Abstract
We report the characterization of three novel flaviviruses isolated in Spain. Marisma Mosquito virus, a novel mosquito borne virus, was isolated from Ochlerotatus caspius mosquitoes; Spanish Ochlerotatus flavivirus and Spanish Culex flavivirus, two novel insect flaviviruses, were isolated from Oc. caspius and Culex pipiens, respectively. During this investigation, we designed a sensitive RT-nested polymerase chain reaction method that amplifies a 1019bp fragment of the flavivirus NS5 gene and could be directly used in clinical or environmental samples for flavivirus characterization and surveillance. Analysis of the sequence generated from that amplicon contains enough phylogenetic information for proper taxonomic studies. Moreover, the use of this tool allowed the detection of additional flavivirus DNA forms in Culex, Culiseta, and Ochlerotatus mosquitoes.
Key Words: Flavivirus, Mosquitoes, Mosquito-only flavivirus
Introduction
The genus Flavivirus (family Flaviviridae) comprises more than 70 viruses, most of them zoonotic and transmitted by arthropods. The genus includes major human pathogens such as Yellow fever virus (YFV), Dengue virus (DENV), West Nile virus (WNV), Japanese encephalitis virus (JEV), or Tick-borne encephalitis virus (TBEV) (Monath and Heinz 1996). The viruses in the Flavivirus genus are classified in three different groups: tick-borne viruses (TBV), mosquito borne viruses (MBV), and the third group consisting of viruses with not-known arthropod vector; finally, Cell Fusing Agent (CFAV) and Tamana bat virus are considered “tentative species in the genus”(Calisher and Gould 2003). This classification is strongly supported not only by ecological, epidemiological, and disease associations but also by intensive phylogenetic studies performed mainly in the NS5 gene, which encodes the viral polymerase (Gaunt et al. 2001). In Spain, previous field studies suggested the existence of a large number of uncharacterized flaviviruses (Aranda et al. 2009, Sánchez-Seco et al. 2009), although the short nature of the amplification product obtained did not allow complete phylogenetic characterization.
Given that they contain several human pathogens, the MBV and TBV flavivirus groups are the focus of most of the research in the genus. Several novel MBV were recently described: Nounane and Tai forest viruses in Cote d'Ivoire (Junglen et al. 2009), T'Ho virus in Mexico, (Farfan-Ale et al. 2009); and Lammi virus (LAMV) in Finland (Huhtamo et al. 2009). In this article, we will describe another novel flavivirus, related with some of these novels MBV agents, detected in mosquitoes collected in Spain. To achieve its characterization, we designed and validated a generic reverse transcription (RT)-Nested-polymerase chain reaction (PCR) in the NS5 gene that is sensitive enough to allow amplification directly from biological samples, thus avoiding the requirement of cultivation for proper classification.
CFAV was isolated from a cell line established from the mosquito Aedes aegypti (Stollar and Thomas 1975; Cammisa-Parks et al. 1992), and detected in field mosquitoes from Puerto Rico (Cook et al. 2006) and Thailand (Kihara et al. 2007). It was the first “insect flavivirus” (e.g viruses that propagate only in mosquito cells but not in mammalian cells) discovered. To date, six other “insect flaviviruses” have been described: Kamiti River virus (KRV), isolated from field collected Aedes macintoshi in Africa in 2003 (Crabtree et al. 2003); Culex Flavivirus (CxFV), isolated mainly from Culex mosquitoes in Japan, Indonesia (Hoshino et al. 2007), Guatemala (Morales-Betoulle et al. 2008), México (Farfan-Ale et al. 2009), United States, Trinidad (Kim et al. 2009), and Uganda (Cook et al. 2009); Aedes Flavivirus (AEFV), isolated from Aedes albopictus mosquito in Japan (Hoshino et al. 2009); Quang Binh virus (QBV) isolated from Culex tritaeniorhynchus in Vietnam (Crabtree et al. 2009); Nakiwogo virus (NAKV) isolated from Mansonia africana nigerrima in Uganda (Cook et al. 2009); and Calbertado virus detected mainly in Cx. tarsalis mosquitoes in Canada (Pabbaraju et al. 2009). Interestingly, no insect-only flavivirus had been isolated in Europe, although genomic sequences had been already detected in Ochlerotatus caspius, Aedes Vexans, and Ae. albopictus in Italy (Roiz et al. 2009, Calzolari et al. 2010), Portugal (unpublished), and Spain (Aranda et al. 2009, Sánchez-Seco et al. 2009). In this article, we describe the characterization and isolation of two novel insect-only flavivirus recovered from Oc. caspius, Culex pipiens, and Culex theileri in Spain. Integrated sequences for CFAV and KRV had been described in the genome of Aedes mosquitoes (Crochu et al. 2004). These findings have major implications regarding evolution, as they represent an entirely different mechanism by which genetic diversity may be generated in eukaryotic cells. Interestingly, we also demonstrated that long genomic fragments of these viruses were present as DNA forms. So far, these integration events were restricted to Aedes mosquitoes, and in this article, we will also describe putative additional genomic integration events of flaviviral sequences in Ae. vexans, Oc. caspius, Oc. detritus, and Culiseta annulata.
