Abstract
Band 3 aggregation in the plane of the red blood cell (RBC) membrane is postulated to be important in the pathophysiology of hemolysis of dense sickle and normal RBCs. We used the fluorescence photobleaching recovery and polarized fluorescence depletion techniques to measure the lateral and rotational mobility of band 3, glycophorins, and phospholipid analogues in membranes of density-separated intact RBCs from seven patients with sickle cell disease and eight normal controls. The fractions of laterally mobile band 3 and glycophorin decreased progressively as sickle RBC density increased. Normal RBCs also showed a progressive decrease in band 3 fractional mobility with increasing buoyant density. Rapidly rotating, slowly rotating, and rotationally immobile forms of band 3 were observed in both sickle and normal RBC membranes. The fraction of rapidly rotating band 3 progressively decreased and the fraction of rotationally immobile band 3 progressively increased with increasing sickle RBC density. Changes in the fraction of rotationally immobile band 3 were not reversible upon hypotonic swelling of dense sickle RBCs, and normal RBCs osmotically shrunken in sucrose buffers failed to manifest band 3 immobilization at median cell hemoglobin concentration values characteristic of dense sickle RBCs. We conclude that dense sickle and normal RBCs acquire irreversible membrane abnormalities that cause transmembrane protein immobilization and band 3 aggregation. Band 3 aggregates could serve as cell surface sites of autologous antibody binding and thereby lead to removal of dense sickle and normal (senescent) RBCs from the circulation.
Full text
PDF

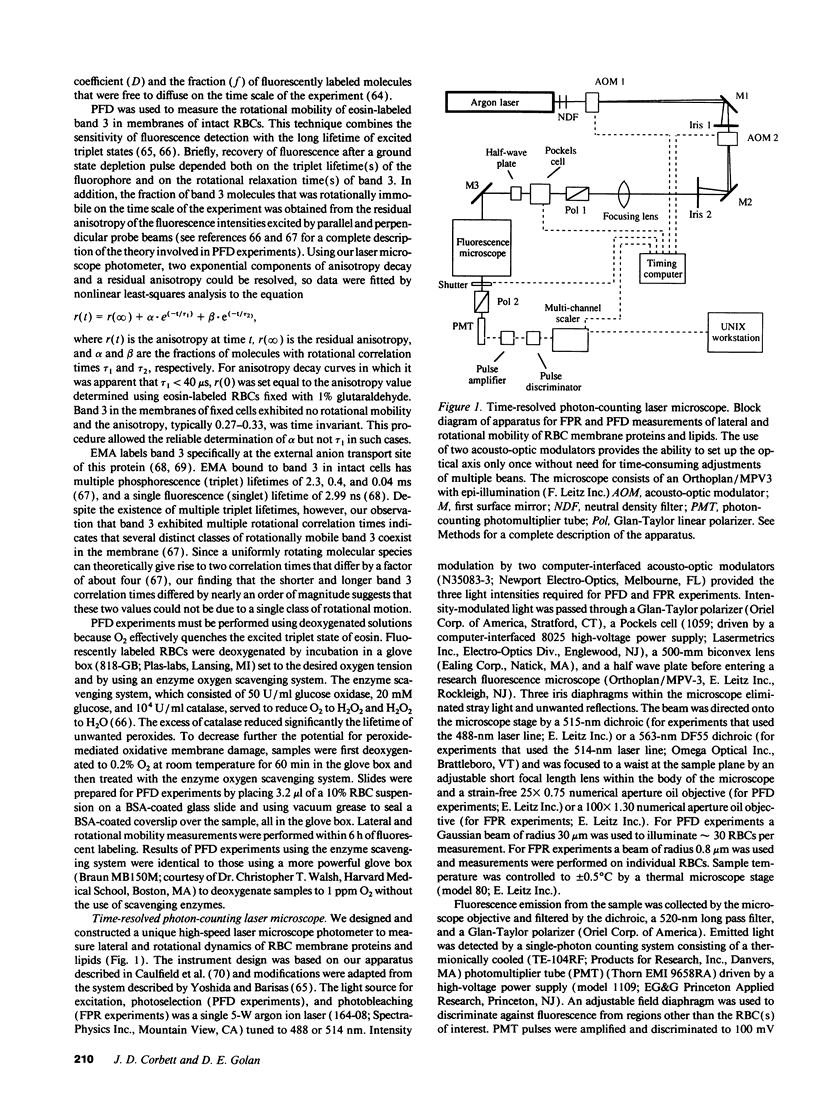
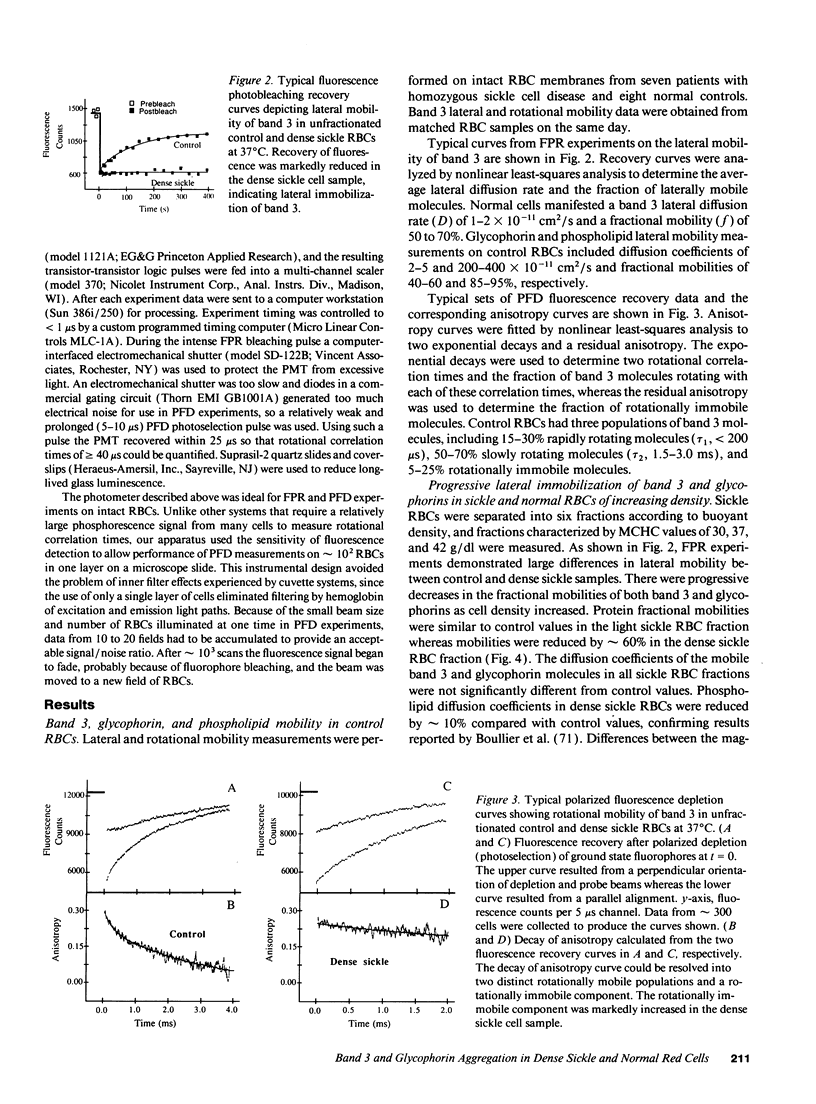
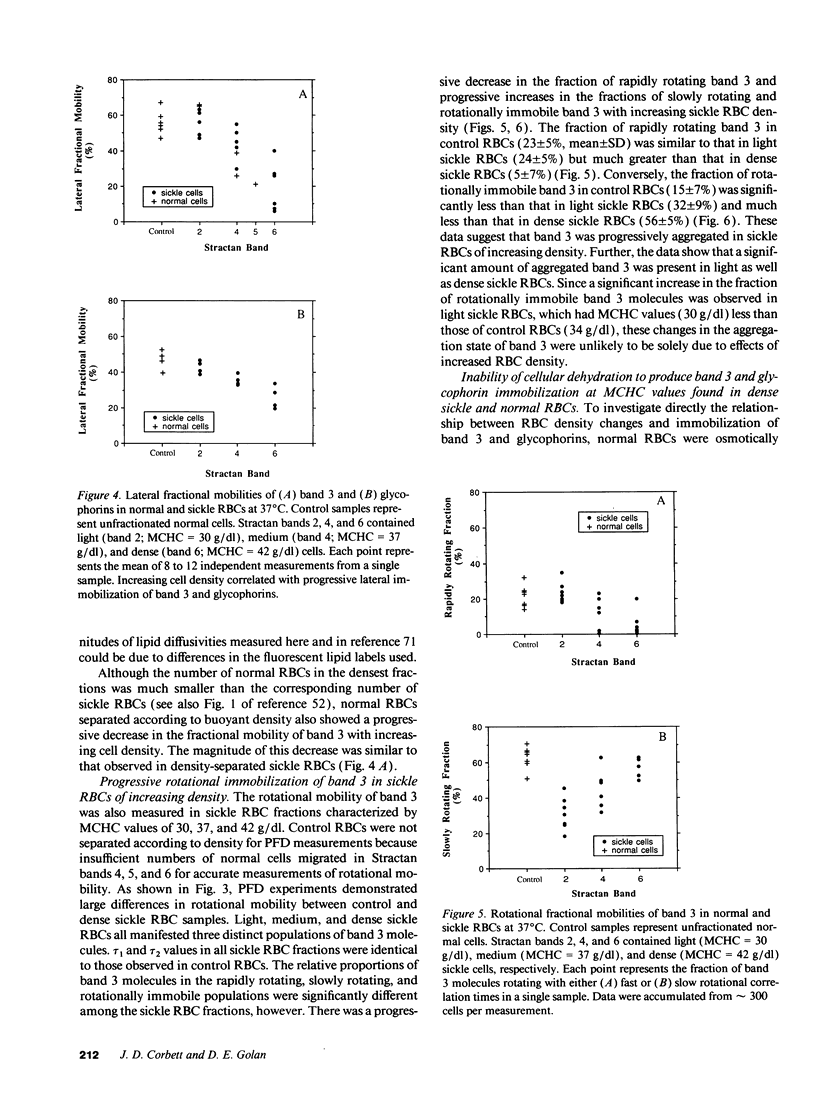
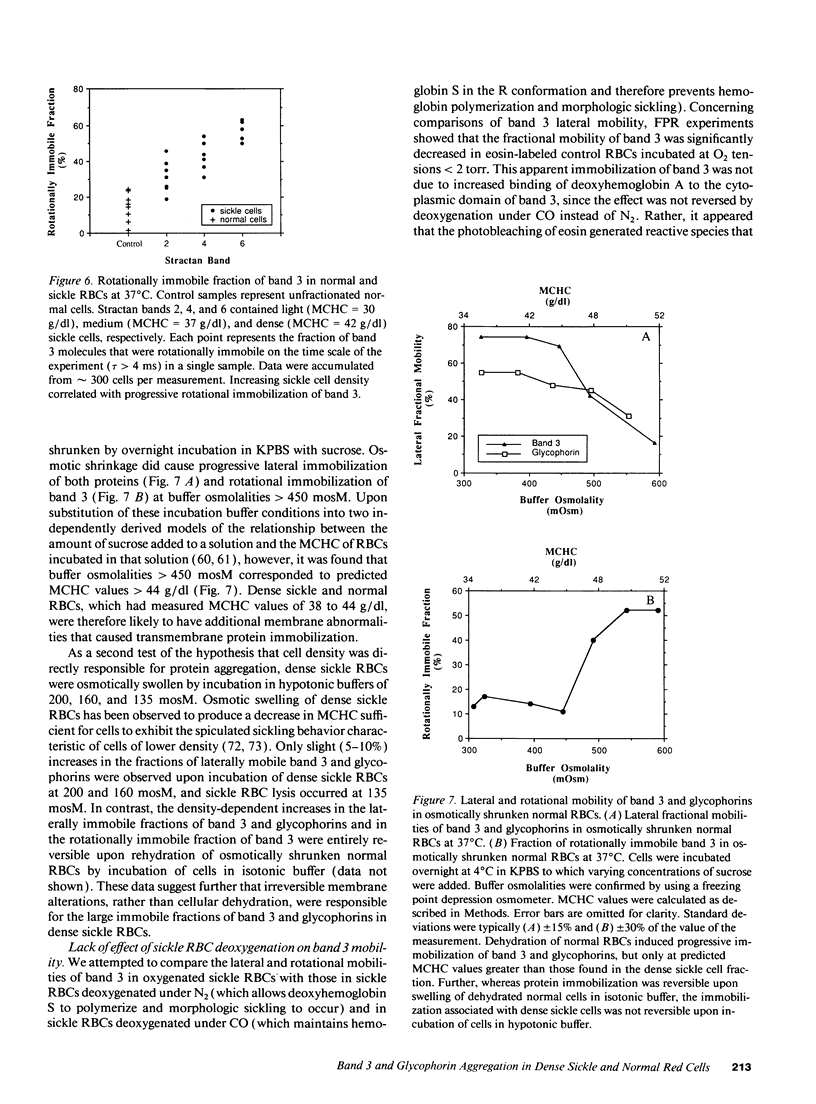




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderman E. M., Fudenberg H. H., Lovins R. E. Isolation and characterization of an age-related antigen present on senescent human red blood cells. Blood. 1981 Aug;58(2):341–349. [PubMed] [Google Scholar]
- Aminoff D. The role of sialoglycoconjugates in the aging and sequestration of red cells from circulation. Blood Cells. 1988;14(1):229–257. [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V. Purification of an active proteolytic fragment of the membrane attachment site for human erythrocyte spectrin. J Biol Chem. 1978 Apr 10;253(7):2292–2299. [PubMed] [Google Scholar]
