Abstract
Non-technical summary
Obesity is the result of a disruption in the maintenance of energy balance such that energy intake exceeds expenditure. Why this occurs is unknown. We show that after food deprivation or consumption of a high fat diet gastric vagal afferent responses to distension are reduced. In addition, the effect of the orexigenic peptide ghrelin is enhanced. Thus the satiety signal is reduced not only after food deprivation but also after a high fat diet. This reduction in satiety signalling may explain the increase in energy intake and disruption in maintenance of energy balance in obesity.
Abstract
Afferent signals from the stomach play an important role in inhibition of food intake during a meal. The gastric hormone ghrelin can influence gastric satiety signalling by altering the sensitivity of gastric vagal afferents. Changes in diet, including food restriction and high fat diet (HFD) alter satiety signalling. We hypothesised that the function of gastric vagal afferent endings are affected by both a period of food restriction and a high fat diet, and that the inhibitory effect of ghrelin on vagal afferents is influenced by the different feeding conditions. We found that both fasting and HFD reduced the responses of gastric vagal tension receptors to distension, but not responses of mucosal receptors to mucosal contact. We traced vagal afferents anterogradely to their terminals in the mucosa where we found they were in close apposition to ghrelin-containing cells. Ghrelin receptor mRNA was expressed in vagal afferent cell bodies of the nodose ganglia, and increased in response to caloric restriction, but decreased in HFD mice. In control mice, ghrelin decreased the sensitivity of tension but not mucosal receptors. After caloric restriction or high fat diet, ghrelin inhibited mucosal receptors, and the inhibition of mechanosensitive tension receptors was enhanced. Therefore, both caloric restriction and HFD decrease mechanosensory vagal afferent signals, and augment the inhibitory effect of ghrelin on vagal afferents, but different mechanisms mediate the short- and longer-term changes.
Introduction
It is well established that signals generated in the gastrointestinal tract are able to influence food intake (Bray, 2000). One of these signals is mechanical distension of the stomach which inhibits feeding via stimulation of vagal afferents (Deutsch, 1983). However, there are two distinct subtypes of vagal afferent mechanoreceptors, mucosal receptors and tension receptors (Iggo, 1955; Page & Blackshaw, 1998; Page et al. 2002), which are classified according to their response to specific types of mechanical stimulation. Mucosal receptors respond to fine tactile stimulation whereas tension receptors respond to stretch (Page et al. 2002). The role of gastric distension in the control of food intake is clear but that of mucosal contact is less obvious. In addition to generating sensations of nausea and vomiting in response to chemical stimuli, (Andrews & Sanger, 2002) mucosal receptors may be important in detecting particle density, a factor which influences the rate of gastric emptying, which in turn relates inversely to satiety (McIntyre et al. 1997; Tuleu et al. 1999). In response to nutrients in the small intestine, enteroendocrine cells release peptides that activate adjacent mucosal vagal afferents to elicit satiation (Cummings & Overduin, 2007). There are also enteroendocrine cells in the gastric mucosa that contain and release peptides known to affect appetite (Bado et al. 1998; Sobhani et al. 2000; Moesgaard et al. 2004). The afferents innervating the gastric mucosa are in an ideal position to detect both mechanical stimuli and locally released hormones such as ghrelin.
Ghrelin is a 28 amino acid orexigenic peptide released from exocrine X/A cells in the stomach (Kojima et al. 1999; Hosoda et al. 2000; Gnanapavan et al. 2002). The acute effect of ghrelin administration is a rapid increase in food intake (Tschop et al. 2000; Wren et al. 2000, 2001a; Asakawa et al. 2001; Nakazato et al. 2001; Druce et al. 2006) and chronic administration of ghrelin increases body weight in rodents (Tschop et al. 2000). The data suggest that ghrelin is involved in both the initiation of food intake and the signalling of energy deficit and thus plays an important role in regulation of energy homeostasis (Tschop et al. 2000; Asakawa et al. 2001; Wren et al. 2001b). At least part of the signalling mechanism for ghrelin involves the vagus nerve and brain stem nuclei that ultimately signal to the hypothalamus (Asakawa et al. 2001; Date et al. 2002). There is controversy about the role of the vagus nerves in the orexic effects of ghrelin. Date et al. (2002) showed that either truncal or selective gastric vagotomy or perivagal capsaicin abolished its action when given intravenously in rats. Asakawa et al. (2001) showed that ghrelin inhibited the resting discharge in whole vagal nerve recordings and that lesion of vagal afferent fibres inhibited the appetite-stimulating actions of ghrelin, whereas Arnold et al. (2006) showed no effect of vagotomy on the acute effects of ghrelin administered intraperitoneally. The receptor for ghrelin, growth hormone secretagogue (GHS) 1 receptor, is found in vagal afferents that project to the stomach (Sakata et al. 2003) and is expressed in mouse nodose ganglia (Page et al. 2007b). We have reported that ghrelin selectively inhibited a subpopulation of mechanosensitive gastric vagal afferents (Page et al. 2007b). Therefore there is an integral relationship between ghrelin and gastric vagal afferent satiety signals.
In addition to the acute effect of gastrointestinal peptides on vagal afferent satiety signals it is becoming apparent that they undergo longer-term changes in neurochemical phenotype with changes in nutritional status (Dockray & Burdyga, 2011). For example, it has been shown that withdrawal of food is responsible for changes in expression of G-protein-coupled receptors such as melanin-concentrating hormone receptor-1 in vagal afferent neurons projecting to the gastrointestinal tract (Burdyga et al. 2006a). High fat diets have also been reported to change the expression in nodose ganglia of transcripts encoding receptors known to be involved in appetite regulation, such as cholecystokinin (CCK1) and cannabinoid (CB1) receptors (Nefti et al. 2009; Paulino et al. 2009). We have shown that ghrelin modulates the mechanosensitivity of gastric vagal afferents in vitro (Page et al. 2007b) but it has not been established whether feeding state affects this relationship between ghrelin and gastric vagal afferents. Ghrelin release and plasma ghrelin levels depend significantly on the feeding state; fasting causes increased plasma ghrelin levels, whereas a high fat diet reduces plasma ghrelin levels in mice (Moesgaard et al. 2004).
