Abstract
Oncolytic measles virus (MV) encoding the human thyroidal sodium iodide symporter (MV-NIS) has proved to be safe after intraperitoneal or intravenous administration in patients with ovarian cancer or multiple myeloma, respectively, but it has not yet been administered through intratumoral injection in humans. Squamous cell carcinoma (SCC) of the head and neck (SCCHN) usually is locally invasive and spreads to the cervical lymph nodes, which are suitable for the intratumoral administration of oncolytic viruses. To test whether oncolytic MV is an effective treatment for SCCHN, we used oncolytic MV-NIS to infect SCCHN in vitro and in vivo. The data show that SCCHN cells were infected and killed by MV-NIS in vitro. Permissiveness of the tumor cells to MV infection was not affected by irradiation after viral addition. Monitored noninvasively through radioiodine-based single-photon emission computed tomography/computed tomography, intratumorally virus-delivered NIS has concentrated the radioiodine in the MV-NIS–treated tumors in the FaDu mouse xenograft model of human SCCHN, and the antitumor effect could be boosted significantly (p<0.05) either with concomitant cyclophosphamide therapy or with appropriately timed administration of radioiodine 131I. MV-NIS could be a promising new anticancer agent that may substantially enhance the outcomes of standard therapy after intratumoral administration in patients with locally advanced SCCHN.
Li and colleagues investigate the use of oncolytic measles virus encoding human thyroidal sodium iodide symporter (MV-NIS) to treat squamous cell carcinoma of the head and neck (SCCHN) in vitro and in vivo. MV-NIS-treated tumors are able to concentrate administered radioiodine in a mouse xenograft model of human SCCHN, and the antitumor effect is significantly boosted by cyclophosphamide therapy.
Introduction
Epithelial carcinomas of the head and neck typically are squamous cell in origin and arise from the mucosal surfaces in the paranasal sinuses, oral cavity, nasopharynx, oropharynx, hypopharynx, or larynx. Approximately 25,000 new cases of squamous cell carcinoma (SCC) of the head and neck (SCCHN) are diagnosed each year in the United States, accounting for about 3% of male adult cancers. Worldwide, the annual incidence is approximately 900,000, making SCCHN the sixth most common cancer (Sankaranarayanan et al., 1998; Chin et al., 2006; Jemal et al., 2010). Clinical presentations differ according to the precise site of origin of the cancer and the extent of local tissue invasion, spread to regional lymph nodes, and distant metastasis. Treatment options include surgery, radiotherapy, and chemotherapy (including monoclonal antibody therapy).
Several chemoradiotherapy regimens have been developed for treatment of locally and regionally advanced SCCHN (Blanchard et al., 2011). Radiotherapy is typically administered in a dose of 1.8–2.0 Gy 5 days per week for 6 or 7 weeks, but it can be hyperfractionated (two or three doses daily) or accelerated to administer the same total dose in a shorter time. However, the disease of most patients remains incurable even with the best available therapy, and the search continues to identify new, nontoxic agents with activity against SCCHN for use in combination with existing chemoradiotherapy protocols (Lefebvre, 2005; Morganti et al., 2011).
For decades, gene therapy has been conceived to tackle human malignancy. One current strategy is to engineer viruses to replicate selectively in neoplastic tissues. Many such oncolytic therapeutic viruses are being investigated currently in both preclinical animal studies and human clinical trials (T.C. Liu et al., 2007; Ayllón Barbellido et al., 2008; Liu and Kirn, 2008; Nguyen et al., 2009). For disseminated cancers and metastases, oncolytic viruses ideally should be delivered systemically through the bloodstream, and therefore must be both accurate—recognizing and destroying only tumor tissue—and efficient. Unfortunately, preformed antiviral antibodies present a formidable obstacle to efficient virus delivery through the bloodstream (Russell and Peng, 2007, 2008). However, in contrast to many other malignant tumors, SCCHN only infrequently disseminates through the bloodstream, and therefore may be curable by locoregional therapy.
Oncolytic adenoviruses and herpes simplex viruses (HSVs) have been administered by intratumoral (IT) injection to patients with SCCHN (Galanis et al., 2005; Mace et al., 2008). In November 2005, Chinese government regulators approved the E1B 55-kDa–deleted adenovirus H101 for the treatment of nasopharyngeal carcinoma in combination with cisplatin chemotherapy. Approval was based on a trial in which IT H101 plus chemotherapy produced a 79.0% response rate (41/52) compared with 39.6% in patients treated with chemotherapy alone (Garber, 2006).