Materials and Methods
Mosquito collection and flavivirus screening
The area of study included four Spanish wetlands [previously described for Sánchez-Seco et al. (2009) and Aranda et al. (2009)]. Adult mosquito specimens were captured from 2001 to 2007, from a variety of locations and using different methods to maximize species diversity. Mosquitoes were pooled by species, sex, collection site, and date, with a maximum number of 100 individuals per pool. The screening was performed with a generic nested RT-PCR to detect flavivirus genome (Sánchez-Seco et al. 2005). Here, in this work, 100 representative mosquitoes' pools were selected for further analysis by using the new generic amplification method. The pool selection was realized depending on the group of virus to which it belongs, species, sex, site, and date of collection.
Cell culture, virus isolation, and electron microscopy studies
Virus isolation was attempted in C6/36, Vero, and BHK-21 cell lines incubated at 33°C or 37°C (insect or mammalian cells respectively). Cells were observed daily for cytopathic effects (CPE), and the culture supernatants were collected after, at least, three blind passages and stored as viral stocks at −80°C until tested by RT-PCR. The studies by electron microscopy were achieved in both fresh supernatants and cells from CPE positive cultures. The supernatants were fixed at a final concentration of 2% glutaraldehyde, clarified by low-speed centrifugation, ultracentrifugated at 35,000 rpm for 60 min in a Ty 50 Ti Beckman rotor at 4°C, and negative stained with PTA (phosphotungstic acid). The cells' monolayers were fixed with 2% glutaraldehyde, put together with the cell pellets from the supernatant clarifications, dehydrated in serial ethanols, and embedded in epoxydic resin for ultrathin sectioning. Viral particles were identified based on their ultrastructural characteristics in a Tecnai 12 or a Philips CM12 electron microscope.
Generic NS5 RT-nested-PCR
Nucleotide sequences of complete NS5 genes of different flaviviruses were obtained from GenBank (National Institute of Health, Bethesda, MD) and aligned by using the algorithm Clustal X as implemented in the MEGA 4.0 software (Tamura et al. 2007). Degenerated primers were designed based on conserved motifs of the NS5 gene; primers selected were 1NS5F: 5′9035-GCATCTAYAWCAYNATGGG-9053 3′, 1NS5Re: 5′10129- CCANACNYNRTTCCANAC -10146 3′, 2NS5F: 5′9103-GCNATNTGGTWYATGTGG-91203′ and 2NS5Re: 5′10103- CATRTCTTCNGTNGTCATCC-101223′. Indicated positions correspond to the sequence of WNV strain NY99-flamingo382–99 (accession number: AF196835).
Viral RNA was extracted from mosquito pools or cell culture supernatants by using a QIAamp Viral RNA Mini Kit (QIAGEN). RT-PCR was conducted by using One-Step RT-PCR kit (QIAGEN) using degenerated primer set 1NS5F/1NS5Re. First amplification profile was 50°C for 45 min and 95°C 15 min, followed by 40 cycles of 94°C for 1 min, 50°C for 4 min, and 72°C for 1 min, with a final extension for 10 min at 72°C. Second amplification was carried out in a final volume of 50 μL and contained 5 mM MgCl2 (Perkin Elmer-Cetus), 0.1 mM of each dNTP (Amersham Pharmacia Biotech), 60 pmol of each primer, 2.5 U of AmpliTaq DNA Polymerase (Applied Biosystems), and 1 μL of the first amplification product. Second amplification profile was 94°C for 5 min, followed by 40 cycles of 94°C for 1 min, 50°C for 3 min, and 72°C for 1 min, with a final extension for 10 min at 72°C. The reactions were performed in a Peltier Thermal Cycler (PTC-200; MJ Research, Watertown). The amplified products were visualized by ethidium bromide staining after electrophoresis on a 1.5% high-resolution agarose gel (MS8; Hispanlab).
To assess relative and absolute sensitivity, the method was tested with dilutions of a wide range of flavivirus species available in the laboratory (JEV strain K94P05 AF045551, WNV strain Eg101 AF260968, YFV Vaccine strain 17DD U17066, DENV type 1 Singapore strain S275/90 M87512, DENV type 2 strain New Guinea C M29095, DENV type 3 strain H87 M93130, DENV type 4 strain 814669 AF326573, TBEV strain Neudoerfl U27495, Murray Valley encephalitis virus (MVEV) strain MVE-1–51 AF161266, Saint Louis encephalitis virus (SLEV) strain 78v6507 AF205481, USUV strain SAAR AY453412, and Kunjin virus strain MRM61C D00246) and several dilutions of a WNV stock with 4×106 TCID50/ml. We were able to detect about 40 TCID50 per tube by using the nested reaction. Samples of whole blood, taken from four viremic patients infected with dengue, were used to validate the method for use in clinical samples (Domingo et al. 2004).
Study for the presence of virus-specific DNA
To test whether the positive pools were the results of genomic RNA amplification or DNA forms, nucleic acid extracts were treated with RNAsa A before amplification (Sánchez-Seco et al. 2009).