Using an in vitro gastro-oesophageal vagal afferent preparation to accurately evaluate different populations of mechanosensitive vagal afferent fibres, we investigated the effect of feeding state on the mechanosensitivity of gastric vagal afferents. In addition, we determined the effect of three feeding states on the neuromodulatory action of ghrelin on gastric vagal afferents. To establish if any changes in function of ghrelin could be the result of changes in receptor expression we determined the relative expression of ghrelin receptors in the vagal afferent cell bodies in the nodose ganglia.
Methods
All experimental studies were performed with the approval of the animal ethics committee of the Institute of Medical and Veterinary Science and the University of Adelaide, and in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The experiments also conform to the principles of UK regulations, as described in Drummond (2009).
Short-term restriction of food intake
Eight-week-old female C57/BL6 mice were randomly assigned to one of two groups. The first group (controls) were fed ad libitum and allowed free access to a standard laboratory diet. The second group were fasted for 14 h (fasted). Food was removed but they still had free access to water.
High fat diet model
Eight-week-old female C57/BL6 mice were randomly assigned to one of two groups. The first group (controls) were fed ad libitum and allowed free access to a standard laboratory diet (SLD) comprising 7% energy from fat. The second group were fed ad libitum and allowed free access to a high fat diet (HFD; Specialty Feeds, Glen Forrest, Western Australia) comprising 60% energy from fat. Water was freely available for both SLD- and HFD-fed mice. The mice were kept on either the SLD or the HFD for a period of 12 weeks during which time food intake was measured. The weight of the mice was monitored weekly. Blood was collected from a subset of these mice at the 12 week point, for determination of plasma glucose and insulin concentration.
In vitro mouse gastro-oesophageal afferent preparation
C57/BL6 mice were humanely killed via CO2 inhalation and the thorax was opened by a midline incision. The stomach and oesophagus with attached vagal nerves were removed and placed in a modified Krebs solution containing (mm): NaCl 118.1, KCl 4.7, NaHCO3 25.1, NaH2PO4 1.3, MgSO4.7H2O 1.2, CaCl2 1.5, citric acid 1.0 and glucose 11.1, bubbled with 95% O2–5% CO2 at 4°C during dissection to prevent metabolic degradation. After further dissection, the preparation was opened out longitudinally along the oesophagus and greater curve of the stomach. The preparation was then placed mucosal side up in the organ bath. This preparation has been described in detail previously (Page et al. 2002, 2005). Nifedipine (1 μm) was also added to the Krebs solution to prevent smooth muscle contraction. In a previous preliminary study we have shown that nifedipine has no effect on the mechanical sensitivity of gastro-oesophageal vagal afferents (Page et al. 2006).
Characterisation of gastro-oesophageal vagal afferent properties
Two types of mechanosensitive afferent fibre were studied, those responding to mucosal stroking but not circular tension (mucosal receptors) and those responding to mucosal stroking and circular tension (tension receptors) as reported previously (Page et al. 2002).
Location of receptive fields of all types of afferent fibre was determined by mechanical stimulation throughout the preparation with a brush. Accurate quantification of mechanical responses was performed differently according to the primary adequate stimulus for the type of fibre. Mechanical thresholds of both types of fibre were determined using calibrated von Frey hairs. The most reproducible, stimulus-dependent responses of these afferents to mucosal stroking were evoked when the probe was moved at a rate of 5 mm s−1 across the receptive field rather than being kept static. Due to the fact that the receptive fields were small (<1 mm2), a single test at each intensity is prone to missing the centre of the receptive field on some occasions. Therefore, we minimised experimenter error by measuring the mean response to the middle eight of ten standard strokes given at 1 s intervals. Because the von Frey hair was bent throughout the stroking stimulus, the receptive field was subjected to an even force as the hair passed over it. Tension–response curves were also obtained and used in combination with von Frey thresholds to determine whether the receptive fields of fibres were located in the mucosa or the muscle layer. Tension stimuli were applied via fine suture silk attached via a hook to an unpinned point adjacent to the mechanoreceptive fields. The thread was attached to a cantilever via a pulley close to the preparation. Reference standard weights were then placed on the opposite end of the cantilever. Each weight was applied as a step and maintained for 1 min, and the response was measured as the mean discharge evoked over this period. Due to the fact that all responses to tension adapted slowly, this method of assessment was considered representative of physiological responsiveness. The tension–response curves were produced by applying weights to the cantilever system in the range of 1–5 g. A recovery period of at least 1 min was allowed between each tension stimulus. We compared the effects of diet and mechanical stimuli on the mechanosensitivity of tension and mucosal receptors using a two-way ANOVA to establish if diet had an effect on the response to mechanical stimuli. For some animals the mechanosensitivity of multiple receptors was determined. For analysis these were averaged to provide one replicate per receptor type per animal.
Effect of ghrelin on the mechanosensitivity of vagal afferents
After mechanical sensitivity of the gastric and oesophageal vagal afferents had been established, the effect of ghrelin on mechanical sensitivity was determined. Ghrelin (1 nm) was added to the superfusing solution and allowed to equilibrate for 20 min after which time the tension–response and stroke–response curves were redetermined. This equilibration period was observed so as to ensure penetration of the drug into all layers of the tissue. After this time the tension–response and stroke–response curves were redetermined. This procedure was repeated for ghrelin at increasingly higher doses (3–10 nm). Time-controlled experiments were performed in which there was no significant change in the mechanical responses over a comparable duration. We compared the effects of ghrelin and mechanical stimuli on the mechanosensitivity of tension and mucosal receptors using a two-way ANOVA, to see if ghrelin affected the response to mechanical stimulation. The effect of diet on the modulatory action of ghrelin on responses to mechanical stimulation was determined by assessing the response to either stroking (50 mg von Frey hair; mucosal receptors) or tension (3 g; tension receptors) at different concentrations of ghrelin (0, 1, 3 and 10 nm). Significant differences between diets and ghrelin concentration were assessed using a two-way ANOVA to determine if diet caused a change in ghrelin effect.
Drugs
Stock solution of the peptide ghrelin (0.1 mm; Sigma, Australia) was kept frozen (–80°C) and diluted to its final concentration in Krebs solution on the day of the experiment.
Quantitative reverse-transcription polymerase chain reaction
Nodose ganglia were removed bilaterally from the same animals used for the in vitro mouse gastro-oesophageal vagal afferent preparation. RNA was isolated using an RNeasy Micro RNA extraction kit according to the manufacturer's instructions (Qiagen, Sydney, Australia). This process utilised RNeasy MinElute SpinElute Columns, allowing adsorption of RNA to the silica membrane, an on-column DNase digestion treatment, removal of contaminants with simple wash steps and elution of the RNA with RNase-free water. RNA quantification was determined by measuring the absorbance at 260 nm (A260) via a spectrophotometer (Bio-Rad, Regents Park, New South Wales, Australia). RNA quality was estimated by the A260 nm and A280 nm ratio.
