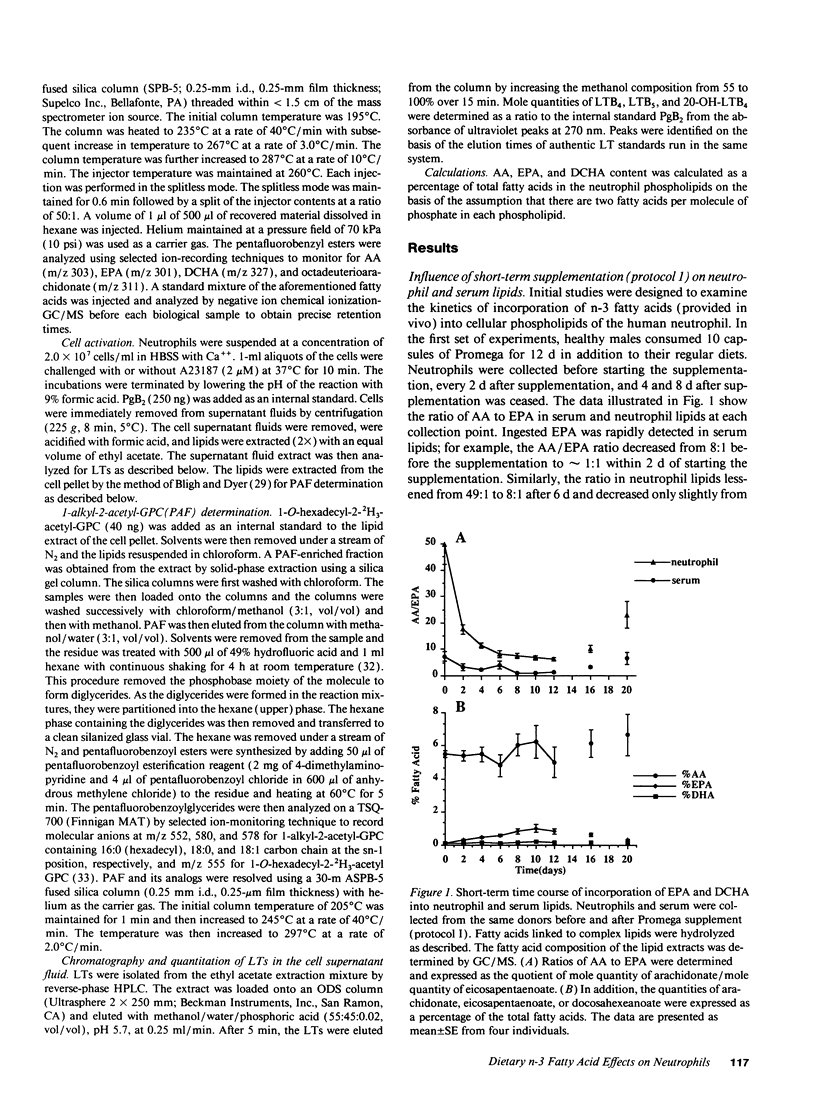
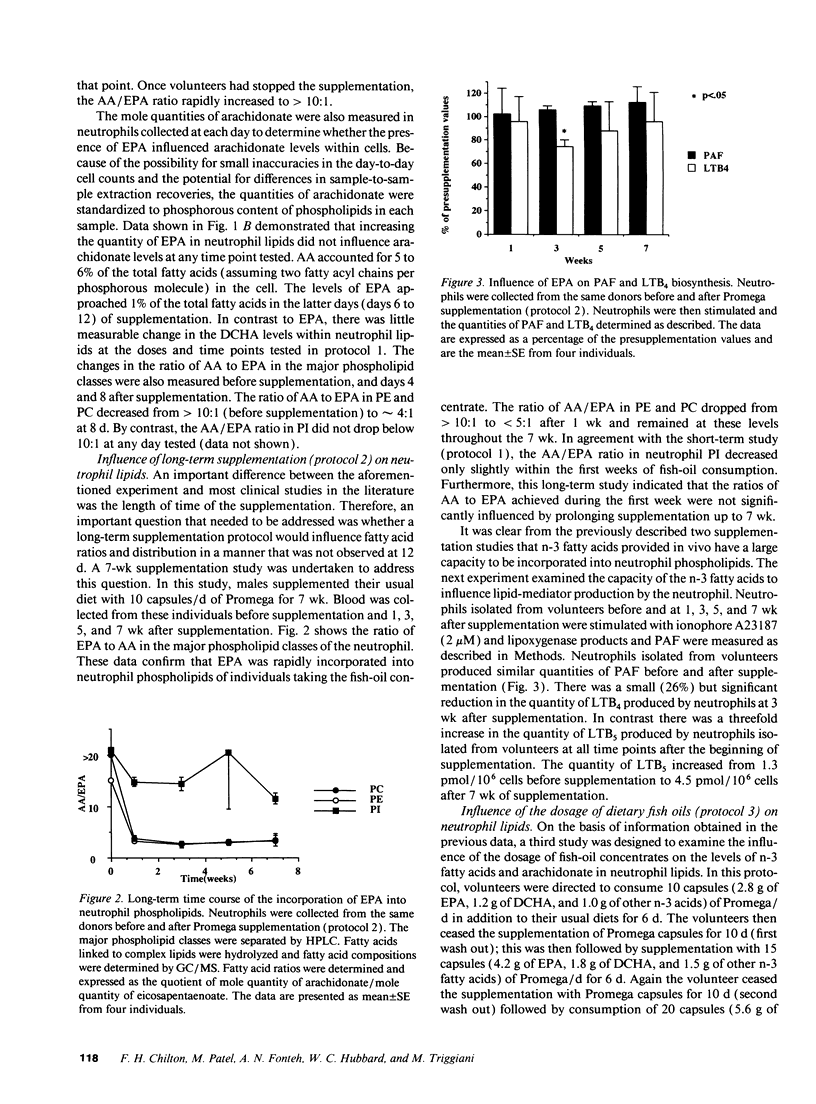
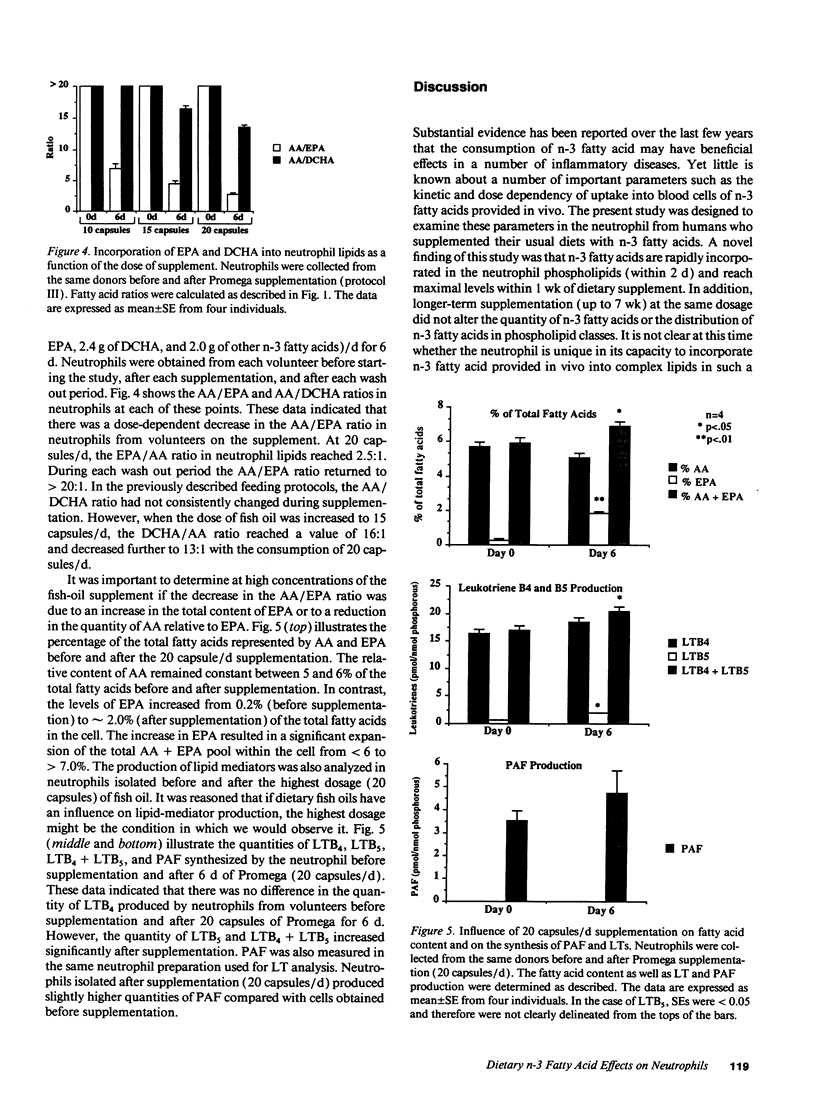
Healthy volunteers supplemented their usual Western diets with Promega fish oil supplement (eicosapentaenoic acid [EPA], 0.28 g; docosahexaenoic acid [DCHA], 0.12 g; other n-3 fatty acids 0.10 g per capsule) using three protocols. Initial experiments (protocol 1 and 2) investigated the kinetics of incorporation of n-3 fatty acids into serum and neutrophil lipids after 10 capsules/d of Promega. EPA was rapidly detected in both serum and neutrophil lipids; the arachidonic acid (AA) to EPA ratio in neutrophil phospholipids showed a maximal reduction of 49:1 to 8:1 within 1 wk of beginning supplementation. EPA was preferentially incorporated into phosphatidyl-ethanolamine and phosphatidylcholine but not phosphatidylinositol. Long-term supplementation for up to 7 wk did not influence the AA/EPA ratio or the distribution of EPA among neutrophil phospholipids in a manner that was not observed after the first week. Neutrophils produced similar quantities of platelet-activating factor and slightly lower quantities of leukotriene B4 during long-term supplementation when compared with presupplementation values. Experiments examining the influence of Promega dosage indicated that the AA/EPA ratio in neutrophil lipids decreased in a dose-dependent manner. Only when the dose was increased to 15 capsules/d was there a reduction in the AA/DCHA ratio in neutrophil lipids. The quantity of AA in neutrophil lipids remained relatively constant at all supplement doses. Taken together, the current study demonstrates the capacity of n-3 fatty acids provided with a Western diet to be rapidly incorporated into neutrophil lipids. However, dietary n-3 fatty acids appear not to significantly reduce arachidonate content within neutrophil phospholipids. Constant arachidonate levels may account for the lack of large reductions in the biosynthesis of lipid mediators by neutrophils after fish-oil supplementation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S. B., Leszczynska-Piziak J., Weissmann G. Arachidonic acid as a second messenger. Interactions with a GTP-binding protein of human neutrophils. J Immunol. 1991 Jul 1;147(1):231–236. [PubMed] [Google Scholar]
