Abstract
Abstract
The time course of excitatory synaptic currents, the major means of fast communication between neurons of the central nervous system, is encoded in the dynamic behaviour of post-synaptic glutamate-activated channels. First-pass attempts to explain the glutamate-elicited currents with mathematical models produced reaction mechanisms that included only the most basic functionally defined states: resting vs. liganded, closed vs. open, responsive vs. desensitized. In contrast, single-molecule observations afforded by the patch-clamp technique revealed an unanticipated kinetic multiplicity of transitions: from microseconds-lasting flickers to minutes-long modes. How these kinetically defined events impact the shape of the synaptic response, how they relate to rearrangements in receptor structure, and whether and how they are physiologically controlled represent currently active research directions. Modal gating, which refers to the slowest, least frequently observed ion-channel transitions, has been demonstrated for representatives of all ion channel families. However, reaction schemes have been largely confined to the short- and medium-range time scales. For glutamate receptors as well, modal gating has only recently come under rigorous scrutiny. This article reviews the evidence for modal gating of glutamate receptors and the still developing hypotheses about the mechanism(s) by which modal shifts occur and the ways in which they may impact the time course of synaptic transmission.
Gabriela K. Popescu(Associate Professor, University at Buffalo, School of Medicine and Biomedical Sciences) is curious and passionate about how brains work! A native of Bucharest, Romania, she moved with her family to Buffalo in 1992, earned a PhD in Biochemistry in 1999, and established her independent research laboratory in 2006. Her scientific programme focuses on mechanisms for fast synaptic transmission and plasticity in the central nervous system, with particular emphasis on the molecular physiology of NMDA receptors. In 2003, she re-discovered and characterized modal gating of NMDA receptors, which helped her to develop direct quantitative correlations between microscopic and macroscopic channel behaviours and to ascertain a new form of short-term frequency-dependent facilitation. Current projects in the Popescu laboratory aim to match kinetically identified functional states with structurally defined NMDA conformers and to delineate mutual relationships between receptor-mediated fluxes and the function or dysfunction of excitatory synapses in brain and spinal cord.
 |
Introduction
All life forms rely on oligomeric ion-channel proteins for fast communication across biological membranes. The signals detected and transduced span a broad range of physical and chemical inputs, including mechanical and electrical forces, temperature and concentration gradients. In all cases, though, the biologically relevant output is the passive flux of ions across the cellular membrane. The information-bearing temporal signature of the ionic current is a direct expression of the free-energy space occupied by the protein channel after it absorbs the energizing signal. Detailed quantitative knowledge of the quasi-stable conformations that make up this landscape, their relative occupancies and the rates at which they interconvert is of particular relevance to understanding how information circulates, is processed, and is integrated in the nervous system.
Communication between nerve cells occurs primarily across chemical synapses and relies on the activation of chemically activated ion channels. Present understanding of how chemical transmission occurs has deep roots in the receptor theory born from classical pharmacological studies as well as the initial breakthrough insights into the cellular architecture of nervous tissue. Cajal's observations that neurons are physically separated by synaptic clefts argued against the competing hypothesis of direct electrical communication and instead, strongly favoured a chemical process, where diffusion could be invoked (Albright et al. 2000). Earlier studies had already invoked the existence of ‘cellular side chains’, or receptive substances, on the surface of sensitive cells as mediators of drug actions, thus laying the conceptual framework for how extracellular chemicals could interact with cellular membranes (Maehle, 2004). Together, these fundamentally novel insights led to the hypothesis that direct binding of transmitter molecules to membrane-embedded receptors represents an essential component of the signal transduction process. The binding postulate provided the basis for applying the law of mass action to the observable effects of drugs on cells and resulted in the first occupancy-based numerical models of chemical transmission (Kenakin & Christopoulos, 2011). However, to fill in the ‘black box’ of signal transduction with insight into how agonist binding produces an electrical signal required experiments with a temporal resolution afforded only later by modern electrophysiology.
Quantitative examination of the changes in the membrane potential of muscle fibres elicited by the simultaneous application of various drugs provided the first conclusive evidence that the observed response is controlled not only by the nature and concentration of the applied drug but also by a response function characteristic of every drug-receptor ensemble (Del Castillo & Katz, 1957). Thus, chemical transmission began to be described with state models comprising discrete binding and isomerisation equilibria, akin to those used to describe enzyme-catalysed chemical reactions (Michaelis & Menten, 1913). The direct observation of single-molecule signals and their intrinsic complexity provided unequivocal evidence that models comprising merely functionally defined states, such as resting, open or desensitized, represent theoretical simplifications of a much larger range of conformations accessible to agonist–receptor complexes and are thus intrinsically limited in the mechanistic insight they can provide. Since then, the goal has been to achieve an as complete as possible description of the microscopic signal, one that explains the biologically relevant characteristics of the macroscopic signal and informs about the structural changes responsible for function.
The ability to watch in real time (and to record for extended periods) currents produced by individual channels brought profoundly satisfying validation for the existence of discrete membrane-embedded pores. Indeed, as had been expected, single-channel traces consisted of randomly spaced square pulses of constant amplitudes, providing definitive proof that membrane ionic currents result from the opening and closing of discrete pores (Neher & Sakmann, 1976). However, the multiplicity of kinetic behaviours revealed by these traces was unexpected and perplexing (Neher & Steinbach, 1978; Conti & Neher, 1980; Sakmann et al. 1980). As a rule, single-channel records showed openings and closures whose durations had complex distributions. In addition, many of the observed traces displayed kinetic heterogeneity in time that was indicative of spontaneous but lasting shifts in opening pattern referred to as modal gating (Fig. 1A–D) (Patlak et al. 1979; Hess et al. 1984; Auerbach & Lingle, 1986; Patlak & Ortiz, 1986; Silberberg et al. 1996). The routine observation of complex event durations and sometimes of modes provided definitive evidence that channel-forming proteins populate multiple ion-conducting (open) and non-conducting (closed) conformations and that these conformations differed widely in their stabilities. Thus it was immediately understood that realistic reaction mechanisms must include transition that span a much wider range of time scales, with the fastest events limited by the response frequency of the electronic amplifier and the slowest events limited by the observation period.
Figure 1. Single-channel current recordings reveal modal gating.
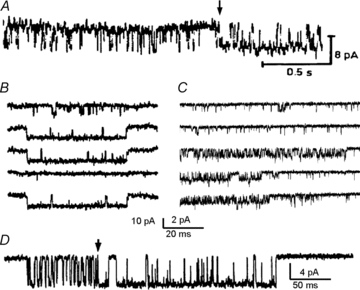
A, glutamate-activated chloride-channel activity recorded from denervated locust muscle (extracellular patch clamp). From Patlak et al. (1979); reprinted by permission from Macmillan Publishers Ltd: Nature, ©1979. B, voltage-activated calcium-channel sweeps recorded from dissociated guinea pig ventricle cells (cell attached voltage-clamp). From Hess et al. (1984); reprinted by permission from Macmillan Publishers Ltd: Nature, ©1984. C, voltage-activated sodium-channel current sweeps from Rana sartorius muscle fragments (from Patlak & Ortiz 1986). D, acetylcholine-activated channels in cultured Xenopus myocytes (cell-attached voltage-clamp) (from Auerbach & Lingle, 1986). Arrows mark sudden changes in channel kinetics that occur during a cluster of openings.
Even in advance of tight-seal recordings, which were perfected just 2 years later (Sigworth & Neher, 1980; Hamill et al. 1981), Neher and Steinbach illustrated both the unprecedented power and the frustrating limitations that kinetic analyses of single-channel currents would bring. In their first paper reporting single-molecule recordings, the authors displayed the current traces on a storage oscilloscope, marked events manually and, directly from the display screen, measured the length of all openings and closures observed and the frequencies with which these occurred. (Neher & Steinbach, 1978). Frequency histograms revealed exponentially distributed open and closed durations as expected for a stochastic process. Given a reaction mechanism, this analysis provided rate constants for each elementary step postulated, an extraordinary opportunity for mechanistic insight. On the other hand, limited time resolution, unknown number of proteins contributing to the observed signal, and the kinetic complexity of the recorded trace brought to the fore a new set of challenges and stimulated additional theoretical and technological developments. Statistical approaches became necessary to describe the random nature of single-molecule transitions, and because statistics demand by definition large data sets, automation and computational methods were called upon as well (Colquhoun & Hawkes, 1981, 1982; Qin et al. 1996, 1997, 2000; Qin, 2004; Shelley & Magleby, 2008). Together these and other advances permitted state models to be fitted directly to single-channel data and made it feasible to develop and test reaction mechanisms of increasing complexity.
In addition to bringing the recording resolution to single molecule levels, the development of patch clamp techniques also eliminated the need for separate intracellular current and voltage electrodes, thus greatly expanding the range of biological samples amenable to electrophysiological investigations. Thus the three classic preparations, the frog neuromuscular junction used by Galvani as early as the 1700s, the electric organ of the Torpedo fish introduced by Matteucci in 1838, and the giant axon from the stellar nerve of the squid discovered by Young in 1937, could now be joined by the much smaller cells of the mammalian central nervous system (Marco, 1998; Albright et al. 2000). Importantly, the ability to record currents across minuscule bits of membrane sealed onto the tip of a glass pipette revealed that ion channels are not restricted to excitable membranes, but rather represent a ubiquitous fixture of transmembrane signalling mechanisms and are present in all cell types, including unicellular organisms, and even in intracellular membranes.
Successful efforts to prepare and maintain tissue slices and dissociated cells from brain and spinal cord paved the way for a new wave of single channel investigations, which revealed the microscopic behaviours of channels native to central nervous tissue (Crain & Bornstein, 1964; McKhann et al. 1969; Scott et al. 1969; Godfrey et al. 1975; Prochiantz et al. 1979). It was immediately apparent that in central neurons, the principal excitatory neurotransmitter glutamate gated several conductances and each conductance produced events with composite time distributions (Nowak et al. 1984; Cull-Candy & Ogden, 1985; Cull-Candy & Usowicz, 1987a,b; Jahr & Stevens, 1987; Cull-Candy et al. 1988; Howe et al. 1988). Initial efforts focused on organizing and explaining the faster, more abundant transitions. In recent years, attempts to delineate kinetic and structural models of channel activation have begun to also incorporate slower, less frequent transitions such as desensitization and modal gating. Here, I review the current evidence for modal gating in glutamate receptor-channels aiming to delineate similarities and differences between NMDA- and AMPA-sensitive receptors and to raise awareness about roles that slow transitions in synaptic receptors may play in fast neurotransmission and thus in brain function.
