Abstract
Abstract
During development of the central nervous system (CNS), precise synaptic connections between pre- and postsynaptic neurons are formed that ultimately give rise to higher order cognitive skills such as learning and memory. Previously, my group identified a novel type II transmembrane protein, synapse differentiation induced gene 1 (SynDIG1), that regulates synaptic AMPA receptor content in dissociated rat hippocampal neurons. The magnitude of this effect matches that of the prototypical scaffold postsynaptic density protein of 95 kDa (PSD-95) identifying SynDIG1 as a previously unknown central regulator of excitatory synaptic strength. SynDIG1-mediated regulation of synaptic AMPA receptor targeting shares characteristics related to two distinct classes of transmembrane synaptic proteins: (1) ion channel auxiliary factors such as transmembrane AMPA receptor regulatory proteins (TARPs) important for AMPA receptor surface expression and channel gating properties; and (2) trans-synaptic organizing molecules such as leucine rich repeat transmembrane protein 2 (LRRTM2) that influence synapse maturation by recruitment of AMPA receptors to nascent synapses. An interesting aspect of SynDIG1 is that its distribution at excitatory synapses is regulated by activity, suggesting that SynDIG1 might also play a role in synaptic plasticity.
Elva Diaz is an Associate Professor in the Department of Pharmacology at the University of California–Davis School of Medicine. Research in the Diaz lab focuses on understanding molecular mechanisms of neural development with a focus on two main areas: synapse formation and neural proliferation. Her background is in molecular cell biology, biochemistry and functional genomics. In this symposium review article, she describes the discovery and characterization of a novel transmembrane protein that is a critical regulator of excitatory synapse development.
 |
Introduction
Synapses contain several specialized domains including the presynaptic bouton containing hundreds of synaptic vesicles (SVs), the presynaptic active zone where SVs dock and fuse with the plasma membrane, and the juxtaposed postsynaptic density (PSD) composed of an electron dense meshwork of proteins including glutamate receptors, other ion channels and various signalling components (Waites et al. 2005). Cell adhesion molecules (CAMs) extend across the synaptic cleft to stabilize this macromolecular complex (Scheiffele, 2003; Waites et al. 2005; McAllister, 2007). AMPA type glutamate receptors comprise tetramers of different combinations of four subunits (GluA1–4) and are responsible for fast excitatory synaptic transmission in the CNS (Bredt & Nicoll, 2003; Malenka, 2003). NMDA type glutamate receptors are composed of GluN1 and GluN2 subunits and play a critical role in synaptic plasticity (Cull-Candy & Leszkiewicz, 2004).
Excitatory synapse development
During development, synapse formation is directed by signalling between pre- and postsynaptic neurons and the recruitment of SVs, scaffolds and ion channels to sites of axodendritic contact (Scheiffele, 2003; Waites et al. 2005; McAllister, 2007; see Fig 1). A number of synaptogenic molecules are capable of promoting presynaptic or postsynaptic differentiation when presented to axons or dendrites. It has recently been shown that such factors are components of multimeric trans-synaptic adhesion complexes that participate in bidirectional signalling to organize the structure and function of central synapses (Siddiqui & Craig, 2011). Synaptic activity then directs whether synapses will be stabilized, eliminated or strengthened (Waites et al. 2005; McAllister, 2007). While a large number of studies were undertaken to dissect the mechanisms of AMPA receptor trafficking during synaptic plasticity (Barry & Ziff, 2002; Bredt & Nicoll, 2003; Malenka, 2003; Sheng & Hyoung Lee, 2003; Chen et al. 2006; Nicoll et al. 2006), it is unclear if similar or distinct molecular mechanisms underlie AMPA receptor recruitment during the initial stages of synaptogenesis. One popular model suggests that synapses develop via an NMDA receptor only intermediate (so-called ‘silent synapses’) with subsequent conversion of silent synapses upon NMDA receptor activity to mature synapses containing AMPA receptors (Liao et al. 1999; Petralia et al. 1999). Indeed, NMDA receptor or Ca2+–calmodulin-dependent protein kinase II (CaMKII) activation leads to AMPA receptor recruitment to CAM-induced sites of postsynaptic specializations (Nam & Chen, 2005), consistent with this model. While several classes of synaptic organizing complexes have been identified that function during the initial stages of synapse formation (Siddiqui & Craig, 2011), molecular mechanisms underlying the later stages of synapse development are less well understood. However, in recent years, several molecules, including SynDIG1, have been discovered that appear to play a role in synapse maturation by the recruitment of AMPA receptors to nascent synapses (McMahon & Diaz, 2011). Some of these synaptic organizing complexes interact directly with AMPA receptors to mediate their recruitment to synapses while other complexes promote synapse maturation through indirect mechanisms.