Phylogenetic analysis
The sequences obtained in this study from different species of mosquitoes were compared with those available in public databases, and representative flavivirus sequences were used in the phylogenetic analysis. The Program MEGA version 4.0 (Tamura et al. 2007) was used to align the sequences with manual adjustment to maintain a correct reading frame. One first tree was built with the neighbor-joining (NJ) method and distance-p model, which calculated confidence values of 1000 bootstrapping trials. ModelTest3.7 and PAUP4.0 (Posada 2003) programs were used to select the best-fit model of nucleotide substitution for the construction of phylogenetic trees. Data were separately analysed for each flavivirus group (mosquito-borne and insect flavivirus). The displayed phylogeny was estimated by using the program MEGA4, employing the NJ algorithm. The reliability of different phylogenetic groupings was evaluated by using the bootstrap test (1000 bootstrap replications).
Results
In our previous report (Aranda et al. 2009, Sánchez-Seco et al. 2009), we showed the presence of several potential novel flavivirus in Spanish mosquitoes. However, the short nature of the amplification products that we had obtained during that analysis allowed only for preliminary characterization. In the current study, we further studied those positive mosquito pools by using viral cell culture and molecular methods. With the goal of obtaining enough phylogenetic information for proper analysis, we developed a new generic NS5 amplification method. Representative pools previously positive were selected while taking into consideration the species and sex of the mosquitoes, geographical location of the collection site, and date of isolation. The positive samples in our new PCR assay yielded an information fragment of 960 bp of the NS5 gene. Representative pools of each group (taking into consideration the species of mosquitoes, geographical location of the collection site, and date) were selected for RNase A digestion analysis. Of 22 samples, we demonstrated that 12 pools were positive as RNA forms, and 8 were detected in DNA form; in the other 2 samples, the RNA was degraded (data not shown).
Seventy seven new sequences were obtained in this study directly from pools from different species of mosquitoes. Phylogenetic analysis performed including sequences of different flavivirus demonstrated that the sequences detected in this work were grouped in five novel clusters, three of them containing only sequences obtained in the RNA form, the remaining two being detected only as DNA (Figs. 1 and 2). Representative virus strains were obtained in C6/36 cell culture for each of the “RNA-form” clusters (Table 1), no virus was detected from the “DNA-form” clusters. The nucleotide sequences reported in this paper article been submitted to the GenBank data bank.
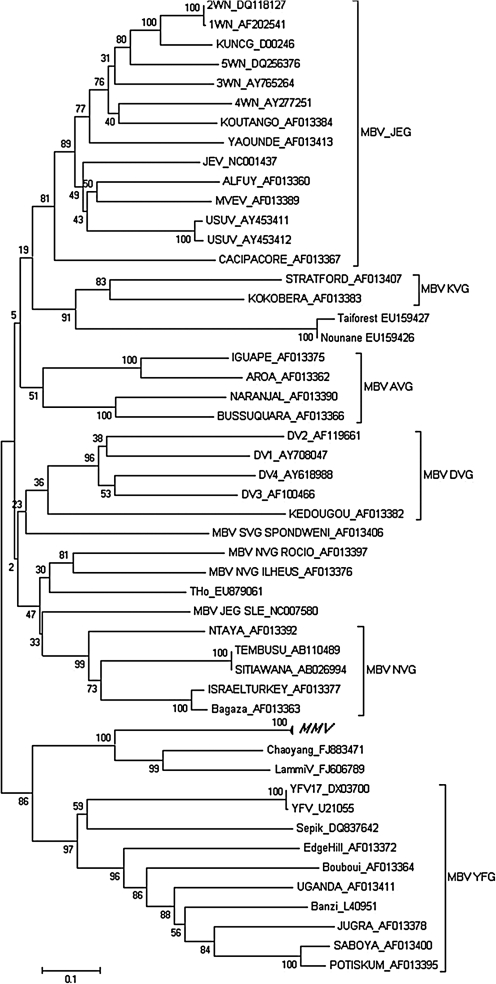
FIG. 1.
Phylogenetic tree based on partial NS5 gene of new group Marisma Mosquito virus (MMV) detected in Ochlerotatus caspius mosquitoes from Spain. Phylogenetic analysis is based on the 860 nucleotides fragment of 54 sequences. The tree is unrooted and was displayed by using the program Mega4, Neighbor-Joining method and Tamura-Nei model with gamma parameter: 0.6990 and 1000 bootstrap replicates. GenBank accession numbers for the different groups of mosquito-borne flavivirus (MBV) sequences are indicated in the tree. Accession numbers for MMV sequences: JF737835, JF737836, JF737837, JF737838, JF737839, and JN603190.
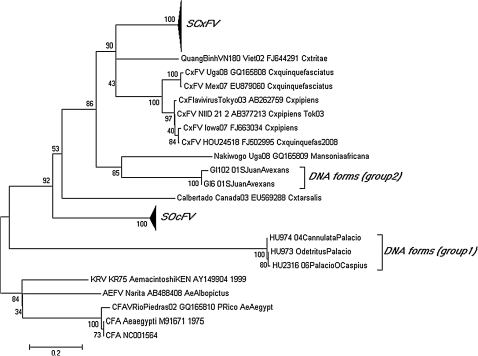
FIG. 2.
