Abstract
The spread of avian influenza viruses (AIV) in nature is intrinsically linked with the movements of wild birds. Wild birds are the reservoirs for the virus and their migration may facilitate the circulation of AIV between breeding and wintering areas. This cycle of dispersal has become widely accepted; however, there are few AIV studies that present cross-seasonal information. A flyway perspective is critical for understanding how wild birds contribute to the persistence of AIV over large spatial and temporal scales, with implications for how to focus surveillance efforts and identify risks to public health. This study characterized spatio-temporal infection patterns in 10,389 waterfowl at two important locations within the Pacific Flyway—breeding sites in Interior Alaska and wintering sites in California's Central Valley during 2007–2009. Among the dabbling ducks sampled, the northern shoveler (Anas clypeata) had the highest prevalence of AIV at both breeding (32.2%) and wintering (5.2%) locations. This is in contrast to surveillance studies conducted in other flyways that have identified the mallard (Anas platyrhynchos) and northern pintail (Anas acuta) as hosts with the highest prevalence. A higher diversity of AIV subtypes was apparent at wintering (n=42) compared with breeding sites (n=17), with evidence of mixed infections at both locations. Our study suggests that wintering sites may act as an important mixing bowl for transmission among waterfowl in a flyway, creating opportunities for the reassortment of the virus. Our findings shed light on how the dynamics of AIV infection of wild bird populations can vary between the two ends of a migratory flyway.
Key Words: Alaska, California, Northern shoveler, Surveillance, Waterfowl
Introduction
Understanding the long-distance movement of wild migratory birds between breeding and wintering grounds is crucial in explaining the circulation of avian influenza virus (AIV). Upon arrival at their breeding and wintering sites, migratory birds are thought to introduce AIV into naïve populations facilitating exchange and reassortment of AIV subtypes (Webster et al. 1992, Stallknecht and Brown 2008). This proposed AIV cycle, where birds act as agents for the spread of the virus along a migratory flyway has become widely accepted (Stallknecht and Brown 2008). However, evidence for this cycle of dispersal is fragmented because surveillance studies of waterfowl have been conducted independently at either wintering (Stallknecht et al. 1990c, Sharp et al. 1993, Hanson et al. 2005, Ferro et al. 2008) or breeding grounds (Hinshaw et al. 1980, 1985, Ito et al. 1995, Runstadler et al. 2007, Ip et al. 2008) but never at both. Studies of geese (genus: Anser) conducted throughout the annual cycle have provided insights into the antibody dynamics of AIV (Hoye et al. 2010). However, no study of AIV has systematically investigated infection patterns across a range of waterfowl species at both ends of a migratory flyway.
A flyway perspective is critical for understanding how waterfowl contribute to the persistence of AIV throughout annual cycles and ultimately the movement of the virus globally where flyways overlap. Surveillance studies conducted in North America, Europe, and Australia point to contrasting infection patterns according to stage of the migratory bird cycle. Birds sampled before fall migration typically demonstrate a higher prevalence of AIV infection (Hanson et al. 2003, Munster et al. 2007, Hansbro et al. 2010) compared with wintering counterparts (Sharp et al. 1993, Ferro et al. 2010a). This has been attributed to the high ratio of juvenile birds at breeding grounds that provide a large population of immunonaïve hosts for AIV infection (Webster et al. 1992, Guberti et al. 2007). Other aspects of the AIV infection cycle remain less clear, such as which waterfowl species influence the seasonal epizootiology of viral strains. The mallard (Anas platyrhynchos) is typically reported as having high prevalence in North America (Hinshaw et al. 1980, Deibel et al. 1985, Alfonso et al. 1995) and to a lesser degree the blue-winged teal (Anas discors) (Stallknecht et al. 1990c, Hanson et al. 2005, Ferro et al. 2010a) and the northern shoveler (Anas clypeata) (Hill et al. 2010, Goekjian et al. 2011). However, infection patterns associated with age and species have not been verified through cross-seasonal studies within a migratory flyway.
The Pacific Flyway encompasses the migration route from breeding grounds in Alaska to wintering areas extending from California to Mexico. Within this flyway the dispersal of AIV has potential public health risks. Of the four migratory flyways that encompass North America, the Pacific Flyway has the greatest potential for the introduction of the highly pathogenic AIV of the lethal H5N1 subtype by wild birds due to overlap with birds from Asia where outbreaks occur frequently (Winker et al. 2007, Koehler et al. 2008, Pearce et al. 2009). Although trans-hemispheric gene flow appears to be relatively rare, invasion of a Eurasian subtype hemagglutinin gene has been documented within the Pacific Flyway. This event occurred in the 1990s when migratory birds infected with Eurasian-origin H6 viruses introduced a new lineage of AIV to North America (Bahl et al. 2009, zu Dohna et al. 2009). Knowledge of the patterns defining AIV circulation among breeding and wintering waterfowl populations in the Pacific Flyway is needed to achieve predictive understanding of the risks of virus spread. Our study characterized the prevalence of AIV and circulating subtypes at breeding sites in Interior Alaska and wintering areas in California's Central Valley (CCV) between 2007 and 2009 to gain insights into the mechanisms of viral perpetuation in the waterfowl reservoir.
Materials and Methods
Sampling sites
Our sampling sites were selected on the basis of strong migratory connectivity between Alaska and California by waterfowl. This has been demonstrated by the number of Alaska banded species recovered in California, which is higher than any other U.S. state in the Pacific Flyway (Pacific Flyway Council 2006). Satellite tracking of northern pintail from the CCV similarly highlights that Alaska is a favored breeding ground (Miller et al. 2005). While a clear migratory link exists between these two regions, not all waterfowl conform to this migratory corridor, with differences in migratory routes between waterfowl species. Banding returns indicate that species that breed in Alaska fly to wintering grounds in California, Oregon, Washington, or the prairie potholes of Montana and Idaho (Bellrose 1976). Likewise, many species overwintering in California breed in Alaska, but others have breeding grounds in British Columbia, Alberta, and Saskatchewan (Bellrose 1976).
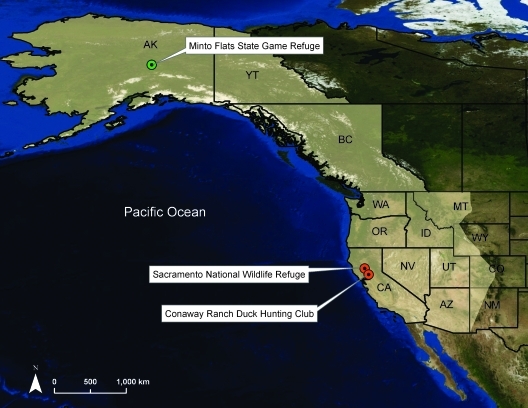
On the breeding grounds, waterfowl were captured at Minto Flats State Game Refuge (Minto Flats), near the Alaskan native village of Minto (64° 53′N, 148° 46′W) (Fig. 1). This site has significance as the first waterfowl breeding ground where AIV surveillance was conducted in the Pacific Flyway (Ito et al. 1995). Minto Flats is one of the most densely populated breeding grounds for waterfowl in Alaska, supporting 213.2 ducks per square mile during the breeding season (Conant and Hodges 1985). The refuge is an expanse of remote shallow ponds and waterways drained by the Chatanika and Tolovana Rivers in the boreal forest of Interior Alaska. Ducks were either live-trapped (August 2007 and 2008) or hunter-harvested (September 2007 and 2008). Live-trapping was conducted using swim-in traps made of 30×60 cm welded wire fencing baited with corn and barley (Hunt and Dahlka 1953). Traps were checked twice per day to ensure no bird remained in a trap for longer than 12 h. This period between August and September coincides with the fledging of hatch-year birds and staging of waterfowl at high densities before fall migration.
