Abstract
Erythroid differentiation is accompanied by dramatic alterations in morphology and membrane mechanical properties resulting, in large part, from reorganization of the membrane skeletal protein network. The 80-kD protein 4.1 is an important organizational component of this membrane skeleton. Recently, it has been recognized that multiple structural isoforms of 4.1 are encoded by a single gene via alternative pre-mRNA splicing, and that an upstream ATG can be spliced in and used for translation of high molecular weight 4.1. We are exploring the hypothesis that differentiation-associated switches in protein 4.1 structure play an important role in membrane reorganization. To study changes in 4.1 gene expression during normal human differentiation, we analyzed 4.1 protein and mRNA structure at various developmental stages. Using immunofluorescence microscopy, we observed high molecular weight 4.1 isoforms in preproerythroblasts producing punctate, predominantly cytoplasmic staining with a perinuclear area of intense fluorescence, while mature red cells expressed very little high molecular weight 4.1. Isoforms containing an alternatively expressed 102-nucleotide exon near the COOH terminus were abundant in both preproerythroblasts and mature cells but produced a punctate distribution of fluorescence over the entire preproerythroblast and intense membrane-associated fluorescence in the erythrocyte. Characterization of RNA by polymerase chain reaction and nuclease protection assays revealed a differentiation-associated