Phylogenetic tree based on partial NS5 gene of new groups Spanish Ochlerotatus Flavivirus (SOcFV) and Spanish Culex Flavivirus (SCxFV) detected in Oc. caspius and Culex mosquitoes from Spain. Phylogenetic analysis is based on the 860 nucleotides fragment of 85 sequences. The tree is un-rooted and was displayed by using the program Mega4, Neighbor-Joining method, and Tamura-Nei model with gamma parameter: 0.6617 and 1000 bootstrap replicates. GenBank accession numbers for the insect flavivirus sequences are indicated in the tree. Accession numbers for SOcFV sequences: JF707790, JF707791, JF707792, JF707793, JF707794, JF707795, JF707796, JF707797, JF707798, JF707799, JF707800, JF707800, JF707801, JF707802, JF707803, JF707804, JF707805, JF707806, JF707807, JF707808, JF707809, and JF707810; accession numbers for SCxFV sequences: JF707811, JF707812, JF707813, JF707814, JF707815, JF707816, JF707817, JF707818, JF707819, JF707820, JF707821, JF707822, JF707823, JF707824, JF707825, JF707826, JF707827, JF707828, JF707829, JF707830, JF707831, JF707832, JF707833, JF707834, JF707835, JF707836, JF707837, JF707838, JF707839, JF707840, JF707841, JF707842, JF707843, JF707844, JF707845, JF707846, JF707847, JF707848, JF707849, JF707850, JF707851, JF707852, JF707853, JF707854, and JF707855; accession numbers for DNA forms sequences: JF707856, JF707857, JF707858, JF707859, and JF707860.
Table 1.
Summary of Novel Flaviviruses Detected in Mosquitoes in Spain
| Groups | Mosquito species | No. sequences obtained | Pools inoculated | Pools isolated | Prototype strain |
|---|---|---|---|---|---|
| MMV | Ochlerotatus caspius | 6 | 4 | 1 (females) | HU4528/07 (captured: 01/08/2007) |
| SOcFV | Oc. caspius | 21 | 17 | 1 (males) | HU3737/06 (captured: 29/11/2006) |
| SCxFV | Culex sp. | 45 | 4 | 4 (females) | HU2549/06 (captured: 23/05/2006) |
MMV, Marisma Mosquito virus; SOcFV, Spanish Ochlerotatus Flavivirus; SCxFV, Spanish Culex Flavivirus.
The first cultured virus, henceforth tentatively named Marisma Mosquito virus (MMV, prototype strain: HU4528/07), was isolated from a pool of Oc. caspius collected in southern Spain (Huelva, Andalusian) and belonged to the MBV group. The phylogenetic analysis showed that the NS5 sequence clustered in a clade with LAMV (Huhtamo et al. 2009) and Chaoyang (unpublished) (Fig. 1). LAMV was isolated in Finland in 2004, whereas Chaoyang was isolated in China in 2008. Their degree of divergence in comparison with the rest of the members of the MBV group is similar to that observed between viruses belonging to different flavivirus antigenic groups. Thus, these three viruses might define a new antigenic group. The concurrent recent discovery of these related viruses and their widespread geographical distribution merit forward studies. Monolayers of the first passage of C6/36, Vero and BHK-21 cells inoculated with MMV resulted in the appearance of CPE (cell detachment) 5–7 days postinfection in the three cell lines. Viral RNA was successfully amplified from supernatant at 1, 3, 5 and 7 days postinfection. We assumed that this RNA detection was due to MMV infection in the first passage and that the PCR wasn't detecting residual RNA from the inoculum, because we could observe CPE in the culture cell. However, neither CPE nor positive PCR amplification on cell culture supernatant was observed after the first passage on Vero and BHK-21 cells.
The two other isolated viruses were also captured in southern Spain (Huelva, Andalusian) and broadly clustered with the “insect flavivirus” CFAV and KRV (Fig. 2). One of the sequence clusters, henceforth named Spanish CxFV (SCxFV, prototype strain: HU2549/06), contained sequences obtained from Cx. theileri mainly and Cx. pipiens. It also included partial sequences detected from Cx. theileri mosquitoes captured in Portugal (Genbank Accession number EU716420). The second cluster, from now on named Spanish Ochlerotatus Flavivirus (SOcFV, prototype strain: HU3737/06), was obtained from Oc. caspius mosquitoes. This cluster also included partial sequences obtained from Oc. caspius mosquitoes captured in Italy and Portugal (Calzolari et al. 2010). Each cluster of sequences was highly homologous with nucleotide dissimilarities of less than 3% (SOcFV: 2.63%, SCxFV: 1.85%). Table 2 shows the degree of similarity among all members of the insect flavivirus group including CxFV, CFAV, KRV, QuangBinh, Nakiwogo, C.albertado, and AEFV. SOcFV sequences shares 64.9% identity with CxFV and only 32.9% with AEFV. SCxFV sequences shares 74.7% identity with CxFV but only 36.1% with AEFV. These data strongly suggest that the SOcFV and SCxFV mosquito sequences correspond to new insect flaviviruses that are related to but distinct from CFAV, KRV, CxFV, and AEFV. In culture, some pools of SOcFV and SCxFV developed moderate CPEs (cell aggregation) on C6/36 cells 5–7 days post-infection (data not shown). No CPE was observed in Vero or BHK-21 cells, supernatants of these cultures were negative for flavivirus. Flavivirus-like particles were seen by transmission electron microscopy of infected C636 cells in both groups. The enveloped virions were approximately 40–50 nm in diameter.