FIG. 1.
The Pacific Flyway (highlighted in white) and study sites in Alaska (breeding habitat=green) and in California (wintering habitat=orange) where sampling took place during 2007–2009.
On the wintering sites, waterfowl were sampled at Sacramento National Wildlife Refuge in Glenn County (39° 24′ 31″N, 122° 9′54″W) and Conaway Ranch Duck Club in Yolo County (38° 38′52″N, 121° 40′1″W) in the CCV (Fig. 1). These sites are ∼120 km apart. The CCV supports 60% of the migratory waterfowl in the Pacific Flyway during the winter and nearly 20% of waterfowl in North America (Gilmer et al. 1982). The dominant land use surrounding both sites is agriculture, specifically rice fields that are flooded postharvest to speed straw decomposition, a practice that provides forage such as waste grain and benthic invertebrates for waterfowl (Miller et al. 1989). Waterfowl were collected for sampling from hunters when they exited check stations at Sacramento NWR and Conaway Ranch. Sampling occurred up to three times per week during October 2007–January 2008, and October 2008–January 2009.
Sample collection
The species and sex of all waterfowl were determined and, where possible, age was determined as either hatch-year (HY) or after hatch-year (AHY) on the basis of plumage. To collect AIV samples, a rayon- or polyester-tipped swab (MicroPur™; PurFybr Inc.) was inserted into the cloaca of the bird. The tip of the swab was removed and preserved in cryovial tubes (Remel Inc.) containing viral transport media (VTM). There were differences in the VTM used in Alaska (Remel M4RT VTM) compared with California. Samples were kept on ice for<8 h or transferred to a liquid nitrogen vapor shipper before storage in a −80°C freezer prior to laboratory analysis.
Laboratory analysis
Samples from Alaska were screened by rRT-polymerase chain reaction (PCR) and those that were positive were inoculated into embryonating chicken eggs for subsequent virus isolation. In brief, viral RNA was extracted from VTM (M4RT; Remel) using the MagMAX-96 Viral Isolation Kit (Ambion Inc.). RNA was screened for AIV with a two-step rRT-PCR targeting the matrix gene (Runstadler et al. 2007). PCR assays were run on an ABI 7500 real-time PCR System (Applied Biosystems). PCR-positive VTM samples were subjected to an H5/H7-specific one-step RT-PCR to identify the subtypes associated with highly pathogenic AIV (Wang et al. 2008). Positive VTM (100 μL) was inoculated into the allantoic cavity of 9–11-day-old embryonating-specific pathogen free (SPF) chicken eggs (SPAFAS) and incubated at 37.5°C for 72 h or until embryo death, as detected by daily candling. RNA was extracted from virus allantoic fluid (VAF) and the matrix rRT-PCR was repeated. A total of three passages were attempted to determine if virus was present.
In contrast, samples from California were screened for AIV by virus isolation in embryonating chicken eggs followed by testing for hemagglutinating activity with chicken red blood cells. In brief, 150 μL of VTM was inoculated into the allantoic cavity of 9–11-day-old embryonating-SPF chicken eggs (SPAFAS) and incubated at 37.5°C for 6 days or until embryo death, as detected by daily candling. VTM consisted of tissue culture medium 199 containing 0.5% bovine serum albumin, penicillin G (2×106 U/L), streptomycin (200 mg/L), polymyxin B (2×106 U/L), gentamicin (250 mg/L), nystatin (0.5×106 U/L), ofloxacin HCl (60 mg/L), and sulfamethoxazole (0.2 g/L). Three passages were attempted to determine the presence of virus. The VAF from live embryos was tested for hemagglutinating activity with chicken red blood cells following standard methods (Swayne et al. 1998). RNA was extracted from VAF harvested from all dead embryos and the hemagglutinating VAF from live embryos using the MagMAX-96 Viral Isolation Kit (Ambion Inc.). RNA was tested for AIV with a one-step rRT-PCR targeting the matrix gene and H5/H7 (Spackman et al. 2003).
Positive samples from Alaska were defined as those that were PCR positive (0–45 CT) after a maximum of three passages in embryonating eggs. For California, positive samples were defined as those that were PCR positive (0–35 CT) after demonstrating hemagglutinating activity. Differences in the screening methods precluded a direct comparison of prevalence between Alaska and California. Genetic subtyping was performed by characterizing the hemagglutinin (HA) and neuraminidase (NA) gene using rRT-PCR, with universal primers (Hoffman et al. 2001, Phipps et al. 2004). Amplicons from Alaska were purified using ExoSAP-IT (USB), whereas those from California were purified with Microcon cleanup columns (Millipore) and submitted for sequencing. Sequences were aligned (VectorNTI, Sequencher; Invitrogen) followed by NCBI Blast search to determine subtype. Mixed infections were detected when (a) two bands were visible on the electrophoresis gel or (b) two sequences with competing signals were apparent on the fluorogram. All candidates for mixed infections were then cloned to verify that isolates were genetically distinct.
Statistical analysis
Prevalence was defined as the number of infected individuals expressed as a percentage of the total number of individuals sampled (Bush et al. 1997). For statistical analysis, we applied the criterion that the sample size from each species must be large enough to account for the low prevalence of AIV, with the assumption that a random sample of 100 individuals are required to detect AIV in a study population with a prevalence of 3% (U.S. Avian Influenza Interagency Working Group 2006). This resulted in five waterfowl species being included in analyses, all of which were dabbling ducks (genus: Anas); American green-winged teal (Anas crecca; AGWT), American wigeon (Anas americana; AMWI), mallard (MALL), northern pintail (NOPI), and northern shoveler (NSHO).
To test whether AIV prevalence varied according to the factors: age (HY or AHY), sex (M or F) or species (AGWT, AMWI, MALL, NOPI, or NSHO), a binomial logistic model (link function=logit) for main effects was constructed. Two-way interactions between age and species were also investigated and date was applied as a covariate for all models. When significant differences were detected, a pairwise comparison of estimated marginal means was run to identify which species were different from one another. Since AIV screening techniques differed between Alaska and California and detection limits vary between virus isolation and RT-PCR (Spackman et al. 2002, Runstadler et al. 2007, Munster et al. 2009), we did not statistically compare infection patterns. Models were constructed separately for Alaska and California.
Subtype diversity was defined as the number of unique subtype combinations (HA-NA) hosted by a species. Analysis of subtype diversity necessitated aggregating the data by species, and therefore precluded analysis of demographics (age and sex) derived from an individual. To test whether subtype diversity varied in the five species of dabbling ducks, a Poisson distribution model (link function=log) was constructed and sampling year (2007–2008 or 2008–2009) was applied as a covariate. As above, pairwise comparison of means was performed to identify the cause of significance and models were constructed separately for Alaska and California. Mixed infections were not statistically analyzed owing to the small number of cases.