Glutamate receptor activation is incompletely understood
In the central nervous system most excitatory synaptic transmission is mediated by ionotropic glutamate receptors (iGluRs). iGluRs are critical to normal formation, maintenance and plasticity of central excitatory synapses and mediate higher brain functions including cognition, memory and learning processes, and certain behaviours. Their inappropriate activation, either insufficient or excessive, has been implicated in the aetiology of pernicious brain and spinal cord disorders such as intellectual disabilities, epilepsy, addiction, chronic pain and neurodegenerative conditions (Kemp & McKernan, 2002; Papadia & Hardingham, 2007; Bowie, 2008; Mellor, 2010). Three classes of glutamate-activated channels can be distinguished by their sequence homology, pharmacology and kinetics: AMPA-, KA- and NMDA-sensitive channels (Lodge, 2009). Despite wide interest, the activation mechanism of iGluRs is only beginning to be described in molecular detail and is clearly still behind the level of understanding reached for the receptors mediating excitatory transmission at the neuromuscular junction (Auerbach, 2010). The development of realistic mathematical models for central excitatory currents has been held up by challenges that fall into at least three categories: the excitatory postsynaptic current reflects the simultaneous activation of several iGluR isoforms; synaptic receptors are practically inaccessible at the single-molecule level; and iGluRs have complex activation mechanisms.
At a canonical central excitatory synapse, AMPA and NMDA receptors are often co-localized and generate a dual-component response to synaptically released glutamate (Fagg & Matus, 1984; Dale & Roberts, 1985; Bekkers & Stevens, 1989; Keller et al. 1991). Native channels are hetero-tetramers of the homologous subunits GluA1–4 and GluN1–3, respectively (see Traynelis et al. 2010 for comprehensive review). They are firmly anchored in the postsynaptic membrane where they represent the cores of large and dynamic protein complexes (Kennedy, 2000; Sheng & Lee, 2000; Nicoll et al. 2006). Although rarely explicitly stated, and despite decades of intense multidisciplinary scrutiny, the exact molecular composition of synaptic channels, their kinetic behavioural repertoire, and how their responses are controlled by extracellular, intracellular and intra-membrane forces are largely unknown. In addition, the architecture of excitatory synapses denies direct access to individual channels in native synaptic environments, with only occasional exceptions (Silver et al. 1992). Instead, the strategy has been to observe and characterize the single-molecule activities of either neuronal receptors residing in somatic membranes or recombinant receptors expressed in heterologous cells. Each of these two approaches has advantages and drawbacks. Most importantly, in neuronal preparations the molecular composition of the receptor observed is uncertain, whereas in heterologous cells, protein partners and chemical modulators critical to native behaviours may be absent. The ultimate goal is to reach an as accurate as possible description of synaptic receptors, and in this respect, observations in both systems have proved valuable.
For both native and recombinant preparations the single channel signal is complex. In all cases, the distributions of open and closed intervals have multiple components and their exact number and individual lifetimes often depend on experimental conditions and analytical methodology. For these reasons the early literature on single glutamate-activated channels was largely descriptive, was difficult to reconcile across laboratories, and often served only the most specialized audience. It proved far from trivial to address immediate questions such as: How many kinetic states can be resolved? In what order are the kinetically defined states accessed during synaptic transmission? How do states differ structurally from one another? What functions do each serve? To various degrees these questions are still awaiting definitive answers.
Within single-channel current traces, modal shifts separate periods during which channels gate with distinct kinetics and are by definition the least frequent and the most elusive to statistical approaches. Nevertheless, modal transitions have been demonstrated for most ion channels investigated in detail at the single-channel level. Modal shifts have been demonstrated for several AMPA and NMDA receptor isoforms and current evidence points to a potentially prominent role of modes in shaping excitatory transmission. Evidence for modal gating in KA receptors is at present lacking. This is not surprising given the limited number of single channel studies reported for KA receptors, but it may emerge as such investigations continue to accumulate (Cull-Candy & Usowicz, 1989; Swanson et al. 1996; Zhang et al. 2009; Contractor et al. 2011). It is important to keep in mind that although modal gating may turn out to be a common characteristic of all ion channel proteins, it need not arise by the same mechanism, nor serve the same function. Rather modal shifts may simply reflect the wide dynamic range of these multimeric, often modular proteins. Therefore, to establish the mechanism, structural correlates and physiological significance of modal gating, these aspects must be examined separately for each ion-channel.
Slow transitions in glutamate receptors
Evidence for modal shifts in iGluRs emerged very early, almost simultaneously with single-channel recordings from central neurons. Jahr & Stevens (1987) obtained current traces that clearly illustrated that the largest glutamate-activated conductance in cultured hippocampal neurons has (at least) two modes of gating (Fig. 2A). In retrospect, this observation was quite remarkable given that at the time the existence of separate classes of glutamate-gated channels was still a matter of debate with definitive evidence just emerging (Cull-Candy & Ogden, 1985; Cull-Candy & Usowicz, 1987b; Usowicz et al. 1989). Coincidentally, these current traces were published in the same issue of Nature as the discovery that glycine potentiates NMDA receptor currents (Johnson & Ascher, 1987). In fact, glycine is required for NMDA receptor activation and thus the NMDA receptor recordings predating this report must have relied on the trace levels of glycine that commonly contaminate laboratory solutions and glassware and on glycine released from adjacent cells (Kleckner & Dingledine, 1988; Johnson & Ascher, 1987).
Figure 2. Neuronal glutamate receptor-channels have several gating modes.
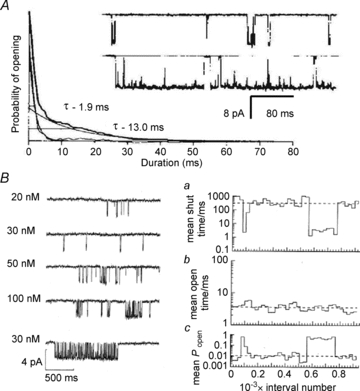
A, glutamate-elicited currents in dissociated rat hippocampal neurons (>2 weeks in culture; outside-out patch-clamp). From Jahr & Stevens, (1987); reprinted by permission from Macmillan Publishers Ltd: Nature, ©1987. The large conductance (50 pS) produced bursts with short (∼2 ms) or long (∼13 ms) openings indicative of two modes of gating. B, glutamate-elicited currents in cultured rat hippocampal cells (outside-out patch-clamp). Left, traces recorded at several applied glutamate concentrations, as labelled; the lowest trace shows an example of ‘high Po’ periods that occurred at all concentrations; these were excluded from kinetic analyses. Right, stability plot for mean closed time/ms (top), mean open time/ms (middle) and open probability (bottom) for an entire record (957 intervals) obtained with 20 nm glutamate (from Gibb & Colquhoun, 1991 with permission of The Royal Society).
Despite early observations of modes in iGluRs, the phenomenon of modal gating did not become the focus of quantitative investigations for another decade. Instead accumulating reports of the complex and heterogeneous kinetics of native iGluRs led to the realization that comprehensive mechanistic understanding of their single-channel behaviours may be a formidable task (Fig. 2B) (Cull-Candy & Ogden, 1985; Cull-Candy & Usowicz, 1987a; Ascher et al. 1988; Cull-Candy et al. 1988; Gibb & Colquhoun, 1991, 1992; Howe et al. 1991; Edmonds & Colquhoun, 1992). In central neurons, glutamate produces several conductances with mixed pharmacology. Even for a given conductance level, frequency distributions have multiple components which may vary with time (Howe et al. 1988). Goals for deducing the activation mechanism from microscopic observations had to be scaled down, aiming first to establish which conductance belonged to which channel-type and then to determine how many kinetic components could be reliably discerned for each conductance (Howe et al. 1988, 1991; Gibb & Colquhoun, 1992). Rigorous attribution of observed conductances and kinetic components to a particular receptor had to await the availability of cloned receptors such that recordings could be made from channels of defined molecular composition.
The preferred heterologous expression systems for electrophysiological investigations of recombinant channels are the human epithelial cell line HEK 293 and the frog oocyte (Miledi et al. 2002; Thomas & Smart, 2005). When seeking to record whole-cell currents, the smaller HEK 293 cells are favoured for kinetic studies whereas the much larger oocytes are chosen for pharmacological characterization and chemical modifications. When aiming for single-channel currents, HEK 293 cells are the preparation of choice because they lack endogenous glutamate receptor subunits and also have the ability to produce mammalian proteins that are properly processed, folded and glycosylated (Thomas & Smart, 2005). Frog oocytes express endogenous iGluR subunits and while quantitatively limited, this uncontrolled expression raises the risk that some of the few channels recorded and laboriously analysed may contain amphibian subunits (Schmidt & Hollmann, 2009; Schmidt et al. 2009). Studies of recombinant receptors expressed in HEK 293 cells exposed class- and isoform-specific properties that are far from being fully characterized. This is principally because in most cases, even when recording from receptors of defined subunit composition, the single-channel trace displays considerable complexity.
Two successful approaches to simplify the recorded signal and thus render microscopic data more amenable to mechanistic interpretation have been to manipulate agonist concentrations and to use allosteric modulators. One popular strategy has been to record stationary currents at subsaturating concentrations of agonists. By limiting the frequency with which glutamate binds to a receptor, two channels will rarely open simultaneously regardless of how many channels reside in the clamped membrane patch. In these conditions, a burst of closely spaced openings can be reasonably assumed to originate from the same receptor. The opposite approach has been to maximally increase the channel activity by using high agonist concentrations and/or allosteric modulators. By increasing the frequency with which glutamate binds to the receptor, the lifetimes of unliganded or partially liganded species become shorter than the experimental detection limit. As a consequence, the corresponding closed events are imperceptible and the detected intervals confidently reflect only liganded conformations. Additionally, when channel activity is high, long records in which no double openings occur originate most likely from a patch containing exactly one channel. These one-channel records are of particular value because all the events recorded, including the long gaps that separate bursts of openings, represent transitions experienced by the same receptor and are documented in the recorded trace in the exact order in which they occurred.