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Boullier J. A., Brown B. A., Bush J. C., Jr, Barisas B. G. Lateral mobility of a lipid analog in the membrane of irreversible sickle erythrocytes. Biochim Biophys Acta. 1986 Apr 14;856(2):301–309. doi: 10.1016/0005-2736(86)90040-4. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Campwala H. Q., Desforges J. F. Membrane-bound hemichrome in density-separated cohorts of normal (AA) and sickled (SS) cells. J Lab Clin Med. 1982 Jan;99(1):25–28. [PubMed] [Google Scholar]
- Caulfield J. P., Chiang C. P., Yacono P. W., Smith L. A., Golan D. E. Low density lipoproteins bound to Schistosoma mansoni do not alter the rapid lateral diffusion or shedding of lipids in the outer surface membrane. J Cell Sci. 1991 May;99(Pt 1):167–173. doi: 10.1242/jcs.99.1.167. [DOI] [PubMed] [Google Scholar]
- Chasis J. A., Shohet S. B. Red cell biochemical anatomy and membrane properties. Annu Rev Physiol. 1987;49:237–248. doi: 10.1146/annurev.ph.49.030187.001321. [DOI] [PubMed] [Google Scholar]
- Chiu D., Lubin B., Shohet S. B. Erythrocyte membrane lipid reorganization during the sickling process. Br J Haematol. 1979 Feb;41(2):223–234. doi: 10.1111/j.1365-2141.1979.tb05851.x. [DOI] [PubMed] [Google Scholar]
- Chétrite G., Cassoly R. Affinity of hemoglobin for the cytoplasmic fragment of human erythrocyte membrane band 3. Equilibrium measurements at physiological pH using matrix-bound proteins: the effects of ionic strength, deoxygenation and of 2,3-diphosphoglycerate. J Mol Biol. 1985 Oct 5;185(3):639–644. doi: 10.1016/0022-2836(85)90076-2. [DOI] [PubMed] [Google Scholar]
- Clague M. J., Harrison J. P., Cherry R. J. Cytoskeletal restraints of band 3 rotational mobility in human erythrocyte membranes. Biochim Biophys Acta. 1989 May 19;981(1):43–50. doi: 10.1016/0005-2736(89)90080-1. [DOI] [PubMed] [Google Scholar]
- Clark L. J., Chan L. S., Powars D. R., Baker R. F. Negative charge distribution and density on the surface of oxygenated normal and sickle red cells. Blood. 1981 Apr;57(4):675–678. [PubMed] [Google Scholar]
- Clark M. R., Guatelli J. C., Mohandas N., Shohet S. B. Influence of red cell water content on the morphology of sickling. Blood. 1980 May;55(5):823–830. [PubMed] [Google Scholar]
- Cohen N. S., Ekholm J. E., Luthra M. G., Hanahan D. J. Biochemical characterization of density-separated human erythrocytes. Biochim Biophys Acta. 1976 Jan 21;419(2):229–242. doi: 10.1016/0005-2736(76)90349-7. [DOI] [PubMed] [Google Scholar]
- Corash L., Shafer B., Perlow M. Heterogeneity of human whole blood platelet subpopulations. II. Use of a subhuman primate model to analyze the relationship between density and platelet age. Blood. 1978 Oct;52(4):726–734. [PubMed] [Google Scholar]