The p-values≤0.05 were considered statistically significant. All statistical analyses were conducted using SPSS version 16 software for Macintosh (SPSS 2007)
Results
AIV prevalence
In total, 10,389 migratory waterfowl were sampled for AIV; 2962 at breeding grounds in Alaska (12 species) and 7427 at wintering areas in California (17 species). The average prevalence of AIV among waterfowl in Alaska was 9.8% (289/2962, 95% CI: 7.8% to 11.1%). In California, the average prevalence of AIV among waterfowl was 2.5% (182/7427, 95% CI: 1.9% to 3.0%; Table 1). In Alaska, HY birds had a significantly higher prevalence (11.8%, 95% CI: 9.9% to 13.7%) than AHY birds (6.2%, 95% CI: 4.6% to 8.0%) (Wald χ2=15.27, n=3096, p<0.001). Similarly in California, HY birds had a significantly higher prevalence (3.8%, 95% CI: 3.2% to 5.6%) than AHY birds (2.0%, 95% CI: 1.2% to 2.7%: Wald χ2=10.50, n=2827, p=0.001). There was no significant two-way interaction between age and species in either Alaska (Wald χ2=5.42, p=0.247) or California (Wald χ2=7.39, p=0.116). The sex of ducks was not significantly related to AIV prevalence in either Alaska (Wald χ2=0.20, p=0.656) or California (Wald χ2=1.33, p=0.249).
Table 1.
Prevalence of Avian Influenza Virus and Sample Size of Waterfowl in Alaska and California During 2007–2009
| |
Alaska |
California |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Time period |
2007 |
|
2008 |
|
Total |
2007–08 |
|
2008–09 |
|
Total |
| Host species | AIV positive | Sampled | AIV positive | Sampled | AIV prevalence (%) | AIV positive | Sampled | AIV positive | Sampled | AIV prevalence (%) |
| Northern shoveler (Anas clypeata)a | 30 | 96 | 8 | 22 | 32.20 | 28 | 550 | 19 | 351 | 5.22 |
| American wigeon (Anas americana)a | 6 | 193 | 1 | 48 | 2.90 | 9 | 351 | 18 | 393 | 3.63 |
| Greater white-fronted goose (Anser albifrons) | 0 | 1 | 0 | 2 | 0.00 | 2 | 82 | 4 | 105 | 3.21 |
| Bufflehead (Bucephala albeola) | 0 | 23 | 0 | 6 | 0.00 | 1 | 40 | 1 | 24 | 3.13 |
| Mallard (Anas platyrhynchos)a | 46 | 360 | 78 | 644 | 12.35 | 2 | 867 | 48 | 1422 | 2.18 |
| Northern pintail (Anas acuta)a | 22 | 477 | 79 | 814 | 7.82 | 3 | 355 | 11 | 355 | 1.97 |
| Green-winged teal (Anas crecca)a | 10 | 96 | 9 | 119 | 8.84 | 3 | 468 | 18 | 665 | 1.85 |
| Ring-necked duck (Aythya collaris) | 0 | 16 | 0 | 0 | 0.00 | 3 | 114 | 0 | 58 | 1.74 |
| Gadwall (Anas strepera) | 0 | 0 | 0 | 1 | 0.00 | 2 | 390 | 9 | 436 | 1.33 |
| Cinnamon teal (Anas cyanoptera) | 0 | 0 | 0 | 0 | 0.00 | 1 | 58 | 0 | 100 | 0.63 |
| Wood duck (Aix sponsa) | 0 | 0 | 0 | 0 | 0.00 | 0 | 9 | 0 | 9 | 0.00 |
| Lesser scaup (Aythya affinis) | 0 | 11 | 0 | 8 | 0.00 | 0 | 36 | 0 | 12 | 0.00 |
| Canada goose (Branta canadensis) | 0 | 0 | 0 | 0 | 0.00 | 0 | 7 | 0 | 7 | 0.00 |
| Snow goose (Chen caerulescens) | 0 | 2 | 0 | 0 | 0.00 | 0 | 55 | 0 | 8 | 0.00 |
| Ross's goose (Chen rossii) | 0 | 0 | 0 | 0 | 0.00 | 0 | 14 | 0 | 5 | 0.00 |
| Ruddy duck (Oxyura jamaicensis) | 0 | 0 | 0 | 0 | 0.00 | 0 | 30 | 0 | 7 | 0.00 |
| Canvasback (Aythya valisineria) | 0 | 20 | 0 | 3 | 0.00 | 0 | 44 | 0 | 0 | 0.00 |
| Grand total | 114 | 1295 | 175 | 1667 | 9.76 | 54 | 3470 | 128 | 3957 | 2.45 |
The five species of dabbling duck included in statistical analysis.
AIV, avian influenza virus.
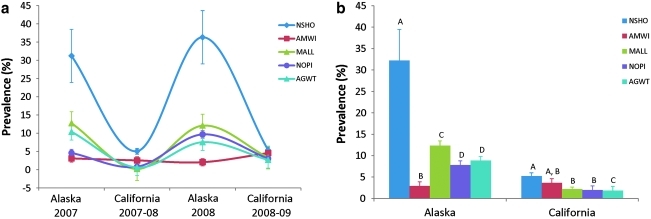
The prevalence of AIV was significantly different among the five dabbling species in both Alaska (Wald χ2=54.64, p<0.001) and California (Wald χ2=26.44, p<0.001) (Fig. 2a). In Alaska, NSHO (32.2%, 95% CI: 22.8% to 41.6%) had a significantly higher prevalence than MALL (12.4%, 95% CI: 10.1% to 14.6%), AGWT (8.8%, 95% CI: 4.1% to 11.3%), NOPI (7.8%, 95% CI: 6.2% to 9.2%), and AMWI (2.9%, 95% CI: 0.5% to 5.2%; Fig. 2b). In California, NSHO (5.2% 95% CI: 4.0% to 6.4%) and AMWI (3.6%, 95% CI: 2.5% to 4.7%) had a significantly higher prevalence than MALL (2.2%, 95% CI: 1.7% to 2.8%), NOPI (2.0%, 95% CI: 1.1% to 3.4%), and AGWT (1.9%, 95% CI: 1.2% to 2.5%; Fig. 2b). The mean monthly prevalence in Alaska was higher in September (17.9%, 95% CI: 11.6% to 23.7%) than in August (4.1%, 95% CI: 2.7% to 5.1%; Table 2). In California, the mean monthly prevalence was highest in October (5.6%, 95% CI: 3.3% to 6.8%) and lowest in January (0.8%, 95% CI: 0.1% to 1.2%) (Table 2).
FIG. 2.
The prevalence of avian influenza virus (with standard error bars) in dabbling ducks from Alaska and California according to (a) sampling year and (b) sampling site (significant differences between species at each location are indicated by nonmatching letters). AGWT, American green-winged teal; AMWI, American wigeon; MALL, mallard; NOPI, northern pintail; NSHO, northern shoveler.
Table 2.