Figure 1. Composition of excitatory synapses.
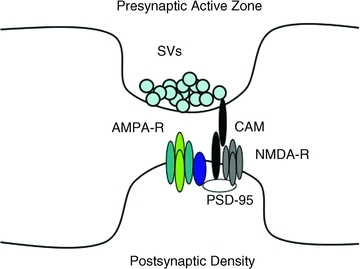
The presynaptic bouton contains hundreds of synaptic vesicles (SVs) which dock and fuse with the plasma membrane at the presynaptic active zone. The juxtaposed postsynaptic density is composed of an electron dense meshwork of proteins including glutamate receptors (NMDA-R and AMPA-R) that are stabilized at the synapse by interaction with scaffolds such as PSD-95. In the case of AMPA-Rs, synaptic localization is mediated via interaction with PSD-95 through a TARP intermediate (dark blue). Cell adhesion molecules (CAMs) extend across the synaptic cleft to stabilize this macromolecular complex.
A growing number of accessory transmembrane proteins that interact with AMPA receptors to regulate synaptic strength have been identified (Diaz, 2010; Jackson & Nicoll, 2011). The best studied class of accessory proteins are transmembrane AMPA receptor regulatory proteins (TARPs), which control synaptic AMPA receptor targeting and channel gating properties (Kato et al. 2010; Jackson & Nicoll, 2011). TARPs are now recognized as auxiliary subunits for AMPA receptors (Chen et al. 2000; Schnell et al. 2002; Tomita et al. 2003) that copurify with AMPA receptors from brain (Tomita et al. 2004), comigrate with AMPA receptor complexes under native gel electrophoresis conditions (Vandenberghe et al. 2005) and are evolutionarily conserved (Wang et al. 2008). The prototypical TARP, stargazin, is mutated in stargazer mice (Letts et al. 1998). Cerebellar granule cells from stargazer mice show selective loss of synaptic AMPA receptors (Chen et al. 2000). TARPs function by: (1) mediating AMPA receptor translocation from intracellular sites to the cell surface; and (2) binding to postsynaptic density protein of 95 kDa (PSD-95) and related scaffolds to promote synaptic targeting of surface AMPA receptor (Chen et al. 2000).
SynDIG1: a novel regulator of excitatory synapse maturation
My group recently showed that knock-down of synapse differentiation induced gene 1 (SynDIG1) in dissociated rat hippocampal neurons reduces AMPA receptor content at developing synapses by ∼50% as determined by immunocytochemistry and electrophysiology (Kalashnikova et al. 2010). SynDIG1 is a brain specific protein that is enriched at synapses and the PSD as demonstrated by immunocytochemistry and biochemical fractionation, respectively, with anti-SynDIG1 antibodies. Synapse density (defined by the number of colocalized vGlut1 (presynaptic vesicular glutamate transporter) and PSD-95 puncta per dendrite length) was decreased by 25% upon knockdown of SynDIG1 with short hairpin RNA (shRNA) compared with control shRNA (Kalashnikova et al. 2010). Furthermore, the density of AMPA receptor containing synapses (defined by the number of colocalized vGlut1 and GluA1 or GluA2 colocalized puncta) was significantly decreased by 50% compared with control neurons. As expected, synapse function was correspondingly decreased such that the mean amplitude and frequency of AMPA receptor-mediated miniature excitatory postsynaptic currents (mEPSCs) were reduced (Fig. 2A). The decrease in AMPA receptor-mediated mEPSC frequency is likely to reflect, in part, a decreased ability to detect mEPSCs due to decreased mEPSC amplitude. Alternatively, loss of SynDIG1 in the postsynaptic neuron could reflect a decrease in total synapse number; however, loss of SynDIG1 did not influence density of vGlut1 puncta and the frequency and amplitude of NMDA receptor-mediated mEPSCs were unchanged (Kalashnikova et al. 2010; see Fig. 2B). Together, these data suggest that SynDIG1 is necessary for the presence of AMPA receptors and PSD-95 but not NMDA receptors at excitatory synapses.
