Abstract
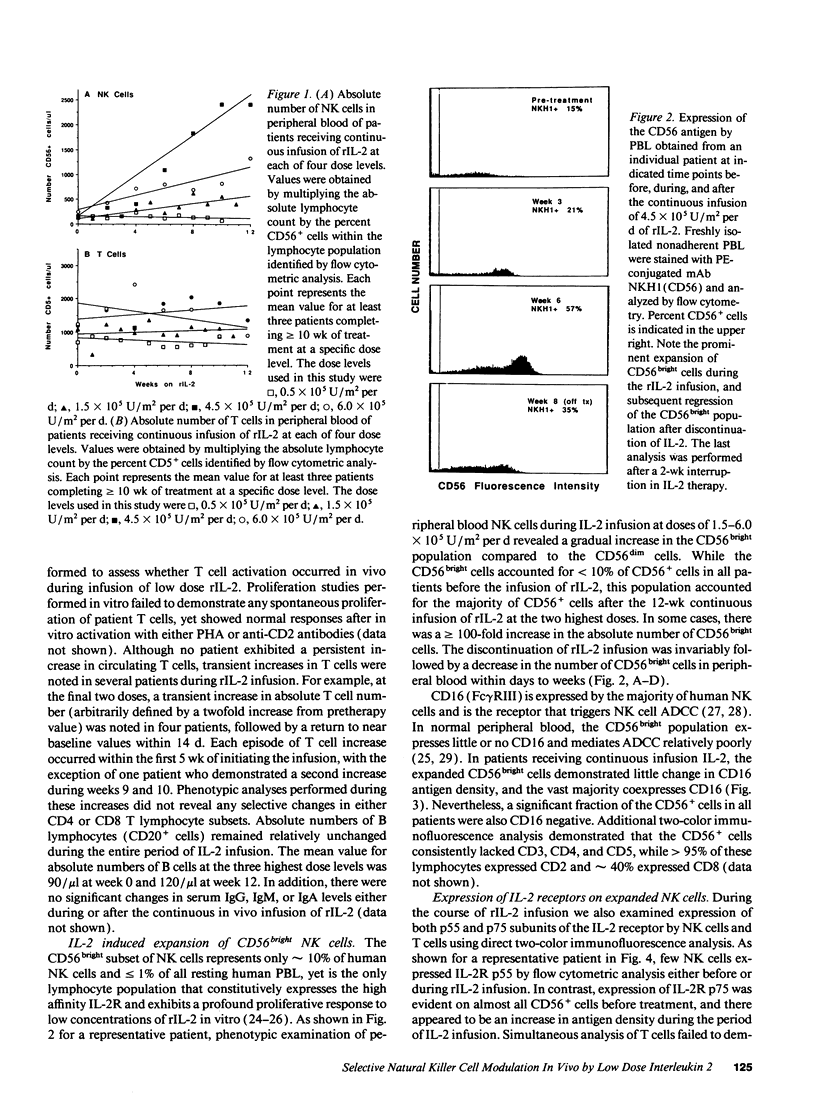
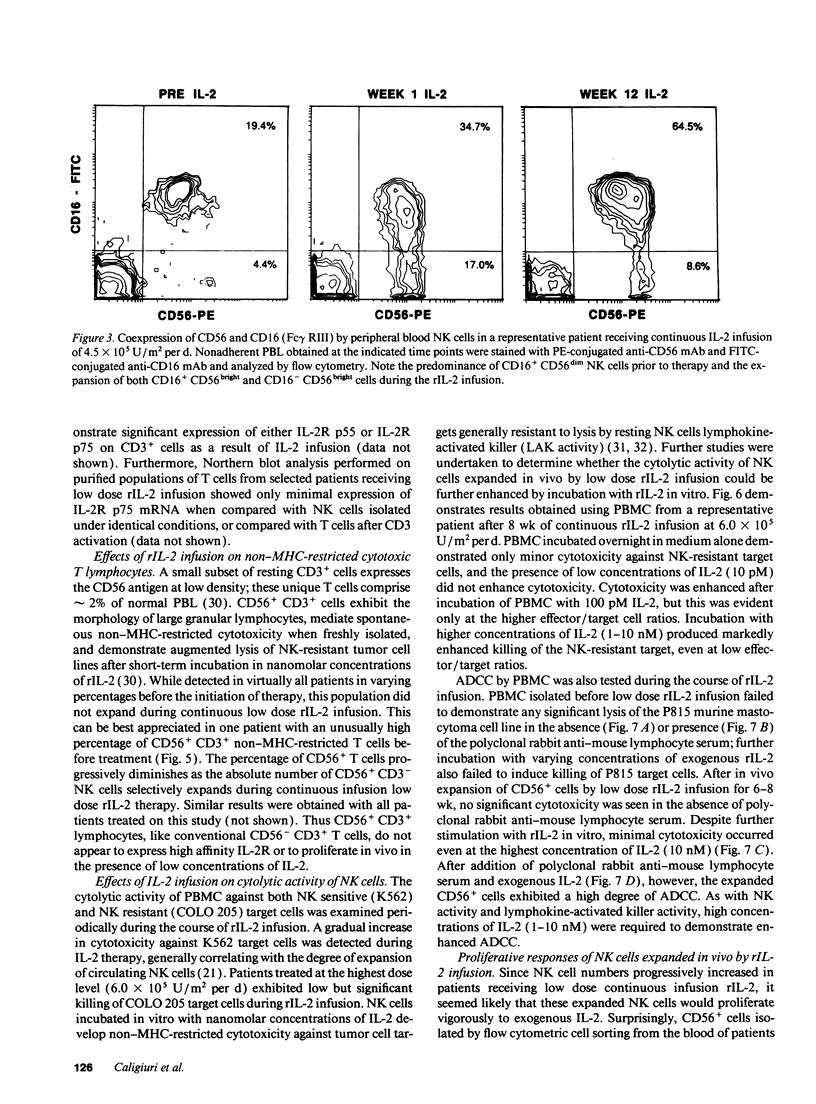
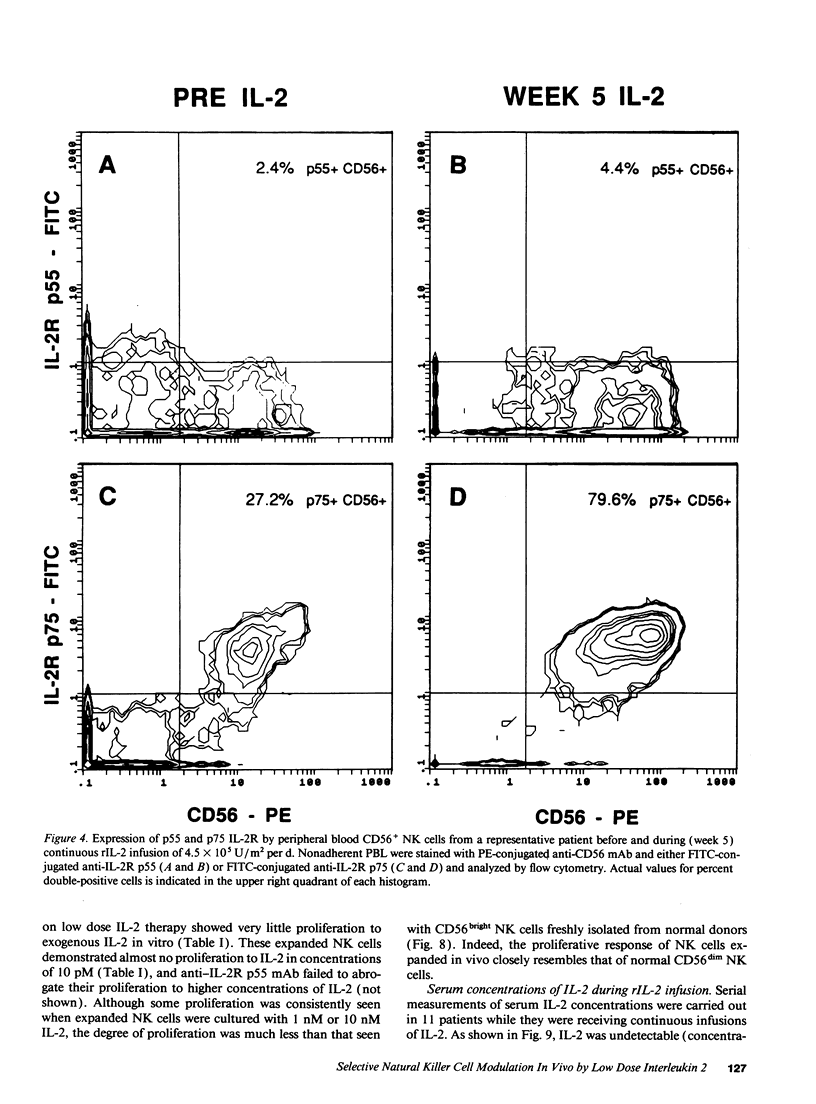
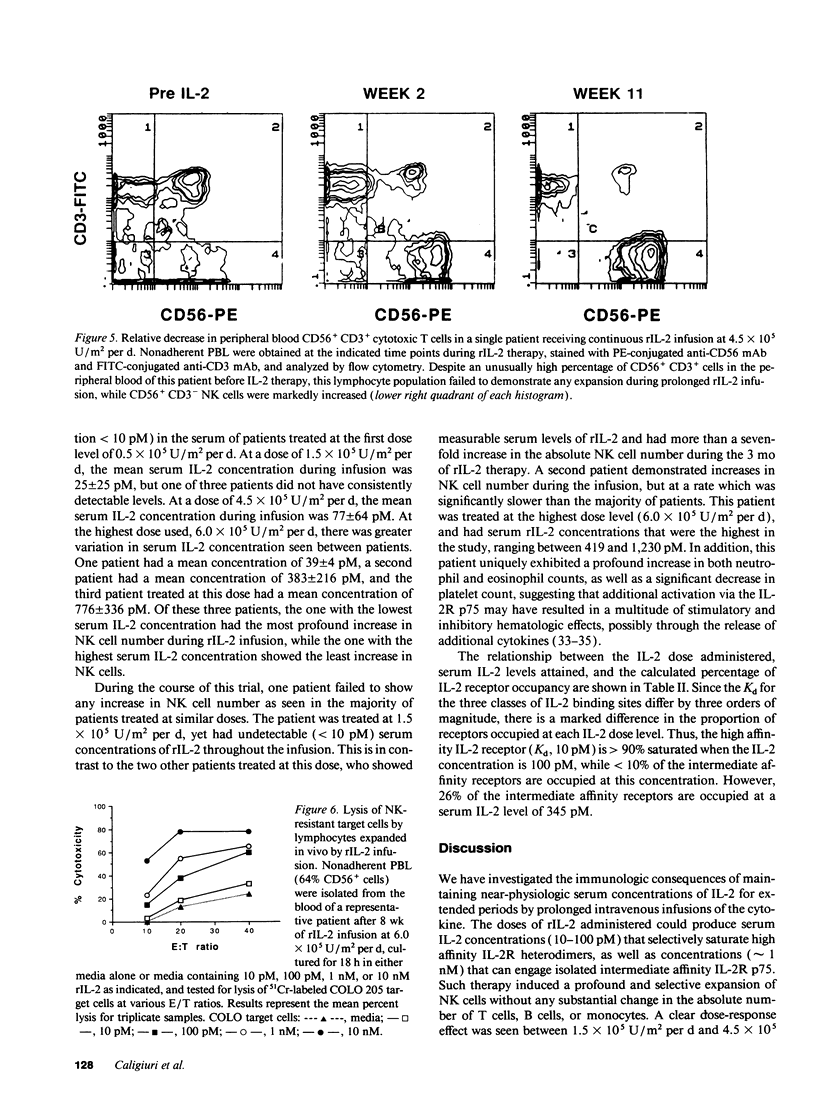
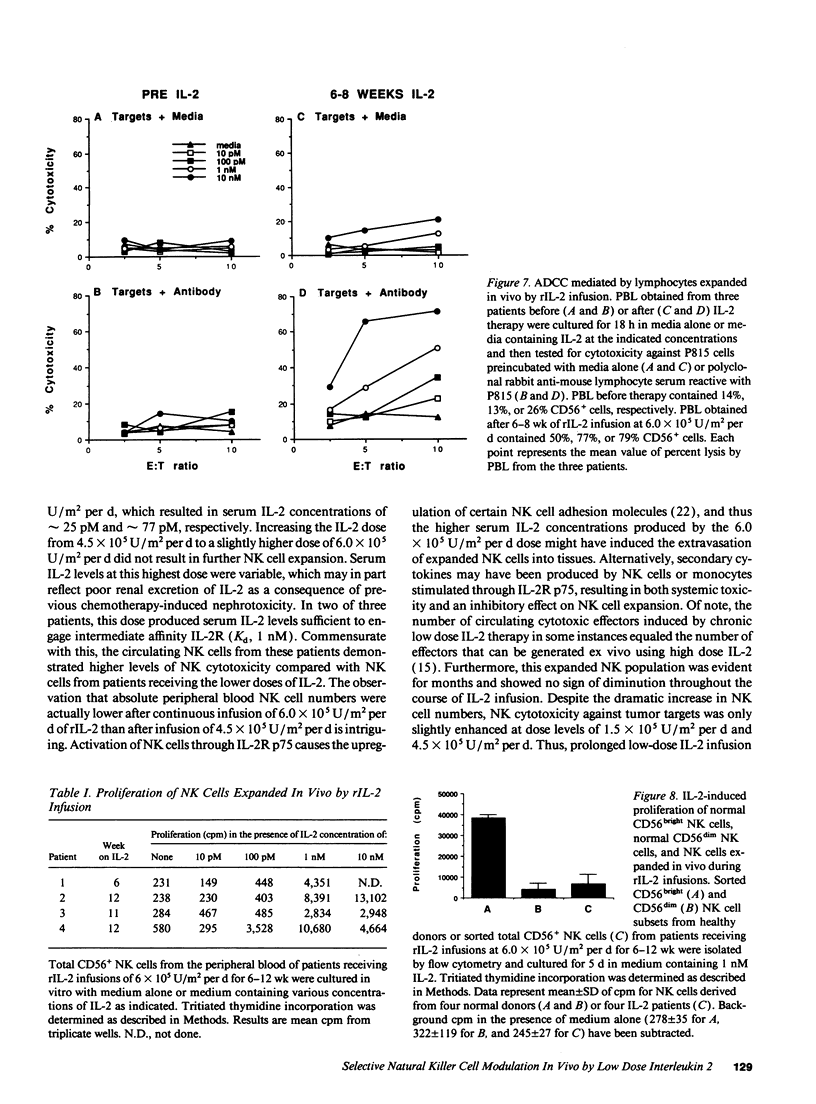
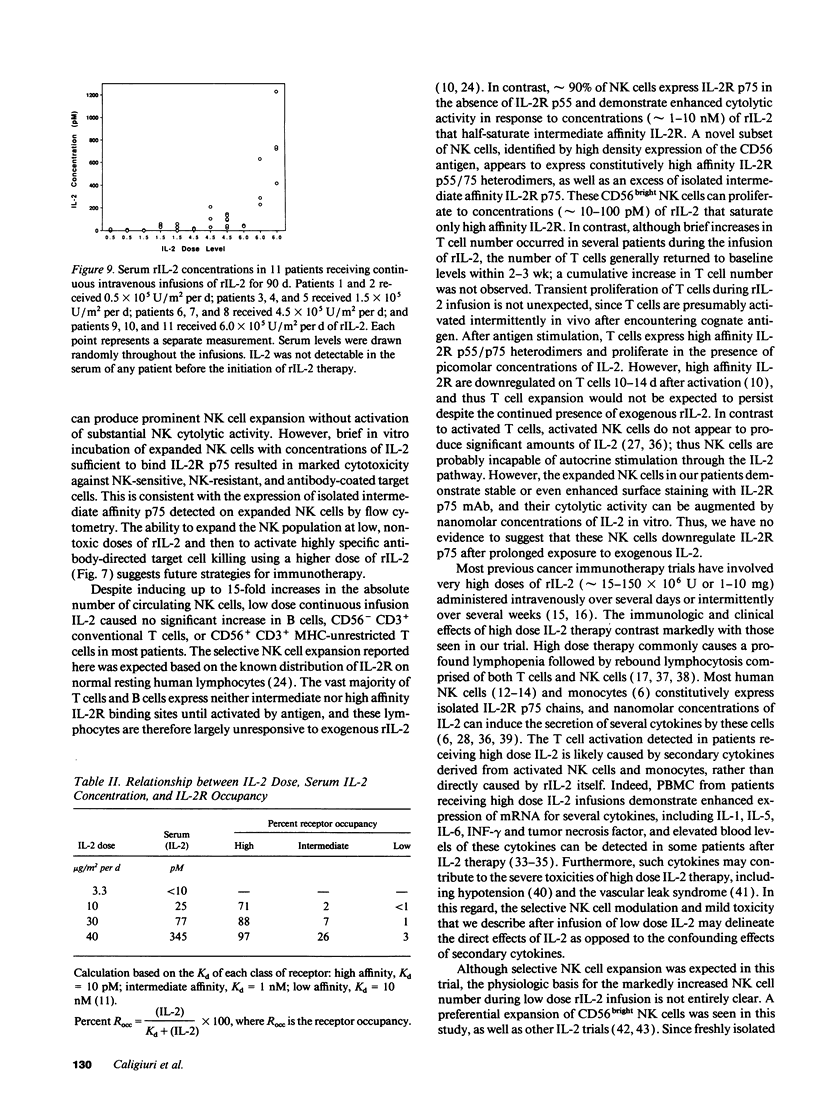
The immunologic consequences of prolonged infusions of rIL-2 in doses that produce physiologic serum concentrations of this cytokine were investigated. rIL-2 in doses of 0.5-6.0 x 10(6) U/m2 per d (3.3-40 micrograms/m2 per d) was administered by continuous intravenous infusion for 90 consecutive days to patients with advanced cancer. IL-2 concentrations (25 +/- 25 and 77 +/- 64 pM, respectively) that selectively saturate high-affinity IL-2 receptors (IL-2R) were achieved in the serum of patients receiving rIL-2 infusions of 10 micrograms/m2 per d and 30 micrograms/m2 per d. A gradual, progressive expansion of natural killer (NK) cells was seen in the peripheral blood of these patients with no evidence of a plateau effect during the 3 mo of therapy. A preferential expansion of CD56bright NK cells was consistently evident. NK cytotoxicity against tumor targets was only slightly enhanced at these dose levels. However, brief incubation of these expanded NK cells with IL-2 in vitro induced potent lysis of NK-sensitive, NK-resistant, and antibody-coated targets. Infusions of rIL-2 at 40 micrograms/m2 per d produced serum IL-2 levels (345 +/- 381 pM) sufficient to engage intermediate affinity IL-2R p75, which is constitutively expressed by human NK cells. This did not result in greater NK cell expansion compared to the lower dose levels, but did produce in vivo activation of NK cytotoxicity, as evidenced by lysis of NK-resistant targets. There was no consistent change in the numbers of