Such strategies to control and to simplify the recorded trace coupled with growing computational power and with increasingly faster signal detection and kinetic analyses have been instrumental in establishing that modal transitions represent an integral component of the activation repertoire of glutamate-activated ion channels. Such transitions have been characterized in detail for the two principal NMDA receptor isoforms, 2A and 2B, and have been reported recently for homo-tetramers of all AMPA receptor subtypes, GluA1–4.
Modes in NMDA receptors
Relative to other neurotransmitter-gated channels, NMDA receptors have large uniform unitary conductances and gate slowly, features that render this receptor type attractive to single-channel studies (McBain & Mayer, 1994). Further, the availability of selective agonists and antagonists allowed these channels to be identified and studied even in native preparations where additional glutamate-gated conductances are usually co-expressed. Thus, as soon as neurons of the central nervous system became amenable to single-channel recordings, the largest glutamate-activated conductance (∼50 pS) was found to be NMDA sensitive (Nowak et al. 1984; Cull-Candy & Usowicz, 1987a,b; Ascher et al. 1988; Kleckner & Pallotta, 1995). Single-channel traces produced by NMDA receptor revealed multiple closed and open time components for which neither function nor mechanism was obvious (Jahr & Stevens, 1987; Howe et al. 1988, 1991; Cull-Candy & Usowicz, 1989; Gibb & Colquhoun, 1991, 1992). The observed channel open probability (Po) was heterogeneous along a recorded trace, with sporadic ‘high Po’ periods even when recordings were obtained at very low (nanomolar) glutamate concentrations (Howe et al. 1988; Gibb & Colquhoun, 1991). In contrast to the ‘high Po’ gating mode reported previously by Jahr and Stevens, where high Po originated primarily from markedly longer openings, these sporadic changes in gating seemed to correlate better with shorter closed durations (Fig. 2B). However, these periods of increased activity were relatively infrequent and were typically excluded from additional kinetic analyses.
Recordings from recombinant receptors validated knowledge obtained earlier from native preparations and demonstrated considerable kinetic differences between the four (A–D) NMDA receptor isoforms (Stern et al. 1994; Wyllie et al. 1996, 1998; Cheffings & Colquhoun, 2000; Cull-Candy et al. 2001). Still, periods with ‘atypical’ kinetics continued to pepper traces of otherwise uniform appearance, evidence that such behaviours, even if sporadic, represent intrinsic aspects of these receptors’ activation mechanism. The initial thrust has been to characterize the most prevalent mode of activity: to establish the number, durations and frequencies of its closed and open components. Even so, within periods with stable kinetics, the multiplicity of event distributions signalled the presence of at least two open and up to five closed states (Wyllie et al. 1996, 1998; Anson et al. 1998, 2000). How to arrange these states into a gating sequence was not immediately apparent.
The first success at representing NMDA receptor single-channel activity with state models came from focusing on a specific region of the activation landscape. Banke and Traynelis expressed 2B-type NMDA receptors in HEK 293 cells and recorded currents from one-channel patches exposed only briefly (1–4 ms) to maximally effective concentrations of glutamate (in the presence of saturating concentrations of glycine) (Banke & Traynelis, 2003). In this experimental set-up, by precluding glutamate re-binding during a sweep, and by largely precluding desensitization, each sweep reflected primarily the sequence of openings and closures that occurred immediately after binding glutamate. Experiments with low-efficacy agonists indicated that two closed states are briefly populated after glutamate binds but before the channel opens. With this approach the most likely route between freshly liganded closed and open conformations was distilled to the sequential occupancy of two principal pre-open states and an open state, which could flicker (Banke & Traynelis, 2003). A significant advance in understanding the operation of NMDA receptors, the resulting model described quantitatively the opening pathway and simultaneously accounted for substantial aspects of the microscopic and macroscopic signals.
Independently, Popescu and Auerbach expressed 2A-type NMDA receptors in HEK 293 cells and recorded tens of minutes of continuous activity from on-cell one-channel patches exposed to high concentrations of glutamate and glycine. Two relatively stable pre-open states were prominent in these recordings as well. In addition, when contamination by ambient channel blockers such as Zn2+, Mg2+ and other divalent cations was eliminated (by EDTA chelation), shifts in opening pattern were immediately apparent. Consistent with modal gating, these shifts were abrupt, spontaneous and reversible (Popescu & Auerbach, 2003, 2004) (Fig. 3A). Kinetic analyses of activity separated by open durations revealed that regardless of kinetic mode, NMDA receptors gate with the same mechanism in which some rates are different: two pre-open states lead initially into a flickering open state, followed by transitions into a more stable open state whose lifetime varied spontaneously in time (Popescu & Auerbach, 2003). Although a kinetic model for 2D receptors has not been developed yet, subsequent work established that a similar reaction mechanism was likely to be valid for all NMDA receptor isoforms (Popescu & Auerbach, 2003, 2004; Auerbach & Zhou, 2005; Erreger et al. 2005; Dravid et al. 2008; Amico-Ruvio & Popescu, 2010; Vance et al. 2010). More recently, modal gating was characterized in detail for 2B-type NMDA receptors and also for NMDA receptors native to cortical neurons (Zhang et al. 2008). These studies established that modal shifts, which may be difficult to detect unequivocally under physiological concentrations of Ca2+, Mg2+ and Zn2+, represent the basis for the multiple kinetic components and patch-to-patch variability that had hindered the quantitative modelling of NMDA receptor single-channel traces.
Figure 3. NMDA receptors have three gating modes.
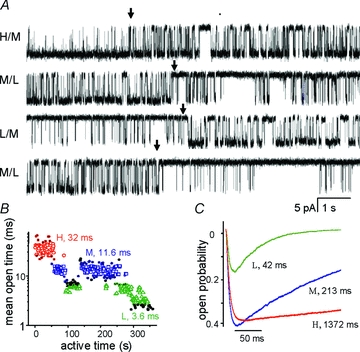
A, four consecutive (non-contiguous) single-channel current traces (10 s each, GluN1/GluN2A receptors, cell-attached) illustrate shifts in opening pattern (arrows); openings are downward. B, segments (1 s each) of active time (desensitization gaps removed) sorted into three categories mirror the four switches in current pattern illustrated in A. Numbers indicate the mean for each population in the patch illustrated. C, predicted macroscopic responses for channels in each mode, elicited by a 1 ms pulse of 1 mm glutamate. For channels in each mode, peak Po is about half the equilibrium Po. Numbers indicate time constants of single exponential fits to the decay phase of the currents. Red, high (H); blue, medium (M); green, low (L); black symbols, excluded. Adapted from Popescu (2005) with permission from Springer.
The most prevalent NMDA receptor isoform in adult brain, the 2A type, generates traces in which three modes (high, H, medium, M, and low, L) can be identified and separated by their characteristic mean open times (MOT: ∼30, ∼10 and ∼4 ms) (Popescu & Auerbach, 2003, 2004) (Fig. 3B). When desensitized periods are excluded from analyses, the remaining active periods contain closures of similar mean duration regardless of gating mode (MCT =∼5 ms) and differ in open probabilities by <2.5-fold (Po: 0.97, 0.83 and 0.4). These kinetic features inferred from single channel analyses predict that macroscopic responses generated by 2A receptors in H, M and L mode will differ substantially in amplitude, time course and total charge transferred (Fig. 3C). Similarly, the 2B type of NMDA receptors, which is prevalent during development and at nascent synapses, produces three modes of activity that differ in their mean open times for H, M and L modes (MOT: ∼10 ms, ∼5 and ∼2 ms). However, for these receptors modes also differ in mean closed times (MCT: 7, 14 and 83 ms), resulting in open probabilities that vary by >6-fold (∼0.67, 0.51 and 0.11) across modes (when desensitized periods are omitted). This mechanism provides a simple explanation for the much wider kinetic repertoire previously reported for recombinant 2B receptors and for neuronal preparations expressing this NMDA receptor type (Vicini et al. 1998; Banke et al. 2005; Erreger et al. 2005; Zhang et al. 2008; Amico-Ruvio & Popescu, 2010).
The recognition that modal transitions are intrinsic components of the NMDA receptor gating repertoire led to the development of tiered reaction mechanisms, with each arm representing gating transitions in one mode (Popescu & Auerbach, 2004; Zhang et al. 2008). Subsequent studies measured microscopic rate constants for glutamate binding and dissociation and demonstrated that for a given receptor type, these are similar for all modes (Popescu et al. 2004; Amico-Ruvio & Popescu, 2010). Finally, desensitization transitions were incorporated in the model resulting in comprehensive mathematical description of all transitions observed in single channel traces and providing means to account mechanistically for macroscopic behaviours such as response time course, peak open probability, dose–response relationships, etc. (Kussius et al. 2009; Kussius & Popescu, 2009, 2010). Although still an approximation of the myriad different conformations that a receptor visits during gating, the tiered activation model has already proved instrumental in generating new knowledge regarding the structures responsible for the kinetic transitions discerned, for identifying transitions sensitive to allosteric ligands, and for testing how these transitions are physiologically important (Kussius et al. 2010; Popescu et al. 2010; Amico-Ruvio et al. 2011; Borschel et al. 2011; Talukder & Wollmuth, 2011).
Modes in AMPA receptors
The activation mechanism of AMPA receptors has been even more difficult to pin down. Even in heterologous expression systems, AMPA receptors can assemble as homo- or hetero-tetramers of four related subunits, GluA1–4, resulting in isoforms with distinct biophysical properties (Traynelis et al. 2010). Moreover, native receptors are most likely hetero-tetramers whose exact molecular composition is uncertain. To further complicate matters, synaptic AMPA receptors are often associated with auxiliary subunits and the resulting protein complexes, which can have variable stoichiometry, also have distinct kinetics (Tomita et al. 2005; Nicoll et al. 2006; Cho et al. 2007; Shi et al. 2009, 2010; Jackson et al. 2011). Despite these challenges, single-channel studies of native AMPA receptors have been successful in establishing that each receptor can generate several current levels (Cull-Candy & Ogden, 1985; Jahr & Stevens, 1987; Traynelis et al. 1993; Wyllie et al. 1993; Jin et al. 2003).