switch in pre-mRNA splicing in the spectrin-actin binding domain. Since this domain plays a critical role in regulating membrane material properties, we speculate that this switch may be crucial to reorganization of the skeletal network during erythropoiesis. We conclude that 4.1 isoforms are differentially expressed and differentially localized during erythropoiesis, and that this isoform family is likely to have diverse functions during terminal differentiation.
Full text
PDF

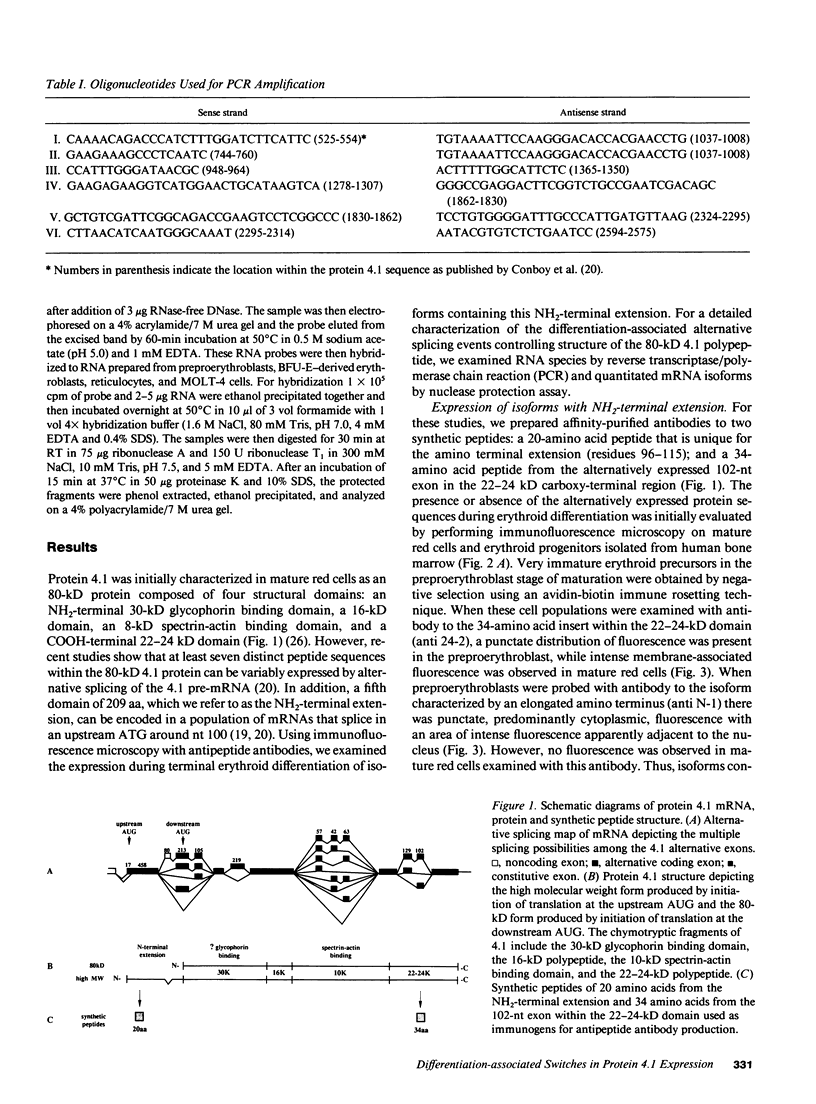
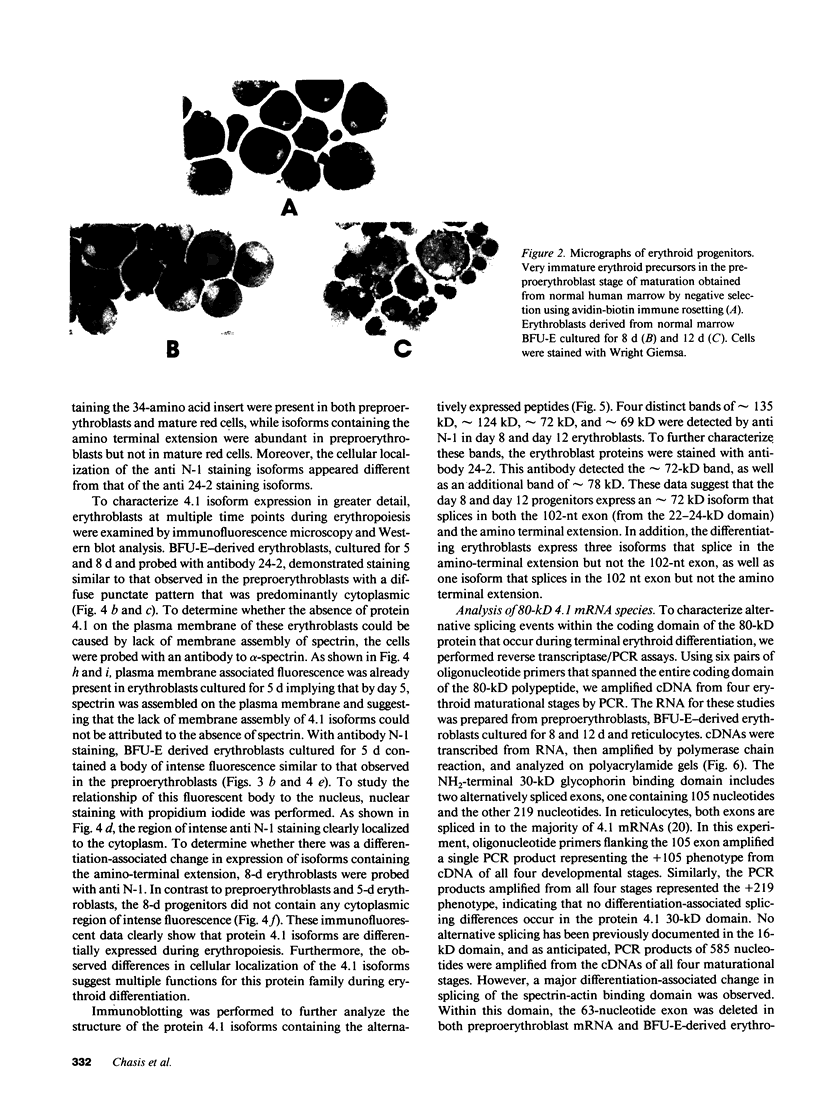
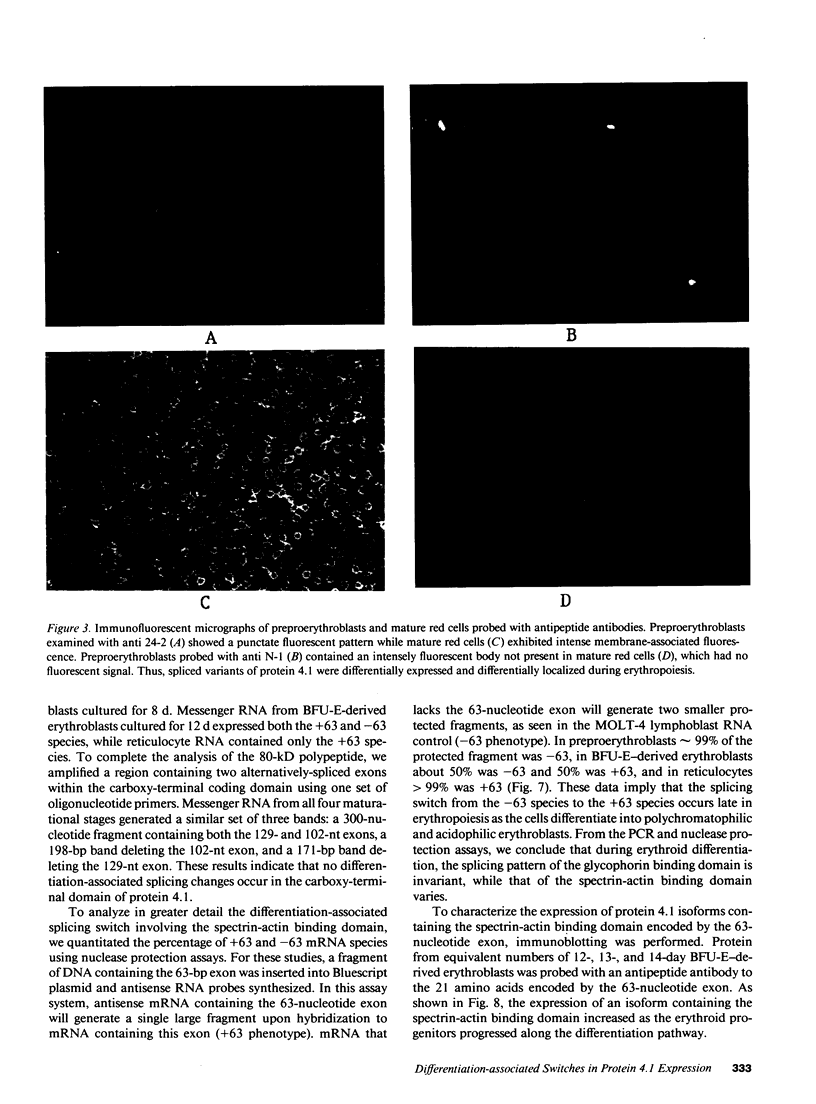
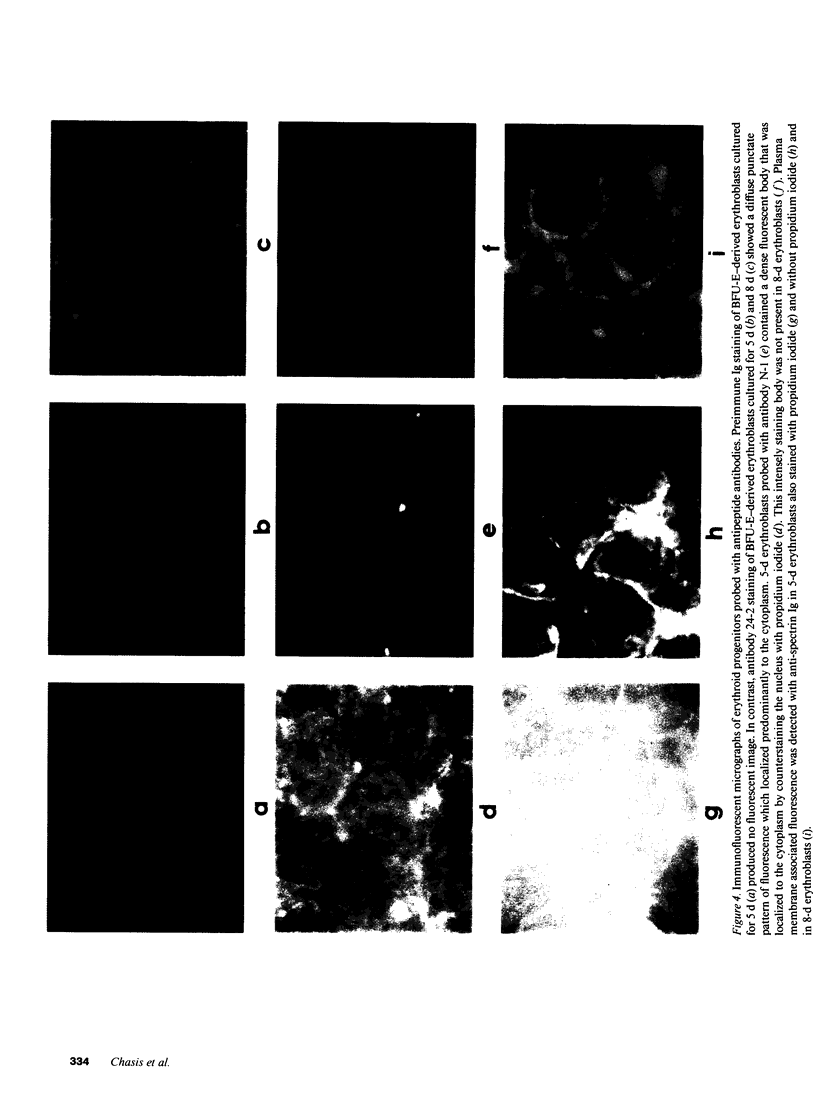

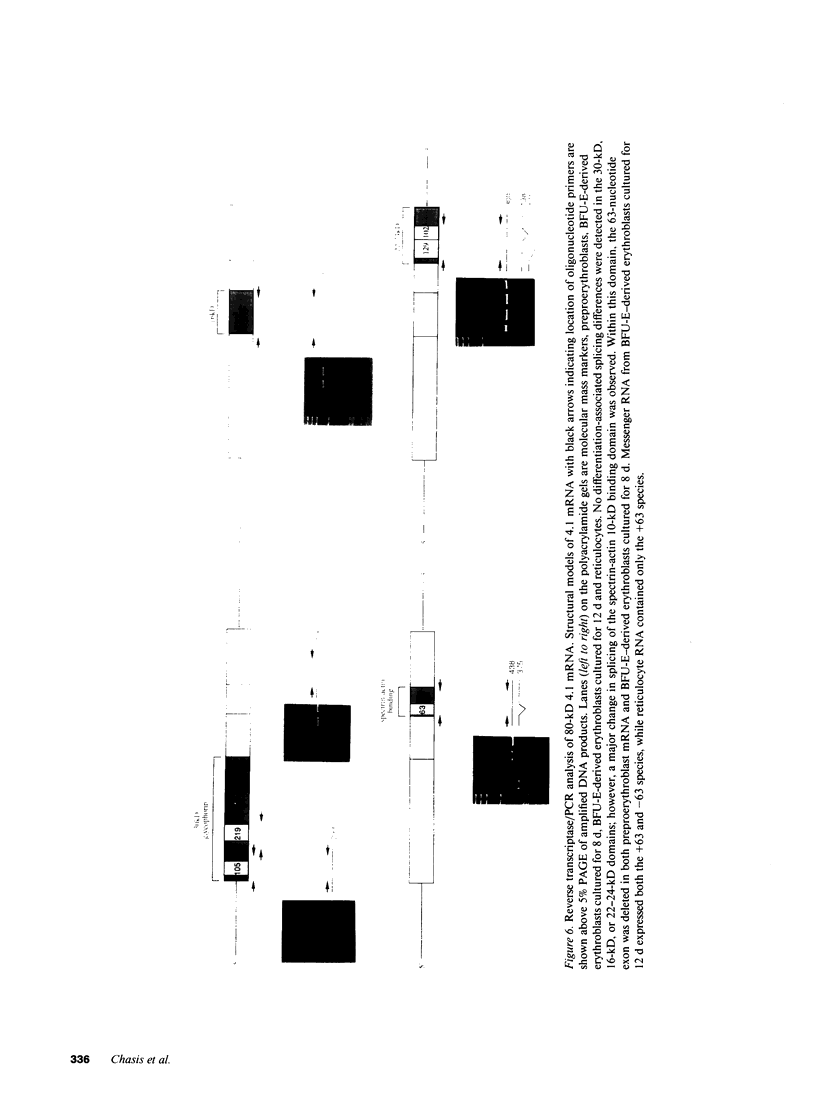
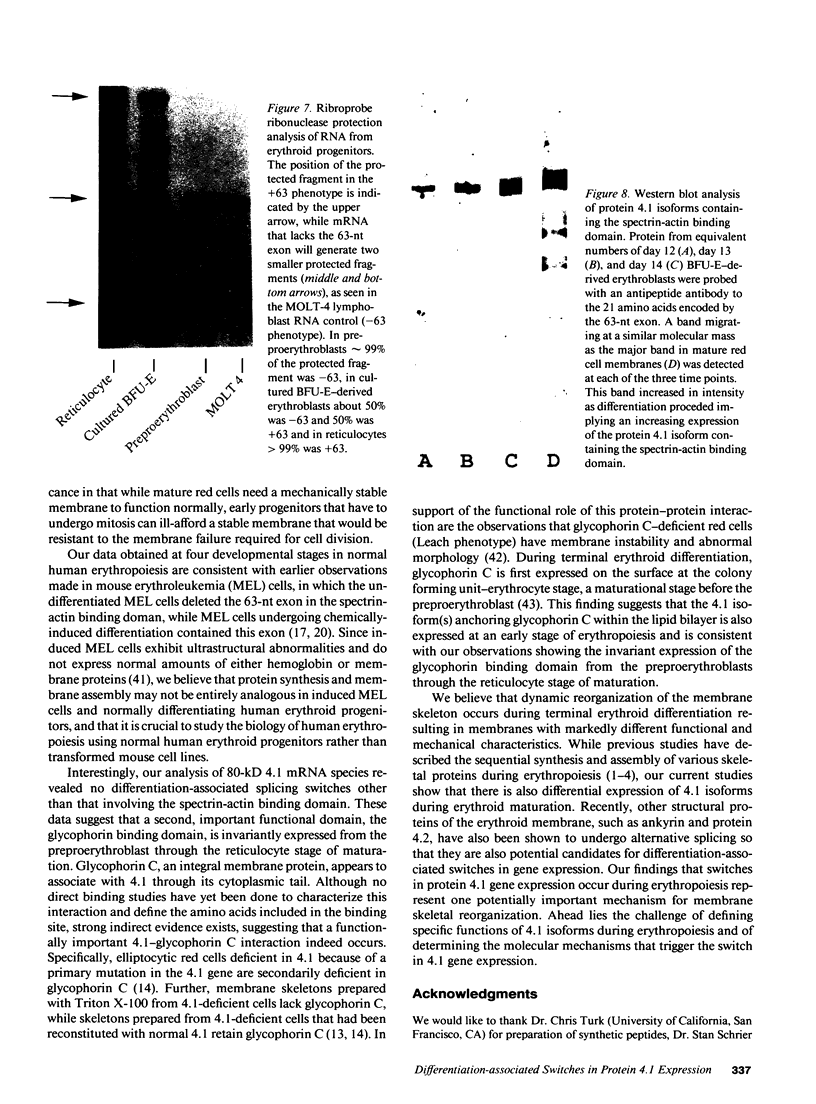

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chang H., Langer P. J., Lodish H. F. Asynchronous synthesis of erythrocyte membrane proteins. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3206–3210. doi: 10.1073/pnas.73.9.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N. Erythrocyte membrane deformability and stability: two distinct membrane properties that are independently regulated by skeletal protein associations. J Cell Biol. 1986 Aug;103(2):343–350. doi: 10.1083/jcb.103.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasis J. A., Prenant M., Leung A., Mohandas N. Membrane assembly and remodeling during reticulocyte maturation. Blood. 1989 Aug 15;74(3):1112–1120. [PubMed] [Google Scholar]