Quantitative RT-PCR (QRT-PCR) reactions were performed as described in detail previously (Hughes et al. 2007). Briefly, QRT-PCR reactions were performed by using a Chromo4 (MJ Research, Bio-Rad) real-time instrument attached to a PTC-200 Peltier thermal cycler (MJ Research) and analysed with Opticon Monitor software (MJ Research). QRT-PCR reactions were performed with a QuantiTect SYBRgreen RT-PCR one-step RT-PCR kit (Qiagen) according to the manufacturer's specifications, with specific QuantiTect Primer Assays (Qiagen) optimised for the detection of the known sequence of mouse ghrelin receptor and β-tubulin transcripts contained in the NCBI reference sequence database (http://www.ncbi.nlm.nih.gov/RefSeq). These primer assays were used under the following conditions: reverse transcription, 50°C for 30 min; initial PCR activation, 95°C for 15 min; PCR cycles 94°C for 15 s, 55°C for 30 s, and 72°C for 30 s repeated for 50 cycles. A melting curve programme verified the specificity and identity of the RT-PCR products and no primer dimers were observed. Confirmation of the amplified products was resolved by 3% agarose gel electrophoresis and visualised via ethidium bromide staining. Each assay was run in at least triplicate in separate experiments. Control PCRs were performed by substituting the RNA template with distilled RNase-free water. All assays were validated for linearity of amplification efficiency and quantitative standard curves obtained by serial dilutions of RNA. Calculations for relative mRNA transcripts expression were performed by the comparative CT method, using the internal reference gene β-tubulin and the equation ΔCT (CT of target transcript – CT of β-tubulin). To determine the relative expression of these transcripts in whole nodose ganglia from high fat-fed mice compared to standard laboratory-fed mice and fasted mice compared to mice fed ad libitum, the ΔΔCT was calculated by using the formula ΔΔCT=ΔCT(ghrelin receptor in HFD or fasted mice) –ΔCT(ghrelin receptor in SLD or mice fed ad libitum, respectively). As such, fold differences were calculated relative to the levels of β-tubulin RNA in the same sample. The relative fold differences were calculated by using the modified version of 2−ΔΔCT (Pfaffl et al. 2002; Page et al. 2007a) correcting for PCR efficiencies. Quantitative data are expressed as means ± SEM; transcript expression was compared between groups using the Mann–Whitney test, with P < 0.05 considered significant.
Tracing studies
Adult (8 weeks old) female C57 BL 6 mice had ad libitum access to water and a SLD and were fasted overnight before experimentation.
Anterograde tracing
The mice were anaesthetised with isoflurane (1–1.5% in oxygen). The left nodose ganglion was exposed and 0.5 μl of wheat-germ agglutinin-conjugated horseradish peroxidase (WGA-HRP (4 mg ml−1); Vector Laboratories) was pressure injected into the nodose ganglia via a glass micropipette (i.d. = 25 μm). The injection site was then dried, the skin incision closed and antibiotics (terramycin; 10 mg kg−1) and analgesic (butorphanol; 5 mg kg−1) administered subcutaneously. Two days following injection, mice were anaesthetised with pentobarbitone (60 mg kg−1, i.p.) and transcardially perfused with heparinised saline at 40°C, then 4% paraformaldehyde in 0.1 m PBS (PFA-PBS) at 4°C. The stomach was then removed and cryoprotected with 30% sucrose for a minimum of 18 h. The stomach was then frozen and 10 μm transverse sections cut. Permanent visualisation of WGA-HRP was achieved using tyramide signal amplification (TSA). Briefly, the tissue sections were rinsed in TNT buffer (0.05% Tween 20, 0.15 m NaCl, and 0.1 m Tris-HCl, pH 7.5), blocked for 30 min in TNB is 0.1 m Tris-HCl, pH 7.5; 0.15 m NaCl; 0.5% blocking reagent (provided with the Perkin-Elmer TSA biotin reagent kit), and then incubated for 20 min with tyramide–biotin, diluted 1:50 in amplification diluent. The tissue was then rinsed in TNT buffer and reacted with streptavidin conjugated to Alexa Fluor 647 (Invitrogen) for 2 h at room temperature. Following ghrelin immunohistochemistry (below) sections were coverslipped using ProLong antifade mounting media (Invitrogen).
Immunohistochemistry
Immunoreactivity for ghrelin was detected in anterogradely traced stomach sections using a chicken polyclonal antibody to ghrelin (Abcam). Primary antibody was visualised using goat anti-chicken secondary antibody conjugated to Alexa Fluor 488 (Invitrogen). Briefly, sections were air dried at room temperature and rinsed in PBS + 0.2% Triton X-100 (Sigma-Aldrich; PBS-T, pH 7.4) to facilitate antibody penetration. Primary antibody was diluted 1:1000 in PBS-T and incubated at 4°C overnight. Unbound antibody was then removed with PBS-T washes and sections were incubated for 1 h at room temperature with secondary antibody (1:200 in PBS-T). The sections were given final PBS-T washes, drained and mounted with ProLong antifade. Slides where the primary antibody was omitted showed no labelling and served as negative controls.
Visualisation
Slide sections were visualised using an epifluorescence microscope (BX-51, Olympus) equipped with filters for AF647 and AF488, with images acquired by a Cool-Snapfx monochrome digital camera (Roper Scientific). Individual fluorochromes were pseudo-coloured using Olympus imaging analySis software; however, the luminance of images was not adjusted.
Results
Short-term restriction of food intake reduces mechanosensitivity of vagal afferents
The effect of mechanical stimulation on gastric and oesophageal vagal afferents from control and fasted mice is illustrated in Fig. 1. The mechanosensitivity of mucosal receptors was similar in both groups of mice (Fig. 1Aa and b). In contrast, the responses of gastric tension receptors from fasted mice were significantly smaller than those in control mice (P < 0.0001: diet effect, two-way ANOVA; Fig. 1Ba). Oesophageal tension receptors were more variably affected, and although there appears to be a similar reduction in the responses of oesophageal tension receptors from fasted mice the difference in response did not differ significantly from those in control mice (Fig. 1Bb: P = 0.26: diet effect, two-way ANOVA).
