Abstract
Leptin is a hormone that crosses the blood-brain barrier and regulates numerous CNS functions. The hippocampus in particular is an important site for leptin action. Indeed, leptin markedly influences excitatory synaptic transmission and synaptic plasticity in this brain region. Recent studies indicate that leptin modulation of hippocampal excitatory synaptic transmission is age-dependent however the cellular basis for this is unclear. Here we show that early in development leptin evokes a transient (P11-18) or persistent (P5-8) depression of synaptic transmission, whereas leptin evokes a long lasting increase (LTP) in synaptic strength in adulthood. The synaptic depressions induced by leptin required activation of NMDA receptor GluN2B subunits and the ERK signalling cascade. Conversely, leptin-induced LTP in adult was mediated by GluN2A subunits and involved PI 3-kinase dependent signalling. In addition, low-frequency stimulus (LFS)-evoked LTD occluded the persistent effects of leptin at P5-8 and vice versa. Similarly, synaptically-induced LTP occluded the persistent increase in synaptic transmission induced by leptin, indicating that similar expression mechanisms underlie leptin-induced LTD and LFS-induced LTD at P5-8, and leptin-induced LTP and HFS-induced LTP in adult. These findings have important implications for the role of leptin in hippocampal synaptic function during early neuronal development and in aging.
Keywords: Leptin, synaptic transmission, NMDA receptor, synaptic plasticity, PI 3-kinase, MAPK
Introduction
Synaptic plasticity is one of the most widely studied phenomena of the mammalian central nervous system (CNS). There is compelling evidence that activity-dependent forms of synaptic plasticity such as hippocampal long-term potentiation (LTP) and long-term depression (LTD) are cellular correlates for information storage within the CNS (Bliss and Collingridge, 1993). However, recent studies have focused on the modulation of synaptic strength by various endogenous hormonal systems, such as insulin (Huang et al., 2004; Man et al., 2000; Man et al., 2003; van der Heide et al., 2005), estrogens (McEwen, 2002) and leptin (Harvey et al., 2006). Leptin is a 167 kDa protein that circulates in the plasma at concentrations relative to body fat. Extensive research has identified a role for leptin in regulating satiety and energy homeostasis via its hypothalamic actions (Spiegelman and Flier, 2001). However the effects of leptin are not restricted to the hypothalamus. Indeed, recent evidence indicates that leptin plays an important role in modulating many neuronal functions including hippocampal synaptic plasticity and glutamate receptor trafficking (Durakoglugil et al., 2005; Irving et al., 2006; Moult et al., 2010; Moult et al., 2009; Shanley et al., 2001). Moreover, it has been shown that leptin-insensitive rodents display impaired hippocampal LTP, LTD and spatial memory (Li et al., 2002), and administration of leptin into rodent hippocampus improves memory processing (Wayner et al., 2004). In addition, leptin has the ability to facilitate hippocampal LTP (Shanley et al., 2001) and promote the induction of a novel form of de novo hippocampal LTD (Durakoglugil et al., 2005). Recent evidence indicates that leptin promotes an increase in the synaptic expression of GluA2-lacking AMPA receptors in adult hippocampal slices resulting in a persistent increase in the efficacy of excitatory synaptic transmission (Moult et al., 2010). In contrast to its effects in adult, leptin evokes a transient depression of excitatory synaptic transmission in juvenile hippocampus (Shanley et al., 2001; Xu et al., 2008), suggesting that leptin modulates excitatory synaptic transmission in an age-dependent manner. In order to determine the cellular basis for this age-dependence, we have systematically examined the effects of leptin on excitatory synaptic transmission in hippocampal slices at four distinct ages. Here we show that the direction of synaptic modulation by leptin is age-dependent such that at early stages of postnatal development leptin results in either a transient (P11-18) or persistent (P5-8) depression of synaptic transmission, whereas in adult hippocampus (12-16 week and 12-14 month) leptin evokes a long lasting increase in excitatory synaptic strength. Although there are distinct age-dependent differences in the polarity and duration of synaptic modulation induced by leptin, the effects of leptin required NMDA receptor activation at all ages examined. Moreover, the ability of leptin to alter excitatory synaptic transmission displayed subunit-specific NMDA receptor dependence such that at early stages of postnatal development the effects of leptin required the activation of GluN2B NMDA subunits, whereas in adult hippocampus the leptin-driven persistent increase in synaptic strength was mediated by GluN2A subunits. In addition, divergent signalling pathways were found to mediate the effects of leptin at different ages. Thus, both the long lasting (P5-8) and transient (P11-18) synaptic depressions induced by leptin involved the activation of the ERK signalling cascade, whereas the leptin-driven persistent increase in synaptic strength in adult was mediated by a PI 3-kinase-dependent mechanism. Moreover, occlusion studies show that synaptically induced LTP occludes the persistent synaptic potentiation induced by leptin in adult hippocampus and vice versa, suggesting that HFS-induced LTP and leptin-induced LTP share similar expression mechanisms. Similarly, synaptically induced LTD occludes the persistent depression induced by leptin early in developement (P5-8) and vice versa, indicating that analogous expression mechanisms also underlie leptin-induced LTD and LFS-induced LTD. These findings have important implications for the role of leptin in regulating hippocampal synaptic strength.
Materials & Methods
Hippocampal slices (350 μm) were prepared from P5-8, P11-18, 12-16 week or 12-14 month old, male Sprague-Dawley rats. Animals were killed by cervical dislocation (P5-8, P11-18 and 12-16 week) or deep anaesthesia with isofluorane followed by decapitation (12-14 month) according to UK (Scientific Procedures Act 1986) legislation. Brains were rapidly removed and placed in ice-cold artificial cerebrospinal fluid (aCSF; bubbled with 95% O2 and 5% CO2) containing (in mM): 124 NaCl, 3 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgSO4, and 10 D-glucose. Once prepared, parasagittal slices (350μm) were allowed to recover at room temperature in oxygenated aCSF for at least 1 hr before use. Slices were transferred to a submerged chamber maintained at 30-31°C and perfused with aCSF containing the GABAA receptor antagonist, picrotoxin (50 μM), at a rate of 2-3 mlmin−1. Blind whole-cell patch clamp recordings from stratum pyramidale of area CA1 were obtained using electrodes (4–7 MΩ) containing (in mM): 130 Cs+ methanesulphonate, 5 NaCl, 1 CaCl2, 5 HEPES, 1 EGTA, 5 Mg-ATP, 0.5 Na-GTP, and 5 QX-314, pH 7.3. Cells were voltage clamped at –60 mV. The Schaffer collateral-commissural pathway was stimulated at 0.033 Hz, using a stimulus intensity that evoked peak EPSC amplitudes of ~50% of the maximum. Synaptic currents were low pass filtered at 2 kHz and digitally sampled at 10 kHz. The mean series resistance for all cells was 24 ± 4 MΩ (n = 308).
To isolate the NMDA receptor-mediated component of the EPSC, NBQX (5 μM) and picrotoxin (50 μM) were included in the bath solution and the cell was voltage clamped at −30 mV. The weighted decay time constant of NMDA receptor-mediated EPSCs was measured offline, from 2 min averages (4 trials per average). The weighted time constant of the synaptic current was calculated by fitting the decay of the synaptic current with 2 exponentials (A1 e−t/τ1 + A2 e−t/τ2) as a bi-exponential curve provides a better fit than a single exponential. The value of the weighted time constant (τw) was calculated using the expression:
The values for τ1 and τ2 were consistent across all cells recorded within each age group.
The paired pulse ratio (PPR) was calculated as ratio of amplitude of second EPSC to first EPSC. To ensure measurement of second EPSC was not contaminated, the residual component of first EPSC was removed (Moult et al., 2010). The coefficient of variation (CV) was calculated as before (Moult et al., 2010). Briefly, the mean and standard deviation (SD) were calculated for EPSC amplitudes recorded during successive 5 min epochs (SDEPSC and meanEPSC). The SD of background noise was calculated for each 5 min epoch using a period immediately before electrical stimulation (SDnoise). The CV for each 5 min epoch was calculated as (SDEPSC – SDnoise) / meanEPSC.