- Conboy J. G., Chan J. Y., Chasis J. A., Kan Y. W., Mohandas N. Tissue- and development-specific alternative RNA splicing regulates expression of multiple isoforms of erythroid membrane protein 4.1. J Biol Chem. 1991 May 5;266(13):8273–8280. [PubMed] [Google Scholar]
- Conboy J. G., Chan J., Mohandas N., Kan Y. W. Multiple protein 4.1 isoforms produced by alternative splicing in human erythroid cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9062–9065. doi: 10.1073/pnas.85.23.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy J. G., Chasis J. A., Winardi R., Tchernia G., Kan Y. W., Mohandas N. An isoform-specific mutation in the protein 4.1 gene results in hereditary elliptocytosis and complete deficiency of protein 4.1 in erythrocytes but not in nonerythroid cells. J Clin Invest. 1993 Jan;91(1):77–82. doi: 10.1172/JCI116203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy J., Marchesi S., Kim R., Agre P., Kan Y. W., Mohandas N. Molecular analysis of insertion/deletion mutations in protein 4.1 in elliptocytosis. II. Determination of molecular genetic origins of rearrangements. J Clin Invest. 1990 Aug;86(2):524–530. doi: 10.1172/JCI114739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correas I., Leto T. L., Speicher D. W., Marchesi V. T. Identification of the functional site of erythrocyte protein 4.1 involved in spectrin-actin associations. J Biol Chem. 1986 Mar 5;261(7):3310–3315. [PubMed] [Google Scholar]