- Devaux P. F. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991 Feb 5;30(5):1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- Eaton J. W., Skelton T. D., Swofford H. S., Kolpin C. E., Jacob H. S. Elevated erythrocyte calcium in sickle cell disease. Nature. 1973 Nov 9;246(5428):105–106. doi: 10.1038/246105a0. [DOI] [PubMed] [Google Scholar]
- Elson E. L. Membrane dynamics studied by fluorescence correlation spectroscopy and photobleaching recovery. Soc Gen Physiol Ser. 1986;40:367–383. [PubMed] [Google Scholar]
- Embury S. H. The clinical pathophysiology of sickle cell disease. Annu Rev Med. 1986;37:361–376. doi: 10.1146/annurev.me.37.020186.002045. [DOI] [PubMed] [Google Scholar]
- Freedman J. C., Hoffman J. F. Ionic and osmotic equilibria of human red blood cells treated with nystatin. J Gen Physiol. 1979 Aug;74(2):157–185. doi: 10.1085/jgp.74.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Clark M. R., Shohet S. B. Excessive binding of natural anti-alpha-galactosyl immunoglobin G to sickle erythrocytes may contribute to extravascular cell destruction. J Clin Invest. 1986 Jan;77(1):27–33. doi: 10.1172/JCI112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Flechner I., Knyszynski A., Danon D., Rachmilewitz E. A. The natural anti-alpha-galactosyl IgG on human normal senescent red blood cells. Br J Haematol. 1986 Feb;62(2):317–324. doi: 10.1111/j.1365-2141.1986.tb02935.x. [DOI] [PubMed] [Google Scholar]
- Galili U., Korkesh A., Kahane I., Rachmilewitz E. A. Demonstration of a natural antigalactosyl IgG antibody on thalassemic red blood cells. Blood. 1983 Jun;61(6):1258–1264. [PubMed] [Google Scholar]
- Glass G. A., Gershon H., Gershon D. The effect of donor and cell age on several characteristics of rat erythrocytes. Exp Hematol. 1983 Nov;11(10):987–995. [PubMed] [Google Scholar]
- Golan D. E., Brown C. S., Cianci C. M., Furlong S. T., Caulfield J. P. Schistosomula of Schistosoma mansoni use lysophosphatidylcholine to lyse adherent human red blood cells and immobilize red cell membrane components. J Cell Biol. 1986 Sep;103(3):819–828. doi: 10.1083/jcb.103.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golan D. E., Veatch W. Lateral mobility of band 3 in the human erythrocyte membrane studied by fluorescence photobleaching recovery: evidence for control by cytoskeletal interactions. Proc Natl Acad Sci U S A. 1980 May;77(5):2537–2541. doi: 10.1073/pnas.77.5.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. A., Rehn M. M., Kalra V. K. Cell-bound autologous immunoglobulin in erythrocyte subpopulations from patients with sickle cell disease. Blood. 1985 May;65(5):1127–1133. [PubMed] [Google Scholar]
