Abstract
Beta 1- and beta 2-adrenergic receptor (beta-ARs) expression in the thick ascending limb of rat kidney was studied at the level of mRNA and receptor coupling to adenylyl cyclase. Absolute quantitation of beta 1- and beta 2-AR mRNAs in microdissected nephron segments was performed with an assay based on reverse transcription and polymerase chain reaction, using in vitro transcribed mutant RNAs as internal standards. In the cortical thick ascending limb (CTAL), the number of mRNA molecules/mm of tubular length was 2,806 +/- 328 (n = 12) for beta 1-AR and 159 +/- 26 for beta 2-AR (P < 0.01). Lower levels were obtained in the medullary thick ascending, beta 1-AR mRNA still being predominant. The pharmacological properties of beta-ARS was also studied in the CTAL. Cyclic AMP accumulation was stimulated by beta-agonist with a rank order of potency of isoproterenol > norepinephrine > epinephrine. This observation, and the higher efficiency of a beta 1 than of a beta 2 antagonist to inhibit isoproterenol-induced cAMP accumulation, establish the typical beta 1-AR sensitivity of the CTAL. No detectable contribution of atypical or beta 3-ARs to adenylyl cyclase stimulation could be found. In conclusion, this study, which shows markedly different levels of beta 1- and beta 2-AR mRNAS in the CTAL, provides a molecular basis for the predominant expression of the beta 1 receptor subtype in this nephron segment.
Full text
PDF


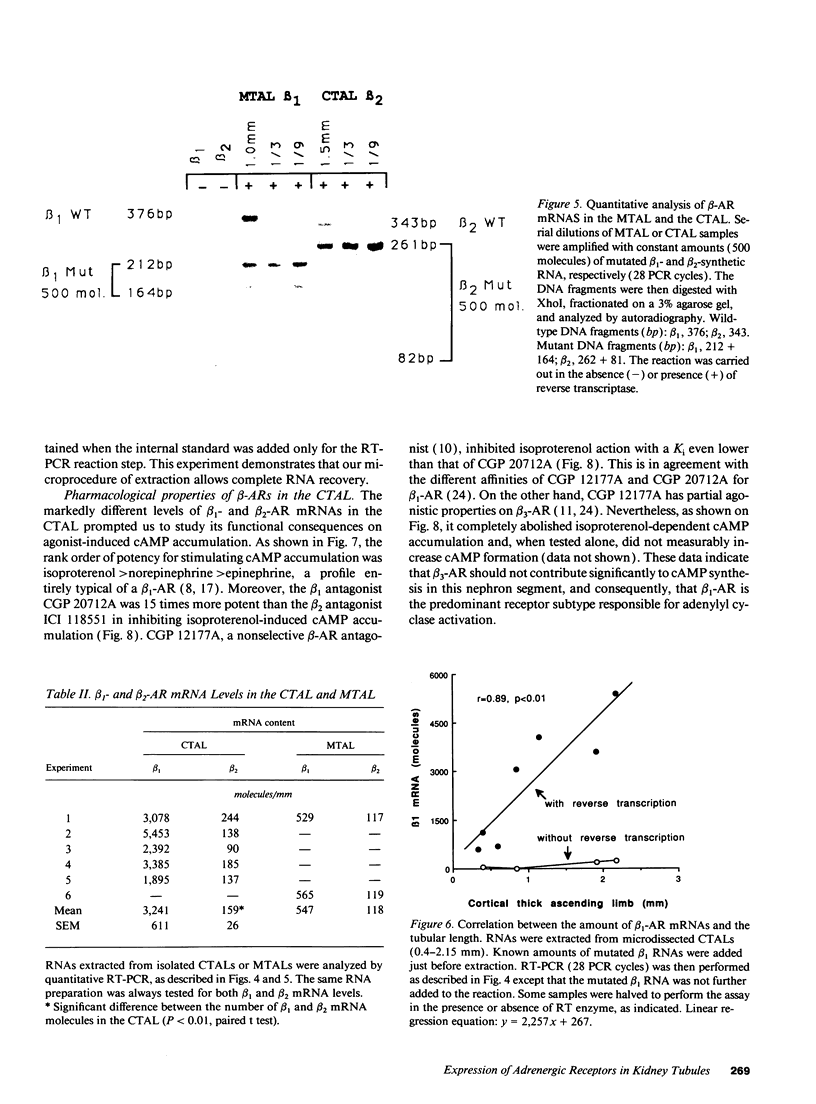
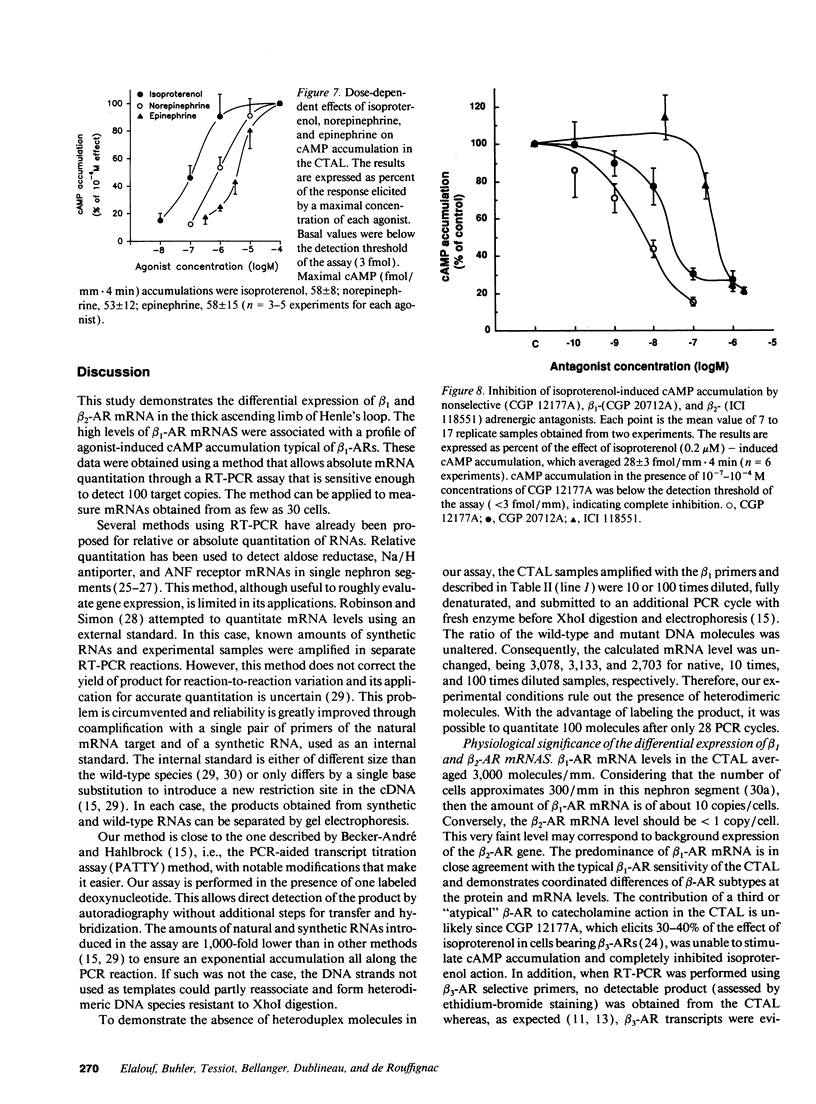


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailly C., Imbert-Teboul M., Roinel N., Amiel C. Isoproterenol increases Ca, Mg, and NaCl reabsorption in mouse thick ascending limb. Am J Physiol. 1990 May;258(5 Pt 2):F1224–F1231. doi: 10.1152/ajprenal.1990.258.5.F1224. [DOI] [PubMed] [Google Scholar]
- Barajas L., Powers K. V. Innervation of the thick ascending limb of Henle. Am J Physiol. 1988 Aug;255(2 Pt 2):F340–F348. doi: 10.1152/ajprenal.1988.255.2.F340. [DOI] [PubMed] [Google Scholar]
- Becker-André M., Hahlbrock K. Absolute mRNA quantification using the polymerase chain reaction (PCR). A novel approach by a PCR aided transcript titration assay (PATTY). Nucleic Acids Res. 1989 Nov 25;17(22):9437–9446. doi: 10.1093/nar/17.22.9437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch W. A biochemical perspective of the polymerase chain reaction. Biochemistry. 1991 Mar 19;30(11):2735–2747. doi: 10.1021/bi00225a001. [DOI] [PubMed] [Google Scholar]