For recombinant AMPA receptors, the majority of single-channel studies have been done on homo-tetrameric channels. These, too, generate multi-level single-channel currents. Transitions between levels represent the stochastic and independent gating of a receptor subunit, with each subunit contributing an equal increment to the observed current amplitude (Rosenmund et al. 1998; Smith & Howe, 2000; Smith et al. 2000; Jin et al. 2003). As an additional experimental difficulty, AMPA receptors are fast to open and desensitize, with the equilibrium current accounting for less than 2% of the initial brief peak. Together these features have rendered single-channel records exceedingly challenging to obtain and to interpret, such that the bulk of the kinetic information available has been derived from macroscopic current recordings. Since modal behaviour can be identified only in single-channel traces, evidence for modal gating in AMPA type receptors has just begun to emerge. Two groups have demonstrated recently that AMPA receptors display modal gating (Poon et al. 2010, 2011; Prieto & Wollmuth, 2010). To render the recorded signal amenable to statistical analyses, both groups increased channel activity by pharmacologically reducing desensitization with cyclothiazide (CTZ) treatment but each group used different recording protocols, excised or attached patches, with short or long agonist exposure, and different receptor isoforms. Regardless, both groups observed sporadic changes in channel open probabilities indicative of modal behaviour.
Prieto & Wollmuth (2010) focused on a portion of the activation landscape of AMPA receptors (GluA1, 2 or 4) by recording current sweeps that follow brief applications of glutamate (200 ms, with 30 μm CTZ). They exposed excised patches repeatedly to high (5 mm) or low (60 nm) concentrations of glutamate, at positive or negative membrane potentials. In these conditions the recorded activity was relatively high (>1000 events per 200 ms sweep). In 5 mm glutamate, four conductance levels were apparent and these occurred in accordance to a state model where each subunit binds glutamate and opens independently of the others. The calculated open probability for each subunit was high (Po= 0.58). In contrast, in ∼100-fold lower glutamate concentration (60 nm), two opening patterns were apparent: a normal mode with the expected low subunit probability (Po= 0.007); and a high mode, when occasionally the subunit open probability was much higher than expected for the low glutamate used (Po= 0.53), and very similar to that observed in 5 mm glutamate (Prieto & Wollmuth, 2010) (Fig. 4A).
Figure 4. High mode of gating in AMPA receptors.
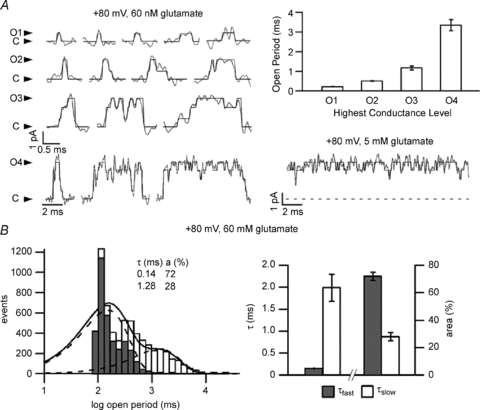
Currents from one GluA2(Q) channel (outside-out patches) were elicited by 200 ms applications of glutamate. Desensitization and polyamine block were reduced with CTZ (30 μm external) and ATP (internal), respectively. A, traces obtained in 60 nm glutamate illustrate openings to the four open levels (O1–4). Openings to the highest conductance level O4 were substantially longer (note different time scale); occurred at higher frequencies (bar graph) than predicted by binomial distribution; and were similar in appearance with the openings produced by 5 mm glutamate. B, left, histogram of all open durations in one patch (open bins) illustrates two exponential components (inset: time constants and areas); the fast component overlaps with the distribution of openings to the lowest conductance level, O1 (filled bins). Right, summary of time constants and areas of the fast and slow open components obtained from five patches. From Prieto & Wollmuth (2010).
These initial observations of AMPA receptor modal gating were made in patches clamped at –80 mV, but held true regardless of membrane polarity. At +80 mV, the percentage of high mode activity was enhanced from 8% to 22% and subunit open probabilities were slighter higher (subunit Po, 0.78 for 5 mm glutamate and 0.002 or 0.71 for 60 nm glutamate). Examining activity at positive potentials, where the high mode was more abundant, the authors were able to assign the increased subunit open probability that marks the high mode to longer openings (Fig. 4B). In 60 nm glutamate, open distributions had two discernible components with each component originating from a separate type of activity. The fast component (0.15 ms, 72%) represented mostly openings to the lowest conductance level (O1), as expected for this low glutamate concentration. The slow component (1.99 ms) resembled the single-component distribution observed in 5 mm glutamate and reflected openings to the highest conductance level (O4) (Prieto & Wollmuth, 2010).
Poon and her colleagues studied the single-molecule behaviour of homomeric GluA3-type AMPA receptors (Poon et al. 2010, 2011). They recorded stationary single-channel activity in several concentrations of either the physiological agonist glutamate (50 μm–5 mm) or a series of partial agonists (FW, NO2W, ClW, 5–500 μm) (Patneau et al. 1992). They reduced channel desensitization with CTZ (100–150 μm) and selected for analyses only records that originated from one-channel patches. In all records, which ranged in duration from 2 to 10 min, the authors observed only three current levels: 14, 26 and 35 pS, as if the opening of a single subunit was too low to be detected. These data were well fit by a linear model where three closed states preceded three open states of increasing conductances. Although all agonists produced the same conductance levels, glutamate more prevalently produced openings to the largest conductance level even when all agonists were used at maximally effective concentrations. In most records, occupancies of the three open levels followed binomial distributions, as expected from a mechanism of independently gating subunits. In a minority of records, only one conductance level (O3 in glutamate or O1 in FW) was prevalent, as if subunits were highly coupled or constrained in some way. Given the many differences in experimental conditions and analytical approaches, it is difficult to ascertain whether this non-binomial mode of gating resulted from the type of slow transitions documented by Prieto & Wollmuth, (2010). Clearly, the transitions that affect subunit coupling, if this is what they represent, were quite infrequent in both studies. Instead, Poon and her colleagues selected for in-depth kinetic analyses the more prevalent records, those with binomial distributions (Poon et al. 2010; Poon et al. 2011).
The analyses were initiated by first selecting active periods within each record (the longest closures representing desensitized intervals were removed). Further, since the Po for each conductance level was variable and agonist dependent, the authors measured the probability of the channel was closed, Pc= 1 –Po. According to Pc, the active periods segregated into five modes, with open probabilities (to either conductance level) ranging from 0.95 for the very high (VH) mode to less than 0.02 for the very low (VL) mode (Fig. 5A). Most records had more than one mode and several records had all five modes. Successive segments were more likely to reflect activity in the same mode than to change gating mode (Fig. 5B), an indication that modal shifts are less frequent than the long closures that flank active periods (i.e. desensitized intervals). These same five modes were also observed in the files where channels appeared to gate with a mechanism of highly coupled subunits (all openings were at same conductance level). This observation may indicate that the transitions that cause ‘coupled-gating’ are even less frequent than those that change segment Pc (Poon et al. 2010).
Figure 5. Five gating modes in GluA3 receptors.
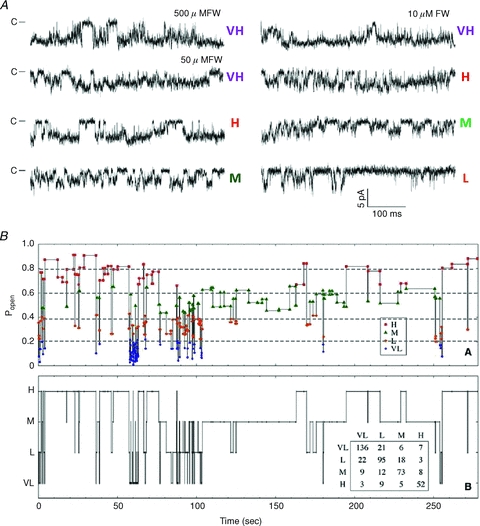
Currents from one GluA3(G) channel (on-cell patches) were recorded with the indicated agonists, and 100 μm CTZ in the pipette. Regardless of agonist and its concentration, channels opened to three conductance levels (downward). During active periods (longest closed intervals removed), using a Po criterion segments fell into five categories: VH, H, M, L and VL. A, examples of single channel traces in VH, H, M and L modes. B, for one patch obtained with 50 μm FW, segments are represented in the order in which they occurred in the record and are plotted as a horizontal line according to segment Po (upper panel) or assigned gating mode (lower panel). Inset, transition matrix between modes shows that consecutive segments are more likely to have the same mode of gating than to switch mode, an indication that modal transitions occur on a much slower time scale than transitions associated with channel activation. From Poon et al. (2010), reprinted with permission from Elsevier/the Biophysical Society.
Clearly, comprehensive reaction mechanisms for AMPA receptors remain elusive. Still, given the complexity of the recorded signal, we should applaud and encourage efforts to further sort out the different types of behaviours, to describe them with quantitative models, and to re-assemble these, ideally, into a unified understanding of AMPA receptor activation. Although admittedly challenging, such analyses are essential if we are to understand how the experimentally observed single-channel patterns come together in a macroscopic signal and which, when and how modes contribute to the synaptic signal. These studies will also be indispensable to discovering the structures responsible for modal behaviour in AMPA receptors. However, for iGluRs modal gating is still an emerging theme and efforts to identify intramolecular interactions that control these sporadic shifts in receptor opening pattern are in their infancy.
Structural correlates of modal gating
Atomic resolution models emerging over the past decade have been immensely valuable in formulating structural hypotheses for channel function. However, for all ion channels investigated to date, the spectrum of kinetic behaviours observed in single channel records far outweighs the number of structural conformations for which knowledge is available. Thus, even though most of the kinetic models presently in use explain only select regions of the free energy landscape populated by any ion channel protein, the set of resolved structures is even more severely limited. Identifying structural correlates of kinetically defined conformations represents a matter of intense current investigation. The goal is to unify emerging mathematical models of channel activation, which should account quantitatively for and predict macroscopic function, with the dynamic fluctuations in receptor structure, which represent the physical basis for changes in function. To date, two of the structurally identified fluctuations may account for modal transitions in iGluRs. The first involves interactions in the ligand binding domain (LBD) that may physically trap the agonist on the receptor to cause extended activations. The second involves rearrangements in the pore that may serve to lock the channel in open conformations and cause longer than usual openings.