Using the same recording setup as above standard extracellular recording techniques were also used to monitor evoked field responses from stratum radiatum. The slope of the evoked field excitatory postsynaptic potentials (fEPSPs) was measured and expressed relative to the pre-conditioning baseline. Baseline responses were set to approximately 50% of the maximal response. Data were monitored online and analyzed off-line using the WINltp program (Anderson and Collingridge, 2007). The degree of long term potentiation or depression was calculated 30–35 min after addition of leptin and expressed as a percentage of baseline ± SEM. In order to quantify the magnitude of transient effects, the peak effect over a 5 min period was calculated and expressed as a percentage of baseline ± SEM. All data are expressed as means ± SEM, and statistical analyses were performed using paired t test (two-tailed; 95% confidence interval) for comparison of means within subject or two-way ANOVA with Tukey post hoc test for comparisons between multiple groups . P < 0.05 was considered significant.
Results
Leptin modulates excitatory synaptic transmission in an age-dependent manner
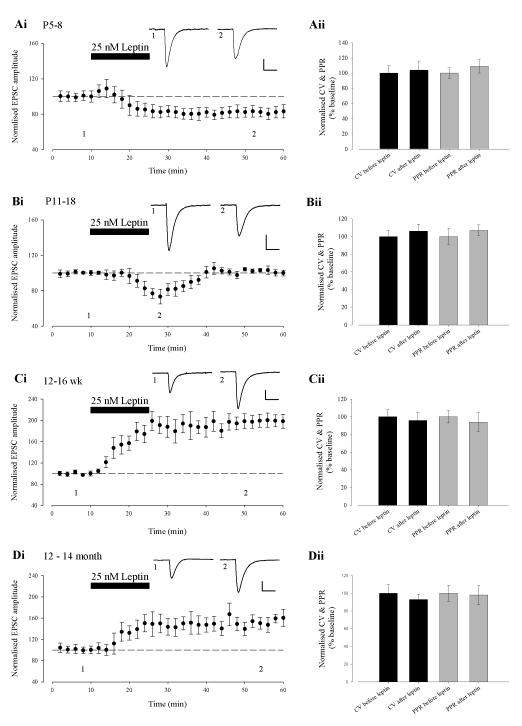
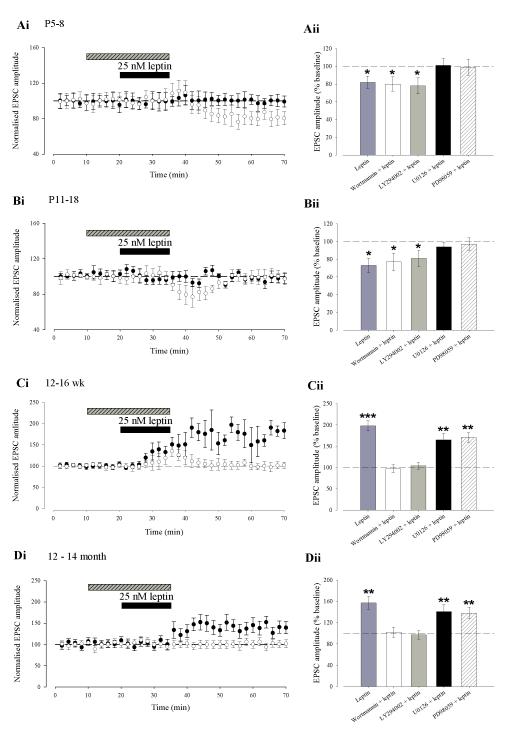
We have recently shown that leptin results in a persistent increase in excitatory synaptic transmission in adult hippocampal slices (12-16 week old; Moult et al., 2010). In contrast, we and others have demonstrated that leptin transiently depresses excitatory synaptic transmission in juvenile hippocampus (Shanley et al., 2001; Xu et al 2008). In order to determine the cellular basis for the bi-directional modulation of synaptic transmission by leptin, we examined the effects of leptin (25nM) in hippocampal slices at four different ages; P5-8, P11-18, 12-16 week and 12-14 month old (Fig. 1A-D). In agreement with previous studies (Shanley et al, 2001; Xu et al, 2008), application of leptin (25nM; 15 min) to slices at P11-18, resulted in a rapid depression in EPSC amplitude (to 73 ± 8 % of baseline; n=15; P < 0.05; Fig 1Bi), that returned to baseline levels on washout of leptin. In contrast, at P5-8, application of leptin (25nM; 15 min) induced a robust decrease in EPSC amplitude (to 82 ± 7 % of baseline; n=14; P < 0.05; Fig 1Ai) that persisted on leptin washout for the duration of recordings (up to 90 min). In accordance with our previous studies (Moult et al, 2010), addition of leptin (25nM; 15 min) to adult hippocampal slices (12-16 week) resulted in a rapid increase in EPSC amplitude (to 198 ± 12% of baseline; n=13; P < 0.001; Fig 1Ci) that was sustained for the duration of recordings. Similarly, addition of leptin (25 nM; 15 min) induced a long-lasting increase in EPSC amplitude (to 157 ± 12% of baseline; n=14; P < 0.01; Fig 1Di) in slices from 12-14 month old animals, however, the magnitude of enhancement at this age was significantly less than that observed at 12-16 weeks (P<0.05). Thus our data indicate that leptin bi-directionally regulates synaptic transmission in an age-dependent manner.
Figure 1. The effects of leptin on excitatory synaptic transmission vary with age.
Ai-Di, Plots of pooled and normalized data illustrating the effects of leptin (25nM; 15 min) on excitatory synaptic transmission evoked in hippocampal slices at P5-8 (A), P11-18 (B), 12-16 weeks (C) and 12-14 months of age (D), respectively. In this and subsequent figures, each point is the average of four consecutive excitatory postsynaptic currents (EPSCs). Representative synaptic records for each experiment are shown above each plot and for the time points indicated. Aii-Dii Histograms of pooled data illustrating the corresponding normalised PPR and CV data obtained before and after addition of leptin in the experiments in Ai-Di. The effects of leptin on synaptic transmission were not accompanied by any significant change in PPR or CV at all ages examined.
Previously, we have shown high levels of leptin receptor expression at both pre- and post-synaptic sites on hippocampal neurons (Shanley et al., 2002). Therefore leptin receptors located at either locus could mediate the age-dependent effects on excitatory synaptic function. In order to identify the locus of leptin’s effects, we analysed the paired pulse ratio (PPR; two pulses delivered at an interval of 50ms) and coefficient of variation (CV) in the above experiments as changes in these parameters classically reflect alterations in release probability (Kullmann, 1994). The ability of leptin to induce the long-lasting depression of synaptic transmission at P5-8 and the transient synaptic depression at P11-18 were not accompanied by any change in PPR or CV (n=6 and P>0.5 for each; Fig 1Aii and Fig 1Bii). In addition, the leptin-induced persistent increase in synaptic strength was not associated with any significant change in PPR or CV in slices from either 12-16 week and 12-14 month animals (n=6 and P>0.05 for each; Fig 1Cii and Fig 1Dii, respectively) which is in agreement with our previous studies (Moult et al., 2010). Thus these data indicate that the ability of leptin to modulate excitatory synaptic transmission in the developing and adult hippocampus involves a postsynaptic expression mechanism.
