Abstract
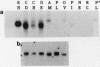
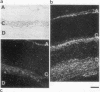
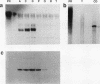
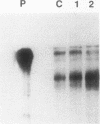
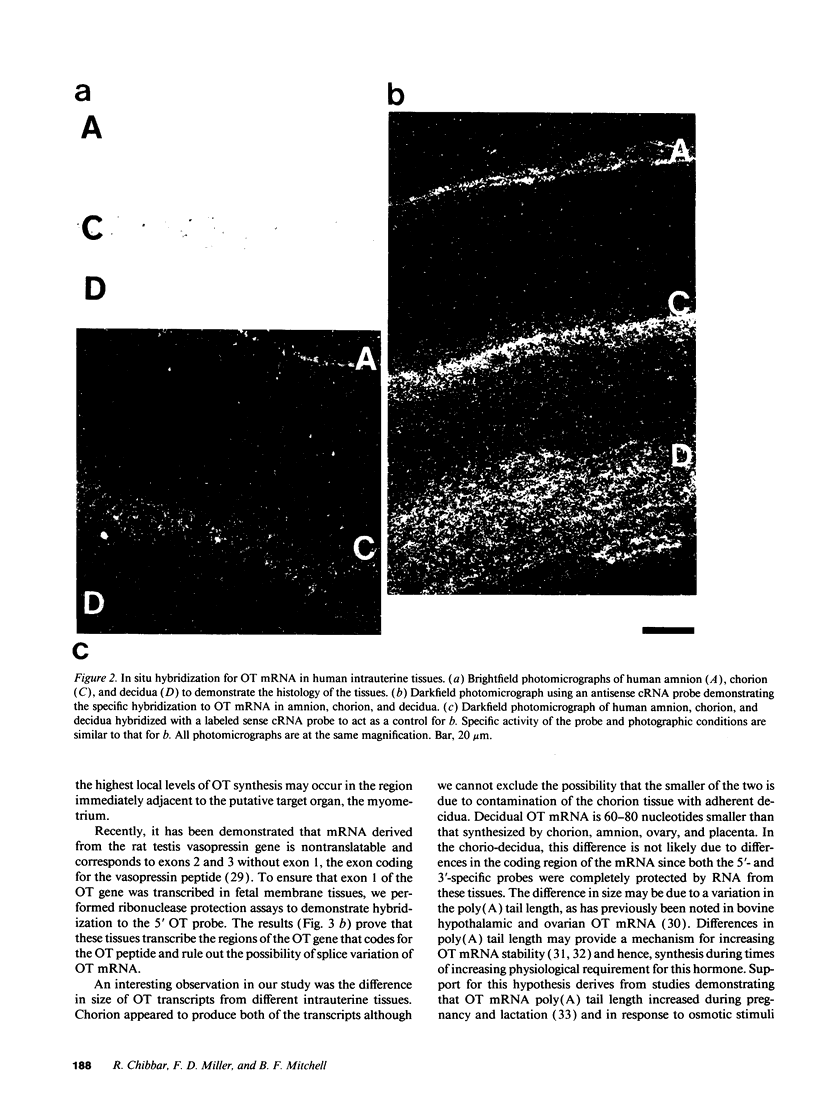
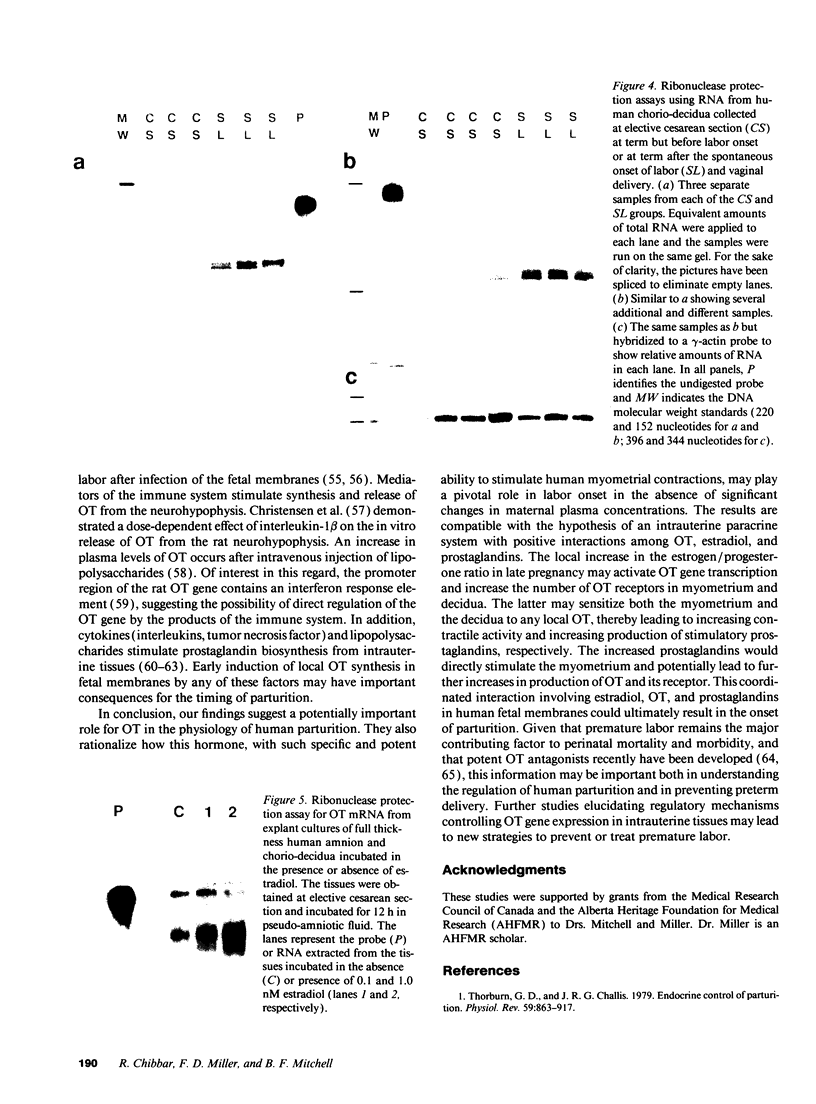
Despite the widespread clinical use of oxytocin (OT) as a potent and specific stimulant of labor, previous research data have not supported a role for OT in the physiology of normal human parturition. We have demonstrated synthesis of OT mRNA in amnion, chorion, and decidua using Northern blot analysis, ribonuclease protection assays, and in situ hybridization. Probes directed towards both the 3' and 5' ends of the gene have been used. Levels were highest in decidua with considerably less in chorion and amnion and very low levels in placenta. The transcript size in decidua appears to be 60-80 nucleotides smaller than the transcripts in amnion and chorion. OT gene expression in chorio-decidual tissues increased three- to fourfold around the time of labor onset. Estradiol stimulated synthesis of OT mRNA during in vitro incubation. These results support the hypothesis of a paracrine system involving OT and sex steroids within intrauterine tissues wherein significant changes could occur without being reflected in the maternal circulation. Such a paracrine system could rationalize a long-sought role for oxytocin in the physiology of human labor. These data may lead to novel approaches towards prevention or treatment or preterm labor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerlund M., Strömberg P., Hauksson A., Andersen L. F., Lyndrup J., Trojnar J., Melin P. Inhibition of uterine contractions of premature labour with an oxytocin analogue. Results from a pilot study. Br J Obstet Gynaecol. 1987 Nov;94(11):1040–1044. doi: 10.1111/j.1471-0528.1987.tb02287.x. [DOI] [PubMed] [Google Scholar]
- Amico J. A., Seitchik J., Robinson A. G. Studies of oxytocin in plasma of women during hypocontractile labor. J Clin Endocrinol Metab. 1984 Feb;58(2):274–279. doi: 10.1210/jcem-58-2-274. [DOI] [PubMed] [Google Scholar]
- Bernstein P., Ross J. Poly(A), poly(A) binding protein and the regulation of mRNA stability. Trends Biochem Sci. 1989 Sep;14(9):373–377. doi: 10.1016/0968-0004(89)90011-x. [DOI] [PubMed] [Google Scholar]
- Brent G. A., Hershman J. M. Thyroxine therapy in patients with severe nonthyroidal illnesses and low serum thyroxine concentration. J Clin Endocrinol Metab. 1986 Jul;63(1):1–8. doi: 10.1210/jcem-63-1-1. [DOI] [PubMed] [Google Scholar]
- Carter D. A., Murphy D. Independent regulation of neuropeptide mRNA level and poly(A) tail length. J Biol Chem. 1989 Apr 25;264(12):6601–6603. [PubMed] [Google Scholar]