- Cox J. V., Stack J. H., Lazarides E. Erythroid anion transporter assembly is mediated by a developmentally regulated recruitment onto a preassembled membrane cytoskeleton. J Cell Biol. 1987 Sep;105(3):1405–1416. doi: 10.1083/jcb.105.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Gordon S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med. 1989 Apr 1;169(4):1333–1346. doi: 10.1084/jem.169.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A., Hochmuth R. M. A solid-liquid composite model of the red cell membrane. J Membr Biol. 1977 Jan 28;30(4):351–362. doi: 10.1007/BF01869676. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Comoglio P. M., Tarone G. Fibronectin-plasma membrane interaction in the adhesion of hemopoietic cells. J Cell Biol. 1986 Aug;103(2):429–437. doi: 10.1083/jcb.103.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanspal M., Palek J. Synthesis and assembly of membrane skeletal proteins in mammalian red cell precursors. J Cell Biol. 1987 Sep;105(3):1417–1424. doi: 10.1083/jcb.105.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S. Q., Spencer T., Miller K., Hopkins C., Trowbridge I. S. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990 Feb;110(2):283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone R. M., Adam M., Hammond J. R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987 Jul 5;262(19):9412–9420. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laski F. A., Alzner-DeWeerd B., RajBhandary U. L., Sharp P. A. Expression of a X. laevis tRNATyr gene in mammalian cells. Nucleic Acids Res. 1982 Aug 11;10(15):4609–4626. doi: 10.1093/nar/10.15.