CD56- CD3+ T cells, CD56+ CD3+ MHC-unrestricted T cells, or B cells during infusions of rIL-2 at any of the dosages used. This study demonstrates that prolonged infusions of rIL-2 in doses that saturate only high affinity IL-2R can selectively expand human NK cells for an extended period of time with only minimal toxicity. Further activation of NK cytolytic activity can also be achieved in vivo, but it requires concentrations of IL-2 that bind intermediate affinity IL-2R p75. Clinical trials are underway attempting to exploit the differing effects of various concentrations of IL-2 on human NK cells in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins M. B., Gould J. A., Allegretta M., Li J. J., Dempsey R. A., Rudders R. A., Parkinson D. R., Reichlin S., Mier J. W. Phase I evaluation of recombinant interleukin-2 in patients with advanced malignant disease. J Clin Oncol. 1986 Sep;4(9):1380–1391. doi: 10.1200/JCO.1986.4.9.1380. [DOI] [PubMed] [Google Scholar]
- Baume D. M., Robertson M. J., Levine H., Manley T. J., Schow P. W., Ritz J. Differential responses to interleukin 2 define functionally distinct subsets of human natural killer cells. Eur J Immunol. 1992 Jan;22(1):1–6. doi: 10.1002/eji.1830220102. [DOI] [PubMed] [Google Scholar]
- Boldt D. H., Mills B. J., Gemlo B. T., Holden H., Mier J., Paietta E., McMannis J. D., Escobedo L. V., Sniecinski I., Rayner A. A. Laboratory correlates of adoptive immunotherapy with recombinant interleukin-2 and lymphokine-activated killer cells in humans. Cancer Res. 1988 Aug 1;48(15):4409–4416. [PubMed] [Google Scholar]