- Casey M. L., MacDonald P. C. Biomolecular processes in the initiation of parturition: decidual activation. Clin Obstet Gynecol. 1988 Sep;31(3):533–552. doi: 10.1097/00003081-198809000-00005. [DOI] [PubMed] [Google Scholar]
- Chan W. Y. Enhanced prostaglandin synthesis in the parturient rat uterus and its effects on myometrial oxytocin receptor concentrations. Prostaglandins. 1987 Dec;34(6):889–902. doi: 10.1016/0090-6980(87)90069-4. [DOI] [PubMed] [Google Scholar]
- Chan W. Y. Enhanced prostaglandin synthesis in the parturient rat uterus and its effects on myometrial oxytocin receptor concentrations. Prostaglandins. 1987 Dec;34(6):889–902. doi: 10.1016/0090-6980(87)90069-4. [DOI] [PubMed] [Google Scholar]
- Chard T. Fetal and maternal oxytocin in human parturition. Am J Perinatol. 1989 Apr;6(2):145–152. doi: 10.1055/s-2007-999566. [DOI] [PubMed] [Google Scholar]
- Chibbar R., Toma J. G., Mitchell B. F., Miller F. D. Regulation of neural oxytocin gene expression by gonadal steroids in pubertal rats. Mol Endocrinol. 1990 Dec;4(12):2030–2038. doi: 10.1210/mend-4-12-2030. [DOI] [PubMed] [Google Scholar]
- Christensen J. D., Hansen E. W., Fjalland B. Influence of interleukin-1 beta on the secretion of oxytocin and vasopressin from the isolated rat neurohypophysis. Pharmacol Toxicol. 1990 Jul;67(1):81–83. doi: 10.1111/j.1600-0773.1990.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Dawood M. Y., Ylikorkala O., Trivedi D., Fuchs F. Oxytocin in maternal circulation and amniotic fluid during pregnancy. J Clin Endocrinol Metab. 1979 Sep;49(3):429–434. doi: 10.1210/jcem-49-3-429. [DOI] [PubMed] [Google Scholar]
- Foo N. C., Carter D., Murphy D., Ivell R. Vasopressin and oxytocin gene expression in rat testis. Endocrinology. 1991 Apr;128(4):2118–2128. doi: 10.1210/endo-128-4-2118. [DOI] [PubMed] [Google Scholar]
- Fuchs A. R., Fuchs F., Husslein P., Soloff M. S., Fernström M. J. Oxytocin receptors and human parturition: a dual role for oxytocin in the initiation of labor. Science. 1982 Mar 12;215(4538):1396–1398. doi: 10.1126/science.6278592. [DOI] [PubMed] [Google Scholar]
- Fuchs A. R., Fuchs F., Husslein P., Soloff M. S. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984 Nov 15;150(6):734–741. doi: 10.1016/0002-9378(84)90677-x. [DOI] [PubMed] [Google Scholar]
- Fuchs A. R., Husslein P., Fuchs F. Oxytocin and the initiation of human parturition. II. Stimulation of prostaglandin production in human decidua by oxytocin. Am J Obstet Gynecol. 1981 Nov 15;141(6):694–697. doi: 10.1016/s0002-9378(15)33313-5. [DOI] [PubMed] [Google Scholar]