Mean Monthly Prevalence of Avian Influenza Virus and Stages of the Annual Cycle for Dabbling Ducks in Alaska and California
| |
Alaska |
California |
|||||
|---|---|---|---|---|---|---|---|
| Host species | August | September | October | November | December | January | |
| American wigeon | 0 (1:5.0) | 3.0 (1:5.9) | 11.0 (1:2.1) | 7.1 (1:3.2) | 0.3 (1:1.3) | 0.6 (1:0.3) | |
| American green-winged teal | 4.8 (1:0.9) | 15.3 (1:4.8) | 3.0 (1:1) | 2.7 (1:0.4) | 1.4 (1:0.6) | 1.1 (1:0.3) | |
| Mallard | 9.1 (1:0.5) | 23.9 (1:2.1) | 4.1 (1:0.2) | 3.1 (1:0.4) | 1.5 (1:1.8) | 0.4 (1:1.1) | |
| Northern pintail | 6.6 (1:1.7) | 15.0 (1:3.5) | 3.2 (1:0.9) | 4.0 (1:0.9) | 0.4 (0:0.1) | 0.7 (1:0.2) | |
| Northern shoveler | — | 32.2 (1:7.0) | 6.4 (1:3.0) | 4.9 (1:1.6) | 6.2 (1:1.3) | 1.0 (1:0.6) | |
| Mean monthly prevalence | 4.1 (1:1.1) | 17.9 (1:3.8) | 5.6 (1:0.9) | 4.4 (1:0.9) | 2.0 (1:1.1) | 0.8 (1:0.5) | |
| Premigration staging | |||||||
| Fall migration | |||||||
| Overwintering | |||||||
The ratio of after hatch-year to hatch-year birds sampled is shown in parentheses. The highest monthly prevalence of AIV and ratio of hatch-year birds are highlighted in bold.
AIV subtypes
Viruses were isolated and complete HA-NA subtype information was obtained from 2.7% of the Alaskan samples (80/2962) and 2.2% of samples from California (164/7427). Twelve of the 16 known HA subtypes (H1–H12) and all 9 NA subtypes were detected during the study. In Alaska, six of the HA subtypes (H1, H3, H4, H8, H9, and H12) and eight of the NA subtypes (N1–N9) were isolated. In California, 10 of the HA subtypes (H1–H7 and H10–H12) and all 9 NA subtypes were detected. The subtype H5N1 was not detected and all H5 and H7 viruses were characterized as low pathogenic by the National Veterinary Services Laboratory.
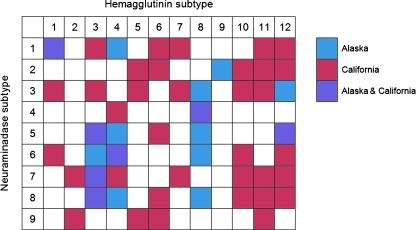
A total of 52 different AIV subtype combinations were identified from the five dabbling duck species in this study. Most viral subtypes (67.3%, 35/52) were found exclusively in California, whereas 19.2% (10/52) were found only in Alaska, and 13.5% (7/52) were found in both Alaska and California (Fig. 3). Among the five dabbling ducks species, the mean number of subtypes detected in California (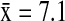 ) was double the number detected in Alaska (
) was double the number detected in Alaska (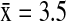 ). In Alaska, NSHO (
). In Alaska, NSHO (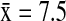 ) hosted a significantly higher mean diversity of subtypes (Wald χ2=27.07, p<0.001) than NOPI (
) hosted a significantly higher mean diversity of subtypes (Wald χ2=27.07, p<0.001) than NOPI (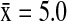 ), MALL (
), MALL (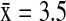 ), AGWT (
), AGWT (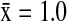 ), and AMWI (
), and AMWI (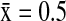 ). In California, NSHO (
). In California, NSHO (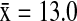 ) and MALL (
) and MALL (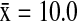 ) hosted a significantly higher number of subtypes (Wald χ2=9.92, p=0.043) than the AGWT (
) hosted a significantly higher number of subtypes (Wald χ2=9.92, p=0.043) than the AGWT (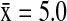 ), AMWI (
), AMWI (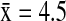 ) and NOPI (
) and NOPI (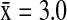 ).
).
FIG. 3.
Distribution of hemagglutinin and neuraminidase subtypes in the five dabbling duck species from Alaska (n=17 virus subtypes) and California (n=42 virus subtypes) during 2007–2009.
In Alaska, the most common subtype was H3N8 (68.8%), followed by H4N6 (18.3%) and H4N8 (4.9%). In California, different subtypes dominated the virus pool, including H6N1 (39.4%), H10N7 (7.9%), and H11N9 (3.6%). The most prevalent subtypes in each location also demonstrated the broadest host range. In Alaska, H3N8 showed the broadest host range, infecting five species; in California, H6N1 had the broadest host range, infecting eight species. The eight subtypes identified as common to both Alaska and California were H1N1, H3N5, H3N7, H3N8, H4N6, H4N8, H8N4, and H12N5. The most prevalent subtype common to both breeding and wintering habitat among waterfowl was H3N8.
Mixed infections
A total of nine cases of mixed infections were identified in this study based on the presence of multiple bands or sequences during genetic subtyping by Sanger sequencing (Table 3). Of these, four occurred in Alaska (44.0%) and five occurred in California (56.0%). Northern shovelers hosted three cases of mixed infections (H3N8–H4N8, H3N5–H3N8, and H3N6–H3N8). Mallards hosted two cases of mixed infections (H11N2–H11N7 and H12N7–H12N8), as did NOPI (H6N1–H6N8 and H8N5–H8N6). In terms of age, seven cases of mixed infections were from HY (77.8%) and two were from AHY (22.2%).
Table 3.
The Nine Cases of Mixed Infections of Avian Influenza Virus Detected in Waterfowl from Alaska and California
| State | Sampling date | Species | Age | Sex | HA subtype | NA subtype |
|---|---|---|---|---|---|---|
| AK | September 2, 2007 | Northern shoveler | HY | F | H3 | N6 and N8 |
| AK | September 5, 2007 | Northern shoveler | HY | F | H3 and H4 | N8 |
| AK | August 17, 2008 | Northern pintail | HY | M | H8 | N5 and N6 |
| AK | September 13, 2008 | Northern shoveler | HY | F | H3 | N5 and N8 |
| CA | October 25, 2008 | Bufflehead | AHY | F | H4 | N7 and N8 |
| CA | November 5, 2008 | Mallard | HY | M | H12 | N7 and N8 |
| CA | November 15, 2008 | Northern pintail | AHY | M | H6 | N1 and N8 |
| CA | November 15, 2008 | American wigeon | HY | F | H11 | N1 and N8 |
| CA | December 6, 2008 | Mallard | HY | M | H11 | N2 and N7 |
AHY, after hatch-year; AK, Alaska; CA, California; F, female; HA, hemagglutinin; HY, hatch-year; M, male; NA, neuraminidase.
Discussion
Our cross-seasonal study within the Pacific Flyway revealed that NSHO was an important host for AIV throughout the year, reaching a prevalence of 32.2% in Alaska and 5.2% in California—the highest of all waterfowl species at each site. The number of NSHO swabbed in Alaska was small and could only be sampled in September, when hunting was permitted. There are biases implicit with hunter-harvesting because young birds are more vulnerable to being shot (Cox et al. 1998, Pace and Afton 1999); therefore, susceptible individuals may have been over-represented in the sample. This situation warrants continued sampling of NSHO at the breeding grounds. Unlike the majority of studies that report the MALL as the waterfowl species with highest prevalence of AIV (Kocan et al. 1980, Hinshaw et al. 1986, Hanson et al. 2003, Runstadler et al. 2007), this species was of secondary importance in Alaska relative to the NSHO. The MALL has long been a focus of surveillance studies owing to its wide distribution and relative abundance across North America and Europe. In addition, its popularity as a table bird for consumption may explain why it is favored in hunter-harvested sampling. The emergence of the NSHO as an important host suggests that sample sizes may have been too low in earlier studies to accurately detect AIV in this species. This may be related to the distribution of this species across migratory flyways. The NSHO frequents wintering grounds in the Pacific and Central Flyways, with less than 3% of the continental population wintering east of the Mississippi River (Bellrose 1976). Unique ecological traits such as the filter feeding habits of this species may heighten exposure to AIV particles in the surface film (Hill et al. 2010). Owing to high lamellar density, the NSHO is efficient at filtering fine particles from the water compared with other dabblers (Gurd 2007), potentially increasing the risk of AIV infection during foraging.