HSV oncolytics were administered by direct IT injection in two published SCCHN trials. In one trial, conducted in Glasgow, UK, 20 patients received a single IT injection of HSV1716 at a dose of 105 pfu or 5×105 pfu (Mace et al., 2008). The injections were well tolerated with no adverse biological effects, but evidence for IT virus amplification or biological activity was lacking. In another UK study of intrapatient dose escalation, 30 patients with subcutaneous deposits of breast, gastrointestinal, or head and neck cancer or with malignant melanoma (five patients with SCCHN), each patient received one to three IT doses of an oncolytic HSV expressing granulocyte macrophage colony stimulating factor (OncoVEXGM-CSF; Amgen, Thousand Oaks, CA) (Harrington et al., 2010). In each case, the first dose was 106 pfu, followed by second and third doses of 107 and 108 pfu, respectively. Pain, inflammation, or ulceration of the injected tumor, or a combination of these, was observed in four of the five patients with SCCHN, and HSV antigen was detected in necrotic areas of two posttreatment tumor biopsies.
The thyroidal sodium iodide symporter (NIS) on thyroid follicular cells is a membrane ion channel for transporting iodine into the cells required for thyroxine production (Dai et al., 1996). The human NIS gene is located on chromosome 19 and codes for an intrinsic membrane protein predicted to have 13 transmembrane segments, with the amino terminus being extracellular. NIS belongs to the solute carrier family 5A and functions as a symporter transporting two sodium ions (Na+) into the cell for each single iodide anion (I–) transported. The steep Na+ concentration gradient that drives this process is maintained by the sodium/potassium adenosine triphosphatase. Perchlorate (ClO4–) is a potent and specific inhibitor of NIS-mediated I– uptake. Numerous ions in addition to iodide are transported efficiently by the NIS protein, including pertechnetate, which allows NIS expression imaging with the readily available radioisotopes iodine-123 (123I), iodine-125 (125I), or technetium-99m (99mTc; as pertechnetate) (Zuckier et al., 2004). NIS has been used clinically for thyroid imaging (with 123I) or ablation (with 131I) and for systemic therapy of thyroid malignancies (Bushnell et al., 1992; Sisson and Carey, 2001).
An attenuated measles virus (MV) has been engineered to express the human NIS, designated as MV-NIS (Dingli et al., 2004). The cellular receptor CD46 is used by oncolytic MV to mediate virus entry (Anderson et al., 2004). Accordingly, the oncolytic efficacy of MV-NIS is highly correlated to the density of CD46 receptors on target cells. MV-NIS can specifically enter and replicate in the tumor cells expressing high-density CD46 receptor, but spare the normal tissues that have low-density CD46 receptors (Anderson et al., 2004). When MV-NIS was administered to rodents bearing human tumor xenografts, IT spread of the virus could be monitored noninvasively through radioiodine imaging (the virus does not propagate in normal mouse tissues), and the antitumor effect could be boosted considerably by appropriately timed administration of 131I (Dingli et al., 2004; Penheiter et al., 2010). In the present study, the feasibility of MV-NIS use for SCCHN radiovirotherapy was investigated. In addition, the combination therapy of MV-NIS with cyclophosphamide (CPA) was administered to enhance the radiovirotherapy.
Materials and Methods
Cell lines and viruses
Human head oral SCC cell lines SCC-25 and SCC-15 were maintained in Dulbecco's modified Eagle's medium and Ham F-12 medium (in 1:1 ratio) with 10% fetal bovine serum (FBS) supplemented with hydrocortisone (400 ng/ml). A rapidly growing cell line of anaplastic human thyroid carcinoma SW579 was maintained in Dulbecco's modified Eagle's medium with 10% FBS; a human hypopharyngeal carcinoma FaDu was maintained in RPMI 1640 with 10% FBS. All cell lines were purchased from the American Type Culture Collection (Manassas, VA).
MV-NIS was engineered as previously described (Dingli et al., 2004). The MV-NIS preparation and measles virus–green fluorescent protein (MV-GFP) used in all experiments of our study were produced by the Mayo Clinic Viral Vector Production Laboratory, met the demands for clinical usage, and contained a 3.5×107/ml Vero cell tissue culture infective dose (TCID50) (Langfield et al., 2011).