- Fuchs A. R., Husslein P., Kofler E., Grünberger W., Rasmussen A., Rehnström J. Effect of cervical application of prostaglandin (PG) E2 on plasma 13,14-dihydro-15-keto-PGF2 alpha and oxytocin in pregnant women at term. Br J Obstet Gynaecol. 1983 Jul;90(7):612–617. doi: 10.1111/j.1471-0528.1983.tb09276.x. [DOI] [PubMed] [Google Scholar]
- Fuchs A. R., Periyasamy S., Alexandrova M., Soloff M. S. Correlation between oxytocin receptor concentration and responsiveness to oxytocin in pregnant rat myometrium: effects of ovarian steroids. Endocrinology. 1983 Aug;113(2):742–749. doi: 10.1210/endo-113-2-742. [DOI] [PubMed] [Google Scholar]
- Fuchs A. R., Romero R., Keefe D., Parra M., Oyarzun E., Behnke E. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol. 1991 Nov;165(5 Pt 1):1515–1523. doi: 10.1016/0002-9378(91)90399-c. [DOI] [PubMed] [Google Scholar]
- Fukai H., Den K., Sakamoto H., Kodaira H., Uchida F., Takagi S. Study of oxytocin receptor: II. oxytocin and prostaglandin F2 alpha receptors in human myometria and amnion-decidua complex during pregnancy and labor. Endocrinol Jpn. 1984 Oct;31(5):565–570. doi: 10.1507/endocrj1954.31.565. [DOI] [PubMed] [Google Scholar]
- Garfield R. E., Daniel E. E., Dukes M., Fitzgerald J. D. Changes of gap junctions in myometrium of guinea pig at parturition and abortion. Can J Physiol Pharmacol. 1982 Mar;60(3):335–341. doi: 10.1139/y82-047. [DOI] [PubMed] [Google Scholar]
- Garfield R. E., Rabideau S., Challis J. R., Daniel E. E. Hormonal control of gap junction formation in sheep myometrium during parturition. Biol Reprod. 1979 Nov;21(4):999–1007. doi: 10.1095/biolreprod21.4.999. [DOI] [PubMed] [Google Scholar]
- Guillon G., Balestre M. N., Roberts J. M., Bottari S. P. Oxytocin and vasopressin: distinct receptors in myometrium. J Clin Endocrinol Metab. 1987 Jun;64(6):1129–1135. doi: 10.1210/jcem-64-6-1129. [DOI] [PubMed] [Google Scholar]
- Guldenaar S. E., Wathes D. C., Pickering B. T. Immunocytochemical evidence for the presence of oxytocin and neurophysin in the large cells of the bovine corpus luteum. Cell Tissue Res. 1984;237(2):349–352. doi: 10.1007/BF00217155. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluska G. J., West N. B., Novy M. J., Brenner R. M. Uterine estrogen receptors are increased by RU486 in late pregnant rhesus macaques but not after spontaneous labor. J Clin Endocrinol Metab. 1990 Jan;70(1):181–186. doi: 10.1210/jcem-70-1-181. [DOI] [PubMed] [Google Scholar]
- Husslein P., Fuchs A. R., Fuchs F. Oxytocin and the initiation of human parturition. I. Prostaglandin release during induction of labor by oxytocin. Am J Obstet Gynecol. 1981 Nov 15;141(6):688–693. doi: 10.1016/s0002-9378(15)33312-3. [DOI] [PubMed] [Google Scholar]
