Abstract
Cochlear gene therapy can be a new avenue for the treatment of severe hearing loss by inducing regeneration or phenotypic rescue. One necessary step to establish this therapy is the development of a safe and feasible inoculation surgery, ideally without drilling the bony cochlear wall. The round window membrane (RWM) is accessible in the middle-ear space, but viral vectors placed on this membrane do not readily cross the membrane to the cochlear tissues. In an attempt to enhance permeability of the RWM, we applied hyaluronic acid (HA), a nontoxic and biodegradable reagent, onto the RWM of guinea pigs, prior to delivering an adenovirus carrying enhanced green fluorescent protein (eGFP) reporter gene (Ad-eGFP) at the same site. We examined distribution of eGFP in the cochlea 1 week after treatment, comparing delivery of the vector via the RWM, with or without HA, to delivery by a cochleostomy into the perilymph. We found that cochlear tissue treated with HA-assisted delivery of Ad-eGFP demonstrated wider expression of transgenes in cochlear cells than did tissue treated by cochleostomy injection. HA-assisted vector delivery facilitated expression in cells lining the scala media, which are less accessible and not transduced after perilymphatic injection. We assessed auditory function by measuring auditory brainstem responses and determined that thresholds were significantly better in the ears treated with HA-assisted Ad-eGFP placement on the RWM as compared with cochleostomy. Together, these data demonstrate that HA-assisted delivery of viral vectors provides an atraumatic and clinically feasible method to introduce transgenes into cochlear cells, thereby enhancing both research methods and future clinical application.
Shibata and colleagues report that pre-treating the round window membrane (RWM) of the middle-ear space with hyaluronic acid (HA) leads to wider transgene expression in guinea pig cochlear cells upon adenoviral vector (Ad) delivery to the same site. HA-assisted Ad treatment on the RWM also improved thresholds of auditory function.
Introduction
Future Therapies for preventing or curing deafness due to inner-ear diseases will likely require introduction of viral vectors and other large therapeutic reagents into the cochlea. Efficient and safe delivery of reagents into the cochlea is a challenge, because the cochlea is encased in the temporal bone and cannot be readily approached without surgery. Delivering a meaningful concentration of a reagent requires access to the cochlear fluids, which is a surgical procedure in both humans and experimental animals. In the human ear, that access involves drilling the mastoid bone and opening a cochleostomy in the lateral wall of the cochlea or penetrating the round window membrane (RWM). These invasive procedures pose risks of perilymphatic fistula and other mechanical damage to the cochlea.
Placing reagents in the middle ear using an intratympanic approach would be much less traumatic, with the most effective placement being in the round window niche. The RWM separates the middle-ear cavity and the scala tympani (the cochlear compartment that contains sodium-rich perilymph) and serves as a semipermeable membrane that can selectively absorb and/or secrete molecules, depending on factors such as size, electrical charge, and concentration (Goycoolea and Lundman, 1997; Goycoolea, 2001). The membrane functions as a barrier that most likely provides protection for the inner ear, and may be one of the reasons why recurrent otitis media does not usually lead to inner-ear damage. Although the barrier provided by the RWM is imperative for maintenance and protection, it can also be a viable route for atraumatic diffusion of therapeutic reagents into the inner ear. Development of a novel drug-delivery system that efficiently delivers a meaningful concentration of therapeutic reagents into the inner-ear fluids via the RWM could become a new avenue for the treatment of otological diseases.
Therapeutic reagents could be introduced into the cochlea for treating both hearing and balance diseases. The targets could be sensory cells, nonsensory epithelial cells, neurons, or any other cellular or extracellular element in the cochlea or vestibular organs that can be manipulated for preventing degeneration or enhancing repair and regeneration. The types of reagents may include anti-inflammatory drugs, growth factors (Iwai et al., 2006; Lee et al., 2007), small therapeutic molecules (Maeda et al., 2009), and gene vectors (Staecker et al., 1998; Van de Water et al., 1999; Jero et al., 2001; Aarnisalo et al., 2006). Some of these therapeutic reagents have been found to traverse the RWM when applied onto the round window niche, either directly or infiltrated through an absorbent such as Gelfoam. Anti-inflammatory drugs such as dexamethasone are found at higher concentrations in the perilymph of animals when injected intratympanically into the middle ear than when injected by traditional systemic routes, which is most likely explained by diffusion across the RWM (Salt and Plontke, 2005; Bird et al., 2007; Plontke et al., 2008). Other reagents such as growth factors and gene vectors do not readily cross the RWM (Stover et al., 1999; Jero et al., 2001; Duan et al., 2004; Wimmer et al., 2004), and therefore require the assistance of an absorbent that maintains the contact of the reagent with the RWM and/or induces a transient increase in the permeability of the RWM.
Hyaluronic acid (HA) is a viscous, high-molecular-weight, nonsulfated glycosaminoglycan polysaccharide that is present throughout epithelial, connective, and neural tissue. HA is a nontoxic compound that is biodegradable over time. A concentrated form of HA is used clinically in cosmetic and ophthalmic surgeries and is used pharmaceutically as a substrate for drug delivery. In the inner ear, HA has also been used for cochlear implant surgeries as a lubricant to decrease friction and facilitate safer and deeper insertion of the electrode into the scala tympani (Lehnhardt, 1992; Donnelly et al., 1995). More recently, HA has been used as a substrate to deliver therapeutic molecules such as dexamethasone into the inner ear via the RWM (Selivanova et al., 2003, 2005; Barkdull et al., 2007; Borden et al., 2010). The exact mechanism of this enhanced delivery is not clearly identified. Its hypothesized mechanisms include increased osmotic pressure above the RWM (Bjurström et al., 1987; Bagger-Sjöbäck, 1991; Bagger-Sjöbäck et al., 1993), enhanced membrane permeability (Selivanova et al., 2003), and prolonged contact due to the high viscosity of HA allowing reagents to traverse the RWM (Chandrasekhar et al., 2000). Intratympanic injection of dexamethasone with HA produced a higher concentration of dexamethasone in perilymph than did intratympanic or systemic injection of dexamethasone alone (Chandrasekhar et al., 2000; Borden et al., 2010). Clinical trials using intratympanic administration of dexamethasone with HA for the treatment of refractory idiopathic sudden deafness or Ménière's disease have shown significant improvement of auditory function without systemic or local side effects (Gouveris et al., 2005; Selivanova et al., 2005). These trials indicate that HA can be used safely and efficiently as a drug-delivery system in the ear.