125I uptake
Iodide uptake assay was used as described previously (Dingli et al., 2004; Penheiter et al., 2010). The 12-well plates were seeded with 1.5×105 SCC-15 cells or FaDu cells. The MVs were added to the plates 24 hr later at a multiplicity of infection (MOI) of 0.1 or 1.0. After incubation for 2 hr at 37°C (98.6°F), cells were washed with phosphate-buffered saline (PBS); the medium was changed to complete culture medium and incubated for an additional 48 hr before 125I uptake testing. Containing an activity of 7×105 counts per minute, 125I was added to the wells and incubated for 45 min. In the control wells, 100 mM potassium perchlorate was used to block NIS-mediated iodide influx. After incubation, the cells were washed with medium and lysed with 1 M sodium hydroxide. Iodide activity remaining in the lysis buffer was measured with a γ counter. All data points were measured in triplicate and displayed as means.
Clonogenic assays
Clonogenic assays were performed as described. Head and neck cancer cells were seeded into six-well plates at a density of 100,000 per well. Cells were infected with MV-NIS 24 hr before irradiation. Six hours after radiation, the cells were transferred to six-well plates at densities ranging from 100 to 40,000 cells per well and were put in an incubator for 10–14 days. After incubation at 37°C, cells were fixed with glutaraldehyde and stained with crystal violet. Colony (containing at least 50 cells) formation for each condition was compared with that of untreated control cells. Results were obtained from four replicate wells, and each experiment was repeated twice.
In vivo studies
Athymic nu/nu mice (Harlan Sprague Dawley, Inc., Indianapolis, IN) were used for the animal studies. For subcutaneous tumors, mice received a right-flank implant of FaDu cells in 100 μl of PBS. Tumor growth was measured with a caliper in two dimensions. In our study, small tumors (diameter, 0.3–0.4 cm) and larger tumors (diameter, 0.6–0.8 cm) were treated intratumorally with MVs. The mice were injected with 125I (100 μCi) intraperitoneally, and tumor imaging was done serially with a high-resolution micro single-photon emission computed tomography/computed tomography (SPECT/CT) system (X-SPECT; Gamma Medica-Ideas, Inc., Northridge, CA). Uptakes were calculated with the software PMOD (PMOD Technologies, Ltd., Zurich, Switzerland) (Penheiter et al., 2011). In the combined treatment groups, mice received CPA (100 mg/kg) at 24 hr before viral administration and received 131I (1 μCi) at 48 hr after the viral injection. Tumor growth was monitored closely and calculated with the following formula: length×width2×0.52. A mouse was euthanized when its tumor grew to more than 10% of its weight or the mouse was unable to eat or drink. All animal studies were approved by the Mayo Clinic Institutional Animal Care and Use Committee and were performed in accordance with guidelines approved by the Assessment and Accreditation of Laboratory Animal Care.
Statistical analysis
Data were processed with the software GraphPad Prism 4.0 (GraphPad Software, Inc., San Diego, CA). The Kaplan-Meier method was used for graphic representation of survival curves. Iodide uptake was analyzed with a t test. A p value less than 0.05 was considered statistically significant.
Results
MV-NIS propagated in human head and neck cancer cells
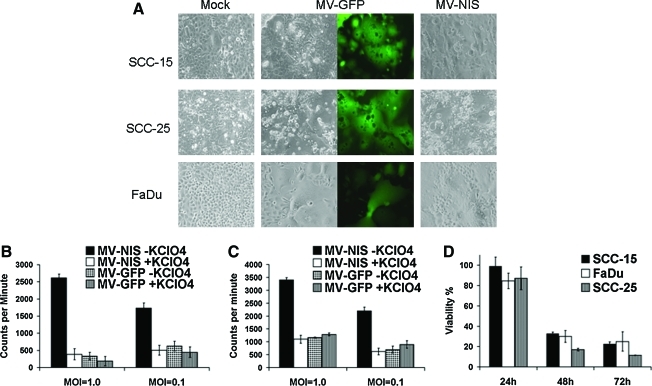
To check the infectivity of MVs in head and neck cancer cells, several SCCHN cells were tested using 12-well plates after infection with MVs at an MOI of 1.0. Trypan blue staining was done at 24, 48, and 72 hr after infection. All the head and neck cancer cell lines checked were susceptible to MV infection and formed syncytia (Fig. 1A). Cell viability decreased substantially within 48 hr. About 70% of the SCCHN cells were killed in most of the cell lines in vitro 72 hr after infection (Fig. 1D).
FIG. 1.