Figure 1. The effect of short-term restriction in food intake on the response of gastric and oesophageal mechanosensitive vagal afferents to mechanical stimulation.

Stimulus–response functions of mucosal (A) and tension-sensitive (B) gastric (a) and oesophageal (b) vagal afferents from mice fed ad libitum (•) and mice fasted for 14 h (○). ***P < 0.001 compared with fed mice (diet effect, two-way ANOVA).
Effects of long-term alterations in diet
Mice on the HFD increased in weight by 6.57 ± 0.3 g (N = 51) over the 12-week period that they were on the diet (N = 51) and gained significantly more weight (P < 0.001; unpaired t test) than SLD mice who gained 5.22 ± 0.12 g (N = 52). This is likely to be due to the significant increase (P < 0.0001; unpaired t test) in energy intake, over the 12 week period, of the HFD mice (4798.97 ± 149.49 MJ; N = 11) compared to the SLD mice (3434.82 ± 99.72 MJ; N = 12) in accord with previous reports (Gardiner et al. 2010). The gonadal fat pad weight was significantly heavier (P < 0.01; unpaired t test) in HFD mice (0.55 ± 0.07 g; N = 17) compared to SLD mice (0.32 ± 0.03 g; N = 16). Blood glucose and insulin levels were not significantly different between SLD (7.34 ± 0.64 mmol l−1 and 0.41 ± 0.08 pmol l−1, respectively; N = 11) and HFD (5.96 ± 0.48 mmol l−1 and 0.66 ± 0.15 pmol l−1, respectively; N = 11) indicating that the HFD group did not develop diabetes over the 12 week diet period.
The effect of mechanical stimulation on gastric and oesophageal vagal afferents from both groups of mice is illustrated in Fig. 2. The mechanosensitivity of mucosal receptors was not significantly different between SLD and HFD mice (Fig. 2Aa and b). However, the response of gastric and oesophageal tension receptors of mice fed a HFD was significantly less than the responses of mice fed a SLD (P < 0.001: diet effect, two-way ANOVA; Fig. 2Ba and b). Thus the effect of a HFD on the mechanosensitivity of gastro-oesophageal tension and mucosal receptors appears similar to the effect of fasting (Fig. 1).
Figure 2. The effect of a high fat diet on the response of gastric and oesophageal mechanosensitive vagal afferents to mechanical stimulation.
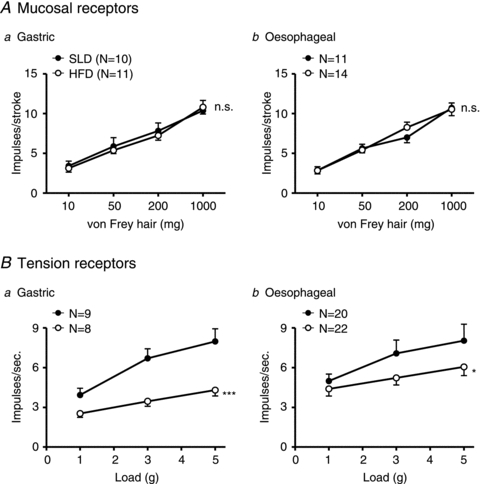
Stimulus–response functions of mucosal (A) and tension-sensitive (B) gastric (a) and oesophageal (b) vagal afferents from mice fed a standard laboratory diet (SLD; •) and mice fed a high fat diet (HFD; ○). *P < 0.05, ***P < 0.001 compared with mice fed a standard laboratory diet (diet effect, two-way ANOVA).
Anatomy of vagal afferent endings and ghrelin-containing cells in the gastric mucosa
We determined the anatomical relationship of ghrelin-immunopositive cells in the gastric mucosa with identified vagal afferent endings. Ghrelin-like immunoreactivity was restricted to isolated cells at the base of submucosal glands with apical surfaces in the lumen (Fig. 3Ab) which had morphology consistent with enteroendocrine X/A cells. Anterograde tracing of vagal afferents from the nodose ganglion revealed intraepithelial fibres within the mucosal layer of the stomach (Fig. 3Aa), as previously described in the small intestine (Williams et al. 1997). These were closely apposed (within a few micrometres) to ghrelin-containing cells (Fig. 3Ac). Identified vagal endings also surrounded myenteric ganglia, consistent with previously characterised intraganglionic laminar endings (Phillips & Powley, 2000). These endings were located at a distance from ghrelin-containing cells separated by the lamina propria, submucosa, muscularis mucosae and circular smooth muscle (data not shown).
Figure 3. Relationship between vagal afferents and ghrelin-containing cells in the gastric mucosa.
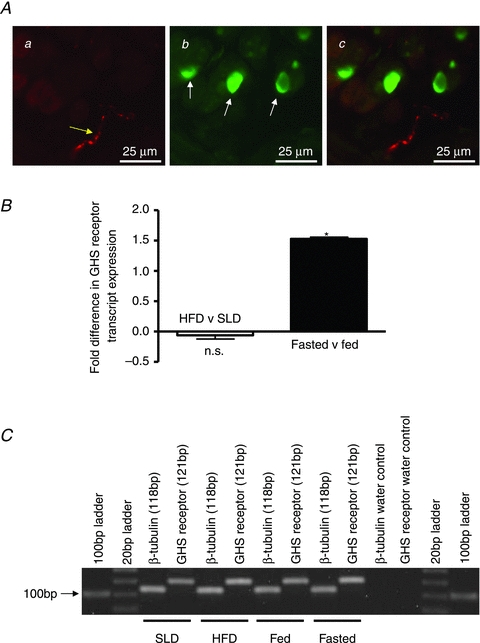
Combined ghrelin immunohistochemistry and anterogradely traced vagal afferent fibres in the mouse stomach (Aa–c). The stomach sections show a cross section through a gland. Aa, anterogradely traced (WGA-HRP) vagal afferent fibre (yellow arrow). Ab, epithelial cells immunopositive for ghrelin in stomach villi (white arrows). Ac, overlay of a and b showing close proximity of ghrelin-containing epithelial cells and anterogradely labelled vagal afferent fibres. B, relative expression of GHS 1 receptor mRNA in nodose ganglia from HFD mice compared with SLD mice (open bar) and fasted mice compared with fed mice (filled bar). The relative expression values were calculated using ΔΔCT (ΔCT HFD –ΔCT SLD or ΔCT fasted –ΔCT fed ad libitum) of ghrelin receptor transcript. The fold difference in transcript expression in HFD nodose ganglia relative to SLD and nodose ganglia from fasted mice compared to mice fed ad libitum was calculated using the formula 2−ΔΔCT. In nodose ganglia from mice fasted for 14 h there is a 1.53-fold increase in transcript compared to fed mice (filled bar, *P < 0.05; Mann–Whitney test). In nodose ganglia from HFD mice there is a small but not significant 0.06-fold decrease in transcript compared to SLD mice (open bar). C, agarose gel electrophoresis confirmed the amplified product sizes.