- Havell T. C., Hillman D., Lessin L. S. Deformability characteristics of sickle cells by microelastimetry. Am J Hematol. 1978;4(1):9–16. doi: 10.1002/ajh.2830040103. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P. Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood. 1991 Jan 15;77(2):214–237. [PubMed] [Google Scholar]
- Hebbel R. P., Boogaerts M. A., Eaton J. W., Steinberg M. H. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980 May 1;302(18):992–995. doi: 10.1056/NEJM198005013021803. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Eaton J. W., Balasingam M., Steinberg M. H. Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest. 1982 Dec;70(6):1253–1259. doi: 10.1172/JCI110724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbel R. P., Schwartz R. S., Mohandas N. The adhesive sickle erythrocyte: cause and consequence of abnormal interactions with endothelium, monocytes/macrophages and model membranes. Clin Haematol. 1985 Feb;14(1):141–161. [PubMed] [Google Scholar]
- Hebbel R. P. The sickle erythrocyte in double jeopardy: autoxidation and iron decompartmentalization. Semin Hematol. 1990 Jan;27(1):51–69. [PubMed] [Google Scholar]
- Hebbel R. P., Yamada O., Moldow C. F., Jacob H. S., White J. G., Eaton J. W. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest. 1980 Jan;65(1):154–160. doi: 10.1172/JCI109646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanishi H., Nakai T., Abe T., Takino T. Glutathione metabolism in red cell aging. Mech Ageing Dev. 1985 Oct 14;32(1):57–62. doi: 10.1016/0047-6374(85)90035-1. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Shohet S. B. A novel phospholipid in irreversibly sickled cells: evidence for in vivo peroxidative membrane damage in sickle cell disease. Blood. 1984 Feb;63(2):362–367. [PubMed] [Google Scholar]
- Johnson P., Garland P. B. Depolarization of fluorescence depletion. A microscopic method for measuring rotational diffusion of membrane proteins on the surface of a single cell. FEBS Lett. 1981 Sep 28;132(2):252–256. doi: 10.1016/0014-5793(81)81172-6. [DOI] [PubMed] [Google Scholar]