Cells of SCCHN are permissive to MV infection in vitro. Human SCCHN cell lines SCC-15, SCC-25, and FaDu were infected with MV-GFP or MV-NIS at MOI of 0.1. Images were taken 48 hr later (A). Sodium iodide (125I) uptake assays were carried out in SCC-15 (B) and FaDu (C) cells 72 hr after MV infection (MOIs of 1.0 and 0.1). Cell viability was compared with mock infection in SCC-15, SCC-25, and FaDu cells after trypan blue staining (D). “KClO4” indicates potassium perchlorate. Color images available online at www.liebertonline.com/hum
Iodide uptake assays were performed to test whether NIS protein expressed by oncolytic MV was bioactive after viral infection, as previously described (Dingli et al., 2004; Penheiter et al., 2010). Human SCC-15 and FaDu cells were infected with MV-NIS or MV-GFP. Both cell lines showed significant iodide uptake after 125I incubation when first infected with MV-NIS. More viruses led to better iodide uptake in both cell lines because of more virus replication and increasing NIS expression (Fig. 1B and C). Perchlorate, a specific NIS inhibitor, and MV-GFP were used to test whether the iodide uptake was NIS-specific. As expected, iodide uptake was blocked by perchlorate, and radioiodine could not be concentrated after MV-GFP infection (Fig. 1B and C). These data showed that NIS protein expression driven by oncolytic MV was integrated into cell membrane properly in virus-infected tumor cells.
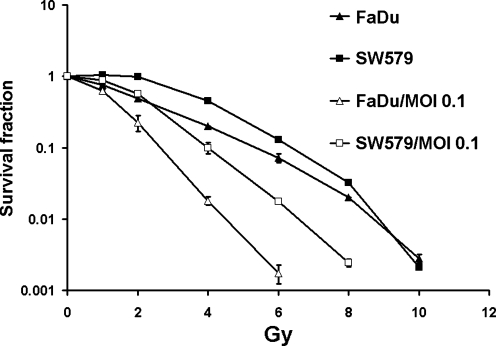
To assess the effect of oncolytic virotherapy and radiation treatment methods on cell proliferation and survival, we performed clonogenic assays on SCCHN FaDu and SW579 cell lines. Irradiation, followed 2 hr later by the addition of MV-NIS or MV-GFP at an MOI of 0.1, resulted in the pronouced increase of cytopathic effect shown on clonogenic assay (Fig. 2). This effect was also observed in SW579 cells. FaDu cells were moderately sensitive to damage from irradiation. At a dose of 4 Gy, irradiation alone resulted in 19.8% cell viability. However, after combination treatment with MV-NIS at an MOI of 0.1 and the same dose of radiation, FaDu cell survival dropped to 1.8%. Similar results were obtained with the SW579 cells (Fig. 2). The data showed that radiation treatment can be added to oncolytic virotherapy to enhance tumor cell killing in vitro, which is consistent with the previous report from C. Liu et al. (2007).
FIG. 2.
MVs and radiation therapy have combined antitumor effect in vitro. SCCHN cell lines FaDu and SW579 were irradiated at different doses, and clonogenic assays were done with or without MV-GFP infection at MOI of 0.1.
MV-NIS–infected SCCHN tumors concentrated radioiodine in vivo
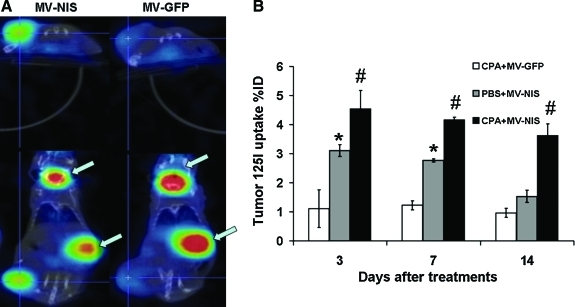
Human SCCHN FaDu cells were injected subcutaneously in female athymic nude mice. When the tumors reached a mean diameter of 0.5 cm, the mice were injected intratumorally with MV-GFP or MV-NIS (2×106 TCID50) with or without CPA (100 mg/kg) at 24 hr before viral injection. At day 3 after virus administration, 125I (100 μCi) was injected intraperitoneally, and SPECT/CT imaging was carried out 1 hr later. On days 7 and 14 after virus administration, the imaging procedure was repeated.