Regulation of synaptic transmission by leptin is dependent upon NMDA receptor activation
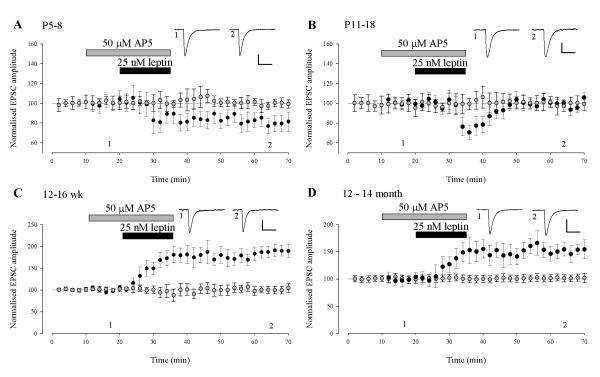
We have shown that the leptin-driven increase in synaptic transmission in adult slices (12-16 week) is dependent upon the synaptic activation of NMDA receptors (Moult et al., 2010). In addition, the ability of leptin to facilitate hippocampal LTP (Shanley et al., 2001), reverse established LTP (Moult et al., 2009) and induce a novel form of de novo LTD (Durakoglugil et al., 2005) are all NMDA receptor-dependent processes. Thus, we examined the role of NMDA receptors in mediating the effects of leptin on synaptic function across the different ages. In the presence of the competitive NMDA receptor antagonist D-AP5 (50 μM), the ability of leptin to modulate excitatory synaptic transmission was inhibited at all ages examined (Fig 2A-D). Thus at P5-8 and P11-18 application of leptin failed to reduce EPSC amplitude (99 ± 4% of baseline; n=6; P > 0.05 at P5-8 and 100 ± 6% of baseline; n=6; P>0.05 at P11-18) in slices exposed to D-AP5. Similarly, leptin failed to enhance synaptic transmission after blockade of NMDA receptors at 12-16 week (99 ± 9% of baseline; n=6; P>0.05) and 12-14 month (102 ± 6% of baseline, n=6; P>0.05), respectively. These data indicate that the ability of leptin to bi-directionally regulate hippocampal excitatory synaptic transmission requires the activation of NMDA receptors.
Figure 2. NMDA receptor activation underlies the age-dependent effects of leptin on synaptic function.
A-D, Plots of pooled and normalized data illustrating the effects of leptin (25nM; 15 min) on EPSC amplitude in the absence (filled circle) and presence of D-AP5 (50 μM; open circle) at P5-8 (A), P11-18 (B), 12-16 weeks (C) and 12-14 months of age (D), respectively. Representative synaptic records are shown above each plot. The ability of leptin to alter excitatory synaptic transmission requires the activation of NMDA receptors at all ages.
Bi-directional regulation of synaptic transmission by leptin requires distinct NMDA receptor subunits
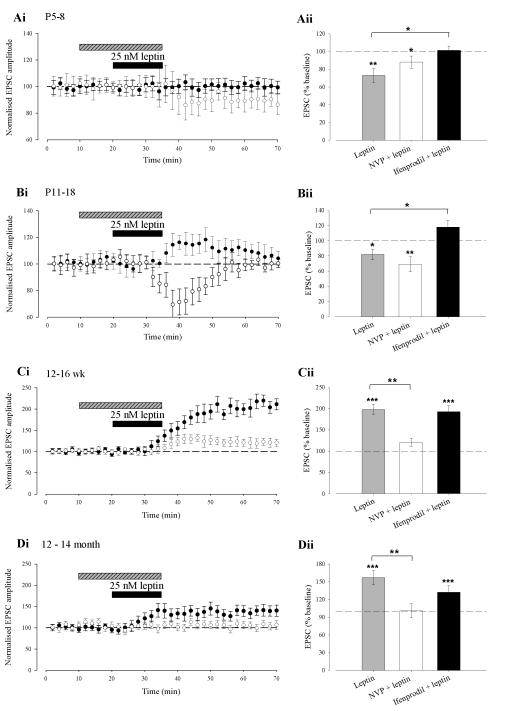
Several lines of evidence indicate that molecularly distinct NMDA receptors underlie different forms of activity-dependent synaptic plasticity in the hippocampus (Liu et al., 2004; Bartlett et al., 2007) and cortex (Massey et al., 2004). It is also known that subunit-specific alterations in the composition and localisation of NMDA receptors occur during development (Monyer et al., 1994). Thus it is feasible that distinct NMDA receptor subunits mediate the bi-directional effects of leptin on excitatory synaptic strength. In order to explore this possibility, we used the quinoxalinedione NVP-AAM077, an NMDA receptor antagonist with preferential selectivity for GluN2A subunits (Auberson et al., 2002) at the concentration (0.1μM) used in this study, or the selective GluN2B antagonists, ifenprodil (3 μM; Williams, 1993). In P5-8 slices exposed to NVP-AAM077 (0.1μM), leptin (25nM; 15 min) depressed synaptic transmission to 88 ± 7% of baseline (n=6; P < 0.05; Fig 3Ai & Aii) which persisted for the duration of recordings (up to 90 min); an effect not significantly different from control (P > 0.05). In contrast, in the presence of the GluN2B antagonist, ifenprodil, leptin failed to significantly alter the amplitude of EPSCs (101 ± 5%; n=6; P > 0.05), suggesting the involvement of NR2B subunits in leptin-induced LTD. Similarly, in P11-18 slices treated with NVP-AAM077, leptin (25nM; 15 min) induced an acute depression of synaptic transmission (to 69 ± 10% of baseline; n=6; P < 0.01; Fig 3Bi & Bii), an effect not significantly different from control (P > 0.05). However, in slices exposed to ifenprodil, leptin (25 nM; 15 min) failed to depress synaptic transmission but rather induced a small, transient increase in EPSC amplitude (118 ± 9%; n=6, P < 0.05).
Figure 3. Distinct NMDA receptors mediate the age-dependent effects of leptin.
Ai-Di, Plots of pooled and normalised data illustrating the effects of leptin on synaptic transmission in the presence of either ifenprodil (3 μM; filled circle) or NVP-AAM077 (0.1 μM; open circle) at P5-8 (Ai), P11-18 (Bi), 12-16 weeks (Ci) and 12-14 months of age (Di), respectively. Aii-Dii, Histograms of the pooled data showing the relative depressions in synaptic transmission induced by leptin in control conditions and in the presence of either ifenprodil or NVP-AAM077. At P5-8 and P11-18, blockade of NR2B subunits, prevented the synaptic depression induced by leptin. Conversely, the leptin-driven increase in synaptic transmission was significantly attenuated following blockade of NR2A subunits. In this and subsequent figures *, ** and *** represent P<0.05, P<0.01 and P<0.001, respectively.
In adult slices (12-16 week), exposure to NVP-AAM077 resulted in a significant reduction (P<0.001) in the degree of potentiation induced by leptin (to 21 ± 9% of baseline; n=6; P < 0.05; Fig 3Ci & Cii), whereas the ability of leptin to increase synaptic transmission was unaffected by application of ifenprodil (increase to 193 ± 14% of baseline; n=6; P < 0.001; Fig 3C). Similarly, in slices from 12-14 month old animals leptin failed to alter EPSC amplitude (Fig 3D; n=6; P > 0.05) in the presence of NVP-AAM077. However, in the presence of ifenprodil, leptin induced a sustained increase in EPSC amplitude (to 132 ± 11% of baseline; n=6; P < 0.001); an effect not significantly different from control (P > 0.05; Fig 3Di & Dii). These data suggest that at early stages of postnatal development both the long lasting (P5-8) and acute (P11-18) synaptic depressions induced by leptin are dependent upon the activation of GluN2B, but not GluN2A, subunits. In contrast, the persistent increase in synaptic transmission induced by leptin in adult hippocampus (12-16 week and 12-14 month) requires the activation of GluN2A, but not GluN2B, subunits.