Figure 2. SynDIG1 modulates synaptic AMPA receptors but not NMDA receptors.
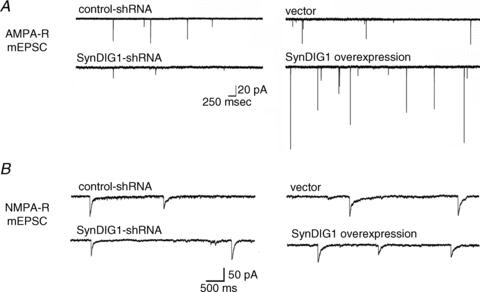
A, the frequency and amplitude of AMPA receptor-mediated miniature excitatory postsynaptic currents (mEPSCs) are decreased or increased upon SynDIG1 knockdown (left traces) or overexpression (right traces) in dissociated cultures of rat hippocampal neurons compared with control neurons. B, the frequency and amplitude of NMDA receptor-mediated miniature excitatory postsynaptic currents (mEPSCs) are unchanged upon SynDIG1 knockdown (left traces) or overexpression (right traces) in dissociated cultures of rat hippocampal neurons compared with control neurons. Modified from Kalashnikova et al. (2010) with permission from Elsevier.
Interestingly, SynDIG1 knockdown resembles that of PSD-95/PSD-93 double knockdown in that decreased amplitude and frequency of mEPSCs were observed (Elias et al. 2006). Loss of PSD-95 or PSD-93 individually silences distinct subsets of excitatory synapses since knockdown of either PSD-95 or PSD-93 leads to decreased mEPSC frequency with no change in amplitude (Elias et al. 2006). Together, these data suggest that SynDIG1-mediated loss of synaptic AMPA receptors could be due to a uniform loss of PSD-95-like membrane associated guanylate kinases (MAGUKs) at all synapses. Because the mechanisms whereby PSD-95-like MAGUKs are anchored at the synapse are at present unclear, SynDIG1 might represent one such molecular mechanism important for synaptic localization of PSD-95-like MAGUKs.
In addition, SynDIG1 is sufficient to induce synapse maturation by increasing AMPA receptor and PSD-95 levels at synapses (Fig. 2A). Overexpression of SynDIG1 increases the density of PSD-95 or GluA1 puncta that colocalize with vGlut1 as assessed by immunocytochemistry and mEPSC frequency and amplitudes were concurrently increased (Kalashnikova et al. 2010). Importantly, this effect was specific for AMPA receptors and PSD-95 as NMDA receptor content at synapses was unchanged as assessed by the synaptic distribution of the NMDA receptor subunit NR1 by immunocytochemistry and NMDA receptor-mediated mEPSCs (Kalashnikova et al. 2010; Fig. 2B). Thus, SynDIG1 overexpression is sufficient to increase synaptic AMPA receptors and PSD-95 but not NMDA receptors.