We anticipated that serial iodide uptake by the tumors would be consistent with increasing NIS expression (Li et al., 2010). The results suggested that viral gene expression and viral replication are coupled. No notable γ photon signal was detected from the tumors injected with MV-GFP with or without CPA (Fig. 3). Radioiodine was concentrated into MV-NIS–treated tumors and visualized by imaging at day 7 (Fig. 3A). With CPA, the oncolytic MV-NISs replicate much better than in the group without CPA, thereby concentrating more 125I into the tumor. The data showed that in vivo monitoring of oncolytic virus propagation can be achieved noninvasively by including human NIS and radioiodine imaging.
FIG. 3.
(A) Representative SPECT/CT images of nude mice with FaDu flank tumor. The only physiologic uptakes (arrows) were detected in areas of endogenous NIS-mediated radioiodide uptake in the thyroid and stomach and in accumulation in the bladder. In contrast, notable iodide accumulation was seen in xenografts infected with MV-NIS. (B) CPA, not PBS, can substantially enhance the radioiodide uptake to the tumor sites since day 3 after virotherapy with MV-NIS. *p<0.05 for PBS+MV-NIS vs. CPA+MV-GFP; #p<0.01 for CPA+MV-NIS vs. PBS+MV-NIS. Color images available online at www.liebertonline.com/hum
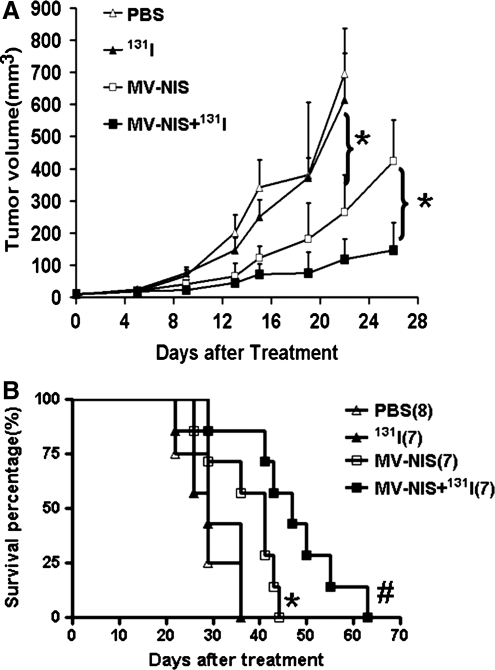
Combined therapy with 131I and MV-NIS was superior to single-agent MV virotherapy in vivo for head and neck cancer xenografts
MVs have been shown to control a wide array of tumors in vivo (Russell and Peng, 2007, 2008; Li et al., 2010), but have not yet been used to treat SCCHN. To test the efficacy of MVs in SCCHN, FaDu cells (1×106) were injected into the flanks of nude mice. When the tumors were palpable, MV or PBS was injected intratumorally, and 131I (1 μCi) was given 48 hr after the viral injection in the combined therapy group. Tumor growth was monitored twice a week. The survival curve was made at the end of the experiments. In PBS-treated mice, rapid tumor growth was observed, whereas tumor growth was inhibited substantially in MV-NIS–treated mice (Fig. 4). The average survival time for mice receiving PBS or 131I alone was about 29 days after treatment. In contrast, the average survival time for mice receiving MV-NIS alone was 41 days (p<0.05); for those receiving both MV-NIS and 131I, it was 47 days (p<0.01) (Fig. 4). Thus, combination therapy of MV-NIS and 131I reduced tumor burden and lengthened survival time.
FIG. 4.
Combined therapy with iodohippurate sodium 131I increased the efficiency of MV-NIS virotherapy. (A) In a xenograft model of human SCCHN, tumor volumes were compared in the different groups. Significant inhibition of tumor growth occurred in the MV-NIS– and in the MV-NIS+131I–treated groups compared with the control groups (p<0.01). (B) Kaplan-Meier survival curves show significant prolongation of survival in MV-NIS–treated mice compared with the PBS or 131I control group. The 131I enhances the MV-NIS virotherapy significantly. *p<0.05; #p<0.01.