- Ahmed A. A., Holub B. J. Alteration and recovery of bleeding times, platelet aggregation and fatty acid composition of individual phospholipids in platelets of human subjects receiving a supplement of cod-liver oil. Lipids. 1984 Aug;19(8):617–624. doi: 10.1007/BF02534720. [DOI] [PubMed] [Google Scholar]
- Arm J. P., Horton C. E., Mencia-Huerta J. M., House F., Eiser N. M., Clark T. J., Spur B. W., Lee T. H. Effect of dietary supplementation with fish oil lipids on mild asthma. Thorax. 1988 Feb;43(2):84–92. doi: 10.1136/thx.43.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arm J. P., Horton C. E., Spur B. W., Mencia-Huerta J. M., Lee T. H. The effects of dietary supplementation with fish oil lipids on the airways response to inhaled allergen in bronchial asthma. Am Rev Respir Dis. 1989 Jun;139(6):1395–1400. doi: 10.1164/ajrccm/139.6.1395. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Benfenati E., Macconi D., Noris M., Icardi G., Bettazzoli L., De Bellis G., Gavinelli M., Rotondo S., Remuzzi G. Development of a mass spectrometric method to quantitate platelet activating factor in mouse urine. J Lipid Res. 1989 Dec;30(12):1977–1981. [PubMed] [Google Scholar]
- Boudreau M. D., Chanmugam P. S., Hart S. B., Lee S. H., Hwang D. H. Lack of dose response by dietary n-3 fatty acids at a constant ratio of n-3 to n-6 fatty acids in suppressing eicosanoid biosynthesis from arachidonic acid. Am J Clin Nutr. 1991 Jul;54(1):111–117. doi: 10.1093/ajcn/54.1.111. [DOI] [PubMed] [Google Scholar]
- Braden G. A., Knapp H. R., Fitzgerald D. J., FitzGerald G. A. Dietary fish oil accelerates the response to coronary thrombolysis with tissue-type plasminogen activator. Evidence for a modest platelet inhibitory effect in vivo. Circulation. 1990 Jul;82(1):178–187. doi: 10.1161/01.cir.82.1.178. [DOI] [PubMed] [Google Scholar]
- Careaga-Houck M., Sprecher H. Effect of a fish oil diet on the composition of rat neutrophil lipids and the molecular species of choline and ethanolamine glycerophospholipids. J Lipid Res. 1989 Jan;30(1):77–87. [PubMed] [Google Scholar]
- Careaga-Houck M., Sprecher H. The effect of a fish oil diet on the fatty acid composition of individual phospholipids and eicosanoid production by rat platelets. Lipids. 1989 Jun;24(6):477–481. doi: 10.1007/BF02535125. [DOI] [PubMed] [Google Scholar]