The first structural arrangement for a functional tetrameric iGluR has been revealed only recently (Sobolevsky et al. 2009) (Fig. 6A). It illustrates an AMPA-type receptor in complex with the antagonist CNQX, and thus it most likely represents an inactive conformation. However, the modular nature of iGluR has allowed N-terminal domains (NTDs) and ligand-binding domains (LBDs) to be expressed separately as soluble globular proteins and to be investigated with crystallographic approaches. The results informed about the atomic arrangements within soluble modules, about possible inter-domain interactions, and about conformational changes produced by ligands (for a recent review see Mayer, 2011). The first such structure solved for iGluRs, the LBD of the GluA2 subunit, revealed two lobes connected with a flexible hinge, helped delineate agonist-dependent changes, and suggested the current model of agonist-dependent iGluR gating (Armstrong et al. 1998; Armstrong & Gouaux, 2000). This model postulates that receptor activation involves the physical association of an agonist molecule first with residues located on the upper lobe (domain 1) of the LBD, followed by interactions with residues located on the lower, membrane-proximal lobe (domain 2). In some cases, ligand-receptor interactions are accompanied by cross-cleft interactions between domain 1 and 2 residues. Plausibly, in the full-length receptor, these movements would induce mechanical strain, which could be released either by pulling on membrane helices to open the pore (activation gating) or by pulling on LBD modules to separate them from functional dimers (desensitization gating) (Gouaux, 2004; Mayer, 2006). Consistent with this model the more efficacious agonists produce larger domain movement than weaker or partial agonists, whereas antagonists produce no movement at all. This hypothesis is beautifully conveyed by superimposing the GluA2-LBD structures obtained in the absence of ligand (apo) or in the presence of either partial (KA) or full agonist (AMPA) (Armstrong & Gouaux, 2000) (Fig. 6B). Relative to the apo structure, in the KA-bound structure, domain 2 rotates 12 deg toward domain 1 to narrow the cleft. In the AMPA-bound structure the cleft is even narrower due to a more robust 20 deg rotation and this compact conformation is further stabilized by additional cross-cleft interactions. Whether such cross-cleft interactions can form or not depends on the position of the protein backbone along the G653–S652–D651 tripeptide, which can be flipped as in the AMPA-bound structure or un-flipped as in the KA-bound structure (Fig. 6B). To date, several structures have been described for LBDs of all four GluA2 isoforms. These include apo conformations as well as well as modules bound with ligands of efficacies covering a broad pharmacologic spectrum.
Figure 6. Structural hypotheses for iGluR modes.
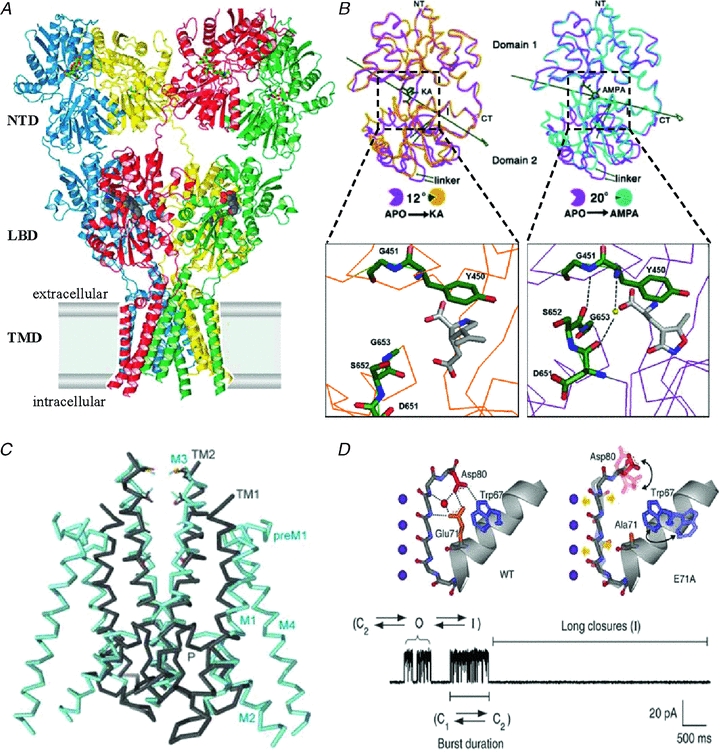
A, structure of a homo-tetrameric AMPA-type receptor in complex with antagonist (CNQX) molecules. Each subunit (blue, red, green and yellow) forms two extracellular globular domains (NTD and LBD) and a transmembrane domain (TMD). From Sobolevsky et al. (2009); reprinted by permission from Macmillan Publishers Ltd: Nature, ©2009. B, isolated GluA2 LBDs organize as two hinged lobes that can adopt slightly different positions relative to one another. Relative to the apo form (magenta), in the KA-bound form (orange) domain 2 is closer to domain 1 by 12 deg whereas in the AMPA-bound form (purple), domain 2 is closer to domain 1 by 20 deg (right panel). Also, in the AMPA-bound form two additional H-bonds form across the cleft between domains 1 and 2 due to an alternative conformation of the protein backbone around the tripeptide G653-S652-D651 (adapted from Armstrong & Gouaux, 2000 with permission from Elsevier). C, Two subunits of the KcsA channel (grey) superimposed with the membrane domain (M1–M4) of two GluA2 subunits; in the GluA2 structure the selectivity filter appears disordered (from Sobolevsky et al. 2009). D, atomic arrangements in the selectivity filter of KcsA. In the wild-type channel (left panel) a network of hydrogen bonds stabilizes the filter in a conformation that can quickly fluctuate between closed (C2) and open (O) conformations, with occasional sojourns in the C-type inactivated state (I); in the E71A KcsA mutant (right panel), increased conformational dynamics of Asp80 prevents its interaction with Trp67 and by averting filter collapse stabilizes the open state. The single channel trace illustrates fluctuations between actively gating and inactivated modes. From Cordero-Morales et al. 2006a; reprinted by permission from Macmillan Publishers Ltd: Nature Structural and Molecular Biology, ©2006.
Using the LBDs of GluA3, Poon and colleagues observed that for a given LBD–agonist complex, the corresponding G-S-D tripeptide can adopt either the flipped or the unflipped conformations (Poon et al. 2011). They proposed that the added stability that the flipped conformation confers to a narrow, compact cleft may represent a high open probability state for that subunit, whereas the unflipped conformation may represent a low open probability state. Given that each of the subunits can adopt either the flipped (high) or the unflipped (low) conformation, this model predicts that a receptor with four independently gating units would produce up to five patterns of activity, as observed by these authors in their single channel data: with the highest mode represented by receptors with all four subunits flipped and the lowest mode represented by receptors with all four subunits unflipped; the intermediate modes would be generated by receptors with three, two or only one subunit flipped (Poon et al. 2010). This hypothesis is consistent with the observation that in all AMPA channels modes are stimulus sensitive: the type of mode observed depends on the type and concentration of agonist used to activate the channel. Alternatively, Prieto and Wollmuth favour a hypothesis where modal transitions change the receptors’ affinity for glutamate and speculate that changes in inter-subunit interactions at the level of LBDs may be the structural basis for the modal gating they observe (Prieto & Wollmuth, 2010). Both mechanisms are plausible and rigorous experimentation will be needed to gather evidence for or against either of these models of AMPA receptor modal gating.
However, a mechanism involving rearrangements in the LBD cleft is unlikely to explain the modal shifts of NMDA receptors. First, a peptide flip has not been described for any of the LBD structures solved to date for NMDA receptor subunits (Furukawa & Gouaux, 2003; Furukawa et al. 2005). Further, NMDA receptors whose LBDs are locked shut, by engineering cysteine residues across clefts, continue to display modal shifts similar to their wild-type counterparts (Kussius & Popescu, 2009). In addition, given that during gating NMDA receptor subunits are highly coupled, a subunit-based mechanism is unlikely to generate three distinct opening patterns (Talukder & Wollmuth, 2011). A plausible hypothesis may involve alternative arrangements in the channel pore as recently demonstrated for the closely related pore of a bacterial potassium-selective channel.
The best characterized mechanism for modal gating so far is that of the bacterial KcsA channel (Chakrapani et al. 2011). This small homotetrameric protein represents the prototypical model for the pore-forming domains of all potassium-selective channels and closely resembles the core transmembrane domains of iGluRs (Wo & Oswald, 1995). A KcsA subunit consists of two transmembrane helices that flank a membrane re-entrant helix/P-loop segment, which are highly homologous to the M1–M3 segments of iGluRs (Fig. 6C). Residues in the P-loop form a highly selective filter for potassium ions and are strictly conserved in Kv channels (Doyle et al. 1998). This region is disordered in the tetrameric iGluR structure solved by the Gouaux group. In contrast, for KcsA several conformations of the selectivity filter have been described with high resolution. Perozo and his colleagues traced modal gating to specific interactions between residues located in this pore domain (Cordero-Morales et al. 2006b). The reaction mechanism of KcsA channels consists of fast activation gating and slower C-type inactivation (Armstrong, 2003). These functional changes correspond to separate structural domains: stimulus-dependent rearrangements at the intersection of transmembrane helices and rearrangements within the re-entrant pore loop, respectively. Single channel traces recorded from KcsA channels display three gating modes characterized by high, intermediate and low Po (Chakrapani et al. 2007a,b). More recently, Chakrapani and her colleagues were successful in identifying substitutions in the pore region of KcsA that trap channels in high or low Po modes (Chakrapani et al. 2011). These experiments add to the accumulating evidence that strong interactions between an aspartate residue situated at the top of the selectivity filter (D80) and a glutamate residue located on the adjacent short helix (E71) promote a structural filter collapse, which functionally represents low Po single channel activity and the C-type inactivation defined in macroscopic currents. Further, structural and functional characterization of a series of mutations in this region revealed that a highly conserved tryptophan residue (W67) promotes a tripartite hydrogen-bond network between D80, E71 and W67 side-chains (Fig. 6D). This network controls the structural stability of the conducting filter and determines the gating modes observed in single KcsA recordings (Cordero-Morales et al. 2011).