The finding that overexpression of SynDIG1 leads to increased AMPA receptors at synapses is particularly intriguing because SynDIG1 is one of a small number of molecules that when overexpressed leads to increased synaptic strength. For example, overexpression of PSD-95 increased mEPSC frequency and amplitude (Stein et al. 2003; Ehrlich & Malinow, 2004). A three-step model for AMPA receptor retention at synapses has recently been proposed to include: (1) exocytosis of the intracellular pool of AMPA receptors at extra/perisynaptic sites; (2) lateral diffusion to synaptic sites; (3) and retention at synapses via scaffold interactions (Opazo & Choquet, 2011). NMDA receptor activation facilitates translocation of CaMKII, resulting in retention of extrasynaptic AMPA receptors at synapses (Opazo et al. 2010). AMPA receptor retention at synapses is dependent on phosphorylation of stargazin by facilitating binding to PSD-95 (Opazo et al. 2010; Sumioka et al. 2010). Opazo & Choquet (2011) note that overexpression of PSD-95, constitutively active CaMKII or phosphomimetic stargazin alone is sufficient to increase levels of synaptic AMPA receptors. A particularly interesting idea is that SynDIG1 might function in the same pathway proposed for CaMKII–pStargazin–PSD-95 to promote retention of AMPA receptors at synapses. Furthermore, because common mechanisms are thought to be involved in controlling synaptic AMPA receptors by PSD-95-like MUGAKs and synaptic plasticity, it is tempting to speculate that SynDIG1 might also play a role in synaptic plasticity. Further experiments are necessary to test these interesting possibilities.
Molecular mechanisms for SynDIG1 regulation of synapse development
The mechanism whereby SynDIG1 regulates excitatory synapse maturation is potentially due to direct interaction with AMPA receptors. SynDIG1 was shown to associate with AMPA receptor complexes in brain lysates and to associate with and cluster the AMPA receptor subunit GluA2 in heterologous cells (other AMPA receptor subunits were not tested) (Kalashnikova et al. 2010). Moreover, structure–function studies demonstrated that the extracellular C-terminal region of SynDIG1 is required for association with GluA2 clusters as well as SynDIG1-mediated increased excitatory synaptic strength (Kalashnikova et al. 2010). While it is tempting to conclude that SynDIG1 effects are selective for AMPA receptors, it is important to note that interaction with other synaptic proteins such as NMDA receptors and synaptic scaffolds has yet to be reported. While SynDIG1 does not possess any recognizable domains that might provide clues to other potential interacting partners, secondary structure predictions suggest that the AMPA receptor interacting domain is helical in nature with a coiled coil region at the extreme C-terminus. In addition, SynDIG1 is capable of forming homodimers and the extracellular C-terminal region is also important for dimerization (Kalashnikova et al. 2010). SynDIG1 regulation of PSD-95 content at synapses suggests that SynDIG1 is important for multiple aspects of synapse maturation; however, lack of a PDZ-binding motif indicates that SynDIG1 is unlikely to interact with PSD-95 via PDZ domain as shown for other synaptic proteins.
SynDIG1-mediated regulation of synaptic AMPA receptor targeting shares characteristics related to two distinct classes transmembrane synaptic proteins: (1) ion channel auxiliary factors such as TARPs important for AMPA receptor surface expression and channel gating properties (Jackson & Nicoll, 2011); and (2) trans-synaptic organizing molecules that influence synapse maturation such as leucine rich repeat transmembrane protein 2 (LRRTM2), which influences synapse maturation by recruitment of AMPA receptors to nascent synapses (McMahon & Diaz, 2011). For example, similar to stargazin, SynDIG1 promotes synaptic localization of AMPA receptor complexes. The fact that loss of SynDIG1 significantly reduces AMPA receptor content at synapses indicates that TARPs are not sufficient to compensate for SynDIG1. One alternative is that SynDIG1 might function on a pool of AMPA receptors that are not associated with TARPs. A second alternative is that decreased synaptic PSD-95 upon SynDIG1 knockdown inhibits TARP-dependent delivery of AMPA receptors to synapses. Additional experiments are necessary to determine the relationship of SynDIG1 function to TARP-associated AMPA receptor complexes and PSD-95.
LRRTM2 is a cell adhesion molecule that interacts with presynaptic neurexins to organize excitatory synapse development (Linhoff et al. 2009) via regulation of AMPA receptor localization and/or function at synapses (de Wit et al. 2009). Interestingly, GluA1 and GluA2, as well as NR1, co-precipitate with LRRTM2 when coexpressed in non-neuronal cells, an interaction requiring the extracellular LRR domain (de Wit et al. 2009). Whether SynDIG1 functions via a trans-synaptic complex to regulate synaptic maturation remains to be determined.