Alterations in NMDA receptor subunit composition parallel the NMDA receptor subunit-dependence of synaptic modulation by leptin
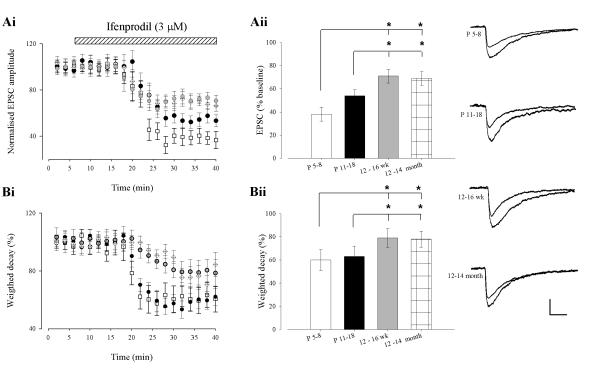
It is well documented that the subunit composition of native NMDA receptors changes during development such that the relative contribution of GluN2A to GluN2B increases with age (Loftis and Janowsky, 2003; Monyer et al., 1994; Sheng et al., 1994). To assess if changes in GluN2 subunit expression parallel the opposing effects on synaptic transmission induced by leptin, we compared the effects of the two antagonists, NVP-AAM077 and ifenprodil, on pharmacologically-isolated NMDA EPSCs in the hippocampal CA1 region. In P5-8 slices NVP-AAM077 reduced the peak amplitude of NMDA EPSCs to 84 ± 5% of control (n=6; P < 0.05; not shown). In accordance with previous studies (Bartlett et al., 2007), application of NVP-AAM077 to P11-18 slices resulted in a significantly greater reduction (to 65 ± 6% of control; n=6; P < 0.05; not shown) in the peak amplitude of NMDA EPSCs than that observed at P5-8 (P < 0.01). Furthermore, NVP-AAM077 reduced the peak amplitude of NMDA EPSCs to an even greater extent in adult tissue (42 ± 6% and 40 ± 6% depression at 12-16 week and 12-14 month, respectively; n=6 and P < 0.001 for each). At all ages, subsequent addition of the selective GluN2B antagonist, ifenprodil after NVP-AAM077 resulted in almost complete inhibition of EPSCs (n=6 and P < 0.001 for each). In agreement with previous findings (Flint et al., 1997; Quinlan et al., 1999; Sheng et al., 1994), these results suggest that native synaptic NMDA receptors contain a greater proportion of GluN2B subunits at earlier developmental stages, with an increase in the contribution of GluN2A subunits to synaptic NMDA receptors with age. In this series of experiments, a low concentration of NVP-AAM077 (0.1 μM) was utilised, to limit effects at GluN2B subunits (Bartlett et al., 2007). However at this concentration there may be incomplete block of all GluN2A containing receptors (Neyton and Paoletti, 2006). Therefore, to confirm our interpretation of the above results we repeated this experiment, but with application of ifenprodil before NVP-AAM077. In P5-8 slices ifenprodil reduced the peak amplitude of NMDA EPSCs to 38 ± 6% of control (n=6; P < 0.001), whereas in P11-18 slices NMDA EPSC peak amplitude was reduced to 54 ± 5% of control (n=6; P < 0.001; Fig 4Ai & 4ii); an effect significantly less than at P5-8 (P < 0.05). As expected the contribution of GluN2B subunits to NMDA EPSCs in adult hippocampus was significantly less, with ifenprodil reducing the peak amplitude to 71 ± 6% (n=6; P < 0.001; Fig 4Ai & 4ii) and 69 ± 7% of baseline (n=6; P < 0.001; Fig 4Ai & 4ii) in 12-16 week and 12-14 month old slices, respectively. Both these decreases were significantly less than that seen at P5-8 and P11-18 (P < 0.05). At all ages examined, application of NVP-AAM077 after ifenprodil resulted in almost complete inhibition of NMDA EPSCs (n=6 for each; P<0.001for each).
Figure 4. Age-dependent changes in the ifenprodil-sensitivity of synaptic NMDARs.
(A) In the presence of NBQX (5 μM), ifenprodil (3 μM) depressed NMDA EPSCs recorded at -30mV at P5-8 (open square), P11-18 (closed circle), 3-4 month (blue circle) and 12-14 month (open diamond). Representative sample traces of NMDA EPSCs from the different age groups before and 30 min after ifenprodil application are shown on the right B, Histogram illustrating the mean percentage of NMDA current remaining after ifenprodil application. C, Treatment with ifenprodil significantly decreased the time constant in young animals (P5-8 (open square); P11-18 (closed circle)) whereas the decrease in decay time constant is less pronounced in older animals (3-4 month (blue circle); 12-14 month (open diamond)). D, Histogram showing the decay time constant of NMDA EPSCs recorded in NBQX at -30 mV at the different ages.
It is well established that NMDA receptors composed of GluN2A or GluN2B subunits display distinct decay kinetics (Cull-Candy et al., 2001; Vicini et al., 1998) and that analysis of mean weighted decay is an alternative method to estimate the relative contribution of each subunit to NMDA EPSCs (Bartlett et al., 2007; Cull-Candy et al., 2001; Vicini et al., 1998). Thus the effects of ifenprodil and NVP-AAM077 on mean weighted decay of NMDA EPSCs were also examined. In agreement with previous studies (Bellone and Nicoll, 2007), application of ifenprodil decreased the weighted decay to 60 ± 9% and 63 ± 9% respectively (n=6; P < 0.001 for each; Fig4Bi & Bii) in P5-8 and P11-18 slices, respectively. Similarly, in adult slices (12-16 week or 12-14 month old), ifenprodil decreased the weighted decay to 79 ± 8% and 81 ± 7% respectively (n=6; P < 0.05 for each; Fig 4B), although this effect was significantly less than that observed in younger slices. (P <0.05; Fig 4Bi & Bii). Application of NVP-AAM077 increased the weighted decay to 152 ± 8% and 163 ± 14% respectively (n=6; P < 0.001 for each) in P5-8 and P11-18 slices, respectively. Similarly, in adult slices (12-16 week or 12-14 month old), NVP-AAM077 increased the weighted decay to 182 ± 16% and 183 ± 8% respectively (n=6; P < 0.05 for each), although this effect was significantly greater than that observed in younger slices. (P <0.05). These results confirm previous studies indicating that GluN2B subunits contribute more than GluN2A subunits to NMDA EPSCs at earlier stages of development and that the contribution of GluN2Asubunits increases with age.
Distinct signalling cascades underlie leptin-driven bi-directional changes in synaptic transmission
We have shown recently that the leptin-induced persistent increase in excitatory synaptic transmission in adult hippocampus involves inhibition of PTEN and subsequent activation of the PI 3-kinase signalling pathway (Moult et al., 2010). Leptin is also capable of signalling via the MAPK (ERK) pathway in hippocampal neurons (Shanley et al., 2001; O’Malley et al, 2007). Moreover, a combination of PI 3-kinase and ERK signalling pathways mediate the leptin-driven facilitation of NMDA responses in cultured hippocampal neurons (Shanley et al, 2001), whereas leptin promotes an increase in GluN2B-mediated responses via an ERK-dependent pathway in cerebellar granule cells (Irving et al., 2006). Thus, as the age-dependent effects of leptin on synaptic transmission are mediated by distinct NMDA receptor subunits, it is feasible that divergent signalling pathways underlie the age-dependent effects of leptin. Thus in order to address this possibility, we examined the role of PI 3-kinase or MAPK signalling at each age. Incubation of hippocampal slices with either LY294002 (50 μM) or wortmannin (50nM) had no effect on synaptic transmission per se at all ages examined (n=6; P>0.05; for each age). In P5-8 slices treated with either one of the two PI 3-kinase inhibitors, leptin induced a long-lasting decrease in EPSC amplitude (wortmannin; 80 ± 8%; n=6; P < 0.05; Fig 5Ai & Aii or LY294002; 78 ± 9%; n=6; P < 0.05; Fig 5 Aii) and these effects of leptin were not significantly different from control (P > 0.05 for each). In contrast at the same stage of development (P5-8), leptin failed to induce any significant change in EPSC amplitude in the presence of either of two inhibitors of MAPK activation, namely U0126 (20 μM; 101 ± 8%; n=6; P > 0.05; Fig 5Ai &Aii) or PD98059 (20 μM); 99 ± 9%; n=6; P > 0.05; Fig 5Aii). Application of either U0126 or PD98059 alone had no effect on basal synaptic transmission at all ages examined (n=24 for each). Similarly, in P11-18 slices treated with either PI 3-kinase inhibitor, leptin induced an acute depression of synaptic transmission (wortmannin; 77 ± 10% of baseline; n=6; P < 0.05; Fig 5Bi & Bii or LY294002; 81 ± 9% of baseline; n=6; P < 0.05; Fig 5Bii), that was not significantly different from control (P > 0.05 for each). However, leptin failed to induce any significant change in EPSC amplitude in the presence of U0126 (94 ± 5% of baseline; n=6; P > 0.05; Fig 5Bi & 5Bii) or PD98059 (97 ± 7% of baseline; n=6; P > 0.05; Fig 5Bii). In contrast, in 12-16 week old slices, leptin induced a robust increase in EPSC amplitude in the presence of either U0126 (165 ± 15% of baseline; n=6; P < 0.01); Fig 5Ci & 5Cii) or PD98059 (171 ± 11% of baseline; n=6; P < 0.001; Fig 5Cii); effects that were not significantly different from control (P > 0.05 for each). In agreement with our previous study (Moult et al., 2010), leptin failed to induce any change in EPSC amplitude in the presence of either wortmannin (97 ± 9% of baseline; n=6; P > 0.05; Fig 5Ci & Cii) or LY294002 (104 ± 8% of baseline; n=6; P > 0.05; Fig 5Cii). Similarly, in slices from 12-14 month old animals leptin induced a persistent increase in EPSC amplitude in the presence of U0126 (141 ± 13% of baseline; n=6; P < 0.01; Fig 5Di & 5Dii) or PD98059 (138 ± 11 of baseline; n=6; P < 0.01; Fig 5Dii), but was unable to induce any significant change in EPSC amplitude in the presence of the PI3K inhibitors (wortmannin; 102 ± 9% of baseline; n=6; P > 0.05; Fig 5Di or LY294002; 97 ± 8% of baseline; n=6; P > 0.05; Fig 5Dii). Taken together these data suggest that both the long lasting (P5-8) and transient (P11-18) synaptic depressions induced by leptin at early stages of postnatal development are mediated by a MAPK (ERK), but not PI 3-kinase, signalling pathway. Conversely, the persistent increase in excitatory synaptic transmission induced by leptin in adult slices (12-16 week &12-14 month) involves the activation of a PI 3-kinase-dependent pathway.