Ghrelin receptor expression in vagal afferent pathways
QRT-PCR was used to determine the expression of ghrelin receptors in vagal afferent neurons in the nodose ganglia. Quantitative analysis revealed that ghrelin receptor mRNA levels in the nodose ganglia of mice fasted for 14 h were 1.53-fold higher than in fed mice (P < 0.05: Mann-Whitney test; Fig. 3B). There was no significant difference in the expression of ghrelin receptor mRNA detected in the nodose ganglia of HFD mice compared to SLD mice (Fig. 3B). Agarose gel electrophoresis confirmed amplification of housekeeper (β-tubulin) and ghrelin receptors with product sizes of 118 and 121 bp, respectively (Fig. 3C).
Vagal afferent responses to ghrelin are altered by changes in food intake
An overnight fast had major effects on the ghrelin sensitivity of gastric mucosal receptors (Fig. 4). The response to mucosal stroking of gastric mucosal receptors was significantly reduced in fed mice, but only at the highest dose of ghrelin (10 nm; Fig. 4Aa; P < 0.05: ghrelin effect, two-way ANOVA). In fasted mice ghrelin (1–10 nm) significantly reduced the responses of gastric mucosal receptors to mucosal stroking with calibrated von Frey hairs (10–1000 mg; P < 0.01: ghrelin effect, two-way ANOVA; Fig. 4Ab). When the response to a 50 mg von Frey hair at varying concentrations of ghrelin (0–10 nm) was plotted (Fig. 4B) it was evident that diet significantly altered the response to mechanical stimulation in the presence of ghrelin (P < 0.01; diet effect, two-way ANOVA). Therefore gastric mucosal receptor sensitivity to ghrelin was significantly increased following an overnight fast.
Figure 4. Fasting increases the effect of ghrelin on the response of gastric mucosal receptors.
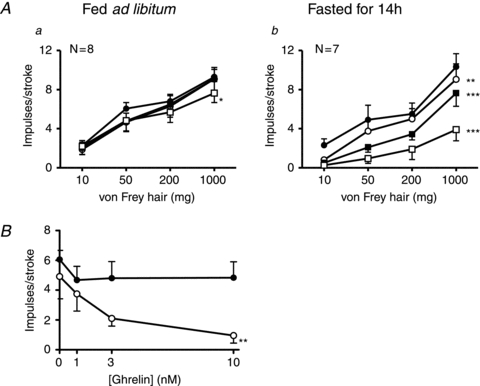
A, the responses of gastric mucosal receptors to mucosal stroking with calibrated von Frey hairs (10–1000 mg) in the absence (•) and presence of ghrelin (1 (○), 3 (▪) and 10 nm (□)). These recordings were taken from fed mice (a) or fasted mice (b) prior to the experiment. *P < 0.05, **P < 0.01, ***P < 0.001 compared with control (•; ghrelin effect, two-way ANOVA). B, the effect of ghrelin on the responses of mucosal receptors to stoking with a 50 mg von Frey hair in mice fed ad libitum (•) and mice fasted (○) for 14 h. Diet significantly enhanced the inhibitory action of ghrelin (**P < 0.01; diet effect, two-way ANOVA).
Ghrelin (3–10 nm) significantly and dose-dependently reduced the response of gastric (Fig. 5Aa and Fig. 5Ba) tension receptors to circular tension (P < 0.05: ghrelin effect, two-way ANOVA; 1–5 g). In fasted mice ghrelin (1–10 nm) also significantly reduced the response of gastric tension receptors (Fig. 5Ab and Bb) to circular tension even at the lowest dose tested of 1 nm (P < 0.001: ghrelin effect, two-way ANOVA). When the response to a 3 g load was plotted against increasing concentrations of ghrelin there was no significant difference between the fed and fasted curves (data not shown). Therefore we found no evidence that a restriction in food intake affected the sensitivity of gastric tension receptors to ghrelin.
Figure 5. The effect of ghrelin on the response of gastric tension receptors.

A, the responses of gastric tension-sensitive receptors to circumferential stretch (1–5 g) in the absence (•) and presence of ghrelin (1 (○), 3 (▪) and 10 nm (□)). These recordings were taken from fed (a) or fasted mice (b). *P < 0.05, **P < 0.01, ***P < 0.001 compared with control (•; ghrelin effect, two-way ANOVA). There was no significant difference in the inhibitory effect of ghrelin between the two diets. B, typical traces of tension receptors from a fed (a) and a fasted (b) mouse.
The effect of longer term changes in food intake on ghrelin's neuromodulatory action on vagal afferent responses to mechanical stimulation is illustrated in Figs 6 and 7. Ghrelin (1–10 nm) did not significantly affect the response of gastric (Fig. 6Aa and Ca) mucosal receptors to mucosal stroking in SLD mice. In contrast, ghrelin significantly reduced the response to mechanical stimulation of gastric (Fig. 6Ab and Cb) mucosal receptors (P < 0.05: ghrelin effect, two-way ANOVA) in HFD mice, thus mimicking responses seen in the fasted state. When the response to stroking with a 50 mg von Frey hair was plotted against varying concentrations of ghrelin (0–10 nm; Fig. 6B) it was evident that diet significantly altered the response to mechanical stimulation in the presence of ghrelin (P < 0.01: diet effect, two-way ANOVA). Therefore gastric mucosal receptor sensitivity to ghrelin was significantly increased after a HFD.
Figure 6. High fat diet increases the effect of ghrelin on the response of gastric (A and C) mucosal receptors.