Table 2.
Genetic Relatedness Between Spanish Flavivirus and Selected Other Flavivruses
| |
SOcFV |
SCxFV |
MMV |
|||
|---|---|---|---|---|---|---|
| Groups | Nt aa | Nt aa | Nt aa | |||
| MBV_JEG | 46.2 | 49.3 | 47.3 | 50.2 | 35 | 29.1 |
| MBV_AVG | 47.1 | 49 | 47.5 | 51.5 | 35 | 29.6 |
| MBV_NVG | 45.6 | 47.8 | 46.7 | 50 | 34.7 | 28.3 |
| MBV_SVG | 45.4 | 47.6 | 48.1 | 49.2 | 36.6 | 31.3 |
| MBV_DVG | 45.2 | 48.2 | 47.9 | 50.5 | 37.5 | 32.3 |
| MBV_KVG | 44.1 | 46.6 | 47.9 | 49.4 | 36.6 | 31.7 |
| MBV_YFG | 46.8 | 48.1 | 47.5 | 48.8 | 35.4 | 28.2 |
| Chaoyang | 44.7 | 48.1 | 45.7 | 50.2 | 27.7 | 16.3 |
| Uranotaenia | 45.9 | 50.4 | 47.7 | 51.8 | 37.5 | 35.6 |
| Tho | 45.1 | 48.5 | 47.6 | 49.2 | 33.8 | 27.1 |
| Lammi | 44.9 | 48.1 | 45 | 50.9 | 27.1 | 18 |
| MMV | 45.4 | 46 | 47.6 | 50.2 | — | — |
| SCxFV | 35 | 32.3 | — | — | 47.6 | 50.2 |
| SOcFV | — | — | 35 | 32.3 | 45.4 | 46 |
| Nakiwogo | 35.9 | 29.9 | 32.1 | 20.9 | 44.6 | 49.1 |
| Calbertado | 34.8 | 30.6 | 36.3 | 32.3 | 48.5 | 51.1 |
| AEFV | 67.1 | 82.4 | 63.9 | 82.4 | 69 | 86.4 |
| CxFV | 35.1 | 30.5 | 25.3 | 12.2 | 46.7 | 50.1 |
| KRV | 35.5 | 31.2 | 38.2 | 34.3 | 46 | 49.1 |
| CFA | 36.1 | 32.3 | 37.7 | 33.7 | 47.2 | 49.8 |
| Tamana | 65.2 | 81 | 65.9 | 80.7 | 64.3 | 80.5 |
Nucleotides distances are based on 874-nucleotide fragment of the non-structural NS5 gene. Amino acid distances are based on a 289-residue region of the NS5 gene, and were calculated using Mega4, Neighbor-Joining method and distancia-p model. The lowest numbers in each column is denoted in bold.
MBV, Mosquito Borne Virus, JEG, Japanese Encephalitis Group, AVG, Aroa Virus Group, NVG, Ntaya SVG DVG KVG YFG, AEFV, Aedes Flavivirus; KRV, Kamiti River virus; CFA, Cell Fusing Agent.
Temporal and geographical co-circulation of SOcFV and MMV in Oc. caspius was observed. Both viruses have been detected in different pools of the same vector (Oc. caspius) captured in the same period of time (between the months of July and August, mainly) and geographic location (Huelva, Andalusian).
The two clusters of sequences that were detected only as DNA-forms were also included in the insect flavivirus group with good bootstrap support. One of the groups, group 2, presented stop codons that abrogated the open reading frame in their sequences. Viruses could not be isolated from these mosquito pools, no positive PCR was obtained from the supernatant of the exposed cells, and neither CPE was observed after three blind passages.
Discussion
In recent years, several novel flaviviruses have been discovered and characterized all over the world. In this study, we describe the characterization of 3 flaviviruses isolated from mosquitoes captured in Spain. For their characterization as a new species, we used a new generic method to amplify partially the flavivirus polymerase gene. We propose the use of this method for initial characterization of flavivirus, thus allowing focusing of efforts in terms of virus evolution and phylogeny.
Among these three new species, two novel insect flavivirus were characterized. Both phylogeny and cell tropism suggest that the viruses belonged to that group, rather than arthropod-borne flavivirus. We obtained SCxFV only from Culex mosquitoes, whereas SOcFV was only detected from Ochlerotatus mosquitoes, thus supporting the hypothesis that each insect flavivirus is maintained in a host genus-specific manner. Nevertheless, this is in contrast with the findings of Cook et al. (2006), where CFAV was isolated mainly from Ae. albopictus and Ae. aegypti, although sequences were also detected in Culex, Aedeomyia, and Uranotaenia mosquitoes. The insect flaviviruses have not been reported as agents of human disease, and they are thought to be restricted to replication in insects and insect cell lines. The study of SCxFV and SOcFV could provide important clues toward understanding the ecological and evolutionary dynamics of insect flaviviruses.