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. From genes to structural morphogenesis: the genesis and epigenesis of a red blood cell. Cell. 1987 Nov 6;51(3):345–356. doi: 10.1016/0092-8674(87)90631-3. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Marchesi V. T. A structural model of human erythrocyte protein 4.1. J Biol Chem. 1984 Apr 10;259(7):4603–4608. [PubMed] [Google Scholar]
- Morris L., Crocker P. R., Gordon S. Murine fetal liver macrophages bind developing erythroblasts by a divalent cation-dependent hemagglutinin. J Cell Biol. 1988 Mar;106(3):649–656. doi: 10.1083/jcb.106.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B. T., Johnstone R. M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983 Jul;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- Pasternack G. R., Anderson R. A., Leto T. L., Marchesi V. T. Interactions between protein 4.1 and band 3. An alternative binding site for an element of the membrane skeleton. J Biol Chem. 1985 Mar 25;260(6):3676–3683. [PubMed] [Google Scholar]
- Patel V. P., Lodish H. F. A fibronectin matrix is required for differentiation of murine erythroleukemia cells into reticulocytes. J Cell Biol. 1987 Dec;105(6 Pt 2):3105–3118. doi: 10.1083/jcb.105.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. P., Lodish H. F. Loss of adhesion of murine erythroleukemia cells to fibronectin during erythroid differentiation. Science. 1984 Jun 1;224(4652):996–998. doi: 10.1126/science.6585955. [DOI] [PubMed] [Google Scholar]