- Caligiuri M. A., Murray C., Soiffer R. J., Klumpp T. R., Seiden M., Cochran K., Cameron C., Ish C., Buchanan L., Perillo D. Extended continuous infusion low-dose recombinant interleukin-2 in advanced cancer: prolonged immunomodulation without significant toxicity. J Clin Oncol. 1991 Dec;9(12):2110–2119. doi: 10.1200/JCO.1991.9.12.2110. [DOI] [PubMed] [Google Scholar]
- Caligiuri M. A., Zmuidzinas A., Manley T. J., Levine H., Smith K. A., Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990 May 1;171(5):1509–1526. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Ellis T. M., Creekmore S. P., McMannis J. D., Braun D. P., Harris J. A., Fisher R. I. Appearance and phenotypic characterization of circulating Leu 19+ cells in cancer patients receiving recombinant interleukin 2. Cancer Res. 1988 Nov 15;48(22):6597–6602. [PubMed] [Google Scholar]
- Espinoza-Delgado I., Ortaldo J. R., Winkler-Pickett R., Sugamura K., Varesio L., Longo D. L. Expression and role of p75 interleukin 2 receptor on human monocytes. J Exp Med. 1990 May 1;171(5):1821–1826. doi: 10.1084/jem.171.5.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henney C. S., Kuribayashi K., Kern D. E., Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981 May 28;291(5813):335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- Heslop H. E., Gottlieb D. J., Bianchi A. C., Meager A., Prentice H. G., Mehta A. B., Hoffbrand A. V., Brenner M. K. In vivo induction of gamma interferon and tumor necrosis factor by interleukin-2 infusion following intensive chemotherapy or autologous marrow transplantation. Blood. 1989 Sep;74(4):1374–1380. [PubMed] [Google Scholar]
- Kaplan G., Kiessling R., Teklemariam S., Hancock G., Sheftel G., Job C. K., Converse P., Ottenhoff T. H., Becx-Bleumink M., Dietz M. The reconstitution of cell-mediated immunity in the cutaneous lesions of lepromatous leprosy by recombinant interleukin 2. J Exp Med. 1989 Mar 1;169(3):893–907. doi: 10.1084/jem.169.