Abstract
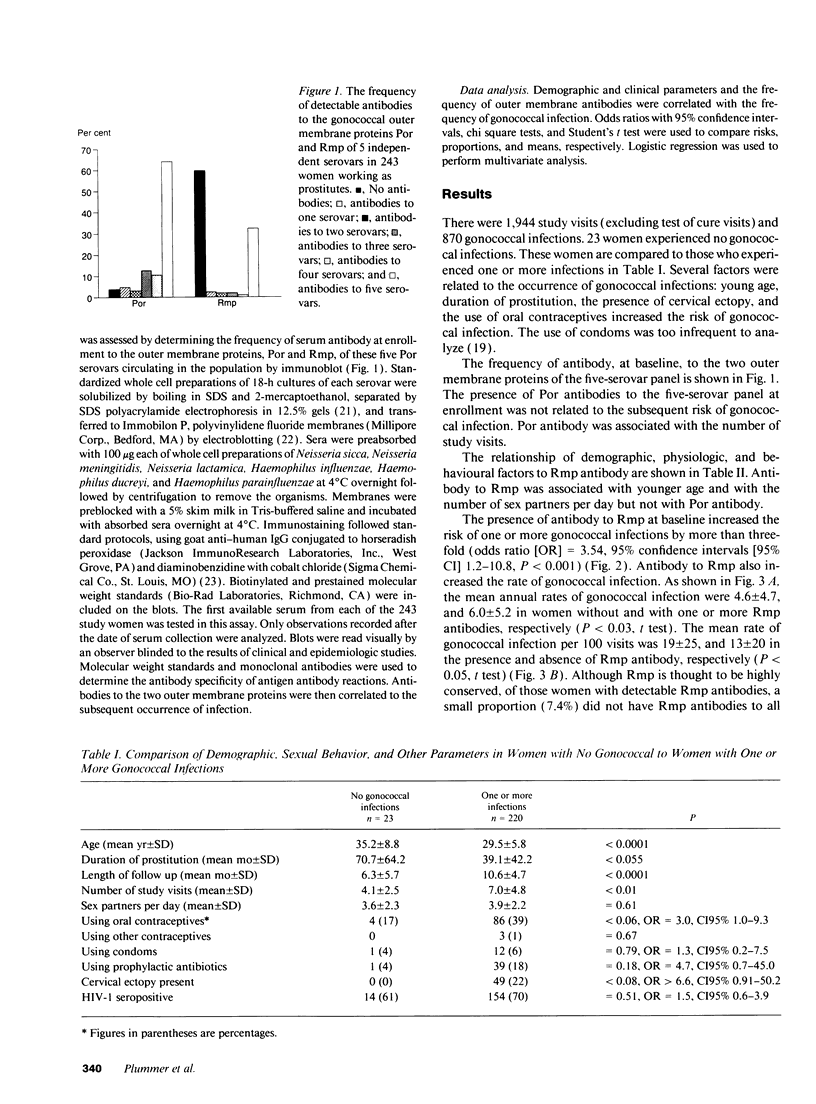
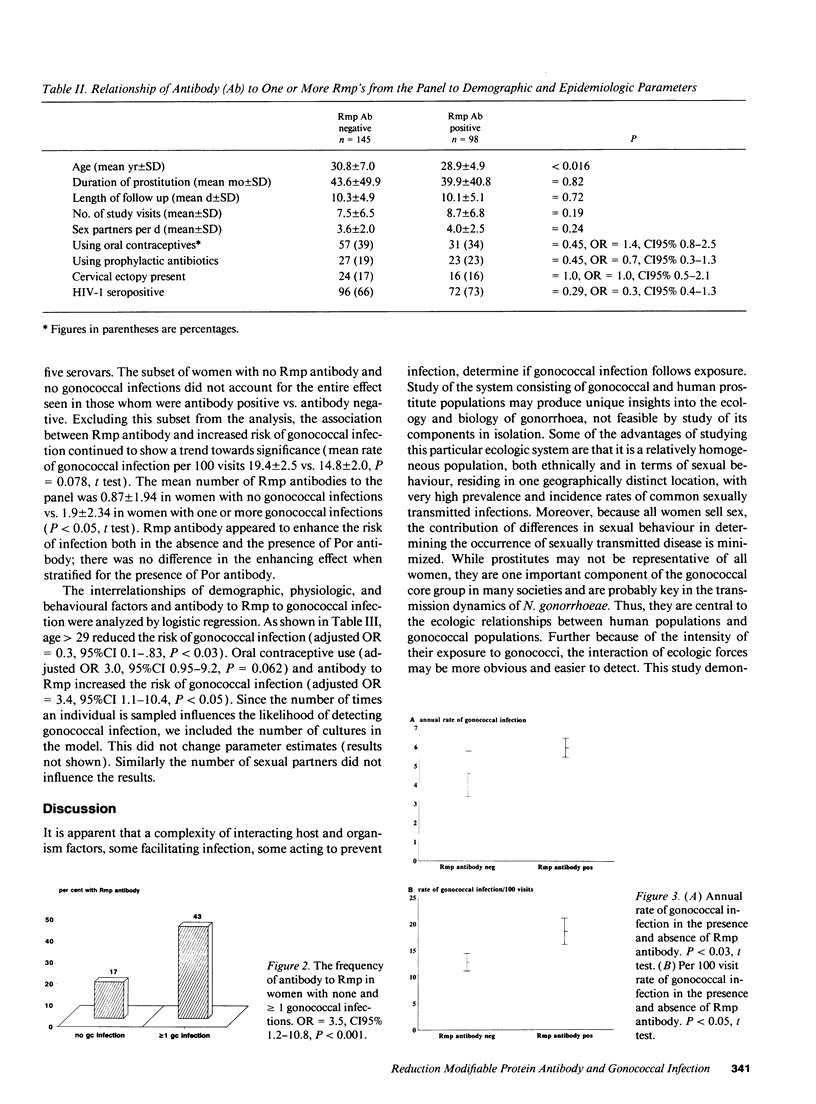
The severe adverse effects of gonococcal infection on human fertility suggests that Neisseria gonorrhoeae would exert powerful selection for the development of a protective immune response in humans. N. gonorrhoeae is an obligate human pathogen and must persist in humans to survive. Since it is an ecologically successful organism, it must have evolved strategies to evade any human immune response it elicits. In a longitudinal study among 243 women working as prostitutes and experiencing frequent gonococcal infection, younger women, women with HIV infection, and women with antibody to the gonococcal outer membrane protein 3 (Rmp) were at increased risk of infection (adjusted odds ratio 3.4, CI95% 1.1-10.4, P < 0.05). Rmp is highly conserved in N. gonorrhoeae and the blocking of mucosal defences may be one of its functions. As similar proteins occur in many gram negative mucosal pathogens, the enhancing effect of such proteins may be a general strategy whereby bacteria evade human immune responses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansar Ahmed S., Penhale W. J., Talal N. Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am J Pathol. 1985 Dec;121(3):531–551. [PMC free article] [PubMed] [Google Scholar]
- Barnes R. C., Holmes K. K. Epidemiology of gonorrhea: current perspectives. Epidemiol Rev. 1984;6:1–30. doi: 10.1093/oxfordjournals.epirev.a036267. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Wetzler L. M., Gotschlich E. C., Rice P. A. Protein III: structure, function, and genetics. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S60–S63. doi: 10.1128/cmr.2.suppl.s60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Hildebrandt J. F. Antigen-specific serotyping of Neisseria gonorrhoeae: characterization based upon principal outer membrane protein. Infect Immun. 1981 Jun;32(3):985–994. doi: 10.1128/iai.32.3.985-994.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. H., Rest R. F. Gonococci possessing only certain P.II outer membrane proteins interact with human neutrophils. Infect Immun. 