CPA improved the radiovirotherapy with MV-NIS and 131I
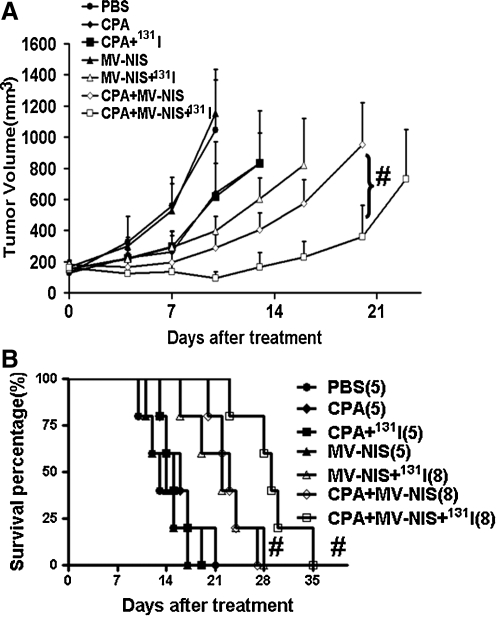
CPA has been used as an immunosuppressant to increase virus replication in tumor cells (Tyminski et al., 2005; Myers et al., 2007; Currier et al., 2008). In the present study, we tested both whether CPA could improve the antitumor effect of oncolytic measles virotherapy and whether it could achieve an enhanced effect when administered with 131I radiotherapy. FaDu cells (5×106) were inoculated into the flank of nude mice in order to achieve a larger tumor to test. When the tumor exceeded 6–8 mm in diameter, the tumor-bearing mice received a single injection of MV-NIS (2×106 TCID50 intratumorally) with either (1) CPA (100 mg/kg intraperitoneally) 24 hr before virus injection, (2) 131I (1 μCi per mouse intraperitoneally) 48 hr after virus injection, or (3) a combination of these regimens (Fig. 5). Administration of CPA with or without 131I produced an antitumor effect similar to that of virotherapy (p=0.26; survival, p=0.30). However, coadministration of MV-NIS and either CPA or 131I increased the antitumor effect substantially, as shown in tumor reductions compared with virotherapy alone (p<0.001 or p<0.002 for tumor growth; p<0.007 or p<0.002 for survival). Most importantly, when added to MV-NIS virotherapy, CPA administration could achieve an enhanced therapeutic effect with 131I radiotherapy. The antitumor effect was significantly pronounced compared with that of any single or double treatment, although the therapeutic regimen was given only once. In summary, CPA as a immunosuppressant increased MV-NIS replication tentatively by reducing immune response against virus infection, which resulted in enhanced NIS expression in FaDu tumors. Thereby, CPA can be used to boost radiovirotherapy using MV-NIS and 131I in vivo.
FIG. 5.
CPA significantly improves the therapeutic effect of MV-NIS–mediated radiovirotherapy. After IT MV-NIS plus intraperitoneal 131I administration, CPA intraperitoneal injection can amplify significantly the inhibition of FaDu tumor growth (A) and mouse survival (B). Tumors were injected with CPA (100 mg/kg) or PBS on day 1 and with MV-NIS (2×106) or PBS on day 0. Mice were euthanized when tumor volume reached 1.5 cm3 or the tumor exhibited severe ulceration due to rapid tumor growth. #p<0.01.
Discussion
MV-NIS has shown safety in ongoing phase 1 clinical studies, activity in preclinical SCCHN models, and potential compatibility with chemoradiotherapy in the present study. We can hypothesize that MV-NIS can enhance significantly the outcomes of therapy for SCCHN alone or when correctly incorporated into existing chemotherapy programs for locally advanced disease.
To our knowledge, our study is the first in which oncolytic MVs for SCCHN were developed and the role of NIS gene transfer in SCCHN was tracked. Several studies published in the past decade (since the original cloning of NIS) have demonstrated the value of NIS as a reporter gene for in vivo imaging applications (Dadachova and Carrasco, 2004; Li et al., 2010; Penheiter et al., 2011). Interest is growing in the use of NIS in preclinical studies designed to monitor the biodistribution of gene expression after in vivo vector administration, the spread of NIS-encoding oncolytic viruses, and the in vivo fate of implanted NIS-expressing cells. In addition to the MV-NIS virus being clinically evaluated at Mayo Clinic, a replication-competent adenovirus encoding human NIS was developed at the Henry Ford Medical Center in Detroit and has been advanced to ongoing clinical testing in patients with prostate cancer (Barton et al., 2008). Already, radioiodine uptake has been documented at sites of virus infection in some of the treated cancer patients. MV-NIS is a highly promising new anticancer agent that may substantially enhance the outcomes of standard therapy after IT administration in patients with locally advanced SCCHN.
