Abstract
Fifty-six Trypanosoma cruzi stocks from Chile and neighboring countries and different hosts, humans, and Triatoma infestans and Mepraia sp., vectors of domiciliary and natural environments were characterized by using three molecular markers. These were cytochrome b (Cyt b) gene sequencing, minicircle DNA blotting, and hybridization with five genotype-specific DNA probes and nuclear analysis of 1f8 and gp72 by polymerase chain reaction–restriction fragment length polymorphism. The results with all three molecular markers are concordant, with minor limitations, grouping T. cruzi stocks into four discrete typing units (DTUs) (TcI, TcII, TcV, and TcVI). TcI and TcII stocks were heterogeneous. TcI and TcII stocks were clustered in two main subgroups determined by Cyt b gene sequencing and minicircle hybridization. However, TcV and TcVI stocks were homogeneous and not differentiated by Cyt b gene sequencing or minicircle DNA hybridization. The discriminatory power and limitations of the molecular markers are discussed, as well as the distribution of the four DTUs in the domiciliary and sylvatic transmission cycles of Chile and the limitations of each marker for molecular epidemiological studies performed with T. cruzi stocks rather than the analysis of direct T. cruzi samples from natural hosts.
Key Words: Cytochrome b gene sequencing, Minicircle hybridization, 1f8 and gp72 PCR-RFLP, Trypanosoma cruzi
Introduction
Trypanosoma cruzi, the etiologic agent of Chagas disease, infects 16–18 million people in Latin America (Moncayo 2003). T. cruzi is mainly transmitted by bloodsucking reduviid bugs of the subfamily Triatominae (Deane 1964). Although >130 species of triatomine bugs have been identified, only some are competent vectors for T. cruzi (Lent and Wygodzinsky 1979).
There is consensus that T. cruzi is a paradigm of the clonal evolution model (Tibayrenc et al. 1986). The geographical heterogeneity of the disease suggests that genetic variation of the host, the parasite, or both are important in establishing the clinical manifestations of the disease (Macedo and Pena 1998). The variability of the parasite has been extensively studied by biological, biochemical, and molecular methods, and it may explain the varying clinical manifestations of Chagas disease (cardiac, megaesophagus, and megacolon) and the geographical differences in morbidity and mortality (Macedo et al. 2004). T. cruzi shows high genetic diversity, and a plethora of genetic markers have been used to stratify the species into various subdivisions, with greater or lesser levels of resolution depending on the markers used (Fernandes et al. 1998, Telleria et al. 2006, Llewellyn et al. 2009).
Different genetic analyses led an expert committee to designate two highly divergent major lineages as T. cruzi I and T. cruzi II (Anonymous 1999). T. cruzi II has been partitioned into five sublineages (TcIIa–e) based on several genetic markers (Brisse et al. 2000, 2001). The discrete typing unit (DTU) was coined to classify T. cruzi populations, which correspond to set of strains that are genetically more similar to each other (Tibayrenc 1998). The modern consensus is that six DTUs exist (TcI-TcVI) (Zingales et al. 2009). It is thought that TcI and TcII are the ancestral DTUs, whereas TcIII, TcIV, TcV, and TcVI are hybrids, the last two being the more recent (Westenberger et al. 2005). In Chile, only TcI, TcII, TcV, and TcVI have been documented in the sylvatic and domestic cycles (Miles et al. 1984, Brenière et al. 1991, Barnabé et al. 2001, Coronado et al. 2006, 2009, Galuppo et al. 2009).
In T. cruzi, the mitochondrial DNA or kinetoplast DNA (kDNA) is composed of maxicircles and minicircles. The ∼22 kb maxicircles are all identical and are present in at least a dozen copies per cell. They carry the mitochondrial genes, including cytochrome genes (Westenberger et al. 2006a). The other components of kDNA are the minicircles, with 10,000–20,000 copies/cell. The size and structure of the minicircles are species specific. T. cruzi minicircles have a size of 1.4 kbp and are composed of four conserved regions and four intercalated hypervariable regions (Avila and Simpson 1995). It has been shown that T. cruzi clones have specific minicircle classes that define each DTU, by means of minicircle sequencing or hybridization (Macina et al. 1986, 1987). In previous studies, the genetic polymorphism of several nuclear loci was analyzed by multilocus analysis (polymerase chain reaction–restriction fragment length polymorphism [PCR-RFLP]) and multilocus sequencing in a sample representative of the diversity within T. cruzi (Machado and Ayala 2001, Brisse et al. 2003, Westenberger et al. 2005, Rozas et al. 2007, Lewis et al. 2009, Cura et al. 2010).
Efforts to understand the genetic composition and population structure of T. cruzi may be justified by correlations with biological properties of the parasite, including geographical distribution, host specificity, and clinical outcome of infection. Although no global correlations have been found so far, there are many instances of local associations between parasite genetics and biomedical parameters (Tibayrenc 1998, Campbell et al. 2004).
In Chile, two triatomine genera transmit T. cruzi. Insects of the genus Mepraia (Mepraia spinolai, Mepraia gajardoi) are widely distributed in the sylvatic cycle, whereas Triatoma infestans transmit to humans and reservoirs (Galuppo et al. 2009).
This study was undertaken to apply different molecular markers to genotype T. cruzi stocks circulating in different transmission cycles of Chile. One of them, cytochrome b (Cyt b) gene sequencing, has a high resolution. This marker has the advantage that many sequences from T. cruzi stocks are available and useful for comparative purposes (Brisse et al. 2003, Spotorno et al. 2008, Marcili et al. 2009, Subileau et al. 2009). The other kDNA marker was the minicircle, characterized by DNA blotting analysis with T. cruzi-specific probes. This method has been proved to be useful provided that different minicircle classes are present in different T. cruzi genotypes (Macina et al. 1987, Coronado et al. 2006, 2009, González et al. 2010). Minicircles represent an abundant target that makes them one of the most sensitive to amplification by PCR. Finally, we studied the nuclear genes 1f8 and gp72; both allow accurate discrimination of the six DTUs (Rozas et al. 2007).