- Buckland P. R., Hill R. M., Tidmarsh S. F., McGuffin P. Primary structure of the rat beta-2 adrenergic receptor gene. Nucleic Acids Res. 1990 Feb 11;18(3):682–682. doi: 10.1093/nar/18.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins S., Bouvier M., Bolanowski M. A., Caron M. G., Lefkowitz R. J. cAMP stimulates transcription of the beta 2-adrenergic receptor gene in response to short-term agonist exposure. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4853–4857. doi: 10.1073/pnas.86.13.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Stanton B., Klein-Robbenhaar G., Giebisch G. Inhibitory effect of epinephrine on renal potassium secretion: a micropuncture study. Am J Physiol. 1983 Sep;245(3):F303–F311. doi: 10.1152/ajprenal.1983.245.3.F303. [DOI] [PubMed] [Google Scholar]
- DiBona G. F., Sawin L. L. Effect of renal nerve stimulation on NaCl and H2O transport in Henle's loop of the rat. Am J Physiol. 1982 Dec;243(6):F576–F580. doi: 10.1152/ajprenal.1982.243.6.F576. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Dublineau I., Pradelles P., de Rouffignac C., Elalouf J. M. Differential short-term desensitization to vasopressin, isoproterenol, glucagon, parathyroid hormone and calcitonin in the thick ascending limb of rat kidney. Pflugers Arch. 1992 Jan;420(1):16–22. doi: 10.1007/BF00378636. [DOI] [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Engel G., Maurer R., Perrot K., Richardson B. P. beta-Adrenoceptor subtypes in sections of rat and guinea-pig kidney. Naunyn Schmiedebergs Arch Pharmacol. 1985 Jan;328(3):354–357. doi: 10.1007/BF00515567. [DOI] [PubMed] [Google Scholar]
- Frielle T., Collins S., Daniel K. W., Caron M. G., Lefkowitz R. J., Kobilka B. K. Cloning of the cDNA for the human beta 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fève B., Emorine L. J., Briend-Sutren M. M., Lasnier F., Strosberg A. D., Pairault J. Differential regulation of beta 1- and beta 2-adrenergic receptor protein and mRNA levels by glucocorticoids during 3T3-F442A adipose differentiation. J Biol Chem. 1990 Sep 25;265(27):16343–16349. [PubMed] [Google Scholar]