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasid A., Director E. P., Rosenberg S. A. Induction of endogenous cytokine-mRNA in circulating peripheral blood mononuclear cells by IL-2 administration to cancer patients. J Immunol. 1989 Jul 15;143(2):736–739. [PubMed] [Google Scholar]
- Keever C. A., Pekle K., Gazzola M. V., Collins N. H., Bourhis J. H., Gillio A. Natural killer and lymphokine-activated killer cell activities from human marrow precursors. II. The effects of IL-3 and IL-4. J Immunol. 1989 Nov 15;143(10):3241–3249. [PubMed] [Google Scholar]
- Kehrl J. H., Dukovich M., Whalen G., Katz P., Fauci A. S., Greene W. C. Novel interleukin 2 (IL-2) receptor appears to mediate IL-2-induced activation of natural killer cells. J Clin Invest. 1988 Jan;81(1):200–205. doi: 10.1172/JCI113295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M. T., Matory Y. L., Ettinghausen S. E., Rayner A. A., Sharrow S. O., Seipp C. A., Custer M. C., Rosenberg S. A. In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol. 1985 Oct;135(4):2865–2875. [PubMed] [Google Scholar]
- Malkovský M., Loveland B., North M., Asherson G. L., Gao L., Ward P., Fiers W. Recombinant interleukin-2 directly augments the cytotoxicity of human monocytes. Nature. 1987 Jan 15;325(6101):262–265. doi: 10.1038/325262a0. [DOI] [PubMed] [Google Scholar]
- Margolin K. A., Rayner A. A., Hawkins M. J., Atkins M. B., Dutcher J. P., Fisher R. I., Weiss G. R., Doroshow J. H., Jaffe H. S., Roper M. Interleukin-2 and lymphokine-activated killer cell therapy of solid tumors: analysis of toxicity and management guidelines. J Clin Oncol. 1989 Apr;7(4):486–498. doi: 10.1200/JCO.1989.7.4.486. [DOI] [PubMed] [Google Scholar]
