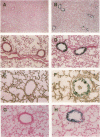
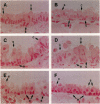
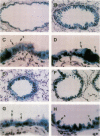
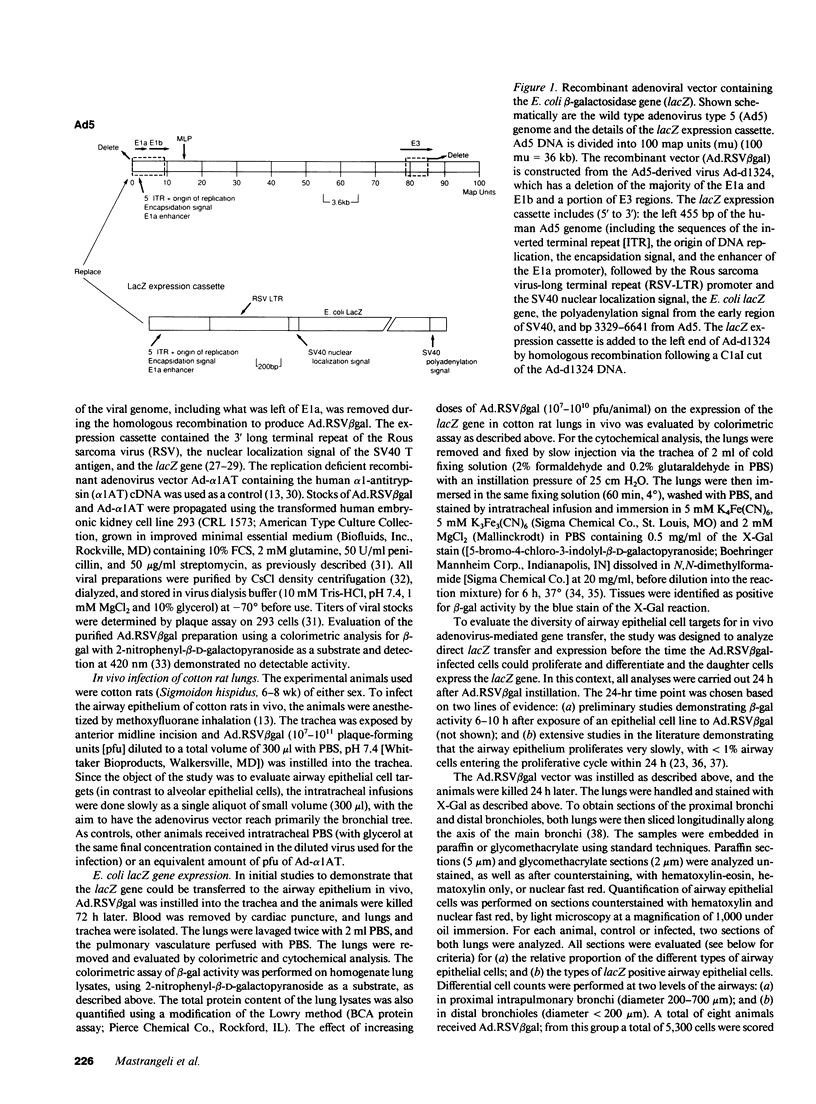
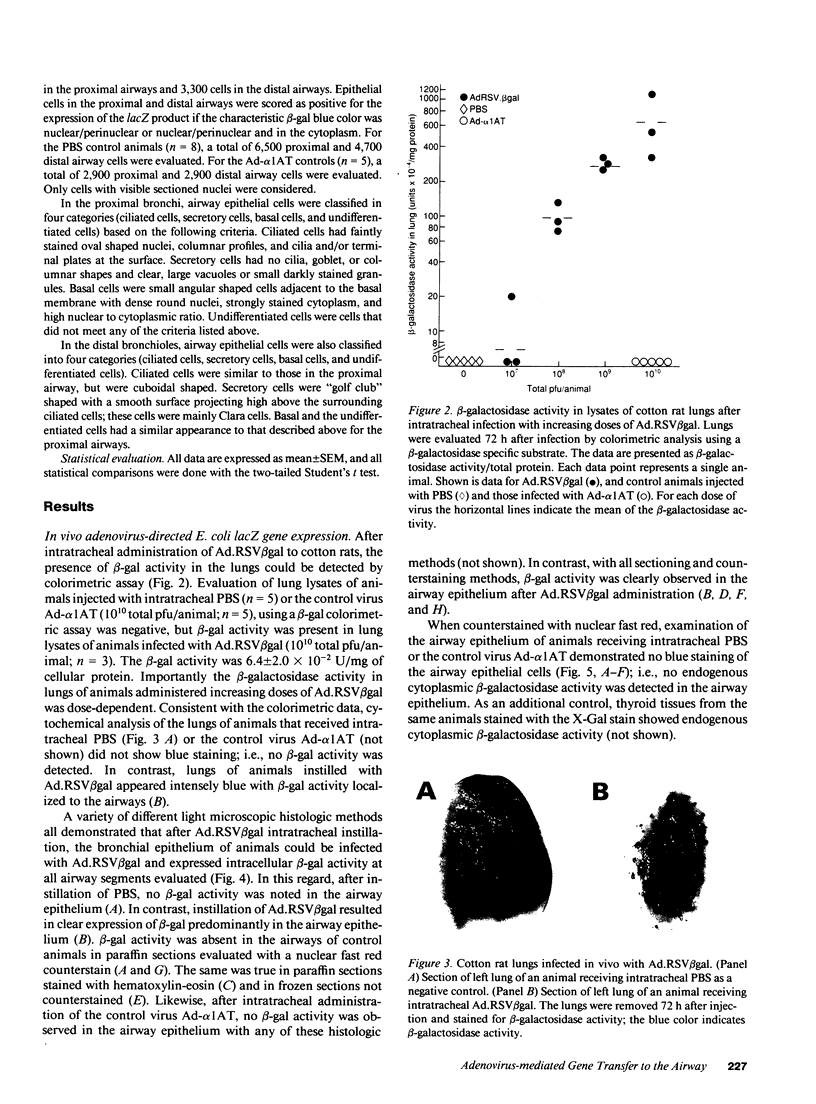
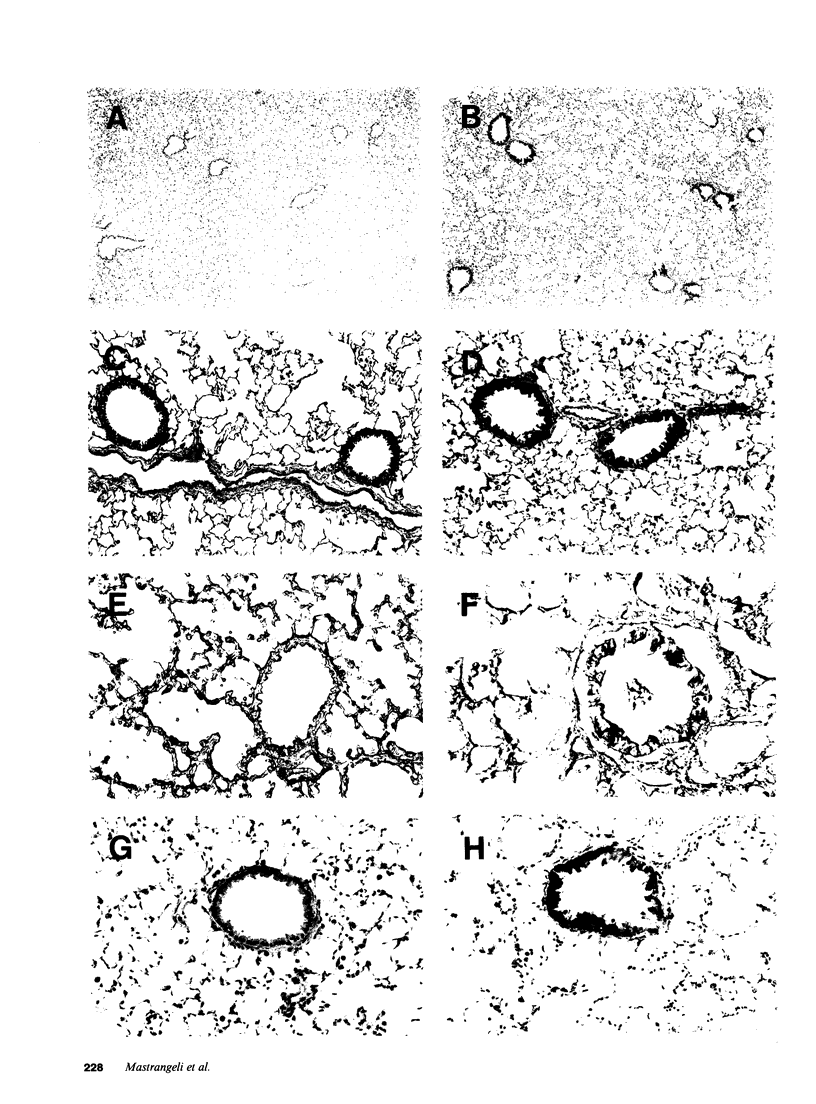
Abstract
A variety of pulmonary disorders, including cystic fibrosis, are potentially amenable to treatment in which a therapeutic gene is directly transferred to the bronchial epithelium. This is difficult to accomplish because the majority of airway epithelial cells replicate slowly and/or are terminally differentiated. Adenovirus vectors may circumvent this problem, since they do not require target cell proliferation to express exogenous genes. To evaluate the diversity of airway epithelial cell targets for in vivo adenovirus-directed gene transfer, a replication deficient recombinant adenovirus containing the Escherichia coli lacZ (beta-galactosidase [beta-gal]) gene (Ad.RSV beta gal) was used to infect lungs of cotton rats. In contrast to uninfected animals, intratracheal Ad.RSV beta gal administration resulted in beta-gal activity in lung lysate and cytochemical staining in all cell types forming the airway epithelium. The expression of the exogenous gene was dose-dependent, and the distribution of the beta-gal positive airway epithelial cells in Ad.RSV beta gal-infected animals was similar to the normal cell differential of the control animals. Thus, a replication deficient recombinant adenovirus can transfer an exogenous gene to all major categories of airway epithelial cells in vivo, suggesting that adenovirus vectors may be an efficient strategy for in vivo gene transfer in airway disorders such as cystic fibrosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayers M. M., Jeffery P. K. Proliferation and differentiation in mammalian airway epithelium. Eur Respir J. 1988 Jan;1(1):58–80. [PubMed] [Google Scholar]