The goal of this study is to test three molecular markers to genotype previously characterized T. cruzi stocks circulating in Chile and nearby countries. The Cyt b gene sequence is universal and easy to compare with available data. Minicircle hybridization, even though complex, has the advantage of working even with low parasite amounts. The method with two nuclear genes is easy to perform and to record the results. The results of the three different genotyping methods used here agree, with minor limitations, in the typing of the 56 T. cruzi isolates studied. Moreover, some genetic markers displayed higher discriminatory power, thus allowing subclassification of some DTUs. The implications for the study of molecular epidemiology of Chagas disease with each genetic marker are discussed.
Materials and Methods
Parasite stocks and extraction of genomic DNA
A panel of 56 T. cruzi stocks representing the major DTUs circulating in Chile and nearby countries was selected. Most of them were previously genotyped by isoenzyme analysis (Table 1). They originated from diverse localities in endemic areas and belong to both the sylvatic and domestic transmission cycles. Parasites were cultivated in Diamond's medium supplemented with 10% fecal calf serum at 28°C as previously described (Diamond 1968). DNA was isolated from epimastigote cultures by phenol extraction.
Table 1.
Identification, Host, and Origin of Trypanosoma cruzi Stocks Studied
| Name of stock | Host or vector | Locality | DTU characteristic |
|---|---|---|---|
| sp161 | Mepraia spinolai | Ramadilla | TCI |
| sp 54 | M. spinolai | Bellavista | TCI |
| sp AII | M. spinolai | Flor del Valle | TCI |
| sp Comb 2 | M. spinolai | Combarbalá | TCI |
| sp 104 | M. spinolai | Ramadilla | TCI |
| sp 31 | M. spinolai | Flor del Valle | TCI |
| sp Guayacan | M. spinolai | Guayacan | TCI |
| sp Inca | M. spinolai | Inca de Oro | TCI |
| sp 130 | M. spinolai | Ramadilla | TCI |
| sp Comb A1 | M. spinolai | Combarbalá | TCI |
| sp Trans | M. spinolai | El Transito | TCI |
| sp col 2 | M. spinolai | Santiago | TCI |
| sp Ti9 | M. spinolai | Santiago | TCI |
| sp Col 108 | M. spinolai | Santiago | TCI |
| sp Til 70 | M. spinolai | Santiago | TCI |
| sp 153 | M. spinolai | Ramadilla | TCII |
| VGM | Triatoma infestans | Iquique | TCI |
| V115 | T. infestans | San Pedro Atacama | TCI |
| V111 | T. infestans | San Pedro Atacama | TCI |
| Coyo-3 | T. infestans | San Pedro Atacama | TCI |
| Peine | T. infestans | San Pedro Atacama | TCI |
| 203 SAB108 | T. infestans | San Pedro Atacama | TCI |
| VMV3 | T. infestans | Francia-Iquique | TCI |
| vQUI I | T. infestans | Iquique | TCI |
| VOV2 | T. infestans | Ovalle-Limari | TCII |
| MCH3 | T. infestans | Arrayan-Limari | TCII |
| V1738 | T. infestans | Las Ramadas de Tulahuén | TCII |
| VTV | T. infestans | Iquique | TCII |
| T | T. infestans | Limarí | TCII |
| vOV6 | T. infestans | Ovalle-Limarí | TCV |
| VCF | T. infestans | San Felix | TCV |
| CH2 | T. infestans | San Pedro Atacama | TCVI |
| Gaj 29 | Mepraia gajardoi | Arica | TCV |
| 143 | Human | Chañaral Alto-Limari | TCI |
| LQ | Human | La Isla | TCI |
| AP | Human | El Salvador | TCI |
| 75 | Human | Chañaral Alto-Limari | TCI |
| LGN | Human | Limarí | TCI |
| MVB | Human | La Ligua de Cogotí | TCII |
| MxCh 46 | Human | Salamanca-Choapa | TCII |
| XhCh 80 | Human | Chañaral-Limari | TCII |
| MxCh 53 | Human | Chañaral Alto-Limari | TCII |
| MxCh 88 | Human | Cuncumesi-Limari | TCII |
| IVV | Human | Limari | TCII |
| MCV | Human | Tulahuén | TCII |
| Xd 143 | Human | Choapa | TCV |
| NT 1 | Human | Chillepin | TCV |
| Xd 97 | Human | Choapa | TCV |
| CE | Human | Chillepin | TCV |
| Xd 141 | Human | Choapa | TCV |
| Xd 103 | Human | Choapa | TCV |
| Xd 189 | Human | Choapa | TCV |
| 108 862039 | Didelphis marsupialis | Bolivia | TCI |
| SE9V | Human | Santiago del Estero_Argentina | TCI |
| CAI/72 | Human | Argentina | TCI |
| 118 2675–1 | Guinea pig | Salta–Argentina | TCVI |
| v15P | T. infestans | Arequipa Perú | TCI |
| v10P | T. infestans | Arequipa Perú | TCI |
DTUs, discrete typing units.
Cytochome b gene sequencing
PCR tests and primers targeting the 5′-half of the Cyt b gene were used. The amplicon size is 573 bp. Sequences of primers were as follows: p18 (50-GAC AGG ATT GAG AAG CGA GAG AG-30) and p20 (50-CAA ACC TAT CAC AAA AAG CAT CTG-30) (Brisse et al. 2003). Amplifications were performed for 35 cycles (94°C, 1 min; 50°C, 30 s; 72°C, 90 s) followed by a final elongation step (7 min, 72°C). The fragment was sequenced by using an external service (information at www.macrogen.com).
The sequences obtained were deposited in GenBank with accession numbers JF267928-JF267940.
Phylogenetic analysis of Cyt b gene
Sequences were edited using the program Bioedit 7.0.8.0 (Hall 1999) and aligned using Clustal W (Thompson et al. 1994) as implemented in Bioedit. After alignment, sites that showed nucleotide substitutions were re-examined by visual inspection of each individual's raw fluorogram data. Phylogenetic trees were inferred by the maximum likelihood (ML) by using the online platform PhyML 3.0 (Guindon and Gascuel 2003). We first searched for the model of DNA substitution that best fitted the data using the Akaike Information Criterion (Akaike 1974) as implemented in the program JmodelTest 0.1.1 (Posada 2008), choosing the model GTR+G (G=0.1130, -lnL=3851.9282). Nodal supports were estimated by the bootstrap method (Felsenstein 1985) with 1000 replicates using PhyML 3.0 (Guindon and Gascuel 2003). Nine previously determined sequences were included in the analysis: AO4F, 24tp18, 26tp18, 58p18, 54p18, and 5p18, with accession numbers EU559323–EU559329 (Spotorno et al. 2008), Esmeraldo (AJ130931), TU18 (AJ130932), and Marinkele (AJ130927), which were used as outgroup.