Mayo Clinic conducted extensive preclinical testing of MV-NIS, demonstrating remarkable efficacy in mouse models of multiple myeloma and ovarian cancer (Dingli et al., 2004; Hasegawa et al., 2006). Comprehensive preclinical pharmacology and toxicology studies were then conducted under good laboratory practice in measles-susceptible transgenic mice and in squirrel monkeys with or without CPA immunosuppression (Myers et al., 2007). Significant toxicities were not observed. Clinical grade MV-NIS was manufactured in the Mayo Clinic Viral Vector Production Laboratory under good manufacturing practice, and phase 1 clinical protocols were developed to evaluate the safety of MV-NIS administered initially through intravenous infusion in patients with multiple myeloma and subsequently through intraperitoneal infusion in patients with ovarian carcinoma. Data from the first of these ongoing clinical trials indicate that MV-NIS can be administered safely to human study participants through intravenous infusion up to the highest tested dose level (1×109 TCID50).
In summary, the efficacy of using oncolytic MV to treat SCCHN has been demonstrated in this study. CPA and radioactive 131I can be incorporated into the treatment to enhance the virotherapy.
Acknowledgments
This work is supported by NIH grant R01CA100634 and the Richard M. Schulze Family Foundation to Stephen J. Russell.
Author Disclosure Statement
Stephen J. Russell, M.D., Ph.D., is a named inventor on patents pertaining to the use of MV as an anticancer drug therapy. These patents are owned by Mayo Clinic.
References
- Anderson B.D. Nakamura T. Russell S.J. Peng K.W. High CD46 receptor density determines preferential killing of tumor cells by oncolytic measles virus. Cancer Res. 2004;64:4919–4926. doi: 10.1158/0008-5472.CAN-04-0884. [DOI] [PubMed] [Google Scholar]
- Ayllón Barbellido S. Campo Trapero J. Cano Sánchez J., et al. Gene therapy in the management of oral cancer: review of the literature. Med. Oral Patol. Oral Cir. Bucal. 2008;13:E15–E21. [PubMed] [Google Scholar]
- Barton K.N. Stricker H. Brown S.L., et al. Phase I study of noninvasive imaging of adenovirus-mediated gene expression in the human prostate. Mol. Ther. 2008;16:1761–1769. doi: 10.1038/mt.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard P. Hill C. Guihenneuc-Jouyaux C., et al. Mixed treatment comparison meta-analysis of altered fractionated radiotherapy and chemotherapy in head and neck cancer. J. Clin. Epidemiol. 2011;64:985–992. doi: 10.1016/j.jclinepi.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Bushnell D.L. Boles M.A. Kaufman G.E., et al. Complications, sequela and dosimetry of iodine-131 therapy for thyroid carcinoma. J. Nucl. Med. 1992;33:2214–2221. [PubMed] [Google Scholar]
- Chin D. Boyle G.M. Porceddu S., et al. Head and neck cancer: past, present and future. Expert Rev. Anticancer Ther. 2006;6:1111–1118. doi: 10.1586/14737140.6.7.1111. [DOI] [PubMed] [Google Scholar]
- Currier M.A. Gillespie R.A. Sawtell N.M., et al. Efficacy and safety of the oncolytic herpes simplex virus rRp450 alone and combined with cyclophosphamide. Mol. Ther. 2008;16:879–885. doi: 10.1038/mt.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadachova E. Carrasco N. The Na/I symporter (NIS): imaging and therapeutic applications. Semin. Nucl. Med. 2004;34:23–31. doi: 10.1053/j.semnuclmed.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Dai G. Levy O. Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- Dingli D. Peng K.W. Harvey M.E., et al. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- Galanis E. Okuno S.H. Nascimento A.G., et al. Phase I–II trial of ONYX-015 in combination with MAP chemotherapy in patients with advanced sarcomas. Gene Ther. 2005;12:437–445. doi: 10.1038/sj.gt.3302436. [DOI] [PubMed] [Google Scholar]
- Garber K. China approves world's first oncolytic virus therapy for cancer treatment. J. Natl. Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- Harrington K.J. Hingorani M. Tanay M.A., et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin. Cancer Res. 2010;16:4005–4015. doi: 10.1158/1078-0432.CCR-10-0196. [DOI] [PubMed] [Google Scholar]