- Ballay A., Levrero M., Buendia M. A., Tiollais P., Perricaudet M. In vitro and in vivo synthesis of the hepatitis B virus surface antigen and of the receptor for polymerized human serum albumin from recombinant human adenoviruses. EMBO J. 1985 Dec 30;4(13B):3861–3865. doi: 10.1002/j.1460-2075.1985.tb04158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K. L. Development of adenovirus vectors for the expression of heterologous genes. Biotechniques. 1988 Jul-Aug;6(7):616–629. [PubMed] [Google Scholar]
- Bolduc P., Reid L. Mitotic index of the bronchial and alveolar lining of the normal rat lung. Am Rev Respir Dis. 1976 Dec;114(6):1121–1128. doi: 10.1164/arrd.1976.114.6.1121. [DOI] [PubMed] [Google Scholar]
- Breeze R. G., Wheeldon E. B. The cells of the pulmonary airways. Am Rev Respir Dis. 1977 Oct;116(4):705–777. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- Chanock R. M., Ludwig W., Heubner R. J., Cate T. R., Chu L. W. Immunization by selective infection with type 4 adenovirus grown in human diploid tissue cultures. I. Safety and lack of oncogenicity and tests for potency in volunteers. JAMA. 1966 Feb 7;195(6):445–452. [PubMed] [Google Scholar]
- Evans M. J., Moller P. C. Biology of airway basal cells. Exp Lung Res. 1991 May-Jun;17(3):513–531. doi: 10.3109/01902149109062862. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Rechkemmer G., Shoemaker R. L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986 Aug 1;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- Gilardi P., Courtney M., Pavirani A., Perricaudet M. Expression of human alpha 1-antitrypsin using a recombinant adenovirus vector. FEBS Lett. 1990 Jul 2;267(1):60–62. doi: 10.1016/0014-5793(90)80287-s. [DOI] [PubMed] [Google Scholar]