At wintering grounds in California, AMWI also showed a high prevalence of AIV relative to other dabbling species. Similar to NSHO, few other surveillance studies have identified this species as an important host of the virus (except see: Alfonso et al. 1995). The Pacific Flyway is the main migratory corridor for AMWI with the majority of the population wintering in the CCV estimated at ∼900,000 birds (U.S. Fish and Wildlife Service 2009). Neither AMWI nor NSHO are early migrants to the CCV, an ecological trait previously associated with high AIV prevalence in wintering bird populations (Stallknecht et al. 1990c, Ferro et al. 2010a). For example, the blue-winged teal (Anas discors) is one of the first species to fly south in the fall (Bellrose 1976), and therefore infected individuals have the potential to continue shedding virus between the breeding and wintering grounds. This species also travels in long, uninterrupted distances and may therefore be more susceptible to infection owing to a demanding migration strategy (Ferro et al. 2010a). The cinnamon teal (Anas cyanoptera) is the Pacific Flyway equivalent of the blue-winged teal, but unfortunately was not sampled in adequate numbers to test this prediction. Other duck species that are among the first to arrive at the CCV include the NOPI and AGWT (Bellrose 1976). The lack of association between early migration and risk of AIV infection suggests other factors may be involved in the pattern of host species observed among overwintering birds.
HY birds were at higher risk of infection than mature birds at both breeding and wintering sites. Prevalence was almost twice as high among HY birds compared with AHY birds in both Alaska and California. HY birds are viewed as important hosts for the virus because they are immunonaïve and lack developed immune systems required to limit AIV infection (Webster et al. 1992, Olsen et al. 2006, Guberti et al. 2007). Prevalence peaked in September coinciding with large numbers of HY birds staging before fall migration. Susceptibility of juvenile birds coupled with their high density during premigration staging may be important factors in facilitating fecal–oral transmission in Alaska. The overall low prevalence in California (2.5%) was consistent with AIV studies of overwintering bird populations [0.4% in Louisiana (Stallknecht et al. 1990c), and 0.8%–2% in Texas (Ferro et al. 2008, 2010a)] and suggested limited circulation on the wintering grounds. In addition, a decline in the mean AIV prevalence between October and January was observed. In overwintering birds, AIV prevalence diminishes as HY acquire resistance to infection (De Marco et al. 2003). The growing number of immune birds in California may explain the decline in mean AIV prevalence of overwintering birds.
Despite the low prevalence at wintering sites in California, the diversity of AIV subtypes was considerably higher than at breeding grounds in Alaska. Dabbling ducks from California shed 42 different AIV subtypes during the 2-year study, compared with 17 AIV subtypes shed by ducks from Alaska. This trend should be interpreted with caution because of the possible differences in sensitivity between the virus isolation methods used in Alaska and California. However, the high diversity of subtypes at wintering grounds has been previously noted by Stallknecht et al. (1990c), who suggested that exchange of AIV subtypes between previously unassociated species and populations was responsible. Wintering grounds may act as migration bottlenecks, attracting a variety of species from different breeding sites, even outside the migratory flyway. Band recoveries indicate that birds that overwinter in the CCV come from a variety of breeding grounds, including Alaska, the prairie pothole regions in northwest Canada, and northern states of the United States (Bellrose 1976). In addition, the sweeping conversion of natural habitat to agriculture in the last century has reduced the availability of wetlands across North America (Dahl and Johnson 1991), resulting in higher densities of overwintering waterfowl in fewer areas (Ankney 1996). The majority of losses have occurred in the CCV with disappearance of 90% of wetlands (Gilmer et al. 1982). This is one of the most important rice-growing regions in the United States where natural habitat is now limited to managed wetlands such as wildlife refuges or rice fields that are flooded postharvest (Elphick and Oring 1998). This concentration of overwintering sites that support over 6 million birds each winter in the CCV (Reid and Heitmeyer 1995) may facilitate intermingling of different species originating from various breeding grounds, resulting in exchange of subtypes. This is consistent with studies that have modeled AIV risk in North America and concluded that agriculture is an important predictor of outbreaks (Fuller et al. 2010). Our data suggest that the CCV may be an important mixing bowl for AIV subtypes that circulate along the Pacific Flyway, with implications for the reassortment and evolution of the virus. Phylogenetic studies are needed to assess the degree of reassortment at wintering (compared with breeding grounds) and clarify whether this stage in the annual cycle is critical for the genesis of new viruses that persist over time.
A unique finding of this study was evidence of mixed infection at both breeding and wintering sites, indicating that conditions at Alaska and California were conducive to reassortment of AIV subtypes. Very few surveillance studies have reported mixed infections in migratory birds, possibly due to the focus on H5 and H7, the main HA subtypes viewed as having public health and agricultural significance. Interestingly, neither H5 nor H7 were associated with mixed infections in our study population. Instead, mixed infections were most commonly associated with the H3 subtype in Alaska and the H11 subtype in California. A phylogenetic study by Dugan et al. (2008) identified a high frequency (26%) of reassortment between AIV from wild birds in North America. In contrast, we only investigated mixed infections associated with the HA and NA genes from samples amplified in eggs, and by excluding analysis of internal genes we likely underestimated the number of mixed infections. The virus is in a constant state of genomic reshuffling wherever wild birds congregate, a hypothesis supported by numerous phylogenetic studies (Spackman et al. 2005, Macken et al. 2006, Dugan et al. 2008, Chen and Holmes 2009). Mixed infections in the Pacific Flyway were most often isolated from HY rather than adult birds, and were more commonly associated with the NSHO. During the wintering period in California, this species hosted 12 and 14 subtypes in 2007–2008 and 2008–2009, respectively. Based on these results, juvenile NSHO would be the most likely candidates for potential reassortment of AIV in dabbling ducks. Similarly, Ferro et al. (2010a) found the highest subtype diversity in the NSHO and the blue-winged teal at overwintering sites in Texas. Whether certain species are predisposed to make better hosts for mixed infections because of ecological, pathobiological, or immunological factors would be an ideal subject for future investigation.