Figure 5. Divergent signalling pathways underlie the age-dependent effects of leptin on synaptic transmission.
Ai-Di, Plots of pooled and normalized data illustrating the effects of leptin on excitatory synaptic transmission in the presence of either the ERK inhibitor, U0126 (filled circle) or the PI 3-kinase inhibitor, wortmannin (open circle) at P5-8 (Ai), P11-18 (Bi), 12-16 weeks (Ci) and 12-14 months (Di) of age, respectively. Aii-Dii, Histograms of the pooled data showing the relative effects of leptin on synaptic transmission in control conditions and in the presence of either LY294002 (10 μM), wortmannin (50 nM), U0126 (20 μM) or PD98059 (20 μM). Both the leptin-induced LTD at P5-8 and the transient synaptic depression at P11-18 are mediated by an ERK-dependent process as inhibition of ERK activation prevented the effects of leptin. Conversely, the ability of leptin to increase synaptic transmission at 12-16 week (C) or 12-14 month (D) involved a PI 3-kinase-dependent process as inhibition of PI 3-kinase signalling completely inhibited the effects of leptin.
Leptin induced long-term potentiation occludes classical Hebbian LTP and vice versa
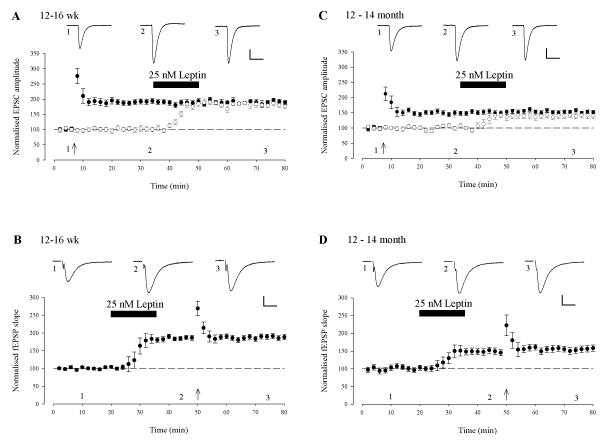
As the long-lasting potentiation of synaptic transmission induced by leptin displays a number of similarities to classical synaptically-induced LTP, we assessed if the two phenomena shared a common expression mechanism by performing occlusion experiments. Thus, two-input experiments were performed and LTP was induced in one pathway in adult slices using a standard high frequency stimulation (HFS) paradigm (100Hz, 1s, 0mV), which resulted in increases in synaptic transmission to 192 ± 8% of baseline (n=6; P<0.01; Fig 6A) and 152 ± 7% of baseline (n=6; P<0.001; Fig 6C), at 12-16 week and 12-14 month respectively. However, subsequent addition of leptin (25 nM;15min) 30 min after the induction of LTP, resulted in no further increase in synaptic transmission at potentiated synapses (12-16 week; 189 ± 6%; n=6; P>0.05; Fig 6A and 12-14 month 156 ± 6%; n=6; P>0.05; Fig 6C). However, leptin did increase synaptic transmission 183 ± 5% of baseline at 12-16 week (n=6; P<0.05; Fig 6A) and 140 ± 8% of baseline at 12-14 month (n=6; P<0.05; Fig 6C) in the control pathway and the magnitude of potentiation was not significantly different to that induced by HFS (P>0.05; Fig 6A & C). We then examined if prior application of leptin occluded HFS-induced LTP. In this series of experiments, extracellular field potential recordings were used in order to prevent washout of LTP during whole cell recordings. Application of leptin increased the fEPSP slope to 185 ± 8% of control (12-16 week; n=6; P<0.001; Fig 6B) and 149 ± 9% of control (12-14 month; n=6; P < 0.001 Fig 6D) respectively. However, subsequent delivery of the HFS paradigm resulted in no further increase in fEPSP slope (188 ± 7% and 153 ± 8% respectively (n = 6 and P > 0.05 for each). Thus these data suggest that leptin induced LTP and synaptically-induced LTP share a common expression mechanism.
Figure 6. Leptin induced LTP and classical Hebbian LTP share similar expression mechanisms.
A, C. Activity-dependent LTP occludes leptin induced LTP. Plot of the pooled data of the normalized EPSC amplitude against time in 12-16 week (A) and 12-14 month (C) old slices. A pairing protocol (100Hz for 1sec paired with postsynaptic depolarisation) was given to the test pathway (filled circle) at the time indicated by the arrow and this resulted in LTP. Subsequent addition of leptin (25 nM; 15 min) failed to induce any further increase in synaptic transmission in this path. In the control pathway (open circle), addition of leptin resulted in LTP and the magnitude of this effect was not significantly different to the potentiated pathway. B,D. Leptin-induced LTP occludes activity-dependent LTP. Plot of the pooled data of the normalized fEPSP slope against time in 12-16 week (B) and 12-14 month (D) old slices. Application of leptin resulted in a persistent increase in synaptic transmission that was unaffected by the subsequent LTP-inducing protocol.
Leptin induced long-term depression occludes de novo LTD and vice versa
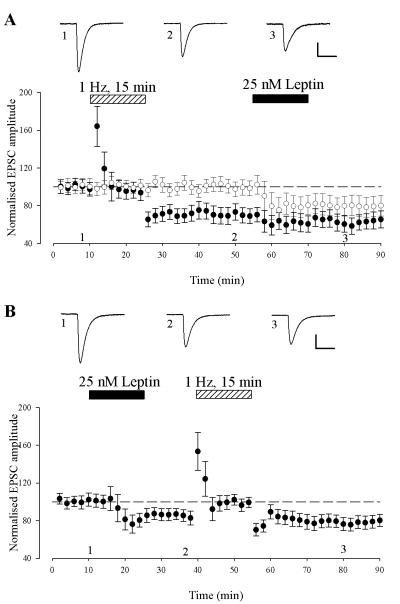
The persistent depression of synaptic transmission induced by leptin at P5-8 also displays similarities to synaptically induced, de novo, LTD as both forms of synaptic plasticity are NMDA receptor-dependent. Thus we examined if these phenomenon displayed similar expression mechanisms by performing occlusion studies. Classical de novo LTD was induced with a low frequency, 1Hz stimulation protocol for 15 min which resulted in a persistent depression of synaptic transmission (to 71 ± 7% of baseline; n=6; P < 0.01; Fig 7A). Subsequent application of leptin, 30 min after LTD induction produced no additional decrease in EPSC amplitude (64 ± 9 % of baseline; n=6; fig 7A). However, leptin reduced synaptic transmission to 80 ± 10% of baseline in the control pathway and the magnitude of depression was not significantly different to that induced by LFS (P>0.05; Fig 7A). Conversely, prior application of leptin resulted in a sustained depression of synaptic transmission (84 ± 7% of baseline; n=6; P < 0.05; Fig 7B); an effect that was unaffected (78 ± 7 % of baseline, n=6; P>0.05) by subsequent delivery of the low frequency stimulation protocol (1Hz; 15 min) 30 min after leptin-induced LTD (Fig 7B). Thus, these data indicate that similar expression mechanisms underlie leptin to induce LTD and LFS-induced LTD at P5-8.