- Chilton F. H., Ellis J. M., Olson S. C., Wykle R. L. 1-O-alkyl-2-arachidonoyl-sn-glycero-3-phosphocholine. A common source of platelet-activating factor and arachidonate in human polymorphonuclear leukocytes. J Biol Chem. 1984 Oct 10;259(19):12014–12019. [PubMed] [Google Scholar]
- Chilton F. H., O'Flaherty J. T., Ellis J. M., Swendsen C. L., Wykle R. L. Selective acylation of lyso platelet activating factor by arachidonate in human neutrophils. J Biol Chem. 1983 Jun 25;258(12):7268–7271. [PubMed] [Google Scholar]
- DeCaterina R., Giannessi D., Mazzone A., Bernini W., Lazzerini G., Maffei S., Cerri M., Salvatore L., Weksler B. Vascular prostacyclin is increased in patients ingesting omega-3 polyunsaturated fatty acids before coronary artery bypass graft surgery. Circulation. 1990 Aug;82(2):428–438. doi: 10.1161/01.cir.82.2.428. [DOI] [PubMed] [Google Scholar]
- Dyerberg J., Bang H. O. A hypothesis on the development of acute myocardial infarction in Greenlanders. Scand J Clin Lab Invest Suppl. 1982;161:7–13. [PubMed] [Google Scholar]
- Dyerberg J., Bang H. O., Stoffersen E., Moncada S., Vane J. R. Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet. 1978 Jul 15;2(8081):117–119. doi: 10.1016/s0140-6736(78)91505-2. [DOI] [PubMed] [Google Scholar]
- Fischer S., von Schacky C., Siess W., Strasser T., Weber P. C. Uptake, release and metabolism of docosahexaenoic acid (DHA, c22:6 omega 3) in human platelets and neutrophils. Biochem Biophys Res Commun. 1984 May 16;120(3):907–918. doi: 10.1016/s0006-291x(84)80193-x. [DOI] [PubMed] [Google Scholar]
- Galloway J. H., Cartwright I. J., Woodcock B. E., Greaves M., Russell R. G., Preston F. E. Effects of dietary fish oil supplementation on the fatty acid composition of the human platelet membrane: demonstration of selectivity in the incorporation of eicosapentaenoic acid into membrane phospholipid pools. Clin Sci (Lond) 1985 Apr;68(4):449–454. doi: 10.1042/cs0680449. [DOI] [PubMed] [Google Scholar]
- Goldman D. W., Pickett W. C., Goetzl E. J. Human neutrophil chemotactic and degranulating activities of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochem Biophys Res Commun. 1983 Nov 30;117(1):282–288. doi: 10.1016/0006-291x(83)91572-3. [DOI] [PubMed] [Google Scholar]