- Mier J. W., Vachino G., van der Meer J. W., Numerof R. P., Adams S., Cannon J. G., Bernheim H. A., Atkins M. B., Parkinson D. R., Dinarello C. A. Induction of circulating tumor necrosis factor (TNF alpha) as the mechanism for the febrile response to interleukin-2 (IL-2) in cancer patients. J Clin Immunol. 1988 Nov;8(6):426–436. doi: 10.1007/BF00916947. [DOI] [PubMed] [Google Scholar]
- Migliorati G., Cannarile L., Herberman R. B., Bartocci A., Stanley E. R., Riccardi C. Role of interleukin 2 (IL 2) and hemopoietin-1 (H-1) in the generation of mouse natural killer (NK) cells from primitive bone marrow precursors. J Immunol. 1987 Jun 1;138(11):3618–3625. [PubMed] [Google Scholar]
- Mingari M. C., Gerosa F., Carra G., Accolla R. S., Moretta A., Zubler R. H., Waldmann T. A., Moretta L. Human interleukin-2 promotes proliferation of activated B cells via surface receptors similar to those of activated T cells. Nature. 1984 Dec 13;312(5995):641–643. doi: 10.1038/312641a0. [DOI] [PubMed] [Google Scholar]
- Musso T., Espinoza-Delgado I., Pulkki K., Gusella G. L., Longo D. L., Varesio L. IL-2 induces IL-6 production in human monocytes. J Immunol. 1992 Feb 1;148(3):795–800. [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Cwirla S., Phillips J. H. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989 Nov 15;143(10):3183–3191. [PubMed] [Google Scholar]
- Nagler A., Lanier L. L., Phillips J. H. Constitutive expression of high affinity interleukin 2 receptors on human CD16-natural killer cells in vivo. J Exp Med. 1990 May 1;171(5):1527–1533. doi: 10.1084/jem.171.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa R., Chatila T., Pahwa S., Paradise C., Day N. K., Geha R., Schwartz S. A., Slade H., Oyaizu N., Good R. A. Recombinant interleukin 2 therapy in severe combined immunodeficiency disease. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5069–5073. doi: 10.1073/pnas.86.13.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Gemlo B. T., Myers W. W., Rayner A. A., Lanier L. L. In vivo and in vitro activation of natural killer cells in advanced cancer patients undergoing combined recombinant interleukin-2 and LAK cell therapy. J Clin Oncol. 1987 Dec;5(12):1933–1941. doi: 10.1200/JCO.1987.5.12.1933. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med. 1986 Sep 1;164(3):814–825. doi: 10.1084/jem.164.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Takeshita T., Sugamura K., Lanier L. L. Activation of natural killer cells via the p75 interleukin 2 receptor. J Exp Med. 1989 Jul 1;170(1):291–296. doi: 10.1084/jem.170.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. J., Caligiuri M. A., Manley T. J., Levine H., Ritz J. Human natural killer cell adhesion molecules. Differential expression after activation and participation in cytolysis. J Immunol. 1990 Nov 15;145(10):3194–3201. [PubMed] [Google Scholar]
- Robertson M. J., Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990 Dec 15;76(12):2421–2438. [PubMed] [Google Scholar]
- Robertson M. J., Soiffer R. J., Wolf S. F., Manley T. J., Donahue C., Young D., Herrmann S. H., Ritz J. Response of human natural killer (NK) cells to NK cell stimulatory factor (NKSF): cytolytic activity and proliferation of NK cells are differentially regulated by NKSF. J Exp Med. 1992 Mar 1;175(3):779–788. doi: 10.1084/jem.175.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Yang J. C., Aebersold P. M., Linehan W. M., Seipp C. A., White D. E. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989 Oct;210(4):474–485. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma M. R., Falkenburg J. H., Landegent J. E., Duinkerken N., Osanto S., Ralph P., Kaushansky K., Wagemaker G., Van Damme J., Willemze R. In vivo production of interleukin-5, granulocyte-macrophage colony-stimulating factor, macrophages colony-stimulating factor, and interleukin-6 during intravenous administration of high-dose interleukin-2 in cancer patients. Blood. 1991 Oct 15;78(8):1981–1987. [PubMed] [Google Scholar]
- Schmidt R. E., Michon J. M., Woronicz J., Schlossman S. F., Reinherz E. L., Ritz J. Enhancement of natural killer function through activation of the T11 E rosette receptor. J Clin Invest. 1987 Jan;79(1):305–308. doi: 10.1172/JCI112800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. E., Murray C., Daley J. F., Schlossman S. F., Ritz J. A subset of natural killer cells in peripheral blood displays a mature T cell phenotype. J Exp Med. 1986 Jul 1;164(1):351–356. doi: 10.1084/jem.164.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J. P., Puri R. K. Interleukin-2 toxicity. J Clin Oncol. 1991 Apr;9(4):694–704. doi: 10.1200/JCO.1991.9.4.694. [DOI] [PubMed] [Google Scholar]
- Siegel J. P., Sharon M., Smith P. L., Leonard W. J. The IL-2 receptor beta chain (p70): role in mediating signals for LAK, NK, and proliferative activities. Science. 1987 Oct 2;238(4823):75–78. doi: 10.1126/science.3116668. [DOI] [PubMed] [Google Scholar]
- Smith K. A. The interleukin 2 receptor. Annu Rev Cell Biol. 1989;5:397–425. doi: 10.1146/annurev.cb.05.110189.002145. [DOI] [PubMed] [Google Scholar]
- Soiffer R. J., Murray C., Cochran K., Cameron C., Wang E., Schow P. W., Daley J. F., Ritz J. Clinical and immunologic effects of prolonged infusion of low-dose recombinant interleukin-2 after autologous and T-cell-depleted allogeneic bone marrow transplantation. Blood. 1992 Jan 15;79(2):517–526. [PubMed] [Google Scholar]