1988 Jun;56(6):1574–1579. doi: 10.1128/iai.56.6.1574-1579.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldacre M. J., Loudon N., Watt B., Grant G., Loudon J. D., McPherson K., Vessey M. P. Epidemiology and clinical significance of cervical erosion in women attending a family planning clinic. Br Med J. 1978 Mar 25;1(6115):748–750. doi: 10.1136/bmj.1.6115.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C. Development of a gonorrhoea vaccine: prospects, strategies and tactics. Bull World Health Organ. 1984;62(5):671–680. [PMC free article] [PubMed] [Google Scholar]
- Gotschlich E. C., Seiff M., Blake M. S. The DNA sequence of the structural gene of gonococcal protein III and the flanking region containing a repetitive sequence. Homology of protein III with enterobacterial OmpA proteins. J Exp Med. 1987 Feb 1;165(2):471–482. doi: 10.1084/jem.165.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman C. J. Regulation of the immune system by sex steroids. Endocr Rev. 1984 Summer;5(3):435–455. doi: 10.1210/edrv-5-3-435. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Scales R., Warren K. A., Frank M. M., Rice P. A. Mechanism of action of blocking immunoglobulin G for Neisseria gonorrhoeae. J Clin Invest. 1985 Nov;76(5):1765–1772. doi: 10.1172/JCI112167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd R. C. 125I-peptide mapping of protein III isolated from four strains of Neisseria gonorrhoeae. Infect Immun. 1982 Aug;37(2):622–631. doi: 10.1128/iai.37.2.622-631.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Tam M. R., Nowinski R. C., Holmes K. K., Sandström E. G. Serological classification of Neisseria gonorrhoeae with use of monoclonal antibodies to gonococcal outer membrane protein I. J Infect Dis. 1984 Jul;150(1):44–48. doi: 10.1093/infdis/150.1.44. [DOI] [PubMed] [Google Scholar]