- Ivell R., Brackett K. H., Fields M. J., Richter D. Ovulation triggers oxytocin gene expression in the bovine ovary. FEBS Lett. 1985 Oct 14;190(2):263–267. doi: 10.1016/0014-5793(85)81296-5. [DOI] [PubMed] [Google Scholar]
- Ivell R., Furuya K., Brackmann B., Dawood Y., Khan-Dawood F. Expression of the oxytocin and vasopressin genes in human and baboon gonadal tissues. Endocrinology. 1990 Dec;127(6):2990–2996. doi: 10.1210/endo-127-6-2990. [DOI] [PubMed] [Google Scholar]
- Ivell R., Richter D. The gene for the hypothalamic peptide hormone oxytocin is highly expressed in the bovine corpus luteum: biosynthesis, structure and sequence analysis. EMBO J. 1984 Oct;3(10):2351–2354. doi: 10.1002/j.1460-2075.1984.tb02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirikowski G. F., Sanna P. P., Bloom F. E. mRNA coding for oxytocin is present in axons of the hypothalamo-neurohypophysial tract. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7400–7404. doi: 10.1073/pnas.87.19.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasting N. W. Characteristics of body temperature, vasopressin, and oxytocin responses to endotoxin in the rat. Can J Physiol Pharmacol. 1986 Dec;64(12):1575–1578. doi: 10.1139/y86-265. [DOI] [PubMed] [Google Scholar]
- Khan-Dawood F. S., Dawood M. Y. Human ovaries contain immunoreactive oxytocin. J Clin Endocrinol Metab. 1983 Dec;57(6):1129–1132. doi: 10.1210/jcem-57-6-1129. [DOI] [PubMed] [Google Scholar]
- Lamont R. F., Rose M., Elder M. G. Effect of bacterial products on prostaglandin E production by amnion cells. Lancet. 1985 Dec 14;2(8468):1331–1333. doi: 10.1016/s0140-6736(85)92628-5. [DOI] [PubMed] [Google Scholar]
- Leake R. D., Weitzman R. E., Glatz T. H., Fisher D. A. Plasma oxytocin concentrations in men, nonpregnant women, and pregnant women before and during spontaneous labor. J Clin Endocrinol Metab. 1981 Oct;53(4):730–733. doi: 10.1210/jcem-53-4-730. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller F. D., Ozimek G., Milner R. J., Bloom F. E. Regulation of neuronal oxytocin mRNA by ovarian steroids in the mature and developing hypothalamus. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2468–2472. doi: 10.1073/pnas.86.7.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B. F., Powell W. A. Progesterone production by human fetal membranes: an in vitro incubation system for studying hormone production and metabolism. Am J Obstet Gynecol. 1984 Feb 1;148(3):303–309. doi: 10.1016/s0002-9378(84)80073-3. [DOI] [PubMed] [Google Scholar]
- Mitchell B., Cruickshank B., McLean D., Challis J. Local modulation of progesterone production in human fetal membranes. J Clin Endocrinol Metab. 1982 Dec;55(6):1237–1239. doi: 10.1210/jcem-55-6-1237. [DOI] [PubMed] [Google Scholar]