SynDIG1 synaptic distribution is activity regulated
A particularly interesting characteristic of SynDIG1 is that the distribution of SynDIG1 protein is activity regulated. Dendritic protrusions called spines are the primary location of excitatory synapses. In control cultures of hippocampal neurons, SynDIG1 is localized to spines as well as dendrite shafts; however, upon activity suppression with tetrotoxin SynDIG1 becomes localized exclusively to spines (Kalashnikova et al. 2010). This phenotype is reminiscent of AMPA receptor trafficking during homeostatic synaptic scaling, a form of synaptic plasticity that adjusts the strength of all of a neuron's excitatory synapses up or down to stabilize firing. Current evidence suggests that neurons detect changes in their own firing rates through a set of calcium-dependent sensors that then regulate receptor trafficking to increase or decrease the accumulation of glutamate receptors at synaptic sites (Turrigiano, 2008). Preliminary studies suggest that SynDIG1 is required for homeostatic synaptic scaling of AMPA receptor upon activity suppression, suggesting that SynDIG1 might be involved in calcium-dependent signalling that underlies homeostatic synaptic scaling. This aspect of SynDIG1 function distinguishes it from the reported roles for auxiliary factors and trans-synaptic organizing complexes. Interestingly, PSD-95 and PSD-93 play critical but distinct roles in synaptic scaling up and down (Sun & Turrigiano, 2011), suggesting that SynDIG1-mediated regulation of synaptic PSD-95 might underlie its potential role in homeostatic plasticity.
Conclusions
Taken together, these data suggest that SynDIG1 is a previously unrecognized critical regulator of excitatory synaptic strength. The magnitude of the effects ascribed to SynDIG1 matches that of PSD-95 highlighting the functional importance of SynDIG1 at excitatory synapses. One possible mechanism is that direct interaction of SynDIG1 with AMPA receptors promotes trafficking to synapses (Fig. 3A). Alternatively, SynDIG1 might promote synapse maturation by ‘priming’ nascent synapses for delivery of AMPA receptors via other molecules such as TARPs (Fig. 3B). Current studies in mice with targeted deletion of the SynDIG1 gene are ongoing in my lab to test the role of SynDIG1 in excitatory synapse development in vivo as well as behaviours dependent on synaptic AMPA receptors such as learning and memory.
Figure 3. Two models for SynDIG1 regulation of synaptic AMPA receptor content.
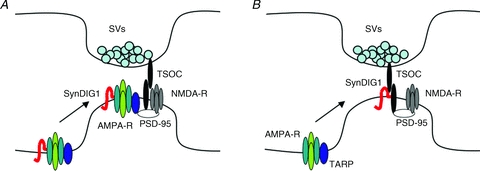
A, auxiliary subunit model. SynDIG1 associates with AMPA receptors (AMPA-R) at extrasynaptic sites and SynDIG1 is required for synaptic localization of AMPA receptors. SynDIG1 would likely recruit AMPA receptors associated with another auxiliary subunit (dark blue oval) capable of anchoring of the receptors at synapses via interaction with a synaptic scaffold such as PSD-95. B, trans-synaptic organizing complex model. SynDIG1 is present at synapses, potentially as a component of a trans-synaptic organizing complex (TSOC), and promotes postsynaptic differentiation via TSOC-dependent interaction with PSD-95 and SynDIG1-dependent interaction with AMPA receptor complexes.
Acknowledgments
I wish to thank past and present members of the Diaz lab for contribution to the results presented in this report. Research in the laboratory is funded by grants from the National Science Foundation and the National Institutes of Health (NIH) Director's New Innovator Award Program.