Delivery of most pharmacological reagents leads to a short-term activity limited by the diffusion, stability, metabolism, and clearance of the drugs. To obtain long-term therapy, gene delivery may have an advantage, as it leads to continued gene expression over time. Gene delivery is most efficient via viral vectors, but these do not readily cross the RWM (Stover et al., 1999; Jero et al., 2001; Duan et al., 2004; Wimmer et al., 2004; Wang et al., 2011). As such, a reagent that can increase the permeability of the RWM to viral vectors is necessary. To investigate the potential use of HA for facilitating the migration of viral vectors across the RWM, we applied HA with an adenovirus carrying enhanced green fluorescent protein (eGFP) reporter gene (Ad-eGFP) to the RWM of normal guinea pigs. Subsequently, we evaluated eGFP expression in cells lining the scala tympani and those lining scala media (the cochlear compartment containing potassium-rich endolymph). We also tested for changes in auditory function. We compared these results with those obtained by placing HA or Ad-eGFP alone on the RWM, and with results from injection of Ad-eGFP into the perilymph via cochleostomy. Our data demonstrate that ears treated with HA-assisted Ad-eGFP transport through the RWM had robust eGFP expression in cells lining the perilymphatic and endolymphatic spaces, whereas little or no eGFP expression was seen in ears treated with HA or Ad-eGFP alone. Animals inoculated with Ad-eGFP into the perilymph via cochleostomy had transgene expression limited to cells lining the perilymphatic space. Auditory function measurements revealed that the combination of HA and Ad-eGFP applied to the RWM induced less hearing loss after surgery than did injection of Ad-eGFP through a cochleostomy.
Materials and Methods
Animals and groups
Animal care and handling were approved by the University of Michigan Institutional Committee on the Use and Care of Animals and performed using accepted veterinary standards. Twenty-five female or male pigmented guinea pigs weighing 300–350 g were purchased from Elm Hill (Chelmsford, MA). Each animal was tested for the presence of Preyer's reflex before being included in the study.
One set of 15 animals was processed for quantitative histological assessment 7 days after inoculation. These animals were divided into three groups according to the inoculation procedure. The first group had HA-assisted Ad-eGFP inoculation via the RWM on the left ear and HA alone applied on the RWM of the contralateral ear (seven animals). The second group had Ad-eGFP alone applied on the RWM (three animals). The third group had Ad-eGFP inoculated without HA via a cochleostomy made in the basal turn (five animals) and no treatment in the contralateral ear.
The other set of 10 animals received physiologic assessment by auditory brainstem response (ABR) threshold measurements before and after inoculation, and was divided into two groups according to the inoculation procedure. One group consisted of guinea pigs receiving Ad-eGFP inoculation with HA via the RWM on the left ear and HA alone on the contralateral ear (five animals). The other group included guinea pigs receiving cochleostomy inoculation of Ad-eGFP without HA on the left ear (five animals). All cochleae in each group were processed for whole-mount (surface preparation) analysis for detecting reporter-gene expression.
ABR assessment
ABRs were assessed for each animal in both ears. The thresholds were measured for three frequencies, 4, 12, and 20 kHz (tone bursts, 15-ms duration, 1-ms cos2-shaped rise-fall times), as described previously (Yamasoba and Dolan, 1997). ABRs were assessed prior to the inoculation surgery (baseline) and 14 days after the inoculation surgery. We compared the threshold shift from baseline and 14 days. Furthermore, we compared the threshold shifts between the HA-assisted Ad-eGFP inoculation, HA alone, and Ad-eGFP without HA inoculated via cochleostomy. The mean at each frequency was tested against an expected mean of zero by t test. For each set of three frequencies for a given treatment group, the observed p values were compared against the appropriate sequential Bonferroni criterion to maintain a criterion of p<0.05 to infer statistical significance.
Inoculation surgeries and viral vectors
General anesthesia was induced with ketamine (40 mg/kg) and xylazine (10 mg/kg). After the guinea pig was fully sedated, postauricular incision was performed and the anteroventral border of the left trapezius was teased away from its insertion on the posterior aspect of the skull. The otic bulla was opened, and the RWM niche could be visualized under the surgical scope. One drop of 14 mg/ml HA (hyaluronic acid sodium salt from rooster comb; Sigma H5388; Sigma, St. Louis, MO) diluted in sterile saline, or commercially provided 14 mg/ml Healon GV (Advanced Medical Optics Inc., Santa Ana, CA) was placed onto the left ear RWM niche. This concentration of HA was shown in a previous in vitro study (Kelly et al., 1999) to be efficient for the gradual release of gentamicin. Ten minutes later, we carefully removed as much HA as possible with absorbents, without perforating the membrane. Subsequently, we applied 5 μl of Ad-eGFP onto the left RWM using a microcannula attached to a Hamilton syringe. This vector solution was not removed from the RWM. The right ear RWM received HA alone. We used this method of application to minimize the dilution of viral particle concentration by mixing with HA. Despite careful removal of the HA from the RWM, small amounts of the reagent remained on the surface of the RWM, allowing it to mix with the viral vector and remain on the RWM surface. The highly viscous nature of 14 mg/ml HA makes it difficult to aspirate and mix evenly with the viral vector without vigorous agitation, which degrades the vector. The adenovector, Adef.11D 1.0383 PU/Ml×1012, was constructed with the transgene driven by the cytomegalovirus promoter (kind gift from GenVec, Inc., Gaithersburg, MD). As controls, we applied Ad-eGFP alone or HA alone likewise onto the RWM. A cochleostomy was made in the basal turn of the cochlea, and the inoculation of 5 μl of Ad-eGFP into the scala tympani was carried out as described previously (Stover et al., 1999), except that a silicon ball was not fixed to the tip of the cannula.