- Herold P. M., Kinsella J. E. Fish oil consumption and decreased risk of cardiovascular disease: a comparison of findings from animal and human feeding trials. Am J Clin Nutr. 1986 Apr;43(4):566–598. doi: 10.1093/ajcn/43.4.566. [DOI] [PubMed] [Google Scholar]
- Kelley V. E., Ferretti A., Izui S., Strom T. B. A fish oil diet rich in eicosapentaenoic acid reduces cyclooxygenase metabolites, and suppresses lupus in MRL-lpr mice. J Immunol. 1985 Mar;134(3):1914–1919. [PubMed] [Google Scholar]
- Kremer J. M., Bigauoette J., Michalek A. V., Timchalk M. A., Lininger L., Rynes R. I., Huyck C., Zieminski J., Bartholomew L. E. Effects of manipulation of dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet. 1985 Jan 26;1(8422):184–187. doi: 10.1016/s0140-6736(85)92024-0. [DOI] [PubMed] [Google Scholar]
- Kremer J. M., Robinson D. R. Studies of dietary supplementation with omega 3 fatty acids in patients with rheumatoid arthritis. World Rev Nutr Diet. 1991;66:367–382. doi: 10.1159/000419305. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Hoover R. L., Williams J. D., Sperling R. I., Ravalese J., 3rd, Spur B. W., Robinson D. R., Corey E. J., Lewis R. A., Austen K. F. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med. 1985 May 9;312(19):1217–1224. doi: 10.1056/NEJM198505093121903. [DOI] [PubMed] [Google Scholar]
- Lee T. H., Menica-Huerta J. M., Shih C., Corey E. J., Lewis R. A., Austen K. F. Characterization and biologic properties of 5,12-dihydroxy derivatives of eicosapentaenoic acid, including leukotriene B5 and the double lipoxygenase product. J Biol Chem. 1984 Feb 25;259(4):2383–2389. [PubMed] [Google Scholar]
- Lennartz M. R., Brown E. J. Arachidonic acid is essential for IgG Fc receptor-mediated phagocytosis by human monocytes. J Immunol. 1991 Jul 15;147(2):621–626. [PubMed] [Google Scholar]
- Leslie C. A., Gonnerman W. A., Ullman M. D., Hayes K. C., Franzblau C., Cathcart E. S. Dietary fish oil modulates macrophage fatty acids and decreases arthritis susceptibility in mice. J Exp Med. 1985 Oct 1;162(4):1336–1349. doi: 10.1084/jem.162.4.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T. A., Codde J. P., Vandongen R., Beilin L. J. New findings in the fatty acid composition of individual platelet phospholipids in man after dietary fish oil supplementation. Lipids. 1987 Oct;22(10):744–750. doi: 10.1007/BF02533975. [DOI] [PubMed] [Google Scholar]