Minicircle PCR
The PCR was performed as previously reported by using primers 121 (AAA TAA TGT ACG G (T/G) GAG ATG CAT GA) and S36 (GGG TTC GATTGG GGT TGG TGT), which anneal with the constant regions present in all minicircles, to amplify the variable regions of minicircle DNA (Sturm et al. 1989, Wincker et al. 1994). We initially heated the lid at 105°C for 2 min, 2 initial cycles of denaturation for 1 min at 98°C and 2 min 64°C, then ran 33 cycles of heating for 1 min at 94°C and 1 min 64°C, and a final extension step of 10 min at 72°C. The PCR products were analyzed by electrophoresis on 2% agarose gels for 2 h and visualized by staining with ethidium bromide. A 330-bp product indicated a positive result (Coronado et al. 2006).
DNA blot hybridization analysis with minicircle DNA probes
These analyses were performed according to Coronado et al. (2006). For T. cruzi genotyping, five different T. cruzi clones isolated in Chile (sp 104 cl1, CBB cl3, IVV cl4, NR cl3, and v195 cl1) corresponding to TcI, TcII, TcII, TcV, and TcVI, respectively, were used to generate genotype-specific probes. These represent the entire collection of variable region minicircle classes present in the T. cruzi clones; therefore, only the probes will hybridize with minicircle amplicons containing very similar minicircle composition. Construction of minicircle probes was performed to eliminate the constant region sequences and radio labeled as described (Veas et al. 1991).
PCR at nuclear loci
Amplification reactions were performed in a final volume of 50 μL. The reaction mixture was amplified using the following conditions: for 1f8, the primers 1F8sen (CTG GAG TTC CGT CTG ATG CTG) and 1F8anti (CAA CAA AGT CCT CGG AGC CCT) were used, we initially heated the lid at 105°C for 2 min, and then for 5 min at 95°C, followed by 35 cycles of 30 s at 94°C, 1 min at 65°C, and 1 min 72°C, with a final extension of 10 min at 72°C. The size of the amplicon is 950 bp. For gp72, the primers GP72sen (GCG GAC AGT GCC AAC AAC CT) and GP72anti (CGC CGA ACT TCC AAC CAT CAG) were used. We initially heated the lid at 105°C for 2 min, and then for 5 min at 95°C, followed by 35 cycles of 30 s at 94°C, 1 min at 60°C, 1.5 min at 72°C, and a final extension step of 10 min at 72°C. The size of amplicon is 1200 bp.
PCR-RFLP 1F8 and gp72 assays
After PCR assays, the digestion of each amplicon was performed in a final volume of 10 μL using 10 U of each restriction enzyme, using the conditions recommended by the manufacturer (MBI-Fermentas; New England Biolab). Ten microliters of PCR-RFLP products were analyzed by electrophoresis in 3% UltraPure™ Agarose 1000 gels for 1 h 30 min. All assays were performed with excess of endonuclease to reduce possible errors in data acquisition caused by incomplete digestions.
Results
Cyt b nucleotide sequences
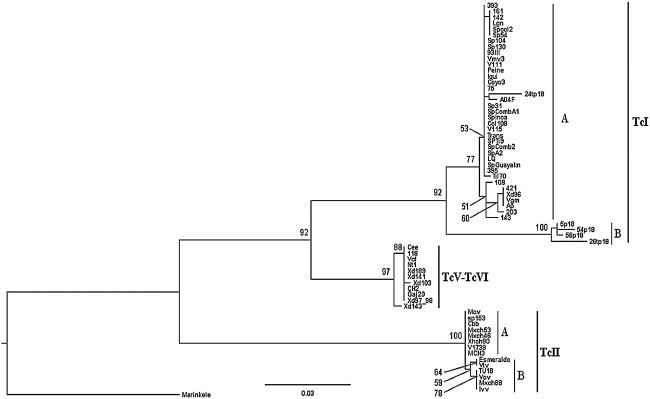
The length of Cyt b sequences amplified by PCR was 516 bp. The sequencing of the Cyt b locus found different alleles among the 56 T. cruzi stocks, samples, and clones under study. The likelihood tree (Fig. 1) showed three defined clusters (TcI, TcII, and TcV together with TcVI) supported by significant bootstrap values. A rather heterogeneous TcI clade was observed, with two major subgroups supported by significant bootstrap values (Fig. 1, subgroups A and B). Subgroup A was detected in T. cruzi stocks studied here, and subgroup B was described earlier from blood samples of reservoir species of Chile (Capra hircus and Octodon degus) (Spotorno et al. 2008). Within subgroup A, there is a high genetic diversity, with two subgroups that have low bootstrap values. A similar situation was found among the TcII stocks; at least, two subgroups of TcII parasites were detected, even though clustered with low bootstrap values; the major group (A) and a minor group composed of the stocks IVV, MxCh88, Vov2, and TU18 (B). However, stock Vtv grouped with the Brazilian Esmeraldo clone. Finally, the third clade of T. cruzi contained the hybrid TcV and TcVI stocks, which represented a homogeneous cluster of parasites. This figure also shows T. cruzi stocks from other countries (see Table 1).
FIG. 1.
Maximum likelihood phylogenetic tree of Trypanosoma cruzi strains based on Cyt b gene sequences inferred with the model GTR+G (G=0.1130, -lnL=3851.9282). Numbers at nodes are the support values derived from 1000 replicates. TcI, TcII, and TcV-TcVI are the three clusters supported by significant bootstrap values representing the genotypes of T. cruzi. A, B, subgroups within each cluster.