Apart from these two new insect flaviviruses, we also characterized two new groups of flaviviral DNA form sequences. Since we only tested for partial segments and we did not utilize primer walking techniques to find integration signals, we cannot differentiate whether these forms resemble the DNA-forms as described by Cook et al. (2006) or integrated sequences as described by Crochu et al. (2004). However, the fact that no viral RNA was detected, and the fact that one of them (group 2) contained codon stops might be analyzed as signals that they are integrated in the mosquitoes genome. Earlier, integrated events were restricted to the Aedes mosquitoes. Here, we describe DNA-forms in Oc. caspius, Oc. detritus, and Culiseta annulata (sequence group 1) and also in Ae. vexans mosquitoes (sequence group 2). Phylogenetic studies cluster these two groups of sequences within the insect flaviviruses group. Although the mechanisms by which flaviviral sequences are integrated in the genome are yet unknown, the integration may be a consequence of co-adaptation between mosquitoes and ancestral insect flaviviruses. It has been posted that mosquitoes were infected by flavivirus-like viruses before integration events (Crochu et al. 2004). Integrated flavivirus sequences obtained from different mosquito species are closely related, which might be interpreted as if integration predates mosquito speciation or, more likely, that integration events are more common than earlier. Therefore, infection by insect flaviviruses or the existence of flaviviral sequences integrated in mosquitoes may affect infection, replication, and propagation of mosquito-borne flaviviruses both in vivo and in vitro, and may have already produced selective pressure on virus susceptibilities and/or vector competence of mosquitoes (Farfan-Ale et al. 2009).
A second significant finding was the discovery of MMV, a flavivirus clustering within the MBV group. Almost all MBV members are transmitted by mosquitoes and could infect humans or other animals, thus causing disease. MMV clusters with LAMV isolated in Finland (Huhtamo et al. 2009) and Chaoyang virus (isolated in China in 2008, unpublished), thus suggesting that they represent different virus species (MMV shared more than 72% homology in the nucleotide sequences with the other two viruses) within a putative new antigenic group. These three viruses seem not to replicate in vertebrate cells. Further, MMV was also detected in the antropophilic Oc. caspius mosquitoes in 2003, 2005, 2006, 2007, 2008, 2009, and 2010 (unpublished data) in Huelva, Spain, thus suggesting active circulation of the virus in this area. Despite its grouping with other mosquito-borne flaviviruses, MMV grew successfully on insect cells, whereas vertebrate cells only support growth of MMV in the first passage. Although this result suggests that MMV is only able to infect insects, primary isolation of viruses could be difficult, and even arboviruses that are isolated from infected humans often do not replicate on mammalian cell cultures (Chen and Tao 1996, Diniz et al. 2006).
Temporal and geographical co-circulation of SOcFV (with high prevalence) and MMV in Oc. caspius was observed. Infection with one flavivirus has been shown to suppress infection and prevent transmission of a second, antigenically similar flavivirus: JEV and MVEV superinfections in Cx. tritaeniorhynchus Giles (Altman 1963), two different strains of WNV in Cx. pipiens form molestus Forskal (Rozeboom and Kassira 1969), and WNV and SLEV in Cx. quinquefasciatus (Pesko and Mores 2009). In contrast, persistent triple-virus co-infections (DEN-2, densovirus, and JEV) of mosquito cells (C6/36) had also been described (Kanthong et al. 2010). Moreover, the replication of an insect-only flavivirus (CxFV strain Izabal) had no effect on the infection, transmission, or dissemination of WNV in C6/36 cells or in Cx. quinquefasciatus (Kent et al. 2010). Thus, it is possible that co-infection with two antigenically similar flavivirus might affect transmission, whereas co-infection with two antigenically different flavivirus would have no effect on growth. Thus, mosquitoes naturally infected with insect-only flaviviruses (SCxFV and SOcFV between others) or MMV could be refractory or more susceptible to superinfection with another flavivirus due to viral interference. In this sense, more data about the possible interactions among the flaviviruses that circulates in Spain (MMV, SOcFV, SCxFV, WNV, and USUV) (Vázquez et al. 2010, 2011) would be highly interesting.
During the past few years, our knowledge of the spectrum of flaviviruses has widened, as new species in the genus have been isolated and characterized. These findings evidence our precarious knowledge about the variety and evolutionary history of flaviviruses. The use of molecular amplification systems employing highly conserved enzymatic motifs of the NS3 and NS5 genes has been crucial in the identification of novel flaviviruses. The method described here constitutes a valuable tool for the discovery, characterization, and phylogenetic analysis of classical and atypical flaviviruses.
Acknowledgments
The authors thank Laboratorio Central de Veterinaria, Algete, Spain, and Laboratorio de Aislamiento y Detección, ISCIII for, providing C636 and BHK-21 cells, respectively. They also thank Cristina Domingo for her help in the laboratory work and Laureano Cuevas and Esperanza Pérez of Unidad de Microscopía Electrónica, ISCIII, for their help and work in microscopy electronic studies. The Spanish Health Ministry via its Thematic Research Net “EVITAR” funded this research (G03=059). The grant FIS PI07/1308, Red de Investigación de Centros de Enfermedaes Tropicales (RICET, RD06/0021), and the agreement signed between the Institute of Health Carlos III and the Spanish Ministry of Health and Social Policy for the surveillance of imported viral hemorrhagic fevers also provided financial support.