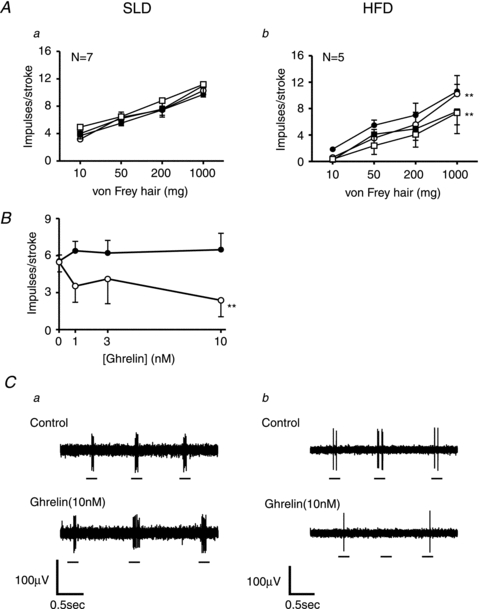
A and C, the responses of gastric mucosal receptors to mucosal stroking with calibrated von Frey hairs (10–1000 mg) in the absence (•) and presence of ghrelin (1 (○), 3 (▪) and 10 nm (□)). These recordings were taken from mice fed a standard laboratory diet (Aa and Ca) or mice fed a high fat diet (Ab and Cb) for 12 weeks prior to the experiment. **P < 0.01 compared with control (•; ghrelin effect, two-way ANOVA). B, the effect of ghrelin on the response of mucosal receptors to stoking with a 50 mg von Frey hair in mice fed a SLD (•) and mice fed a HFD (○). Diet significantly enhanced the inhibitory action of ghrelin (**P < 0.01; diet effect, two-way ANOVA).
Figure 7. The effect of ghrelin on the response of gastric tension receptors.
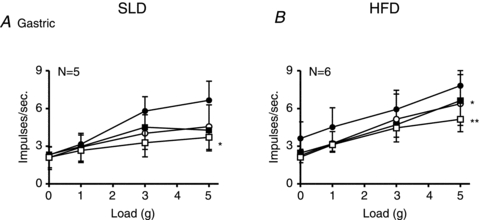
The responses of gastric tension receptors to circumferential stretch (1–5 g) in the absence (•) and presence of ghrelin (1 (○), 3 (▪) and 10 nm (□)). These recordings were taken from mice fed a standard laboratory diet (A) or mice fed a high fat diet (B) for 12 weeks prior to the experiment. *P < 0.05, **P < 0.01 compared with control (•; ghrelin effect, two-way ANOVA). There was no significant difference in the inhibitory effect of ghrelin between the two diets.
Ghrelin significantly reduced the response of gastric tension receptors to circular stretch (1–5 g) in SLD and HFD mice (Fig. 7A and B, respectively; P < 0.05: ghrelin effect, two-way ANOVA). When the response to a 3 g load was plotted against increasing concentrations of ghrelin there was no significant difference between the SLD and HFD curves (data not shown). Therefore we found no evidence that a HFD affected the sensitivity of gastric tension receptors to ghrelin.
Discussion
These data provide the first evidence that signalling of gastric distension by vagal afferents is significantly reduced by both food withdrawal and a high fat diet. Moreover, ghrelin inhibits mucosal vagal afferents that are normally insensitive to it. Thus, not only is the gastric vagal afferent satiety signal reduced by food withdrawal and high fat feeding, but the orexigenic effect of ghrelin is also enhanced.
Short-term restriction of food intake selectively reduced the mechanosensitivity of tension receptors and in particular gastric tension receptors. As these afferents detect distension of the stomach (Berthoud & Neuhuber, 2000; Phillips & Powley, 2000; Berthoud & Morrison, 2008) their reduced sensitivity to stretch may delay satiety signalling, leading to increased food intake of an individual in order to achieve satiation; this would constitute a natural adaptive response after a fast. Importantly we found that in mice chronically fed a high fat diet there was also a reduction in the mechanosensitivity of tension receptors suggesting that the satiety signal would also be attenuated in response to a high fat diet. Such a mechanism may have evolved to maximise assimilation of energy from calorie-rich foods in anticipation of famine. A number of studies have shown that obese humans have increased gastric capacity (Geliebter, 1988; Kim et al. 2001) which results in increased energy intake. This could occur due to a reduction in the gastric distension-stimulated vagal afferent satiety signal. A recent publication has revealed that the sensitivity of jejunal afferents to satiety stimuli is reduced after a HFD (Daly et al. 2011). Further, the major mechanism for this decrease in sensitivity was due to a reduction in excitability of the neuronal cell membrane (Daly et al. 2011). This reduced excitability of all vagal afferent cell membranes in HFD mice may explain the reduced mechanosensitivity of oesophageal afferents observed in the present study.
The mechanisms leading to reduced mechanosensitivity of vagal afferents are currently unknown both in terms of its precise causes and how it is manifested at the molecular level. It has been shown that fasting alters the neurochemical phenotype of vagal afferent neurons that project to the gastrointestinal tract in terms of the G-protein-coupled receptors and peptide neurotransmitters they express (Burdyga et al. 2004, 2006a, 2008; de Lartigue et al. 2007). High fat diets have also been reported to modify vagal afferent transcript expression of specific G-protein-coupled receptors and growth factors (Nefti et al. 2009; Paulino et al. 2009; Zeeni et al. 2009). Together with changes in the levels of plasma satiety hormones these adaptations are likely to profoundly alter vagal satiety signals during a high fat diet. For example, the plasma levels of leptin are significantly elevated in mice fed a high fat diet (Ahren et al. 1997) and this adipokine may directly influence vagal afferent mechanosensitivity while also acting to modulate their responsiveness to ghrelin. Our data now adds new knowledge that diet induces changes in the fundamental mechanosensory properties of gastric vagal afferents. Now that we have demonstrated that reduced mechanosensitivity is a robust outcome in response to a HFD, we are in a good position to investigate the mechanism of this change, which may reveal more empirical targets for the treatment of obesity.
In the present study we confirmed our earlier findings (Page et al. 2007b) that ghrelin selectively and dose-dependently reduces the response of tension receptors in normal control mice fed ad libitum on a SLD. This effect of ghrelin on tension receptors was maintained after food withdrawal and after a HFD. The effect of ghrelin was not selective for gastric vagal afferents as it also reduced the mechanosensitivity of oesophageal tension-sensitive afferents which are distant from the site of ghrelin release (data not shown), suggesting this would be a systemic action in vivo. The concentration of ghrelin used in our in vitro organ bath preparation is comparable with the concentration of ghrelin in mouse plasma which ranges from 1 to 3 nm depending on the feeding state (Moesgaard et al. 2004). Plasma ghrelin levels are significantly higher after withdrawal of food for 18 h and significantly reduced in mice fed a HFD (Moesgaard et al. 2004). Therefore, based on plasma levels and the fact that sensitivity of gastric vagal tension receptors to ghrelin did not significantly change in both conditions, it could be predicted that ghrelin inhibition of tension receptors would be increased after food withdrawal, but decreased in mice fed a high fat diet.