- Ginsberg H. S., Horswood R. L., Chanock R. M., Prince G. A. Role of early genes in pathogenesis of adenovirus pneumonia. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6191–6195. doi: 10.1073/pnas.87.16.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Haj-Ahmad Y., Graham F. L. Development of a helper-independent human adenovirus vector and its use in the transfer of the herpes simplex virus thymidine kinase gene. J Virol. 1986 Jan;57(1):267–274. doi: 10.1128/jvi.57.1.267-274.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery P. K. Morphologic features of airway surface epithelial cells and glands. Am Rev Respir Dis. 1983 Aug;128(2 Pt 2):S14–S20. doi: 10.1164/arrd.1983.128.2P2.S14. [DOI] [PubMed] [Google Scholar]
- Jeffery P. K., Reid L. New observations of rat airway epithelium: a quantitative and electron microscopic study. J Anat. 1975 Nov;120(Pt 2):295–320. [PMC free article] [PubMed] [Google Scholar]
- Jetten A. M., Yankaskas J. R., Stutts M. J., Willumsen N. J., Boucher R. C. Persistence of abnormal chloride conductance regulation in transformed cystic fibrosis epithelia. Science. 1989 Jun 23;244(4911):1472–1475. doi: 10.1126/science.2472008. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Knowles M., Gatzy J., Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981 Dec 17;305(25):1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTMAN B. Measurement of activity of single molecules of beta-D-galactosidase. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1981–1991. doi: 10.1073/pnas.47.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich D. P., Anderson M. P., Gregory R. J., Cheng S. H., Paul S., Jefferson D. M., McCann J. D., Klinger K. W., Smith A. E., Welsh M. J. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature. 1990 Sep 27;347(6291):358–363. doi: 10.1038/347358a0. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rommens J. M., Iannuzzi M. C., Kerem B., Drumm M. L., Melmer G., Dean M., Rozmahel R., Cole J. L., Kennedy D., Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989 Sep 8;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Siegfried W., Yoshimura K., Yoneyama K., Fukayama M., Stier L. E., Päkkö P. K., Gilardi P., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991 Apr 19;252(5004):431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter R. G., Titus J. L., Divertie M. B. Cytodynamics in the respiratory tract of the rat. Thorax. 1966 Jan;21(1):32–37. doi: 10.1136/thx.21.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Makeh I., Perricaudet M., Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992 Aug;90(2):626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Trapnell B. C., Chu C. S., Paakko P. K., Banks T. C., Yoshimura K., Ferrans V. J., Chernick M. S., Crystal R. G. Expression of the cystic fibrosis transmembrane conductance regulator gene in the respiratory tract of normal individuals and individuals with cystic fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6565–6569. doi: 10.1073/pnas.88.15.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezise A. E., Buchwald M. In vivo cell-specific expression of the cystic fibrosis transmembrane conductance regulator. Nature. 1991 Oct 3;353(6343):434–437. doi: 10.1038/353434a0. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Fick R. B. Cystic fibrosis. J Clin Invest. 1987 Dec;80(6):1523–1526. doi: 10.1172/JCI113237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., de Crombrugghe B., Pastan I. Identification of a functional promoter in the long terminal repeat of Rous sarcoma virus. Cell. 1980 Dec;22(3):787–797. doi: 10.1016/0092-8674(80)90555-3. [DOI] [PubMed] [Google Scholar]
- Yoshimura K., Nakamura H., Trapnell B. C., Dalemans W., Pavirani A., Lecocq J. P., Crystal R. G. The cystic fibrosis gene has a "housekeeping"-type promoter and is expressed at low levels in cells of epithelial origin. J Biol Chem. 1991 May 15;266(14):9140–9144. [PubMed] [Google Scholar]