Minicircle DNA blotting and hybridization
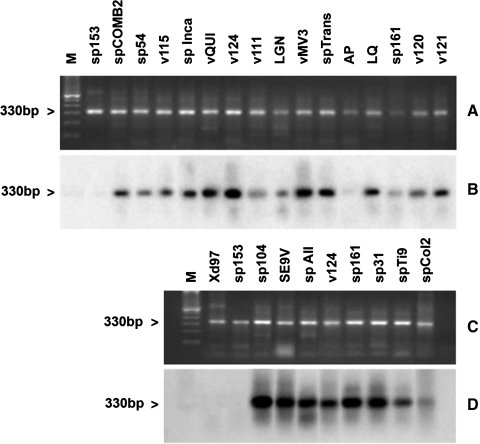
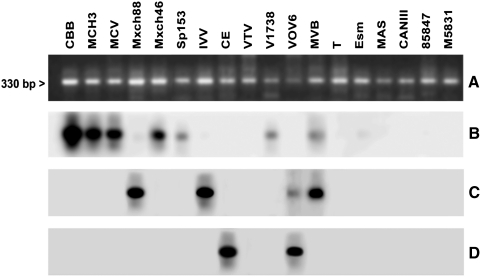
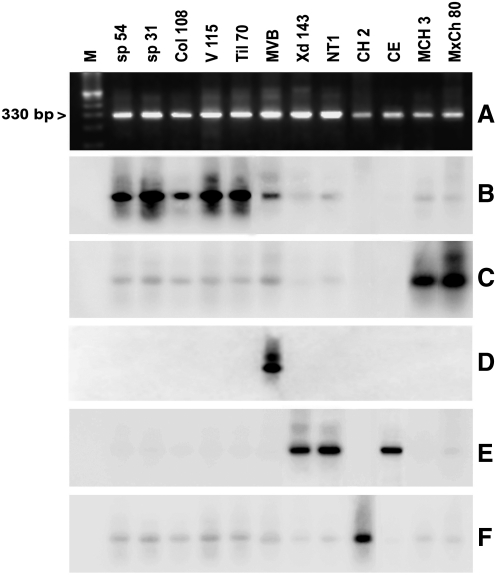
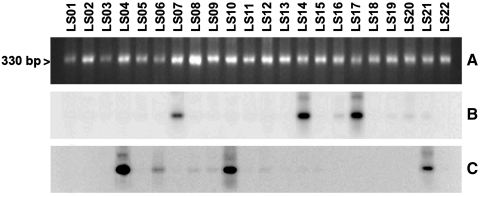
The definition of T. cruzi stocks with five genotype specific DNA probes and hybridization tests allowed the differentiation of a large part of the TcI stocks under study with the probe from T. cruzi clone sp104 (TcI) (Fig. 2). All of the 33 studied TcI stocks showed a hybridization band except for a subgroup of TcI (AP, VGM, xd96, and SAB108). Figure 2 shows a representative result of some TcI stocks of this subgroup and the hybridization results. Similar T. cruzi stocks from Argentina (CAI/72), Bolivia (862039), and Perú (V10P and V15P) were detected by this probe (not shown). Two other probes (CBB cl3 and IVV cl4) derived from TcII clones resulted complementary to subclassify the panel of the 12 TcII stocks studied. The probes derived from T. cruzi clones IVV and CBB cross-hybridized with stocks MxCh88, IVV, and MVB and stocks CBB, MCH3, MCV, MxCh46, sp153, and v1738, respectively (Fig. 3B, C). However, a T. cruzi stock classified as TcII (vTV) by the Cyt b sequence did not cross-hybridize with any TcII or any other probe tested under the same stringency conditions. This figure also shows that these probes do not cross-hybridize with other TcII stocks of Brazil (Esm cl3, MAS1) or with TcIII and TcIV clones (CAN III cl1 and M5631). Figure 4 shows representative results with T. cruzi stocks and probes from the TcI, TcII, and TcV clones. Cross-hybridization was observed with the homologous probe but not with any other probe tested (Fig. 4B–E). This Figure also shows that the MVB stocks corresponded to a mixture of TcI and TcII (IVV), and the vOV6 stock is a mixture of TcII (IVV) and TcV. Finally, the TcVI probe only cross-hybridized with the homologue represented by T. cruzi stock CH2 (Fig. 4F). DNA probes TcV and TcVI also cross-hybridized with the homologous T. cruzi clones (Table 2) under the same hybridization conditions with parasites from other South American countries as shown in Figure 5. These results demonstrate the universal characteristics of the DTUs TcV and TcVI. Finally, all T. cruzi stocks studied were genotyped with this marker except for the vTV stock.
FIG. 2.
Representative results of hybridization patterns of different T. cruzi stocks belonging to TcI and other discrete typing units (DTUs). (A, C) Ethidium bromide staining of a minicircle polymerase chain reaction (PCR) product. (B, D) Hybridization with the TcI probe (sp104cl1). M, molecular marker.
FIG. 3.
Hybridization patterns of different T. cruzi stocks and clones belonging to DTU TcII and other DTUs. (A) Ethidium bromide staining from minicircle PCR products. (B) Hybridization with the TcII probe (CBBcl3). (C) Hybridization with the TcII probe (IVVcl4). (D) Hybridization with the TcV probe (NRcl3).
FIG. 4.
Representative results of hybridization patterns of different T. cruzi stocks belonging to different DTUs. (A) Ethidium bromide staining of a minicircle PCR product. (B) Hybridization with the TcI probe (sp104cl1). (C) Hybridization with the TcII probe (CBBcl3). (D) Hybridization with the TcIV probe (IVVcl4). (E) Hybridization with the TcV probe (NRcl3). (F) Hybridization with the TcVI probe (v195cl1). M, molecular weight marker.
Table 2.