Disclosure Statement
The authors declare that no conflicts or financial interests exist.
References
- Altman RM. The behavior of Murray Valley encephalitis virus in Culex tritaeniorhynchus Giles and Culex pipiens quinquefasciatus Say. Am J Trop Med Hyg. 1963;12:425–434. [PubMed] [Google Scholar]
- Aranda C. Sánchez-Seco MP. Cáceres F. Escosa R, et al. Detection and monitoring of mosquito flaviviruses in Spain between 2001 and 2005. Vector Borne Zoonotic Dis. 2009;9:171–178. doi: 10.1089/vbz.2008.0073. [DOI] [PubMed] [Google Scholar]
- Calisher CH. Gould EA. Taxonomy of the virus family Flaviviridae. Adv Virus Res. 2003;59:1–19. doi: 10.1016/s0065-3527(03)59001-7. [DOI] [PubMed] [Google Scholar]
- Calzolari M. Bonilauri P. Bellini R. Caimi M, et al. Arboviral Survey of Mosquitoes in Two Northern Italian Regions in 2007 and 2008. Vector Borne Zoonotic Dis. 2010;10:875–884. doi: 10.1089/vbz.2009.0176. [DOI] [PubMed] [Google Scholar]
- Cammisa-Parks H. Cisar LA. Kane A. Stollar V. The complete nucleotide sequence of cell fusing agent (CFA): homology between the nonstructural proteins encoded by CFA and the nonstructural proteins encoded by arthropod-borne flaviviruses. Virology. 1992;189:511–524. doi: 10.1016/0042-6822(92)90575-a. [DOI] [PubMed] [Google Scholar]
- Chen B. Tao S. Arbovirus survey in China in recent ten years. Chin Med J. 1996;109:13–15. [PubMed] [Google Scholar]
- Cook S. Bennett SN. Holmes EC. De Chesse R, et al. Isolation of a new strain of the flavivirus cell fusing agent virus in a natural mosquito population from Puerto Rico. J Gen Virol. 2006;87:735–748. doi: 10.1099/vir.0.81475-0. [DOI] [PubMed] [Google Scholar]
- Cook S. Moureau G. Harbach R. Mukwaya L, et al. Isolation of a new species of flavivirus and a novel strain of Culex flavivirus (Flaviviridae), from a natural mosquito population in Uganda. J Gen Virol. 2009;90:2669–2678. doi: 10.1099/vir.0.014183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree MB. Nga PT. Miller BR. Isolation and characterization of a new mosquito flavivirus, Quang Binh virus, from Vietnam. Arch Virol. 2009;154:857–860. doi: 10.1007/s00705-009-0373-1. [DOI] [PubMed] [Google Scholar]
- Crabtree MB. Sang RC. Stollar V. Dunster LM. Miller BR. Genetic and phenotypic characterization of the newly described insect flavivirus. Kamiti River virus. Arch Virol. 2003;148:1095–1118. doi: 10.1007/s00705-003-0019-7. [DOI] [PubMed] [Google Scholar]
- Crochu S. Cook S. Attoui H. Charrel RN, et al. Sequences of flavivirus-related RNA viruses persist in DNA form integrated in the genome of Aedes spp. mosquitoes. J Gen Virol. 2004;85:1971–1980. doi: 10.1099/vir.0.79850-0. [DOI] [PubMed] [Google Scholar]
- Diniz JA. Nunes MR. Travassos da Rosa AP. Cruz AC, et al. Characterization of two new rhabdoviruses isolated from midges (Culicoides SPP) in the Brazilian Amazon: proposed members of a new genus, Bracorhabdovirus. Arch Virol. 2006;151:2519–2527. doi: 10.1007/s00705-006-0812-1. [DOI] [PubMed] [Google Scholar]
- Domingo C. Palacios G. Niedrig M. Cabrerizo M, et al. A New Tool for the Diagnosis and Molecular Surveillance of Dengue Infections in Clinical Samples. Dengue Bull. 2004;28:87–95. [Google Scholar]
- Farfan-Ale J. Loroño-Pino M. Garcia-Rejon J. Hovav E, et al. Detection of RNA from a novel West Nile-like virus and high prevalence of an insect-specific flavivirus in mosquitoes in the Yucatan Peninsula of Mexico. Am J Trop Med Hyg. 2009;80:85–95. [PMC free article] [PubMed] [Google Scholar]
- Gaunt MW. Sall AA. de Lamballerie X. Falconar AK, et al. Phylogenetic relationships of flaviviruses correlate with their epidemiology, disease association and biogeography. J Gen Virol. 2001;82:1867–1876. doi: 10.1099/0022-1317-82-8-1867. [DOI] [PubMed] [Google Scholar]