Several features of modal gating are similar between KcsA and NMDA receptor. Published data illustrate that both channels have three gating modes, each with characteristic open durations. Notably in KcsA modes are insensitive to the nature of the stimulus and so are NMDA receptor modes (Kussius & Popescu, 2009; Kussius & Popescu, 2010). The observation that a number of allosteric modulators that influence NMDA receptor gating have little or no effect on modes is also consistent with separate structural determinants for activation gating and modal gating (Kussius et al. 2009; Amico-Ruvio et al. 2011). In addition, truncation of the NTDs of NMDA receptors does not abolish modal gating (Amico-Ruvio, personal communication) nor does truncation of C-terminal domains (Maki & Popescu, 2011). Although these observations do not preclude the possibility that perturbations within these distal domains can substantially modulate the kinetics with which modes happen, the structural substrates of modal shifts are likely to reside within the core receptor structure, perhaps as observed for KcsA. Whether dynamic fluctuations in the structure of the selectivity filter underlie the mechanism of modal gating in NMDA receptors or other iGluRs is difficult to predict. The three residues involved in the modal gating of KcsA are largely conserved across many K+ channels and are likely to mediate modal gating of the K+ channel family members. However, these residues are less well conserved in iGluR channels. The residues corresponding to W67 of KcsA are strictly conserved in both AMPA and NMDA receptors, but the conservation of residues corresponding to E71 and D80 is weaker.
The examples discussed above illustrate two types of structural changes that may lead to changes in the observed gating pattern of iGluRs. Rules to connect particular types of kinetic transitions to types of protein motions or to the perturbations that cause these motions have not yet emerged. Thus, to extrapolate from one channel family to another and even within the same channel family is unwarranted. In the absence of a unifying principle, facts will have to be established the hard way, by examining one molecule and one channel type at a time. As difficult a task as this may be, evidence that modes may have a prominent role in determining the time course of synaptic transmission make a compelling case for taking on this challenge.
Physiologic relevance of slow gating transitions
If modal transitions are too slow to occur with substantial probability during a synaptic episode, the question arises whether modes are of any relevance to the time course of the synaptic response and thus to brain function. They may well be just peculiarities of single-molecule dynamics, inconsequential remnants of aeons of protein evolution. Strong evidence for how modal gating can affect the amplitude and time course of excitatory postsynaptic currents came from experiments where an NMDA receptor of the 2A type was exposed repeatedly to brief synaptic-like pulses of glutamate, for several minutes in a row (Fig. 7) (Zhang et al. 2008).
Figure 7. Modal gating controls NMDA receptor macroscopic time course.
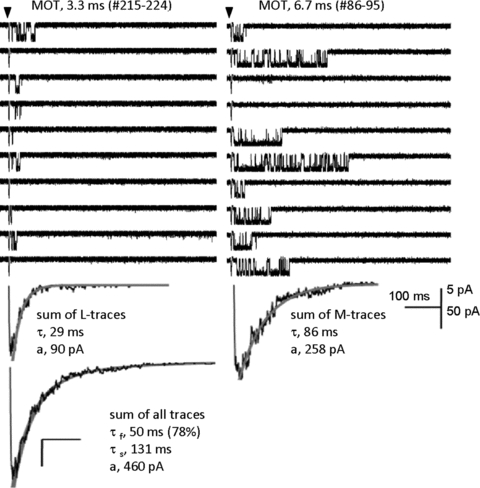
About 1000 consecutive sweeps were recorded from one channel (GluN1/GluN2A receptor, outside-out patch) following brief pulses (arrow, 1 ms) of 1 mm glutamate applied at 0.2 Hz, in the presence of glycine (0.1 mm). Sweeps with similar kinetics (MOT and burst length) clustered together. Upper panels illustrate two runs of 10 consecutive sweeps, recorded within the same one-channel patch at ∼10 min interval (sweep number and MOT are indicated for each run). Below each run, is the sum trace for L- and M-sweeps, and at bottom the sum trace for all sweeps. Grey lines superimposed over summed current traces represent fits to declining exponential functions. The characteristic bi-exponential decay of an NMDA receptor macroscopic response reflects the merging of two distinct behaviours each declining with mono-exponential time course and interconverting on a min time scale. From Zhang et al. (2008).
These experiments were prompted by the puzzling observation that NMDA receptor reaction mechanisms that did not explicitly include modal transitions failed to predict the well documented bi-phasic decay of this receptor's macroscopic response (Lester et al. 1990; Keller et al. 1991; Vicini et al. 1998; Wyllie et al. 1998). In addition, the experimental currents decayed with more variable kinetics than predicted for a populations of channels that gated with homogeneous kinetics (Wyllie et al. 1998; Banke & Traynelis, 2003; Popescu & Auerbach, 2003; Popescu et al. 2004; Auerbach & Zhou, 2005; Schorge et al. 2005). Inspection of series of activations elicited from the same one-channel patch for a period long enough to include at least one modal shift (>5 min) showed that, indeed, the summed activations in each patch decayed with biphasic kinetics (Fig. 7). This time course matched well with values measured in multi-channel patches under similar experimental conditions, an indication that taken together the single-channel sweeps, even though recorded one at a time, represent well the unison response observed in a macro-patch. Importantly, when individual activations were selected by open time, a kinetic marker for modes in 2A and 2B channels, they also had similar open probabilities and activation lengths. Sweeps with similar kinetics also tended to occur in runs as would be expected if the change in kinetics was on a longer time scale than activation and desensitization gating (Fig. 7).
These features were well captured with a tiered model where each arm represented the linear model derived from activations grouped by kinetic mode. The tiered model posits that the biphasic deactivation of NMDA receptors reflects short and long activations of kinetically distinct receptor populations, and these can interconvert on a minute time scale through modal transitions. Thus, although too slow to occur with measurable probability during a synaptic response, modal transitions may influence the shape of the synaptic response by controlling how resting receptors are pre-distributed across distinct kinetic modes. Neuronal NMDA receptors have modal gating as well, and together these result support the view that the biphasic decay of the NMDA receptor-mediate synaptic response is controlled by modal gating.
Conclusion
In summary, modal gating designates low probability rearrangements in protein structure that produce a substantial change in the overall pattern of channel opening. Modal switches can be directly observed only in single channel traces and contribute substantial complexity to a signal that is already difficult to interpret. However, the effort required to identify, sort and characterize single molecule behaviours is well worth investing. Such efforts have already generated new knowledge and are likely to provide additional clarity into how each microscopic transition shapes the excitatory synaptic response; which transitions are modulated by physiological and pharmacological agents; and which structures are responsible for these observed transitions. Without doubt, since the first observation of an electrical signal passing from one cell to another, we have come a long way in understanding how the postsynaptic currents arise and are controlled. However, the unknowns remain formidable. For the excitatory glutamate-activated channels of the mammalian central nervous system, we know the primary sequence of the building units but not which ones come together to make functional receptors at real synapses; we know atomic architectures for some structural modules but not how all atoms organize in intact receptors; and as outlined in this review, we have only begun to chart their gating mechanisms, including their ability to change modes. Although in iGluRs, the kinetics and mechanism(s) of modal gating have just begun to be investigated systematically, emerging evidence that modal shifts may control key features of the excitatory current, such as peak amplitude, time course and charge transferred, make the case that modes deserve the same attention and curiosity that the activation and desensitization of iGluRs have attracted all along.
Acknowledgments
Acknowledgements go to Symposium participants and manuscript reviewers for valuable input; Dr. Linda Nowak for critical feedback, Swetha E. Murthy and Eileen M. Kasperek for their help with manuscript and figure preparations; and NIH for funding (NS052669).
References
- Albright TD, Jessell TM, Kandel ER, Posner MI. Neural science: a century of progress and the mysteries that remain. Neuron. 2000;25:S1–S55. doi: 10.1016/s0896-6273(00)80912-5. [DOI] [PubMed] [Google Scholar]
- Amico-Ruvio S, Popescu G. Stationary gating of GluN1/GluN2B receptors in intact membrane patches. Biophys J. 2010;98:1160–1169. doi: 10.1016/j.bpj.2009.12.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico-Ruvio SA, Murthy SE, Smith TP, Popescu GK. Zinc Effects on NMDA receptor gating kinetics. Biophys J. 2011;100:1910–1918. doi: 10.1016/j.bpj.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson LC, Chen PE, Wyllie DJA, Colquhoun D, Schoepfer R. Identification of amino acid residues of the NR2A subunit that control glutamate potency in recombinant NR1/NR2A NMDA receptors. J Neurosci. 1998;18:581–589. doi: 10.1523/JNEUROSCI.18-02-00581.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson LC, Schoepfer R, Colquhoun D, Wyllie DJ. Single-channel analysis of an NMDA receptor possessing a mutation in the region of the glutamate binding site. J Physiol. 2000;527:225–237. doi: 10.1111/j.1469-7793.2000.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM. Voltage-gated K channels. Sci STKE. 2003;2003:re10. doi: 10.1126/stke.2003.188.re10. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. doi: 10.1016/s0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Armstrong N, Sun Y, Chen GQ, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. doi: 10.1038/27692. [DOI] [PubMed] [Google Scholar]
- Ascher P, Bregestovski P, Nowak L. NMDA-activated channels of mouse central neurones in magnesium-free solutions. J Physiol. 1988;399:207–226. doi: 10.1113/jphysiol.1988.sp017076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A. The gating isomerization of neuromuscular acetylcholine receptors. J Physiol. 2010;588:573–586. doi: 10.1113/jphysiol.2009.182774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A, Lingle CJ. Heterogeneous kinetic properties of acetylcholine receptor channels in Xenopus myocytes. J Physiol. 1986;378:119–140. doi: 10.1113/jphysiol.1986.sp016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A, Zhou Y. Gating reaction mechanisms for NMDA receptor channels. J Neurosci. 2005;25:7914–7923. doi: 10.1523/JNEUROSCI.1471-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke TG, Dravid SM, Traynelis SF. Protons trap NR1/NR2B NMDA receptors in a nonconducting state. J Neurosci. 2005;25:42–51. doi: 10.1523/JNEUROSCI.3154-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banke TG, Traynelis SF. Activation of NR1/NR2B NMDA receptors. Nat Neurosci. 2003;6:144–152. doi: 10.1038/nn1000. [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Stevens CF. NMDA and non-NMDA receptors are co-localized at individual excitatory synapses in cultured rat hippocampus. Nature. 1989;341:230–233. doi: 10.1038/341230a0. [DOI] [PubMed] [Google Scholar]