- Mitchell M. D., Edwin S., Romero R. J. Prostaglandin biosynthesis by human decidual cells: effects of inflammatory mediators. Prostaglandins Leukot Essent Fatty Acids. 1990 Sep;41(1):35–38. doi: 10.1016/0952-3278(90)90128-8. [DOI] [PubMed] [Google Scholar]
- Mitchell M. D., Romero R. J., Avila C., Foster J. T., Edwin S. S. Prostaglandin production by amnion and decidual cells in response to bacterial products. Prostaglandins Leukot Essent Fatty Acids. 1991 Mar;42(3):167–169. doi: 10.1016/0952-3278(91)90152-u. [DOI] [PubMed] [Google Scholar]
- Mohr E., Bahnsen U., Kiessling C., Richter D. Expression of the vasopressin and oxytocin genes in rats occurs in mutually exclusive sets of hypothalamic neurons. FEBS Lett. 1988 Dec 19;242(1):144–148. doi: 10.1016/0014-5793(88)81003-2. [DOI] [PubMed] [Google Scholar]
- Mohr E., Zhou A., Thorn N. A., Richter D. Rats with physically disconnected hypothalamo-pituitary tracts no longer contain vasopressin-oxytocin gene transcripts in the posterior pituitary lobe. FEBS Lett. 1990 Apr 24;263(2):332–336. doi: 10.1016/0014-5793(90)81407-f. [DOI] [PubMed] [Google Scholar]
- Moore J. J., Moore R. M., Vander Kooy D. Protein kinase-C activation is required for oxytocin-induced prostaglandin production in human amnion cells. J Clin Endocrinol Metab. 1991 May;72(5):1073–1080. doi: 10.1210/jcem-72-5-1073. [DOI] [PubMed] [Google Scholar]
- Okazaki T., Casey M. L., Okita J. R., MacDonald P. C., Johnston J. M. Initiation of human parturition. XII. Biosynthesis and metabolism of prostaglandins in human fetal membranes and uterine decidua. Am J Obstet Gynecol. 1981 Feb 15;139(4):373–381. [PubMed] [Google Scholar]
- Olson D. M., Skinner K., Challis J. R. Estradiol-17 beta and 2-hydroxyestradiol-17 beta-induced differential production of prostaglandins by cells dispersed from human intrauterine tissues at parturition. Prostaglandins. 1983 May;25(5):639–651. doi: 10.1016/0090-6980(83)90118-1. [DOI] [PubMed] [Google Scholar]
- Olson D. M., Skinner K., Challis J. R. Prostaglandin output in relation to parturition by cells dispersed from human intrauterine tissues. J Clin Endocrinol Metab. 1983 Oct;57(4):694–699. doi: 10.1210/jcem-57-4-694. [DOI] [PubMed] [Google Scholar]
- Rehbein M., Hillers M., Mohr E., Ivell R., Morley S., Schmale H., Richter D. The neurohypophyseal hormones vasopressin and oxytocin. Precursor structure, synthesis and regulation. Biol Chem Hoppe Seyler. 1986 Aug;367(8):695–704. doi: 10.1515/bchm3.1986.367.2.695. [DOI] [PubMed] [Google Scholar]
- Richard S., Zingg H. H. The human oxytocin gene promoter is regulated by estrogens. J Biol Chem. 1990 Apr 15;265(11):6098–6103. [PubMed] [Google Scholar]