- Patton G. M., Fasulo J. M., Robins S. J. Separation of phospholipids and individual molecular species of phospholipids by high-performance liquid chromatography. J Lipid Res. 1982 Jan;23(1):190–196. [PubMed] [Google Scholar]
- Payan D. G., Wong M. Y., Chernov-Rogan T., Valone F. H., Pickett W. C., Blake V. A., Gold W. M., Goetzl E. J. Alterations in human leukocyte function induced by ingestion of eicosapentaenoic acid. J Clin Immunol. 1986 Sep;6(5):402–410. doi: 10.1007/BF00915380. [DOI] [PubMed] [Google Scholar]
- Pickett W. C., Nytko D., Dondero-Zahn C., Ramesha C. The effect of endogenous eicosapentaenoic acid on PMN leukotriene and PAF biosynthesis. Prostaglandins Leukot Med. 1986 Aug;23(2-3):135–140. doi: 10.1016/0262-1746(86)90176-9. [DOI] [PubMed] [Google Scholar]
- Prescott S. M. The effect of eicosapentaenoic acid on leukotriene B production by human neutrophils. J Biol Chem. 1984 Jun 25;259(12):7615–7621. [PubMed] [Google Scholar]
- Prickett J. D., Robinson D. R., Steinberg A. D. Dietary enrichment with the polyunsaturated fatty acid eicosapentaenoic acid prevents proteinuria and prolongs survival in NZB x NZW F1 mice. J Clin Invest. 1981 Aug;68(2):556–559. doi: 10.1172/JCI110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesha C. S., Pickett W. C. Measurement of sub-picogram quantities of platelet activating factor (AGEPC) by gas chromatography/negative ion chemical ionization mass spectrometry. Biomed Environ Mass Spectrom. 1986 Mar;13(3):107–111. doi: 10.1002/bms.1200130302. [DOI] [PubMed] [Google Scholar]
- Ramesha C. S., Pickett W. C. Platelet-activating factor and leukotriene biosynthesis is inhibited in polymorphonuclear leukocytes depleted of arachidonic acid. J Biol Chem. 1986 Jun 15;261(17):7592–7595. [PubMed] [Google Scholar]
- Randriamampita C., Trautmann A. Arachidonic acid activates Ca2+ extrusion in macrophages. J Biol Chem. 1990 Oct 25;265(30):18059–18062. [PubMed] [Google Scholar]
- Rosenthal M. D., Hill J. R. Elongation of arachidonic and eicosapentaenoic acids limits their availability for thrombin-stimulated release from the glycerolipids of vascular endothelial cells. Biochim Biophys Acta. 1986 Feb 12;875(2):382–391. doi: 10.1016/0005-2760(86)90189-x. [DOI] [PubMed] [Google Scholar]