Immunohistochemistry and analysis
Seven days after inoculation of Ad-eGFP, the temporal bones were extracted and cochlear tissues locally perfused with 4% paraformaldehyde (PFA) and fixed further for 1 hr in PFA. After a rinse in phosphate-buffered saline (PBS), tissues were permeabilized with 0.3% Triton X-100 for 10 min. Alexa Fluor 594 phalloidin (Molecular Probes, Eugene, OR) was used to stain F-actin (1:200 dilution in PBS) for 30 min to observe cytoarchitecture. After the tissue was washed with PBS, the tissue was cut into four turns, mounted onto glass slides, and cover-slipped with Fluoro-Gel with TES buffer (Electron Microscopy Sciences, Hatfield, PA). Slides were observed with a Leica DMRB epifluorescence microscope (Leica, Eaton, PA), Zeiss LSM 510 confocal microscope (Carl Zeiss AG, Oberkochen, Germany), or Leica MZ10F fluorescence stereomicroscope (Leica). Confocal images were acquired and processed with LSM Image Browser (Carl Zeiss, Germany). Fluorescence stereomicroscopy was used to observe the lateral wall tissues (stria vascularis and spiral ligament areas).
Results
Auditory function
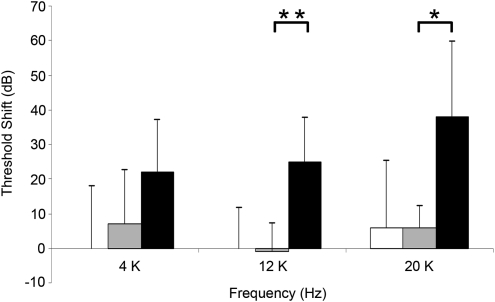
We evaluated auditory function using ABR threshold measurement following inoculation of Ad-eGFP with HA-assisted RWM inoculation as compared with Ad-eGFP via cochleostomy inoculation without HA. At 2 weeks after treatment, we compared auditory thresholds to baseline (Fig. 1). Additionally, ABRs were measured after HA application to the RWM (with no viral vector). Threshold shifts were significantly greater at the higher frequencies (12 and 24 kHz) in ears inoculated with Ad-eGFP via cochleostomy in comparison with ears inoculated with Ad-eGFP with HA via the RWM. HA alone or Ad-eGFP with HA caused a mild threshold shift of less than 10 dB at all tested frequencies (Fig. 1, white and gray bars). There were no statistical differences between the ears treated by Ad-eGFP with HA and those treated with HA alone. In ears inoculated with Ad-eGFP via cochleostomy, the threshold shifts ranged from 20 to 40 dB throughout all frequencies. These data suggest that (a) HA alone, when applied on the RWM, does not induce significant threshold shift, (b) the vector does not induce a threshold shift when placed on the RWM with HA, and (c) in contrast, the process of infusing a viral vector via a cochleostomy does induce a significant threshold shift.
FIG. 1.
Threshold shifts (means + standard error) of groups treated with Ad-eGFP inoculation via cochleostomy (black bars), HA alone on RWM (white bars), and HA-assisted Ad-eGFP inoculation via RWM (gray bars), and measured 2 weeks after inoculation, at 4, 12, and 20 kHz. Ears inoculated with Ad-eGFP via cochleostomy had average threshold shifts greater than 20 dB at each tested frequency, with larger shifts at higher frequencies, and all three averages were significantly greater than zero (p<0.05 after sequential Bonferroni adjustment). Average threshold shifts of ears treated with HA alone or HA with Ad-eGFP were less than 10 dB and were not significantly different from zero. Moreover, the average shifts induced by HA-assisted Ad-eGFP inoculation were not significantly different from those induced by HA alone, even at 4 kHz where the difference between means was somewhat larger (p>0.5). In addition, threshold shifts for HA-assisted Ad-eGFP inoculation via RWM were statistically lower than in animals inoculated with Ad-eGFP via cochleostomy at 12 kHz (p<0.05) and 20 kHz (p<0.01). For all groups, n=5.
The influence of HA on transgene expression
Transgene expression of eGFP in ears inoculated with Ad-eGFP with HA via the RWM was observed in cells residing in both the endolymphatic and perilymphatic compartments. In contrast, in ears inoculated with Ad-eGFP via a cochleostomy with no HA, expression of eGFP was limited to cells lining the scala tympani. A summary of transduced cells and tissues is given in Table 1. The data show that HA-RWM application of the viral vector resulted in gene expression in all four cochlear turns as compared with cochleostomy, which only facilitated expression in the lower two turns, and that only HA-RWM application reached cells lining the scala media.
Table 1.