Figure 7. Similar expression mechanisms underlie leptin induced long-term depression and de novo hippocampal LTD.
A. Low frequency stimulation (LFS)-induced LTD occludes leptin-induced LTD. Plot of the pooled data of the normalized EPSC amplitude against time in P5-8 slices. A LFS (1Hz for 900 sec) protocol was given to the test pathway (filled circle) at the time indicated by the bar, and this resulted in LTD of excitatory synaptic transmission. Subsequent addition of leptin (25 nM; 15 min) failed to induce any further inhibition of synaptic transmission in this path. In the control pathway (open circle), addition of leptin resulted in LTD and the magnitude of this depression was not significantly different to that obtained in the test pathway. B. Leptin-induced LTD occludes LFS-induced LTD. Application of leptin resulted in a persistent depression of synaptic transmission that was unaffected by the subsequent LFS protocol.
Discussion
It is well established that leptin regulates a number of hypothalamic-driven functions including energy balance, reproduction and the control of bone formation (Caprio et al., 2001; Spiegelman and Flier, 2001; Takeda and Karsenty, 2001). However, evidence is accumulating that leptin is a multi-faceted hormone with widespread actions in the CNS. Indeed, leptin receptors are highly expressed in the hippocampal formation (Mercer et al., 1996; Shanley et al., 2002) and several lines of evidence indicate that leptin is a potent regulator of hippocampal synaptic function (Harvey, 2007; Irving et al., 2006; Solovyova et al., 2009). In particular, recent studies have shown that leptin has the capacity to transiently depress excitatory synaptic transmission in juvenile hippocampus (Shanley et al., 2001; Xu et al., 2008), whereas leptin promotes a persistent increase in excitatory synaptic strength in adult (Moult et al., 2010), suggesting that the direction of synaptic modulation by leptin is age-dependent. Here we confirm these findings to show that leptin promotes a rapid but transient depression of EPSCs in juvenile hippocampus. Moreover, we have extended these findings to show that at an earlier stage of postnatal development (P5-8) leptin results in a long lasting depression (LTD) of synaptic transmission. In agreement with our recent studies (Moult et al., 2010), leptin induced a persistent increase in excitatory synaptic transmission in adult hippocampus in this study. Leptin also increased the efficacy of excitatory synaptic transmission in the aging hippocampus (12-14 month old), however the magnitude of increase induced by leptin was markedly attenuated at this age.
It is known that leptin receptors are located at both pre- and postsynaptic sites on hippocampal neurons (Shanley et al., 2002). Consequently, activation of leptin receptors at either locus could mediate the effects of leptin on excitatory synaptic transmission. Here we show that both the persistent and transient synaptic depressions induced by leptin in the developing hippocampus were not accompanied by any significant change in PPR or CV indicating the involvement of a postsynaptic expression mechanism. In contrast, however, Xu et al (2008) observed a small alteration in PPR following the reversible depression of excitatory synaptic transmission induced by leptin in juvenile (3 week old) murine slices. The same study also provided evidence for a postsynaptic expression mechanism as leptin selectively attenuated AMPA, but not NMDA, receptor-dependent synaptic transmission (Xu et al., 2008). Differences in the expression mechanisms proposed to underlie the leptin-dependent synaptic depressions in juvenile hippocampus may be attributed to species-dependent differences as Xu et al (2008) utilised murine tissue in all their studies. Alternatively differences in recording conditions may contribute to the reported differences in these studies. In agreement with our recent studies (Moult et al., 2010), the leptin-driven increase in excitatory synaptic transmission in adult hippocampus involves a postsynaptic mechanism rather than a change in the probability of release as this effect of leptin was not accompanied by any change in either PPR or CV. In support of a postsynaptic expression mechanism, we have shown that trafficking of AMPA receptors to hippocampal synapses underlies the leptin-driven increase in synaptic strength at this age (Moult et al., 2010).
We have shown that NMDA receptors are pivotal for the effects of leptin on hippocampal excitatory synaptic function. Indeed, the synaptic activation of NMDA receptors underlies the ability of leptin to facilitate the induction of LTP, depotentiate hippocampal CA1 synapses and induce a novel form of de novo LTD (Durakoglugil et al., 2005; Moult et al., 2009; Shanley et al., 2001). Moreover NMDA receptor activation also mediates the alterations in dendritic morphology and AMPA receptor trafficking to hippocampal synapses by leptin (Moult et al., 2010; O’Malley et al., 2007). Similarly in this study, the competitive NMDA receptor antagonist, D-AP5 blocked both the long lasting and transient synaptic depressions induced by leptin at early stages of postnatal development, indicating the involvement of an NMDA receptor-dependent process. Furthermore, in agreement with our previous studies (Moult et al., 2010), an NMDA receptor-driven mechanism mediates the effects of leptin in adult hippocampus as blockade of NMDA receptors also prevented the leptin-driven increase in excitatory synaptic strength in slices from 12-16 week and 12-14 month old animals. It is well documented that the subunit composition of NMDA receptors varies during development (Monyer et al., 1994) and differences exist in the molecular composition of synaptic and extrasynaptic NMDA receptors (Rumbaugh and Vicini, 1999). In accordance with these studies, the contribution of GluN2B subunits to synaptic NMDA receptors, assayed as the sensitivity of NMDA EPSCs to ifenprodil, was significantly greater early in postnatal development, whereas the contribution of GluN2A subunits increases with age in this study. Moreover, the direction of synaptic modulation by leptin depended on the subunit composition of NMDA receptors at different developmental stages. Thus, at P5-8 and P11-18, when the synaptic GluN2B subunit expression is higher than GluN2A, both the persistent and transient synaptic depressions induced by leptin were completely blocked by the GluN2B antagonist, ifenprodil, but were unaffected by exposure to NVP-AAM077. Together these data suggest that the ability of leptin to depress synaptic transmission at early stages of postnatal development required the activation of GluN2B-containing NMDA receptors.