- Kadlubowski M. The effect of in-vivo ageing of the human erythrocyte on the protein of the plasma membrane. A characterisation. Int J Biochem. 1978;9(2):67–78. doi: 10.1016/0020-711x(78)90014-9. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Bosman G. J., Lawrence C. Functional topography of band 3: specific structural alteration linked to functional aberrations in human erythrocytes. Proc Natl Acad Sci U S A. 1988 Jan;85(2):492–496. doi: 10.1073/pnas.85.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M., Flowers N., Goodman J., Bosman G. Alteration in membrane protein band 3 associated with accelerated erythrocyte aging. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5834–5838. doi: 10.1073/pnas.86.15.5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M., Goodman S. R., Sorensen K., Whitfield C. F., Wong P., Zaki L., Rudloff V. Senescent cell antigen is immunologically related to band 3. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1631–1635. doi: 10.1073/pnas.80.6.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Localization of senescent cell antigen on band 3. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5753–5757. doi: 10.1073/pnas.81.18.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khansari N., Springer G. F., Merler E., Fudenberg H. H. Mechanisms for the removal of senescent human erythrocytes from circulation: specificity of the membrane-bound immunoglobulin G. Mech Ageing Dev. 1983 Jan;21(1):49–58. doi: 10.1016/0047-6374(83)90015-5. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Sheetz M. P., Schindler M. Matrix control of protein diffusion in biological membranes. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3576–3580. doi: 10.1073/pnas.78.6.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuross S. A., Rank B. H., Hebbel R. P. Excess heme in sickle erythrocyte inside-out membranes: possible role in thiol oxidation. Blood. 1988 Apr;71(4):876–882. [PubMed] [Google Scholar]
- Lew V. L., Bookchin R. M. Volume, pH, and ion-content regulation in human red cells: analysis of transient behavior with an integrated model. J Membr Biol. 1986;92(1):57–74. doi: 10.1007/BF01869016. [DOI] [PubMed] [Google Scholar]
- Low P. S. Structure and function of the cytoplasmic domain of band 3: center of erythrocyte membrane-peripheral protein interactions. Biochim Biophys Acta. 1986 Sep 22;864(2):145–167. doi: 10.1016/0304-4157(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Low P. S., Waugh S. M., Zinke K., Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985 Feb 1;227(4686):531–533. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- Low P. S., Willardson B. M., Mohandas N., Rossi M., Shohet S. Contribution of the band 3-ankyrin interaction to erythrocyte membrane mechanical stability. Blood. 1991 Apr 1;77(7):1581–1586. [PubMed] [Google Scholar]