Number of Guinea Pig (GP) Ears with Positive eGFP Transgene Expression
| |
Method of delivery (number of animals) |
|
|---|---|---|
| RWM with HA (n=7) | Cochleostomy (n=5) | |
| GFP distribution by turns | ||
| All | 7 | 0 |
| 1 and 2, only | 0 | 5 |
| GFP distribution by tissue or cell type | ||
| Reissner's membrane | 7 | 5 |
| Mesothelial cells | 7 | 5 |
| Spiral ligament cells | 3 | 5 |
| Organ of Corti cells | ||
| Deiters' | 3 | 0 |
| Pillars | 3 | 0 |
| Hensen's | 2 | 0 |
| Inner sulcus | 3 | 0 |
| Interdental | 3 | 0 |
| Outer hair cell | 3 | 0 |
| Inner hair cell | 0 | 0 |
The extent of eGFP-positive cells is broader with HA-assisted Ad-eGFP inoculation compared with cochleostomy inoculation. The transgene expression of HA-assisted Ad-eGFP inoculation in the organ of Corti is distributed mainly in the basal turn. Inner hair cells were not transduced. Ad-eGFP inoculated via cochleostomy demonstrated expression predominantly in the cells lining the scala tympani.
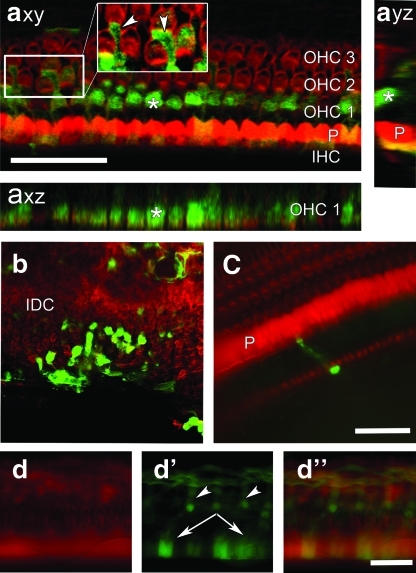
In ears inoculated with Ad-eGFP with HA via the RWM, eGFP-positive cells in the lining of scala media were detected mostly in the basal turn, with more robust transduction toward the RWM (Fig. 2axy, axz, ayz, and b). Transgene expression observed in the second turn was minimal (Fig. 2c), and little or no gene expression could be observed in the higher turns. Cell types demonstrating eGFP expression in the scala media included outer hair cells, Deiters' cells, inner and outer pillar cells, and interdental cells (Fig. 2a–c). Inner hair cells were not transduced. Transduction of cells and structures lining the scala tympani and scala vestibuli was seen not only in the basal and second turns, but in most cases also in the third turn (Fig. 3a) or higher (Table 1; images not shown).
FIG. 2.
Whole mounts of auditory epithelium from the basal (a, b, d) and second (c) cochlear turn after HA-assisted Ad-eGFP inoculation via the RWM. eGFP-positive cells (green) indicate transduction, and phalloidin (red) was used to stain F-actin. (axy) Confocal images showing outer hair cells (OHC), Deiters' cells (arrowheads), and pillar cells (P) with robust eGFP expression. (axz) Tangential optical section through the first row of outer hair cells showing eGFP expression. (ayz) A radial section showing an eGFP-positive outer hair cell (*) adjacent to a pillar cell (P). (b) Confocal stack of images showing eGFP expression in interdental cells (IDC) in basal turn. (c) Epifluorescence image showing the second turn organ of Corti with intact cells and normal organization pattern and a transduced inner sulcus cell. (d) Epifluorescence for actin (d), eGFP (d′), and merged (d″) showing eGFP in Deiters' cells (arrowheads) and pillar cells (arrows). Scale bars=50 μm in a–c and 40 μm in d.
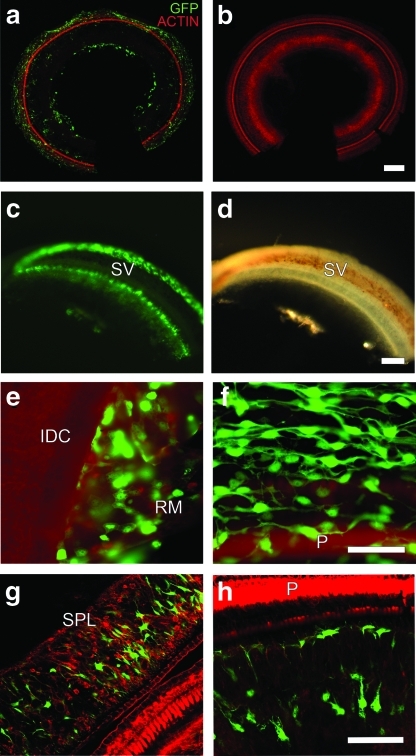
FIG. 3.
Transgene expression of eGFP after HA-assisted Ad-eGFP inoculation via the RWM (a, c–h) or Ad-eGFP inoculation via cochleostomy (b) in cells lining the scala tympani. (a) Mesothelial cells and fibrocytes with eGFP expression, third turn. (b) No eGFP expression was observed in ears with Ad-eGFP inoculated via cochleostomy (no HA) in upper turns (third turn shown here). (c) Low-magnification image of the stria vascularis and spiral ligament. (d) Light microscopy image of (c). (e) Reissner's membrane (RM) shows eGFP expression in the basal turn. (f) Mesothelial cells line the scala tympani; focal plane is lower than the luminal surface of the organ of Corti. (g) Connective tissue in spiral ligament shows eGFP expression, third turn. (h) Fibrocytes within the osseous spiral lamina with eGFP expression, second turn. P, pillar cells; IDC, interdental cells; SPL, spiral ligament; SV, stria vascularis. Scale bars=50 μm for all images (paired images are at same scale).
In contrast, ears inoculated with Ad-eGFP alone (no HA) via cochleostomy exhibited transgene expression only in mesothelial cells and fibrocytes. Cells in the scala media were not transduced, and the distribution of eGFP was limited to the first two turns (Fig. 3b, third turn).