Isolation of AIV from waterfowl indicated that subtype pools were relatively distinct between Alaska and California, with only 13.5% of subtypes common to both regions. It should be clarified that our study did not prove direct connectivity between the migratory bird population sampled at Alaska and California. Marking birds with satellite transmitters could shed light on the precise migratory movements of the sampled population, an activity beyond the scope of the present study. However, our study provides preliminary evidence that there is little overlap in subtypes between these two important bird habitats within the Pacific Flyway. Within Alaska, H3N8 was the predominate subtype (68.8%) followed by H4N6 (18.3%). These two subtypes appear to be fixtures among the virus pool in Alaska, having been identified as common in wild bird studies conducted in 1995 (Ito et al. 1995) and 2005 to 2010 (Runstadler et al. 2007; and unpublished data). These subtypes have also been identified as commonly circulating subtypes in the Central Flyway from Texas (Ferro et al. 2010a) to Alberta (Krauss et al. 2004, Pasick et al. 2010). Within California, H6N1 was the dominant subtype (39.4%) among overwintering birds followed by H10N7 (7.9%). While H6N1 has been a recent fixture of subtype pools in California (Siembieda et al. 2010) and Texas (Ferro et al. 2010), before 1986 the more common subtype in wild duck populations in North America was H6N2 (Krauss et al. 2004), suggesting that H6N1 may have outcompeted its predecessor. Of interest, the two prevailing subtypes in Alaska (H3N8) and California (H6N1) possessed broad host ranges infecting eight and five species, respectively. Our findings are consistent with the notion that there has been relative stability in the circulation of H3N8 and H6N1 subtypes over the last decade in the Pacific Flyway, potentially due to their broad host range.
In view of the low prevalence of AIV during the overwintering period in California, our findings raise the question: is prevalence during the late winter sufficient to support northward transport of the virus to the breeding grounds by birds undertaking spring migration? In a study of AIV in common teal (Anas crecca) from Camargue, France (Lebarbenchon et al. 2009), the circulation of subtypes among the overwintering population reached a critically low level just before spring migration, such that the authors predicted few subtypes would be exported to the breeding grounds. Shorebirds show a south–north gradient of AIV prevalence and have been suggested to play a role in the infection of waterfowl that overlap in breeding distribution at northern latitudes (Krauss et al. 2004). Shorebirds harbor a wider variety of subtypes than waterfowl and may be the primary host of subtypes such as H9, H10, and H11 that are only occasionally isolated from waterfowl (Krauss et al. 2004, Hanson et al. 2008). However, populations sampled along the Pacific Coast show very low AIV prevalence (Iverson et al. 2008). Alternatively, environmental persistence of the virus at breeding grounds during the winter (Ito et al. 1995) may also be responsible for the infection of spring migrants arriving in Alaska, particularly in view of experimental studies that demonstrate that cold conditions extend the period of infectivity (Stallknecht et al. 1990a, 1990b). Sampling at stopover sites during the spring migration may elucidate the prevalence and subtypes hosted by waterfowl and shorebirds along the migratory flyway. Stopover sites are of particular importance in view of the possibility that AIV may be spread by relay transmission with exchange between birds at wetlands during spring or fall migration, rather than a single uninterrupted dispersal event (Olsen et al. 2006, Latorre-Margalef et al. 2009, Gaidet et al. 2010).
In conclusion, sampling at both ends of a migratory flyway provided insights into the spatio-temporal heterogeneity in AIV infection patterns—a hallmark of the virus (Olsen et al. 2006, Munster et al. 2007). We found a high diversity of subtypes in California compared with Alaska that may be a product of exchange between waterfowl species from a variety of breeding grounds that are concentrated during the winter in the wetlands of the CCV. The conversion of natural wetlands to agriculture in recent decades has concentrated the available roosting habitat for species such as the greater white-fronted goose (Anser albifrons frontalis) that overwinter in the CCV (Ackerman et al. 2006), and may provide conditions conducive to the reassortment and evolution of AIV in waterfowl. In view of the high prevalence of AIV and diversity of subtypes hosted by the NSHO, surveillance efforts in the Pacific Flyway should not be limited to the MALL and NOPI. Our understanding of how waterfowl species contribute to the dispersal of AIV is in its infancy and future research should be directed at more thoroughly investigating this question at the flyway scale.
Acknowledgments
We wish to thank staff at UC Davis, including Julie Nelson for assistance with waterfowl sampling and Nichole Anchell, Nguyet Dao, Tracey Goldstein, Leann Lindsay, and Magdalena Plancarte for virological analysis of Californian samples. At UAF, we thank Florian Aldehoff, Danielle Dillon, Paige Gingrich, Lori Gildehaus, Nancy Gundlach, and Jynene Black for conducting analyses of Alaskan samples. At the USGS, we thank Annie Shultz, Kyle Spragens, Brittany Wensky, and Garth Herring for help with sampling waterfowl and Lacy Smith for assistance with GIS mapping. Sampling at the Minto Flats State Game Refuge was conducted in collaboration with Mike Petrula of Alaska Department of Fish and Game. Our thanks to all staff at Sacramento NWR, in particular Michael Wolder, Kevin Foerster, and Greg Mensik. We also appreciate the assistance of Dan Yparraguirre, Andy Atkinson, Tim Caldwell, and California Department of Fish and Game check station employees. Owners of Conaway Ranch Duck Club granted permission to collect samples—particular thanks to Mike Hall, the Wildlife Manager of Conaway Ranch and Preservation Group. We thank Ken Richkus, Robert Trost, and the staff at the Pacific Flyway Wingbee for help with aging duck wings from California. This study was funded by the National Institute of Allergy and Infectious Diseases (contracts HHSN266200700007C and HHSN266200700009C) and by the U.S. Fish and Wildlife Service, Alaska Region. Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. government.
Disclosure Statement
No competing financial interests exist.