- Lubin B., Chiu D., Bastacky J., Roelofsen B., Van Deenen L. L. Abnormalities in membrane phospholipid organization in sickled erythrocytes. J Clin Invest. 1981 Jun;67(6):1643–1649. doi: 10.1172/JCI110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., John K. M., Karnovsky M. J. Irreversible deformation of the spectrin-actin lattice in irreversibly sickled cells. J Clin Invest. 1976 Oct;58(4):955–963. doi: 10.1172/JCI108549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara I. G., Kuo S., Cantley L. C. Evidence that inhibitors of anion exchange induce a transmembrane conformational change in band 3. J Biol Chem. 1983 Feb 10;258(3):1785–1792. [PubMed] [Google Scholar]
- Marchesi V. T., Furthmayr H., Tomita M. The red cell membrane. Annu Rev Biochem. 1976;45:667–698. doi: 10.1146/annurev.bi.45.070176.003315. [DOI] [PubMed] [Google Scholar]
- Marikovsky Y., Danon D. Electron microscope analysis of young and old red blood cells stained with colloidal iron for surface charge evaluation. J Cell Biol. 1969 Oct;43(1):1–7. doi: 10.1083/jcb.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matayoshi E. D., Jovin T. M. Rotational diffusion of band 3 in erythrocyte membranes. 1. Comparison of ghosts and intact cells. Biochemistry. 1991 Apr 9;30(14):3527–3538. doi: 10.1021/bi00228a025. [DOI] [PubMed] [Google Scholar]
- Matayoshi E. D., Sawyer W. H., Jovin T. M. Rotational diffusion of band 3 in erythrocyte membranes. 2. Binding of cytoplasmic enzymes. Biochemistry. 1991 Apr 9;30(14):3538–3543. doi: 10.1021/bi00228a026. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Groner W. Cell membrane and volume changes during red cell development and aging. Ann N Y Acad Sci. 1989;554:217–224. doi: 10.1111/j.1749-6632.1989.tb22423.x. [DOI] [PubMed] [Google Scholar]
- Mohandas N., Rossi M. E., Clark M. R. Association between morphologic distortion of sickle cells and deoxygenation-induced cation permeability increase. Blood. 1986 Aug;68(2):450–454. [PubMed] [Google Scholar]
- Mühlebach T., Cherry R. J. Influence of cholesterol on the rotation and self-association of band 3 in the human erythrocyte membrane. Biochemistry. 1982 Aug 31;21(18):4225–4228. doi: 10.1021/bi00261a006. [DOI] [PubMed] [Google Scholar]