When HA was used along with the adenovirus vector, transduced cell types and tissues consisted of mesothelial cells, Reissner's membrane, and fibrocytes in the osseous spiral lamina and the spiral ligament (Fig. 3c–h). However, the epithelium of the stria vascularis remained negative (Fig. 3c). Transduction was consistent and robust in the mesothelial cells (Fig. 3f). We assessed the outer hair cell loss in five of the seven guinea pigs treated with HA-assisted Ad-eGFP via the RWM. Outer hair cell loss was minimal and averaged 3.5 cells in half of the basal turn of the cochlea. Inner hair cells were mostly intact. Contralateral ears and ears treated with HA alone (no viral vector) had no eGFP expression. Animals that had Ad-eGFP alone applied to the RWM showed no expression of eGFP transgene in any cell type of the inner ear (data not shown). These observations demonstrate that HA-assisted transgene delivery via the RWM was most effective in transducing cells lining the scala tympani. Nevertheless, the moderate degree of transduction of cells in the scala media should provide an attractive target for research involving transgene expression in supporting cells and outer hair cells using the intratympanic approach.
Discussion
This study compared transgene expression of Ad-eGFP in cochlear tissue after two gene-delivery methods: HA-assisted delivery via the RWM and delivery via cochleostomy without HA. Our results demonstrate that relative to the traditional cochleostomy method, which disperses viral particles directly into the perilymph, HA-assisted gene delivery caused less ABR threshold shift and greater transduction of cochlear cells. With HA, eGFP expression could be observed not only in the cells lining the scala tympani, but also in the scala media. Biological side effects were minimal or absent in ears with inoculated HA via the intratympanic approach.
Influence of HA-assisted inoculation via the RWM on auditory function
Threshold shifts measured in the ears inoculated with Ad-eGFP and HA on the RWM (intratympanic approach) were less than 10 dB and not significantly greater than zero, comparable to that in ears that had HA applied alone (control). This result, along with the intact hair cell morphology we observed, indicated that the Ad-eGFP vector had little or no side effects on auditory function. This is consistent with previous observations of minimal disturbance of auditory function caused by one application of adenovirus vectors (Stover et al., 1999; Yagi et al., 1999; Venail et al., 2007). In the ears inoculated with Ad-eGFP via cochleostomy, the threshold shift was significantly elevated in comparison with the shift with the HA-assisted inoculation of Ad-eGFP, with larger shifts at the higher frequencies. Because the Ad-eGFP itself has little effect on the ABR measurements, mechanical damage inflicted by the cochleostomy is likely responsible for the threshold shifts. This is consistent with previous reports that have illustrated a correlation between the mechanical damage caused by the cochleostomy and elevated threshold shifts (Carvalho and Lalwani, 1999; Stover et al., 1999; Kanzaki et al., 2007; Venail et al., 2007). Therefore, the HA-assisted inoculation is a substantially safer delivery modality than traditional inoculations via cochleostomy. Additionally, ears that will likely receive treatments using HA will have severe hearing loss to begin with, and therefore a potential for mild threshold shift caused by HA could be considered an acceptable risk. However, more caution may be necessary when HA is used in treatments that aim to protect residual hearing.
Distribution of transgene expression in the cochlea, HA versus cochleostomy
In ears inoculated with HA and Ad-eGFP via the RWM, eGFP expression was observed in cells lining the perilymph throughout all cochlear turns and in cells of the membranous labyrinth in the lower two turns. In contrast, transgene expression in ears inoculated via cochleostomy showed a limited distribution of eGFP expression in the scala tympani in the first two turns. These data suggest that the inoculation via the RWM with HA allows the viral vector to spread further into the inner ear than a cochleostomy inoculation. The absence of transgene expression in the higher turns with cochleostomy inoculation may be due to the opposing flow of perilymph and cerebrospinal fluid toward the leaking fenestra (Stover et al., 1999; Salt and Plontke, 2009). HA-assisted inoculation may allow vectors to be distributed throughout the cochlea without altering the natural passive diffusion of the cochlear fluids. Therefore, the modality and site of inoculation could determine the extent of transgene expression in the inner ear. Modification of the cochleostomy, such as sealing the fenestra with HA (Salt and Plontke, 2009) or using a slow regulated injection flow, may allow the viral vector to proceed more efficiently into the higher turns.
The transduction of cells in the organ of Corti and other cells lining the scala media after HA-assisted delivery was concentrated in the basal turn, but could be seen at low efficiency in the second turn. This may suggest that the anatomical barrier between perilymph and endolymph is more permeable near the hook area adjacent to the RWM in guinea pigs, allowing higher titers of the vector to reach the basal turn. The data could also reflect the concentration gradient in the perilymph after inoculation, which is greatest near the RWM; therefore, the highest likelihood of diffusion across the basilar membrane will be at this end.
Defining endolymphatic barriers using HA
Transduction of cells that line the scala media, including the organ of Corti, is an important outcome due to the functional significance of these cells. Previous reports and our data suggest that adenovirus-mediated transgene expression is absent in the organ of Corti after perilymphatic inoculation (Stover et al., 1999; Venail et al., 2007; Konishi et al., 2008). The spread of the vector from the perilymph to the endolymphatic regions of the cochlea could be explained by the ability of HA to enhance membrane permeability (Selivanova et al., 2003), which may allow direct penetration of the viral particles through the basilar membrane to reach the endolymph. Another explanation could be that HA facilitates vectors crossing via the canaliculi perforantes into the modiolus and from there to the endolymph. The finding that the Reissner's membrane can be transduced suggests that the vectors reach the scala vestibuli. This could occur by spread across the connective tissue in the spiral ligament. The radial connection of the fluids between the scala tympani and scala vestibuli has been demonstrated in previous reports using ionic tracers (Salt et al., 1991a,b). Vectors entering the scala vestibuli may be able to cross the Reissner's membrane with the assistance of HA. The exact mechanism or pathway of viral vector spread in the cochlea in conjunction with HA still needs to be elucidated.