Identification, Origin, and Lineage of Trypanosoma cruzi Clones Studied
| Code | T. cruzi clones | DTU | Origin |
|---|---|---|---|
| LS01 | Esm cl3 | TcII | Sao Felipe Brazil |
| LS02 | Chile C22 | Tcl | Flor de Valle, Chile |
| LS03 | Can III cl1 | TcIV | Belem Brazil |
| LS04 | Chaco 17 col1 | TcVI | Chaco, Paraguay |
| LS05 | 10 R26 | TcIV | Santa Cruz, Bolivia |
| LS06 | ARMA 18 cl13 | TcIII | Campo Lorro, Paraguay |
| LS07 | 92 80 cl2 | TcV | Santa Cruz, Bolivia |
| LS08 | P251 cl7 | TcI | Cochabamba, Bolivia |
| LS09 | M5631 cl5 | TcIII | Marajo Brazil |
| LS10 | CL Brener | TcVI | Rio Grande do Sul. Brazil |
| LS11 | P I (CJ007) | Tcl | Carajas Brazil |
| LS12 | JA2 cl2.2 | TcIII | Amazonas Brazil |
| LS13 | 92101601P cl1 | TcI | Georgia, United States |
| LS14 | Vinch 101 cl1 | TcV | Limari, Chile |
| LS15 | 92122102R | TcIV | Georgia, United States |
| LS16 | Rita cl5 | TcII | Sao Felipe Brazil |
| LS17 | Para4 cl3 | TcV | Paraguari, Paraguay |
| LS18 | X10/1 | Tcl | Belém, Brazil |
| LS19 | ARMA 13 cl1 | TcIII | Campo Lorro, Paraguay |
| LS20 | Pot7a cl1 | TcII | San Martín, Paraguay |
| LS21 | Chaco9 col15 | TcVI | Chaco, Paraguay |
| LS22 | Chaco23 col4 | TcII | Chaco, Paraguay |
FIG. 5.
Hybridization patterns of different T. cruzi clones belonging to different DTUs from different countries. (A) Ethidium bromide staining of a minicircle PCR amplification product. (B) Hybridization with the TcV probe (NRcl3). (C) Hybridization with the TcVI probe (v195cl1).
1f8 and gp72 PCR-RFLP
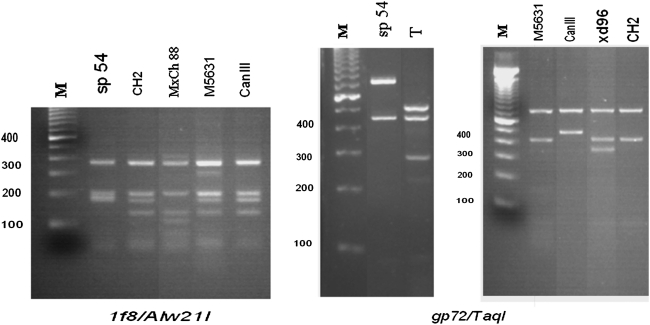
The two nuclear genes (1f8 and gp72) studied by means of PCR-RFLP proved to be necessary and complementary to differentiate the four DTUs present in this sample. The 1f8 gene amplicon digested with Alw21I differentiated the six DTUs into three groups (a. TcI, b. TcII, c. TcIII, TcIV, TcV, and TcVI); the amplicon of the gp72 gene digested with TaqI differentiated three groups (a. TcII, b. TcI, TcIII, TcIV, and TcVI, and c.TcV). The algorithm to classify Chilean DTUs with this method was TcI by 1f8, TcII by both markers, TcV by gp 72, and TcVI by both markers.
Results for representative T. cruzi stocks are shown in Figure 6. TcI, TcII, TcV, and TcVI generated a characteristic RFLP with 1f8/Alw21I. The fragment pattern for TcIII and TcIV was similar to that of TcV and TcVI; since it was not possible to distinguish them with this molecular marker, an additional marker, gp72/TaqI, was needed. The corresponding RFLP for the same group of parasites from gp72/TaqI generated characteristic fragments specific for TcI, TcII, and the hybrids TcV and TcVI. All T. cruzi stocks TcI, TcII, TcV, and TcVI studied here corresponded to the profiles just described. As for 1f8/Alw21I, this molecular marker alone did not differentiate between TcIII, TcIV, and TcVI; thus, two markers were required to genotype T. cruzi stocks.
FIG. 6.
PCR–restriction fragment length polymorphism patterns of representative T. cruzi stocks belonging to different DTUs: sp54 (TcI); T and MxCh88 (TcII); M5631 (TcIII); CanIII (TcIV); Xd96 (TcV); and CH2 (TcVI). Ten or 15 μL of amplified PCR digested with the endonuclease were analyzed on a 3% agarose gel and stained with ethidium bromide. M, molecular weight marker.
Discussion
An understanding of the genetic diversity of any microbial pathogen is crucial to define the species, especially for epidemiologic research diagnostics, evolutionary and biomedical studies. The phylogeny of the Chilean T. cruzi stocks by means of Cyt b nucleotide sequence revealed three major mitochondrial clades as previously described (de Freitas et al. 2006): clade A of T. cruzi stocks corresponds to T. cruzi I; clade B to hybrids (TcV and TcVI); and clade C is exclusively T. cruzi II. Parasites described here belonging to Clade A proved to be heterogeneous by both Cyt b nucleotide sequence and minicircle hybridization, but homogeneous for 1f8 and gp72 PCR-RFLP. Two major TcI haplotypes were detected in this and a previous sample by means of Cyt b nucleotide sequence. Interestingly, the direct analysis of T. cruzi from Chilean reservoir blood samples revealed a genetically distant subgroup of TcI not present in the stocks studied here (Spotorno et al. 2008). Minicircle hybridization with the DNA probe (TcI) detected 29 out 33 of the T. cruzi stocks studied, except for a few TcI stocks that appear to have a rather different minicircle class composition, as they not cross-hybridize with the probe. The detection of only one major T. cruzi clone TcI by the Cyt b sequence or minicircle hybridization is probably due to the strong parasite selection that occurs during isolation and culture (Bosseno et al. 2000). By contrast, other genotyping methods based on multilocus enzyme electrophoresis, and molecular karyotype analyses applied to equivalent panels of Chilean T. cruzi stocks, generated several subdivisions within TcI (Henriksson et al. 1993, Venegas et al. 1997, Solari et al. 1998, Barnabé et al. 2001). This observation indicates the high resolution power of these complex analytical methods compared with the more simple method used here.
Clade B is composed of a homogeneous group of parasites for the Cyt b gene sequence, while minicircle hybridization allows detection of two different DTUs using selected DNA probes generated from TcV and TcVI clones. These probes were universal; they recognized homologous DTUs from other countries. This result was also obtained by nuclear loci PCR-RFLP, which distinguished TcV from TcVI in this sample by the nuclear markers 1f8 and gp72 but not by either alone. It is worth mentioning that genotyping of TcV and TcVI in the Chilean sample was only possible, as TcIII and TcIV have not been documented in Chile, because the marker Cyt b does not resolve DTUs TcV and TcVI. Probably since these are recent and given the evolution taxa of the Cyt b gene, this does not allow a clear distinction among these hybrids.