- Fève B., Emorine L. J., Lasnier F., Blin N., Baude B., Nahmias C., Strosberg A. D., Pairault J. Atypical beta-adrenergic receptor in 3T3-F442A adipocytes. Pharmacological and molecular relationship with the human beta 3-adrenergic receptor. J Biol Chem. 1991 Oct 25;266(30):20329–20336. [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocayne J., Robinson D. A., FitzGerald M. G., Chung F. Z., Kerlavage A. R., Lentes K. U., Lai J., Wang C. D., Fraser C. M., Venter J. C. Primary structure of rat cardiac beta-adrenergic and muscarinic cholinergic receptors obtained by automated DNA sequence analysis: further evidence for a multigene family. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8296–8300. doi: 10.1073/pnas.84.23.8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadcock J. R., Malbon C. C. Down-regulation of beta-adrenergic receptors: agonist-induced reduction in receptor mRNA levels. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5021–5025. doi: 10.1073/pnas.85.14.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino Y., Troy J. L., Brenner B. M. Effects of catecholamines on electrolyte transport in cortical collecting tubule. J Membr Biol. 1981;61(2):67–73. doi: 10.1007/BF02007632. [DOI] [PubMed] [Google Scholar]
- Kimmel P. L., Goldfarb S. Effects of isoproterenol on potassium secretion by the cortical collecting tubule. Am J Physiol. 1984 Jun;246(6 Pt 2):F804–F810. doi: 10.1152/ajprenal.1984.246.6.F804. [DOI] [PubMed] [Google Scholar]
- Krapf R., Solioz M. Na/H antiporter mRNA expression in single nephron segments of rat kidney cortex. J Clin Invest. 1991 Sep;88(3):783–788. doi: 10.1172/JCI115377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Machida C. A., Bunzow J. R., Searles R. P., Van Tol H., Tester B., Neve K. A., Teal P., Nipper V., Civelli O. Molecular cloning and expression of the rat beta 1-adrenergic receptor gene. J Biol Chem. 1990 Aug 5;265(22):12960–12965. [PubMed] [Google Scholar]
- Morel F., Imbert-Teboul M., Chabardès D. Distribution of hormone-dependent adenylate cyclase in the nephron and its physiological significance. Annu Rev Physiol. 1981;43:569–581. doi: 10.1146/annurev.ph.43.030181.003033. [DOI] [PubMed] [Google Scholar]
- Moriyama T., Murphy H. R., Martin B. M., Garcia-Perez A. Detection of specific mRNAs in single nephron segments by use of the polymerase chain reaction. Am J Physiol. 1990 May;258(5 Pt 2):F1470–F1474. doi: 10.1152/ajprenal.1990.258.5.F1470. [DOI] [PubMed] [Google Scholar]
- Muzzin P., Revelli J. P., Kuhne F., Gocayne J. D., McCombie W. R., Venter J. C., Giacobino J. P., Fraser C. M. An adipose tissue-specific beta-adrenergic receptor. Molecular cloning and down-regulation in obesity. J Biol Chem. 1991 Dec 15;266(35):24053–24058. [PubMed] [Google Scholar]