Transfer of genes into hair cells is important for several research goals, such as manipulating gene expression to understand the roles of gene products in these cells. Gene transfer into hair cells is also of clinical importance for developing procedures for hearing protection or for phenotypic rescue in hereditary deafness cases. Previous work with adenovirus vectors in mature normal mammals or prenatal culture conditions has shown that the transduction was restricted to supporting cells (Kanzaki et al., 2001; Ishimoto et al., 2002; Excoffon et al., 2006; Venail et al., 2007). The reason for the lack of hair cell transduction in these studies is not clear and could be partly attributed to the inability of adenoviruses to reach the receptors (e.g., coxsackie and adenovirus receptor and integrins) on the luminal surface of the hair cells. Our present data demonstrate that with HA-assisted gene delivery, the first row of outer hair cells in the basal turn could be transduced efficiently. One possible explanation of this result could be that HA may partially contribute to the removal or attenuation of components on the apical surface of hair cells that prevent the attachment of adenovirus to its receptors.
The transduction of supporting cells in the auditory epithelium is especially important because these cells would likely be the primary target for inducing hair cell regeneration via transdifferentiation in deaf patients in the future (White et al., 2006) and because more than half of hereditary deafness patients have supporting cell mutations (Hildebrand et al., 2008). The mesothelial cells lining the scala tympani and fibrous tissue in the osseous spiral lamina could also become vital targets for gene therapy, because they can express neurotrophic factor genes and secrete neurotrophins to induce neurite growth and/or enhance survival of spiral ganglion cells, outcomes that could enhance the function of the cochlear implant (Shibata et al., 2010; Wise et al., 2010).
Trans-RWM as a delivery route in humans and animals
The trans-RWM route of gene delivery requires a minimally invasive surgical manipulation via the ear canal, leading to no systemic side effects, minimal cochlear side effects, and moderately efficient gene expression. The application of the therapeutic reagent can be repeated if necessary. However, although introducing reagents into the middle-ear side of the RWM is possible, the membrane is rather impermeable and, in most cases, does not allow substances to reach the perilymph on the other side (Goycoolea and Lundman, 1997; Goycoolea, 2001). Some facilitating reagents, such as endotoxins or exotoxins (Ikeda and Morizono, 1988; Selivanova et al., 2003), histamine (Chandrasekhar et al., 2000), and benzyl alcohol, or suction near the RWM (Mikulec et al., 2008), have been shown to enhance RWM permeability and allow reagents to reach the cochlear fluids. Many of the facilitating reagents have been used for the purpose of delivering drugs such as dexamethasone or aminoglycosides. Our data together with those of another group using Gelfoam (Jero et al., 2001; Aarnisalo et al., 2006) demonstrate the potential for enhanced efficiency of transgene delivery by the trans-RWM route. When clinical use of this method is considered, it will be necessary to evaluate the effects of the procedure on the middle-ear tissues and the outcome of gene expression of the therapeutic genes on the RWM and surrounding middle-ear structures.
The choice of viral vectors for therapy is influenced by several parameters. Adeno-associated viral vector (AAVs) gene expression lasts for a long time and is ideal for secreting neurotrophins or expressing other transgenes needed over a long time. Additional studies are needed to determine the influence of HA on the delivery of AAVs on the trans-RWM delivery of genes in the cochlea. Some therapeutic applications do not need sustained gene expression over time, and actually work better with a pulse of very efficient expression that does not last for more than days or weeks. The use of developmental genes for inducing transdifferentiation is an example of short-term gene expression. The present study uses adenovirus vectors, which are the vectors of choice for transient gene expression. Future improvement in these vectors will likely reduce their side effects and make them more acceptable for clinical use.
Conclusions
In summary we present an atraumatic method to introduce transgenes into cochlear cells. An important outcome in this study is the wide delivery of transgenes into the cells lining the scala tympani and moderate transduction in the scala media of guinea pigs. No substantial side effects were observed after surgery, and neither hair cell loss nor granulation tissue proliferation was apparent in the whole-mount analysis of the HA- and Ad-eGFP-treated ears, which indicates that both had minimal ototoxicity.
Acknowledgments
This work was supported by the A. Alfred Taubman Medical Research Institute, the Williams Professorship, and NIH/NIDCD grants T32-DC005356, DC-007634, DC001634, and P-30-DC05188.
Author Disclosure Statement
No competing financial interests exist.