The third clade described here shows some heterogeneity among T. cruzi II (clade C). At least two groups of parasites were detected by means of the Cyt b gene sequence and minicircle hybridization, which resulted in two perfectly corresponding subgroups of parasites. The Tc II stocks analysed here were similarly grouped by means of Cyt b gene sequence and minicircle hybridization with two DNA probes (CBB and IVV). These TcII have been described before (clonets 33 and 32) in Chile and Bolivia (Tibayrenc et al. 1986). These subgroups were also previously characterized by molecular karyotype analysis (Henriksson et al. 1993) and appear to be the ancestors of TcV and TcVI (Westenberger et al. 2006b). Finally, a third subgroup appears to exist, even though only one T. cruzi stock (vTV) was detected in this study, which clustered with the Esmeraldo clone of Brazil.
Thus far, the amount of sequence variance among the maxicircles in the different DTUS does not distinguish between the hybrid DTUs (Sturm and Campbell 2010). The phylogenetic analysis of all available Cyt b gene sequences, thus, allows a robust definition and unambiguous characterization of most T. cruzi clades and subgroups previously described, and the identification and distinction of all Chilean samples from reservoirs (Spotorno et al. 2008). These include clades C and A, which were also the most divergent in all trees based on cytochrome sequences, as well with microsatellite data (de Freitas et al. 2006). This finding, together with the comparison of two complete maxicircle sequences (Westenberger et al. 2006a), and the very recent identification of new T. cruzi I haplotypes (Herrera et al. 2007, Cura et al. 2010) indicate that there is still much genetic variation to uncover in the complex variability, especially among the ancestral DTU TcI of T. cruzi. It is worth mentioning that all the studied T. cruzi stocks were unambiguously genotyped with all the genetic markers used. All seem to be homogeneous and have a clonal characteristic, even though they were not cloned before the analysis. This characteristic is not observed in T. cruzi from Chilean biological samples, where mixed infections (over 40%) are frequent in vertebrate and invertebrate hosts studied by minicircle hybridization (Coronado et al. 2006, 2009). However, mixed infections from T. cruzi stocks are detected but not as frequent (5%); only two cases were detected in this study, in the vOV6 and MVB stocks (Torres et al. 2004). It has been described that processes such as T. cruzi isolation and culture favor the selection of some T. cruzi clones from a mixture of T. cruzi, thus allowing the permanence of only some T. cruzi clones after prolonged culture (Deane et al. 1984, Bosseno et al. 2000). Two of the three molecular markers used here present some limitations for epidemiological studies. The detection limit to study the nuclear genes 1f8 and gp72 is close to 5–50 parasite equivalents/assay, and the detection limit for Cyt b gene sequencing is 5 parasite equivalents/assay. Minicircles are more easily amplified by PCR due to their high copy number/parasite (0.01 parasite equivalents/assay); therefore, they may be used for direct genotyping without the bias of parasite isolation and culture. We did not observe any genetic isolation with geographical distance in the T. cruzi populations under study.
In the current study, we confirmed with different characterization methods that the DTUs circulating in the sylvatic and domestic transmission cycles of Chile are TcI, TcII, TcV, and TcVI. This study also showed that DTUs TcI and TcII are heterogeneous and that TcV and TcVI are homogeneous. However, some molecular characterization techniques have higher resolution power, such as minicircle hybridization and Cyt b gene sequencing; thus, the importance of selecting the appropriate molecular tools to determine DTUs in T. cruzi populations from parasite stocks or direct biological samples. Future studies should include other more powerful markers to evaluate their merits for taxonomic purposes.
Acknowledgments
This study was financed by FONDECYT 1085154 and the European Community's Seventh Framework Programme (FP7) under grant agreement 223034. The authors also thank Dr. M. Tibayrenc for the supply of T. cruzi clones used as international reference strains and G. Audewa and I. Maes for providing optimal conditions to genotype with the nuclear genes.
Disclosure Statement
No competing financial interests exist.
References
- Akaike H. A new look at the statistical model identification. IEEE Trans Automat. Contr. 1974;19:716–723. [Google Scholar]
- Anonymous. Recommendations from a satellite meeting. Mem Inst Oswaldo Cruz. 1999;194:429–432. doi: 10.1590/s0074-02761999000700085. [DOI] [PubMed] [Google Scholar]
- Avila HA. Simpson L. Organization and complexity of minicircle-encoded guide RNAs in Trypanosoma cruzi. RNA. 1995;1:939–947. [PMC free article] [PubMed] [Google Scholar]
- Barnabé C. Neubauer K. Solari A. Tibayrenc M. Trypanosoma cruzi: presence of the two major phylogenetic lineages and of several lesser discrete typing units (DTUs) in Chile and Paraguay. Acta Trop. 2001;78:127–137. doi: 10.1016/s0001-706x(00)00183-2. [DOI] [PubMed] [Google Scholar]
- Bosseno MF. Yacsik N. Vargas F. Brenière SF. Selection of Trypanosoma cruzi clonal genotypes (clonet 20 and 39) isolated from Bolivian triatomines following subculture in liquid medium. Mem Inst Oswaldo Cruz. 2000;95:601–607. doi: 10.1590/s0074-02762000000500002. [DOI] [PubMed] [Google Scholar]
- Brenière SF. Braquemond P. Solari A. Agnèse JF, et al. An isoenzyme study of naturally occurring clones of Trypanosoma cruzi isolated from both sides of the West Andes highland. Trans R Soc Trop Med Hyg. 1991;85:62–66. doi: 10.1016/0035-9203(91)90160-z. [DOI] [PubMed] [Google Scholar]