References
- Ackerman J. Takekawa JY. Orthmeyer DL. Fleskes JP, et al. Spatial use by wintering greater white-fronted geese relative to a decade of habitat change in California's Central Valley. J Wildl Manag. 2006;70:965–976. [Google Scholar]
- Alfonso CP. Cowen BS. van Campen H. Influenza A viruses isolated from waterfowl in two wildlife management areas of Pennsylvania. J Wildl Dis. 1995;31:179–185. doi: 10.7589/0090-3558-31.2.179. [DOI] [PubMed] [Google Scholar]
- Ankney CD. An embarrassment of riches: too many geese. Wildl Soc Bull. 1996;60:217–223. [Google Scholar]
- Bahl J. Vijaykrishna D. Holmes EC. Smith GJ, et al. Gene flow and competitive exclusion of avian influenza A virus in natural reservoir hosts. Virology. 2009;390:289–297. doi: 10.1016/j.virol.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellrose FC. Ducks, Geese and Swans of North America. Harrisburg: Stackpole Books; 1976. [Google Scholar]
- Bush AO. Lafferty KD. Lotz JM. Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- Chen R. Holmes EC. Frequent inter-species transmission and geographic subdivision in avian influenza viruses from wild birds. Virology. 2009;383:156–161. doi: 10.1016/j.virol.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant B. Hodges JI. Minto Flats Waterfowl Resources. Anchorage, AL: U.S. Fish and Wildlife Service; 1985. pp. 1–7. [Google Scholar]
- Cox RR., Jr. Afton AD. Pace RMI. Survival of female northern pintails wintering in southwestern Louisiana. J Wildl Manag. 1998;62:1512–1521. [Google Scholar]
- Dahl TE. Johnson CE. Status and Trends of Wetlands in the Conterminous United States, mid-1970s to mid-1980s. Washington, D.C.: U.S. Fish and Willdife Service; 1991. [Google Scholar]
- De Marco MA. Foni E. Campitelli L. Raffini E, et al. Long-term monitoring for avian influenza viruses in wild bird species in Italy. Vet Res Commun. 2003;27(Suppl 1):107–114. doi: 10.1023/b:verc.0000014126.72654.22. [DOI] [PubMed] [Google Scholar]
- Deibel R. Emord DE. Dukelow W. Hinshaw VS, et al. Influenza viruses and paramyxoviruses in ducks in the Atlantic flyway, 1977–1983, including an H5N2 isolate related to the virulent chicken virus. Avian Dis. 1985;29:970–985. [PubMed] [Google Scholar]
- Dugan VG. Chen R. Spiro DJ. Sengamalay N, et al. The evolutionary genetics and emergence of avian influenza viruses in wild birds. PLoS Pathog. 2008;4:e1000076. doi: 10.1371/journal.ppat.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elphick CS. Oring LW. Winter management of Californian rice fields for waterbirds. J Appl Ecol. 1998;35:95–108. [Google Scholar]
- Ferro PJ. Budke CM. Peterson MJ. Cox D, et al. Multiyear surveillance for avian influenza virus in waterfowl from wintering grounds, Texas coast, USA. Emerg Infect Dis. 2010a;16:1224–1230. doi: 10.3201/eid1608.091864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro PJ. Peterson MJ. Merendino T. Nelson M, et al. Comparison of real-time reverse transcription-PCR and virus isolation for estimating prevalence of avian influenza virus in hunter-harvested wild birds at waterfowl wintering grounds along the Texas mid-Gulf Coast (2005–2006 through 2008–2009) Avian Dis. 2010b;54:655–659. doi: 10.1637/8810-040109-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Fuller TL. Saatchi SS. Curd EE. Toffelmier E, et al. Mapping the risk of avian influenza in wild birds in the US. BMC Infect Dis. 2010;10:187. doi: 10.1186/1471-2334-10-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidet N. Cappelle J. Takekawa JY. Prosser DJ, et al. Potential spread of highly pathogenic avian influenza H5N1 by wildfowl: dispersal ranges and rates determined from large-scale satellite telemetry. J Appl Ecol. 2010;47:1169–1179. [Google Scholar]
- Gilmer DS. Miller MR. Bauer RD. LeDonne JR. California's Central Valley wintering waterfowl: concerns and challenges. Transactions N Am Wildl Nat Resour Conf. 1982;47:441–452. [Google Scholar]
- Goekjian VH. Smith JT. Howell DL. Senne DA, et al. Avian influenza viruses and avian paramyxoviruses in wintering and breeding waterfowl populations in North Carolina, USA. J Wildl Dis. 2011;47:240–245. doi: 10.7589/0090-3558-47.1.240. [DOI] [PubMed] [Google Scholar]
- Guberti V. Scremin M. Busani L. Bonfanti L, et al. A simulation model for low-pathogenicity avian influenza viruses in dabbling ducks in Europe. Avian Dis. 2007;51:275–278. doi: 10.1637/7633-042806R1.1. [DOI] [PubMed] [Google Scholar]
- Gurd DB. Predicting resource partitioning and community organization of filter-feeding dabbling ducks from functional morphology. Am Nat. 2007;169:334–343. doi: 10.1086/510924. [DOI] [PubMed] [Google Scholar]
- Hansbro PM. Warner S. Tracey JP. Arzey KE, et al. Surveillance and analysis of avian influenza viruses, Australia. Emerg Infect Dis. 2010;16:1896–1904. doi: 10.3201/eid1612.100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson BA. Luttrell MP. Goekjian VH. Niles L, et al. Is the occurrence of avian influenza virus in Charadriiformes species and location dependent? J Wildl Dis. 2008;44:351–361. doi: 10.7589/0090-3558-44.2.351. [DOI] [PubMed] [Google Scholar]
- Hanson BA. Stallknecht DE. Swayne DE. Lewis LA, et al. Avian influenza viruses in Minnesota ducks during 1998–2000. Avian Dis. 2003;47:867–871. doi: 10.1637/0005-2086-47.s3.867. [DOI] [PubMed] [Google Scholar]
- Hanson BA. Swayne DE. Senne DA. Lobpries DS, et al. Avian influenza viruses and paramyxoviruses in wintering and resident ducks in Texas. J Wildl Dis. 2005;41:624–628. doi: 10.7589/0090-3558-41.3.624. [DOI] [PubMed] [Google Scholar]
- Hill NJ. Takekawa JY. Cardona CJ. Ackerman JT, et al. Waterfowl ecology and avian influenza in California: do host traits inform us about viral occurrence? Avian Dis. 2010;54:426–432. doi: 10.1637/8912-043009-Reg.1. [DOI] [PubMed] [Google Scholar]
- Hinshaw VS. Nettles VF. Schorr LF. Wood JM, et al. Influenza virus surveillance in waterfowl in Pennsylvania after the H5N2 avian outbreak. Avian Dis. 1986;30:207–212. [PubMed] [Google Scholar]
- Hinshaw VS. Webster RG. Turner B. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can J Microbiol. 1980;26:622–629. doi: 10.1139/m80-108. [DOI] [PubMed] [Google Scholar]
- Hinshaw VS. Wood JM. Webster RG. Deibel R, et al. Circulation of influenza viruses and paramyxoviruses in waterfowl originating from two different areas of North America. Bull World Health Organ. 1985;63:711–719. [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. Stech J. Guan Y. Webster RG, et al. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol. 2001;146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- Hoye BJ. Munster VJ. Nishiura H. Fouchier RAM, et al. Reconstructing an annual cycle of interaction: natural infection and antibody dynamics to avian influenza along a migratory flyway. Oikos. 2010;120:748–755. [Google Scholar]
- Hunt GS. Dahlka KJ. Live trapping of diving ducks. J Wildl Manag. 1953;17:92–97. [Google Scholar]
- Ip HS. Flint PL. Franson JC. Dusek RJ, et al. Prevalence of Influenza A viruses in wild migratory birds in Alaska: patterns of variation in detection at a crossroads of intercontinental flyways. Virol J. 2008;5:71. doi: 10.1186/1743-422X-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T. Okazaki K. Kawaoka Y. Takada A, et al. Perpetuation of influenza A viruses in Alaskan waterfowl reservoirs. Arch Virol. 1995;140:1163–1172. doi: 10.1007/BF01322743. [DOI] [PubMed] [Google Scholar]
- Iverson SA. Takekawa JY. Schwarzbach SE. Cardona CJ, et al. Low prevalence of avian influenza virus in shorebirds on the Pacific Coast of North America. Waterbirds. 2008;31:602–610. [Google Scholar]
- Kocan AA. Hinshaw VS. Daubney GA. Influenza A viruses isolated from migrating ducks in Oklahoma. J Wildl Dis. 1980;16:281–285. doi: 10.7589/0090-3558-16.2.281. [DOI] [PubMed] [Google Scholar]
- Koehler AV. Pearce JM. Flint PL. Franson JC, et al. Genetic evidence of intercontinental movement of avian influenza in a migratory bird: the northern pintail (Anas acuta) Mol Ecol. 2008;17:4754–4762. doi: 10.1111/j.1365-294X.2008.03953.x. [DOI] [PubMed] [Google Scholar]
- Krauss S. Walker D. Pryor SP. Niles L, et al. Influenza A viruses of migrating wild aquatic birds in North America. Vector Borne Zoonotic Dis. 2004;4:177–189. doi: 10.1089/vbz.2004.4.177. [DOI] [PubMed] [Google Scholar]
- Latorre-Margalef N. Gunnarsson G. Munster VJ. Fouchier RAM, et al. Effects of influenza A virus infection on migrating mallard ducks. Proc Roy Soc B. 2009;276:1029–1036. doi: 10.1098/rspb.2008.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebarbenchon C. Albespy F. Brochet AL. Grandhomme V, et al. Spread of avian influenza viruses by common teal (Anas crecca) in Europe. PLoS One. 2009;4:e7289. doi: 10.1371/journal.pone.0007289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macken CA. Webby RJ. Bruno WJ. Genotype turnover by reassortment of replication complex genes from avian influenza A virus. J Gen Virol. 2006;87:2803–2815. doi: 10.1099/vir.0.81454-0. [DOI] [PubMed] [Google Scholar]
- Miller MR. Sharp DE. Gilmer DS. Mulvaney WR. Rice available to waterfowl in harvested fields in the Sacramento Valley of California. California Fish and Game. 1989;75:113–123. [Google Scholar]
- Miller MR. Takekawa JY. Fleskes JP. Orthmeyer DL, et al. Spring migration of Northern Pintails from California's Central Valley wintering area tracked with satellite telemetry: routes, timing, and destinations. Can J Zoolog. 2005;83:1314–1332. [Google Scholar]
- Munster VJ. Baas C. Lexmond P. Bestebroer TM, et al. Practical considerations for high-throughput influenza A virus surveillance studies of wild birds by use of molecular diagnostic tests. J Clin Microbiol. 2009;47:666–673. doi: 10.1128/JCM.01625-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ. Baas C. Lexmond P. Waldenstrom J, et al. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. PLoS Pathog. 2007;3:e61. doi: 10.1371/journal.ppat.0030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B. Munster VJ. Wallensten A. Waldenstrom J, et al. Global patterns of influenza a virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- Pace RMI. Afton AD. Direct recovery rates of lesser scaup banded in northwestern Minnesota: sources of heterogeneity. J Wildl Manag. 1999;63:389–395. [Google Scholar]
- Pacific Flyway Council. Surveillance for early detection of highly pathogenic avian influenza H5N1 in migratory birds—a strategy for the Pacific Flyway. 2006. p. 21.