References
- Aarnisalo A.A. Aarnisalo P. Pietola L., et al. Efficacy of gene transfer through the round window membrane: an in vitro model. ORL J. Otorhinolaryngol. Relat. Spec. 2006;68:220–227. doi: 10.1159/000092123. [DOI] [PubMed] [Google Scholar]
- Bagger-Sjöbäck D. Sodium hyaluronate application to the open inner ear: an ultrastructural investigation. Am. J. Otol. 1991;12:35–39. [PubMed] [Google Scholar]
- Bagger-Sjöbäck D. Holmquist J. Mendel L. Mercke U. Hyaluronic acid in middle ear surgery. Am. J. Otol. 1993;14:501–506. doi: 10.1097/00129492-199309000-00016. [DOI] [PubMed] [Google Scholar]
- Barkdull G.C. Hondarrague Y. Meyer T., et al. AM-111 reduces hearing loss in a guinea pig model of acute labyrinthitis. Laryngoscope. 2007;117:2174–2182. doi: 10.1097/MLG.0b013e3181461f92. [DOI] [PubMed] [Google Scholar]
- Bird P.A. Begg E.J. Zhang M., et al. Intratympanic versus intravenous delivery of methylprednisolone to cochlear perilymph. Otol. Neurotol. 2007;28:1124–1130. doi: 10.1097/MAO.0b013e31815aee21. [DOI] [PubMed] [Google Scholar]
- Bjurström S. Slepecky N. Angelborg C. A histopathological study of the inner ear after the administration of hyaluronan into the middle ear of the guinea pig. Acta Otolaryngol. 1987;(Suppl. 442):62–65. doi: 10.3109/00016488709102841. [DOI] [PubMed] [Google Scholar]
- Borden R.C. Saunders J.E. Berryhill W.E., et al. Hyaluronic acid hydrogel sustains the delivery of dexamethasone across the round window membrane. Audiol. Neurootol. 2010;16:1–11. doi: 10.1159/000313506. [DOI] [PubMed] [Google Scholar]
- Carvalho G.J. Lalwani A.K. The effect of cochleostomy and intracochlear infusion on auditory brain stem response threshold in the guinea pig. Am. J. Otol. 1999;20:87–90. [PubMed] [Google Scholar]
- Chandrasekhar S.S. Rubinstein R.Y. Kwartler J.A., et al. Dexamethasone pharmacokinetics in the inner ear: comparison of route of administration and use of facilitating agents. Otolaryngol. Head Neck Surg. 2000;122:521–528. doi: 10.1067/mhn.2000.102578. [DOI] [PubMed] [Google Scholar]
- Donnelly M.J. Cohen L.T. Clark G.M. Initial investigation of the efficacy and biosafety of sodium hyaluronate (Healon) as an aid to electrode array insertion. Ann. Otol. Rhinol. Laryngol. 1995;(Suppl. 166):45–48. [PubMed] [Google Scholar]
- Duan M. Venail F. Spencer N. Mezzina M. Treatment of peripheral sensorineural hearing loss: gene therapy. Gene Ther. 2004;11(Suppl 1):S51–S56. doi: 10.1038/sj.gt.3302369. [DOI] [PubMed] [Google Scholar]
- Excoffon K.J. Avenarius M.R. Hansen M.R., et al. The coxsackievirus and adenovirus receptor: a new adhesion protein in cochlear development. Hear. Res. 2006;215:1–9. doi: 10.1016/j.heares.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Gouveris H. Selivanova O. Mann W. Intratympanic dexamethasone with hyaluronic acid in the treatment of idiopathic sudden sensorineural hearing loss after failure of intravenous steroid and vasoactive therapy. Eur. Arch. Otorhinolaryngol. 2005;262:131–134. doi: 10.1007/s00405-004-0772-6. [DOI] [PubMed] [Google Scholar]
- Goycoolea M.V. Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol. 2001;121:437–447. doi: 10.1080/000164801300366552. [DOI] [PubMed] [Google Scholar]
- Goycoolea M.V. Lundman L. Round window membrane. Structure function and permeability: a review. Microsc. Res. Tech. 1997;36:201–211. doi: 10.1002/(SICI)1097-0029(19970201)36:3<201::AID-JEMT8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Hildebrand M.S. Newton S.S. Gubbels S.P., et al. Advances in molecular and cellular therapies for hearing loss. Mol. Ther. 2008;16:224–236. doi: 10.1038/sj.mt.6300351. [DOI] [PubMed] [Google Scholar]
- Ikeda K. Morizono T. Changes of the permeability of round window membrane in otitis media. Arch. Otolaryngol. Head Neck Surg. 1988;114:895–897. doi: 10.1001/archotol.1988.01860200079023. [DOI] [PubMed] [Google Scholar]
- Ishimoto S. Kawamoto K. Kanzaki S. Raphael Y. Gene transfer into supporting cells of the organ of Corti. Hear. Res. 2002;173:187–197. doi: 10.1016/s0378-5955(02)00579-8. [DOI] [PubMed] [Google Scholar]
- Iwai K. Nakagawa T. Endo T., et al. Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope. 2006;116:529–533. doi: 10.1097/01.mlg.0000200791.77819.eb. [DOI] [PubMed] [Google Scholar]
- Jero J. Mhatre A.N. Tseng C.J., et al. Cochlear gene delivery through an intact round window membrane in mouse. Hum. Gene Ther. 2001;12:539–548. doi: 10.1089/104303401300042465. [DOI] [PubMed] [Google Scholar]
- Kanzaki S. Karolyi I.J. Raphael Y. Midwinter Meeting of the Association for Research in Otolaryngology. St. Petersburg, FL: 2001. Adenoviral mediated gene transfer to the inner ear of shaker 2 mice in vitro. [Google Scholar]
- Kanzaki S. Shiotani A. Inoue M., et al. Sendai virus vector-mediated transgene expression in the cochlea in vivo. Audiol. Neurootol. 2007;12:119–126. doi: 10.1159/000097798. [DOI] [PubMed] [Google Scholar]
- Kelly R.M. Meyer J.D. Matsuura J.E., et al. In vitro release kinetics of gentamycin from a sodium hyaluronate gel delivery system suitable for the treatment of peripheral vestibular disease. Drug Dev. Ind. Pharm. 1999;25:15–20. doi: 10.1081/ddc-100102137. [DOI] [PubMed] [Google Scholar]