- Borschel FW, Murthy SE, Kasperek EM, Popescu GK. MDA receptor activation requires remodeling of inter-subunit contacts within ligand-binding heterodimers. Nat Commun. 2011;2:498. doi: 10.1038/ncomms1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D. Ionotropic glutamate receptors & CNS disorders. CNS Neurol Disord Drug Targets. 2008;7:129–143. doi: 10.2174/187152708784083821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrapani S, Cordero-Morales JF, Jogini V, Pan AC, Cortes DM, Roux B, Perozo E. On the structural basis of modal gating behavior in K+ channels. Nat Struct Mol Biol. 2011;18:67–74. doi: 10.1038/nsmb.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrapani S, Cordero-Morales JF, Perozo E. A quantitative description of KcsA gating I: macroscopic currents. J Gen Physiol. 2007a;130:465–478. doi: 10.1085/jgp.200709843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrapani S, Cordero-Morales JF, Perozo E. A quantitative description of KcsA gating II: single-channel currents. J Gen Physiol. 2007b;130:479–496. doi: 10.1085/jgp.200709844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheffings CM, Colquhoun D. Single channel analysis of a novel NMDA channel from Xenopus oocytes expressing recombinant NR1a, NR2A and NR2D subunits. J Physiol. 2000;526:481–491. [PubMed] [Google Scholar]
- Cho CH, St-Gelais F, Zhang W, Tomita S, Howe JR. Two families of TARP isoforms that have distinct effects on the kinetic properties of AMPA receptors and synaptic currents. Neuron. 2007;55:890–904. doi: 10.1016/j.neuron.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981;211:205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Hawkes AG. On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci. 1982;300:1–59. doi: 10.1098/rstb.1982.0156. [DOI] [PubMed] [Google Scholar]
- Conti F, Neher E. Single channel recordings of K+ currents in squid axons. Nature. 1980;285:140–143. doi: 10.1038/285140a0. [DOI] [PubMed] [Google Scholar]
- Contractor A, Mulle C, Swanson GT. Kainate receptors coming of age: milestones of two decades of research. Trends Neurosci. 2011;34:154–163. doi: 10.1016/j.tins.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Morales JF, Cuello LG, Zhao Y, Jogini V, Cortes DM, Roux B, Perozo E. Molecular determinants of gating at the potassium-channel selectivity filter. Nat Struct Mol Biol. 2006a;13:311–318. doi: 10.1038/nsmb1069. [DOI] [PubMed] [Google Scholar]
- Cordero-Morales JF, Cuello LG, Perozo E. Voltage-dependent gating at the KcsA selectivity filter. Nat Struct Mol Biol. 2006b;13:319–322. doi: 10.1038/nsmb1070. [DOI] [PubMed] [Google Scholar]
- Cordero-Morales JF, Jogini V, Chakrapani S, Perozo E. A multipoint hydrogen-bond network underlying KcsA C-type inactivation. Biophys J. 2011;100:2387–2393. doi: 10.1016/j.bpj.2011.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain SM, Bornstein MB. Bioelectric activity of neonatal mouse cerebral cortex during growth and differentiation in tissue culture. Exp Neurol. 1964;10:425–450. doi: 10.1016/0014-4886(64)90034-2. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Howe JR, Ogden DC. Noise and single channels activated by excitatory amino acids in rat cerebellar granule neurones. J Physiol. 1988;400:189–222. doi: 10.1113/jphysiol.1988.sp017117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy SG, Ogden DC. Ion channels activated by L-glutamate and GABA in cultured cerebellar neurons of the rat. Proc R Soc Lond B Biol Sci. 1985;224:367–373. doi: 10.1098/rspb.1985.0038. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Usowicz MM. Glutamate and aspartate activated channels and inhibitory synaptic currents in large cerebellar neurons grown in culture. Brain Res. 1987a;402:182–187. doi: 10.1016/0006-8993(87)91065-1. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Usowicz MM. Multiple-conductance channels activated by excitatory amino acids in cerebellar neurons. Nature. 1987b;325:525–528. doi: 10.1038/325525a0. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Usowicz MM. On the multiple-conductance single channels activated by excitatory amino acids in large cerebellar neurones of the rat. J Physiol. 1989;415:555–582. doi: 10.1113/jphysiol.1989.sp017736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale N, Roberts A. Dual-component amino-acid-mediated synaptic potentials: excitatory drive for swimming in Xenopus embryos. J Physiol. 1985;363:35–59. doi: 10.1113/jphysiol.1985.sp015694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J, Katz B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957;146:369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Dravid SM, Prakash A, Traynelis SF. Activation of recombinant NR1/NR2C NMDA receptors. J Physiol. 2008;586:4425–4439. doi: 10.1113/jphysiol.2008.158634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds B, Colquhoun D. Rapid decay of averaged single-channel NMDA receptor activations recorded at low agonist concentration. Proc R Soc Lond B Biol Sci. 1992;250:279–286. doi: 10.1098/rspb.1992.0160. [DOI] [PubMed] [Google Scholar]
- Erreger K, Dravid SM, Banke TG, Wyllie DJ, Traynelis SF. Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. J Physiol. 2005;563:345–358. doi: 10.1113/jphysiol.2004.080028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagg GE, Matus A. Selective association of N-methyl aspartate and quisqualate types of L-glutamate receptor with brain postsynaptic densities. Proc Natl Acad Sci U S A. 1984;81:6876–6880. doi: 10.1073/pnas.81.21.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Gouaux E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. EMBO J. 2003;22:2873–2885. doi: 10.1093/emboj/cdg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- Gibb AJ, Colquhoun D. Glutamate activation of a single NMDA receptor-channel produces a cluster of channel openings. Proc R Soc Lond B Biol Sci. 1991;243:39–45. doi: 10.1098/rspb.1991.0007. [DOI] [PubMed] [Google Scholar]
- Gibb AJ, Colquhoun D. Activation of N-methyl-D-aspartate receptors by L-glutamate in cells dissociated from adult rat hippocampus. J Physiol. 1992;456:143–179. doi: 10.1113/jphysiol.1992.sp019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey EW, Nelson PG, Schrier BK, Breuer AC, Ransom BR. Neurons from fetal rat brain in a new cell culture system: a multidisciplinary analysis. Brain Res. 1975;90:1–21. doi: 10.1016/0006-8993(75)90679-4. [DOI] [PubMed] [Google Scholar]
- Gouaux E. Structure and function of AMPA receptors. J Physiol. 2004;554:249–253. doi: 10.1113/jphysiol.2003.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P, Lansman JB, Tsien RW. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984;311:538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Howe JR, Colquhoun D, Cull-Candy SG. On the kinetics of large-conductance glutamate-receptor ion channels in rat cerebellar granule neurons. Proc R Soc Lond B Biol Sci. 1988;233:407–422. doi: 10.1098/rspb.1988.0030. [DOI] [PubMed] [Google Scholar]
- Howe JR, Cull-Candy SG, Colquhoun D. Currents through single glutamate receptor channels in outside-out patches from rat cerebellar granule cells. J Physiol. 1991;432:143–202. doi: 10.1113/jphysiol.1991.sp018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Milstein AD, Soto D, Farrant M, Cull-Candy SG, Nicoll RA. Probing TARP modulation of AMPA receptor conductance with polyamine toxins. J Neurosci. 2011;31:7511–7520. doi: 10.1523/JNEUROSCI.6688-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr CE, Stevens CF. Glutamate activates multiple single channel conductances in hippocampal neurons. Nature. 1987;325:522–525. doi: 10.1038/325522a0. [DOI] [PubMed] [Google Scholar]
- Jin R, Banke TG, Mayer ML, Traynelis SF, Gouaux E. Structural basis for partial agonist action at ionotropic glutamate receptors. Nat Neurosci. 2003;6:803–810. doi: 10.1038/nn1091. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Keller BU, Konnerth A, Yaari Y. Patch clamp analysis of excitatory synaptic currents in granule cells of rat hippocampus. J Physiol. 1991;435:275–293. doi: 10.1113/jphysiol.1991.sp018510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nat Neurosci. 2002;5(Suppl):1039–1042. doi: 10.1038/nn936. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Christopoulos A. Analytical pharmacology: the impact of numbers on pharmacology. Trends Pharmacol Sci. 2011;32:189–196. doi: 10.1016/j.tips.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. Signal-processing machines at the postsynaptic density. Science. 2000;290:750–754. doi: 10.1126/science.290.5492.750. [DOI] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–837. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Kleckner NW, Pallotta BS. Burst kinetics of single NMDA receptor currents in cell-attached patches from rat brain cortical neurons in culture. J Physiol. 1995;486:411–426. doi: 10.1113/jphysiol.1995.sp020822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussius CL, Kaur N, Popescu GK. Pregnanolone sulfate promotes desensitization of activated NMDA receptors. J Neurosci. 2009;29:6819–6827. doi: 10.1523/JNEUROSCI.0281-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussius CL, Popescu AM, Popescu GK. Agonist-specific gating of NMDA receptors. Channels. 2010;4:1–5. doi: 10.4161/chan.4.2.10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussius CL, Popescu GK. Kinetic basis of partial agonism at NMDA receptors. Nat Neurosci. 2009;12:1114–1120. doi: 10.1038/nn.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kussius CL, Popescu GK. NMDA receptors with locked glutamate-binding clefts open with high efficacy. J Neurosci. 2010;30:12474–12479. doi: 10.1523/JNEUROSCI.3337-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RA, Clements JD, Westbrook GL, Jahr CE. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990;346:565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Lodge D. The history of the pharmacology and cloning of ionotropic glutamate receptors and the development of idiosyncratic nomenclature. Neuropharmacology. 2009;56:6–21. doi: 10.1016/j.neuropharm.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Maehle AH. “Receptive substances”: John Newport Langley (1852–1925) and his path to a receptor theory of drug action. Med Hist. 2004;48:153–174. doi: 10.1017/s0025727300000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki BE, Popescu GK. Contributions of carboxy-terminal domains to NMDA receptor gating. Biophys J. 2011;100:269a–270a. doi: 10.1016/j.bpj.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco P. Animal electricity and the birth of electrophysiology: the legacy of Luigi Galvani. Brain Res Bul. 1998;46:381–407. doi: 10.1016/s0361-9230(98)00026-4. [DOI] [PubMed] [Google Scholar]