- Pasick J. Berhane Y. Kehler H. Hisanaga T, et al. Survey of influenza A viruses circulating in wild birds in Canada 2005 to 2007. Avian Dis. 2010;54:440–445. doi: 10.1637/8800-040109-Reg.1. [DOI] [PubMed] [Google Scholar]
- Pearce JM. Ramey AM. Flint PL. Koehler AV, et al. Avian influenza at both ends of a migratory flyway: characterizing viral genomic diversity to optimize surveillance plans for North America. Evol Appl. 2009;2:457–468. doi: 10.1111/j.1752-4571.2009.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps LP. Essen SC. Brown IH. Genetic subtyping of influenza A viruses using RT-PCR with a single set of primers based on conserved sequences within the HA2 coding region. J Virol Methods. 2004;122:119–122. doi: 10.1016/j.jviromet.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Reid FA. Heitmeyer ME. Waterfowl and rice in the California's Central Valley. Calif Agri. 1995;49:62. [Google Scholar]
- Runstadler JA. Happ GM. Slemons RD. Sheng Z-M, et al. Using RRT-PCR analysis and virus isolation to determine the prevalence of avian influenza virus infections in ducks in Minto Flats State Game Refuge, Alaska, during August 2005. Arch Virol. 2007;152:1901–1910. doi: 10.1007/s00705-007-0994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp GB. Kawaoka Y. Wright SM. Turner B, et al. Wild ducks are the reservoir for only a limited number of influenza A subtypes. Epidemiol Infec. 1993;110:161–176. doi: 10.1017/s0950268800050780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siembieda JL. Johnson CK. Cardona C. Anchell N, et al. Influenza A Viruses in Wild Birds of the Pacific Flyway, 2005–2008. Vector Borne Zoonotic Dis. 2010;10:793–800. doi: 10.1089/vbz.2009.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E. Senne DA. Bulaga LL. Myers TJ, et al. Development of real-time RT-PCR for the detection of avian influenza virus. Avian Dis. 2003;47:1079–1082. doi: 10.1637/0005-2086-47.s3.1079. [DOI] [PubMed] [Google Scholar]
- Spackman E. Senne DA. Myers TJ. Bulaga LL, et al. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spackman E. Stallknecht DE. Slemons RD. Winker K, et al. Phylogenetic analyses of type A influenza genes in natural reservoir species in North America reveals genetic variation. Virus Res. 2005;114:89–100. doi: 10.1016/j.virusres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- SPSS. SPSS for Macintosh, 16.0. Chicago, IL: SPSS Inc.; 2007. [Google Scholar]
- Stallknecht DE. Brown JD. Ecology of avian influenza in wild birds. In: Swayne D, editor. Avian Influenza. Iowa: Blackwell Publishing; 2008. pp. 43–58. [Google Scholar]
- Stallknecht DE. Kearney MT. Shane SM. Zwank PJ. Effects of pH, temperature, and salinity on persistence of avian influenza viruses in water. Avian Dis. 1990a;34:412–418. [PubMed] [Google Scholar]
- Stallknecht DE. Shane SM. Kearney MT. Zwank PJ. Persistence of avian influenza viruses in water. Avian Dis. 1990b;34:406–411. [PubMed] [Google Scholar]
- Stallknecht DE. Shane SM. Zwank PJ. Senne DA, et al. Avian influenza viruses from migratory and resident ducks of coastal Louisiana. Avian Dis. 1990c;34:398–405. [PubMed] [Google Scholar]
- Swayne DE. Senne DA. Beard CW. Avian Influenza. In: Glisson JR, editor; Swayne DE, editor; Jackwood MW, editor; Pearson JE, editor; Reed WM, editor. A Laboratory Manual for the Isolation and Identification of Avian Pathogens. Kennett Square: American Association of Avian Pathologists; 1998. pp. 150–155. [Google Scholar]
- U.S. Avian Influenza Interagency Working Group. An Early Detection System for Highly Pathogenic H5N1 Avian Influenza in Wild Migratory Birds. US Department of Agriculture; Washington, DC: 2006. pp. 1–90. [Google Scholar]
- U.S. Fish and Wildlife Service. Winter Waterfowl Survey, Pacific Flyway 2005–2009. Sacramento: U.S. Department of the Interior, Fish and Wildlife Service; 2009. [Google Scholar]
- Wang RX. Soll L. Dugan V. Runstadler J, et al. Examining the hemagglutinin subtype diversity among wild duck-origin influenza A viruses using ethanol-fixed cloacal swabs and a novel RT-PCR method. Virology. 2008;375:182–189. doi: 10.1016/j.virol.2008.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG. Bean WJ. Gorman OT. Chambers TM, et al. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winker K. McCracken KG. Gibson DD. Pruett CL, et al. Movements of birds and avian influenza from Asia into Alaska. Emerg Infect Dis. 2007;13:547–552. doi: 10.3201/eid1304.061072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zu Dohna H. Li J. Cardona CJ. Miller J, et al. Invasions by Eurasian avian influenza virus H6 genes and replacement of the virus' North American clade. Emerg Infect Dis. 2009;15:1040–1045. doi: 10.3201/eid1507.090245. [DOI] [PMC free article] [PubMed] [Google Scholar]