The response of mucosal receptors to mucosal stroking was unaffected by both a short-term restriction in food intake and a high fat diet. However, where these particular vagal afferent fibres become relevant is their innervation of the gastric mucosa, where they are also likely to detect locally released hormones such as ghrelin. Indeed, we observed that vagal afferents innervating the gastric mucosa are in close apposition to ghrelin-containing epithelial cells. However, in agreement with our earlier observations (Page et al. 2007b), mucosal receptors were unaffected by ghrelin in control mice other than at the highest dose used. After food withdrawal ghrelin potently reduced the response of gastric vagal mucosal receptors to mucosal stroking. This effect was seen also after a high fat diet, so mucosal receptors acquire ghrelin sensitivity. Gastric mucosal receptors are considered to be important in detecting particle size, which reduces gastric emptying and food intake (McIntyre et al. 1997; Tuleu et al. 1999), so ghrelin could act to reduce this signal in fasting and obesity. In fasted healthy volunteers, ghrelin administration has been shown to increase the rate of gastric emptying in addition to elevating hunger ratings (Falken et al. 2010); this may be a consequence of the decreased mechanosensitivity of gastric mucosal receptors by ghrelin.
The expression of the ghrelin receptor transcript in the nodose ganglia was significantly increased in mice that were fasted for 14 h. An increase in ghrelin receptor expression may explain the increase in effect of ghrelin on gastric mucosal receptors. Correspondingly it has been established that GHS receptor-containing vagal neurons project to the stomach (Sakata et al. 2003) although reports vary on changes in ghrelin receptor expression after food withdrawal. One group has reported that the transcript levels of ghrelin receptor in the rat nodose ganglia are unaltered (Burdyga et al. 2006b) while another group has shown a doubling of transcript expression in fasted compared to fed rats (Sato et al. 2007). Previously we reported that ghrelin reduced the mechanosensitivity of mucosal receptors in the ferret and concluded that this was a possible species difference (Page et al. 2007b). The difference is most likely due to the fact that ferrets were fasted prior to experimentation. The expression of ghrelin receptor in the nodose ganglia of mice fed a HFD was not significantly different from mice fed a SLD, and therefore the increased effect of ghrelin on gastric mucosal receptors cannot be explained by an increase in ghrelin receptor expression unlike the effect of fasting. It is possible that in the longer term, changes occur in the coupling of ghrelin receptors to specific intracellular pathways, rather than in absolute levels of ghrelin receptor expression.
In conclusion, we have established that the basic mechanosensitivity of gastro-oesophageal vagal afferents, particularly gastric afferents, is significantly reduced by both restriction and excess of energy intake. A consequence of this decreased mechanosensitivity would be an undesirable dampening of the satiety signal in obesity. In addition, the inhibitory effect of ghrelin on vagal afferents becomes less selective after food withdrawal and a high fat diet and is therefore amplified. The additional inhibitory effect of ghrelin on gastric mucosal receptors would reinforce the inhibitory effect of ghrelin on tension receptors and enhance its orexigenic effect. These data indicate the plasticity in the mechanism of action of ghrelin under different feeding states, and the complex role of gastric mechanisms in food intake regulation.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (NHMRC) project grant no. 565186 and NHMRC Principal Research Fellowship to L. Ashley Blackshaw. We would also like to thank Kylie Lange for her statistical expertise. Kylie is supported by the Centre for Clinical Excellence in Nutritional Physiology, Interventions and Outcomes funded by the NHMRC.
Glossary
- GHS
growth hormone secretagogue
- HFD
high fat diet
- SLD
standard laboratory diet
- WGA-HRP
wheat-germ agglutinin-conjugated horseradish peroxidase
Author contributions
A.J.P. performed electrophysiology experiments, quantitative RT-PCR, analysis and wrote the manuscript. S.K., A.J.P., G.A.W. and L.A.B. contributed to conception and design of the experiments and interpretation of data. S.K. performed the majority of the electrophysiological experiments. H.L. and L.K.P. monitored mice weight, food intake and well being. T.A.O'D. performed electrophysiological experiments. N.J.I. performed the immunohistochemistry experiments. R.L.Y. performed the anterograde tracing surgery. All authors contributed to revising the article and evaluating it critically for important intellectual content. All authors approved the final version of the manuscript.