- Nash G. B., Wyard S. J. Changes in surface area and volume measured by micropipette aspiration for erythrocytes ageing in vivo. Biorheology. 1980;17(5-6):479–484. [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Anchorage of a band 3 population at the erythrocyte cytoplasmic membrane surface: protein rotational diffusion measurements. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4702–4706. doi: 10.1073/pnas.77.8.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Influence of temperature and cholesterol on the rotational diffusion of band 3 in the human erythrocyte membrane. Biochemistry. 1979 Aug 7;18(16):3457–3465. doi: 10.1021/bi00583a004. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Gahmberg C. G., Cherry R. J. Rotational diffusion of band 3 proteins in membranes from En(a-) and neuraminidase-treated normal human erythrocytes. Biochim Biophys Acta. 1980 Aug 14;600(3):636–642. doi: 10.1016/0005-2736(80)90467-8. [DOI] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Seaman G. V., Knox R. J., Nordt F. J., Regan D. H. Red cell aging. I. Surface charge density and sialic acid content of density-fractionated human erythrocytes. Blood. 1977 Dec;50(6):1001–1011. [PubMed] [Google Scholar]
- Shaklai N., Sharma V. S., Ranney H. M. Interaction of sickle cell hemoglobin with erythrocyte membranes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):65–68. doi: 10.1073/pnas.78.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Koppel D. E. Membrane damage caused by irradiation of fluorescent concanavalin A. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3314–3317. doi: 10.1073/pnas.76.7.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P. Membrane skeletal dynamics: role in modulation of red cell deformability, mobility of transmembrane proteins, and shape. Semin Hematol. 1983 Jul;20(3):175–188. [PubMed] [Google Scholar]
- Sheetz M. P., Schindler M., Koppel D. E. Lateral mobility of integral membrane proteins is increased in spherocytic erythrocytes. Nature. 1980 Jun 12;285(5765):510–511. doi: 10.1038/285510a0. [DOI] [PubMed] [Google Scholar]
- Smalley C. E., Tucker E. M. Blood group A antigen site distribution and immunoglobulin binding in relation to red cell age. Br J Haematol. 1983 Jun;54(2):209–219. doi: 10.1111/j.1365-2141.1983.tb02089.x. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOSTESON D. C., CARLSEN E., DUNHAM E. T. The effects of sickling on ion transport. I. Effect of sickling on potassium transport. J Gen Physiol. 1955 Sep 20;39(1):31–53. doi: 10.1085/jgp.39.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOSTESON D. C., SHEA E., DARLING R. C. Potassium and sodium of red blood cells in sickle cell anemia. J Clin Invest. 1952 Apr;31(4):406–411. doi: 10.1172/JCI102623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley L., Foley M., Anders R. F., Dluzewski A. R., Gratzer W. B., Jones G. L., Sawyer W. H. Rotational dynamics of the integral membrane protein, band 3, as a probe of the membrane events associated with Plasmodium falciparum infections of human erythrocytes. Biochim Biophys Acta. 1990 Jun 27;1025(2):135–142. doi: 10.1016/0005-2736(90)90090-b. [DOI] [PubMed] [Google Scholar]