- Münzel P. A., Healy D. P., Insel P. A. Autoradiographic localization of beta-adrenergic receptors in rat kidney slices using [125I]iodocyanopindolol. Am J Physiol. 1984 Feb;246(2 Pt 2):F240–F245. doi: 10.1152/ajprenal.1984.246.2.F240. [DOI] [PubMed] [Google Scholar]
- Nahmias C., Blin N., Elalouf J. M., Mattei M. G., Strosberg A. D., Emorine L. J. Molecular characterization of the mouse beta 3-adrenergic receptor: relationship with the atypical receptor of adipocytes. EMBO J. 1991 Dec;10(12):3721–3727. doi: 10.1002/j.1460-2075.1991.tb04940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee D. A., Wang A., Mark D., Werb Z. Novel method for studying mRNA phenotypes in single or small numbers of cells. J Cell Biochem. 1989 Jan;39(1):1–11. doi: 10.1002/jcb.240390102. [DOI] [PubMed] [Google Scholar]
- Robinson M. O., Simon M. I. Determining transcript number using the polymerase chain reaction: Pgk-2, mP2, and PGK-2 transgene mRNA levels during spermatogenesis. Nucleic Acids Res. 1991 Apr 11;19(7):1557–1562. doi: 10.1093/nar/19.7.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster V. L. Cyclic adenosine monophosphate-stimulated bicarbonate secretion in rabbit cortical collecting tubules. J Clin Invest. 1985 Jun;75(6):2056–2064. doi: 10.1172/JCI111925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers R. J., Stephenson J. A., Kuhar M. J. Localization of beta adrenoceptor subtypes in rat kidney by light microscopic autoradiography. J Pharmacol Exp Ther. 1985 Feb;232(2):561–569. [PubMed] [Google Scholar]
- Terada Y., Moriyama T., Martin B. M., Knepper M. A., Garcia-Perez A. RT-PCR microlocalization of mRNA for guanylyl cyclase-coupled ANF receptor in rat kidney. Am J Physiol. 1991 Dec;261(6 Pt 2):F1080–F1087. doi: 10.1152/ajprenal.1991.261.6.F1080. [DOI] [PubMed] [Google Scholar]
- Vandewalle A. Heterogeneity of uridine incorporation along the rabbit nephron. II. Effect of DOCA. Am J Physiol. 1984 Apr;246(4 Pt 2):F427–F436. doi: 10.1152/ajprenal.1984.246.4.F427. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X. M., Fishman P. H. Desensitization of the human beta 1-adrenergic receptor. Involvement of the cyclic AMP-dependent but not a receptor-specific protein kinase. J Biol Chem. 1991 Apr 25;266(12):7462–7468. [PubMed] [Google Scholar]