- Brisse S. Barnabe C. Tibayrenc M. Identification of six Trypanosoma cruzi phylogenetic lineages by random amplified polymorphic DNA and multilocus enzyme electrophoresis. Int J Parasitol. 2000;30:35–44. doi: 10.1016/s0020-7519(99)00168-x. [DOI] [PubMed] [Google Scholar]
- Brisse S. Henriksson J. Barnabé C. Douzery EJ, et al. Evidence for genetic exchange and hybridization in Trypanosoma cruzi based on nucleotide sequences and molecular karyotype. Infect Genet Evol. 2003;2:173–183. doi: 10.1016/s1567-1348(02)00097-7. [DOI] [PubMed] [Google Scholar]
- Brisse S. Verhoef J. Tibayrenc M. Characterisation of large and small Subunit rRNA and mini-exon genes further supports the distinction of six Trypanosoma cruzi lineages. Int J Parasitol. 2001;31:1218–1226. doi: 10.1016/s0020-7519(01)00238-7. [DOI] [PubMed] [Google Scholar]
- Campbell DA. Westenberger SJ. Sturm NR. The determinants of Chagas disease: connecting parasite and host genetics. Curr Mol Med. 2004;4:549–562. doi: 10.2174/1566524043360249. [DOI] [PubMed] [Google Scholar]
- Coronado X. Rozas M. Botto-Mahan C. Ortíz S, et al. Molecular epidemiology of Chagas disease in the wild transmission cycle: the evaluation in the sylvatic vector Mepraia spinolai from an endemic area of Chile. Am J Trop Med Hyg. 2009;81:656–659. doi: 10.4269/ajtmh.2009.09-0053. [DOI] [PubMed] [Google Scholar]
- Coronado X. Zulantay I. Albrecht H. Rozas M, et al. Variation in Trypanosoma cruzi clonal composition detected in blood patients and xenodiagnosis triatomines: implications in the molecular epidemiology of Chile. Am J Trop Med Hyg. 2006;74:1008–1012. [PubMed] [Google Scholar]
- Cura CI. Mejía-Jaramillo AM. Duffy T. Burgos JM, et al. Trypanosoma cruzi I genotypes in different geographical regions and transmission cycles based on a microsatellite motif of the intergenic spacer of spliced-leader genes. Int J Parasitol. 2010;40:1599–1607. doi: 10.1016/j.ijpara.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane LM. Animal reservoirs of Trypanosoma cruzi in Brazil. Rev Bras Malariol Doencas Trop. 1964;16:27–48. [PubMed] [Google Scholar]
- Deane MP. Mangia RH. Pereira NM. Momen H, et al. Trypanosoma cruzi: strain selection by different schedules of mouse passage of an initially mixed infection. Mem Inst Oswaldo Cruz. 1984;79:495–497. doi: 10.1590/s0074-02761984000400016. [DOI] [PubMed] [Google Scholar]
- de Freitas J. Augusto-Pinto L. Pimenta JR. Bastos-Rodrigues L, et al. Ancestral genomes, sex, and the population structure of Trypanosoma cruzi. PLoS Pathog. 2006;2:226–235. doi: 10.1371/journal.ppat.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond LS. Improved method for the monoxenic cultivation of Entamoeba histolytica schaudinn, 1903 and E. histolytica-like amebae with trypanosomatids. J Parasitol. 1968;54:715–719. [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fernandes O. Souto R. Castro J. Pereira J, et al. Brazilian isolates of Trypanosoma cruzi from humans and triatomines classified into two lineages using miniexon and ribosomal RNA sequences. Am J Trop Med Hyg. 1998;58:807–811. doi: 10.4269/ajtmh.1998.58.807. [DOI] [PubMed] [Google Scholar]
- Galuppo S. Bacigalupo A. García A. Ortiz S, et al. Predominance of Trypanosoma cruzi genotypes in two reservoirs infected by sylvatic Triatoma infestans of an endemic area of Chile. Acta Trop. 2009;111:90–93. doi: 10.1016/j.actatropica.2009.02.010. [DOI] [PubMed] [Google Scholar]
- González CI. Ortiz S. Solari A. Colombian Trypanosoma cruzi major genotypes circulating in patients: minicircle homologies by cross-hybridization analysis. Int J Parasitol. 2010;40:1685–1692. doi: 10.1016/j.ijpara.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Guindon S. Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Hall T. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Henriksson J. Pettersson U. Solari A. Trypanosoma cruzi: correlation between karyotype variability and isoenzyme classification. Exp Parasitol. 1993;77:334–48. doi: 10.1006/expr.1993.1091. [DOI] [PubMed] [Google Scholar]
- Herrera C. Bargues MD. Fajardo A. Montilla M, et al. Identifying four Trypanosoma cruzi I isolate haplotypes from different geographic regions in Colombia. Infect Genet Evol. 2007;7:535–539. doi: 10.1016/j.meegid.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Lent H. Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vector of Chagas disease. Bull Am Mus Nat History. 1979;163:123–520. [Google Scholar]
- Llewellyn MS. Miles MA. Carrasco HJ. Lewis MD, et al. Genome-scale multilocus microsatellite typing of Trypanosoma cruzi discrete typing unit I reveals phylogeographic structure and specific genotypes linked to human infection. PLoS Pathog. 2009;5:e1000410. doi: 10.1371/journal.ppat.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MD. Ma J. Yeo M. Carrasco HJ, et al. Genotyping of Trypanosoma cruzi: systematic selection of assays allowing rapid and accurate discrimination of all known lineages. Am J Trop Med Hyg. 2009;81:1041–1049. doi: 10.4269/ajtmh.2009.09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo AM. Machado CR. Oliveira RP. Pena SD. Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of Chagas disease. Mem Inst Oswaldo Cruz. 2004;99:1–12. doi: 10.1590/s0074-02762004000100001. [DOI] [PubMed] [Google Scholar]
- Macedo AM. Pena SDJ. Genetic variability of Trypanosoma cruzi: implications for the pathogenesis of Chagas disease. Parasitol Today. 1998;14:119–124. doi: 10.1016/s0169-4758(97)01179-4. [DOI] [PubMed] [Google Scholar]