Conversely, the leptin-driven increase in synaptic efficacy in adult hippocampus was not significantly attenuated by ifenprodil, suggesting that the ability of leptin to enhance synaptic transmission at this stage of development involves a GluN2B-independent mechanism. As the potency of ifenprodil at antagonising GluN2B is regulated by the heteromeric composition of NMDA receptors (Barria and Malinow, 2002), we cannot rule out the possibility that the lack of effect of ifenprodil on the leptin-driven increase in synaptic transmission is due to insufficient block of GluN2B subunits by ifenprodil. However, this is unlikely as ifenprodil substantially reduced NMDA receptor-mediated synaptic currents and decreased the weighted decay of NMDA EPSCs in this study suggesting significant blockade of GluN2B subunits. In contrast, however, the effects of leptin in adult hippocampus were blocked following treatment with NVP-AAM077 which is most likely due to a GluN2A-mediated process. Indeed, the effects of NVP-AAM077 on NMDA EPSCs increased with age and NVP-AAM077 increased the weighted decay of NMDA EPSCs in this study which is consistent with GluN2A antagonism. As NVP-AAM007 displays only about a 10 fold greater potency at GluN2A versus GluN2B subunits (Barlett et al, 2007) and other studies have shown it to be difficult to differentiate GluN2A-mediated effects in slices (Frizelle et al, 2006) we cannot completely rule out a small effect of NVP-AAM007 on GluN2B subunits. However as the GluN2B-selective antagonist ifenprodil failed to attenuate the effects of leptin in adult, it is most likely that activation of GluN2B subunits is not required at this stage of development. Previous studies have shown that the pattern of GluN2 expression is important functionally. Indeed in adult forebrain, GluN2A subunits have been implicated in LTP in some studies (Liu et al., 2004; Massey et al., 2004) whereas GluN2B is thought to underlie LTD (Kutsuwada et al., 1996; Liu et al., 2004; Massey et al., 2004; but also note Clayton et al., 2002; Tang et al., 1999). However there is controversy in this area as a number of studies have also found that GluN2B subunits are pivotal for hippocampal LTP (Barria and Malinow, 2005; Foster et al, 2010; Gardoni et al, 2009). Thus although further experiments are required to identify the precise nature of the GluN2 subunits mediating different forms of synaptic plasticity, it is clear that molecularly distinct NMDA receptors play different functional roles in the hippocampus. Thus in accordance with this functional diversity, the molecular composition of NMDA receptors plays a key role in determining the polarity of synaptic modulation by leptin.
Distinct signalling pathways
Our results suggest that both the persistent and transient synaptic depressions induced by leptin early in postnatal development are mediated by an ERK-dependent process as the effects of leptin were completely blocked by inhibitors of ERK, but not PI 3-kinase. Conversely blockade of PI 3-kinase signalling with either LY 294002 or wortmannin prevented the leptin-driven increase in excitatory synaptic strength in adult. We have shown previously that the ability of leptin to facilitate NMDA responses in cerebellar granule cells varies with age. Moreover, leptin selectively increases GluN2B mediated responses via an ERK mediated pathway in cerebellar granule cells (Irving et al., 2006), suggesting that distinct signalling pathways connect leptin receptors to different NMDA receptors. Thus it is feasible that early in postnatal development leptin promotes activation of the ERK signalling pathway which in turn facilitates GluN2B-mediated responses with a resultant depression in synaptic transmission. In contrast, in adult hippocampus leptin increases GluN2A-mediated responses via a PI 3-kinase-dependent process, resulting in a persistent increase in the strength of synaptic transmission.
Leptin-driven changes in synaptic efficacy
In this study, application of leptin resulted in a novel form of NMDA receptor-dependent hippocampal LTD at P5-8. The ability of leptin to induce LTD was dependent on the activation of NMDA receptors comprised of GluN2B subunits as ifenprodil completely blocked leptin-induced LTD at this stage of development. Moreover the expression mechanisms underlying leptin-induced LTD were similar to those underlying activity-dependent LTD as LTD induced by leptin occluded LFS-induced LTD and vice versa. The involvement of GluN2B subunits in leptin-induced LTD parallels previous studies that have implicated GluN2B in activity-dependent LTD. Indeed, mice with knockout of this subunit lack LTD (Kutsuwada et al., 1996), whereas pharmacological blockade of GluN2B subunits with subtype-specific antagonists completely blocks the induction of hippocampal LTD (Liu et al., 2004). However, other studies have failed to show a role for GluN2B subunits in hippocampal LTD (Morishita et al., 2007) , which may be due to differences in recording conditions or the stage of development examined (Collingridge et al., 2010). In this study the ability of leptin to induce LTD early in development was not accompanied by any change in either the PPR or CV indicating a postsynaptic expression mechanism. Most studies have shown that removal of AMPA receptors from hippocampal synapses underlie NMDA receptor-dependent forms of LTD (Collingridge et al., 2004). Thus it is feasible that alterations in AMPA receptor trafficking processes also contribute to leptin-induced LTD at P5-8, however this remains to be determined.
We have shown previously that leptin induces an NMDA receptor-dependent form of LTD in juvenile (P14-18) hippocampus (Durakoglugil et al, 2005). However, in contrast to the present study, the ability of leptin to induce LTD at P14-18 only occurs under conditions of enhanced excitability (Durakoglugil et al, 2005). Although both forms of leptin-induced LTD require the synaptic activation of NMDA receptors, divergent signalling cascades underlie leptin-induced LTD at the different ages, such that an ERK mediated pathway mediates leptin-induced LTD at P5-8, whereas at P14-18 leptin-induced LTD is independent of ERK activation and is negatively regulated by PI 3-kinase (Durakoglugil et al, 2005).
In accordance with our previous studies (Moult et al, 2010), leptin evoked a persistent increase in excitatory synaptic strength (leptin-induced LTP) in adult hippocampus. Leptin also induced LTP in the aging hippocampus however the magnitude of enhancement was significantly less than that observed at 12-16 weeks of age. The ability of leptin to induce LTP displayed parallels to activity-dependent hippocampal LTP as it not only required the synaptic activation of NMDA receptors but also similar magnitudes of enhancement were induced by leptin and HFS at the two ages. Moreover analogous expression mechanisms are likely to underlie the synaptic potentiation induced by leptin and HFS-induced LTP as HFS-evoked LTP occluded the effects of leptin and vice versa. In support of similar expression mechanisms, we have shown previously that insertion of GluA2-lacking AMPA receptors into hippocampal CA1 synapses mediates the leptin-driven increase in excitatory synaptic strength in adult (Moult et al, 2010), which parallels the alterations in the GluA2 content of synaptic AMPA receptors that have been reported following LTP induction at hippocampal CA1 synapses (Plant et al, 2006). Our recent studies have shown that leptin promotes the insertion of GluA2-lacking AMPA receptors into hippocampal synapses via a process involving inhibition of PTEN, which indirectly results in increased PI 3-kinase signalling (Moult et al, 2010). In agreement with these findings, the present study demonstrates that an increase in PI 3-kinase activity underlies leptin-induced LTP in adult hippocampus as the ability of leptin to enhance EPSC amplitude is completely blocked by structurally distinct inhibitors of PI 3-kinase. Activation of PI 3-kinase is also required for the trafficking of AMPA receptors to synapses during hippocampal LTP (Man et al, 2003), which supports the possibility that analogous expression mechanisms underlie LTP induced by either HFS or leptin.
The hormone leptin circulates in the plasma and is transported via the blood brain barrier to most brain regions. As leptin mRNA and protein are expressed in numerous brain regions (Morash et al, 1999) and recent studies have suggested leptin production by the brain (Eikelis et al, 2007), the concentration of leptin reaching the hippocampus may arise from local as well as peripheral sources. Several lines of evidence indicate that leptin regulates hippocampal excitatory synaptic transmission and markedly influences the cellular events underlying spatial learning and memory (Harvey, 2007). The ability of leptin to rapidly and bi-directionally alter excitatory synaptic strength in an age-dependent manner not only provides additional evidence supporting the pivotal role leptin plays in regulating hippocampal synaptic function, but also has important implications for the role of this hormone during early neuronal development and in aging.
It is well documented that circulating hormones exert widespread effects on brain development and accumulating evidence indicates that the hormone leptin is an important development signal. Indeed, there is a surge in leptin levels during a restricted critical period of postnatal development such that plasma leptin levels increase 5-10 fold during the second postnatal week but drop significantly at weaning (Ahima et al., 1999). In addition, studies have shown that leptin-deficiency or insensitivity results in decreases in protein content, the levels of some synaptic proteins and brain weight (Ahima et al., 1999; Steppan and Swick, 1999); effects that are normalized by postnatal leptin administration (Ahima et al., 1999). Furthermore, leptin deficiency has been shown to effect the development of hypothalamic circuits, as arcuate nucleus projections are reduced in ob/ob mice (Bouret et al., 2004) and these deficits are restored by leptin replacement. As dynamic regulation of synaptic strength is thought to be pivotal for the assembly of neuronal connections, the ability of leptin to promote a novel form of LTD during a critical stage of postnatal development is likely to be important for modifying and shaping the neural circuitry within the hippocampus during postnatal development.