- Kreiss J. K., Koech D., Plummer F. A., Holmes K. K., Lightfoote M., Piot P., Ronald A. R., Ndinya-Achola J. O., D'Costa L. J., Roberts P. AIDS virus infection in Nairobi prostitutes. Spread of the epidemic to East Africa. N Engl J Med. 1986 Feb 13;314(7):414–418. doi: 10.1056/NEJM198602133140704. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Louv W. C., Austin H., Perlman J., Alexander W. J. Oral contraceptive use and the risk of chlamydial and gonococcal infections. Am J Obstet Gynecol. 1989 Feb;160(2):396–402. doi: 10.1016/0002-9378(89)90456-0. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Meyer T. F., van Putten J. P. Genetic mechanisms and biological implications of phase variation in pathogenic neisseriae. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S139–S145. doi: 10.1128/cmr.2.suppl.s139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngugi E. N., Plummer F. A., Simonsen J. N., Cameron D. W., Bosire M., Waiyaki P., Ronald A. R., Ndinya-Achola J. O. Prevention of transmission of human immunodeficiency virus in Africa: effectiveness of condom promotion and health education among prostitutes. Lancet. 1988 Oct 15;2(8616):887–890. doi: 10.1016/s0140-6736(88)92480-4. [DOI] [PubMed] [Google Scholar]
- Plummer F. A., Brunham R. C. Gonococcal recidivism, diversity, and ecology. Rev Infect Dis. 1987 Jul-Aug;9(4):846–850. doi: 10.1093/clinids/9.4.846. [DOI] [PubMed] [Google Scholar]
- Plummer F. A., Simonsen J. N., Chubb H., Slaney L., Kimata J., Bosire M., Ndinya-Achola J. O., Ngugi E. N. Epidemiologic evidence for the development of serovar-specific immunity after gonococcal infection. J Clin Invest. 1989 May;83(5):1472–1476. doi: 10.1172/JCI114040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P. A., Vayo H. E., Tam M. R., Blake M. S. Immunoglobulin G antibodies directed against protein III block killing of serum-resistant Neisseria gonorrhoeae by immune serum. J Exp Med. 1986 Nov 1;164(5):1735–1748. doi: 10.1084/jem.164.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A., Brown M., Nickel P., Meyer T. F. Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell. 1986 Oct 10;47(1):61–71. doi: 10.1016/0092-8674(86)90366-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]