- Thijs L. G., Hack C. E., Strack van Schijndel R. J., Nuijens J. H., Wolbink G. J., Eerenberg-Belmer A. J., Van der Vall H., Wagstaff J. Activation of the complement system during immunotherapy with recombinant IL-2. Relation to the development of side effects. J Immunol. 1990 Mar 15;144(6):2419–2424. [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G., Matsumoto-Kobayashi M., Clark S. C., Seehra J., London L., Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984 Oct 1;160(4):1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. M., Smith K. A. The interleukin 2 receptor. Functional consequences of its bimolecular structure. J Exp Med. 1987 Oct 1;166(4):1055–1069. doi: 10.1084/jem.166.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil-Hillman G., Fisch P., Prieve A. F., Sosman J. A., Hank J. A., Sondel P. M. Lymphokine-activated killer activity induced by in vivo interleukin 2 therapy: predominant role for lymphocytes with increased expression of CD2 and leu19 antigens but negative expression of CD16 antigens. Cancer Res. 1989 Jul 1;49(13):3680–3688. [PubMed] [Google Scholar]
- Weinberg K., Parkman R. Severe combined immunodeficiency due to a specific defect in the production of interleukin-2. N Engl J Med. 1990 Jun 14;322(24):1718–1723. doi: 10.1056/NEJM199006143222406. [DOI] [PubMed] [Google Scholar]
- van den Brink M. R., Boggs S. S., Herberman R. B., Hiserodt J. C. The generation of natural killer (NK) cells from NK precursor cells in rat long-term bone marrow cultures. J Exp Med. 1990 Jul 1;172(1):303–313. doi: 10.1084/jem.172.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]