- Mayer ML. Glutamate receptors at atomic resolution. Nature. 2006;440:456–462. doi: 10.1038/nature04709. [DOI] [PubMed] [Google Scholar]
- Mayer ML. Emerging models of glutamate receptor ion channel structure and function. Structure. 2011;19:1370–1380. doi: 10.1016/j.str.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-Methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994;74:723–760. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Ho W, Raiborn C, Varon S. The isolation of neurons from normal and abnormal human cerebral cortex. Arch Neurol. 1969;20:542–547. doi: 10.1001/archneur.1969.00480110106011. [DOI] [PubMed] [Google Scholar]
- Mellor IR. The AMPA receptor as a therapeutic target: current perspectives and emerging possibilities. Future Med Chem. 2010;2:877–891. doi: 10.4155/fmc.10.27. [DOI] [PubMed] [Google Scholar]
- Michaelis L, Menten ML. Mechanism for the catalysis of biological chemical reactions (as translated and excerpted in Mikulás Teich, A Documentary History of Biochemistry, 1770–1940 (Fairleigh Dickinson University Press, Rutherford, NJ, 1992)) Biochemische Zeitschrift. 1913;49:333–369. [Google Scholar]
- Miledi R, Eusebi F, MartÃ-nez-Torres Al, Palma E, Trettel F. Expression of functional neurotransmitter receptors in Xenopus oocytes after injection of human brain membranes. Proc Natl Acad Sci U S A. 2002;99:13238–13242. doi: 10.1073/pnas.192445299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976;260:799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E, Steinbach JH. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Papadia S, Hardingham GE. The dichotomy of NMDA receptor signaling. Neuroscientist. 2007;13:572–579. doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak JB, Gration KA, Usherwood PN. Single glutamate-activated channels in locust muscle. Nature. 1979;278:643–645. doi: 10.1038/278643a0. [DOI] [PubMed] [Google Scholar]
- Patlak JB, Ortiz M. Two modes of gating during late Na+ channel currents in frog sartorius muscle. J Gen Physiol. 1986;87:305–326. doi: 10.1085/jgp.87.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML, Jane DE, Watkins JC. Activation and desensitization of AMPA/kainate receptors by novel derivatives of willardiine. J Neurosci. 1992;12:595–606. doi: 10.1523/JNEUROSCI.12-02-00595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K, Ahmed AH, Nowak LM, Oswald RE. Mechanisms of modal activation of GluA3 receptors. Mol Pharmacol. 2011 doi: 10.1124/mol.111.071688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K, Nowak LM, Oswald RE. Characterizing single-channel behavior of GluA3 receptors. Biophys J. 2010;99:1437–1446. doi: 10.1016/j.bpj.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu G. Mechanism-based targeting of NMDA receptor functions. Cell Mol Life Sci. 2005;62:2100–2111. doi: 10.1007/s00018-005-5227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu G, Auerbach A. Modal gating of NMDA receptors and the shape of their synaptic response. Nat Neurosci. 2003;6:476–483. doi: 10.1038/nn1044. [DOI] [PubMed] [Google Scholar]
- Popescu G, Auerbach A. The NMDA receptor gating machine: lessons from single channels. Neuroscientist. 2004;10:192–198. doi: 10.1177/1073858404263483. [DOI] [PubMed] [Google Scholar]
- Popescu G, Robert A, Howe JR, Auerbach A. Reaction mechanism determines NMDA receptor response to repetitive stimulation. Nature. 2004;430:790–793. doi: 10.1038/nature02775. [DOI] [PubMed] [Google Scholar]
- Popescu GK, Murthy S, Borschel WF. Allosteric inhibitors of NMDA receptor functions: a next generation in CNS therapies. Pharmaceuticals. 2010;3:3240. [Google Scholar]
- Prieto ML, Wollmuth LP. Gating modes in AMPA receptors. J Neurosci. 2010;30:4449–4459. doi: 10.1523/JNEUROSCI.5613-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochiantz A, di Porzio U, Kato A, Berger B, Glowinski J. In vitro maturation of mesencephalic dopaminergic neurons from mouse embryos is enhanced in presence of their striatal target cells. Proc Natl Acad Sci U S A. 1979;76:5387–5391. doi: 10.1073/pnas.76.10.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F. Restoration of single-channel currents using the segmental k-means method based on hidden Markov modeling. Biophys J. 2004;86:1488–1501. doi: 10.1016/S0006-3495(04)74217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J. 1996;70:264–280. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. Maximum likelihood estimation of aggregated Markov processes. Proc R Soc Lond B Biol Sci. 1997;264:375–383. doi: 10.1098/rspb.1997.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F, Auerbach A, Sachs F. A direct optimization approach to hidden Markov modeling for single channel kinetics. Biophys J. 2000;79:1915–1927. doi: 10.1016/S0006-3495(00)76441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Sakmann B, Patlak J, Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980;286:71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Hollmann M. Molecular and functional characterization of Xenopus laevis NMDA receptors. Mol Cell Neurosci. 2009;42:116–127. doi: 10.1016/j.mcn.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Klein C, Hollmann M. Xenopus laevis oocytes endogenously express all subunits of the ionotropic glutamate receptor family. J Mol Biol. 2009;390:182–195. doi: 10.1016/j.jmb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Schorge S, Elenes S, Colquhoun D. Maximum likelihood fitting of single channel NMDA activity with a mechanism composed of independent dimers of subunits. J Physiol. 2005;569:395–418. doi: 10.1113/jphysiol.2005.095349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BS, Engelbert VE, Fisher KC. Morphological and electrophysiological characteristics of dissociated chick embryonic spinal ganglion cells in culture. Exp Neurol. 1969;23:230–248. doi: 10.1016/0014-4886(69)90060-0. [DOI] [PubMed] [Google Scholar]
- Shelley C, Magleby KL. Linking exponential components to kinetic states in Markov models for single-channel gating. J Gen Physiol. 2008;132:295–312. doi: 10.1085/jgp.200810008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Lee SH. Growth of the NMDA receptor industrial complex. Nat Neurosci. 2000;3:633–635. doi: 10.1038/76576. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lu W, Milstein AD, Nicoll RA. The stoichiometry of AMPA receptors and TARPs varies by neuronal cell type. Neuron. 2009;62:633–640. doi: 10.1016/j.neuron.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Suh YH, Milstein AD, Isozaki K, Schmid SM, Roche KW, Nicoll RA. Functional comparison of the effects of TARPs and cornichons on AMPA receptor trafficking and gating. Proc Natl Acad Sci U S A. 2010;107:16315–16319. doi: 10.1073/pnas.1011706107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth FJ, Neher E. Single Na+ channel currents observed in cultured rat muscle cells. Nature. 1980;287:447–449. doi: 10.1038/287447a0. [DOI] [PubMed] [Google Scholar]
- Silberberg SD, Lagrutta A, Adelman JP, Magleby KL. Wanderlust kinetics and variable Ca2+ sensitivity of dSlo [correction of Drosophila], a large conductance Ca2+-activated K+ channel, expressed in oocytes. Biophys J. 1996;71:2640–2651. [PMC free article] [PubMed] [Google Scholar]
- Silver RA, Traynelis SF, Cull-Candy SG. Rapid-time-course miniature and evoked excitatory currents at cerebellar synapses in situ. Nature. 1992;355:163–166. doi: 10.1038/355163a0. [DOI] [PubMed] [Google Scholar]
- Smith TC, Howe JR. Concentration-dependent substate behavior of native AMPA receptors. Nat Neurosci. 2000;3:992–997. doi: 10.1038/79931. [DOI] [PubMed] [Google Scholar]
- Smith TC, Wang LY, Howe JR. Heterogeneous conductance levels of native AMPA receptors. J Neurosci. 2000;20:2073–2085. doi: 10.1523/JNEUROSCI.20-06-02073.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern P, Cik M, Colquhoun D, Stephenson FA. Single channel properties of cloned NMDA receptors in a human cell line: comparison with results from Xenopus oocytes. J Physiol. 1994;476:391–397. doi: 10.1113/jphysiol.1994.sp020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG. Effect of RNA editing and subunit coassembly single-channel properties of recombinant kainate receptors. J Physiol. 1996;492:129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder I, Wollmuth LP. Local constraints in either the GluN1 or GluN2 subunit equally impair NMDA receptor pore opening. J Gen Physiol. 2011;138:179–194. doi: 10.1085/jgp.201110623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Smart TG. HEK293 cell line: A vehicle for the expression of recombinant proteins. J Pharmacol Toxicol Methods. 2005;51:187–200. doi: 10.1016/j.vascn.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Silver RA, Cull-Candy SG. Estimated conductance of glutamate receptor channels activated during EPSCs at the cerebellar mossy fiber-granule cell synapse. Neuron. 1993;11:279–289. doi: 10.1016/0896-6273(93)90184-s. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels:structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usowicz MM, Gallo V, Cull-Candy SG. Multiple conductance channels in type-2 cerebellar astrocytes activated by excitatory amino acids. Nature. 1989;339:380–383. doi: 10.1038/339380a0. [DOI] [PubMed] [Google Scholar]
- Vance KM, Hansen KB, Ogden KK, Traynelis SF. Activation of recombinant rat GluN1/GluN2D NMDA receptors. Biophys J. 2010;98:525a. [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Wo ZG, Oswald RE. Unraveling the modular design of glutamate-gated ion channels. Trends Neurosci. 1995;18:161–168. doi: 10.1016/0166-2236(95)93895-5. [DOI] [PubMed] [Google Scholar]
- Wyllie DJ, Behe P, Colquhoun D. Single-channel activations and concentration jumps: comparison of recombinant NR1a/NR2A and NR1a/NR2D NMDA receptors. J Physiol. 1998;510:1–18. doi: 10.1111/j.1469-7793.1998.001bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJ, Behe P, Nassar M, Schoepfer R, Colquhoun D. Single-channel currents from recombinant NMDA NR1a/NR2D receptors expressed in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1996;263:1079–1086. doi: 10.1098/rspb.1996.0159. [DOI] [PubMed] [Google Scholar]
- Wyllie DJ, Traynelis SF, Cull-Candy SG. Evidence for more than one type of non-NMDA receptor in outside-out patches from cerebellar granule cells of the rat. J Physiol. 1993;463:193–226. doi: 10.1113/jphysiol.1993.sp019591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Howe JR, Popescu GK. Distinct gating modes determine the biphasic relaxation of NMDA receptor currents. Nat Neurosci. 2008;11:1373–1375. doi: 10.1038/nn.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, St-Gelais F, Grabner CP, Trinidad JC, Sumioka A, Morimoto-Tomita M, Kim KS, Straub C, Burlingame AL, Howe JR, Tomita S. A transmembrane accessory subunit that modulates kainate-type glutamate receptors. Neuron. 2009;61:385–396. doi: 10.1016/j.neuron.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