Glossary
- CAM
cell adhesion molecule
- CaMKII
Ca2+–calmodulin-dependent protein kinase II
- LRRTM2
leucine rich repeat transmembrane protein 2
- MAGUK
membrane associated guanylate kinase
- mEPSC
miniature excitatory postsynaptic current
- PDZ
PSD-95/Discs large/zona occludens-1
- PSD
postsynaptic density
- PSD-95
postsynaptic density protein of 95 kDa
- SV
synaptic vesicle
- SynDIG1
synapse differentiation induced gene 1
References
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Chen L, Tracy T, Nam CI. Dynamics of postsynaptic glutamate receptor targeting. Curr Opin Neurobiol. 2006;17:53–58. doi: 10.1016/j.conb.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004:re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- de Wit J, Sylwestrak E, O'Sullivan ML, Otto S, Tiglio K, Savas JN, Yates JR, 3rd, Comoletti D, Taylor P, Ghosh A. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz E. Regulation of AMPA receptors by transmembrane accessory proteins. Eur J Neurosci. 2010;32:261–268. doi: 10.1111/j.1460-9568.2010.07357.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalashnikova E, Lorca RA, Kaur I, Barisone GA, Li B, Ishimaru T, Trimmer JS, Mohapatra DP, Diaz E. SynDIG1: an activity-regulated, AMPA-receptor-interacting transmembrane protein that regulates excitatory synapse development. Neuron. 2010;65:80–93. doi: 10.1016/j.neuron.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato AS, Gill MB, Yu H, Nisenbaum ES, Bredt DS. TARPs differentially decorate AMPA receptors to specify neuropharmacology. Trends Neurosci. 2010;33:241–248. doi: 10.1016/j.tins.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett FS, 2nd, Mori Y, Campbell KP, Frankel WN. The mouse stargazer gene encodes a neuronal Ca2+-channel γ subunit. Nat Genet. 1998;19:340–347. doi: 10.1038/1228. [DOI] [PubMed] [Google Scholar]
- Liao D, Zhang X, O'Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci. 1999;2:37–43. doi: 10.1038/4540. [DOI] [PubMed] [Google Scholar]
- Linhoff MW, Lauren J, Cassidy RM, Dobie FA, Takahashi H, Nygaard HB, Airaksinen MS, Strittmatter SM, Craig AM. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SA, Diaz E. Mechanisms of excitatory synapse maturation by trans-synaptic organizing complexes. Curr Opin Neurobiol. 2011;21:221–227. doi: 10.1016/j.conb.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci U S A. 2005;102:6137–6142. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- Opazo P, Choquet D. A three-step model for the synaptic recruitment of AMPA receptors. Mol Cell Neurosci. 2011;46:1–8. doi: 10.1016/j.mcn.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P, Choquet D. CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron. 2010;67:239–252. doi: 10.1016/j.neuron.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci. 1999;2:31–36. doi: 10.1038/4532. [DOI] [PubMed] [Google Scholar]
- Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Hyoung Lee S. AMPA receptor trafficking and synaptic plasticity: major unanswered questions. Neurosci Res. 2003;46:127–134. doi: 10.1016/s0168-0102(03)00040-3. [DOI] [PubMed] [Google Scholar]
- Siddiqui TJ, Craig AM. Synaptic organizing complexes. Curr Opin Neurobiol. 2011;21:132–143. doi: 10.1016/j.conb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein V, House DR, Bredt DS, Nicoll RA. Postsynaptic density-95 mimics and occludes hippocampal long-term potentiation and enhances long-term depression. J Neurosci. 2003;23:5503–5506. doi: 10.1523/JNEUROSCI.23-13-05503.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumioka A, Yan D, Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66:755–767. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Turrigiano GG. PSD-95 and PSD-93 play critical but distinct roles in synaptic scaling up and down. J Neurosci. 2011;31:6800–6808. doi: 10.1523/JNEUROSCI.5616-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Fukata M, Nicoll RA, Bredt DS. Dynamic interaction of stargazin-like TARPs with cycling AMPA receptors at synapses. Science. 2004;303:1508–1511. doi: 10.1126/science.1090262. [DOI] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe W, Nicoll RA, Bredt DS. Stargazin is an AMPA receptor auxiliary subunit. Proc Natl Acad Sci U S A. 2005;102:485–490. doi: 10.1073/pnas.0408269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- Wang R, Walker CS, Brockie PJ, Francis MM, Mellem JE, Madsen DM, Maricq AV. Evolutionary conserved role for TARPs in the gating of glutamate receptors and tuning of synaptic function. Neuron. 2008;59:997–1008. doi: 10.1016/j.neuron.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]