- Konishi M. Kawamoto K. Izumikawa M., et al. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J. Gene Med. 2008;10:610–618. doi: 10.1002/jgm.1189. [DOI] [PubMed] [Google Scholar]
- Lee K.Y. Nakagawa T. Okano T., et al. Novel therapy for hearing loss: delivery of insulin-like growth factor 1 to the cochlea using gelatin hydrogel. Otol. Neurotol. 2007;28:976–981. doi: 10.1097/MAO.0b013e31811f40db. [DOI] [PubMed] [Google Scholar]
- Lehnhardt E. [Placement of intracochlear electrodes with Healon] HNO. 1992;40:86–89. [PubMed] [Google Scholar]
- Maeda Y. Sheffield A.M. Smith R.J. Therapeutic regulation of gene expression in the inner ear using RNA interference. Adv. Otorhinolaryngol. 2009;66:13–36. doi: 10.1159/000218205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulec A.A. Hartsock J.J. Salt A.N. Permeability of the round window membrane is influenced by the composition of applied drug solutions and by common surgical procedures. Otol. Neurotol. 2008;29:1020–1026. doi: 10.1097/MAO.0b013e31818658ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plontke S.K. Biegner T. Kammerer B., et al. Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol. Neurotol. 2008;29:401–406. doi: 10.1097/MAO.0b013e318161aaae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt A.N. Plontke S.K. Local inner-ear drug delivery and pharmacokinetics. Drug Discov. Today. 2005;10:1299–1306. doi: 10.1016/S1359-6446(05)03574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt A.N. Plontke S.K. Principles of local drug delivery to the inner ear. Audiol. Neurootol. 2009;14:350–360. doi: 10.1159/000241892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt A.N. Ohyama K. Thalmann R. Radial communication between the perilymphatic scalae of the cochlea. I. Estimation by tracer perfusion. Hear. Res. 1991a;56:29–36. doi: 10.1016/0378-5955(91)90150-8. [DOI] [PubMed] [Google Scholar]
- Salt A.N. Ohyama K. Thalmann R. Radial communication between the perilymphatic scalae of the cochlea. II. Estimation by bolus injection of tracer into the sealed cochlea. Hear. Res. 1991b;56:37–43. doi: 10.1016/0378-5955(91)90151-x. [DOI] [PubMed] [Google Scholar]
- Selivanova O. Maurer J. Ecke U. Mann W.J. [The effects of streptolysin-O and sodium hyaluronate on the permeability of the round window membrane in guinea pigs—an electrophysiologic study] Laryngorhinootologie. 2003;82:235–239. doi: 10.1055/s-2003-38937. [DOI] [PubMed] [Google Scholar]
- Selivanova O.A. Gouveris H. Victor A., et al. Intratympanic dexamethasone and hyaluronic acid in patients with low-frequency and Ménière's-associated sudden sensorineural hearing loss. Otol. Neurotol. 2005;26:890–895. doi: 10.1097/01.mao.0000185050.69394.48. [DOI] [PubMed] [Google Scholar]
- Shibata S.B. Cortez S.R. Beyer L.A., et al. Transgenic BDNF induces nerve fiber regrowth into the auditory epithelium in deaf cochleae. Exp. Neurol. 2010;223:464–472. doi: 10.1016/j.expneurol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staecker H. Gabaizadeh R. Federoff H. Van de Water T.R. Brain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell loss. Otolaryngol. Head Neck Surg. 1998;119:7–13. doi: 10.1016/S0194-5998(98)70194-9. [DOI] [PubMed] [Google Scholar]
- Stover T. Yagi M. Raphael Y. Cochlear gene transfer: round window versus cochleostomy inoculation. Hear. Res. 1999;136:124–130. doi: 10.1016/s0378-5955(99)00115-x. [DOI] [PubMed] [Google Scholar]
- Van de Water T.R. Staecker H. Halterman M.W. Federoff H.J. Gene therapy in the inner ear. Mechanisms and clinical implications. Ann. N.Y. Acad. Sci. 1999;884:345–360. doi: 10.1111/j.1749-6632.1999.tb08653.x. [DOI] [PubMed] [Google Scholar]
- Venail F. Wang J. Ruel J., et al. Coxsackie adenovirus receptor and ανβ3/ανβ5 integrins in adenovirus gene transfer of rat cochlea. Gene Ther. 2007;14:30–37. doi: 10.1038/sj.gt.3302826. [DOI] [PubMed] [Google Scholar]
- Wang H. Murphy R. Taaffe D., et al. Efficient cochlear gene transfection in guinea-pigs with adeno-associated viral vectors by partial digestion of round window membrane. Gene Ther. 2011 doi: 10.1038/gt.2011.91. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P.M. Doetzlhofer A. Lee Y.S., et al. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441:984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- Wimmer C. Mees K. Stumpf P., et al. Round window application of D-methionine, sodium thiosulfate, brain-derived neurotrophic factor, and fibroblast growth factor-2 in cisplatin-induced ototoxicity. Otol. Neurotol. 2004;25:33–40. doi: 10.1097/00129492-200401000-00007. [DOI] [PubMed] [Google Scholar]
- Wise A.K. Hume C.R. Flynn B.O., et al. Effects of localized neurotrophin gene expression on spiral ganglion neuron resprouting in the deafened cochlea. Mol. Ther. 2010;18:1111–1122. doi: 10.1038/mt.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M. Magal E. Sheng Z., et al. Hair cell protection from aminoglycoside ototoxicity by adenovirus-mediated overexpression of glial cell line-derived neurotrophic factor. Hum. Gene Ther. 1999;10:813–823. doi: 10.1089/10430349950018562. [DOI] [PubMed] [Google Scholar]
- Yamasoba T. Dolan D.F. Chronic strychnine administration into the cochlea potentiates permanent threshold shift following noise exposure. Hear. Res. 1997;112:13–20. doi: 10.1016/s0378-5955(97)00092-0. [DOI] [PubMed] [Google Scholar]