References
- Ahren B, Mansson S, Gingerich RL, Havel PJ. Regulation of plasma leptin in mice: influence of age, high-fat diet, and fasting. Am J Physiol Regul Integr Comp Physiol. 1997;273:R113–R120. doi: 10.1152/ajpregu.1997.273.1.R113. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol. 2002;2:650–656. doi: 10.1016/s1471-4892(02)00227-8. [DOI] [PubMed] [Google Scholar]
- Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–11060. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Bray GA. Afferent signals regulating food intake. Proc Nutr Soc. 2000;59:373–384. doi: 10.1017/s0029665100000422. [DOI] [PubMed] [Google Scholar]
- Burdyga G, de Lartigue G, Raybould HE, Morris R, Dimaline R, Varro A, Thompson DG, Dockray GJ. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J Neurosci. 2008;28:11583–11592. doi: 10.1523/JNEUROSCI.2493-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Lal S, Varro A, Dimaline R, Thompson DG, Dockray GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci. 2004;24:2708–2715. doi: 10.1523/JNEUROSCI.5404-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurones. Neuroscience. 2006a;137:1405–1415. doi: 10.1016/j.neuroscience.2005.10.057. [DOI] [PubMed] [Google Scholar]
- Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006b;290:G1289–G1297. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly DM, Park SJ, Valinsky WC, Beyak MJ. Impaired intestinal afferent nerve satiety signalling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol. 2011;589:2857–2870. doi: 10.1113/jphysiol.2010.204594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, Kangawa K, Nakazato M. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci. 2007;27:2876–2882. doi: 10.1523/JNEUROSCI.5508-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch JA. Dietary control and the stomach. Prog Neurobiol. 1983;20:313–332. doi: 10.1016/0301-0082(83)90007-2. [DOI] [PubMed] [Google Scholar]
- Dockray GJ, Burdyga G. Plasticity in vagal afferent neurones during feeding and fasting: mechanisms and significance. Acta Physiol (Oxf) 2011;201:313–321. doi: 10.1111/j.1748-1716.2010.02219.x. [DOI] [PubMed] [Google Scholar]
- Druce MR, Neary NM, Small CJ, Milton J, Monteiro M, Patterson M, Ghatei MA, Bloom SR. Subcutaneous administration of ghrelin stimulates energy intake in healthy lean human volunteers. Int J Obes (Lond) 2006;30:293–296. doi: 10.1038/sj.ijo.0803158. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falken Y, Hellstrom PM, Sanger GJ, Dewit O, Dukes G, Gryback P, Holst JJ, Naslund E. Actions of prolonged ghrelin infusion on gastrointestinal transit and glucose homeostasis in humans. Neurogastroenterol Motil. 2010;22:e192–200. doi: 10.1111/j.1365-2982.2009.01463.x. [DOI] [PubMed] [Google Scholar]
- Gardiner JV, Campbell D, Patterson M, Kent A, Ghatei MA, Bloom SR, Bewick GA. The hyperphagic effect of ghrelin is inhibited in mice by a diet high in fat. Gastroenterology. 2010;138:2468–2476. doi: 10.1053/j.gastro.2010.02.012. 2476.e1. [DOI] [PubMed] [Google Scholar]
- Geliebter A. Gastric distension and gastric capacity in relation to food intake in humans. Physiol Behav. 1988;44:665–668. doi: 10.1016/0031-9384(88)90333-2. [DOI] [PubMed] [Google Scholar]
- Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- Hughes PA, Brierley SM, Young RL, Blackshaw LA. Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J Comp Neurol. 2007;500:863–875. doi: 10.1002/cne.21204. [DOI] [PubMed] [Google Scholar]
- Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955;128:593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Camilleri M, Murray JA, Stephens DA, Levine JA, Burton DD. Is there a role for gastric accommodation and satiety in asymptomatic obese people? Obes Res. 2001;9:655–661. doi: 10.1038/oby.2001.89. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- McIntyre A, Vincent RM, Perkins AC, Spiller RC. Effect of bran, ispaghula, and inert plastic particles on gastric emptying and small bowel transit in humans: the role of physical factors. Gut. 1997;40:223–227. doi: 10.1136/gut.40.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moesgaard SG, Ahren B, Carr RD, Gram DX, Brand CL, Sundler F. Effects of high-fat feeding and fasting on ghrelin expression in the mouse stomach. Regul Pept. 2004;120:261–267. doi: 10.1016/j.regpep.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Nefti W, Chaumontet C, Fromentin G, Tome D, Darcel N. A high-fat diet attenuates the central response to within-meal satiation signals and modifies the receptor expression of vagal afferents in mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1681–R1686. doi: 10.1152/ajpregu.90733.2008. [DOI] [PubMed] [Google Scholar]
- Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol. 1998;512:907–916. doi: 10.1111/j.1469-7793.1998.907bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Hughes PA, Blackshaw LA. Acid sensing ion channels 2 and 3 are required for inhibition of visceral nociceptors by benzamil. Pain. 2007a;133:150–160. doi: 10.1016/j.pain.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Page AJ, Martin CM, Blackshaw LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol. 2002;87:2095–2103. doi: 10.1152/jn.00785.2001. [DOI] [PubMed] [Google Scholar]
- Page AJ, O'Donnell TA, Blackshaw LA. Inhibition of mechanosensitivity in visceral primary afferents by GABAB receptors involves calcium and potassium channels. Neuroscience. 2006;137:627–636. doi: 10.1016/j.neuroscience.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Page AJ, Slattery JA, Milte C, Laker R, O'Donnell T, Dorian C, Brierley SM, Blackshaw LA. Ghrelin selectively reduces mechanosensitivity of upper gastrointestinal vagal afferents. Am J Physiol Gastrointest Liver Physiol. 2007b;292:G1376–G1384. doi: 10.1152/ajpgi.00536.2006. [DOI] [PubMed] [Google Scholar]
- Page AJ, Slattery JA, O'Donnell TA, Cooper NJ, Young RL, Blackshaw LA. Modulation of gastro-oesophageal vagal afferents by galanin in mouse and ferret. J Physiol. 2005;563:809–819. doi: 10.1113/jphysiol.2004.075291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulino G, Barbier de la Serre C, Knotts TA, Oort PJ, Newman JW, Adams SH, Raybould HE. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab. 2009;296:E898–E903. doi: 10.1152/ajpendo.90796.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev. 2000;34:1–26. doi: 10.1016/s0165-0173(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Sakata I, Yamazaki M, Inoue K, Hayashi Y, Kangawa K, Sakai T. Growth hormone secretagogue receptor expression in the cells of the stomach-projected afferent nerve in the rat nodose ganglion. Neurosci Lett. 2003;342:183–186. doi: 10.1016/s0304-3940(03)00294-5. [DOI] [PubMed] [Google Scholar]
- Sato M, Nakahara K, Miyazato M, Kangawa K, Murakami N. Regulation of GH secretagogue receptor gene expression in the rat nodose ganglion. J Endocrinol. 2007;194:41–46. doi: 10.1677/JOE-06-0078. [DOI] [PubMed] [Google Scholar]
- Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau JP, Attoub S, Lehy T, Henin D, Mignon M, Lewin MJ. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47:178–183. doi: 10.1136/gut.47.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Tuleu C, Andrieux C, Boy P, Chaumeil JC. Gastrointestinal transit of pellets in rats: effect of size and density. Int J Pharm. 1999;180:123–131. doi: 10.1016/s0378-5173(98)00400-1. [DOI] [PubMed] [Google Scholar]
- Williams RM, Berthoud HR, Stead RH. Vagal afferent nerve fibres contact mast cells in rat small intestinal mucosa. Neuroimmunomodulation. 1997;4:266–270. doi: 10.1159/000097346. [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001a;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Abbott CR, Dhillo WS, Seal LJ, Cohen MA, Batterham RL, Taheri S, Stanley SA, Ghatei MA, Bloom SR. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001b;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- Zeeni N, Chaumontet C, Moyse E, Fromentin G, Tardivel C, Tome D, Jean A, Darcel N. A positive change in energy balance modulates TrkB expression in the hypothalamus and nodose ganglia of rats. Brain Res. 2009;1289:49–55. doi: 10.1016/j.brainres.2009.06.076. [DOI] [PubMed] [Google Scholar]