It is known that leptin-insensitive rodents display deficits in hippocampal learning tasks (Li et al., 2002). Cognitive impairments are also prevalent in obesity-related diseases like type II diabetes (Gispen and Biessels, 2000). Moreover, recent studies have suggested that the incidence of neurodegenerative diseases such as Alzheimer’s disease is linked to the circulating levels of leptin (Lieb et al., 2009; Power et al., 2001). In addition, leptin significantly reduces amyloid β levels in murine models of Alzheimer’s disease. As leptin resistance is a feature of obesity and related disorders, resistance to this hormone is likely to contribute to the cognitive deficits reported in diabetics and neurodegenerative disorders. Thus, the marked age-dependent reduction in the ability of leptin to increase excitatory synaptic efficacy observed in this study may be important not only for the role of leptin in normal brain aging but also in neurological disorders associated with leptin resistance or insensitivity.
Acknowledgements
This work was supported by the Wellcome Trust (075821) and Medical Research Scotland.
References
- Ahima RS, Bjorbaek C, Osei S, Flier JS. Regulation of neuronal and glial proteins by leptin: implications for brain development. Endocrinology. 1999;140:2755–2762. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- Anderson WW, Collingridge GL. Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J Neurosci Methods. 2007;162:346–356. doi: 10.1016/j.jneumeth.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. doi: 10.1016/s0960-894x(02)00074-4. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Bellone C, Nicoll RA. Rapid bidirectional switching of synaptic NMDA receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Caprio M, Fabbrini E, Isidori AM, Aversa A, Fabbri A. Leptin in reproduction. Trends Endocrinol Metab. 2001;12:65–72. doi: 10.1016/s1043-2760(00)00352-0. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J Neurosci. 2002;22:3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Peineau S, Howland JG, Wang YT. Long-term depression in the CNS. Nat Rev Neurosci. 2010;11:459–473. doi: 10.1038/nrn2867. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Durakoglugil M, Irving AJ, Harvey J. Leptin induces a novel form of NMDA receptor-dependent long-term depression. J Neurochem. 2005;95:396–405. doi: 10.1111/j.1471-4159.2005.03375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelis N, Wiesner G, Lambert G, Esler M. Brain leptin resistance in human obesity revisited. Regul Pept. 2007;139:45–51. doi: 10.1016/j.regpep.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K.A, McLaughlin, N., Edbauer D, Phillips M, Bolton A, Constantine-Paton M, Sheng M. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci. 2010;30:2676–85. doi: 10.1523/JNEUROSCI.4022-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizelle PA, Chen PE, Wyllie DJ. Equilibrium constants for (R)-[(S)-1-(4-bromo-phenyl)-ethylamino]-(2,3-dioxo-1,2,3,4-tetrahydroquinoxalin-5-yl)-methyl]-phosphonic acid (NVP-AAM077) acting at recombinant NR1/NR2A and NR1/NR2B N-methyl-D-aspartate receptors: Implications for studies of synaptic transmission. Mol Pharmacol. 2006;70:1022–32. doi: 10.1124/mol.106.024042. [DOI] [PubMed] [Google Scholar]
- Gardoni F, Mauceri D, Malinverno M, Polli F, Costa C, Tozzi A, Siliquini S, Picconi B, Cattabeni F, Calabresi P, Di Luca M. Decreased NR2B subunit synaptic levels cause impaired long-term potentiation but not long-term depression. J Neurosci. 2009;29(3):669–77. doi: 10.1523/JNEUROSCI.3921-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gispen WH, Biessels GJ. Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci. 2000;23:542–549. doi: 10.1016/s0166-2236(00)01656-8. [DOI] [PubMed] [Google Scholar]
- Harvey J. Leptin: a diverse regulator of neuronal function. J Neurochem. 2007;100:307–313. doi: 10.1111/j.1471-4159.2006.04205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res. 2006;45:369–378. doi: 10.1016/j.plipres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CC, Lee CC, Hsu KS. An investigation into signal transduction mechanisms involved in insulin-induced long-term depression in the CA1 region of the hippocampus. J Neurochem. 2004;89:217–231. doi: 10.1111/j.1471-4159.2003.02307.x. [DOI] [PubMed] [Google Scholar]
- Irving AJ, Wallace L, Durakoglugil D, Harvey J. Leptin enhances NR2B-mediated N-methyl-D-aspartate responses via a mitogen-activated protein kinase-dependent process in cerebellar granule cells. Neuroscience. 2006;138:1137–1148. doi: 10.1016/j.neuroscience.2005.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM. Amplitude fluctuations of dual-component EPSCs in hippocampal pyramidal cells: implications for long-term potentiation. Neuron. 1994;12:1111–1120. doi: 10.1016/0896-6273(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, et al. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113:607–615. doi: 10.1016/s0306-4522(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. Jama. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D’Souza S, Wong TP, Taghibiglou C, Lu J, et al. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/s0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387:113–116. doi: 10.1016/0014-5793(96)00473-5. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–5998. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- Morishita W, Lu W, Smith GB, Nicoll RA, Bear MF, Malenka RC. Activation of NR2B-containing NMDA receptors is not required for NMDA receptor-dependent long-term depression. Neuropharmacology. 2007;52:71–76. doi: 10.1016/j.neuropharm.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Moult PR, Cross A, Santos SD, Carvalho AL, Lindsay Y, Connolly CN, Irving AJ, Leslie NR, Harvey J. Leptin regulates AMPA receptor trafficking via PTEN inhibition. J Neurosci. 2010;30:4088–4101. doi: 10.1523/JNEUROSCI.3614-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moult PR, Milojkovic B, Harvey J. Leptin reverses long-term potentiation at hippocampal CA1 synapses. J Neurochem. 2009;108:685–696. doi: 10.1111/j.1471-4159.2008.05810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley D, MacDonald N, Mizielinska S, Connolly CN, Irving AJ, Harvey J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol Cell Neurosci. 2007;35:559–572. doi: 10.1016/j.mcn.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- Power DA, Noel J, Collins R, O’Neill D. Circulating leptin levels and weight loss in Alzheimer’s disease patients. Dement Geriatr Cogn Disord. 2001;12:167–170. doi: 10.1159/000051252. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF. Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci U S A. 1999;96:12876–12880. doi: 10.1073/pnas.96.22.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbaugh G, Vicini S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J Neurosci. 1999;19:10603–10610. doi: 10.1523/JNEUROSCI.19-24-10603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley LJ, O’Malley D, Irving AJ, Ashford ML, Harvey J. Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI 3-kinase-driven activation of BK channels. J Physiol. 2002;545:933–944. doi: 10.1113/jphysiol.2002.029488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Solovyova N, Moult PR, Milojkovic B, Lambert JJ, Harvey J. Bi-directional modulation of fast inhibitory synaptic transmission by leptin. J Neurochem. 2009;108:190–201. doi: 10.1111/j.1471-4159.2008.05751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Swick AG. A role for leptin in brain development. Biochem Biophys Res Commun. 1999;256:600–602. doi: 10.1006/bbrc.1999.0382. [DOI] [PubMed] [Google Scholar]
- Takeda S, Karsenty G. Central control of bone formation. J Bone Miner Metab. 2001;19:195–198. doi: 10.1007/s007740170042. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem. 2005;94:1158–1166. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Wayner MJ, Armstrong DL, Phelix CF, Oomura Y. Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides. 2004;25:991–996. doi: 10.1016/j.peptides.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil discriminates subtypes of the N-methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol. 1993;44:851–859. [PubMed] [Google Scholar]
- Xu L, Rensing N, Yang XF, Zhang HX, Thio LL, Rothman SM, Weisenfeld AE, Wong M, Yamada KA. Leptin inhibits 4-aminopyridine- and pentylenetetrazole-induced seizures and AMPAR-mediated synaptic transmission in rodents. J Clin Invest. 2008;118:272–280. doi: 10.1172/JCI33009. [DOI] [PMC free article] [PubMed] [Google Scholar]