- Hoshino K. Isawa H. Tsuda Y. Sawabe K. Kobayashi M. Isolation and characterization of a new insect flavivirus from Aedes albopictus and Aedes flavopictus mosquitoes in Japan. Virology. 2009;15:119–129. doi: 10.1016/j.virol.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Hoshino K. Isawa H. Tsuda Y. Yano K, et al. Genetic characterization of a new insect flavivirus isolated from Culex pipiens mosquito in Japan. Virology. 2007;359:405–414. doi: 10.1016/j.virol.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Huhtamo E. Putkuri N. Kurkela S. Manni T, et al. Characterization of a novel flavivirus from mosquitoes in northern Europe that is related to mosquito-borne flaviviruses of the tropics. J Virol. 2009;83:9532–9540. doi: 10.1128/JVI.00529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junglen S. Kopp A. Kurth A. Pauli G, et al. A new flavivirus and a new vector: characterization of a novel flavivirus isolated from uranotaenia mosquitoes from a tropical rain forest. J Virology. 2009;83:4462–4468. doi: 10.1128/JVI.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthong N. Khemnu N. Pattanakitsakul SN. Malasit P. Flegel TW. Persistent, triple-virus co-infections in mosquito cells. BMC Microbiol. 2010;20(10):14. doi: 10.1186/1471-2180-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent RJ. Crabtree MB. Miller BR. Transmission of West Nile Virus by Culex quinquefasciatus say infected with Culex Flavivirus Izabal. PLoS Negl Trop Dis. 2010;4:e671. doi: 10.1371/journal.pntd.0000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara Y. Satho T. Eshita Y. Sakai K, et al. Rapid determination of viral RNA sequences in mosquitoes collected in the field. J Virol Methods. 2007;146:372–374. doi: 10.1016/j.jviromet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Kim D. Guzman H. Bueno RJ. Dennett J, et al. Characterization of Culex Flavivirus (Flaviviridae) strains isolated from mosquitoes in the United States and Trinidad. Virology. 2009;30:154–159. doi: 10.1016/j.virol.2008.12.034. [DOI] [PubMed] [Google Scholar]
- Monath TP. Heinz FX. Flaviviruses. In: Fields BN, editor; Knipe DM, editor; Howley PM, editor. Fields Virology. 3rd. Philadelphia, NY: Lippincott–Raven; 1996. pp. 961–1034. [Google Scholar]
- Morales-Betoulle M. Monzón-Pineda M. Sosa S. Panella N, et al. Culex flavivirus isolates from mosquitoes in Guatemala. J Med Entomol. 2008;45:1187–1190. doi: 10.1603/0022-2585(2008)45[1187:cfifmi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Pabbaraju K. Ho KC. Wong S. Fox JD, et al. Surveillance of mosquito-borne viruses in Alberta using reverse transcription polymerase chain reaction with generic primers. J Med Entomol. 2009;46:640–648. doi: 10.1603/033.046.0332. [DOI] [PubMed] [Google Scholar]
- Pesko K. Mores CN. Effect of sequential exposure on infection and dissemination rates for West Nile and St. Louis encephalitis viruses in Culex quinquefasciatus. Vector Borne Zoonot Dis. 2009;9:281–286. doi: 10.1089/vbz.2007.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada D. Using MODELTEST and PAUP* to select a model of nucleotide substitution. Curr Protoc Bioinformatics. 2003 doi: 10.1002/0471250953.bi0605s00. Unit 6.5. [DOI] [PubMed] [Google Scholar]
- Roiz D. Vázquez A. Seco M. Tenorio A. Rizzoli A. Detection of novel insect flavivirus sequences integrated in Aedes albopictus (Diptera: Culicidae) in Northern Italy. Virol J. 2009;5:93. doi: 10.1186/1743-422X-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozeboom LE. Kassira EN. Dual infections of mosquitoes with strains of West Nile virus. J Med Entomol. 1969;6:407–411. doi: 10.1093/jmedent/6.4.407. [DOI] [PubMed] [Google Scholar]
- Sánchez-Seco MP. Rosario D. Domingo C. Hernandez L, et al. Generic RT-nested-PCR for detection of flaviviruses using degenerated primers and internal control followed by sequencing for specific identification. J Virol Methods. 2005;126:101–109. doi: 10.1016/j.jviromet.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Sánchez-Seco MP. Vázquez A. Collao X. Hernández L, et al. Surveillance of Arboviruses in Spanish Wetlands: Detection of New Flavi- and Phleboviruses. Vector Borne Zoonot Dis. 2009;10:203–206. doi: 10.1089/vbz.2008.0188. [DOI] [PubMed] [Google Scholar]
- Stollar V. Thomas V. An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology. 1975;64:367–377. doi: 10.1016/0042-6822(75)90113-0. [DOI] [PubMed] [Google Scholar]
- Tamura K. Dudley J. Nei M. Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Vázquez A. Ruiz S. Herrero L. Moreno J, et al. West Nile and Usutu viruses in mosquitoes in Spain, 2008–2009. Am J Trop Med Hyg. 2011;85:178–181. doi: 10.4269/ajtmh.2011.11-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez A. Sanchez-Seco MP. Ruiz S. Molero F, et al. Putative new lineage of west nile virus, Spain. Emerg Infect Dis. 2010;16:549–552. doi: 10.3201/eid1603.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]