- Machado CA. Ayala FJ. Nucleotide sequences provide evidence of genetic exchange among distantly related lineages of Trypanosoma cruzi. Proc Natl Acad Sci U S A. 2001;98:7396–7401. doi: 10.1073/pnas.121187198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macina RA. Arauzo S. Reyes MB. Sanchez DO. Trypanosoma cruzi isolates from Argentina and Chile grouped with the aid of DNA probes. Mol Biochem Parasitol. 1987;25:45–53. doi: 10.1016/0166-6851(87)90017-x. [DOI] [PubMed] [Google Scholar]
- Macina RA. Sanchez DO. Gluschankof DA. Burrone OR, et al. Sequence diversity in the kinetoplast DNA minicircles of Trypanosoma cruzi. Mol Biochem Parasitol. 1986;21:25–32. doi: 10.1016/0166-6851(86)90075-7. [DOI] [PubMed] [Google Scholar]
- Marcili A. Lima L. Cavazzana M. Junqueira AC, et al. A new genotype of Trypanosoma cruzi associated with bats evidenced by phylogenetic analyses using SSU rDNA, cytochrome b and Histone H2B genes and genotyping based on ITS1 rDNA. Parasitology. 2009;136:641–655. doi: 10.1017/S0031182009005861. [DOI] [PubMed] [Google Scholar]
- Miles MA. Apt BW. Widmer G. Povoa MM, et al. Isozyme heterogeneity and numerical taxonomy of Trypanosoma cruzi stocks from Chile. Trans R Soc Trop Med Hyg. 1984;78:526–535. doi: 10.1016/0035-9203(84)90076-2. [DOI] [PubMed] [Google Scholar]
- Moncayo A. Chagas disease:current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Mem Inst Oswaldo Cruz. 2003;98:577–591. doi: 10.1590/s0074-02762003000500001. [DOI] [PubMed] [Google Scholar]
- Posada D. ModelTest: phylogenetic model averaging. Mol Biol Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Rozas M. De Doncker S. Adaui V. Coronado X, et al. Multilocus polymerase chain reaction restriction fragment–length polymorphism genotyping of Trypanosoma cruzi (Chagas disease): taxonomic and clinical applications. J Infect Dis. 2007;195:1381–1388. doi: 10.1086/513440. [DOI] [PubMed] [Google Scholar]
- Solari A. Wallace A. Ortiz S. Venegas J, et al. Biological characterization of Trypanosoma cruzi stocks from Chilean insect vectors. Exp Parasitol. 1998;89:312–322. doi: 10.1006/expr.1998.4289. [DOI] [PubMed] [Google Scholar]
- Spotorno A. Cordova L. Solari A. Differentiation of Trypanosoma cruzi I subgroups through characterization of cytochrome b gene sequences. Infect Genet Evol. 2008;8:898–900. doi: 10.1016/j.meegid.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Sturm NR. Campbell DA. Alternative lifestyles: the population structure of Trypanosoma cruzi. Acta Trop. 2010;115:35–43. doi: 10.1016/j.actatropica.2009.08.018. [Review.] [DOI] [PubMed] [Google Scholar]
- Sturm NR. Degrave W. Morel C. Simpson L. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol Biochem Parasitol. 1989;33:205–214. doi: 10.1016/0166-6851(89)90082-0. [DOI] [PubMed] [Google Scholar]
- Subileau M. Barnabé C. Douzery EJ. Diosque P, et al. Trypanosoma cruzi: new insights on ecophylogeny and hybridization by multigene sequencing of three nuclear and one maxicircle genes. Exp Parasitol. 2009;122:328–337. doi: 10.1016/j.exppara.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Telleria J. Lafay B. Virreira M. Barnabe C, et al. Trypanosoma cruzi: sequence analysis of the variable region of kinetoplast minicircles. Exp Parasitol. 2006;114:279–288. doi: 10.1016/j.exppara.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Thompson JD. Higgins DG. Gibson TJ. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibayrenc M. Genetic epidemiology of parasitic protozoa and other infectious agents: the need for an integrated approach. Int J Parasitol. 1998;28:85–104. doi: 10.1016/s0020-7519(97)00180-x. [DOI] [PubMed] [Google Scholar]
- Tibayrenc M. Wards P. Moya A. Ayala F. Natural populations of Trypanosoma cruzi, the agent of Chagas disease, have a complex multiclonal structure. Proc Natl Acad Sci U S A. 1986;83:115–119. doi: 10.1073/pnas.83.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres JP. Ortiz S. Muñoz S. Solari A. Trypanosoma cruzi isolates from Chile are heterogeneous and composed of mixed populations when characterized by schizodeme and Southern analyses. Parasitology. 2004;128:161–168. doi: 10.1017/s0031182003004475. [DOI] [PubMed] [Google Scholar]
- Veas F. Breniere SF. Cuny G. Brengues C, et al. General procedure to construct highly specific kDNA probes for clones of Trypanosoma cruzi for sensitive detection by polymerase chain reaction. Cell Mol Biol. 1991;37:73–84. [PubMed] [Google Scholar]
- Venegas J. Ortiz S. Muñoz S. Solari A. Molecular karyotype and schizodeme analyses of Trypanosoma cruzi stocks from Chilean triatomines. Parasitology. 1997;115:41–46. doi: 10.1017/s0031182097001066. [DOI] [PubMed] [Google Scholar]
- Westenberger SJ. Barnabé C. Campbell DA. Sturm NR. Two hybridisation events define the population structure of Trypanosoma cruzi. Genetics. 2005;171:527–543. doi: 10.1534/genetics.104.038745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenberger SJ. Cerqueira GC. El-Sayed NM. Zingales B, et al. Trypanosoma cruzi mitochondrial maxicircles display species and strain-specific variation and a conserved element in the non-coding region. BMC Genomics. 2006a;22:7–60. doi: 10.1186/1471-2164-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenberger SJ. Sturm NR. Campbell DA. Trypanosoma cruzi 5S rRNA arrays define five groups and indicate the geographic origins of an ancestor of the heterozygous hybrids. Int J Parasitol. 2006b:337–346. doi: 10.1016/j.ijpara.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wincker P. Britto C. Pereira JB. Cardoso MA, et al. Use of a simplified polymerase chain reaction procedure to detect Trypanosoma cruzi on blood samples from chronic chagasic patients in a rural endemic area. Am J Trop Med Hyg. 1994;51:771–777. doi: 10.4269/ajtmh.1994.51.771. [DOI] [PubMed] [Google Scholar]
- Zingales B. Andrade SG. Briones MR. Campbell DA, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]