- Tilley L., Nash G. B., Jones G. L., Sawyer W. H. Decreased rotational diffusion of band 3 in Melanesian ovalocytes from Papua, New Guinea. J Membr Biol. 1991 Apr;121(1):59–66. doi: 10.1007/BF01870651. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Kawasaki K., Ohnishi S., Merkle H., Kusumi A. Regulation of band 3 mobilities in erythrocyte ghost membranes by protein association and cytoskeletal meshwork. Biochemistry. 1988 Sep 20;27(19):7447–7452. doi: 10.1021/bi00419a041. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Ohnishi S. Restriction of the lateral motion of band 3 in the erythrocyte membrane by the cytoskeletal network: dependence on spectrin association state. Biochemistry. 1986 Oct 7;25(20):6133–6139. doi: 10.1021/bi00368a045. [DOI] [PubMed] [Google Scholar]
- Walder J. A., Chatterjee R., Steck T. L., Low P. S., Musso G. F., Kaiser E. T., Rogers P. H., Arnone A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J Biol Chem. 1984 Aug 25;259(16):10238–10246. [PubMed] [Google Scholar]
- Waugh S. M., Low P. S. Hemichrome binding to band 3: nucleation of Heinz bodies on the erythrocyte membrane. Biochemistry. 1985 Jan 1;24(1):34–39. doi: 10.1021/bi00322a006. [DOI] [PubMed] [Google Scholar]
- Waugh S. M., Willardson B. M., Kannan R., Labotka R. J., Low P. S. Heinz bodies induce clustering of band 3, glycophorin, and ankyrin in sickle cell erythrocytes. J Clin Invest. 1986 Nov;78(5):1155–1160. doi: 10.1172/JCI112696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C., Batt R. D. The uptake of plasma fatty acids into human red cells and its relationship to cell age. Biochim Biophys Acta. 1970 Feb 10;202(1):9–20. doi: 10.1016/0005-2760(70)90213-4. [DOI] [PubMed] [Google Scholar]
- Yoshida T. M., Barisas B. G. Protein rotational motion in solution measured by polarized fluorescence depletion. Biophys J. 1986 Jul;50(1):41–53. doi: 10.1016/S0006-3495(86)83437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachowski A., Craescu C. T., Galacteros F., Devaux P. F. Abnormality of phospholipid transverse diffusion in sickle erythrocytes. J Clin Invest. 1985 May;75(5):1713–1717. doi: 10.1172/JCI111880. [DOI] [PMC free article] [PubMed] [Google Scholar]