- Patel V. P., Lodish H. F. The fibronectin receptor on mammalian erythroid precursor cells: characterization and developmental regulation. J Cell Biol. 1986 Feb;102(2):449–456. doi: 10.1083/jcb.102.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M. E., Chasis J. A., Mohandas N. Identification of a functional role for human erythrocyte sialoglycoproteins beta and gamma. Blood. 1987 Apr;69(4):1068–1072. [PubMed] [Google Scholar]
- Reid M. E., Takakuwa Y., Conboy J., Tchernia G., Mohandas N. Glycophorin C content of human erythrocyte membrane is regulated by protein 4.1. Blood. 1990 Jun 1;75(11):2229–2234. [PubMed] [Google Scholar]
- Rosemblatt M., Vuillet-Gaugler M. H., Leroy C., Coulombel L. Coexpression of two fibronectin receptors, VLA-4 and VLA-5, by immature human erythroblastic precursor cells. J Clin Invest. 1991 Jan;87(1):6–11. doi: 10.1172/JCI115002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberger S., Iacopetta B. J., Kühn L. C. Endocytosis of the transferrin receptor requires the cytoplasmic domain but not its phosphorylation site. Cell. 1987 May 8;49(3):423–431. doi: 10.1016/0092-8674(87)90295-9. [DOI] [PubMed] [Google Scholar]
- Tang T. K., Leto T. L., Correas I., Alonso M. A., Marchesi V. T., Benz E. J., Jr Selective expression of an erythroid-specific isoform of protein 4.1. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3713–3717. doi: 10.1073/pnas.85.11.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. K., Qin Z., Leto T., Marchesi V. T., Benz E. J., Jr Heterogeneity of mRNA and protein products arising from the protein 4.1 gene in erythroid and nonerythroid tissues. J Cell Biol. 1990 Mar;110(3):617–624. doi: 10.1083/jcb.110.