- Sperling R. I., Weinblatt M., Robin J. L., Ravalese J., 3rd, Hoover R. L., House F., Coblyn J. S., Fraser P. A., Spur B. W., Robinson D. R. Effects of dietary supplementation with marine fish oil on leukocyte lipid mediator generation and function in rheumatoid arthritis. Arthritis Rheum. 1987 Sep;30(9):988–997. doi: 10.1002/art.1780300905. [DOI] [PubMed] [Google Scholar]
- Stenius-Aarniala B., Aro A., Hakulinen A., Ahola I., Seppälä E., Vapaatalo H. Evening primose oil and fish oil are ineffective as supplementary treatment of bronchial asthma. Ann Allergy. 1989 Jun;62(6):534–537. [PubMed] [Google Scholar]
- Strasser T., Fischer S., Weber P. C. Leukotriene B5 is formed in human neutrophils after dietary supplementation with icosapentaenoic acid. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1540–1543. doi: 10.1073/pnas.82.5.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terano T., Salmon J. A., Moncada S. Biosynthesis and biological activity of leukotriene B5. Prostaglandins. 1984 Feb;27(2):217–232. doi: 10.1016/0090-6980(84)90075-3. [DOI] [PubMed] [Google Scholar]
- Thompson P. J., Misso N. L., Passarelli M., Phillips M. J. The effect of eicosapentaenoic acid consumption on human neutrophil chemiluminescence. Lipids. 1991 Dec;26(12):1223–1226. doi: 10.1007/BF02536536. [DOI] [PubMed] [Google Scholar]
- Triggiani M., Connell T. R., Chilton F. H. Evidence that increasing the cellular content of eicosapentaenoic acid does not reduce the biosynthesis of platelet-activating factor. J Immunol. 1990 Oct 1;145(7):2241–2248. [PubMed] [Google Scholar]
- von Schacky C., Fischer S., Weber P. C. Long-term effects of dietary marine omega-3 fatty acids upon plasma and cellular lipids, platelet function, and eicosanoid formation in humans. J Clin Invest. 1985 Oct;76(4):1626–1631. doi: 10.1172/JCI112147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schacky C., Siess W., Fischer S., Weber P. C. A comparative study of eicosapentaenoic acid metabolism by human platelets in vivo and in vitro. J Lipid Res. 1985 Apr;26(4):457–464. [PubMed] [Google Scholar]
- von Schacky C., Weber P. C. Metabolism and effects on platelet function of the purified eicosapentaenoic and docosahexaenoic acids in humans. J Clin Invest. 1985 Dec;76(6):2446–2450. doi: 10.1172/JCI112261. [DOI] [PMC free article] [PubMed] [Google Scholar]