- Romero R., Manogue K. R., Mitchell M. D., Wu Y. K., Oyarzun E., Hobbins J. C., Cerami A. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989 Aug;161(2):336–341. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- Romero R., Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988 Sep;31(3):553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- Romero R., Sirtori M., Oyarzun E., Avila C., Mazor M., Callahan R., Sabo V., Athanassiadis A. P., Hobbins J. C. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989 Sep;161(3):817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- Schwartz A. L., Forster C. S., Smith P. A., Liggins G. C. Human amnion metabolism. I. In vitro maintenance. Am J Obstet Gynecol. 1977 Mar 1;127(5):470–474. doi: 10.1016/0002-9378(77)90437-9. [DOI] [PubMed] [Google Scholar]
- Shukovski L., Healy D. L., Findlay J. K. Circulating immunoreactive oxytocin during the human menstrual cycle comes from the pituitary and is estradiol dependent. J Clin Endocrinol Metab. 1989 Feb;68(2):455–460. doi: 10.1210/jcem-68-2-455. [DOI] [PubMed] [Google Scholar]
- Skinner K. A., Challis J. R. Changes in the synthesis and metabolism of prostaglandins by human fetal membranes and decidua at labor. Am J Obstet Gynecol. 1985 Feb 15;151(4):519–523. doi: 10.1016/0002-9378(85)90281-9. [DOI] [PubMed] [Google Scholar]
- Thorburn G. D., Challis J. R. Endocrine control of parturition. Physiol Rev. 1979 Oct;59(4):863–918. doi: 10.1152/physrev.1979.59.4.863. [DOI] [PubMed] [Google Scholar]
- Van Tol H. H., Bolwerk E. L., Liu B., Burbach J. P. Oxytocin and vasopressin gene expression in the hypothalamo-neurohypophyseal system of the rat during the estrous cycle, pregnancy, and lactation. Endocrinology. 1988 Mar;122(3):945–951. doi: 10.1210/endo-122-3-945. [DOI] [PubMed] [Google Scholar]
- Wilson L., Jr, Parsons M. T., Flouret G. Inhibition of spontaneous uterine contractions during the last trimester in pregnant baboons by an oxytocin antagonist. Am J Obstet Gynecol. 1990 Dec;163(6 Pt 1):1875–1882. doi: 10.1016/0002-9378(90)90767-2. [DOI] [PubMed] [Google Scholar]
- Zeevi M., Nevins J. R., Darnell J. E., Jr Newly formed mRNA lacking polyadenylic acid enters the cytoplasm and the polyribosomes but has a shorter half-life in the absence of polyadenylic acid. Mol Cell Biol. 1982 May;2(5):517–525. doi: 10.1128/mcb.2.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingg H. H., Lefebvre D. L. Oxytocin mRNA: increase of polyadenylate tail size during pregnancy and lactation. Mol Cell Endocrinol. 1989 Aug;65(1-2):59–62. doi: 10.1016/0303-7207(89)90165-2. [DOI] [PubMed] [Google Scholar]