- Hasegawa K. Pham L. O'Connor M.K., et al. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin. Cancer Res. 2006;12:1868–1875. doi: 10.1158/1078-0432.CCR-05-1803. [DOI] [PubMed] [Google Scholar]
- Jemal A. Siegel R. Xu J. Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Langfield K.K. Walker H.J. Gregory L.C. Federspiel M.J. Manufacture of measles viruses. Methods Mol. Biol. 2011;737:345–366. doi: 10.1007/978-1-61779-095-9_14. [DOI] [PubMed] [Google Scholar]
- Lefebvre J.L. Current clinical outcomes demand new treatment options for SCCHN. Ann. Oncol. 2005;16(Suppl 6):vi7–vi12. doi: 10.1093/annonc/mdi452. [DOI] [PubMed] [Google Scholar]
- Li H. Peng K.W. Dingli D., et al. Oncolytic measles viruses encoding interferon beta and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 2010;17:550–558. doi: 10.1038/cgt.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. Sarkaria J.N. Petell C.A., et al. Combination of measles virus virotherapy and radiation therapy has synergistic activity in the treatment of glioblastoma multiforme. Clin. Cancer Res. 2007;13:7155–7165. doi: 10.1158/1078-0432.CCR-07-1306. [DOI] [PubMed] [Google Scholar]
- Liu T.C. Kirn D. Gene therapy progress and prospects cancer: oncolytic viruses. Gene Ther. 2008;15:877–884. doi: 10.1038/gt.2008.72. [DOI] [PubMed] [Google Scholar]
- Liu T.C. Galanis E. Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- Mace A.T. Ganly I. Soutar D.S. Brown S.M. Potential for efficacy of the oncolytic Herpes simplex virus 1716 in patients with oral squamous cell carcinoma. Head Neck. 2008;30:1045–1051. doi: 10.1002/hed.20840. [DOI] [PubMed] [Google Scholar]
- Morganti A.G. Mignogna S. Deodato F., et al. Feasibility study of moderately accelerated intensity-modulated radiotherapy plus concurrent weekly cisplatin after induction chemotherapy in locally advanced head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011;79:1073–1080. doi: 10.1016/j.ijrobp.2009.12.020. [DOI] [PubMed] [Google Scholar]
- Myers R.M. Greiner S.M. Harvey M.E., et al. Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide. Clin. Pharmacol. Ther. 2007;82:700–710. doi: 10.1038/sj.clpt.6100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.L. Tumilasci V.F. Singhroy D., et al. The emergence of combinatorial strategies in the development of RNA oncolytic virus therapies. Cell. Microbiol. 2009;11:889–897. doi: 10.1111/j.1462-5822.2009.01317.x. [DOI] [PubMed] [Google Scholar]
- Penheiter A.R. Wegman T.R. Classic K.L., et al. Sodium iodide symporter (NIS)-mediated radiovirotherapy for pancreatic cancer. AJR Am. J. Roentgenol. 2010;195:341–349. doi: 10.2214/AJR.09.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penheiter A.R. Griesmann G.E. Federspiel M.J., et al. Pinhole micro-SPECT/CT for noninvasive monitoring and quantitation of oncolytic virus dispersion and percent infection in solid tumors. Gene Ther. 2011 doi: 10.1038/gt.2011.107. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S.J. Peng K.W. Viruses as anticancer drugs. Trends Pharmacol. Sci. 2007;28:326–333. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S.J. Peng K.W. The utility of cells as vehicles for oncolytic virus therapies. Curr. Opin. Mol. Ther. 2008;10:380–386. [PubMed] [Google Scholar]
- Sankaranarayanan R. Masuyer E. Swaminathan R., et al. Head and neck cancer: a global perspective on epidemiology and prognosis. Anticancer Res. 1998;18:4779–4786. [PubMed] [Google Scholar]
- Sisson J.C. Carey J.E. Thyroid carcinoma with high levels of function: treatment with 131I. J. Nucl. Med. 2001;42:975–983. [PubMed] [Google Scholar]
- Tyminski E. Leroy S. Terada K., et al. Brain tumor oncolysis with replication-conditional herpes simplex virus type 1 expressing the prodrug-activating genes, CYP2B1 and secreted human intestinal carboxylesterase, in combination with cyclophosphamide and irinotecan. Cancer Res. 2005;65:6850–6857. doi: 10.1158/0008-5472.CAN-05-0154. [DOI] [PubMed] [Google Scholar]
- Zuckier L.S. Dohan O. Li Y., et al. Kinetics of perrhenate uptake and comparative biodistribution of perrhenate, pertechnetate, and iodide by NaI symporter-expressing tissues in vivo. J. Nucl. Med. 2004;45:500–507. [PubMed] [Google Scholar]