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple G. F., Chang J. C., Kan Y. W. Authentic beta-globin mRNA sequences in homozygous betaO-thalassemia. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3047–3051. doi: 10.1073/pnas.74.7.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeval J. L., Le Van Kim C., Bettaieb A., Debili N., Colin Y., el Maliki B., Blanchard D., Vainchenker W., Cartron J. P. Early expression of glycophorin C during normal and leukemic human erythroid differentiation. Cancer Res. 1989 May 15;49(10):2626–2632. [PubMed] [Google Scholar]
- Vuillet-Gaugler M. H., Breton-Gorius J., Vainchenker W., Guichard J., Leroy C., Tchernia G., Coulombel L. Loss of attachment to fibronectin with terminal human erythroid differentiation. Blood. 1990 Feb 15;75(4):865–873. [PubMed] [Google Scholar]
- Waugh R. E. Temperature dependence of the yield shear resultant and the plastic viscosity coefficient of erythrocyte membrane. Implications about molecular events during membrane failure. Biophys J. 1982 Sep;39(3):273–278. doi: 10.1016/S0006-3495(82)84517-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe L. C., John K. M., Falcone J. C., Byrne A. M., Lux S. E. A genetic defect in the binding of protein 4.1 to spectrin in a kindred with hereditary spherocytosis. N Engl J Med. 1982 Nov 25;307(22):1367–1374. doi: 10.1056/NEJM198211253072203. [DOI] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]










